Abstract
Three-dimensional (3D) cell culture by engineering spheroids has gained increasing attention in recent years because of the potential advantages of such systems over conventional two-dimensional (2D) tissue culture. Benefits include the ability of 3D to provide a more physiologically relevant environment, for the generation of uniform, size-controlled spheroids with organ-like microarchitecture and morphology. In recent years, different techniques have been described for the generation of cellular spheroids. Here, we have compared the efficiency of four different methods of islet cell aggregation. Rat pancreatic islets were dissociated into single cells before reaggregation. Spheroids were generated either by (i) self-aggregation in nonadherent petri dishes, (ii) in 3D hanging drop culture, (iii) in agarose microwell plates or (iv) using the Sphericalplate 5D™. Generated spheroids consisted of 250 cells, except for the self-aggregation method, where the number of cells per spheroid cannot be controlled. Cell function and morphology were assessed by glucose stimulated insulin secretion (GSIS) test and histology, respectively. The quantity of material, labor intensity, and time necessary for spheroid production were compared between the different techniques. Results were also compared with native islets. Native islets and self-aggregated spheroids showed an important heterogeneity in terms of size and shape and were larger than spheroids generated with the other methods. Spheroids generated in hanging drops, in the Sphericalplate 5D™, and in agarose microwell plates were homogeneous, with well-defined round shape and a mean diameter of 90 µm. GSIS results showed improved insulin secretion in response to glucose in comparison with native islets and self-aggregated spheroids. Spheroids can be generated using different techniques and each of them present advantages and inconveniences. For islet cell aggregation, we recommend, based on our results, to use the hanging drop technique, the agarose microwell plates, or the Sphericalplate 5D™ depending on the experiments, the latter being the only option available for large-scale spheroids production.
Keywords: islets, spheroid, 3D cell culture, type 1 diabetes, dissociation
Introduction
During embryonic development, cells undergo biological self-assembly to form complex tissues with three dimensional (3D) architecture and extensive cell–cell contacts, which are mandatory for maintaining intracellular functions1. However, cells are mostly studied in two-dimensional (2D) monolayer models, since they provide a well-controlled and homogeneous environment, facilitate microscopic analysis and medium changes, and sustain cell proliferation for most cell types. This characteristic makes 2D platforms attractive for simplicity and efficiency considerations. Nevertheless, these methods are unable to mimic the complexity of the in vivo architecture and environment, which makes 2D-cultured cells different from cells growing in vivo in terms of morphology, proliferation, cell–cell and cell–matrix interactions, signal transduction, differentiation, and other aspects2. To better reproduce physiological conditions, there is a rapidly evolving trend toward the engineering of cell spheroids and their use as building blocks for functional tissue assembly. One of the main research fields using spheroids is oncology, where anticancer drugs are studied on 3D-cultured cancer cells3,4. Spheroids are also used for tissue regeneration using mesenchymal stem cells (MSCs)5. It has indeed been demonstrated that MSCs performed better when cultured in a 3D manner6. Among many other fields using spheroids, pancreatic islet transplantation benefits greatly from this 3D multicellular research tool7–9.
In recent years, many techniques have been developed to generate spheroids using the principle of self-assembly10. Most widely used techniques can be separated into non-microfluidic and microfluidic methods. The first category is composed mainly of hanging drop cultures11, cultures on low-attachment substrates12, and cultures using microwell-containing culture plates13. Spheroids generation using microfluidic methods is a dynamic technique, where cells are exposed to a continuous, controlled pressure perfusion14,15. Although this technique demonstrated several improvements such as spheroid morphology and viability16,17 in comparison to non-microfluidic techniques, it is used in “organ-on-a-chip” models, in opposition to generation of large quantities of functional elements for cell therapy and will therefore not be addressed in this study.
An essential prerequisite is the high-yield fabrication of spheroids of controlled size and composition. Spheroid size is an important parameter, as cells in the core of the spheroid heavily depend on oxygen supplied by diffusion. Consequently, overall spheroid size should not exceed a few hundred micrometers to avoid necrosis. In the field of pancreatic islet transplantation, larger human islets showed an increasing percentage of necrosis when exposed to 24 h of hypoxia in comparison with smaller islets18. Furthermore, central necrotic cores appear in islets >100 μm, in normoxic culture condition, after 48 h. In addition to an improved viability, it has been reported that small islets performed better in terms of function in comparison to larger islets13,19,20. Thus, in order to replace damaged organ function, thousands to millions of spheroids would be necessary. Therefore, there is a need to develop methods that simultaneously allows efficient generation of spheroids and their large-scale production.
