Abstract
Although 90% of clinical islet transplantations are performed via the portal vein approach, it is still far from the ideal transplant site. Alternative islet transplant sites are promising to reduce the islet dose required to reverse hyperglycemia, thereby improving the efficiency of islet transplantation. The aim of this study was to compare the differences in survival and metabolic function of islet grafts transplanted into the hepatic sinus tract (HST) and the splenic parenchyma (SP). Approximately 300 syngeneic mouse islets were transplanted into the HST (n = 6) and the SP (n = 6) of recipient diabetic mice, respectively. After transplantation, the glycemic control, glucose tolerance, and morphology of islet grafts were evaluated and compared in each group. The nonfasting blood glucose of the two groups of mice receiving islet transplantation gradually decreased to the normal range and sustained for more than 100 d. There is no significant difference in the time required to restore normoglycemia (P > 0.05). The results of the glucose tolerance test showed that the SP group presented a smaller area under the curve than the HST group (P < 0.05). Histopathological results showed that islet grafts in the HST and the SP were characterized with normal islet morphology and robust insulin production. Compared with the HST, islet transplantation in the SP presents better blood glucose regulation, although there is no significant difference in the time required to restore normoglycemia.
Keywords: islet transplantation, diabetes, transplantation site, hepatic sinus tract, spleen
Introduction
Currently, diabetes has become a worldwide public health problem that seriously threatens human health and imposes substantial economic burden on society. According to World Health Organization statistics1, there are currently approximately 425 million diabetic patients worldwide, and it is estimated to increase further to 629 million by 2045. Islet transplantation is currently considered to be an effective method for the treatment of type 1 diabetes and certain insulin-dependent type 2 diabetes. Islet transplantation sites reported by literature mainly include the portal vein2, subrenal capsule3, splenic parenchyma (SP)4,5, omentum6, muscle7,8, subcutaneous9,10, gastrointestinal tract11, bone marrow12, and so on. These transplantation sites each have their own characteristics and show more or less advantages, but the optimal islet transplantation site is still controversial. The search for the ideal transplantation site has never stopped, because a more optimized transplantation site is expected to significantly improve the efficiency of islet transplantation and make limited organ resources benefit more patients. In the previous study13, our team successfully established a model of islet transplantation in the hepatic sinus tract (HST), and achieved a therapeutic effect comparable to that of subrenal islet transplantation. Recent studies have reported that the spleen is an ideal islet transplantation site due to its reduced inflammation and expansion of the islet graft14,15. However, we have no idea of which islet transplantation site is better between the HST and SP. Therefore, it is necessary to conduct a direct comparative study of the two clinical promising islet transplantation sites.
Materials and Methods
Animals
Specific pathogen-free (SPF) grade, male C57bl/6 mice, weighing 20 to 25 g, were purchased from the Experimental Animal Resource Center of Liaoning Province and used as recipients and donors. Recipient mice were randomly divided into four groups and exposed to different treatments. Schematic representation of the experimental protocol is available in the Supplemental Fig. 1. All animals were raised under SPF conditions. All experimental protocols were approved by Institutional Animal Care and Use Committee (IACUC) of China Medical University (No. 2019014).
Establishment of Diabetes Model
The mice were intraperitoneally injected with Streptozotocin (Sigma-Aldrich, Shanghai, China) at 180 mg/kg 1 wk before transplantation. Then, mice were considered to be diabetic when their nonfasting blood glucose levels reached at least 19.4 mmol/l for two consecutive daily readings. Mice with nonfasting blood glucose in the range of 20 to 30 mmol/l were screened for subsequent studies.
Isolation and Purification of Islets
Isolation and purification of islets were performed according to the previous method13. Briefly, mice were sacrificed by cervical dislocation and then immersed in 75% alcohol to sterilize. Then V-shaped laparotomy was performed, and the end of the common bile duct was ligated with 6-0 silk thread. Thereafter, collagenase V (1 mg/ml) (Sigma-Aldrich) was injected through the common bile duct by a 5-ml syringe connected to a 31G needle. After the pancreas was fully swelled, the pancreas was completely removed and placed in a 37°C water bath for 10 to 20 min. Immediately after the digestion was completed, precooled Hank’s solution (Beyotime, Shanghai, China) containing 10% fetal bovine serum (Clark, China) was added to discontinue digestion. After filtering digested pancreas through a 30-mesh filter, the islets were washed twice and then purified by Ficoll (Sigma-Aldrich) density gradient centrifugation. About 300 islets were hand-picked and cultured in the precooled 199 medium (Gibco, USA) for subsequent experiments.
