Abstract
Clear cell renal cell carcinoma (ccRCC) is the prominent histological subtype of renal cell carcinoma (RCC) with high incidence of local recurrence and distant metastasis. It has been documented that circular ribonucleic acids (circRNAs) play crucial roles in the development of cancers; however, study on exploring the role of circRNAs in ccRCC still remains limited. In the present study, we aimed to evaluate the biological function of a novel circRNA UBAP2 (circUBAP2) in ccRCC and the underlying mechanism. Our results showed that circUBAP2 expression was significantly down-regulated in ccRCC tissues and cell lines. Overexpression of circUBAP2 significantly inhibited the proliferation, migration, and invasion of ccRCC cells. MiR-148a-3p was a target miRNA of circUBAP2 in ccRCC cells, and its expression levels in ccRCC tissues and cell lines were negatively correlated with circUBAP2 levels. Moreover, miR-148a-3p reversed the inhibitory effects of circUBAP2 on cell proliferation, migration, and invasion in ccRCC cells. Additionally, forkhead box K2 (FOXK2) was found to be a target gene of miR-148a-3p and regulated by miR-148a-3p in ccRCC cells. Furthermore, knockdown of FOXK2 reversed the inhibitory effects of miR-148a-3p inhibitor on ccRCC cells. In conclusion, these findings indicated that circUBAP2 functioned as a novel tumor suppressor in ccRCC through regulating the miR-148a-3p/FOXK2 axis. Therefore, circUBAP2 might serve as a potential therapeutic target for the treatment of ccRCC.
Keywords: clear cell renal cell carcinoma (ccRCC), circUBAP2, miR-148a-3p, FOXK2, tumor suppressor
Introduction
Renal cell carcinoma (RCC) is the most common type of urological malignant tumors in the kidney1. Clear cell RCC (ccRCC) is the prominent histological subtype of RCC, accounting for about 80% to 90% of all RCC cases2. Although the recent efforts have devoted to developing therapeutic approaches, the prognosis of patients with ccRCC remains poor3. Local recurrence and distant metastasis are commonly occurred in ccRCC patients after treatments4,5. Therefore, developing new therapies are imperatively needed for patients with ccRCC.
Non-coding ribonucleic acids (ncRNAs) are endogenous RNA molecules that are not translated into proteins6. In recent years, several types of ncRNAs such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) have attracted many attentions7,8. CircRNAs are a peculiar group of RNA consisting of at least a few hundred nucleotides with a covalently closed continuous loop9. Growing evidence has confirmed that circRNAs regulate gene expression at the transcriptional or post-transcriptional level through binding to target miRNAs or other molecules. A number of studies have unveiled the participation of circRNAs in the regulation of malignant biological processes including tumor cells proliferation, differentiation, apoptosis, invasion, and metastasis10,11. Thus, many circRNAs are considered as therapeutic targets for the treatment of cancers. However, the exact role of circRNAs in ccRCC still remains largely unclear.
CircUBAP2 is an identified cancer-related circRNA and has been found to be abnormally expressed in several types of cancers. CircUBAP2 expression is significantly increased in human osteosarcoma tissue and correlated with osteosarcoma progression and prognosis12. High circUBAP2 expression has been observed in triple-negative breast cancer (TNBC) tissues and promotes TNBC progression13. These findings suggest that abnormal expression of circUBAP2 is involved in the development and progression of tumors. However, the role of circUBAP2 in ccRCC has not been reported.
In the present study, we attempted to investigate the expression levels and roles of circUBAP2 in ccRCC. The relevant molecular mechanisms of circUBAP2 were also illustrated in vitro.
Materials and Methods
Clinical Tissues and Cell Culture
The ccRCC tissues (n = 24) and paired normal tissues (n = 24) were obtained from ccRCC patients after surgery at the First Affiliated Hospital of Medical College, Xi’an Jiaotong University (Xi’an, China). Informed consent was obtained from all participants. The samples were used for the analysis of circUBAP2 expressions with quantitative real-time polymerase chain reaction (qRT-PCR). The usage of the clinical samples in the present study was approved by the Ethics Committee at the First Affiliated Hospital of Medical College, Xi’an Jiaotong University.
A normal human renal tubular epithelial cell line HK-2 and four human ccRCC cell lines (786-O, A498, ACHN, and Caki-1) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (HyClone Laboratories, Logan, UT, USA) with 10% heat-inactivated fetal bovine serum (FBS). All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Oligonucleotides, Plasmids, and Cell Transfection
The full-length sequence of circUBAP2 was inserted into the pcDNA3.1 vector to construct the circUBAP2 overexpressing vector pcDNA3.1-circUBAP2. Small interfering RNA (siRNA) oligonucleotide targeting forkhead box K2 (si-FOXK2) and negative control siRNA (si-NC) were chemically synthesized by Guangzhou Ribobio Co., Ltd. (Guangzhou, China). The miR-148a-3p mimics, control miRNA mimics (miR-NC), miR-148a-3p inhibitor (miR-in-148a-3p), and control miRNA inhibitor (miR-in-NC) were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Caki-1 cells were seeded into six-well plates and incubated for 24 h prior to the transfection. Cell transfections were performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in accordance with the manufacturer’s protocol. Forty-eight hours after transfection, cells were collected for subsequent experiments.
