Abstract
Spinal cord injury (SCI) is considered as one of the most problematic neurological conditions requiring specialized clinical intervention. Taking into account that SCI is characterized by extensive loss of nerve cells, stem cell–based therapy seems to be a reasonable modern strategy to the treatment of SCI. The presented case report describes for the first time experimental treatment with the use of autologous adipose tissue–derived mesenchymal stem cells (ADSCs) of the chronic posttraumatic SCI in a domestic ferret patient with paresis of back legs. It should be noted that most reports in the available literature concern ADSC-based therapies for acute or subacute SCI treatment in other species. Application of ADSC-based therapy did not cause any adverse reactions and resulted in significant improvement of neurological and motor functions. Based on these outcomes, it may be concluded that this form of therapy is promising and may be potentially translated into clinical veterinary practice.
Keywords: neural tissue regeneration, anti-inflammatory effect, analgesic action, paraplexis, stem cell–based therapy
Introduction
Spinal cord injury (SCI) is considered as one of the most problematic neurological conditions requiring specialized clinical intervention. Traumatic injury to the spinal cord may result from lacerations, compressions, stretching, contusions, or direct destruction (e.g., kinking), causing a broad spectrum of neurological symptoms like mobility dysfunction, sensory disorder, autonomic deficits, autonomic dysreflexia, neuropathic pain, as well as bowel and bladder dysfunction1,2. The primary injury occurs as a result of immediate structural damage to the spinal cord, which may be accompanied by fractures or dislocation of the vertebral column. The trauma causes damage to the neurons, oligodendrocytes, and other components that are crucial for neuronal transmission. Moreover, vascular components along with the blood–spinal cord barrier are disrupted, inducing inflammatory cells’ infiltration followed by postinjury inflammation1. The secondary injury, which may be defined as uncontrolled and destructive multifaceted pathological process, occurs after primary injury as a consequence of further chemical and physical damage to the spinal cord. The secondary injury leads to the proapoptotic signaling, prolonged inflammation, vascular changes, ischemia, and further breakdown of the blood–spinal cord barrier1–3. Three months after SCI, the chronic phase occurs, which is characterized by limitation of the axon regeneration across the lesion due to fibrous glial scar formation and deposition of the extracellular matrix4.
Current therapeutic strategies for SCI include surgical decompression, systemic hypothermia, and pharmacotherapy. Taking into account that SCI is characterized by extensive loss of nerve cells, transplantation of the cells into the injured area to induce regeneration process seems to be a reasonable strategy to the treatment of SCI. Stem cells, which have the ability to differentiate into various cell types, are commonly used in SCI treatment to replace damaged cells. Importantly, stem cells were also demonstrated to produce anti-inflammatory cytokines and growth factors, inhibiting inflammation in the damaged tissue and promoting regeneration process4–6. Therefore, stem cell–based therapy provides not only neuroprotective therapy limiting secondary damage, but also neuroregenerative strategy aiming to rebuild the damaged neural tissue, axons, and circuits.
Bone marrow–derived mesenchymal stem cells (BMDSCs) are the most commonly used cells in the SCI treatment. So far, BMDSCs were successfully applied in both preclinical7–10 and clinical trials11–15, improving motor and sensory functions of the patients. Nevertheless, the use of BMDSCs in SCI treatment has some limitations, like invasive and painful procedure for bone marrow harvesting and potential risk related to complications, including infections16,17. In contrast to bone marrow, adipose tissue is not only easily accessible, but also an abundant source of stem cells. Interestingly, it was demonstrated that ADSCs maintain high proliferation potential regardless of the age of the organism16, indicating that ADSCs might be an ideal cell type for stem cell–based treatment of SCI.
The presented case report describes the experimental treatment with the use of ADSCs of chronic posttraumatic SCI in a domestic ferret patient. The domestic ferret was found abandoned in a forest and rescued by an Animal Care Foundation, being aggressive and in general poor condition. Paresis of its back legs was one of the SCI symptoms. This case report presents for the first time successful isolation of the ADSCs from the fat tissue of the ferret and their direct administration by injection into the damaged area to treat chronic SCI. It should be noted that most reports in the available literature concern ADSC-based therapies for acute or subacute SCI treatment in other species.
