Abstract
In this study, we assessed the ability of miR-26b-5p to regulate T cell factor 4 (TCF-4) expression and thereby control human adipose-derived mesenchymal stem cell (hADMSC) adipogenic differentiation. Adipogenic medium was used to induce hADMSC differentiation over a 6-d period. The ability of miR-26b-5p to interact with the TCF-4 mRNA was confirmed through both predictive bioinformatics analyses and luciferase reporter assays. Immunofluorescent staining was used to visualize the impact of miR-26b-5p inhibition or overexpression on TCF-4 and β-catenin levels in hADMSCs. Further functional analyses were conducted by transfecting these cells with siRNAs specific for TCF-4 and β-catenin. Adipogenic marker and Wnt/β-catenin pathway gene expression levels were assessed via real-time polymerase chain reaction and western blotting. β-catenin localization was assessed via immunofluorescent staining. As expected, our adipogenic media induced the adipocytic differentiation of hADMSCs. In addition, we confirmed that TCF-4 is an miR-26b-5p target gene in these cells, and that protein levels of both TCF-4 and β-catenin were reduced when these cells were transfected with miR-26b-5p mimics. Overexpression of this microRNA also enhanced hADMSC adipogenesis, whereas TCF-4 and β-catenin overexpression inhibited this process. The enhanced hADMSC adipogenic differentiation that was observed following TCF-4 or β-catenin knockdown was partially reversed when miR-26b-5p expression was inhibited. We found that miR-26b-5p serves as a direct negative regulator of TCF-4 expression within hADMSCs, leading to inactivation of the Wnt/β-catenin pathway and thereby promoting the adipogenic differentiation of these cells in vitro.
Keywords: hADMSCs, adipogenic differentiation, Wnt/β-catenin pathway, miRNA-26b-5p, TCF-4
Introduction
Facial soft tissue defects in oral and maxillofacial regions can arise as a consequence of tumors or other trauma and can be challenging to reconstruct. The most common maxillofacial reconstruction techniques involve flap transplantation, adipose tissue transplantation, or the use of artificial materials1,2. There are certain limitations to these approaches, however, as flap transplantation can induce local tissue damage3, while artificial implant materials are often rejected4, and transplanted adipose tissues can be rapidly absorbed such that patients may require multiple rounds of surgery5. Therefore, reducing fat tissue absorption has become an important research focus.
The use of adipose tissue transplantation as a means of repairing soft tissue defects is a relatively recent approach6, with many studies to date having sought means of reducing associated absorption of transplanted tissue7. Adipose stem cells are well known to be multipotent and can differentiate into a range of cell types including adipocytes, osteoblasts, chondrocytes, and myocytes, such that influencing their differentiation may be a key strategy for reducing rates of tissue absorption8. Adipose-derived mesenchymal stem cells (ADMSCs) are promising therapeutic tools as they can be readily acquired, proliferate rapidly, are multipotent, and exhibit immunomodulatory properties owing to their ability to secrete specific cytokines and chemokines capable of attracting specific cell types9. Further study of methods capable of promoting the adipogenic differentiation of ADMSCs in transplanted adipose tissue is thus required.
Many researchers have sought to understand how to influence the adipogenic differentiation of ADMSCs while minimizing fat absorption. The Wnt/β-catenin signaling pathway is known to be a key regulator of this differentiation process, and Wnt3a suppresses the expression of peroxisome proliferator-activated receptor γ (PPARγ) in order to block the adipocytic differentiation of murine 3T3-L1 cells10. Murine fibroblasts that are deficient in LPR6, in contrast, undergo spontaneous adipocytic differentiation10. Wnt/β-catenin signaling thus appears to suppress adipogenic differentiation. The canonical Wnt/β-catenin pathway represses the expression of PPARγ at the mRNA level, whereas the noncanonical Wnt pathway activates histone methyltransferases that inhibit PPARγ transactivation via the histone H3 lysine 9 methylation of its target genes11.
A number of different microRNAs (miRNAs) have been shown to influence ADMSC adipogenesis, such as hsa-miR-15a-5p, hsa-miR-27a-3p, hsa-miR-106b-5p, miR-17-5p, and miR-17, all of which have been found to modulate specific signaling pathways in a pro-adipogenic manner, including the Wnt/β-catenin signaling pathway12–14. While multiple miRNAs capable of controlling this differentiation process by altering the expression of components of the Wnt/β-catenin signaling pathway have been identified, further research is still needed in order to firmly identify all of the relevant miRNAs and target genes that can influence this therapeutically important process.
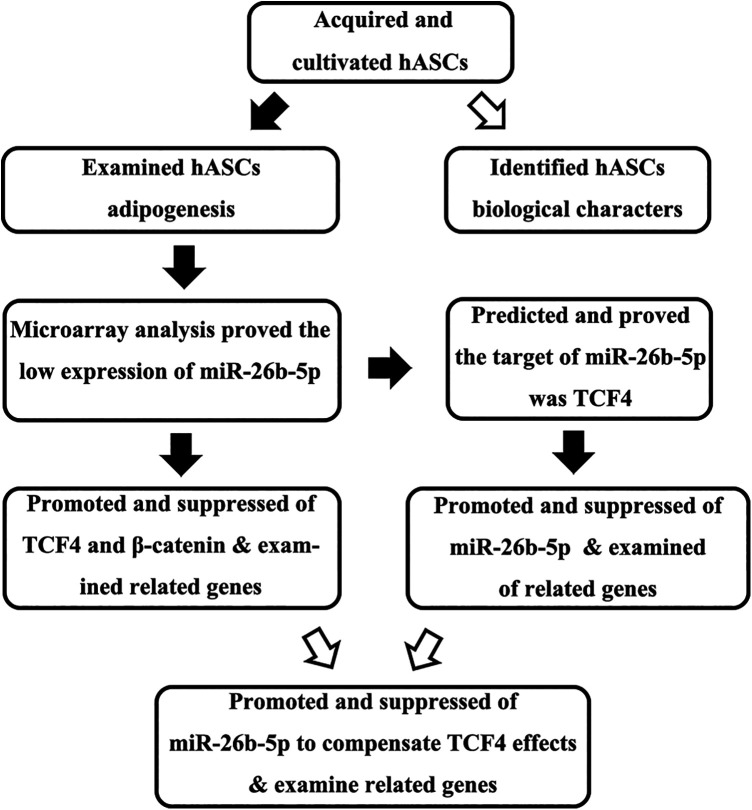
A previous microarray-based study has demonstrated that human adipose-derived mesenchymal stem cell (hADMSCs) upregulate hsa-miR-26b-5p during adipogenic differentiation13, with predictive bioinformatics analyses suggesting that the most likely target of this miRNA was the Wnt/β-catenin pathway component T cell factor 4 (TCF-4, encoded by TCF7L2). We, therefore, hypothesized that the downregulation of has-miR-26b-5p would lead to activation of the Wnt/β-catenin pathway, thereby inhibiting hADMSC adipogenesis. In the present study, we employed a number of appropriate methods in order to test this hypothesis (Fig. 1).
Figure 1.
Study overview.
Materials and Methods
Collection, Characterization, and Differentiation of hADMSCs
For details regarding how hADMSCs were collected and for information regarding their characterization, see the Supplemental Materials. In order to induce adipogenic differentiation, we plated hADMSCs in six-well plates (9,000 cells/cm2) and allowed the cells to rest for 48 h. Adipogenic media (10% fetal bovine serum [FBS], 1 μM dexamethasone, 200 μM indomethacin, 10 mg/l insulin, and 0.5 mM 3-isobutyl-1-methylxanthine in α-MEM) was then added to each appropriate well, with media being exchanged every other day. Control cells were cultured in α-MEM (Gibco, New York, USA) containing 10% FBS (Gibco, New York, USA). All cells were cultured for 4 d at 37°C in a 5% CO2 incubator, after which cells were isolated for downstream experiments.
Bioinformatics Analysis
Complementarity between miR-26b-5p and putative targets, including TCF7L2, was assessed using TargetScan (http://www.targetscan.org), miRWalk (http://mirwalk.umm.uni-heidelberg.de), and miRBase (http://www.mirbase.org).