Cell Proliferation Assay
Transfected Caki-1 cells were inoculated on 96-well plates at a cell density of 1 × 103 cells per well. Cell proliferation assay was performed using a Cell Counting Kit-8 (CCK8; Dojindo Molceular Technologies, Kumamoto, Japan). After 0, 24, 48, or 72 h, 10 µL CCK-8 solution was added to each well and incubated for 3 h at 37°C. The optical density of each well was monitored at the wavelength of 450 nm with a spectrophotometer.
Cell Cycle Assay
Transfected Caki-1 cells were harvested and washed by cold PBS. The cells were further fixed with 70% ice-cold ethanol at 4°C overnight and resuspended in staining solution included with the cell cycle detection kit (Nanjing KeyGen Biotech. Co. Ltd., Nanjing, China). After incubation for 1 h at 37°C in the dark, the stained cells were subsequently analyzed by flow cytometer fluorescence-activated cell sorting (FACS) using the BD FACSCalibur™ Cell Analyzer system (BD Biosciences, San Jose, CA, USA).
Cell Apoptosis Assay
After transfection, Caki-1 cells were detached with EDTA-free trypsin, collected, and centrifuged at 1,000 rpm/min for 5 min at 4°C and the supernatant was discarded. Then, harvested Caki-1 cells were double-stained with propidium iodide (PI) according to the protocol of a FITC-Annexin V cell apoptosis assay kit (BD Biosciences). The cells were then analyzed using a flow cytometer (FACScan; BD Biosciences).
Cell Migration and Invasion Assays
Transwell assays were carried out to assess the migration and invasion abilities of Caki-1 cells using Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA). For the detection of invasion ability, the filters were pre-coated with Matrigel (BD Biosciences). A total of 200 µL FBS-free Dulbecco’s Modified Eagle Medium (DMEM) containing 5 × 104 transfected cells was seeded into the upper chambers, while 500 µL DMEM containing 20% FBS was added into the lower chambers to serve as a chemoattractant. Twenty-four hours later, cells remained on the upper side of the filters were gently removed using a cotton swab. The cells on the lower side of the filters were fixed with 95% ethanol for 15 min and stained with 0.1% crystal violet (Beyotime Institute of Biotechnology, Inc., Shanghai, China) for 20 min at room temperature. The numbers of stained cells in five randomly visual fields were counted under an inverted microscope.
qRT-PCR Analysis
The surgical specimens and cultured cells were collected for the isolation of total RNA using the TRIzol reagent (Invitrogen). The miScript Reverse Transcription kit (Qiagen GmbH, Hilden, Germany) and miScript SYBR Green PCR kit (Qiagen GmbH) were applied to quantify the expression of miR-148a-3p. To determine the expression of circUBAP2, PrimeScript RT Reagent Kit (Takara Biotechnology, Dalian, China) and SYBR Premix Ex Taq™ Kit (Takara) were used on a Roche Light Cycler 480 Real-Time PCR System (Roche Diagnostics, Basel, Switzerland). Small nuclear RNA U6 was served as endogenous controls to normalize the expression levels of target genes. All data were analyzed using the 2-ΔΔCt method.
Western Blot
Caki-1 cells lysates were prepared using radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich, St Louis, MO, USA) containing a protease inhibitor cocktail (Promega, Madison, WI, USA). The total protein concentration of each sample was quantified using a bicinchoninic acid assay (Sangon Biotech, Shanghai, China). Equivalent amounts (30 μg) of protein were separated via electrophoresis on a 10% SDS–PAGE gel and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% skim milk at room temperature for 2 h, the membranes were incubated with primary antibodies against FOXK2 (1:1000 dilution; Abcam, Cambridge, UK, USA) or GAPDH (1:1000 dilution; Abcam) for 8 h at 4°C. Next, a horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000 dilution; Abcam) was added to the cells and incubated at room temperature for 2 h. Finally, the immunoreactive protein bands were visualized using an Enhanced Chemiluminescence Reagent (Bio-Rad Laboratories, Hercules, CA, USA).
Bioinformatics Prediction
StarBase 3.0 (http://starbase.sysu.edu.cn/) was applied to search the miRNAs that may be sponged by circUBAP2. The putative target genes of miR-148a-3p were predicted using TargetScan (http://www.targetscan.org/).
Luciferase Reporter Assay
The wild-type (Wt) or mutant (Mut) sequences of circUBAP2 were cloned into the pMIR-REPORT luciferase vector (Ambion) and named as pMIR-circUBAP2-Wt and pMIR-circUBAP2-Mut, respectively. The Wt or Mut 3’-UTR fragments of FOXK2 containing the miR-148a-3p binding sites were amplified and inserted into pMIR-REPORT to construct pMIR-FOXK2-Wt and pMIR-FOXK2-Mut, respectively. Cells were seeded in 24-well plates and cotransfected with the indicated luciferase reporter plasmid and miR-148a-3p mimics or miR-NC using Lipofectamine 2000 according to the manufacturer’s protocol. At 48 h after transfection, luciferase activity was measured using a Dual-Luciferase Reporter System (Promega).
Statistical Analysis
All analyses are performed using SPSS version 21.0 (SPSS Inc., Chicago, MO, USA) and represent data from three individual experiments. Two-tailed Student’s t-test or one-way analysis of variance was used for evaluating the significance of differences between subgroups. Statistical significance was set when P < 0.05.