Materials and Methods
Case Presentation
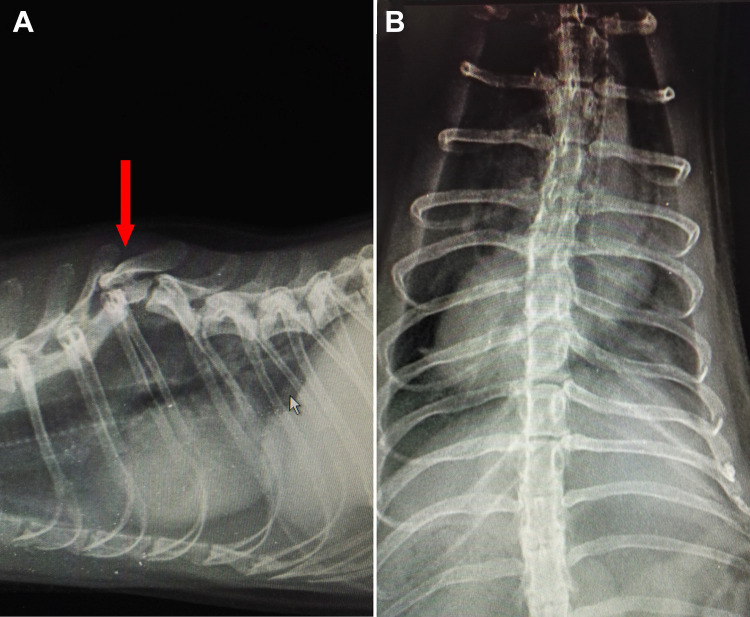
A 7-yr-old male ferret was admitted to Pulawian Veterinary Center (Pulawy, Poland) with chronic SCI. The ferret was overweight but alert with hind legs’ paresis. General examination showed muscles’ atrophy of the ferret’s back starting from the lumbar area of the body to the back. Weakness of the back legs was observed—the ferret was not weight bearing, showing crawling movements (supplemental Video S1). In neurological examination, conscious proprioception (paw placement test) was slower in both legs, table top test was negative in both hind legs, wheelbarrow test was correct for front legs, and hopping test was negative for back legs. When it comes to spinal reflexes, the ferret showed correct withdrawer reflexes in front paws and slower withdrawer reflexes in back legs; patellar reflexes were slower in both legs (details regarding the neurological examination are described in Diagnostic Methods and Outcome Measures section). Full blood work and radiographic examination were also performed. Blood tests showed no abnormalities. Strong kyphosis of Th6–Th8 was discovered on radiographs; Th7 had a character of hemivertebrae (Fig. 1). Congenital or traumatic background was suggested by radiologist. Degenerative process of hip joint was suggested at the same time.
Fig. 1.
X-ray radiographs presenting (red arrow) damaged area within spinal cord of the ferret patient: (A) lateral view; (B) ventrodorsal view.
Adipose Tissue Harvesting
Both collection of adipose tissue and ADSC-based therapy applied for the domestic ferret patient were performed after obtaining written informed consent from the pet’s owner from Animal Care Foundation. The ferret was sedated with a mixture of Cepetor (CP-Pharma Handelsgesellschaft MbH, Burgdorf, Germany) and Vetaketam (VETAKETAM 100 mg/ml, VET-AGRO Sp. z o.o., Lublin, Poland), given by intramuscular injection. After intubation, anesthesia was maintained with Aerane (AErrane, 100% inhalation anesthetic, Baxter Polska Sp. z o. o., Warsaw, Poland). Tolfine (Tolfedine 4%/40 mg/ml injection solution, Vétoquinol UK Limited, Northamptonshire, UK) was also administered by subcutaneous injection. The ferret’s vital parameters were monitored through the procedure and were all the time within their physiological limits. Anesthesia was uneventful. Mesenteric adipose tissue was collected by midline laparotomy from the area of R kidney. The wound was closed with three layers of absorbable 4-0 monofilament suture (Monosyn, B. Braun, Hessen, Germany). No antibiotics were given postoperatively. The procedure of fat tissue collection was performed in accordance with Good Veterinary Practice prepared under the auspices of the Federation of Veterinarians of Europe (FVE).
Harvested adipose tissue (about 20 ml) was placed in a 100 ml Simax bottle filled with 50 ml of phosphate-buffered saline (PBS, Pan-Biotech GmbH, Aidenbach, Bavaria, Germany) enriched with 300 U/ml penicillin, 300 μg/ml streptomycin, and 0.75 μg/ml amphotericin B solution (Sigma-Aldrich Chemicals, Warsaw, Poland). The collected fat tissue was immediately transferred to the Medical University of Lublin (Poland) to perform ADSC isolation.
Isolation and Expansion of ADSCs
ADSCs were isolated from the ferret’s fat tissue according to the procedure described elsewhere18 with own modifications described in our previous research19. The following complete culture medium was applied for ADSC isolation and expansion: 1:1 mixture of Dulbecco’s Modified Eagle’s Medium/Ham’s F12 (DMEM/Ham's F12) medium without phenol red (Sigma-Aldrich Chemicals, Warsaw, Poland) enriched with 2.5 mM l-glutamine (Sigma-Aldrich Chemicals, Warsaw, Poland), 10% fetal bovine serum (FBS, Pan-Biotech GmbH, Bavaria, Germany), 100 μg/ml streptomycin, and 100 U/ml penicillin (both from Sigma-Aldrich Chemicals, Warsaw, Poland). Briefly, adipose tissue was cut using surgical scissors into 3 to 5 mm pieces and washed several times with PBS. Then, the fat was digested using 0.1% collagenase solution in PBS (Gibco, Life technologies, Carlsbad, CA, USA) for 1 h at 37 °C on a shaker (165 rpm). The obtained fat suspension was then subjected to four centrifuge steps at 300 × g at 21 °C for 4 min to obtain the pellet, which was a stromal vascular fraction (SVF). The SVF was resuspended in the complete culture medium and the obtained cell suspension was filtered using cell strainer (BD Falcon, Sigma-Aldrich Chemicals, Warsaw, Poland) to remove tissue debris. The SVF was then plated onto T75 culture flasks and cultured in an incubator at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was replaced with fresh portion (half a volume) every 2 d until the cells reached 90% confluence. At this point the cells were trypsinized (0.05% trypsin–0.02% ethylenediaminetetraacetic acid (EDTA) in PBS without calcium or magnesium dedicated for primary cells was used, ATCC-LGC Standards, Teddington, UK), washed with fresh medium by centrifugation at 125 × g for 5 min, and collected for cryopreservation in liquid nitrogen until use. The freezing medium contained 10% dimethyl sulfoxide (DMSO, Sigma-Aldrich Chemicals, Warsaw, Poland) and 30% FBS. Before freezing, the number of isolated ADSCs was determined using CountessTM II Automated Cell Counter (ThermoFischer Scientific, Waltham, MA, USA). During the isolation procedure, the ADSC morphology and growth was constantly monitored using inverted optical microscope (Olympus CKX53, Olympus Corporation, Tokyo, Japan).