Transfection
In order to modulate gene expression, we prepared plasmids to facilitate TCF7L2 overexpression (EX-TCF7L2) or TCF7L2 siRNA-mediated knockdown (siTCF7L2), with appropriate control plasmids also being purchased (EX-Ctrl and siR-Ctrl). We additionally purchased miR-26b-5p mimics, inhibitors, and appropriate control constructs. For luciferase reporter assay experiments, wild type (WT) and mutated (Mut) versions of the TCF7L2 3′-untranslated region (UTR) were also created. These constructs were transfected into appropriate hADMSCs (from the fourth passage) using Lipofectamine 2000 (Invitrogen, USA) based on provided directions. All miRNA and siRNA constructs were synthesized by GenePharma Corporation (Shanghai, China), while all other constructs were purchased from GeneCopoeia Corporation (Guangzhou, China). For details regarding the sequences of these constructs, see Table 1.
Table 1.
The Constructed Sequences Used in This Study.
| Genes | Sequence | |
|---|---|---|
| miR-26b-5p mimic | 5′-UUCAAGUAAUUCAGGAUAGGU dTdT-3′ | |
| miR-Ctrl mimic | 5′-UUGUACUACACAAAAGUACUG dTdT-3′ | |
| miR-26b-5p inhibitor | 5′-ACCUAUCCUGAAUUACUUGAA dTdT-3′ | |
| miR-Ctrl inhibitor | 5′-CAGUACUUUUGUGUAGUACAA dTdT-3′ | |
| siTCF7L2 | Sense | 5′-GGGACAUGCAUGGAAUCAUTT dTdT-3′ |
| Antisense | 5′-AUGAUUCCAUGCAUGUCCCTT dTdT-3′ | |
| siR-Ctrl | Sense | 5′-UUCUCCGAACGUGUCACGUdT dT-3′ |
| Antisense | 5′-ACGUGACACGUUCGGAGAA dTdT-3′ | |
Luciferase Reporter Assay
The WT and MUT versions of the TCF7L2 3′-UTR were inserted into the psiCHECK-2 vector (Promega, WI, USA) using the Not I and Xho I cleavage sites. HEK293 T cells were then grown in 96-well plates to 70% confluence, after which they were co-transfected with the WT or MUT reporter construct together with miR-26b-5p inhibitors, mimics, or appropriate controls. Cells were incubated for 48 h, after which a Dual-Luciferase Reporter Gene Assay Kit (Beyotime, Shanghai, China) was used based on provided directions, with Renilla luciferase activity being used for normalization purposes.
Immunofluorescent Staining
After being transfected with miR-26b-5p mimic or inhibitors as above, hADMSCs were seeded onto coverslips in six-well plates and incubated for 48 h. These cells were then fixed for 15 min using 4% paraformaldehyde, after which they were washed three times in PBS and permeabilized for 20 min using 0.5% Triton X-100 (Sigma-Aldrich, USA). Cells were then washed again, after which they were blocked for 2 h using goat serum. Cells were next probed overnight with primary anti-TCF-4 (1:1,000, MAB3016, R&D Systems, USA) at 4°C. Cells were then washed and stained with a Cy3-conjugated secondary antibody (1:50, Proteintech, USA) for 1 h at 37°C. Nuclei were then counterstained using 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich), and cells were visualized via fluorescence microscopy (ZEISS, Oberkochen, Germany).
Quantitative polymerase chain reaction RNA was extracted from hADMSCs using Trizol, after which a NanoVue™ Plus spectrophotometer (GE Healthcare Life Sciences, USA) was used to evaluate RNA quality and quantity. The cDNA EcoDry Premix solution (TaKaRa, Japan) was used to prepare cDNA from these RNA samples based upon provided directions, after which an SYBR Premix ExTaq kit (TaKaRa) and an ABI 7300 Real-Time PCR System (Applied Biosystems, UK) were used for quantitative polymerase chain reaction (qPCR) analyses. The comparative delta-delta Ct approach was used to assess relative gene expression, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a normalization control. All primer sequences are shown in Table 2. Each sample was tested three times independently, and the average value was taken as the final result.
Table 2.
The Sequence of Primers Used for Quantitative Polymerase Chain Reaction in This Study.
| Gene | 5′–3′ | Tm (°C) |
|---|---|---|
| C/EBPα | F: CTGATTCTTGCCAAACTGAG | 60 |
| R: GAGGAAGCTAAGACCCACTAC | 60 | |
| PPARγ | F: CTTGACAGGAAAGACAACGG | 60 |
| R: GCTTCTACGGATCGAAACTG | 60 | |
| aP2 | F: AAATCACCGCAGACGACAGG | 60 |
| R: GGCTCATGCCCTTTCATAAAC | 60 | |
| TCF7L2 | F: CGGCGGTGGAGGGGATGAC | 60 |
| R: GGCCGCTTCTTCCAAACTTTCC | 60 | |
| β-catenin | F: AAAATGGCAGTGCGTTTAG | 60 |
| R: TTTGAAGGCAGTCTGTCGTA | 60 | |
| GAPDH | F: GAACGGGAAGCTCACTGG | 60 |
| R: GCCTGCTTCACCACCTTCT | 60 |
GAPDH: glyceraldehyde 3-phosphate dehydrogenase; PPARγ: peroxisome proliferator-activated receptor γ.
Western Blotting
A lysis buffer (Beyotime) was used to extract protein from hADMSCs on ice, after which a BCA Protein Assay Kit (Beyotime) was used to quantify protein levels in each sample. Proteins were next denatured for 5 min at 100°C in 5× SDS loading buffer (Beyotime), after which they were separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (GE Healthcare Life Sciences). These blots were blocked for 2 h with 5% nonfat milk in Tris-buffered saline (TBS) at 37°C, and they were then probed overnight at 4°C with the following primary antibodies (all used at 1:1,000): anti-C/EBPα (ab220813, Abcam, USA), anti-PPARγ (ab45036, Abcam), anti-aP2 (ab92501, Abcam), anti-TCF-4 (MAB3016, R&D Systems, USA), anti-β-catenin (ab32572, Abcam), and anti-GAPDH (#5174, Cell Signaling Technology, USA). Blots were then washed thrice with TBS with 0.1% Tween 20 and probed with appropriate secondary antibodies for 1 h at room temperature. An ECL solution (Thermo Fisher Scientific, Germany) was then used for protein detection, with Quantity One (Bio-Rad, CA, USA) being used for densitometric analysis and with GAPDH as a normalization control. Each sample was tested three times independently, and the average value was taken as the final result.
Statistical Analysis
Data were compared via one- and two-way analyses of variance as appropriate using SPSS v20.0 (IBM SPSS Statistics, NY, USA). P < 0.05 was the significance threshold. The Student–Newman–Keuls method was used for post hoc test.
Results
Induction of hADMSC Adipogenesis
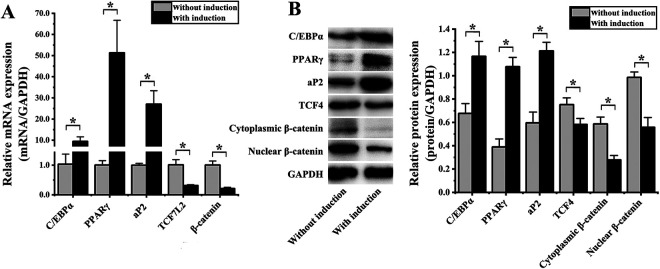
Following a 4-d culture in adipogenic media, we harvested hADMSCs and evaluated the expression of genes associated with adipogenesis and Wnt/β-catenin signaling via qPCR and western blotting. Relative to control hADMSCs grown in non-adipogenic media, cells cultured in adipogenic media exhibited significant mRNA-level increases in the expression of the adipogenesis-related genes C/EBPα (9.13 ± 3.96-fold; P = 0.003), PPARγ (51.01 ± 16.96-fold; P = 0.005), and aP2 (27.03 ± 8.24-fold; P = 0.019). These cells also exhibited significant reductions in the expression of the Wnt/β-catenin pathway genes TCF7L2 (3.22 ± 0.08-fold; P = 0.003) and β-catenin (4.78 ± 0.07-fold; P = 0.001) (Fig. 2A). This was also true at the protein level, with increased levels of C/EBPα (1.72 ± 0.17-fold; P = 0.005), PPARγ (2.76 ± 0.26-fold; P < 0.001), and aP2 (2.03 ± 0.23-fold; P = 0.001), in cells treated with adipogenic media, whereas decreased levels of TCF-4 (1.29 ± 0.07-fold; P = 0.019), cytoplasmic β-catenin (2.11 ± 0.31-fold; P = 0.002), and nuclear β-catenin (1.77 ± 0.21-fold; P = 0.001) (Fig. 2B).
