Abstract
The aim of this study was to investigate claudin-7 (CLDN7) expression in salivary adenoid cystic carcinoma (SACC) and its function in SACC cells. We determined CLDN7 expression in SACC tumors via immunohistochemistry and western blotting and evaluated the association between CLDN7 expression and clinicopathologic variables. Besides this, we constructed a stably transfected CLDN7 knockdown SACC-LM cell line via RNAi and assessed its biological behavior changes (cell viability, migration, and invasion). The correlation between CLDN7 and epithelial-mesenchymal transition (EMT) was analyzed. Additionally, a subcutaneous tumor formation model was used to assess SACC-LM cells tumorigenicity after the CLDN7 knockdown. In the present study, we found the CLDN7 expression of tumor group was lower than that in normal salivary glands and was significantly correlated with lymph node metastasis, recurrence, and gender. CLDN7 knockdown could add the proliferation and metastasis ability of SACC by regulating EMT through Wnt/β-catenin signaling pathway. In addition, CLDN7 knockdown in SACC promoted tumor growth in nude mice. CLDN7 inhibits cell proliferation and metastasis by inactivating the Wnt/β-catenin signaling in SACC. Thus, CLDN7 expression might be a useful marker to identify the potential for progression in SACC.
Keywords: CLDN7, salivary adenoid cystic carcinoma, Wnt/β-catenin signaling, proliferation, metastasis
Introduction
Salivary adenoid cystic carcinoma (SACC) is not rare in salivary gland malignancies. Distant metastasis is common in SACC, and the lung is the most vulnerable1. Thus, it is necessary to better know the mechanisms underlying the malignant potential of SACC in order to develop novel therapeutic strategies to increase survival.
Claudins (CLDNs), the major integral membrane proteins, play an important role in the function of the tight junction (TJ) between epithelial cells. In humans, it has 24 kinds of proteins that are expressed differentially in different tissues2. CLDN7 is part of the TJ protein family. Recent data indicated that CLDN7 is abnormally regulated in various human cancers and CLDN7 is associated with the etiology and progression of cancer3–9. Overexpression of CLDN7 can reversely increase tumor invasion. The upregulation of CLDN7 has been found associated with the poor prognosis in many tumors, including ovarian, breast, and gastric carcinomas6–9.
Conversely, lack of CLDN7 consequently could promote the proliferation because the TJs were destroyed2,10. In vitro, knockdown of CLDN7 promotes tumor migration and invasion in esophageal squamous cell carcinoma11. It is reported that the low expression of CLDN7 is associated with the poor prognosis of many tumors, including colorectal, esophageal, and nasopharyngeal cancers3,4,5,12,13.
The Wnt signaling pathway, a classical signal transduction pathway, plays a key role in controlling lots of aspects of development and cell maintenance in human beings14, such as cell proliferation, migration, invasion, apoptosis, and cell polarity. A previous study reported that CTNNB1, APC, and AXIN1 are mutated during Wnt/β-catenin signal alteration in SACCs15. Moreover, abnormal immunoreactivity of CLDN7 in ductal epithelium cells in many salivary gland tumors was reported, including SACCs16. Currently, there is no evidence demonstrating the correlation between CLDN7 and Wnt/β-catenin signal alteration in SACC. In this research, we examined and compared CLDN7 expression level in SACC tissues and detected the relationship between CLDN7 expression and clinicopathological characteristics. Also, we investigated the effect of CLDN7 knockdown on the Wnt/β-catenin signaling and biological characteristics of SACC cells. Moreover, we explored the in vivo effect of CLDN7 on tumor progression.
Materials and Methods
Patients
In total, 50 patients (29 males and 21 females; range from 27 to 87 years old) who were histopathologically and clinically diagnosed as primary SACC were recruited between 2006 and 2016 from the Jiangsu Stomatological Hospital, the Affiliated Stomatological Hospital of Nanjing Medical University. This study followed-up all patients from 2 to 90 months; however, there are three patients lost during follow-up visits. All experimental protocols were allowed by the Institutional Review Board of the Nanjing Medical University and complied with the Declaration of Helsinki (Approval ID 2019343).
Immunohistochemistry
In short, first, we fixed tissues on glass slides, used xylene and alcohol to deparaffinize and hydrate sections, and washed them with tap water. Second, we blocked endogenous peroxidase activity with 3% H2O2 and preformed antigen retrieval with pressure cooker in 10 mM citrate buffer. Third, we incubated the sections with antibodies against CLDN7 (Sigma, St Louis, MO, USA; 1:100) overnight at 4°C after blocked with nonspecific binding. Last, we used a horseradish peroxidase (HRP)-conjugated secondary antibody (Maixin-Bio, Fujian, China) to bind the primary antibodies and visualized sections with 3,3-diaminobenzidine solution (Maixin-Bio).
Evaluation of Immunoreactivity
CLDN7 immunoreactivity was grouped by Immunoreactive Score (IRS) (IRS = immunointensity score (IS) × proportion score (PS)) selected by staining intensity and distribution. IS was divided into negative (0), weak (1), moderate (2), or strong (3), whereas PS was segmented into negative (0), <10% (1), 11%–50% (2), 51%–80% (3), and >80% (4). The patients were divided into two groups in accordance with the IRS: low expression (0–6) and high expression (>6). Histological tissues were evaluated by two pathologists, respectively.
Cell Culture, Lentiviral Transfection Assay, and Small-Molecule Inhibitor XAV-939
The human SACC-LM cell line, obtained from China Center for Type Culture Collection (Shanghai, China), was cultured with RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Sciencell, California, USA) in incubator with standard 37°C humidified 5% CO2. The cells were transfected with either specific shRNA lentivirus (5′-GAGCTCCTATGCGGGTGACAA-3′) targeting to knock down CLDN7 or scrambled shRNA lentivirus. Stable cells were selected with 2 μg/ml puromycin after transfection. Real-time polymerase chain reaction (PCR) was used to estimate transfection efficiency. XAV-939, resolved in dimethyl sulfoxide, was used to inhibit β-catenin expression in cells.