ADSC Characterization
Immunofluorescent (IF) staining of CD29/integrinβ1 and CD90/Thymocyte differentiation antigen 1 (Thy1) (specific ADSC markers) was performed to prove the successful isolation of ferret’s ADSCs. Briefly, before cell freezing, the sample of isolated cells was seeded into the wells of 48-multiwell plate. After 24 h, the cells were fixed with 3.7% paraformaldehyde (Sigma-Aldrich Chemicals, Warsaw, Poland), permeabilized with 0.2% Triton X-100 (Sigma-Aldrich Chemicals, Warsaw, Poland), and blocked with 1% bovine serum albumin (Pan-Biotech GmbH, Bavaria, Germany). Then, fixed cells were incubated overnight at 4 °C with 10 µg/ml anti-CD29/integrinβ1 and 10 µg/ml anti-CD90/Thy1 primary antibodies (R&D Systems, Abingdon, UK), washed with PBS, and incubated for 1 h at room temperature with 2 µg/ml secondary antibodies conjugated to AlexaFluor647 (Abcam, Cambridge, UK) and NorthernLights(NL)493 (R&D Systems, UK) to detect CD29 and CD90 antigen, respectively. Stained ADSCs were analyzed under confocal laser scanning microscope (Olympus Fluoview equipped with FV1000, Olympus Corporation).
Multipotency of isolated ADSCs was proved by maintenance of the cells in the osteogenic, chondrogenic, and adipogenic media. Briefly, isolated cells were seeded into the wells of 48-multiwell plate in a complete culture medium. After 24 h, the culture medium was replaced with (1) Osteocyte Differentiation Tool (ATCC-LGC Standards, Teddington, UK) to induce osteogenic differentiation, (2) Chondrocyte Differentiation Tool (ATCC-LGC Standards, Teddington, UK) to induce chondrogenic differentiation, and (3) DMEM high glucose (ATCC-LGC Standards, Teddington, UK) supplemented with 10% FBS, 1 µg/ml hydrocortisone (ATCC-LGC Standards, Teddington, UK), 10 µg/ml insulin from bovine pancreas, 1,000 nM dexamethasone, 33 µM biotin, and 17 µM pantothenic acid (all reagents purchased from Sigma-Aldrich Chemicals, Warsaw, Poland) to induce adipogenic differentiation. ADSCs were cultured for 20 d with half medium renewal every 3 to 4 d and then the cells were fixed as described above. Differentiation into osteocytes was demonstrated by staining of mineral deposits using 2% Alizarin Red S solution (ARS, Sigma-Aldrich Chemicals) and IF staining of type I collagen and osteocalcin using primary antibodies applied at a concentration of 5 µg/ml (Abcam, Cambridge, UK). Differentiation into chondrocytes was confirmed by IF staining of type II collagen and aggrecan using primary antibodies applied at a concentration of 5 µg/ml (Abcam, Cambridge, UK). During the above-mentioned experiments, secondary antibodies conjugated to AlexaFluor®532 and AlexaFluor®488 (Abcam, Cambridge, UK) were used. Differentiation into adipocytes was proved by staining of lipids accumulation in the cells using 0.5% Oil Red O dye in 60% isopropanol (Sigma-Aldrich Chemicals, Warsaw, Poland).
Sterility Testing
Sterility of isolated cells was checked by harvesting 100 µl of conditioned medium from the flasks with ADSC culture and then its transfer to Petri dishes with various solid microbiological media: Blood Agar, Mannitol Salt Agar, MacConkey Agar, Müller-Hinton Agar, Anaerobic Blood Agar (all from Sigma-Aldrich Chemicals, Warsaw, Poland). Petri dishes were then incubated at 37 °C for 3 d to detect the growth of both aerobic and anaerobic microorganisms in medium samples.