Figure 2.
Assessment of hADMSC adipogenic differentiation (A) quantitative polymerase chain reaction confirmed that the expression of C/EBPα, PPARγ, and aP2 was significantly increased in hADMSCs following 4 d of adipogenic differentiation, whereas the expression of TCF7L2 and β-catenin was significantly reduced. (B) Western blotting confirmed that the protein level expression of C/EBPα, PPARγ, and aP2 was significantly increased in hADMSCs following adipogenic differentiation, whereas TCF-4, cytoplasmic β-catenin, and nuclear β-catenin levels were decreased at this same time point. *P < 0.05. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; PPARγ: peroxisome proliferator-activated receptor γ; TCF-4: T cell factor 4.
TCF7L2 Is an miR-26b-5p Target Gene
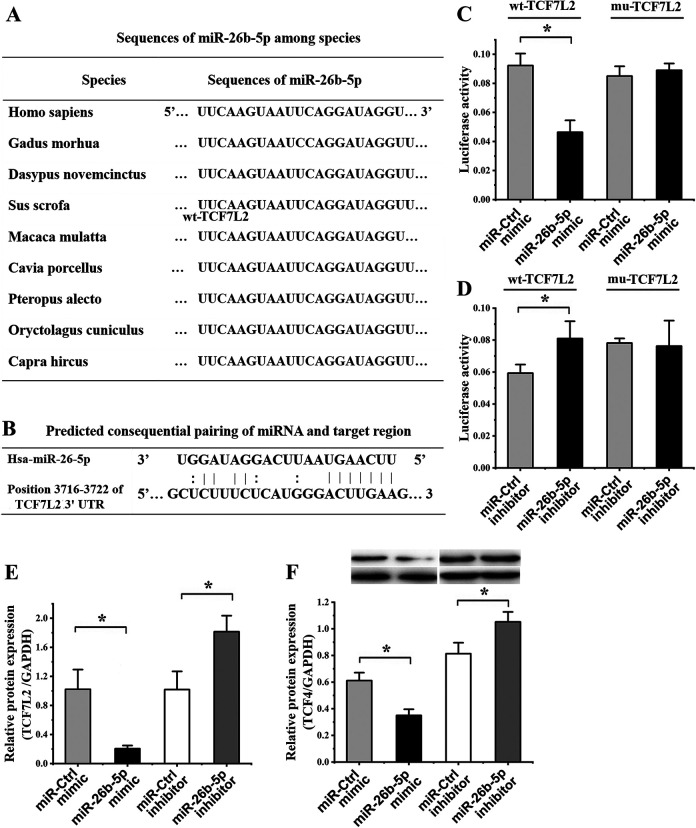
We found that the miR-26b-5p sequence was highly conserved across species (Fig. 3A) and was complementary to the TCF7L2 3′-UTR (Fig. 3B). When miR-26b-5p was overexpressed, this markedly reduced the activity of a luciferase reporter containing the WT TCF7L2 3′-UTR, whereas it had no impact on a reporter in which this putative binding site was mutated (Fig. 3C). Reduced miR-26b-5p expression, in contrast, was associated with significantly increased WT reporter activity, while MUT reporter activity was again unaffected (Fig. 3D).
Figure 3.
TCF7L2 is an miR-26b-5p target gene. (A) Aligned miR-26b-5p sequences from different species. (B) Sequence complementarity between miR-26b-5p and the TCF7L2 3′-UTR. (C) A luciferase reporter assay was used to confirm interactions between miR-26b-5p and TCF7L2, revealing that miR-26b-5p mimic transfection significantly reduced the activity of the WT but not the MUT reporter construct relative to cells transfected with a control. (D) A second luciferase reporter assay revealed that miR-26b-5p inhibitor transfection was associated with a significant increase in the activity of the WT but not the MUT TCF7L2 3′-UTR reporter construct. (E) The expression of TCF7L2 was assessed by quantitative polymerase chain reaction in hADMSCs following miR-26b-5p mimic or inhibitor transfection, resulting in significant decreases and increases in this expression, respectively, relative to cells transfected with control constructs. (F) TCF-4 protein levels were assessed by western blotting in hADMSCs transfected with miR-26b-5p mimics and inhibitors, revealing significant decreases and increases, respectively, in these protein levels relative to cells transfected with control constructs. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; TCF-4: T cell factor 4; UTR: untranslated region; WT: wild type.
When hADMSCs were transfected with an miR-26b-5p mimic, we found that this was associated with a 5.00 ± 0.64-fold reduction in TCLF7L2 expression at the mRNA level relative to cells transfected with a control miRNA (P = 0.007), whereas this expression was increased 1.78 ± 0.57-fold (P = 0.014) when cells were transfected with an miR-26b-5p inhibitor (Fig. 3E). Comparable trends were observed at the protein level, with miR-26b-5p mimic transfection resulting in a 1.75 ± 0.10-fold (P = 0.004) decrease in TCF-4 protein levels, whereas miR-26b-5p inhibitor transfection was associated with 1.29 ± 0.08-fold increase in these levels (P = 0.020) relative to cells transfected with appropriate control constructs (Fig. 3F). These findings thus confirmed that TCF7L2 is an miR-26b-5p target gene.
miR-26b-5p Regulates Intracellular TCF-4 Levels in hADMSCs
Relative to control hADMSCs, we found that cells transfected with an miR-26b-5p mimic exhibited significantly reduced intracellular TCF-4 staining upon immunofluorescent analysis (Fig. 4A). In contrast, this staining intensity was significantly increased in cells transfected with an miR-26b-5p inhibitor (Fig. 4B).
Figure 4.
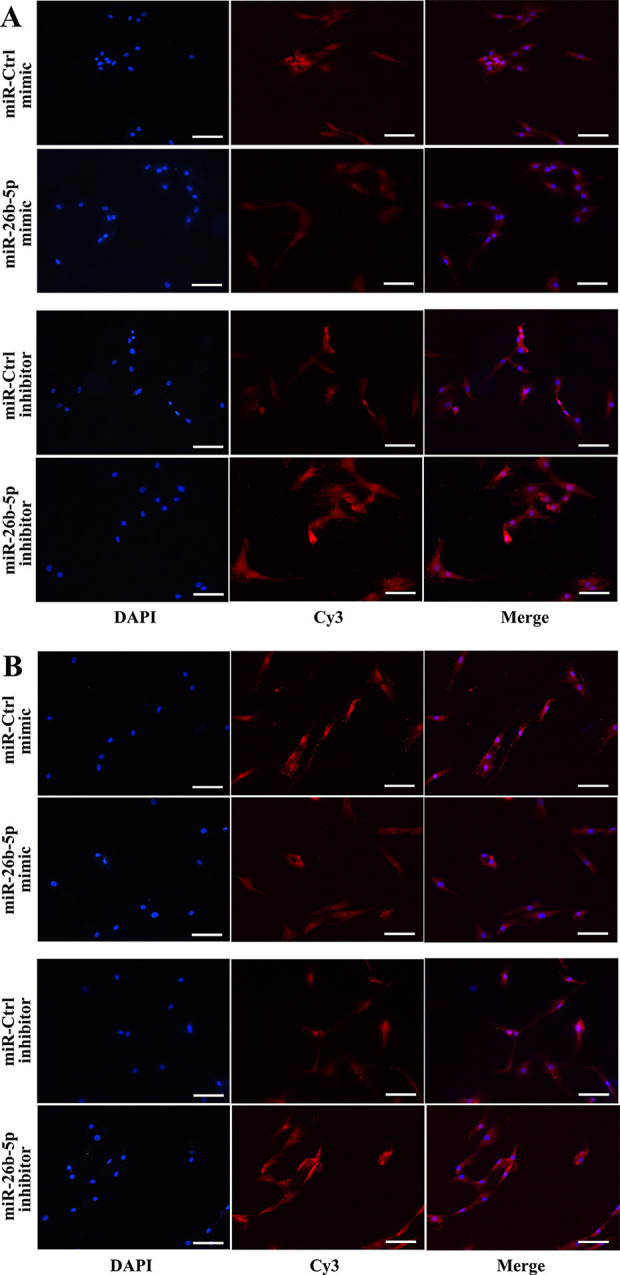
miR-26b-5p reduces intracellular TCF-4 protein levels in hADMSCs. (A) Intracellular TCF-4 levels, as assessed by immunofluorescent microscopy, were reduced in hADMSCs following miR-26b-5p mimic transfection. (B) Intracellular TCF-4 levels were increased in hADMSCs following miR-26b-5p inhibitor transfection (scale bar = 100 μm). hADMSC: human adipose-derived mesenchymal stem cell; TCF-4: T cell factor 4.