Real-Time PCR Analysis
Total RNA from cells and tissues was isolated with TRIzol reagent (Invitrogen, New York, NY, USA). Reverse transcription was performed in the PCR System 7300 (Applied Biosystems, Foster City, CA, USA) with SYBR Green Master Mix (TaKaRa, Shiga, Japan). The primers for CLDN7 were: forward 5′-AGAGCACTTTG GACAGAACCC-3′ and reverse 5′-CTCCGGACTGGATTTCCCTC-3′; E-cadherin: forward 5′-GCCTTATGATTCTCTGCTCGTG-3′ and reverse 5′-GCCCCATTCGT TCAAGTAGTC-3′; N-cadherin: forward 5′-GTGAGCCTGCAGATTTTAAGGTG-3′ and reverse 5′-GTTGGCTTCAGGCTCATTTTACT-3′; Vimentin: forward 5′-CT GGATTCACTCCCTCTGGTT-3′ and reverse 5′-TCGTGATGCTGAGAAGTTTCGTT-3′; and GAPDH: forward 5′-GACGTAGGGAGTGAAGGT C-3′ and reverse 5′-GAGAGTTCAGATGTTGATGG-3′. GAPDH gene expression was given as a normalization and calculations were preformed using the values of the average cycle threshold (ddCt) method.
Western Blotting Analysis
Western blotting was performed in accordance with common methods. In short, protein from tissues and cells was isolated in radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China) mixed with protease inhibitors (Beyotime, Shanghai, China). Then, protein was loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred onto the polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Membranes were incubated with the primary antibody and the protein bands were quantified through exposure to enhanced chemiluminescence reagents (GE Healthcare, Chicago, IL, USA) after incubated with secondary antibodies. Primary antibodies were as follows: GAPDH (1:1,000, Protein-tech, Wuhan, China), CLDN7 (1:500, Invitrogen), E-cadherin (1:1,000, CST, Boston, MA, USA), N-cadherin (1:1,000, CST), Vimentin (1:1,000, CST), β-catenin (1:1,000, CST), and H3 (1:1,000, CST).
Cell Migration and Invasion Assays
A total of 1 × 105 SACC cells were loaded into a transwell insert (8-mm pore size, Costar, Lowell, MA, USA) in migration assay. For invasion assay, cell number is 2 × 105 and the upper chamber was covered with Matrigel (Corning, Bedford, MA, USA). The chemoattractant in the lower chamber for migration was medium containing 10% FBS and for invasion is 20%. After 24 h, the invaded cells were stained and evaluated under a microscope (Tokyo, Japan).
Cell Viability Assay (CCK-8 Assay)
Cell proliferation was evaluated by CCK-8 kits (Dojindo Molecular Technologies, Kumamoto, Japan). A total of 2,000 cells/well were put into culture plates and every 24 hours, CCK-8 reagent (10 μl/well) was seeded and absorbance was measured.
Colony Formation Experiment
A total of 1 × 103 cells were putted on 35-mm culture plates. After 10 days, cells were fixed and stained, and positive colonies were counted.
Immunofluorescence Staining
SACC cells were put onto coverslips. Cells were fixed in paraformaldehyde for 20 min when cells grew to 30%-40% confluency. Then, the slides were incubated in normal goat serum with primary antibody CLDN7 (1:500, Invitrogen), E-cadherin (1:50, CST), N-cadherin (1:50, CST), and Vimentin (1:50, CST) overnight at 4°C. After incubation with goat anti-mouse immunoglobulin G (1:1,000, Life Technologies, New York, NY, USA), the slips were blocked with 4’,6-diamidino-2-phenylindole (Life Technologies) and detected with a FV1000 laser confocal scanning microscope (Olympus, Tokyo, Japan).
Tumor Xenografts
Specific pathogen-free (SPF) BALB/c nude female mice (mean age, 5–6 weeks) were obtained from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). SACC-LM cells (3 × 106) were injected in the right flank of mice, and tumor size and mice body weight were measured every other day. At 26 days, mice were sacrificed and tumors were obtained. All mice were bred and maintained in the SPF Laboratory Animal Center of Nanjing Medical University (Nanjing, China). The use of animals in this study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Approval ID1906018).
Hematoxylin-Eosin Staining
In short, after deparaffinization and rehydration, we put pathological sections in 1% acid ethanol (1% HCl in 70% ethanol) and stained them with hematoxylin solution. Then, we stained the sections with eosin and cleaned in xylene. The slides were evaluated and photographed under a FV1000 laser confocal scanning microscope (Tokyo, Japan).
Statistical Analysis
Data were analyzed with SPSS 22.0 (IBM SPSS Statistics, Armonk, NY, IBM-Corp.) and GraphPad Prism 7 (GraphPad Software Inc.). A paired t-test was applied to compare CLDN7 expression in SACC tissue and normal tissue. The chi-square test and Fisher’s exact probability method were applied to analyze the connection between CLDN7 expression and clinicopathological characteristics among 90 patients with SACC. The P-values of less than 0.05 were considered significant.