Spheroids composed of dissociated islet cells are a typical example of multicellular spheroids. Engineering spheroids from dissociated islet cells allows to create small, homogenous neo-formed islets13. Furthermore, this process offers the possibility to coculture islet cells with stem cells, endothelial cells, or any other cell types that can be beneficial to the islets21.
Here we compare four different techniques commonly used to generate spheroids from islet cells in terms of morphology and function of the resulting spheroids, but also including considerations of technical handling and labor intensivity, in order to provide researchers with information that will allow to select the technique that best suits their planned experiments.
Materials and Methods
Animals
Pregnant female, 10-week-old Lewis rats were purchased from Janvier Laboratory (Le Genest St-Isle, France). Animals were kept and bred in our animal facilities at the University of Geneva School of Medicine. All experiments were performed in compliance with the rules of Geneva Veterinary authorities and according to protocols reviewed and approved by the University of Geneva Institutional Animal Care and Use Committee (GE/34/19).
Islet Isolation and Dissociation
Pancreas digestion was performed by collagenase perfusion (collagenase V, Sigma-Aldrich, Buchs, Switzerland) and islets were purified by a discontinuous Ficoll gradient as previously described22,23. Islets were cultured for 24 h at 37°C, in a 21% O2, 5% CO2 atmosphere, in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Reinach, Switzerland) supplemented with 10% fetal bovine serum (FBS; Merck Millipore, Zug, Switzerland), 2 mmol/l l-glutamin, 100 U/ml penicillin, 0.1 mg/ml of streptomycin, 1 mmol/l sodium pyruvate (Sigma-Aldrich), and 11 mmol/l glucose (Bichsel, Interlaken, Switzerland), hereafter referred to as complete DMEM. At day 1 post-isolation, islets were dispersed into single cells with 0.05% trypsin–ethylenediaminetetraacetic acid (Thermo Fisher) as previously reported24.
Generation of Spheroids
For each experiment, 1 500 spheroids, composed of 250 cells, were generated according to four different techniques:
The self-aggregating technique (petri condition): 375 000 dissociated islet cells were plated in a 35-mm nonadherent petri dish (Falcon) in 2.5 ml complete DMEM. The time needed for cell plating was about 30 s. Medium was changed every 48 h.
The hanging drop technique (drop condition): 375 000 cells were resuspended in 45 ml complete DMEM in a 50 ml conical tube (Falcon). Eleven nonadherent petri dishes of 150 mm diameter (Falcon) were used for this condition. The bottom parts of the petri dishes were filled with 30 ml of phosphate buffered saline (PBS). After resuspension of islet single cells in the conical tube, 30 µl drops were plated on the internal side of the petri dish lid. Lids were then rapidly turned upside down upon the bottom part of the PBS-containing petri dish25. In total, about 1500 drops containing 250 cells each were created. The time needed for drop plating was about 120 min when performed by one operator. Medium was not changed during the culture time.
The agarose 3D microwell technique (mold condition): 500 µl sterile agarose solution at a 2.5% concentration (Promega, Duübendorf, Switzerland), heated at 90°C, was distributed onto autoclaved silicon molds (Microtissues 3D Petri Dish; Sigma-Aldrich), to generate 256-microwell casts (microwells: 300 µm diameter, 800 µm depth). Once solidified, agarose casts were removed from the molds and each cast was placed inside the well of a 12-well cell culture plate (Sigma-Aldrich). Before use, agarose casts were equilibrated for 1 h in complete DMEM at 37°C. Equilibration medium was removed and 375 000 cells were seeded in six agarose casts (62 500 cells/agarose cast) in a final cell suspension volume of 150 µl per cast. A resting period of 30 min was observed in order to allow the cells to sediment inside the microwells before adding 2 ml complete DMEM per well. Medium was changed every 48 h. The time needed for plating was about 35 min (2.5 min for plating, 30 min for cell sedimentation, and 2.5 min for medium addition). This time does not include the casting of the agarose microwells and their equilibration.
Spheroids using the Sphericalplate 5D™ (Kugelmeiers, Erlenbach, Switzerland) (Kugel condition): the Sphericalplate 5D™ is a 24-well plate containing 9 000 pyramidal microwells (500 µm edge) distributed in 12 of the plate wells (750 microwell/well). The other 12 conventional wells were not used in this study. Complete medium (1 ml/well) was used to remove air bubbles and equilibrate the plate before seeding. A total of 375 000 cells was plated in two microwell-containing wells, in a total final volume of 2 ml complete DMEM per well. The time needed for cell plating was about 30 s. Medium was changed every 48 h.
As a control, intact islets (300 IEQ) were plated in a 3.5 cm nonadherent culture petri dish (Falcon), in 2.5 ml complete DMEM, hereafter referred to as IEQ condition. The time needed for cell plating was about 30 s. Medium was changed every 48 h.