Results
CircUBAP2 Expression is Down-Regulated in ccRCC Tissues and Cell Lines
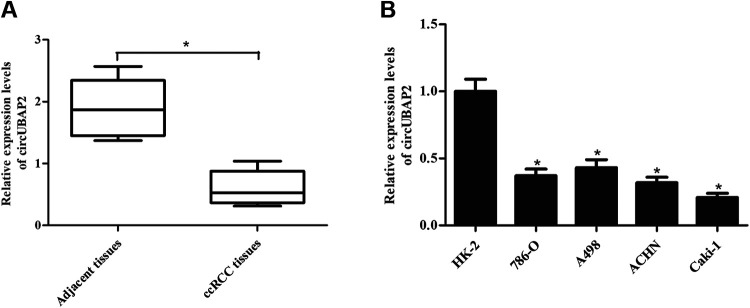
To investigate the role of circUBAP2 in ccRCC, we delineated the expression profile of circUBAP2 in ccRCC tissues and adjacent tissues. As shown in Fig. 1A, the expression levels of circUBAP2 were significantly decreased in the ccRCC tissues compared with that in the adjacent tissues. Next, we detected circUBAP2 expression in human renal tubular epithelial cell line HK-2 and four human ccRCC cell lines (786-O, A498, ACHN, and Caki-1). The results demonstrated that circUBAP2 was downregulated in all four ccRCC cell lines, especially in Caki-1 cells (Fig. 1B).
Fig. 1.
Downregulation of circUBAP2 expression in ccRCC tissues and cell lines. (A) CircUBAP2 expression in 24 paired ccRCC tissues and adjacent tissues. *P < 0.05, compared with adjacent tissues. (B) CircUBAP2 expression in human renal tubular epithelial cell line HK-2 and four human ccRCC cell lines (786-O, A498, ACHN, and Caki-1). *P < 0.05, compared with HK-2 cells. qRT-PCR: quantitative real-time polymerase chain reaction; ccRCC: clear cell renal cell carcinoma.
CircUBAP2 Inhibits the Proliferation, Migration, and Invasion of ccRCC Cells
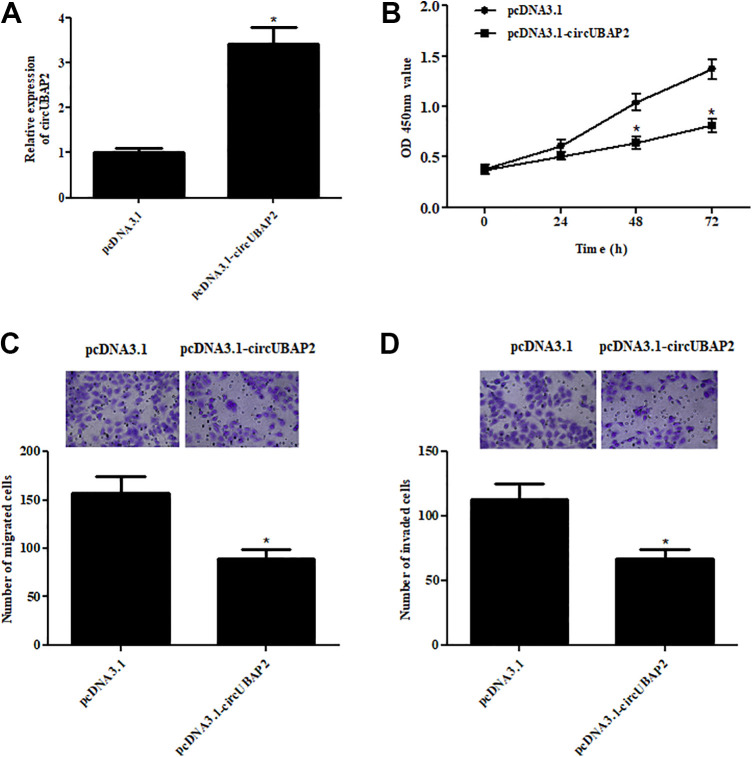
We selected Caki-1 cells for further analyzing the function of circUBAP2 in ccRCC. Caki-1 cells were transfected with pcDNA3.1-circUBAP2 or pcDNA3.1. After 48 h, circUBAP2 expression was markedly increased in pcDNA3.1-circUBAP2 transfected Caki-1 cells (Fig. 2A). To examine the effect of circUBAP2 on cell proliferation, CCK-8 assay was performed. The results showed that cell proliferation was significantly decreased after transfection with pcDNA3.1-circUBAP2, as compared with the pcDNA3.1 group (Fig. 2B). We next explored the effects of circUBAP2 on cell migration and invasion. Transwell assays proved that overexpression of circUBAP2 resulted in significant decreases in cell migration and invasion of Caki-1 cells (Fig. 2C,D).
Fig. 2.
The effects of circUBAP2 on proliferation, migration, and invasion of ccRCC cells. (A) Caki-1 cells were transfected with pcDNA3.1-circUBAP2 or pcDNA3.1, at 48 h post-transfection and the qRT-PCR was performed to detect the circUBAP2 expression. (B) CCK-8 assay was performed to examine the cell proliferation. (C,D) Transwell assay was carried out to detect cell migration and invasion. *P < 0.05, compared with pcDNA3.1-transfected Caki-1 cells. qRT-PCR: quantitative real-time polymerase chain reaction; ccRCC: clear cell renal cell carcinoma.