Dithizone Staining
The islet suspension was transferred to a 15-ml centrifuge tube for centrifugation (300 × g for 3 min), and the supernatant was discarded after centrifugation. Then, the pellet was resuspended and transferred to a six-well plate with 2 ml Hank’s solution (Beyotime). About 20 µl of dithizone staining solution (0.67 mg/ml) (Solarbio, Beijing, China) was added in each well and incubated in dark for 10 min at room temperature, and then images were captured under an inverted microscope (Nikon, Japan).
AO-EB Staining
The collected islets were transferred to the six-well plate as previously described. Then, the prepared AO-EB dyeing working solution (Solarbio) was added according to the instructions. After incubating at room temperature for 2 min, the photos were recorded after excitation with 490 and 510 nm fluorescence. The living cells emitted green fluorescence, while the dead cells emitted red fluorescence. After capturing images, the islet viability was analyzed and calculated by Image J software.
Islet Transplantation
The islet suspension was centrifuged, resuspended with a small amount of 199 medium (Gibco), transferred to a PE50 tube, and kept on ice for subsequent experiments.
Procedures of islet transplantation in the SP group: The mice were anesthetized with continuous isoflurane (Yimeining, Shandong, China) inhalation through an animal anesthesia machine (MIDMARK, USA) and fixed in a lateral position. The spleen was exposed through the left inferior costal incision, and the prepared islet suspension was slowly pushed into the SP with a PE50 tube from the lower pole of the spleen. After the injection was completed, the spleen tail was ligated 3-0 silk (ETHICON, Shanghai, China) circularly, and the abdomen was closed when no active bleeding was seen. Tramadol hydrochloride (30 mg/kg) (Qimaite, Hebei, China) and cefazolin sodium (90 mg/kg) (Lukang, Shandong, China) were given subcutaneously in the first 3 d after the operation.
Procedures for islet transplantation in the HST group: According to the previous method13, the HST was created by temporarily placing a nylon material in the liver parenchyma for 4 wk, then the prepared islet suspension was slowly injected into the HST with a micro-syringe (Gaoge, Shanghai, China). After injection, the liver capsule near the entrance of the HST was sutured with 11-0 suture (Chenghe, Ningbo, China). The postoperative treatment was the same as that of the SP group.
Evaluation of Islet Graft Metabolic Function
The islet graft function was evaluated by measuring nonfasting blood glucose (ACCU-CHEK Performa, Roche, USA) every 3 d after islet transplantation13. If two consecutive blood glucose levels were less than 11.1 mmol/l, it can be determined to reverse the hyperglycemia. If the blood glucose level was less than 11.1 mmol/l for four times, the blood glucose will be monitored once a week. If the blood glucose level was less than 11.1 mmol/l for four consecutive weeks, the blood glucose will be measured once a month. Two consecutive blood glucose measurements more than 19.4 mmol/l indicated loss of graft function.
An intraperitoneal glucose tolerance test (IPGTT) was performed on the 60th day after transplantation. Mice were fasted overnight, and a 50% glucose solution (Kelun, Hunan, China) was injected intraperitoneally at 2 g/kg of body weight. Tail blood glucose was measured at 0, 15, 30, 60, 90, and 120 min after glucose injection. Graphpad Prism 8.0 software was used to draw the glucose tolerance curve and calculate the area under the curve (AUC) to evaluate the glucose tolerance of each group of mice.
On day 120 after transplantation, the mice were anesthetized for splenectomy or left hepatic lobe resection. After excision of the graft, the abdomen was closed after hemostasis, and the mice were bred for 1 wk to observe whether the blood glucose would rise to the preoperative level. The resected specimens were fixed with 4% paraformaldehyde solution (Biosharp, Anhui, China) for 24 h, dehydrated, embedded, and sectioned for hematoxylin and eosin (H&E) staining and immunohistochemical staining of insulin (Abcam, UK), and then images were acquired under a light microscope (Nikon, Japan).
Statistical Analysis
Data analysis and charting were performed using Graphpad Prism 8.0 software. Measurement data were described by mean ± SD. One-way analysis of variance and Tukey’s test were used for comparison between groups. P <0.05 was considered as a statistically significant difference. The AUC was calculated by Graphpad Prism 8.0 software using trapezoidal rules16.