For each condition, native islets or islet cell spheroids were cultured for 5 days at 37°C in a 21% O2 and 5% CO2 atmosphere.
Immunofluorescence Staining
Native islets and spheroids were recovered after 5 days in culture, fixed in 4% paraformaldehyde during 60 min, and suspended in HistoGel (Thermo Fisher) pre-warmed at 70°C. After centrifugation, HistoGels containing native islets or spheroids were left on ice for 15 min. Solidified HistoGels were then recovered and embedded in paraffin. Block sections of 5 µm were cut and mounted on glass slides. Permeabilization was performed with 0.5% Triton X-100 in PBS for 30 min followed by 45 min incubation in 0.5% bovine serum albumin (BSA) in PBS at room temperature to block unspecific sites. Immunofluorescence staining was performed in two sequential steps: slides were first incubated overnight at 4°C with a rabbit anti-somatostatin primary antibody (1:100 dilution; DakoCytomation, Baar, Switzerland). The next day, slides were washed in PBS, and then exposed for 1 h to an anti-rabbit alexa 488 secondary antibody (Jackson ImmunoResearch Laboratories, Rheinfelden, Switzerland). After PBS rinsing, slides were incubated for 2 h with a combination of primary antibodies: guinea pig anti-insulin (1:100 dilution, DakoCytomation) and mouse anti-glucagon (1:4,000, Sigma-Aldrich). Slides were washed in PBS before incubation for 1 h with a combination of fluorescein isothiocyanate goat anti–guinea pig and a Coumarin AMCA donkey anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories). Both primary and secondary antibodies were diluted in PBS containing 0.5% BSA. Pictures were taken using a Zeiss Axioscan.Z1 slide scanner (Zeiss, Feldbach, Germany).
Morphology
Native islets and spheroids were recovered after 5 days in culture. They were placed in nonadherent petri dishes, and pictures were taken for morphology assessment. Diameters were measured on a minimum of 100 native islets or spheroids from four distinct preparations, using the ImageJ software (NIH, Bethesda, MD, USA).
Functional Assessment
Glucose stimulated insulin secretion (GSIS) test was performed after 5 days of culture. Native islets and the newly formed spheroids from the different conditions were plated in triplicates in 24-well plates containing culture inserts (Millipore). A preincubation of 1 h in Krebs–Ringer buffered 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4) with 0.1% BSA (KRB solution) containing 2.8 mmol/l glucose was performed. Native islets and spheroids were then exposed for 1 h to a low glucose KRB solution (2.8 mmol/l), followed by 1 h in a high glucose KRB solution (16.7 mmol/l). Supernatants were recovered and insulin concentrations were measured using an ELISA kit (Mercodia, Uppsala, Sweden) for rat insulin. Native islet and spheroid capacity to respond to glucose was expressed as the ratio of insulin concentration in high to low glucose medium, referred to as the stimulation index (SI). Finally, native islets and spheroids were incubated for 1 h in acid ethanol for evaluation of total insulin content. Insulin secretion was further estimated as a percentage of total insulin contents.
Statistical Analysis
Variables are presented as mean ± standard deviation (SD). Comparisons between two groups were performed with the Mann–Whitney U-test. Statistical analyses were performed using Prism software 8.0 (GraphPad, La Jolla, CA, USA), and a P value of <0.05 was considered statistically significant.