Results
CLDN7 is Downregulated in SACC Tissues and can be Detected at the Membrane and Cytoplasm
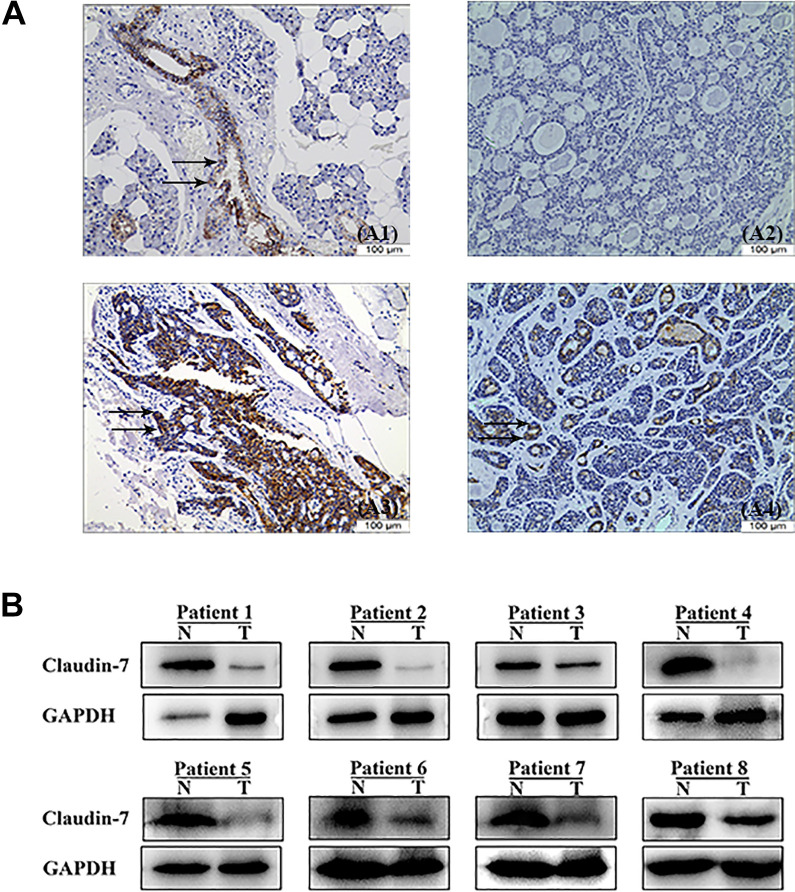
We detected and graded CLDN7 expression in SACC tumors and paired normal peritumoral tissues. Low CLDN7 expression was detected in SACC tissues in 84% (42/50) of cases. All normal salivary glands (100%) were CLDN7 positive, and 83% of cases showed strong immunoexpression. Therefore, CLDN7 expression in SACC tumors was lower than that in paired normal peritumoral tissues (P < 0.05; Fig. 1A).
Figure 1.
Immunoexpression of CLDN7 was observed in SACC. (A1) Positive expression of CLDN7 was observed in normal salivary glands (brown staining as shown by arrows, 200×). (A2, A3, and A4) Representative photomicrographs of negative staining of CLDN7 (200×) and positive staining of CLDN7 (brown staining as shown by arrows, 200×), but some SACC showed evident cytoplasmic expression of CLDN7 (brown staining as shown by arrows, 200×). (B) Western blot analysis of CLDN7 expression in eight pairs of SACC tumor tissues and noncancerous adjacent tissues. SACC: salivary adenoid cystic carcinoma; N: non-cancerous adjacent tissues; T: tumor tissue.
Salivary glands presented a positive reaction to CLDN7 in the cytoplasm of luminal epithelial cells (Fig. 1A1). Immunohistochemical staining showed that 62% specimens (31/50) were negative for CLDN7 (Fig. 1A2). The cribriform and solid patterns showed negative CLDN7 expression, but the tubular/trabecular pattern was CLDN7 moderate (Fig. 1A2, A3). CLDN7 positivity was primarily detected in the glandular epithelial cells, but not in the acinar or myoepithelial cells (Fig. 1A3). CLDN7 positivity was detected as brown membranous and cytoplasmic immunostain in SACC (Fig. 1A4). In normal salivary glands, cytoplasm showed no staining for CLDN7, whereas in SACC tissues, significant cytoplasmic staining appeared (P < 0.05). In this study, SACC group consisted of 24 tubular, 23 cribriform, and 3 solid growth patterns. In this study, SACC group consisted of 24 tubular, 23 cribriform, and 3 solid growth patterns. Besides, we confirmed this phenomenon at the protein level (Fig. 1B).
Association of Clinicopathological Characteristics and CLDN7 Expression in SACC Cancer
CLDN7 expression levels were related to agender, lymph node status, and recurrence (P < 0.05; Table 1). There was no significant connection between CLDN7 expression levels and other clinicopathological characteristics (P > 0.05; Table 1). We found patients with low CLDN7 expression have greater probability in cancer metastasis compared to those with N0 lymph node metastasis (78%, 32/41; P < 0.05). The percentage of samples with low CLDN7 expression was higher in patients with lymph node metastasis (100%, 9/9) than those with N0 lymph node metastasis (78%, 32/41; P < 0.05). The percentage of tissue samples with low CLDN7 expression was also higher in patients with recurrence (100%, 9/9) compared to those without recurrence (78%, 32/41; P < 0.05) and in male patients (93%, 27/29) compared with those in female patients (71%, 15/21; P < 0.05). Although not significant, low CLDN7 expression was also observed in patients with advanced American Joint Committee on Cancer (AJCC) stages (85%, 28/33), advanced T stages (85%, 23/27), distant metastasis (90%, 19/21), nerve invasion (85.7%, 12/14) compared with patients with primary AJCC stages (88%, 15/17; P > 0.05), primary T stages (78.3%, 18/23; P > 0.05), no distant metastasis (72.4%, 21/29; P > 0.05), and no nerve invasion (80.6%, 29/36; P > 0.05), respectively.
Table 1.
