Abstract
We aimed to assess the prevalence of and factors associated with anti- severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) positivity in a large population of adult volunteers from five administrative departments of the Liguria and Lombardia regions. A total of 3609 individuals were included in this analysis. Participants were tested for anti-SARS-CoV-2 antibodies [Immunoglobulin G (IgG) and M (IgM) class antibodies] at three private laboratories (Istituto Diganostico Varelli, Medical Center, and Casa della Salute di Genova). Demographic data, occupational or private exposure to SARS-CoV-2-infected patients, and prior medical history consistent with SARS-CoV-2 infection were collected according to a preplanned analysis. The overall seroprevalence of anti-SARS-CoV-2 antibodies (IgG and/or IgM) was 11.0% [398/3609; confidence interval (CI) 10.0%–12.1%]. Seroprevalence was higher in female inmates than in male inmates (12.5% vs. 9.2%, respectively, p = 0.002), with the highest rate observed among adults aged >55 years (13.2%). A generalized estimating equations model showed that the main risk factors associated with SARS-CoV-2 seroprevalence were the following: an occupational exposure to the virus [Odd ratio (OR) = 2.36; 95% CI 1.59–3.50, p = 0.001], being a long-term care facility resident (OR = 4.53; 95% CI 3.19–6.45, p = 0.001), and reporting previous symptoms of influenza-like illness (OR = 4.86; 95% CI 3.75–6.30, p = 0.001) or loss of sense of smell or taste (OR = 41.00; 95% CI 18.94–88.71, p = 0.001). In conclusion, we found a high prevalence (11.0%) of SARS-CoV-2 infection that is significantly associated with residing in long-term care facilities or occupational exposure to the virus. These findings warrant further investigation into SARS-CoV-2 antibody prevalence among the Italian population.
Keywords: SARS-CoV-2, COVID-19, antibodies, serological test
1. Introduction
In Italy, the first case of pandemic severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection was reported on 20 February, 2020. Since then, the number of cases increased rapidly in the north of the country, with the Lombardia and Liguria regions being heavily affected by the infection [1]. By the end of April 2020, approximately 85,000 laboratory confirmed cases -of SARS-CoV-2 infection were reported in this geographical area of the country [2]. However, these data included only a fraction of the real number of SARS-CoV-2 infections, since not all infected patients were symptomatic [3,4,5], required hospitalizations, or provided specimens for laboratory testing. The extent to which surveillance data reflect the true burden of the disease can also be affected by changes in laboratory testing recommendation [1]. Serology can represent a key element to overcoming these limits and to better understanding the infection statistics at a population level. The primary outcome of this study was to estimate the prevalence of SARS-CoV-2 antibodies. The secondary outcome was to evaluate possible factors associated with anti-SARS-CoV-2 positivity in a large population of individuals from five administrative departments of the Liguria and Lombardia regions.
2. Experimental Methods
This was an observational study designed to evaluate the prevalence and factors associated with SARS-CoV-2 infections among voluntary, unpaid individuals tested for SARS-CoV-2 antibodies in three private institutions (Istituto Diagnostico Varelli, Medical Center, and Casa della Salute di Genova) during March and April 2020. These institutions altogether include approximately 5,784,974 inhabitants living in five administrative departments (Milano, Varese, Pavia, Genova and Savona) of the Liguria and Lombardia regions. Each laboratory process, about 500,000 samples per year, offers a comprehensive range of tests including clinical biochemistry, serology, and genetic analysis.
2.1. Participants
We included non-hospitalized participants (aged > 18 years) who voluntarily tested for SARS-CoV-2 antibodies in an outpatient setting. After providing informed consent, a sample of venous blood was collected from each participant, all of whom also completed a questionnaire on potential risk factors for developing SARS-CoV-2 infection. Recorded data included age, sex. and occupational or private exposure to SARS-CoV-2 infected patients. In addition, information regarding stays at a long-term care facilities or prior medical history consistent with SARS-CoV-2 infection (influenza-like illness defined according to WHO criteria [6] or loss of smell or taste) within the previous month, were also collected.
2.2. Endpoint
The primary goal was to assess the prevalence of SARS-CoV-2 antibodies [either Immunoglobulin M (IgM) and G (IgG)] positivity among the study population. The secondary goal was to investigate the association between positive tests and demographics (age and sex), occupational and private contact with SARS-CoV-2 infected patients, living in long-term care facilities, and prior symptoms consistent with SARS-CoV-2 infection.