Results
Spheroid Generation
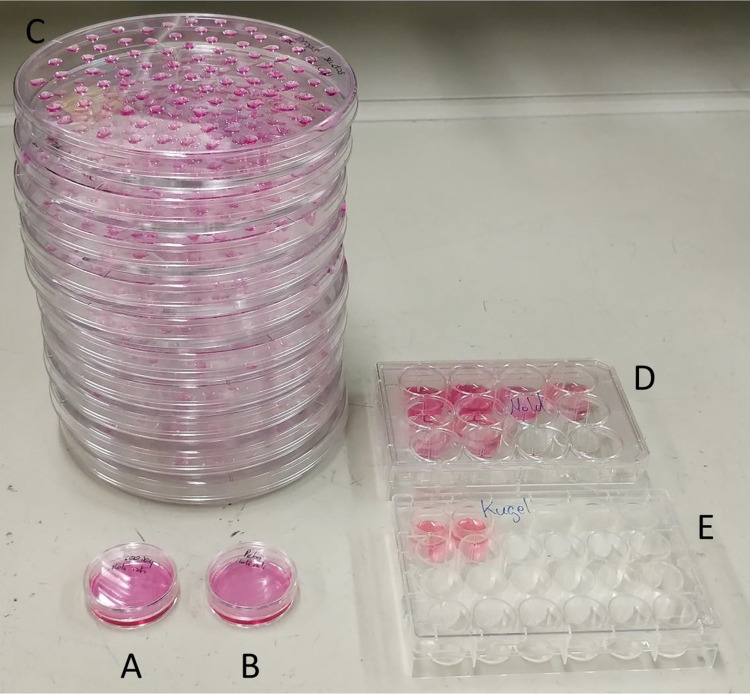
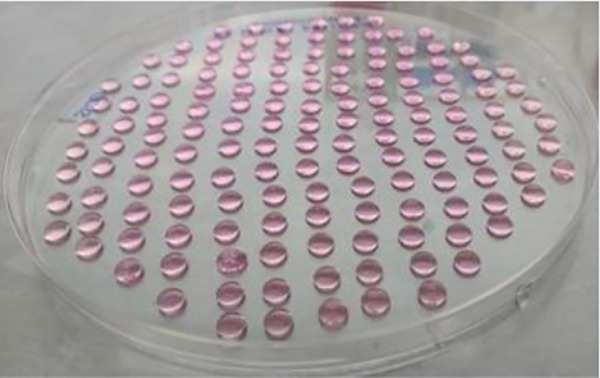
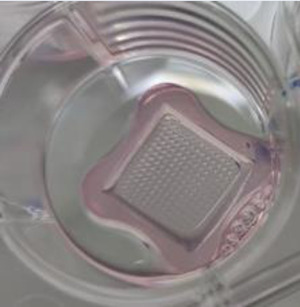
Plating data are summarized in Table 1. The self-aggregation technique in nonadherent petri dishes was the fastest and easiest method and required the fewest amount of material. Only one 35 mm nonadherent petri dish and about 4 ml of complete DMEM (2 ml for the initial seeding and one medium change) were necessary. By contrast, the hanging drop technique required the largest amount of material (eleven 150 mm petri dishes and 45 ml of complete medium) and took the longest time to plate the cells in order to form aggregates. Of note, in order to have a similar distribution of the cell types in the spheroids, cell preparations in the medium had to be homogenized regularly during drops generation. The mold technique was more labor-intensive than the others, except the drop condition, mainly because of the time needed to cast the agarose structures, and medium equilibration and cell sedimentation times. However, agarose casts can be prepared in advance and stored at 4°C in PBS or Hanks. In this study, the mold conditions required 6 wells of a 12-well culture plate, 24 ml complete medium, and 6 agarose structures. The kugel condition was performed using two wells (containing 750 microwells/well) of a Sphericalplate 5D™ and required 8 ml complete DMEM (2 ml for the seeding and one medium change). In contrast to the mold technique where spheroid recovery required inverting the agarose structures upside down before spinning them to make the aggregates fall from the microwells, a simple resuspension of the aggregates with medium allowed to recover all spheroids from the Sphericalplate 5D™. Finally, the control IEQ condition required one 35 mm nonadherent petri dish and about 4 ml complete DMEM (2 ml for the initial seeding and one medium change). Fig. 1 shows the plating substrates just after seeding for the five conditions.
Table 1.
Plating data for spheroids generation.
| Conditions | IEQ | Petri | Drops | Molds | Kugel |
|---|---|---|---|---|---|
| Number of islets or cells | 300 IEQ | 375 000 cells | 375 000 cells | 375 000 cells | 375 000 cells |
| Number of spheroids | — | ∼1 500 | ∼1 500 | ∼1 500 | ∼1 500 |
| Substrate type | Petri 35 mm | Petri 35 mm | Petri 150 mm | 256-well agarose molds in 12-well plate | Engineered microwells in Kugelmeier plate |
| Number of substrate | 1 petri | 1 petri | 11 petri | 6 molds | 2 microwells of the 24-well plate |
| Culture medium volume | 2.5–3 ml | 2.5–3 ml | 45 ml | 12.9 ml | 4 ml |
| Time for seeding | 30" | 30" | 120’ | 2.5’ seeding 30’ sedimentation 2.5’ adding medium |
30" |
| Support devices |
|
|
|
|

|
Table 1 describes the amount of cells, materials, time needed to plate the cells in the five conditions, and the support device used for each condition.
a Picture from https://www.kugelmeiers.com/
Figure 1.
Picture of the results of the five conditions plated: (A) the IEQ condition, (B) the petri condition, (C) the drop condition, (D) the mold condition, and (E) the Kugel condition.
Importantly, 250 cell spheroids were created with the mold, kugel, and drop techniques, whereas for the petri condition, the number of cells per aggregates could not be controlled.