ADSC Injection
After storage in liquid nitrogen, the ADSCs were rapidly thawed by placing the vials into a 37 °C dry heating block. Then, the viability of the cells was checked using CountessTM II Automated Cell Counter (ThermoFischer Scientific, Waltham, MA, USA), which determines cell viability and cell number based on trypan blue exclusion. Proliferation capacity of ADSCs after thawing was determined by Cell Counting kit-8 (WST-8, Sigma-Aldrich Chemicals, Warsaw, Poland). Doubling time (time needed to double cell population expressed in hours) and growth rate (number of doublings occurring per unit of time) for thawed cells were calculated using Doubling Time Computing software (available at: http://www.doubling-time.com/compute.php). To prepare injection suspension, thawed cells were washed 3 times by centrifugation in Hank’s Balanced Salt Solution (HBSS, Pan-Biotech GmbH, Bavaria, Germany) at 125 × g for 5 min. Injection suspension was prepared in HBSS and transferred to sterile vials (two vials containing approx. 4 × 106 ADSCs in 1 ml of HBSS were prepared). Cell number and ADSC viability was determined for the prepared injection suspension using CountessTM II Automated Cell Counter.
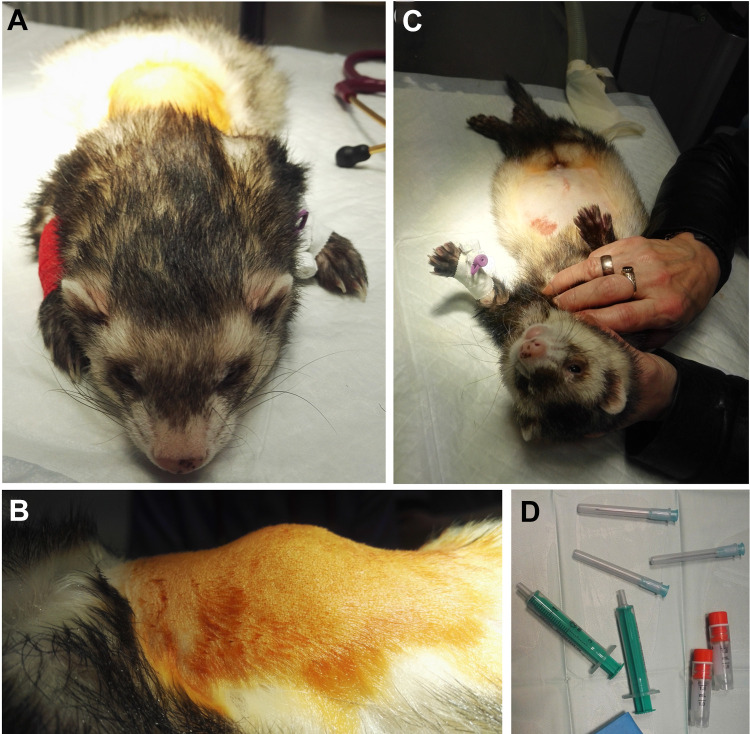
Cell suspension (a total amount of cells approx. 8 × 106 in 2 ml of HBSS) was administered under general anesthesia symmetrically on both sides of the spine—in four injections, 0.5 ml each, in the areas of Th6–Th7 and Th7–Th8 (Fig. 2). Injections’ sites were localized 1 cm laterally from processus spinalis of the affected vertebras (based on the previously performed radiographs—ventrodorsal projection). Anesthetic protocol was the same as in the case of adipose tissue collection. The procedure of ADSC injection was performed in accordance with Good Veterinary Practice prepared under the auspices of the FVE.
Fig. 2.
Images presenting injection procedure: (A, B) the ferret prepared for the ADSC injection; (C) the ferret just after the injection; (D) the vials with ADSC suspension. ADSC: adipose tissue-derived mesenchymal stem cell.
Postinjection Recommendations and Rehabilitation
The owner was informed not to feed the animal 6 h after procedure. The ferret was on follow-up visit 2 d after ADSC injection; his vital parameters were not changed as well as general condition: appetite and body temperature were in their physiological values. From that day, the owner started physiotherapy prescribed by a veterinary surgeon: passive range of motion exercises of back legs 4 times per day (5 min each time), hydrotherapy 2 times per week (15 min each time).
Diagnostic Methods and Outcome Measures
The ferret was scheduled for follow-up medical checkups every 2 wk (2, 4, and 6 wk after ADSC injection) from the date of the procedure. Full general and neurological examination was performed each time. Complete neurological examination included (1) patient’s neurologic history, (2) mentation, (3) posture/gait analysis, (4) cranial nerve examination, (5) proprioception, (6) spinal reflexes, and (7) sensory evaluation. Because of previously performed precise neurolocalization of lesions, during follow-up medical visits neurological examination was focused primarily on proprioception and spinal reflexes. Proprioception assessment involved (1) conscious proprioception (paw placement test), (2) table top test, (3) wheelbarrow test, and (4) hopping test. The following spinal reflexes were checked: (1) withdrawer reflexes and (2) patellar reflexes.