Results
Dithizone Staining and Viability Evaluation of the Purified Islets
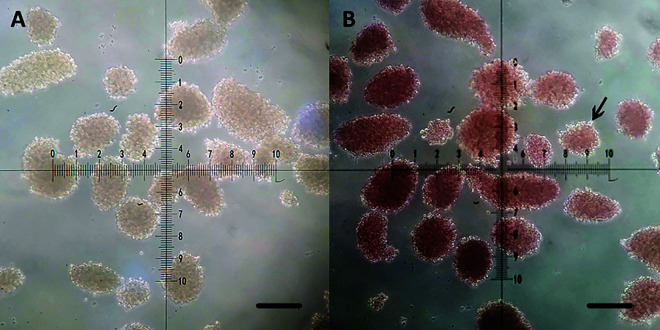
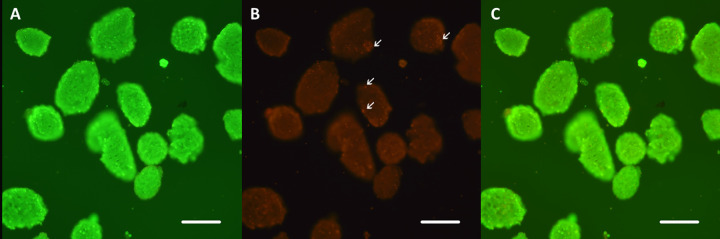
The purified islets were irregularly round or oval in shape, with clear boundaries and different sizes, ranging in diameter from 50 to 300 µm (Fig. 1A). After staining with dithizone, the islets were stained scarlet except for a small number of exocrine cells remaining on the surface (Fig. 1B). AO-EB staining showed that the viability of purified islets was >90%, and only a few scattered dead cells were seen (Fig. 2).
Fig. 1.
Dithizone staining of the purified islets. (A) The purified islets are irregular round or oval yellow masses with distinct boundaries and different sizes, ranging in diameter from 50 to 300 μm. (B) After staining with dithizone, the islets were stained scarlet except for a small number of exocrine cells (arrow) remaining on the surface (B). Scale bars, 100 μm.
Fig. 2.
Viability of the purified islets using live-dead fluorescent method. AO green (A) and EB red staining (B) indicate live and dead cells, respectively. The viability of purified islets was >90% (C), and only a few scattered dead cells (arrows) were seen. Scale bars, 100 μm.
Nonfasting Blood Glucose Measurements
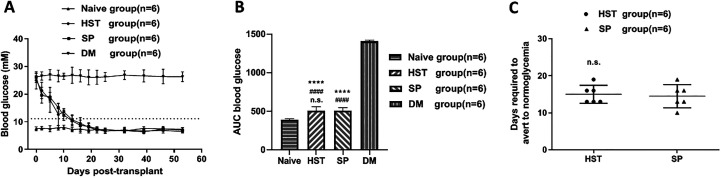
As shown in Fig. 3A, the initial blood glucose levels of the diabetes mellitus (DM) group (n = 6), the HST group (n = 6), and the SP group (n = 6) were 26.3 ± 1.2, 25.0 ± 3.1, and 26.1 ± 1.9 mmol/l, respectively. There was no statistical difference among these three groups (P > 0.05). The average weight of the mice in each group on the day of transplantation was comparable (Supplemental Table 1). After islet transplantation, both the blood glucose of mice in HST group and SP group gradually decreased to below 11.1 mmol/l. The AUC of the two groups were 505.3 ± 53.2 and 508.7 ± 37.5 mmol/l/53 d, respectively (Fig. 3B). There was no significant difference between the two groups (P > 0.05). Furthermore, the mean time required to restore normoglycemia for mice in the HST group was comparable (15.0 ±2.4 d vs 14.5 ± 3.1 d, P > 0.05) with the SP group (Fig. 3C).
Fig. 3.
Nonfasting blood glucose regulated by the syngeneic islet grafts. (A) On day 0, about 300 syngeneic islets were transplanted into the HST (n = 6) or the SP (n = 6) of the recipient mice. Data points represent blood glucose mean ± SD. The dotted straight line represents 11.1 mmol/l (B) AUCs expressed as mmol/l/53 d. The n.s. compared with the SP group (n = 6). #### P < 0.0001 compared with the Naive group (n = 6). ****P < 0.0001 compared with the DM group (n = 6). (C) The mean time required to restore normoglycemia after islet transplantation. The n.s. compared with the SP group (n = 6).