TCF-4 Modulates hADMSC Adipogenic Differentiation
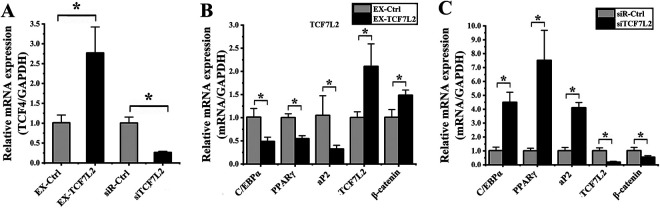
We next conducted gain- and loss-of-function experiments wherein hADMSCs were transfected with constructs to mediate TCF7L2 overexpression (EX-TCF7L2) or knockdown (siTCF7L2). Relative to cells transfected with appropriate control constructs, hADMSCs transfected with EX-TCF7L2 exhibited a 2.74 ± 1.09-fold increase in the expression of this gene (P = 0.011; Fig. 5A), whereas cells transfected with siTCF7L2 exhibited a 3.81 ± 0.54-fold decrease in TCF7L2 expression (P = 0.001; Fig. 5A).
Figure 5.
TCF-4 influences hADMSC adipogenic differentiation. (A) The expression of TCF7L2 was assessed by qPCR in cells transfected with the EX-TCF7L2 or siTCF7L2 vectors, resulting in significant increases and decreases in the expression of this gene, respectively, relative to cells transfected with control constructs. (B) Cells overexpressing TCF7L2 were evaluated via qPCR, revealing significant decreases in the expression of C/EBP, PPARγ, and aP2, and significant increases in the expression of TCF7L2 and β-catenin relative to cells transfected with the control construct. (C) Cells in which TCF7L2 had been knocked down were evaluated via qPCR, revealing significant increases in the expression of C/EBP, PPARγ, and aP2, and significant decreases in the expression of TCF7L2 and β-catenin relative to cells transfected with the control siRNA construct. *P < 0.05. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; PPARγ: peroxisome proliferator-activated receptor γ; qPCR: quantitative polymerase chain reaction; TCF-4: T cell factor 4.
We then evaluated the effect of altered TCF7L2 expression on hADMSC adipogenesis. On day 4 of this differentiation process, we found that cells overexpressing TCF7L2 exhibited significant reductions in the expression of C/EBPα (2.07 ± 0.35-fold; P = 0.012), PPARγ (1.83 ± 0.38-fold; P = 0.002), and aP2 (3.23 ± 1.38-fold; P = 0.043), whereas increased expression of TCF7L2 (2.10 ± 0.68-fold; P = 0.019) and β-catenin (1.47 ± 0.22-fold; P = 0.015) was detected relative to appropriate control cells (Fig. 5B). When TCF7L2 was instead knocked down in these cells, we detected significantly increased expression of C/EBPα (4.40 ± 0.66-fold; P = 0.043), PPARγ (7.03 ± 3.25-fold; P = 0.034), and aP2 (4.04 ± 0.62-fold; P < 0.008) and significantly decreased expression of TCF7L2 (5.11 ± 1.52-fold; P = 0.003) and β-catenin (1.85 ± 0.24-fold; P = 0.037) at this same time point (Fig. 5C). Together, these findings thus indicated that reduced TCF7L2 expression in hADMSCs is associated with their enhanced adipogenic differentiation.
β-Catenin Impacts hADMSC Adipogenic Differentiation
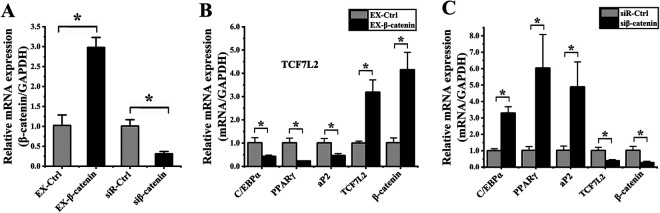
We next conducted gain- and loss-of-function experiments wherein we manipulated β-catenin expression in hADMSCs as above. We confirmed that EX-β-catenin transfection significantly increased mRNA-level β-catenin expression by 2.92 ± 0.84-fold relative to cells transfected with a control construct (P = 0.001; Fig. 6A), while siβ-catenin transfection resulting in a 3.22 ± 0.55-fold decreased in this expression (P = 0.002; Fig. 6A).
Figure 6.
β-catenin influences hADMSC adipogenic differentiation. (A) The expression of β-catenin was assessed by qPCR in cells transfected with the EX-β-catenin or siβ-catenin vectors, resulting in significant increases and decreases in the expression of this gene, respectively, relative to cells transfected with control constructs. (B) Cells overexpressing β-catenin were evaluated via qPCR, revealing significant decreases in the expression of C/EBP, PPARγ, and aP2, and significant increases in the expression of TCF7L2 and β-catenin relative to cells transfected with the EX-Ctrl construct. (C) Cells in which β-catenin had been knocked down were evaluated via qPCR, revealing significant increases in the expression of C/EBP, PPARγ, and aP2, and significant decreases in the expression of TCF7L2 and β-catenin relative to cells transfected with the control siRNA construct. *P < 0.05. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; PPARγ: peroxisome proliferator-activated receptor γ; qPCR: quantitative polymerase chain reaction.
We then evaluated the role of β-catenin in hADMSC adipogenic differentiation via qPCR on day 4, as above. We found that EX-β-catenin transfection was associated with significant decreases in the expression of C/EBPα (2.36 ± 0.78-fold; P = 0.010), PPARγ (4.32 ± 0.98-fold; P = 0.003), and aP2 (2.16 ± 0.87-fold; P = 0.010), together with significant increases in the expression of TCF7L2 (3.18 ± 0.69-fold; P = 0.002) and β-catenin (4.09 ± 0.64-fold; P = 0.002) relative to control cells (Fig. 6B). Consistent with this result, β-catenin knockdown was associated with markedly enhanced expression of C/EBPα (3.28 ± 0.33-fold; P = 0.001), PPARγ (5.93 ± 0.96-fold; P = 0.013), and aP2 (4.78 ± 1.54-fold; P = 0.012) together with significantly decreased expression of TCF7L2 (2.61 ± 0.54-fold; P = 0.007) and β-catenin (3.67 ± 1.73-fold; P = 0.008) relative to control cells (Fig. 6C). These findings thus confirmed that reduced β-catenin expression is associated with enhanced hADMSC adipogenic differentiation.
miR-26b-5p Modulates hADMSC Adipogenic Differentiation
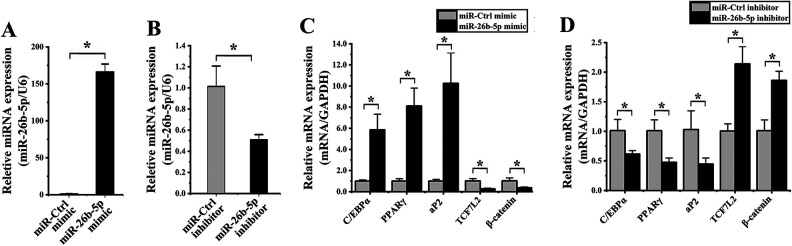
When hADMSCs were transfected with an miR-26b-5p mimic, this resulted in a 161.15 ± 41.26-fold increase in the expression of this miRNA relative to cells transfected with a control construct (P < 0.001; Fig. 7A), whereas transfection with an miR-26b-5p inhibitor induced a 3.88 ± 0.20-fold decrease in the expression of this miRNA (P = 0.012; Fig. 7A).