Morphology and Immunohistological Assessment
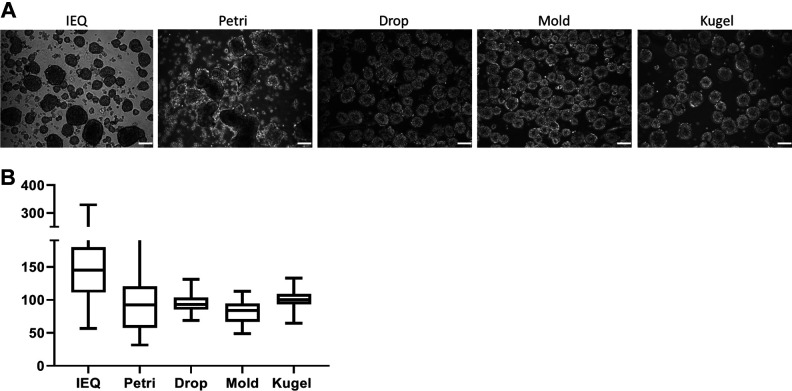
As shown in Fig. 2A, single islet cells showed a good aggregation after 5 days in culture, regardless of the method chosen. Re-aggregated spheroids presented diameters ranging from 40 to 300 μm, with a mean diameter of 96.2 ± 44.3 μm. As expected, heterogeneity in terms of size and shape was important not only in the IEQ condition (146.4 ± 52.2 μm) but also in the petri condition (96.17 ± 44.29 μm). In contrast, spheroids in the other three conditions showed a more homogenous morphology as observed by smaller SDs. Indeed, spheroid mean diameters were 94.5 ± 13.4, 81.3 ± 17.1, and 102.3 ± 14.0 μm, for the drop, mold, and kugel conditions, respectively. Diameter comparison is represented in Fig. 2B.
Figure 2.
Spheroids morphology. (A) Phase-contrast microscopic images of native islets and generated spheroids after 5 days of culture. Scale bar = 100 μm. (B) Diameter values for each condition are presented as mean diameter with SD. SD: standard deviation.
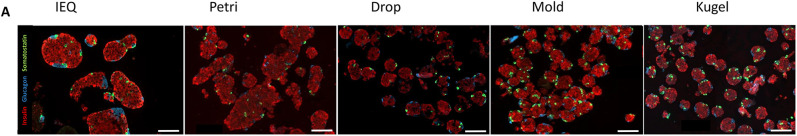
Histology assessment by immunofluorescence is presented in Fig. 3 and demonstrates a similar distribution of the main endocrine islet cell types, with a majority of insulin-positive cells (in red), and the presence of glucagon-positive cells (in blue) and somatostatin-positive cells (in green). In summary, we succeeded to generate spheroids composed of the main endocrine cell types and observed a better homogeneity in terms of size and shape in the drop, mold, and kugel conditions, in comparison to the IEQ and petri conditions.
Figure 3.
Immunohistology. Immunofluorescence staining of islets or spheroids from the five conditions. Insulin is stained in red, glucagon in blue, and somatostatin in green. Scale bar = 100 μm.
In Vitro Function
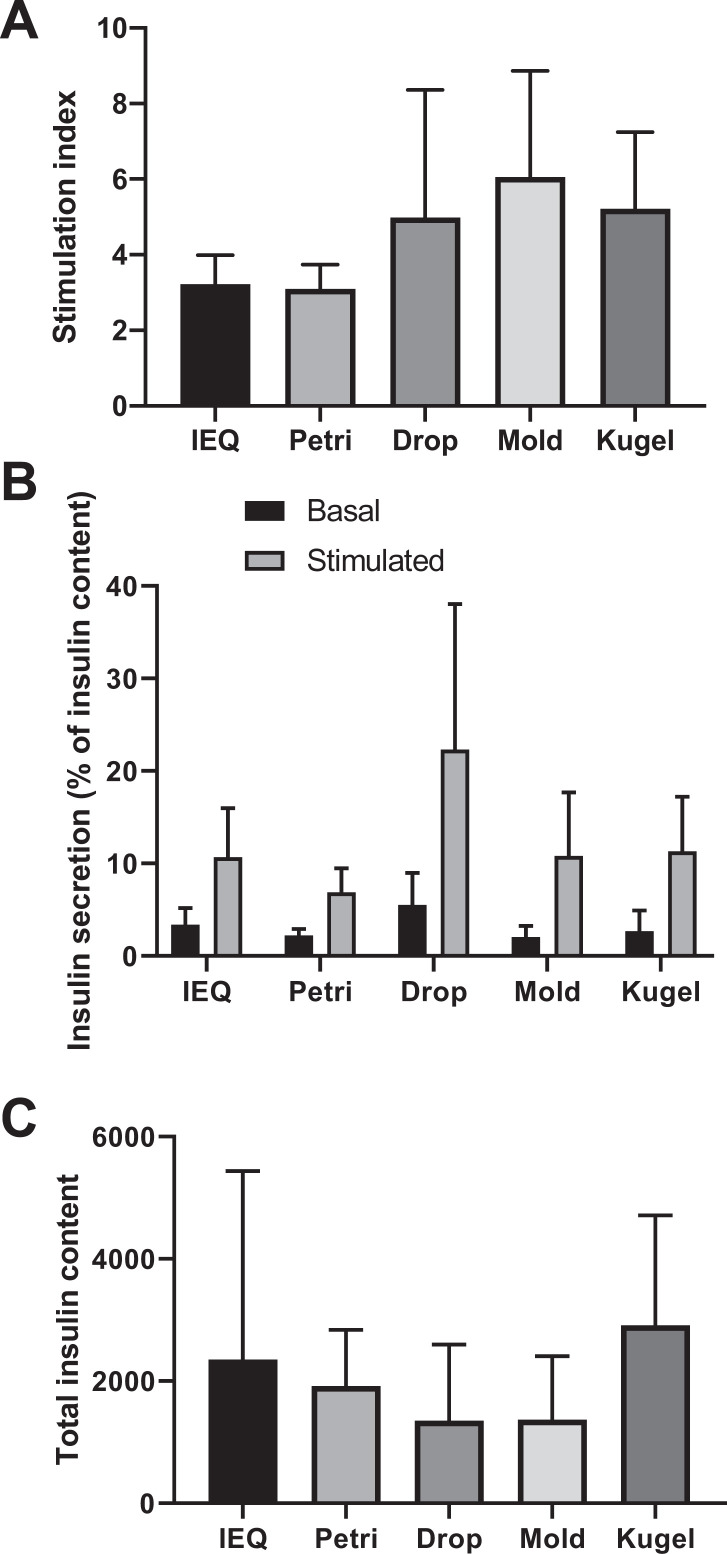
Function of islets and spheroids was determined by a GSIS test. SIs from the different conditions are presented in Fig. 4A. In the petri condition, SI was 3.10 ± 0.64, a value quite similar to that of the IEQ control condition (3.22 ± 0.77; P = 0.791). Spheroids formed by drop, kugel, and mold techniques exhibited higher SIs with mean values of 4.99 ± 3.37, 5.22 ± 2.03, and 6.06 ± 2.80, respectively. A trend toward statistically significant differences when compared to the petri or IEQ conditions was observed for the mold (P = 0.076 and 0.085, respectively) and kugel (P = 0.078 and 0.085, respectively) conditions. Figure 4B presents insulin secretion during the basal and stimulated phases of GSIS expressed as a percentage of total insulin contents, determined by acid ethanol extraction. The drop condition showed higher insulin secretion during the basal and stimulated phases in comparison to the other conditions. Interestingly, this condition showed the lowest insulin content (Fig. 4C). The kugel and IEQ conditions showed the highest amount of insulin contents, but differences failed to reach statistical significance (Fig. 4C).