CircUBAP2 Triggered ccRCC Cell Apoptosis and Disturbed Cell Cycle
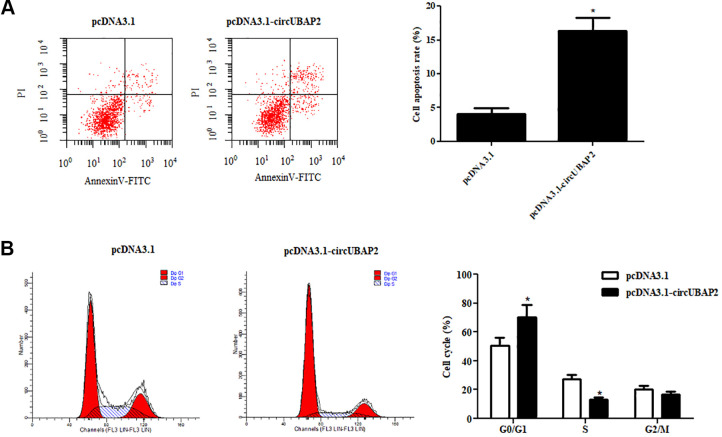
Next, we examined the effects of circUBAP2 on cell apoptosis and cell cycle. As indicated in Fig. 3A, cell apoptosis was obviously increased by overexpression of circUBAP2. Furthermore, the results of cell cycle assay showed that cell cycle was blocked in G1 phase by circUBAP2 overexpression in Caki-1 cells (Fig. 3B).
Fig. 3.
CircUBAP2 triggered ccRCC cell apoptosis and disturbed cell cycle. (A) Flow cytometry assay was used to analyze cell apoptosis. (B) Effects of circUBAP2 on cell cycle. *P < 0.05, compared with pcDNA3.1-transfected Caki-1 cells. ccRCC: clear cell renal cell carcinoma.
CircUBAP2 Targets miR-148a-3p in ccRCC Cells
To further substantiate the underlying mechanism of the above findings, StarBase 3.0 (http://starbase.sysu.edu.cn/) was applied to identify the target miRNAs of circUBAP2. As indicated in Fig. 4A, miR-148a-3p might be a target miRNA of circUBAP2. Luciferase reporter assay demonstrated that luciferase activity of pMIR-circUBAP2-Wt was markedly decreased after cotransfection with miR-148a-3p mimics (Fig. 4B). The results indicated that circUBAP2 might sponge miR-148a-3p in Caki-1 cells.
Fig. 4.
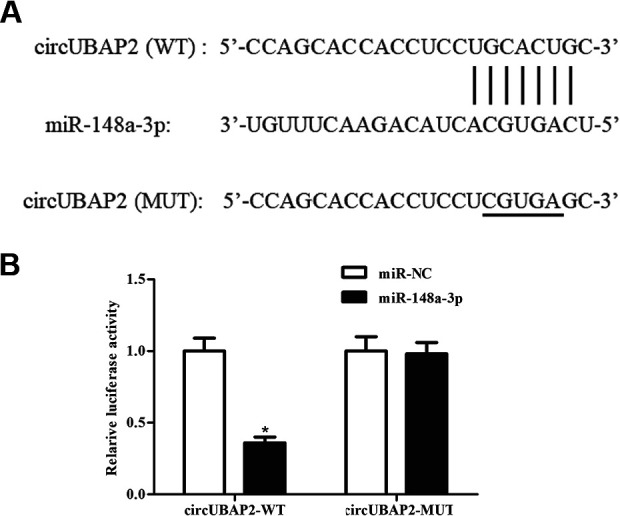
CircUBAP2 sponges miR-148a-3p in Caki-1 cells. (A) MiR-148a-3p might be a target miRNA of circUBAP2. (B) Luciferase reporter assay was performed to validate the interaction between circUBAP2 and miR-148a-3p. *P < 0.05, compared with Caki-1 cells cotransfected with pMIR-circUBAP2-Mut and miR-148a-3p mimics. miRNAs: microRNAs.
MiR-148a-3p Expression Is Up-Regulated in ccRCC Tissues and Cell Lines
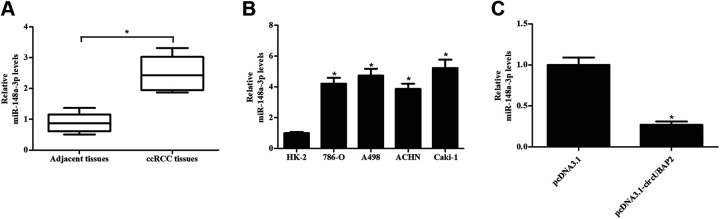
Next, we assessed the expression of miR-148a-3p in clinical tissues and cell lines. The results proved that miR-148a-3p expressions were markedly increased in both ccRCC tissues and cell lines, which indicated a negative correlation between miR-148a-3p and circUBAP2 levels (Fig. 5A,B). To further confirm the relation between miR-148a-3p and circUBAP2, we examine the effect of circUBAP2 overexpression on the expression of miR-148a-3p. A significant decrease in miR-148a-3p expression was observed in circUBAP2-overexpressing Caki-1 cells (Fig. 5C).
Fig. 5.