AUCs: area under the curve; DM: diabetes mellitus; HST: hepatic sinus tract; n.s.: not significant; SP: splenic parenchyma.
Glucose Tolerance Test
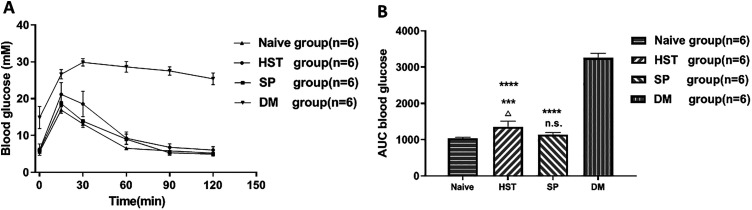
The IPGTT was performed on the 60th day after transplantation. Both the mice in the HST (n = 6) and the SP (n = 6) groups showed pretty glucose tolerance curves (Fig. 4A), but the AUC in the SP group (1143.0 ± 55.3 mmol/l/120 min) was smaller than the HST group (1355.3 ± 156.6 mmol/l/120 min, P < 0.05, Fig. 4B). Actually, the SP group displayed a similar glucose tolerance pattern to the Naive group (1143.0 ± 55.3 mmol/l/120 min vs 1042.0 ± 21.9 mmol/l/120 min, P > 0.05). In contrast, the mice in the DM group were intolerant to glucose stimulation, and the AUC was 3250.7 ± 128.5 mmol/l/120 min.
Fig. 4.
IPGTTs of syngeneic mouse islets transplanted into the HST or the SP, 60 d posttransplantation. Mice were fasted overnight and then 2 g/kg 50% dextrose were administered intraperitoneally. Blood glucose measurements were monitored at t = 0, 15, 30, 60, 90, and 120 min. (A) Blood glucose curve post dextrose bolus. Data points represent blood glucose mean ± SD. (B) AUCs expressed as mmol/l/120 min. △P < 0.05 compared with the SP group (n = 6). ***P < 0.001 and n.s. compared with the Naive group (n = 6). ****P < 0.0001 compared with the DM group (n = 6).
AUCs: area under the curve; DM: diabetes mellitus; HST: hepatic sinus tract; IPGTT: intraperitoneal glucose tolerance test; SP: splenic parenchyma.
Long-Term Islet Graft Retrieval
The results of long-term observation of the islet graft function are shown in Fig. 5. Both the mice in the HST (n = 4) and SP (n = 4) groups sustained normoglycemia for more than 100 d. After the grafts were completely excised on day 120 after transplantation, the blood glucose levels in both groups returned to the pretransplant levels in a week.
Fig. 5.
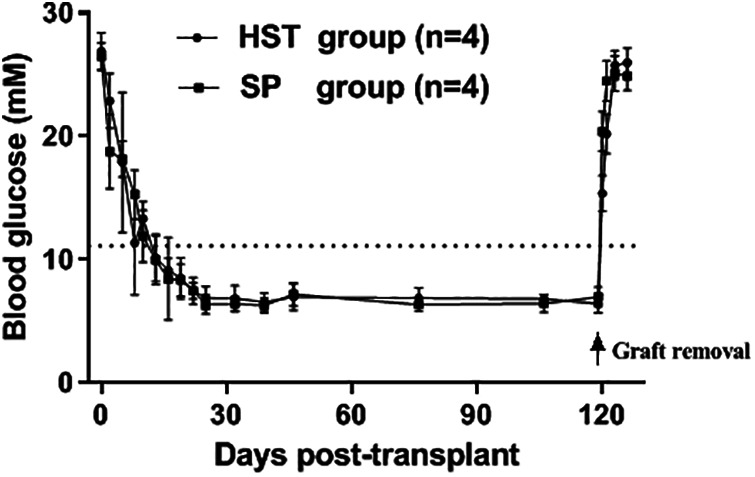
Long-term function of syngeneic islet grafts transplanted into the HST or the SP. Both the mice in the HST (n = 4) and SP (n = 4) groups sustained normoglycemia for more than 100 d. After the grafts were completely excised (arrow) on day 120 after transplantation, the blood glucose levels in both groups returned to the pretransplant levels in a week. The data points represent blood glucose mean ± SD. The dotted straight line represents 11.1 mmol/l.