Results
ADSC Isolation and Characterization
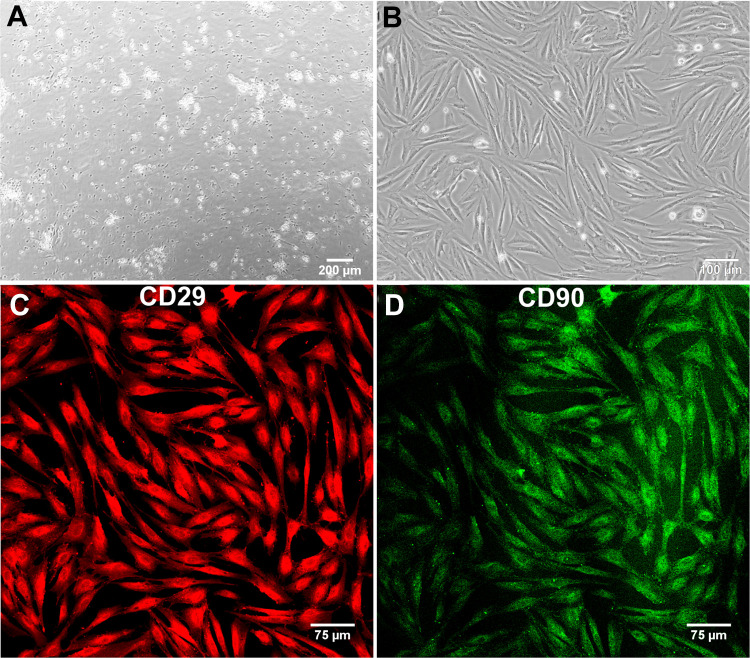
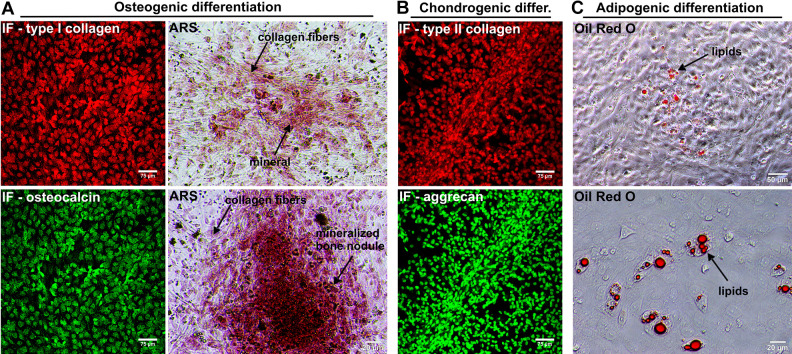
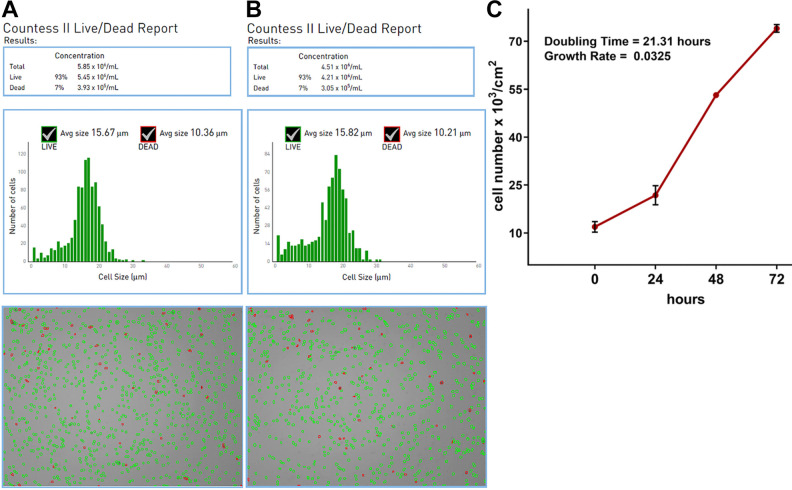
After plating the SVF onto the T75 flasks, the ADSCs attached to the polystyrene surface within first 24 h (Fig. 3A). The cells revealed rapid divisions and required only 4 d to reach over 90% confluence (Fig. 3B). The yield of isolation was 40.5 × 106 ADSCs from approximately 20 ml of the fat tissue. IF staining confirmed that isolated cells were indeed ADSCs since the cells were CD29 and CD90 positive (Fig. 3C, D, respectively). Upon appropriate stimulation, the cells also revealed the ability to differentiate into osteocytes (Fig. 4A), chondrocytes (Fig. 4B), and adipocytes (Fig. 4C), which is typical of multipotent mesenchymal stem cells. Microbiological culture confirmed sterility of the cell suspension. Prior to the preparation of the injection suspension, frozen cells were thawed at 37 °C. Cell viability after thawing was equal to 93%, thus the freezing process did not significantly reduce the number of viable cells (Fig. 5A). Storage in liquid nitrogen did not affect also proliferation capacity of the cells. After thawing, ADSCs showed rapid cell divisions and short doubling time equal to 21.31 h (Fig. 5C). Cell suspension prepared for injection contained 4.21 × 106 of viable cells per 1 ml of HBSS and ADSC viability was determined to be 93% (Fig. 5B).