Figure 4.
Functional assessment of native islets and newly formed spheroids. (A) Insulin secretion, expressed as the stimulation index, visualized as bar plot. (B) Percentage on insulin content secreted during basal and stimulated phase. (C) Total insulin content expressed as pmol/l. N = 5.
Discussion
The generation of cell spheroids has been gaining increasing attention not only in the development of 3D culture systems but also as part of organ bioengineering strategies1. This is epitomized in the field of beta-cell replacement for type 1 diabetes, in which islet cell organoids with potential immunomodulatory features have been generated for transplantation8,9. In this study, we have assessed four different techniques of cell aggregation for the reconstitution of insulin-secreting spheroids from single islet cells. The different methods explored were assessed not only in terms of homogeneity and functionality, but first and foremost in terms of labor intensivity. The nonadherent petri dish technique was easy and fast but did not allow controlling the size or the composition of the cell aggregates. For these reasons, this method cannot be recommended, especially if spheroids of predetermined size and structure, using different cell types, are needed. Furthermore, this technique showed the lowest secretory capacity in response to glucose stimulation.
The drop, kugel, and mold conditions showed similar results in terms of morphology (diameter, homogeneity) and function. Remarkably, insulin secretion was improved compared to native islets or spheroids generated by self-aggregation. Furthermore, these three techniques allow to control islet spheroid size by selecting the number of cells per aggregate and offer the possibility of incorporating cells of different types. The drop method, although effective in terms of morphology and spheroid function, was much more labor intensive, and less cost-efficient given the additional materials and reagents required. Recovery of the newly formed spheroids was time consuming as well. Furthermore, this condition presented an intermediate result in terms of in vitro function but with an important variability, as shown by high SDs. However, this technique can be useful when very small amount of spheroids are required.