Figure 7.
miR-26b-5p alters hADMSC adipogenic differentiation. (A) When hADMSCs were transfected with an miR-26b-5p mimic, significant increases in miR-26b-5p expression were observed relative to cells transfected with an miR-Ctrl construct, whereas this expression was significantly reduced in cells transfected with an miR-26b-5p inhibitor relative to corresponding controls. (B) Cells transfected with an miR-26b-5p mimic were evaluated via qPCR, revealing significant increases in the expression of C/EBP, PPARγ, and aP2, and significant decreases in the expression of TCF7L2 and β-catenin relative to cells transfected with the mimic control construct. (C) Cells transfected with an miR-26b-5p inhibitor were evaluated via qPCR, revealing significant increases in the expression of C/EBP, PPARγ, and aP2, and significant decreases in the expression of TCF7L2 and β-catenin relative to cells transfected with the inhibitor control construct. *P < 0.05. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; PPARγ: peroxisome proliferator-activated receptor γ; qPCR: quantitative polymerase chain reaction.
We found that hADMSCs transfected with an miR-26b-5p mimic exhibit significant increases in the mRNA level expression of C/EBPα (5.83 ± 1.99-fold; P = 0.029), PPARγ (3.99 ± 3.14-fold; P = 0.002), and aP2 (10.17 ± 4.81-fold; P = 0.005) as well as significant decreases in the expression of TCF7L2 (3.80 ± 1.96-fold; P = 0.006) and β-catenin (2.80 ± 1.34-fold; P = 0.016) relative to cells transfected with a control miRNA (Fig. 7B). Consistent with this result, miR-26b-5p inhibitor transfection was associated with significant reductions in the expression of C/EBPα (1.64 ± 0.47-fold; P = 0.026), PPARγ (2.12 ± 0.71-fold; P = 0.009), and aP2 (2.31 ± 0.72-fold; P = 0.039), as well as significant increases in the expression of TCF7 L (2.13 ± 0.36-fold; P = 0.003) and β-catenin (1.84 ± 0.28-fold; P = 0.003) relative to cells transfected with a control inhibitor (Fig. 7C). These results thus suggested that miR-26b-5p overexpression is capable of promoting the adipogenic differentiation of hADMSCs.
miR-26b-5p Regulates TCF-4 and Thereby Influences the Adipogenic Differentiation of hADMSCs
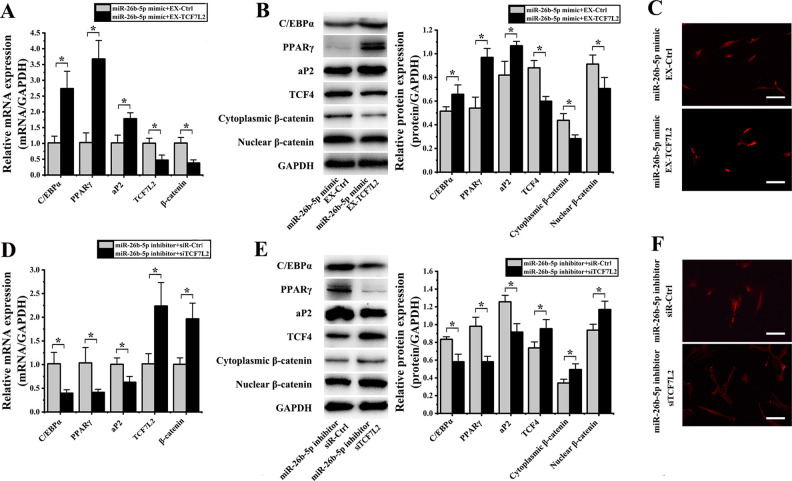
We next co-transfected hADMSCs with both the miR-26b-5p mimic and the EX-TCF7L2 construct, which resulted in significant increases in the expression of C/EBPα (2.69 ± 0.10-fold; P = 0.007), PPARγ (3.58 ± 1.47-fold; P = 0.002), and aP2 (1.75 ± 0.55-fold; P = 0.013) as well as significant decreases in the expression of TCF7L2 (2.13 ± 0.99-fold; P = 0.014) and β-catenin (2.65 ± 0.31-fold; P = 0.006) relative to cells transfected with miR-26b-5p and EX-Ctrl constructs (Fig. 8A). Comparable protein level changes were also observed in these cells, with significant increases in levels of C/EBPα (1.28 ± 0.08-fold; P= 0.048), PPARγ (1.79 ± 0.21-fold; P = 0.004), and aP2 (1.30 ± 0.19-fold; P = 0.025), and significant decreases in levels of TCF-4 (1.47 ± 0.09-fold; P = 0.003), cytoplasmic β-catenin (1.55 ± 0.03-fold; P = 0.015), and nuclear β-catenin (1.29 ± 0.07-fold; P = 0.042) relative to control cells (Fig. 8B). In addition, we observed reduced nuclear β-catenin staining in the nuclei of hADMSCs co-transfected with the miR-26b-5p mimic and EX-TCF7L2 (Fig. 8C).
Figure 8.
miR-26b-5p regulates TCF-4 so as to influence hADMSCs’ adipogenic differentiation. (A) qPCR revealed that when hADMSCs were co-transfected with an miR-26b-5p mimic and EX-TCF7L2, they exhibited significant increases in the expression of C/EBP, PPARγ, and aP2, and significant decreases in the expression of TCF7L2 and β-catenin relative to cells co-transfected with miR-26b-5p and EX-Ctrl constructs. (B) Western blotting revealed that when hADMSCs were co-transfected with an miR-26b-5p mimic and EX-TCF7L2, they exhibited significant increases in C/EBP, PPARγ, and aP2 levels, and significant decreases in levels of TCF-4, cytoplasmic β-catenin, and nuclear β-catenin. (C) Nuclear β-catenin staining intensity was reduced in cells transfected with an miR-26b-5p mimic and EX-TCF7L2. (D) qPCR revealed that when hADMSCs were co-transfected with an miR-26b-5p inhibitor and siTCF7L2 they exhibited significant decreases in the expression of C/EBP, PPARγ, and aP2, and significant increases in the expression of TCF7L2 and β-catenin relative to cells co-transfected with an miR-26b-5p inhibitor and siR-Ctrl constructs. (E) Western blotting revealed that when hADMSCs were co-transfected with an miR-26b-5p inhibitor and siTCF7L2 they exhibited significant decreases in C/EBP, PPARγ, and aP2 levels, as well as significant increases in levels of TCF-4, cytoplasmic β-catenin, and nuclear β-catenin. (F) hADMSCs transfected with miR-26b-5p inhibitor and siTCF7L2 exhibited significant decreases nuclear β-catenin fluorescence intensity. *P < 0.05. GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; PPARγ: peroxisome proliferator-activated receptor γ; qPCR: quantitative polymerase chain reaction; TCF-4: T cell factor 4.