Fig. 3.
Microscopic images presenting isolated ADSCs: (A) phase-contrast microscope image showing adhesion of ADSCs 24 h after plating the SVF onto the T75 flask (magn. 40×); (B) phase-contrast microscope image showing ADSCs 4 d after plating the SVF (magn. 100×); (C, D) confocal microscope images showing CD29 positive (red fluorescence) and CD90 positive (green fluorescence) ADSCs, respectively (magn. 200×). ADSC: adipose tissue-derived mesenchymal stem cell; CD: cluster of differentiation; SVF: stromal vascular fraction.
Fig. 4.
Multipotency of isolated ADSCs: (A) markers of osteogenic differentiation (type I collagen: red fluorescence; osteocalcin: green fluorescence; mineral deposits detected by ARS staining: red color); (B) markers of chondrogenic differentiation (type II collagen: red fluorescence; aggrecan: green fluorescence); (C) marker of adipogenic differentiation (lipids accumulation in ADSCs determined by Oil Red O staining: red color). ADSC: adipose tissue-derived mesenchymal stem cell; ARS: Alizarin Red S staining; IF: immunofluorescent.
Fig. 5.
ADSC characterization after thawing: viability and number of isolated ADSCs determined using CountessTM II Automated Cell Counter just after cell thawing (A) and for injection suspension (B); (C) proliferation capacity of ADSCs determined by WST-8 assay. ADSC: adipose tissue-derived mesenchymal stem cell.
Functional Outcome After ADSC Injection
No adverse reactions were noticed after the procedure of ADSC injection. No functional or neurological changes were noticed at the first two follow-up medical checkups (2 and 4 wk after ADSC injection). However, slight development of muscles was observed at the first follow-up visit (2 wk after procedure). During the next following checkups (4 and 6 wk after the procedure) further development of muscles mass was observed. At the third follow-up visit (6 wk after ADSC injection), the owner of the animal reported increased mobility of the animal. For the first time, the ferret was able to climb up by 20 cm (supplemental Video S2).
In general examination (at the third follow-up visit), the ferret showed better muscles’ tonus in the area previously described as atrophic one. Its weight bearing was much better and the ferret showed less bowie back than before the procedure (Table 1).
Table 1.
General Examination of the Ferret Before and 6 Wk After ADSC-Based Therapy.
| Type of observation | Before ADSC therapy | 6 wk after ADSC injection |
|---|---|---|
| Mobility | Crawling movements | Increased; ability to climb |
| Muscles’ tonus | Atrophic area | Improved; muscles’ development |
| Weight bearing | Weak | Improved |
In neurological examination (at the third follow-up visit), conscious proprioception (paw placement test) improved in both legs, table top test was negative in hind right leg but became gently positive on left side, and hopping test was gently positive for back legs. When it comes to spinal reflexes, the ferret showed improvement in withdrawer reflexes in back legs, but patellar reflexes were still slow in both legs (Table 2).
Table 2.
Neurological Examination of the Ferret Before and 6 Wk After ADSC-Based Therapy.
| Type of neurological examination | Before ADSC therapy | 6 wk after ADSC injection |
|---|---|---|
| Paw placement test | (+) | (++) |
| Table top test | (−) | (+) left (−) right leg |
| Hopping test | (−) | (+) |
| Wheelbarrow test | (+++) | (+++) |
| Withdrawer reflexes | (+) | (++) |
| Patellar reflexes | (+) | (+) |
(−) negative; (+) poorly positive; (++) mildly positive; (+++) strongly positive.
Discussion
In this case report we described for the first time stem cell-based therapy with the use of autologous ADSCs for chronic SCI treatment of domestic ferret patient. Within this study it was demonstrated that direct administration of ADSCs into the damaged area of spinal cord resulted in significant neurological and motor improvements of the ferret patient (Tables 1 and 2, supplemental Video S1 and S2). However, X-ray imaging did not visualize any changes/improvements in the damaged area of spinal cord. Our observation is in agreement with the outcomes presented by other authors9, who also demonstrated that clinical gain after BMDSC therapy performed for SCI was not correlated with alterations in magnetic resonance imaging. Thus, we assumed that observed therapeutic effect of ADSC injection primarily resulted from antioxidant20, anti-inflammatory4–6, and analgesic21 activities of the stem cells, which have been already proved by other authors.
Although ADSC-based therapy is a promising strategy to SCI treatment, majority of available reports concern the use of BMDSCs for improvement of motor and sensory functions after traumatic SCI. So far, a great number of preclinical and clinical trials on the use of BMDSCs for SCI therapy were performed. Importantly, BMDSCs were successfully used for both acute and chronic SCI treatment. Pal et al.8 administered BMDSCs directly to the damaged area of SCI in a rat model and observed significantly improved motor and sensory functions of the treated animals. Similarly, Zurita et al.7 injected (3 mo after SCI) autologous BMDSCs into the lesion zone and adjacent subarachnoid space in paraplegic pigs and demonstrated progressive functional recovery in the treated animals. Penha et al.9 used BMDSC-based therapy for the experimental treatment of naturally injured spinal cord in dog patients and reported significant improvement of the panniculus reflex and reduction in superficial and deep pain response shortly (10 d) after BMDSC transplantation.