HST: hepatic sinus tract; SP: splenic parenchyma.
Histopathological Staining of the Islet Grafts
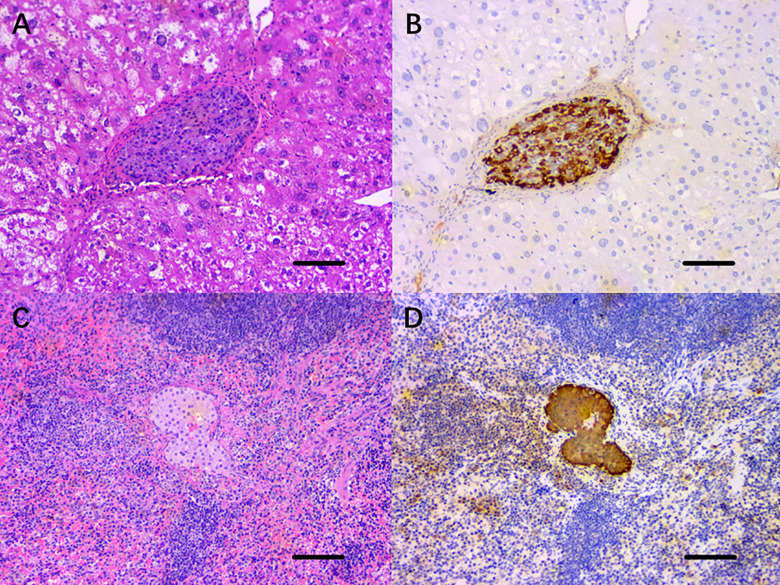
On the 120th day after islet transplantation, the left lobe of the liver where the implanted graft was located was completely excised, and the sections of the islet graft were stained with H&E (Fig. 6A). In the normal form of hepatocytes and hepatic sinusoids, no obvious inflammatory cells infiltration was seen in or around the islet graft. Similarly, the sections of the spleen were stained with H&E (Fig. 6C). It was found that the surviving islet cell clusters were located in the parenchyma of the spleen. There was no significant inflammatory cell infiltration within the grafts. Immunohistochemical staining of the islet grafts sections for insulin in both groups (Fig. 6B,D) showed that insulin was expressed uniformly in the cytoplasm of the islet cell mass.
Fig. 6.
Histopathological staining of the islet grafts transplanted into the HST or the SP, 120 d posttransplantation. (A) H&E staining of the horizontal section of the left lobe of the liver revealed that the islet grafts in the HST were surrounded by normal liver cells and hepatic blood sinuses, with no obvious inflammatory cell infiltration. (C) H&E staining of horizontal section of the spleen showed that the islet graft was located in the splenic parenchyma, and no inflammatory cells infiltrated into the islet graft. (B, D) Immunohistochemical staining of the islet grafts sections for insulin in both groups showed that insulin was expressed uniformly in the cytoplasm of the islet cell mass. Scale bars, 100 μm.
H&E: hematoxylin and eosin; HST: hepatic sinus tract; SP: splenic parenchyma.
Discussion
Since the Edmonton protocol proposed in 200017, more than 1,000 patients worldwide have been treated with islet transplantation18. Currently, 90% of islet transplantations are clinically performed through the portal vein19, but a large amount of literature indicates many defects associated with this approach, including instant blood-mediated inflammatory reaction20,21, the risk of hemorrhage, embolism22,23 and the hepatic tissue necrosis24. Besides, due to the scattered distribution of islets, graft biopsy is rarely performed11, which is not conducive to the early detection of graft rejection. At present, it is believed that an ideal transplantation site should have convenient operation, less complications, lower risk, repeatable operation and biopsy, rich blood flow, and relatively small rejection25. Obviously, the portal vein route is not an ideal site for islet transplantation.
Due to its abundant blood supply, draining into the portal vein, spleen may become a potential transplantation site. Previous studies have suggested that the graft is more likely to be rejected by the recipient when it directly contacts with lymphoid tissue or organs such as spleen, and on the other hand, no special advantages of islet grafts in the SP over the liver were found26,27. However, recent studies14,15 have found that intrasplenic islet transplantation requires less marginal islet mass (50 syngeneic islets) than the portal vein and subrenal capsule route, suggesting that the spleen may have a better survival environment for islet grafts. In other words, the efficiency of intrasplenic islet transplantation remains controversial28–30.