Summary of Expression of CLDN7 and Clinicopathologic Features of SACC Patients.
| Clinical and pathologic variables | Cases (n/%) | CLDN7 expression | P-value | |
|---|---|---|---|---|
| Low | High | |||
| Sex | ||||
| Male | 29 (58%) | 27 | 2 | 0.014 |
| Female | 21 (42%) | 15 | 6 | |
| Age (years) | ||||
| ≤60 | 32 (64%) | 28 | 4 | 0.651 |
| >60 | 18 (36%) | 15 | 3 | |
| Tumor size (cm) | ||||
| <3.5 | 24 (48%) | 18 | 6 | |
| ≥3.5 | 26 (52%) |
24 | 2 | 0.679 |
| AJCC stage | ||||
| I + II | 17 (34%) | 15 | 2 | 0.496 |
| III + IV | 33 (12%) | 28 | 5 | |
| Recurrence | ||||
| Absence | 41 (82%) | 32 | 9 | 0.049 |
| Presence | 9 (18%) | 9 | 0 | |
| T stage | ||||
| T1 + T2 | 23 (46%) | 18 | 5 | 0.496 |
| T3 + T4 | 27 (54%) | 23 | 4 | |
| N stage | ||||
| N0 | 41 (82%) | 32 | 9 | 0.049 |
| N1+N2+N3 | 9 (18%) | 9 | 0 | |
| M stage | ||||
| Positive | 21 (42%) | 19 | 2 | |
| Negative | 29 (58%) | 21 | 7 | 0.696 |
| Vascular invasion | ||||
| Absence | 43 (86%) | 35 | 8 | |
| Presence | 7 (14%) | 6 | 1 | 0.918 |
| Nerve invasion | ||||
| Absence | 36 (72%) | 29 | 7 | |
| Presence | 14 (28%) | 12 | 2 | 0.314 |
SACC: salivary adenoid cystic carcinoma.
CLDN7 Inhibits Cell Growth, Migration, and Invasion In Vitro
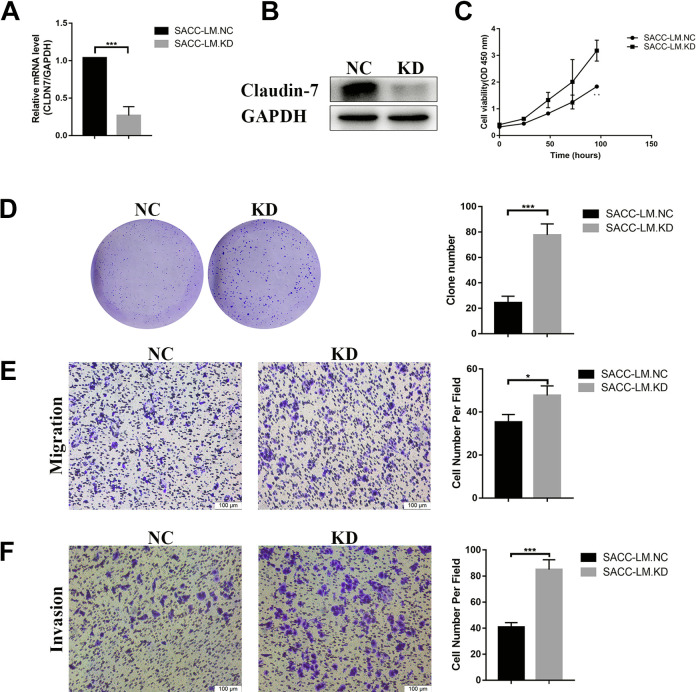
To further study the role of CLDN7 in SACC, we used lentivirus (Genechem Co. Ltd., Shanghai, China) to knock down the expression of CLDN7 in SACC-LM cell line (P < 0.001, Fig. 2A, B). The results showed the viability of the SACC-LM CLDN7 knockdown cells was higher than that of the SACC-LM negative control cells (P < 0.01, Fig. 2C). Beside this, CLDN7 silencing significantly increased the numbers of clones in SACC-LM cells (P < 0.001, Fig. 2D). Meanwhile, transwell assay showed the downregulation of CLDN7 enhanced migratory and invasive ability in SACC-LM cells (P < 0.05, Fig. 2E and P < 0.001, Fig. 2F). Above all, CLDN7 knockdown promoted cell growth, migration, and invasion.
Figure 2.
CLDN7 knockdown in SACC-LM cells promote cell growth and metastasis. (A and B) CLDN7 expression based on real-time RT-PCR and western blotting in SACC-LM.NC and SACC-LM.KD cell line. (C) CCK8 assays of the proliferation of SACC-LM.NC and SACC-LN.KD cells. (D) Clone formation assays: colonies were counted from three individual plates for each sample and were photographed. The number of soft agar colonies presented is the mean of colony counts from three different experiments. (E and F) Representative images of migration and invasion in SACC-LM.NC and SACC-LM.KD cells. CLDN7 silencing enhanced the ability of migration and invasion by transwell assay after incubation for 24 h, scale bar = 50 μm. Data were presented as means ± SD from three independent experiments. *P < 0.05;** P < 0.01; ***P < 0.001, as evaluated using Student’s t-test. RT-PCR: reverse transcription polymerase chain reaction; SACC: salivary adenoid cystic carcinoma; SD: standard deviation.