There are also many case reports presenting outcomes of BMDSC-based therapy, which were obtained with clinical trials. Mendonça et al.15,22 conducted phase I clinical study (ClinicalTrials.gov identifier: NCT01325103) in 14 subjects with chronic traumatic SCI (>6 mo) and demonstrated that intralesional transplantation of autologous BMDSCs may promote neurological improvements. Similarly, Phedy et al.11 performed direct parenchymal injection of BMDSCs to the affected lesion of spinal cord followed by multiple (5 times) intravenous BMDSC injection and observed motor and neurological improvement without any serious adverse reactions. In turn, Araujo et al.23 applied BMDSC-based therapy in the treatment of patients with chronic SCI with residual electrophysiological function and proved significant radiological and electrophysiological improvements along with mild motor functional recovery. Nevertheless, Chotivichit et al.12 showed that intrathecal injection of BMDSCs at the lumbar spine level did not result in significant neurological improvement, but also carried high risk of transient complications (occurring within the first 48 h after transplantation) like unpleasant neuropathic pain, headache, fever, and transient neurological deficit.
In contrary to BMDSC-based therapies for SCI, most preclinical and clinical studies on the use of ADSCs concern acute SCI treatment. Kim et al.24 performed ADSC-based therapy for the treatment of acute thoracolumbar disc disease with no deep pain perception in dog patients. They carried out two treatments to compare clinical outcomes: decompression surgery alone and decompression surgery in combination with transplantation of allogenic ADSCs to the injured spinal cord. They demonstrated that combined therapy resulted in better recovery outcomes compared to decompression surgery alone. They also observed that transplanted ADSCs not only reduced inflammation and glial scar formation, but also supported survival of endogenous nerve cells and had the ability to partially differentiate into neural cells. The same research team proved in their other studies25 that intravenous injection of ADSCs in the acute spinal cord–injured beagle dogs had positive clinical effects on the treatment of damaged area through antioxidant and anti-inflammatory actions. Similarly, Khan et al.20 demonstrated that administration of heme oxygenase-1 (HO-1) and brain-derived neurotrophic factor (BDNF) overexpressed ADSCs to the subacute injured spinal cord of beagle dogs, inhibited the expression of inflammatory cytokines, and promoted neuroregeneration process.
Despite promising results obtained with preclinical studies, there are still very few reports in the available literature describing successful application of ADSCs for SCI treatment in clinical trials. However, Bydon et al.26 published the outcomes obtained with an ongoing phase 1 clinical trial conducted at Mayo Clinic (ClinicalTrials.gov Identifier: NCT03308565)27. The mentioned case report describes the ADSC-based therapy of paralysis after traumatic SCI. Autologous ADSCs were administered by intrathecal injection and clinical signs of improvement in both motor and sensory functions were observed 3, 6, 12, and 18 mo after the injection. Another ongoing clinical trial (ClinicalTrials.gov Identifier: NCT02917291) concerns the use of ADSCs in patients with acute traumatic SCI28; however, no outcomes and publications are available in the literature.
Conclusions
In this case report, we described the successful application without any adverse reactions of autologous ADSCs for the treatment of chronic posttraumatic SCI in a domestic ferret patient. Although these are only preliminary results obtained with experimental treatment of chronic SCI with the use of ADSCs, observed motor and neurological improvements may indicate that this form of therapy is promising and may be potentially translated into clinical veterinary practice. Nevertheless, further studies are needed to be performed in order to provide additional data, which will allow us to better understand the mechanism of action of ADSCs.
Footnotes
Data Availability: The raw/processed data required to reproduce these findings can be obtained from the corresponding author (agata.przekora@umlub.pl) upon reasonable request.
Ethical Approval: Our institution does not require ethical approval for reporting individual animal cases.
Statement of Human and Animal Rights: This article does not contain any studies with human. Cell-based therapy applied for the ferret patient presented in this case study was conducted based on written informed consent obtained from the pet’s owner. The procedure was performed in accordance with Good Veterinary Practice prepared under the auspices of the FVE.
Statement of Informed Consent: Both collection of adipose tissue and ADSC-based therapy applied for the domestic ferret patient were performed after obtaining written informed consent from the pet’s owner from Animal Care Foundation.
Declaration of Conflicting Interests: The author(s) declared no conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The APC was covered by own fund of Medical University of Lublin within statutory activity (DS3/2020 project). The paper was developed using the equipment purchased within agreement no. POPW.01.03.00-06-010/09-00 Operational Program Development of Eastern Poland 2007–2013, Priority Axis I, Modern Economy, Operations 1.3. Innovations Promotion.