When hADMSCs were instead co-transfected with an miR-26b-5p inhibitor and siTCF7L2, we observed significant reductions in the mRNA level expression of C/EBPα (2.56 ± 1.23-fold; P = 0.013), PPARγ (2.51 ± 0.94-fold; P = 0.031), and aP2 (1.60 ± 0.54-fold; P = 0.023) as well as significant increases in the expression of TCF7L2 (2.20 ± 0.09-fold; P = 0.017) and β-catenin (1.95 ± 0.46-fold; P = 0.010) relative to cells transfected with miR-26b-5p inhibitor and siR-Ctrl (Fig. 8D). Similarly, these cells exhibited significant decreases in protein levels of C/EBPα (1.43 ± 0.21-fold; P = 0.008), PPARγ (1.68 ± 0.05-fold; P = 0.004), and aP2 (1.37 ± 0.13-fold; P = 0.008) and significant increases in the levels of TCF-4 (1.29 ± 0.14-fold; P = 0.039), cytoplasmic β-catenin (1.44 ± 0.20-fold; P = 0.028), and nuclear β-catenin (1.25 ± 0.16-fold; P = 0.025) relative to cells transfected with miR-26b-5p inhibitor and siR-Ctrl (Fig. 8E). These co-transfected cells also exhibited significant reductions in levels of both cytoplasmic and nuclear β-catenin (Fig. 8F).
miR-26b-5p Regulates β-Catenin and Thereby Influences the Adipogenic Differentiation of hADMSCs
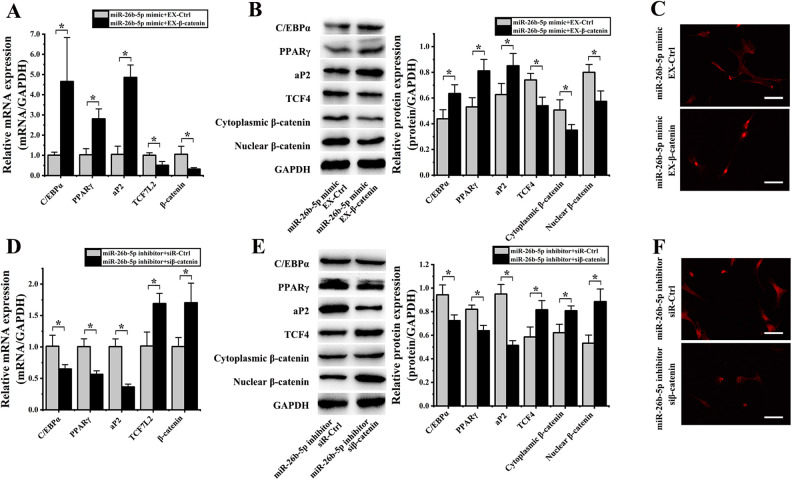
We next co-transfected hADMSCs with an miR-26b-5p mimic and EX-β-catenin, resulting in significant RNA level increases in the expression of C/EBPα (4.62 ± 1.78-fold; P = 0.004), PPARγ (2.73 ± 0.37-fold; P = 0.007), and aP2 (4.64 ± 2.16-fold; P = 0.001) as well as significant decreases in the expression of TCF7L2 (1.97 ± 0.82-fold; P = 0.019) and β-catenin (3.30 ± 0.52-fold; P = 0.036) relative to cells transfected with an miR-26b-5p mimic and EX-Ctrl (Fig. 9A). Similarly, these cells exhibited significant protein-level increases in the expression of C/EBPα (1.45 ± 0.07-fold; P = 0.027), PPARγ (1.53 ± 0.10-fold; P = 0.013), and aP2 (1.36 ± 0.14-fold; P = 0.040) and significant decreases in the protein levels of TCF-4 (1.37 ± 0.19-fold; P = 0.014), cytoplasmic β-catenin (1.45 ± 0.16-fold; P = 0.041), and nuclear β-catenin (1.39 ± 0.12-fold; P = 0.018) relative to control cells (Fig. 9B). These hADMSCs co-transfected with miR-26b-5p mimic and EX-β-catenin constructs also exhibited reduced nuclear β-catenin staining in immunofluorescence analyses (Fig. 9C), highlighting the ability of the miR-26b-5p mimic to partially reverse the inhibitory effects of β-catenin on adipogenic hADMSC differentiation.
Figure 9.
β-catenin miR-26b-5p regulates TCF-4 so as to influence hADMSCs’ adipogenic differentiation. (A) qPCR revealed that when hADMSCs were co-transfected with an miR-26b-5p mimic and EX-β-catenin, they exhibited significant increases in the expression of C/EBP, PPARγ, and aP2, and significant decreases in the expression of TCF7L2 and β-catenin relative to cells co-transfected with miR-26b-5p and EX-Ctrl constructs. (B) Western blotting revealed that when hADMSCs were co-transfected with an miR-26b-5p mimic and EX-β-catenin, they exhibited significant increases in C/EBP, PPARγ, and aP2 levels, and significant decreases in levels of TCF-4, cytoplasmic β-catenin, and nuclear β-catenin. (C) Nuclear β-catenin staining intensity was reduced in cells transfected with an miR-26b-5p mimic and EX- β-catenin. (D) qPCR revealed that when hADMSCs were co-transfected with an miR-26b-5p inhibitor and siβ-catenin they exhibited significant decreases in the expression of C/EBP, PPARγ, and aP2, and significant increases in the expression of TCF7L2 and β-catenin relative to cells co-transfected with an miR-26b-5p inhibitor and siR-Ctrl constructs. (E) Western blotting revealed that when hADMSCs were co-transfected with an miR-26b-5p inhibitor and siβ-catenin they exhibited significant decreases in C/EBP, PPARγ, and aP2 levels, as well as significant increases in levels of TCF-4, cytoplasmic β-catenin, and nuclear β-catenin. (F) hADMSCs transfected with miR-26b-5p inhibitor and siβ-catenin exhibited significant decreases in nuclear β-catenin fluorescence intensity. *P < 0.05.GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hADMSC: human adipose-derived mesenchymal stem cell; PPARγ: peroxisome proliferator-activated receptor γ; qPCR: quantitative polymerase chain reaction; TCF-4: T cell factor 4.
When hADMSCs were co-transfected with an miR-26b-5p inhibitor and siβ-catenin, they exhibited significant decreases in the mRNA expression of C/EBPα (1.56 ± 0.17-fold; P = 0.030), PPARγ (1.78 ± 0.37-fold; P = 0.005), and aP2 (2.76 ± 0.69-fold; P = 0.001) and significant increases in TCF7L2 (1.67 ± 0.31-fold; P = 0.013) and β-catenin (1.69 ± 0.51-fold; P = 0.024) expression relative to cells transfected with an miR-26b-5p inhibitor and siR-Ctrl (Fig. 9D). Similarly, at the protein level these cells exhibited significant reductions in the expression of C/EBPα (1.30 ± 0.05-fold; P = 0.005), PPARγ (1.29 ± 0.03-fold; P = 0.017), and aP2 (1.85 ± 0.02-fold; P = 0.001) as well as significant increases in the levels of TCF-4 (1.39 ± 0.07-fold; P = 0.026), cytoplasmic β-catenin (1.30 ± 0.08-fold; P = 0.018), and nuclear β-catenin (1.66 ± 0.09-fold; P = 0.009) relative to control cells (Fig. 9E). These cells co-transfected with miR-26b-5p inhibitor and siβ-catenin constructs also exhibited significantly reduced nuclear and cytoplasmic β-catenin levels upon immunofluorescence analysis (Fig. 9F). Together, these results suggest that the ability of low β-catenin levels to promote hADMSC adipogenic differentiation can be inhibited by the inhibition of miR-26b-5p expression.
Discussion
Adipose tissue can be readily collected from the human body and is a valuable resource for the repair of soft tissue defects9. Indeed, work by Coleman et al. has led to the development of specific materials used for fat injection15,16. At present, however, autologous adipose tissue samples typically undergo a ∼60% reduction in volume following transplantation owing to tissue necrosis17,18, making it difficult to achieve reliable tissue repair outcomes.
Efforts to reduce adipose tissue absorption are an area of active research, with some studies having found that this absorption can be reduced by transplanting a mixture of ADMSCs and fat particles, resulting in better structural integrity and tissue texture19,20. ADSMCs are able to secrete growth factors and cytokines such as vascular endothelial growth factor, basic fibroblast growth factor, hepatocyte growth factor, and insulin-like growth factor 1 that can support angiogenesis, enabling grafted tissue to receive more blood and to better survive the transplantation process21,22. Indeed, the transplantation of ADMSCs and fat granules together is associated with higher tissue survival rates relative to the transplantation of fat granules alone23,24.