CLDN7 Knockdown Promotes Epithelial-Mesenchymal Transition in SACC-LM Cell
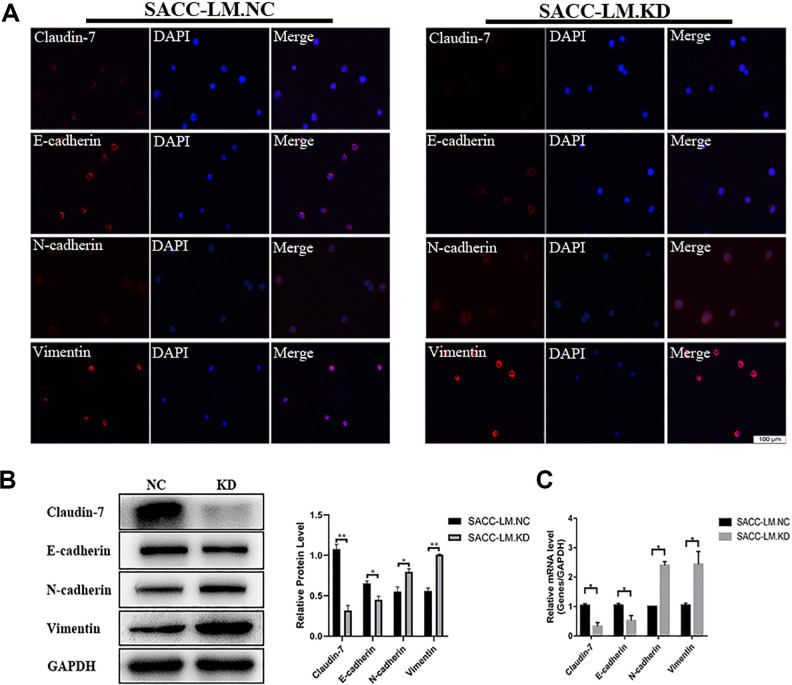
As we know, epithelial-mesenchymal transition (EMT) plays a key role in cancer metastasis, so we investigated whether CLDN7 promotes EMT progression. When CLDN7 is knocked down in SACC-LM cells, the epithelial marker E-cadherin was downregulated, while mesenchymal markers N-cadherin and vimentin were upregulated. Western blotting, real-time PCR, and immunofluorescence analysis all confirmed this phenomenon in SACC-LM knockdown cells than SACC-LM negative control cells (P < 0.05, Fig. 3A–C). Taken together, the downregulation of CLDN7 promoted EMT in SACC.
Figure 3.
CLDN7 knockdown promotes EMT in SACC-LM cells. (A) Immunofluorescence analysis of CLDN7, the EMT marker E-cadherin, N-cadherin, and Vimentin in SACC-LM.KD cells than SACC-LM.NC cells. Red: CLDN7, E-cadherin, N-cadherin, and Vimentin. Blue: DAPI (200×). (B) Protein levels of CLDN7, E-cadherin, N-cadherin, and Vimentin were determined by western blotting. (C) Gene expression of CLDN7, E-cadherin, N-cadherin, and Vimentin was measured by real-time RT-PCR. GAPDH served as loading control. Data represent the mean ± SD in B and D; **P < 0.01. DAPI: 4’,6-diamidino-2-phenylindole; EMT: epithelial-mesenchymal transition; RT-PCR: reverse transcription polymerase chain reaction; SACC: salivary adenoid cystic carcinoma; SD: standard deviation.
CLDN7 Regulated SACC-LM Cells Proliferation and EMT Progression Through the Wnt/β-Catenin Signaling Pathway
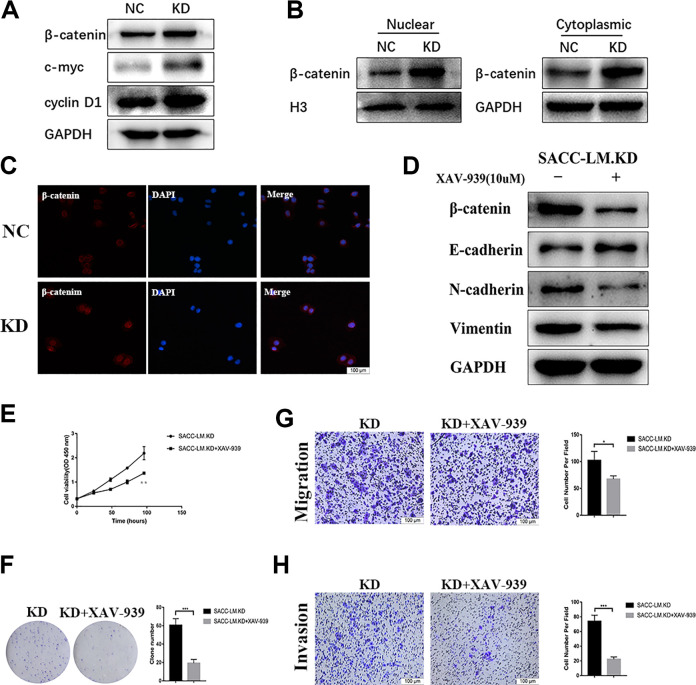
It is well established that Wnt/β-catenin pathway signaling plays a key role in tumor development and progression. In the classic Wnt/β-catenin pathway, β-catenin accumulates in the cytoplasmic and enter into nucleus, thus regulating downstream gene expression and promoting tumor progression. As shown in Fig. 4A–C, silencing CLDN7 also activated Wnt/β-catenin pathway. Both western blotting and immunofluorescence analysis demonstrated β-catenin increased in the nucleus after CLDN7 knockdown (Fig. 4A–C). XAV-939, an β-catenin inhibitor, could silence β-catenin expression. We treated SACC-LM.KD cells with XAV-939 and found that activated EMT process induced by CLDN7 could be reversed. E-cadherin expression was decreased, while N-cadherin and vimentin expression were increased (Fig. 4D). Besides, we detected cell viability, migration, and invasion ability all decreased after treatment with XAV-939 (Fig. 4E–H). Taken together, above findings suggested that CLDN7 downregulation promoted cell proliferation and metastasis in SACC through Wnt/β-catenin signaling pathway.
Figure 4.