The mold technique showed good results in terms of morphology and function, and the quantity of material required was low. It is important to mention that about 20% of the agarose structures presented defects or were damaged when they were removed from their casts. One of the main differences with the Sphericalplate 5D™ is the shape of the microwell bottoms. In the agarose structure, the bottom is flat, whereas the Sphericalplate 5D™ microwells have conical bottoms. This potentially has a big impact because intercellular contacts are impaired in flat bottom wells when cell density is low (<250 cells/microwell), and aggregation can fail. On the contrary, the Sphericalplate 5D™ allows good intercellular contact regardless of the number of cells per microwell. Regarding cost considerations, microtissues silicon molds can be used repeatedly, making this technique very cost effective in the long run. The Sphericalplate 5D™ is very easy to use for cell plating as well as for spheroid recovery and presents good functional results. It allows good control of spheroid size and composition. Its major advantage is that it allows the large-scale production of substantial amounts of spheroids, with minimal effort in 750-microwell plates. This is of critical importance when large numbers of spheroids are needed (for instance in transplant experiments).
Bioengineering of cell aggregates has gained increasing interest in the field of cell transplantation, notably for the treatment of diabetes, but also in other areas of regenerative medicine and in cancer research26. For example, Furuyama et al. used cell re-aggregation after cell reprogramming of non-beta-cells into insulin-secreting cells allowing to correct diabetes in rodents27. We have recently published a study in which organoids composed of re-aggregated islet cells and amniotic epithelial cells were transplanted to diabetic mice and showed improved results in terms of diabetes reversion when compared to native islets8.
In this study, two methods stand out in terms of functional performance of the generated spheroids and minimization of labor intensivity. Islet cell spheroids generated either in locally produced silicon microwells (mold condition) or in the Sphericalplate 5D™ (kugel condition) provide highly functional insulin-producing constructs with minimal labor intensivity. From an economical perspective, the mold condition is more cost effective, but the Sphericalplate 5D™ is the only method that can be easily scaled up to produce large numbers of constructs, as would be required with a translational perspective to preclinical large mammal models or to the human.
We believe that this study will also help researchers working in other fields than beta-cell replacement to select the best method to generate cell aggregates or engineer organoids, depending on the type of their experiments.
Footnotes
Ethical Approval: This study was approved by the University of Geneva Institutional Animal Care and Use Committee, Geneva, Switzerland.
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines and approved by the Institutional Animal Care and Use Committee of the University of Geneva, Switzerland.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Swiss National Science Foundation (Grant #310030_173138, to EB and DB), European Foundation for the Study of Diabetes (to EB), Horizon 2020 Framework Program (VANGUARD 874700 to EB), Juvenile Diabetes Research Foundation (grant # 3-SRA-2020-926-S-B to EB) and the Shota Rustaveli National Science Foundation (FR-19-19760 to EB). The authors thank Thierry Berney for critical review of the manuscript and Patrick Kugelmeier for the provision of Sphericalplates 5D™.
ORCID iD: Charles-Henri Wassmer  https://orcid.org/0000-0002-9018-7088
https://orcid.org/0000-0002-9018-7088
References
- 1. Fitzgerald KA, Malhotra M, Curtin CM, FJ OB, CM OD. Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release. 2015;215:39–54. [DOI] [PubMed] [Google Scholar]
- 2. Weigelt B, Ghajar CM, Bissell MJ. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv Drug Deliv Rev. 2014;69–70:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das T, Meunier L, Barbe L, Provencher D, Guenat O, Gervais T, Mes-Masson AM. Empirical chemosensitivity testing in a spheroid model of ovarian cancer using a microfluidics-based multiplex platform. Biomicrofluidics. 2013;7(1):11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu L, Chen MC, Cheung KC. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip. 2010;10(18):2424–2432. [DOI] [PubMed] [Google Scholar]
- 5. Redondo-Castro E, Cunningham CJ, Miller J, Cain SA, Allan SM, Pinteaux E. Generation of human mesenchymal stem cell 3D spheroids using low-binding plates. Bio Protoc. 2018;8(16):e2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zafar A, Lee J, Yesmin S, Paget MB, Bailey CJ, Murray HE, Downing R. Rotational culture and integration with amniotic stem cells reduce porcine islet immunoreactivity in vitro and slow xeno-rejection in a murine model of islet transplantation. Xenotransplantation. 2019;26(4):e12508. [DOI] [PubMed] [Google Scholar]