The purpose of this experiment is to compare the effects of syngeneic islet transplantation into the HST and the SP, thus providing a reference for the selection of laboratory and clinical islet transplantation sites. It can be seen that glycemic control and graft morphology, including the immunohistochemical staining, showed similar results in the HST and the SP groups. However, in terms of glucose tolerance test, the islet grafts in the SP group showed better blood glucose regulation than the HST group. The reason for this difference may be due to the characteristics of SP. First, the spleen favors the expansion of the islet graft14. Secondly, the spleen acts as a stem cell reservoir, which accelerates the repair of the damage caused by the transplantation procedure15. Furthermore, it has been reported that these splenic mesenchymal stem cells may differentiate directly into islets31.
Although intrasplenic islet transplantation achieved improved results, we still cannot ignore its inherent defects, such as postoperative spleen infarction5, 32 and bleeding. Frankly, no perfect islet transplantation site has been found so far. But it is believed that spleen may prove to be superior than other sites when further researches were done. Continuous exploration of the ideal islet transplantation site will help clarify the necessary conditions for the survival of islet grafts, and obtain new ideas for improving the transplantation site.
Conclusion
Compared with the HST, islet transplantation in the SP presents better blood glucose regulation, although there is no significant difference in the time required to restore normoglycemia.
Supplemental Material
Supplementary for Comparative Study of Two Different Islet Transplantation Sites in Mice: Hepatic Sinus Tract vs Splenic Parenchyma by Feng Li, Yi Lv, Xiaohang Li, Zhaoming Yang, Tingwei Guo and Jialin Zhang in Cell Transplantation
Footnotes
Author Contributions: FL participated in the research design, performance of the research, data analysis, and writing of the manuscript. YL, ZY, and TG participated in the performance of the research and writing of the manuscript. XL and JZ participated in research design and writing of the manuscript.
Ethical Approval: This study was approved by the IACUC of China Medical University, Liaoning Province, China (No. 2019014).
Statement of Human and Animal Rights: All experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines of China Medical University, China. No human rights were involved in this study.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the program of Liaoning Provincial Science and Technology Department (Grant no. 2017225031).
ORCID iD: Jialin Zhang  https://orcid.org/0000-0003-2617-2735
https://orcid.org/0000-0003-2617-2735
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Dadheech N, James Shapiro AM. Human induced pluripotent stem cells in the curative treatment of diabetes and potential impediments ahead. Adv Exp Med Biol. 2019;1144:25–35. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–277. [DOI] [PubMed] [Google Scholar]
- 3. Smood B, Bottino R, Hara H, Cooper DKC. Is the renal subcapsular space the preferred site for clinical porcine islet xenotransplantation? Review article. Int J Surg. 2019;69:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Y, Zhang JL, Liu YF, Li TM, Zhao N. Islet transplantation for diabetic rats through the spleen. Hepatobiliary Pancreat Dis Int. 2005;4(2):203–206. [PubMed] [Google Scholar]
- 5. White SA, London NJ, Johnson PR, Davies JE, Pollard C, Contractor HH, Hughes DP, Robertson GS, Musto PP, Dennison AR. The risks of total pancreatectomy and splenic islet autotransplantation. Cell transplantation. 2000;9(1):19–24. [DOI] [PubMed] [Google Scholar]
- 6. Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C, Pileggi A. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes. 2016;65(5):1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakata N, Aoki T, Yoshimatsu G, Tsuchiya H, Hata T, Katayose Y, Egawa S, Unno M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab Res Rev. 2014;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 8. Svensson J, Lau J, Sandberg M, Carlsson PO. High vascular density and oxygenation of pancreatic islets transplanted in clusters into striated muscle. Cell transplantation. 2011;20(5):738S–783S. [DOI] [PubMed] [Google Scholar]
- 9. Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol. 2015;33(5):518–523. [DOI] [PubMed] [Google Scholar]