ORCID iD: Agata Przekora  https://orcid.org/0000-0002-6076-1309
https://orcid.org/0000-0002-6076-1309
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Katoh H, Yokota K, Fehlings MG. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. 2019;13:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa A. Mesenchymal stem cells for spinal cord injury: current options limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gazdic M, Volarevic V, Randall Harrell C, Fellabaum C, Jovicic N, Arsenijevic N, Stojkovic M. Stem cells therapy for spinal cord injury. Int J Mol Sci. 2018;19(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shao A, Tu S, Lu J, Zhang J. Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res Ther. 2019;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu M, Xue J, Lu S, Yuan Y, Liao Y, Qiu J, Liu C, Liao Q. Anti-inflammatory effect of stromal vascular fraction cells in fat transplantation. Exp Ther Med. 2019;17(2):1435–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Febbo I, Bunnell BA. Immunomodulatory effects of adipose stromal vascular fraction cells promote alternative activation macrophages to repair tissue damage. Stem Cells. 2017;35(10):2198–2207 [DOI] [PubMed] [Google Scholar]
- 7. Zurita M, Vaquero J, Bonilla C, Santos M, Haro JD, Oya S, Aguayo C. Functional recovery of chronic paraplegic pigs after autologous transplantation of bone marrow stromal cells. Transplantation. 2008;86(6):845–853. [DOI] [PubMed] [Google Scholar]
- 8. Pal R, Gopinath C, Rao NM, Banerjee P, Krishnamoorthy V, Venkataramana NK, Tote S. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy. 2010;12(6):792–806. [DOI] [PubMed] [Google Scholar]
- 9. Penha EM, Meira CS, Guimarães ET, Mendonça MVP, Gravely FA, Pinheiro CMB, Pinheiro TMB, Melo SMB, Santos RRD, Soares MBP. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014;2014:437521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hakim R, Covacu R, Zachariadis V, Frostell A, Sankavaram SR, Brundin L, Svensson M. Mesenchymal stem cells transplanted into spinal cord injury adopt immune cell-like characteristics. Stem Cell Res Ther. 2019;10(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phedy P, Djaja YP, Gatam L, Kusnadi Y, Wirawan RP, Tobing IMS, Subakir N, Mappalilu A, Prawira MA, Yauwenas R, Gatam AR. Motoric recovery after transplantation of bone marrow derived mesenchymal stem cells in chronic spinal cord injury: a case report. Am J Case Rep. 2019;20:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chotivichit A, Ruangchainikom M, Chiewvit P, Wongkajornsilp A, Sujirattanawimol K. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: a case report. J Med Case Rep. 2015;9(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533(2013):73–79. [DOI] [PubMed] [Google Scholar]
- 14. Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HAMA, Maadawi ZEMI, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23(6):729–745. [DOI] [PubMed] [Google Scholar]
- 15. Mendonça MVP, Larocca TF, Souza BSDF, Villarreal CF, Silva LFM, André Costa Matos, Novaes MA, Bahia CMP, Martinez ACOM, Kaneto CM, Furtado SBC, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5(6):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liao HT, Chen CT. . Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levi B, Longaker MT. Adipose derived stromal cells for skeletal regenerative medicine. Stem Cells. 2011;29(4):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Estes B, Diekman B, Gimble J, Guilak F. Isolation of adipose derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kazimierczak P, Benko A, Nocun M, Przekora A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int J Nanomedicine. 2019;14:6615–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan IU, Yoon Y, Kim A, Jo KR, Choi KU, Jung T, Kim N, Son YS, Kim WH, Kweon OK. Improved Healing after the Co-transplantation of HO-1 and BDNF Overexpressed mesenchymal stem cells in the subacute spinal cord injury of dogs. Cell Transplant. 2018;27(7):1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han YH, Kim KH, Abdi S, Kim TK. Stem cell therapy in pain medicine. Korean J Pain. 2019;32(4):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ClinicalTrials.gov. Autologous bone marrow stem cell transplantation in patients with spinal cord injury. https://clinicaltrials.gov/ct2/show/NCT01325103. Accessed December 27, 2019. [DOI] [PMC free article] [PubMed]
- 23. Araujo A, Lepski G, Fazzito M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal stem cells in the treatment of patients with Chronic Spinal Cord Injury (ASIA A) with residual electrophysiological function. J Stem Cells Clin Pract. 2015;1(1):101. [Google Scholar]
- 24. Kim Y, Lee SH, Kim WH, Kweon O. Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs. J Vet Sci. 2016;17(1):123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim Y, Jo SH, Kim WH, Kweon OK. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015;6(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bydon M, Dietz AB, Goncalves S, et al. CELLTOP clinical trial: first report from a Phase 1 trial of autologous adipose tissue-derived Mesenchymal stem cells in the treatment of paralysis due to traumatic spinal cord injury. Mayo Clin Proc. 2020;95(2):406–414. doi:10.1016/j.mayocp.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 27. ClinicalTrials.gov. Adipose Stem Cells for Traumatic Spinal Cord Injury (CELLTOP). https://clinicaltrials.gov/ct2/show/NCT03308565. Accessed December 27, 2019.
- 28. ClinicalTrials.gov. Safety and Preliminary Efficacy of FAB117-HC in Patients With Acute Traumatic Spinal Cord Injury (SPINE). https://clinicaltrials.gov/ct2/show/NCT02917291. Accessed December 27, 2019.