Up-regulation of miR-148a-3p expression in ccRCC tissues and cell lines. (A) MiR-148a-3p expression in 24 paired ccRCC tissues and adjacent tissues. *P < 0.05, compared with adjacent tissues. (B) MiR-148a-3p expression in human renal tubular epithelial cell line HK-2 and four human ccRCC cell lines (786-O, A498, ACHN, and Caki-1). *P < 0.05, compared with HK-2 cells. (C) Effect of circUBAP2 overexpression on the expression of miR-148a-3p. *P < 0.05, compared with pcDNA3.1-transfected Caki-1 cells. ccRCC: clear cell renal cell carcinoma.
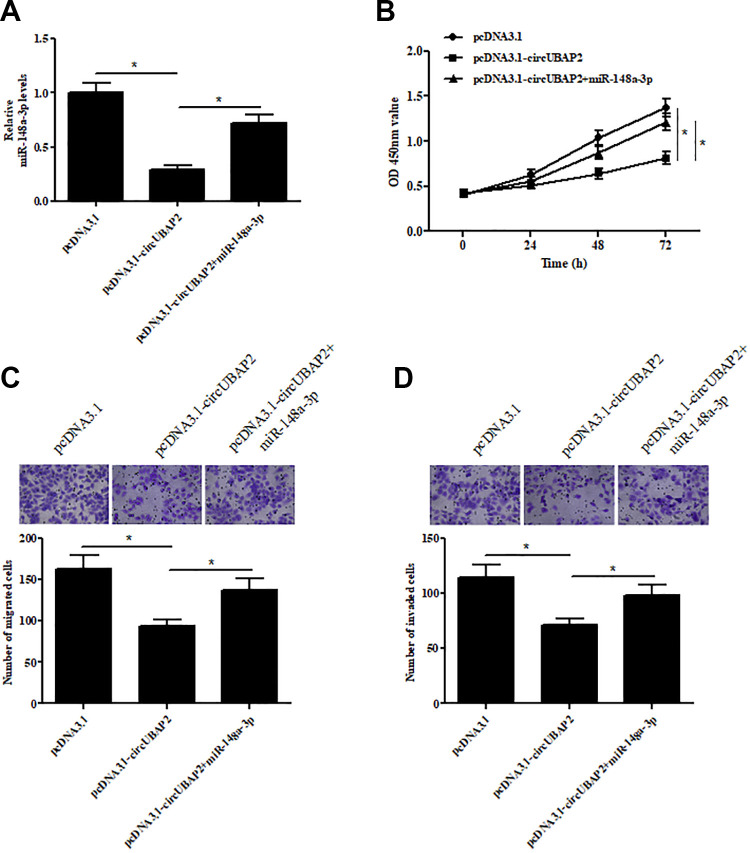
MiR-148a-3p Mimics Reversed the Inhibitory Effects of circUBAP2 on Cell Proliferation, Migration, and Invasion
To validate the role of miR-148a-3p, miR-148a-3p mimics were transfected into circUBAP2-overexpressing Caki-1 cells. As showed in Fig. 6A, the expression of miR-148a-3p was significantly increased in circUBAP2-overexpressing Caki-1 cells. Furthermore, compared to the circUBAP2-overexpressing Caki-1 cells, the proliferative ability of miR-148a-3p mimics-transfected Caki-1 cells was significantly increased (Fig. 6B). Besides, the migrative and invasive abilities were also promoted by transfection with miR-148a-3p mimics (Fig. 6C,D).
Fig. 6.
The effects of miR-148a-3p mimics on cell proliferation, migration, and invasion of Caki-1 cells. (A) qRT-PCR analysis showing the miR-148a-3p levels in Caki-1 cells transfected with pcDNA3.1-circUBAP2 and miR-148a-3p mimics. (B) CCK-8 assay was performed to examine the cell proliferation. (C,D) Transwell assay was carried out to detect cell migration and invasion. *P < 0.05. qRT-PCR: quantitative real-time polymerase chain reaction.
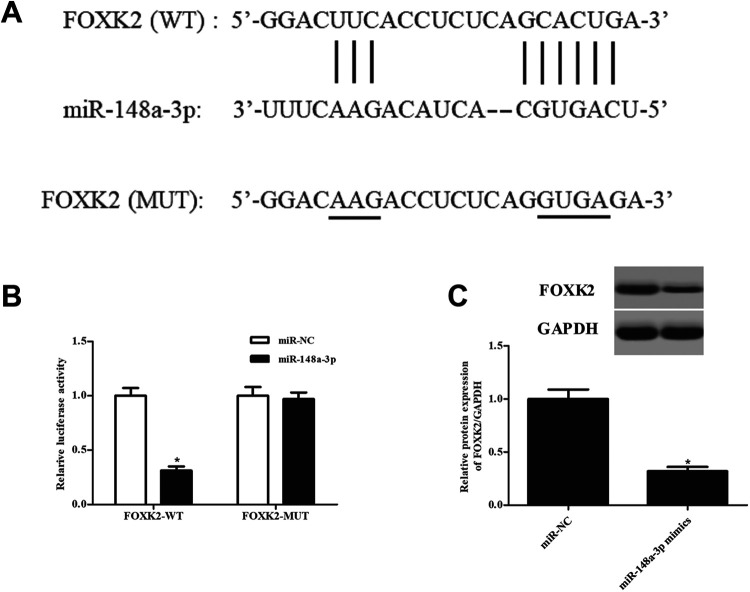
MiR-148a-3p Targets FOXK2 in ccRCC Cells
The downstream gene of miR-148a-3p was predicted by TargetScan (http://www.targetscan.org/). The result indicated that there were complementarities between miR-148a-3p and FOXK2 (Fig. 7A). Cotransfection with pMIR-FOXK2-Wt and miR-148a-3p mimics significantly suppressed the relative luciferase activity of Caki-1 cells, indicating that miR-148a-3p might directly bound to the 3’UTR of FOXK2 (Fig. 7B). In addition, western blot results showed that the protein expression level of FOXK2 was obviously suppressed by miR-148a-3p in Caki-1 cells (Fig. 7C).