- 10. Gebe JA, Preisinger A, Gooden MD, D’Amico LA, Vernon RB. Local, controlled release in vivo of vascular endothelial growth factor within a subcutaneous scaffolded islet implant reduces early islet necrosis and improves performance of the graft. Cell Transplantation. 2018;27(3):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujita M, McGrath KM, Bottino R, Dons EM, Long C, Kumar G, Ekser B, Echeverri GJ, Hata J, Haruma K, Cooper DK. Technique of endoscopic biopsy of islet allografts transplanted into the gastric submucosal space in pigs. Cell transplantation. 2013;22(12):2335–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier RP, Seebach JD, Morel P, Mahou R, Borot S, Giovannoni L, Parnaud G, Montanari E, Bosco D, Wandrey C, Berney T, et al. Survival of free and encapsulated human and rat islet xenografts transplanted into the mouse bone marrow. PLoS One. 2014;9(3):e91268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li F, Jiao A, Li X, Zhang C, Sun N, Zhang J. Survival and metabolic function of syngeneic mouse islet grafts transplanted into the hepatic sinus tract. Transplantation. 2018;102(11):1850–1856. [DOI] [PubMed] [Google Scholar]
- 14. Itoh T, Nishinakamura H, Kumano K, Takahashi H, Kodama S. The spleen is an ideal site for inducing transplanted islet graft expansion in mice. PLoS One. 2017;12(1):e0170899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakata N, Yoshimatsu G, Kodama S. The spleen as an optimal site for islet transplantation and a source of mesenchymal stem Cells. Int J Mol Sci. 2018;19(5):1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiao A, Li F, Zhang C, Lv W, Chen B, Zhang J. βSimulated cholinergic reinnervation of (ins-1) cells: antidiabetic utility of heterotypic pseudoislets containing cell and cholinergic cell. Int J Endocrinol. 2018;2018:1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shapiro AJ, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New Eng J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 18. Vantyghem MC, de Koning EJ, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet (London, England). 2019;394(10205):1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol. 2013;2013:352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–133. [DOI] [PubMed] [Google Scholar]
- 21. Eich T, Eriksson O, Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med. 2007;356(26):2754–2755. [DOI] [PubMed] [Google Scholar]
- 22. Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AM. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–2157. [DOI] [PubMed] [Google Scholar]
- 23. Sakata N, Hayes P, Tan A, Chan NK, Mace J, Peverini R, Sowers L, Pearce WJ, Chinnock R, Obenaus A, Hathout E. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation. 2009;87(6):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melzi R, Sanvito F, Mercalli A, Andralojc K, Bonifacio E, Piemonti L. Intrahepatic islet transplant in the mouse: functional and morphological characterization. Cell transplantation. 2008;17(12):1361–1370. [DOI] [PubMed] [Google Scholar]
- 25. Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11(5):364–374. [DOI] [PubMed] [Google Scholar]
- 26. Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95(12):1449–1461. [DOI] [PubMed] [Google Scholar]
- 27. Sutton R, Gray DW, Burnett M, McShane P, Turner RC, Morris PJ. Metabolic function of intraportal and intrasplenic islet autografts in cynomolgus monkeys. Diabetes. 1989;38(suppl 1):182–184. [DOI] [PubMed] [Google Scholar]
- 28. Evans MG, Warnock GL, Rajotte RV. Comparison of sites for transplantation of canine pancreatic microfragments. Diabetes Res. 1989;10(1):35–41. [PubMed] [Google Scholar]
- 29. Hesse UJ, Meyer GP, Weyer J, Danis J, Pichlmaier H. Islet isolation and autotransplantation in pigs. Zentralbl Chir. 1994;119(9):653–660. [PubMed] [Google Scholar]
- 30. Warnock GL, Dabbs KD, Evans MG, Cattral MS, Kneteman NM, Rajotte RV. Critical mass of islets that function after implantation in a large mammalian. Hormone and metabolic research. Supplement series. 1990;25:156–161. [PubMed] [Google Scholar]
- 31. Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in nod mice. Science. 2003;302(5648):1223–1227. [DOI] [PubMed] [Google Scholar]
- 32. White SA, Robertson GS, Davies JE, Rees Y, London NJ, Dennison AR. Splenic infarction after total pancreatectomy and autologous islet transplantation into the spleen. Pancreas. 1999;18(4):419–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary for Comparative Study of Two Different Islet Transplantation Sites in Mice: Hepatic Sinus Tract vs Splenic Parenchyma by Feng Li, Yi Lv, Xiaohang Li, Zhaoming Yang, Tingwei Guo and Jialin Zhang in Cell Transplantation