CLDN7 regulated SACC-LM cells proliferation and EMT progression through the Wnt/β-catenin signaling pathway. (A) Expression of β-catenin, c-myc, and cyclinD1 protein in SACC-LM.NC and SACC-LM.KD groups. (B) Nuclear and cytoplasmic expression of β-catenin protein in SACC-LM.NC and SACC-LM.KD groups. (C) Immunofluorescence assay shows higher level of nuclear β-catenin protein in the SACC-LM.KD group than in the SACC-LM.NC group. (D) Expression of EMT-related proteins by western blotting in SACC-LM.KD cells after treatment with the XAV-939 (10 μM) for 24 h. (E, F) CCK-8 and cell clone assay to detect cell viability after treatment with the XAV-939 (10 μM) for 24 h. (G, H) Transwell assay with or without Matrigel was used to detect cell migration and invasion ability in cells after treatment with the XAV-939 (10 μM) for 24 h. EMT: epithelial-mesenchymal transition; SACC: salivary adenoid cystic carcinoma.
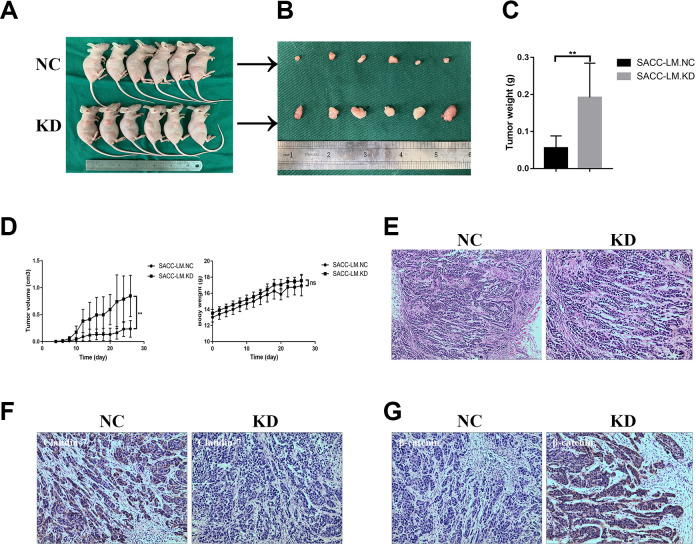
CLDN7 Suppresses Tumor Growth
To study the tumorigenic ability of CLDN7 in SACC in vivo, we used subcutaneous tumor model and injected SACC-LM.NC or SACC-LM.KD cells into the nude mice (n = 6/group). The tumors formed in the SACC-LM.KD group were larger and heavier than SACC-LM.NC group during its development (Fig. 5A–C). There was no significant difference between two groups (P < 0.01, Fig. 5D). Besides, the tumors formed in the nude mice were diagnosed as SACC by HE staining (Fig. 5E). Silencing CLDN7 promoted cell proliferation and metastasis in SACC through the Wnt/β-catenin signaling. Therefore, the expression of CLDN7 and β-catenin was analyzed. We discovered tumors obtained from SACC-LM.NC group presented positive staining of CLDN7 and negative in SACC-LM.KD group (Fig. 5F). Mice in SACC-LM.KD group showed higher β-catenin expression than in SACC-LM.NC group (Fig. 5G).
Figure 5.
CLDN7 suppresses tumor growth in vivo. (A) General observation of tumor-bearing nude mice coinjected with SACC-LM.KD cells and SACC-LM.NC (n = 6). (B and C) Tumor tissues were resected and weighed after 26 days. (D) Volumes of xenograft tumors and body weight measurements in each group. Data represent the mean ± SD in C and D; *P < 0.05, **P < 0.01; ns: no significance. (E) Representative showings of tumors formed in the nude mice were diagnosed as SACC by HE staining (200×; 400×). (F) Representative photomicrographs of positive staining of CLDN7 in SACC-LM.NC cells (200×) and negative staining of CLDN7 in SACC-LM.KD cells (200×). (G) Representative photomicrographs of negative staining of β-catenin in SACC-LM.NC cells (200×) and positive staining of β-catenin in SACC-LM.KD cells (200×).
Discussion
Tumor progression involves multiple factors and steps. This claudin family is crucial for cell polarity and signal transductions. Cell–cell junction loss is said to be an important step in tumor metastasis17. Decreased expression of claudins was considered as a prognostic marker in some cancer types13,14,17. Low CLDN7 expression could be regarded as a molecular characteristic about enhancing cellular disorientation, detachment, and migration of tumor cells. Currently, there is little evidence indicating a correlation between CLDN7 expression and SACC. Our study suggested CLDN7 expression in SACC tissues is clearly lower than that in paired adjacent normal tissues and is correlated with lymph node metastasis, recurrence, and gender. Therefore, CLDN7 expression is a promising prognostic biomarker for SACC.
Our study found that CLDN7 is decreased in most SACC tissues. By PCR and western blot analysis, primary SACC tissues exhibited low expression in 78% tumor tissue samples. Our findings are partly in accordance with the prior studies with few samples18, showing that CLDN7 was expressed in only ducts basal/myoepithelial cells of normal salivary glands. In the present study, CLDN7 was positive for ductal cells, but not for myoepithelial cells in SACC tissues. The fact that CLDN7 was downregulated in SACC tissues may be caused by the increased number of myoepithelial cells, because SACCs are composed of much amounts of myoepithelial cells, compared with ductal cells. It is reported that hypermethylation was related with the downregulation of CLDN7 expression in other cancers17. The underlying mechanism of CLDN7 expression in SACCs should be clarified in the future study.