- 8. Lebreton F, Lavallard V, Bellofatto K, Bonnet R, Wassmer CH, Perez L, Kalandadze V, Follenzi A, Boulvain M, Kerr-Conte J, Goodman DJ, et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat Commun. 2019;10(1):4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lebreton F, Bellofatto K, Wassmer CH, Perez L, Lavallard V, Parnaud G, Cottet-Dumoulin D, Kerr-Conte J, Pattou F, Bosco D, Othenin-Girard V, et al. Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am J Transplant. 2020;20(6):1551–1561. [DOI] [PubMed] [Google Scholar]
- 10. Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12(10):1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eder T, Eder IE. 3D hanging drop culture to establish prostate cancer organoids. Methods Mol Biol. 2017;1612:167–175. [DOI] [PubMed] [Google Scholar]
- 12. Khawar IA, Park JK, Jung ES, Lee MA, Chang S, Kuh HJ. Three dimensional mixed-cell spheroids mimic stroma-mediated chemoresistance and invasive migration in hepatocellular carcinoma. Neoplasia. 2018;20(8):800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu Y, Gamble A, Pawlick R, Pepper AR, Salama B, Toms D, Razian G, Ellis C, Bruni A, Gala-Lopez B, Lu JL, et al. Bioengineered human pseudoislets form efficiently from donated tissue, compare favourably with native islets in vitro and restore normoglycaemia in mice. Diabetologia. 2018;61(9):2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ota H, Kodama T, Miki N. Rapid formation of size-controlled three dimensional hetero-cell aggregates using micro-rotation flow for spheroid study. Biomicrofluidics. 2011;5(3):34105–3410515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moshksayan K, Kashaninejad N, Warkiani M, Lock JG, Moghadas H, Firoozabadi B, Saidi MS, Nguyen NT. Spheroids-on-a-chip: recent advances and design considerations in microfluidi platforms for spheroid formation and culture. Sensor Actuat B-Chem. 2018;263:151–176. [Google Scholar]
- 16. Lee SA, No da Y, Kang E, Ju J, Kim DS, Lee SH. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip. 2013;13(18):3529–3537. [DOI] [PubMed] [Google Scholar]
- 17. Jun Y, Lee J, Choi S, Yang JH, Sander M, Chung S, Lee SH. In vivo-mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci Adv. 2019;5(11):eaax4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R, Gassmann M, Groscurth P, Weber M. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14(1):67–76. [DOI] [PubMed] [Google Scholar]
- 19. Lehmann R, Zuellig RA, Kugelmeier P, Baenninger PB, Moritz W, Perren A, Clavien PA, Weber M, Spinas GA. Superiority of small islets in human islet transplantation. Diabetes. 2007;56(3):594–603. [DOI] [PubMed] [Google Scholar]
- 20. Kin T, Senior P, O’Gorman D, Richer B, Salam A, Shapiro AM. Risk factors for islet loss during culture prior to transplantation. Transpl Int. 2008;21(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 21. Arzouni AA, Vargas-Seymour A, Dhadda PK, Rackham CL, Huang GC, Choudhary P, King AJF, Jones PM. Characterization of the effects of mesenchymal stromal cells on mouse and human islet function. Stem Cells Transl Med. 2019;8(9):935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borot S, Crowe LA, Parnaud G, Ris F, Meier R, Giovannoni L, Muller YD, Lacotte S, Morel P, Toso C, Bosco D, et al. Quantification of islet loss and graft functionality during immune rejection by 3-tesla MRI in a rat model. Transplantation. 2013;96(5):438–444. [DOI] [PubMed] [Google Scholar]
- 23. Sutton R, Peters M, McShane P, Gray DW, Morris PJ. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986;42(6):689–691. [DOI] [PubMed] [Google Scholar]
- 24. Phelps EA, Cianciaruso C, Santo-Domingo J, Pasquier M, Galliverti G, Piemonti L, Berishvili E, Burri O, Wiederkehr A, Hubbell JA, Baekkeskov S. Advances in pancreatic islet monolayer culture on glass surfaces enable super-resolution microscopy and insights into beta cell ciliogenesis and proliferation. Sci Rep. 2017;7:45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cavallari G, Zuellig RA, Lehmann R, Weber M, Moritz W. Rat pancreatic islet size standardization by the “hanging drop” technique. Transplant Proc. 2007;39(6):2018–2020. [DOI] [PubMed] [Google Scholar]
- 26. Chaicharoenaudomrung N, Kunhorm P, Noisa P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J Stem Cells. 2019;11(12):1065–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Furuyama K, Chera S, van Gurp L, Oropeza D, Ghila L, Damond N, Vethe H, Paulo JA, Joosten AM, Berney T, Bosco D, et al. Diabetes relief in mice by glucose-sensing insulin-secreting human alpha-cells. Nature. 2019;567(7746):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]