Fig. 7.
FOXK2 is a target gene of miR-148a-3p in Caki-1 cells. (A) Complementarities between miR-148a-3p and FOXK2 predicted by TargetScan. (B) Luciferase reporter assay was performed to confirm the interaction between miR-148a-3p and FOXK2. *P < 0.05, compared with Caki-1 cells cotransfected with pMIR-FOXK2-Mut and miR-148a-3p mimics. (C) Effect of miR-148a-3p on the expression of FOXK2. *P < 0.05, compared with miR-NC-transfected Caki-1 cells.
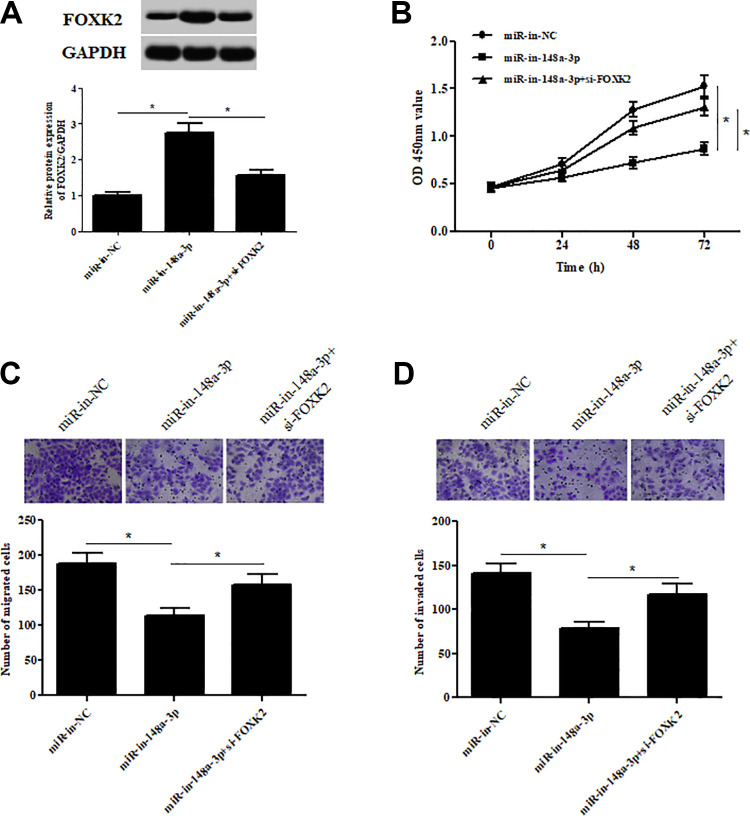
FOXK2 Knockdown Reversed the Inhibitory Effects of miR-148a-3p Inhibitor on Cell Proliferation, Migration, and Invasion
To further investigate the role of FOXK2 in ccRCC, we silenced FOXK2 expression by transfection with si-FOXK2 in Caki-1 cells (Fig. 8A). Moreover, as shown in Fig. 8B–D, inhibition of miR-148a-3p caused significant decreases in cell proliferation, migration, and invasion of Caki-1 cells. However, knockdown of FOXK2 was able to abolish the inhibitory effects of miR-148a-3p inhibitor on cell proliferation, migration, and invasion.
Fig. 8.
FOXK2 knockdown reversed the inhibitory effects of miR-148a-3p inhibitor on cell proliferation, migration, and invasion. (A) Western blotting analysis showing the FOXK2 levels in Caki-1 cells transfected with si-FOXK2 and miR-148a-3p inhibitors. (B) CCK-8 assay was performed to examine the cell proliferation. (C,D) Transwell assay was carried out to detect cell migration and invasion. *P < 0.05.
Discussion
Increasing evidence showed that dysregulation of circRNAs is involved in a variety of diseases, including cancers, through modulating the cancer-related processes such as apoptosis, vascularization, invasion, and metastasis14–18. Zhou et al.19 reported that a novel circRNA circPCNXL2 is significantly upregulated in ccRCC and correlated with poor overall survival in ccRCC patients. Knockdown of circPCNXL2 reduces RCC cells proliferation and invasion in vitro and suppresses tumor growth in vivo. In addition, circHIAT1 has been found to be involved in ccRCC through interacting with androgen receptor20. In the present study, we investigated the role of circUBAP2 in ccRCC. The results showed that circUBAP2 expression was significantly down-regulated in ccRCC tissues and cell lines. Overexpression of circUBAP2 in ccRCC cells inhibited the proliferation and affected cell cycle progression from the G1 phase to the S phase, implying that circUBAP2 affected cell growth by regulating cell cycle distribution. Furthermore, circUBAP2 inhibited the migration and invasion of ccRCC cells. The results indicated that circUBAP2 might be a tumor suppressor of ccRCC.