Interestingly, CLDN7 positivity was observed as brown membranous and cytoplasmic immunostaining in SACC group. The fact that punctate cytoplasmic staining of CLDN7 can be found in ovarian cancer and gastric adenocarcinomas has been reported9,18. It is reported that claudin genes encode proteins in the cytoplasm. The mechanisms of claudin mislocalization remain unclear. Taken together, cytoplasmic localization of CLDN7 seems to be a potential marker of surmising malignant transformation of epithelial cells in salivary glands.
EMT can result in intercellular adhesion and polarity losing and migration and movement obtaining, which finally bring about cancer metastasis, through the transformation of adherent and polarized epithelial cells and disruption of cell–cell adhesion19. EMT have classic features of epithelial adhesion and cytoskeletal markers losing and migratory mesenchymal symbols obtaining, such as E-cadherin, N-cadherin, and Vimentin20,21. According to the studies by Wang et al22, CLDN7 downregulation may enhance the invasive and metastatic ability of colorectal cancer through regulating EMT. Some study has suggested that EMT is a typical event in the metastasis of SACC23,24. However, the relation between CLDN7 and EMT in SACC is rarely studied.
The clinical specimens showed that CLDN7 may play a key role in the migration and metastasis of SACC in our study. When CLDN7 was knocked down, transwell assay showed that downregulation of CLDN7 promoted the metastasis of SACC-LM cells compared with negative control cells. Moreover, the epithelial marker E-cadherin was decreased, while the mesenchymal markers N-cadherin and Vimentin were increased in SACC-LM cells. Furthermore, immunofluorescence also demonstrated this phenomenon. In conclusion, CLDN7 could significantly promote EMT in SACC-LM cells. Nonpalmitoylated CLDN7 was reported to be associated with the formation of intercellular TJs and suppressed tumor progression and metastasis25. More research on the specific expression and function of nonpalmitoylated CLDN7 in SACCs should be further studied in the future.
On the other hand, CLDN7 knockdown significantly increased the numbers of clones in SACC-LM cells. To further support the function of CLDN7, nude mice was used to explore it in vivo. Representative pathological showings of SACC were also discovered in the tumor xenografts. The results showed that the tumor volume in the knockdown SACC-LM group was significantly larger than control group. The fact that lower CLDN7 expression was also demonstrated in the tumor xenografts of the knockdown SACC-LM group. This could be explained by the fact that loss of claudin expression of the cell could directly promote the neoplastic process due to the TJs were destroyed. Furthermore, the downregulation of CLDN7 or less TJs could increase the availability of nutrients and ligands leading to increased proliferation26. Silencing CLDN7 in SACC-LM significantly promoted tumor growth, further indicating that CLDN7 could inhibit tumor growth as a tumor suppressor gene in SACCs.
It is reported that many signaling pathways are associated with EMT, including Wnt/β-catenin signaling19. Aberrant change of the relevant signaling pathways is related to tumorigenesis and metastasis. It is reported that Wnt can activate β-catenin at a downstream location27. The Wnt/β-catenin signaling pathway is reported to have an important role in cell proliferation, differentiation, growth, regeneration, and self-renewal28. It is reported that Wnt signaling may be involved in the regulation and localization of claudins29. In our study, silencing CLDN7 could induce β-catenin expression. Using XAV-939 to control β-catenin activation, the EMT and proliferation of SACC-LM cells were slightly inhibited. Additionally, expression of CLDN7 in SACC-LM cell showed no change. The results in our study showed no regulation of CLDN7 by Wnt/β-catenin.
Therefore, the Wnt/β-catenin signaling pathway has been partly involved in the SACC metastasis and proliferation inhibited by CLDN7. Our study is the first time to indicate that downregulation of CLDN7 expression from normal control to the malignant SACC group. Based on the aforementioned studies, in vitro researches have demonstrated that CLDN7 has multiple carcinogenic effects, including regulating cell proliferation, migration, and prohibiting EMT. In addition, in vivo experiment confirmed the function of the downregulation of CLDN7 in promoting tumor growth. In conclusion, our results are in accordance with the conclusion that CLDN7 knockdown enhances proliferation, migration, and invasion of SACC-LM cells through activating Wnt/β-catenin signaling pathway. The interactions between CLDN7 of cytoplasm and β-catenin of nucleus in SACC cell proliferation and metastasis will be further investigated by our group. These data may provide an explanation for that CLDN7 might be a useful marker in potential therapies in SACC.
Footnotes
Ethical Approval: Ethical approval to this study was obtained from the Institutional Review Board of the Nanjing Medical University and complied with the Declaration of Helsinki (Approval ID 2019343).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institutional Animal Care and Use Committee of Nanjing Medical University (Approval ID 1906018).
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article in accordance with protocols approved by the Human Subjects Institutional Review Board of Nanjing Medical University (Approval ID 2016115).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Natural Science Foundation of Jiangsu Province (Grant No BK2018040793).