Despite these promising properties, there are certain limitations and considerations to the use of ADMSCs for postoperative soft tissue repair in patients with malignant tumors. While these cells are not known to be directly carcinogenic, the growth factors that they secrete can promote endothelial proliferation and neoangiogenesis, thereby potentially supporting tumor growth and metastasis25,26. In clinical contexts, autologous fat grafts are primarily used for tissue repair in breast cancer patients. At present, however, The American Society of Plastic Surgeons offers a level-B recommendation suggesting that such transplantation is not associated with an increased risk of local tumor recurrence (https://www.plasticsurgery.org/Documents/Health-Policy/Principles/principle-2015-post-mastectomy-fat-grafting.pdf). Even so, further research regarding the safety of this technique in cancer patients with soft tissue defects requires further research.
Wnt signaling is known to play a key role in regulating MSC adipogenic differentiation27. For example, one study found that increased Wnt10b expression resulted in 3T3-L1 preadipocytes remaining in an undifferentiated state owing to resultant decreases in the expression of PPARγ and C/EBP-α28,29. Ectopic Wnt1 and Wnt3a expression can also induce Wnt/β-catenin pathway activation, thereby suppressing PPARγ and interfering with MSC adipogenic differentiation27,28,30. Overexpression of Wnt10a and Wnt6 similarly disrupts adipogenesis31. In contrast, PPARγ can partially inhibit the Wnt/β-catenin pathway, although PPARγ and/or C/EBPα overexpression is insufficient to reverse Wnt/β-catenin-mediated inhibition of adipogenesis32,33.
TCF-4 has also previously been shown to be an important regulator of adipogenic differentiation. For example, Singh et al. determined that TCF-4 overexpression impaired the 3T3-L1 cell adipogenesis34, while Zhang et al. found that high-glucose conditions were sufficient to impair β-catenin/TCF-4 nuclear translocation in cardiac stem cells, thereby promoting their adipogenic differentiation35. Embelin has been identified as being capable of preventing weight gain, as it was able to increase nuclear β-catenin and TCF-4 levels and to inhibit proliferation and adipogenesis when used to treat ST2 and C3H10T1/2 cells36. Myostatin also enhances β-catenin nuclear translocation and formation of the Smad3–β-catenin–TCF-4 complex in hMSCs and alters associated gene expression patterns. The impact of myostatin on adipogenic differentiation can be disrupted by β-catenin knockdown or overexpression of a dominant-negative form of TCF-437. Overexpressing such a dominant-negative TCF-4 isoform in pre-adipocytes results in impaired Wnt signaling pathway, leading to adipocytic differentiation27.
Multiple miRNAs have been shown to influence the abovementioned pathways. For example, miR-139 can suppress TCF7L2 expression and β-catenin/TCF-4 signaling in hepatocellular carcinoma cells38. Zhou et al. similarly found that miR-212 can target TCF7L2 so as to inhibit cervical cancer metastasis and progression39. In addition, TCF7L2 is an miR-379 target in laryngeal carcinoma, with this miRNA being able to inhibit tumor cell proliferation, migration, and invasion when overexpressed40. Furthermore, Tian et al. found that miR-17-5p targets TCF7L2 and alters 3T3-L1 adipogenesis14, while Li et al. also found that miR-328 can target TCF7L2 to inhibit cervical cancer cell proliferation41. Therefore, in addition to mir-26b-3p, these miRNAs may also directly regulate TCF-4 and affect fat absorption.
Previous work indicates that miR-26b-3p can influence several physiological and pathological processes. For example, in human umbilical cord-derived MSCs, this miRNA has been shown to be upregulated over time and to correlate with cellular senescence and cell cycle gene expression such that when it was overexpressed the proliferation of these cells was impaired in vitro 42. Lin et al. found that miR-26b-3p was able to inhibit the osteoblastic differentiation of MC3T3-E1 cells owing to its ability to target estrogen receptor α43. Patients with rheumatoid arthritis and their close relative exhibit significant increases in miR-26b-3p expression in whole blood samples, even when asymptomatic44. Ginsenoside Rh2 can suppress miR-26b-3p expression levels in liver cancer cells45. In addition, patients with Alzheimer’s disease exhibit increased miR-26b-3p expression relative to healthy controls46. Therefore, mir-26b-3p may affect these diseases and other physiological processes when it is used as a regulatory target to prevent fat absorption.
Conclusions
In this study, we found that reduced TCF-4 expression was able to promote the adipogenic differentiation of hADMSCs in vitro. In addition, we identified TCF-4 as an miR-26b-5p target gene and found that this miRNA was able to directly suppress TCF-4 expression so as to inactivate Wnt/β-catenin signaling, thereby promoting the adipogenic differentiation of these cells.
Supplemental Material
Supplemental Material, Fig._S1 for miR-26b-5p/TCF-4 Controls the Adipogenic Differentiation of Human Adipose-derived Mesenchymal Stem Cells by Yadong Luo, Huan Ji, Yan Cao, Xu Ding, Meng Li, Haiyang Song, Sheng Li, Chenxing WaTableng, Heming Wu, Jian Meng and Hongming Du in Cell Transplantation
Supplementary_materials for miR-26b-5p/TCF-4 Controls the Adipogenic Differentiation of Human Adipose-derived Mesenchymal Stem Cells by Yadong Luo, Huan Ji, Yan Cao, Xu Ding, Meng Li, Haiyang Song, Sheng Li, Chenxing WaTableng, Heming Wu, Jian Meng and Hongming Du in Cell Transplantation
Acknowledgments
All authors are acknowledged for their contribution to the study.
Footnotes
Ethical Approval: The study was approved by the Ethics Committee of Nanjing Medical University.
Statement of Human and Animal Rights: All procedures in the study involving human subjects were conducted in accordance with the human ethics committee of Nanjing Medical University.
Statement of Informed Consent: We confirm that guidelines on patient consent have been met and any details of informed consent obtained are indicated within the text of the submitted manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our work was funded by the National Natural Science Foundation of China, grant agreement number 81600908 and 81701491; and the Natural Science Foundation of Jiangsu Province, grant agreement number BK20170152.