Recent findings have indicated that a great deal of circRNAs might regulate miRNAs function as microRNA sponges and play a significant role in the regulation of transcription21,22. For instance, circPCNXL2 binds to miR-153 as a miRNA sponge to regulate ZEB2 expression in RCC progression19. CircHIAT1 results in deregulation of the expressions of miR-195-5p, miR-29a-3p, and miR-29c-3p, which increases CDC42 expression to enhance ccRCC cell migration and invasion20. CircUBAP2 plays an important role in the proliferation and invasion of human lung cancer via targeting miR-339-5p, miR-96-3p, and miR-135b-3p23. CircUBAP2 promotes the osteosarcoma progression through acting as a sponge of miR-14312. In the current study, bioinformatics analysis showed that miR-148a-3p might be a target miRNA of circUBAP2, which was confirmed by the luciferase reporter assay.
It has been demonstrated that miR-148a-3p might be an oncogene in several cancers. Increased levels of serum miR-148a-3p have been reported to be associated with prostate cancer24. MiR-148a-3p expression level is significantly higher in bladder cancer tissues than control tissues. MiR-148a-3p promotes proliferation of bladder cancer cells and inhibits apoptosis of the cells, indicating a potential role in the future treatment of bladder cancer25. In addition, Marchionni et al.26 demonstrated that miR-148a-3p is up-regulated in RCC and associates with DNA damage responses, cell cycle progression, and apoptosis. However, there is a gap in the study of miR-148a-3p in the development of ccRCC. Our results showed that miR-148a-3p expression was up-regulated in ccRCC tissues and cell lines, and was negatively correlated with the circUBAP2 levels. Besides, circUBAP2 suppressed miR-148a-3p expression in Caki-1 cells. Additionally, transfection with miR-148a-3p mimics reversed the inhibitory effects of circUBAP2 on cell proliferation, migration, and invasion in Caki-1 cells. These findings suggested that circUBAP2 exerted its tumor suppressive role in ccRCC via sponging miR-148a-3p.
FOXK2 belongs to the forkhead box transcription factor family and has been found to play essential roles in cellular proliferation and survival27. Many studies have investigated the potential role of FOXK2 deregulation in tumorigenesis. FOXK2 expression was found to be reduced in high-grade gastric cancer tissues, and a low expression level of FOXK2 indicates a poor prognosis28. FOXK2 inhibits nonsmall cell lung cancer (NSCLC) epithelial-mesenchymal transition (EMT) and proliferation, which might be regulated by miR-127129. A recent study has proven that FOXK2 inhibits the malignant phenotype of ccRCC and acts as a tumor suppressor possibly through the inhibition of epidermal growth factor receptor (EGFR)30. We found that FOXK2 might be a target gene of miR-148a-3p and regulated by miR-148a-3p in Caki-1 cells. Additionally, knockdown of FOXK2 in Caki-1 cells reversed the inhibitory effects of miR-148a-3p inhibitor on cell proliferation, migration, and invasion, indicating a crucial role of miR-148a-3p/FOXK2 axis underlying the mechanism of circUBAP2 in ccRCC.
In summary, we demonstrated that circUBAP2 functioned as a novel tumor suppressor in ccRCC. Overexpression of circUBAP2 in ccRCC cells suppressed cell proliferation, migration, and invasion through regulating the miR-148a-3p/FOXK2 axis. Taken together, circUBAP2 might serve as a potential therapeutic target for the treatment of ccRCC.
Footnotes
Ethical Approval: This study was approved by the Ethics Committee at the First Affiliated Hospital of Medical College, Xi’an Jiaotong University (Xi’an, China).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the First Affiliated Hospital of Medical College, Xi’an Jiaotong University of Ethics Committee’s or Institutional Review Board’s (Approval Number: 00103) approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of Shaanxi, China (No.2020JM-390).