ORCID iDs: Hongming Du  https://orcid.org/0000-0002-0909-0773
https://orcid.org/0000-0002-0909-0773
References
- 1. Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A, Haigentz M, Jr, Takes RP, Mondin V, et al. Adenoid cystic carcinoma of the head and neck - an update. Oral Oncol. 2015;51(7):652–661. [DOI] [PubMed] [Google Scholar]
- 2. Tsukita S, Yamazaki Y, Katsuno T, Tamura A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27(55):6930–6938. [DOI] [PubMed] [Google Scholar]
- 3. Usami Y, Chiba H, Nakayama F, Ueda J, Matsuda Y, Sawada N, Komori T, Ito A, Yokozaki H. Reduced expression of Claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol. 2006;37(5):569–577. [DOI] [PubMed] [Google Scholar]
- 4. Hsueh C, Chang YS, Tseng NM, Liao CT, Hsueh S, Chang JH, Wu IC, Chang KP. Expression pattern and prognostic significance of claudins 1, 4, and 7 in nasopharyngeal carcinoma. Hum Pathol. 2010;41(7):944–950. [DOI] [PubMed] [Google Scholar]
- 5. Oshima T, Kunisaki C, Yoshihara K, Yamada R, Yamamoto N, Sato T, Makino H, Yamagishi S, Nagano Y, Fujii S, Shiozawa M, et al. Reduced expression of the Claudin-7 gene correlates with venous invasion and liver metastasis in colorectal cancer. Oncol Rep. 2008;19(4):953–959. [PubMed] [Google Scholar]
- 6. Kim CJ, Lee JW, Choi JJ, Choi HY, Park YA, Jeon HK, Sung CO, Song SY, Lee YY, Choi CH, Kim TJ, et al. High Claudin-7 expression is associated with a poor response to platinum-based chemotherapy in epithelial ovarian carcinoma. Eur J Cancer. 2011;47(6):918–925. [DOI] [PubMed] [Google Scholar]
- 7. Bernardi MA, Logullo AF, Pasini FS, Nonogaki S, Blumke C, Soares FA, Brentani MM. Prognostic significance of CD24 and Claudin-7 immunoexpression in ductal invasive breast cancer. Oncol Rep. 2012;27(1):28–38. [DOI] [PubMed] [Google Scholar]
- 8. Park JY, Park KH, Oh TY, Hong SP, Jeon TJ, Kim CH, Park SW, Chung JB, Song SY, Bang S. Up-regulated claudin 7 expression in intestinal-type gastric carcinoma. Oncol Rep. 2007;18(2):377–382. [PubMed] [Google Scholar]
- 9. Dahiya N, Becker KG, Wood WH, Zhang Y, Morin PJ. Claudin-7 is frequently overexpressed in ovarian cancer and promotes invasion. PLoS One. 2011;6(7):e22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, Cao J, Chen YH. Inflammation and disruption of the mucosal architecture in Claudin-7-deficient mice. Gastroenterology. 2012;142(2):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lioni M, Brafford P, Andl C, Rustgi A, El-Deiry W, Herlyn M, Smalley KS. Dysregulation of Claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol. 2007;170(2):709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of Claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. 2014;12(2):156–162. [DOI] [PubMed] [Google Scholar]
- 13. Johnson AH, Frierson HF, Zaika A, Powell SM, Roche J, Crowe S, Moskaluk CA, El-Rifai W. Expression of tight-junction protein Claudin-7 is an early event in gastric tumorigenesis. Am J Pathol. 2005;167(2):577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng LF, Kaur P, Bunnag N, Suresh J, Sung IC, Tan QH, Gruber J, Tolwinski NS. WNT signaling in disease. Cells. 2019;8(8):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daa T, Kashima K, Kaku N, Suzuki M, Yokoyama S. Mutations in components of the Wnt signaling pathway in adenoid cystic carcinoma. Mod Pathol. 2004;17(12):1475–1482. [DOI] [PubMed] [Google Scholar]
- 16. Aoyama T, Takasawa A, Murata M, Osanai M, Takano K, Hasagawa T, Sawada N. Immunoreactivity patterns of tight junction proteins are useful for differential diagnosis of human salivary gland tumors. Med Mol Morphol. 2019;52(1):23–35. [DOI] [PubMed] [Google Scholar]
- 17. Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein Claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22(13):2021–2033. [DOI] [PubMed] [Google Scholar]
- 18. Tassi R A, Bignotti E, Falchetti M, Ravanini M, Calza S, Ravaggi A, Bandiera E, Facchetti F, Pecorelli S, Santin AD. Claudin-7 expression in human epithelial ovarian cancer. Int J Gynecol Cancer. 2010;18(6):1262–1271. [DOI] [PubMed] [Google Scholar]
- 19. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. [DOI] [PubMed] [Google Scholar]
- 21. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev cell. 2008;14(6):818–829. [DOI] [PubMed] [Google Scholar]
- 22. Wang K, Li T, Xu C, Ding Y, Li W, Ding L. Claudin-7 downregulation induces metastasis and invasion in colorectal cancer via thepromotion of epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2019;508(3):797–804. [DOI] [PubMed] [Google Scholar]
- 23. Dong L, Wang YX, Li SL, Yu GY, Gan YH, Li D, Wang CY. TGF-beta1 promotes migration and invasion of salivary adenoid cystic carcinoma. J Dent Res. 2011;90(6):804–809. [DOI] [PubMed] [Google Scholar]
- 24. Gibbons DL, Creighton CJ. Pan-cancer survey of epithelial-mesenchymal transition markers across the cancer genome atlas. Dev Dyn. 2018;247(3):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neetu D, Becker KG, Wood WHI, Zhang Y, Morin PJ. Claudin-7 is frequently overexpressed in ovarian cancer and promotes invasion. Plos One. 2011;6(7):e22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melchers LJ, Bruine dBL, Schnell U, Slagter-Menkema L, Mastik MF, de Bock GH, van Dijk BAC, Giepmans BNG, van der Laan BFAM, van der Wal JE, Roodenburg JLN, et al. Lack of Claudin-7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral Oncology. 2013;49(10):998–1005. [DOI] [PubMed] [Google Scholar]
- 27. Gruber J, Yee Z, Tolwinski NS. Developmental drif and the role of wnt signaling in aging. Cancers. 2016;8(8):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi J, Chi S, Xue J, Yang J, Li F, Liu X. Emerging role and therapeutic implication of Wnt signaling pathways in autoimmune diseases. J Immunol Res. 2016;(2016):9392132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Oliveira SS, de Oliveira IM, De Souza W, Morgado-Diaz JA. Claudins upregulation in human colorectal cancer. FEBS Lett. 2005;579(27):6179–6185. [DOI] [PubMed] [Google Scholar]