ORCID iDs: Meng Li  https://orcid.org/0000-0001-5937-0709
https://orcid.org/0000-0001-5937-0709
Hongming Du  https://orcid.org/0000-0002-0909-0773
https://orcid.org/0000-0002-0909-0773
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bradford BD, Lee JW. Reconstruction of the forehead and scalp. Facial Plast Surg Clin North Am. 2019;27(1):85–94. [DOI] [PubMed] [Google Scholar]
- 2. Saban Y. Rhinoplasty: Lessons from “errors”: from anatomy and experience to the concept of sequential primary rhinoplasty. HNO. 2018;66(1):15–25. [DOI] [PubMed] [Google Scholar]
- 3. Kim RY, Sokoya M, Ducic Y, Williams F. Free-flap reconstruction of the mandible. Semin Plast Surg. 2019;33(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stephan S, Reinisch J. Auricular reconstruction using porous polyethylene implant technique. Facial Plast Surg Clin North Am. 2018;26(1):69–85. [DOI] [PubMed] [Google Scholar]
- 5. Denadai R, Raposo-Amaral CA, Raposo-Amaral CE. Fat grafting in managing craniofacial deformities. Plast Reconstr Surg. 2019;143(5):1447–1455. [DOI] [PubMed] [Google Scholar]
- 6. Giatsidis G, Succar J, Waters TD, Liu W, Rhodius P, Wang C, Nilsen TJ, Chnari E, Orgill DP. Tissue-engineered soft-tissue reconstruction using noninvasive mechanical preconditioning and a shelf-ready allograft adipose matrix. Plast Reconstr Surg. 2019;144(4):884–895. [DOI] [PubMed] [Google Scholar]
- 7. Schweizer R, Taddeo A, Waldner M, Klein HJ, Fuchs N, Kamat P, Targosinski S, Barth AA, Drach MC, Gorantla VS, Cinelli P. et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am J Transplant. 2019;20(5):1272–1284. doi:10.1111/ajt.15726. [DOI] [PubMed] [Google Scholar]
- 8. Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21(11):1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen PN, Huang CJ, Sugii S, Cheong SK, Choo KB. Selective activation of miRNAs of the primate-specific chromosome 19 miRNA cluster (C19MC) in cancer and stem cells and possible contribution to regulation of apoptosis. J Biomed Sci. 2017;24(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ke J, Xu HE, Williams BO. Lipid modification in Wnt structure and function. Curr Opin Lipidol. 2013;24(2):129–133. [DOI] [PubMed] [Google Scholar]
- 11. Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11(3):216–225. [DOI] [PubMed] [Google Scholar]
- 12. Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol. 2015;11(5):276–288. [DOI] [PubMed] [Google Scholar]
- 13. Shi C, Huang F, Gu X, Zhang M, Wen J, Wang X, You L, Cui X, Ji C, Guo X. Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity. Oncotarget. 2016;7(26):40830–40845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian L, Song Z, Shao W, Du WW, Zhao LR, Zeng K, Yang BB, Jin T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017;8(1):e2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coleman SR, Katzel EB. Fat grafting for facial filling and regeneration. Clin Plast Surg. 2015;42(3):289–300. [DOI] [PubMed] [Google Scholar]
- 16. Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24(2):347–367. [PubMed] [Google Scholar]
- 17. Silva ABD, Haupenthal F, Morais AD, Ascenço ASK, Sebastião APM, Cavalcanti MAR, Freitas RS. Relationship between Tamoxifen and the absorption of subfascial autologous fat grafts. Plast Reconstr Surg. 2018;141(6):1408–1415. [DOI] [PubMed] [Google Scholar]
- 18. Kim DY, Ji YH, Kim DW, Dhong ES, Yoon ES. Effects of platelet-rich plasma, adipose-derived stem cells, and stromal vascular fraction on the survival of human transplanted adipose tissue. J Korean Med Sci. 2014;29(Suppl 3):S193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ha KY, Park H, Park SH, Lee BI, Ji YH, Kim TY, Yoon ES. The Relationship of a combination of human adipose tissue-derived stem cells and frozen fat with the survival rate of transplanted fat. Arch Plast Surg. 2015;42(6):677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu F, Li J, Gao J, Ogawa R, Ou C, Yang B, Fu B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg. 2009;124(5):1437–1446. [DOI] [PubMed] [Google Scholar]
- 21. Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–2077. [DOI] [PubMed] [Google Scholar]
- 22. Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo HC. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64(2):222–228. [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375–3382. [DOI] [PubMed] [Google Scholar]
- 24. Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34(9):1178–1185. [DOI] [PubMed] [Google Scholar]
- 25. Salha S, Gehmert S, Brébant V, Anker A, Loibl M, Prantl L, Gehmert S. PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3 K signaling pathway. Clin Hemorheol Microcirc. 2018;70(4):543–551. [DOI] [PubMed] [Google Scholar]
- 26. Preisner F, Leimer U, Sandmann S, Zoernig I, Germann G, Koellensperger E. Impact of human adipose tissue-derived stem cells on malignant melanoma cells in an in vitro co-culture model. Stem Cell Rev Rep. 2018;14(1):125–140. [DOI] [PubMed] [Google Scholar]
- 27. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279(43):45020–45027. [DOI] [PubMed] [Google Scholar]
- 29. Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376(Pt 3):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112(12):3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibañez G, MacDougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50(2):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66(2):236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawai M, Mushiake S, Bessho K, Murakami M, Namba N, Kokubu C, Michigami T, Ozono K. Wnt/Lrp/beta-catenin signaling suppresses adipogenesis by inhibiting mutual activation of PPARgamma and C/EBPalpha. Biochem Biophys Res Commun. 2007;363(2):276–282. [DOI] [PubMed] [Google Scholar]
- 34. Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147(1):141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X, Meng K, Pu Y, Wang C, Chen Y, Wang L. Hyperglycemia altered the fate of cardiac stem cells to Adipogenesis through Inhibiting the β-Catenin/TCF-4 Pathway. Cell Physiol Biochem. 2018;49(6):2254–2263. [DOI] [PubMed] [Google Scholar]
- 36. Gao Y, Li J, Xu X, Wang S, Yang Y, Zhou J, Zhang L, Zheng F, Li X, Wang B. Embelin attenuates adipogenesis and lipogenesis through activating canonical Wnt signaling and inhibits high-fat diet-induced obesity. Int J Obes (Lond). 2017;41(5):729–738. [DOI] [PubMed] [Google Scholar]
- 37. Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/beta-catenin signaling pathways. J Biol Chem. 2008;283(14):9136–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu W, Li X, Wang J. miR-139 regulates the proliferation and invasion of hepatocellular carcinoma through the WNT/TCF-4 pathway. Oncol Rep. 2014;31(1):397–404. [DOI] [PubMed] [Google Scholar]
- 39. Zhou C, Tan DM, Chen L, Xu XY, Sun CC, Zong LJ, Han S, Zhang YZ. Effect of miR-212 targeting TCF7L2 on the proliferation and metastasis of cervical cancer. Eur Rev Med Pharmacol Sci. 2017;21(2):219–226. [PubMed] [Google Scholar]
- 40. Wei JL, Zhang L, Zhao ZM, Zhao YZ, Fu Q, Yang Y. MicroRNA-379 inhibits laryngeal carcinoma cell proliferation and invasion through directly targeting TCF-4. Kaohsiung J Med Sci. 2019;35(12):731–738. [DOI] [PubMed] [Google Scholar]
- 41. Wang X, Xia Y. microRNA-328 inhibits cervical cancer cell proliferation and tumorigenesis by targeting TCF7L2. Biochem Biophys Res Commun. 2016;475(2):169–175. [DOI] [PubMed] [Google Scholar]
- 42. Wang Q, Xu C, Zhao Y, Xu Z, Zhang Y, Jiang J, Yan B, Gu D, Wu M, Wang Y, Liu H. miR-26b-3p regulates human umbilical cord-derived Mesenchymal stem cell proliferation by targeting estrogen receptor. Stem Cells Dev. 2016;25(5):415–426. [DOI] [PubMed] [Google Scholar]
- 43. Lin Y, Xiao L, Zhang Y, Li P, Wu Y, Lin Y. MiR-26b-3p regulates osteoblast differentiation via targeting estrogen receptor α. Genomics. 2019;111(5):1089–1096. [DOI] [PubMed] [Google Scholar]
- 44. Anaparti V, Smolik I, Meng X, Spicer V, Mookherjee N, El-Gabalawy H. Whole blood microRNA expression pattern differentiates patients with rheumatoid arthritis, their seropositive first-degree relatives, and healthy unrelated control subjects. Arthritis Res Ther. 2017;19(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen W, Chu S, Li H, Qiu Y. MicroRNA-146a-5p enhances ginsenoside Rh2-induced anti-proliferation and the apoptosis of the human liver cancer cell line HepG2. Oncol Lett. 2018;16(4):5367–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Satoh J, Kino Y, Niida S. MicroRNA-seq data analysis pipeline to identify blood biomarkers for Alzheimer’s disease from public data. Biomark Insights. 2015;10(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Fig._S1 for miR-26b-5p/TCF-4 Controls the Adipogenic Differentiation of Human Adipose-derived Mesenchymal Stem Cells by Yadong Luo, Huan Ji, Yan Cao, Xu Ding, Meng Li, Haiyang Song, Sheng Li, Chenxing WaTableng, Heming Wu, Jian Meng and Hongming Du in Cell Transplantation
Supplementary_materials for miR-26b-5p/TCF-4 Controls the Adipogenic Differentiation of Human Adipose-derived Mesenchymal Stem Cells by Yadong Luo, Huan Ji, Yan Cao, Xu Ding, Meng Li, Haiyang Song, Sheng Li, Chenxing WaTableng, Heming Wu, Jian Meng and Hongming Du in Cell Transplantation