ORCID iD: Xudong Li  https://orcid.org/0000-0002-6080-8605
https://orcid.org/0000-0002-6080-8605
References
- 1. Perazella MA, Dreicer R, Rosner MH. Renal cell carcinoma for the nephrologist. Kidney Int. 2018;94(3):471–483. [DOI] [PubMed] [Google Scholar]
- 2. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rathmell WK, Chen S. VHL inactivation in renal cell carcinoma: implications for diagnosis, prognosis and treatment. Expert Rev Anticancer Ther. 2008;8(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinzelmann J, Unrein A, Wickmann U, Baumgart S, Stapf M, Szendroi A, Grimm MO, Gajda MR, Wunderlich H, Junker K. MicroRNAs with prognostic potential for metastasis in clear cell renal cell carcinoma: a comparison of primary tumors and distant metastases. Ann Surg Oncol. 2014;21(3):1046–1054. [DOI] [PubMed] [Google Scholar]
- 5. Fujii S, Shimada H, Yamagishi S, Ota M, Ichikawa Y, Kunisaki C, Ike H, Ohki S. Surgical strategy for local recurrence after resection of rectal cancer. Hepatogastroenterology. 2009;56(91–92):667–671. [PubMed] [Google Scholar]
- 6. de Almeida RA, Fraczek MG, Parker S, Delneri D, O’Keefe RT. Non-coding RNAs and disease: the classical ncRNAs make a comeback. Biochem Soc Transact 2016;44(4):1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2016;16(3):167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Z, Wang Y, Shu S, Cai J, Tang C, Dong Z. Non-coding RNAs in kidney injury and repair. Am J Physiol Cell Physiol. 2019;317(2):C177–C188. [DOI] [PubMed] [Google Scholar]
- 9. Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R, Finn SP. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patop IL, Kadener S. circRNAs in cancer. Curr Opin Genet Dev. 2018;48:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xia L, Song M, Sun M, Wang F, Yang C. Circular RNAs as Biomarkers for Cancer. Adv Exp Med Biol. 2018;1087:171–187. [DOI] [PubMed] [Google Scholar]
- 12. Zhang H, Wang G, Ding C, Liu P, Wang R, Ding W, Tong D, Wu D, Li C, Wei Q, Zhang X. et al. Increased circular RNA UBAP2 acts as a sponge of miR-143 to promote osteosarcoma progression. Oncotarget. 2017;8(37):61687–61697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang S, Li Q, Wang Y, Li X, Wang R, Kang Y, Xue X, Meng R, Wei Q, Feng X. Upregulation of circ-UBAP2 predicts poor prognosis and promotes triple-negative breast cancer progression through the miR-661/MTA1 pathway. Biochem Biophys Res Commun. 2018;505(4):996–1002. [DOI] [PubMed] [Google Scholar]
- 14. Bayoumi AS, Aonuma T, Teoh J, Tang Y, Kim I. Circular noncoding RNAs as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol Sin Acta Pharmacol Sin. 2018;39(7):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaiou M. Circular RNAs as potential biomarkers and therapeutic targets for metabolic diseases. Adv Exp Med Biol. 2019;1134:177–191. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Yang J, Zhou P, Le Y, Zhou C, Wang S, Xu D, Lin HK, Gong Z. Circular RNAs in cancer: novel insights into origins, properties, functions and implications. Am J Cancer Res. 2015;5(2):472–480. [PMC free article] [PubMed] [Google Scholar]
- 17. Akhter R. Circular RNA and Alzheimer’s disease. Adv Exp Med Biol. 2018;1087:239–243. [DOI] [PubMed] [Google Scholar]
- 18. Song M, Xia L, Sun M, Yang C, Wang F. Circular RNA in liver: health and diseases. Adv Exp Med Biol. 2018;1087:245–257. [DOI] [PubMed] [Google Scholar]
- 19. Zhou B, Zheng P, Li Z, Li H, Wang X, Shi Z, Han Q. CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle. 2018;17(23):2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang K, Sun Y, Tao W, Fei X, Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. [DOI] [PubMed] [Google Scholar]
- 21. Rong D, Sun H, Li Z, Liu S, Dong C, Fu K, Tang W, Cao H. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8(42):73271–73281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan Y, Jiaoming L, Xiang W, Yanhui L, Shu J, Maling G, Qing M. Analyzing the interactions of mRNAs, miRNAs, lncRNAs and circRNAs to predict competing endogenous RNA networks in glioblastoma. J Neurooncol. 2018;137(3):1–10. [DOI] [PubMed] [Google Scholar]
- 23. Yin Y, Gao H, Guo J, Gao Y. Effect of circular RNA UBAP2 silencing on proliferation and invasion of human lung cancer A549 cells and its mechanism. Zhongguo Fei Ai Za Zhi. 2017;20(12):800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dybos SA, Flatberg A, Halgunset J, Viset T, Rolfseng T, Kvam S, Skogseth H. Increased levels of serum miR-148a-3p are associated with prostate cancer. APMIS. 2018;126(9):722–731. [DOI] [PubMed] [Google Scholar]
- 25. Xiang M, Yuan W, Zhang W, Huang J. Expression of miR-490-5p, miR-148a-3p and miR-608 in bladder cancer and their effects on the biological characteristics of bladder cancer cells. Oncol Lett. 2019;17(5):4437–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchionni L, Hayashi M, Guida E, Ooki A, Munari E, Jabboure FJ, Dinalankara W, Raza A, Netto GJ, Hoque MO, Argani P. MicroRNA expression profiling of Xp11 renal cell carcinoma. Hum Pathol. 2017;67:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heide LP, Van Der, Wijchers PJEC, Lars VO, Burbach JPH, Hoekman MFM, Smidt MP. FoxK2 is required for cellular proliferation and survival. J Cell Physiol. 2015;230(5):1013–1023. [DOI] [PubMed] [Google Scholar]
- 28. Liu X, Wei X, Niu W, Wang D, Wang B, Zhuang H. Downregulation of FOXK2 is associated with poor prognosis in patients with gastric cancer. Mol Med Rep. 2018;18(5):4356–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen S, Jiang S, Hu F, Xu Y, Wang T, Mei Q. Foxk2 inhibits non-small cell lung cancer epithelial-mesenchymal transition and proliferation through the repression of different key target genes. Oncol Rep. 2017;37(4):2335–2347. [DOI] [PubMed] [Google Scholar]
- 30. Zhang F, Ma X, Li H, Zhang Y, Li X, Chen L, Guo G, Gao Y, Gu L, Xie Y, Duan J. et al. FOXK2 suppresses the malignant phenotype and induces apoptosis through inhibition of EGFR in clear-cell renal cell carcinoma. Int J Cancer. 2018;142(12):2543–2557. [DOI] [PubMed] [Google Scholar]