2.3. Detection of Infection
Blood samples were analyzed for serological detection at each participating laboratory by trained staff, unaware of the clinical details of the tested patients. The first laboratory (Istituto Diagnostico Varelli) used a chemiluminescent quantitative immunoassay detecting antibodies against nucleocapsid protein and spike protein (the MaglumiTM 2019) [7]. According to the manufacturer’s recommendations, samples were considered positive above a threshold of 1.1 AU/mL for IgM and IgG. This cut-off resulted in clinical sensitivities/specificities of 78.6%/97.5% and 91.2%/97.3% for IgM and IgG, respectively [7,8]. The second laboratory (Medical Center) applied a rapid chromatographic immunoassay for the qualitative detection of IgG and IgM antibody against spike protein (Realy tech® 2019 nCOV/COVID-19 IgG/IgM Rapid Test Device). The manufacturer’s reported a clinical sensitivity of 92% for IgM; 96% for IgG; and a specificity of 100% for IgM and IgG. The third laboratory (Casa della salute di Genova) assessed anti-SARS-CoV-2 antibodies using a commercially available point-of-care lateral flow immunoassay (Biosynex® Covid-19 BSS, Fribourg, Switzerland) that can simultaneously detect IgM and IgG in human blood, with an overall sensitivity of 88.7% and specificity of 90.6% [9]. This qualitative test detected antibodies against nucleocapsid and spike proteins. All laboratories used internal procedures to validate the diagnostic performance of serological tests. In all cases, the results showed values of sensitivity and specificity consistent with those reported by each manufacturer.
2.4. Statistical Analysis
All statistics were analyzed using SPSS software. Prevalence of anti-SARS-CoV-2 antibodies (IgM or IgG) was calculated and the exact binomial distribution was used to calculate 95% confidence intervals (CIs). The association between positive SARS-CoV-2 antibodies and study variables was estimated in two steps. First, a general linear univariate analysis was performed using a Chi-squared test. The second step used a generalized estimating equation (GEE) model to consider laboratory provenience, with SARS-CoV-2 seropositivity used as a dependent variable. Only differences with a p-values < 0.05 were considered statistically significant.
2.5. Ethical Consideration
The study protocol was approved by the Ethics Committee of Liguria Region (PI Prof. Matteo Bassetti-N. CER Liguria 381/2020-id 10770).
3. Results
3.1. Participant Demographics and Exposures
Between 1 March and 30 April 2020, 3609 individuals agreed to participate in the study. The mean number of screened individuals per administrative department was 721 (52–1430), representing 12 people per 100,000 inhabitants. The patients’ demographics are outlined in Table 1.
Table 1.
Clinical characteristics of the study population.
| Characteristics | N = 3609 (%) |
|---|---|
| Sex | |
| Female | 2007 (55.6) |
| Male | 1602 (44.4) |
| Age groups (Years) | |
| 18–35 | 556 (15.4) |
| 36–45 | 631 (17.4) |
| 46–55 | 929 (25.7) |
| >55 | 1493 (41.4) |
| Region | |
| Lombardia | 3065 (84.9) |
| Liguria | 544 (15.1) |
| Administrative department | |
| Varese | 1430 (39.6) |
| Pavia | 871 (24.1) |
| Milano | 764 (21.2) |
| Genova | 492 (13.6) |
| Savona | 52 (1.4) |
| Resident in a long-term care facility | 207 (5.7) |
Overall, 55.6% (2007/3609) were women and 44.4% were men (1602/3609). The median age was 51 years [interquartile range (IQR) 41–63], with the age group >55 years being most represented (41.4%; n = 1493/3609) and the 18–34 years group being the least represented (15.4%; n = 556/3609). All patients lived in the Lombardia and Liguria regions in the administrative departments of Varese (39.6%; n = 1430/3609), Pavia (24.1%; n = 871/3,609), Milano (21.2%; n = 764/3609), Genova (13.6%; n = 492/3609,) and Savona (1.4%; n = 52/3609;). Approximately 5.7% of the individuals (n = 207/3609) lived in a long-term care facility, whereas 5.0% (n = 179/3609) reported an occupational exposure to infected patients. When asked about recent medical history, 11.8% (n = 427/3609) reported symptoms consistent with influenza-like illness and 0.97% (n = 35/3609) reported loss of smell or taste within the previous month.
3.2. Prevalence of Sars-CoV-2 Antibodies
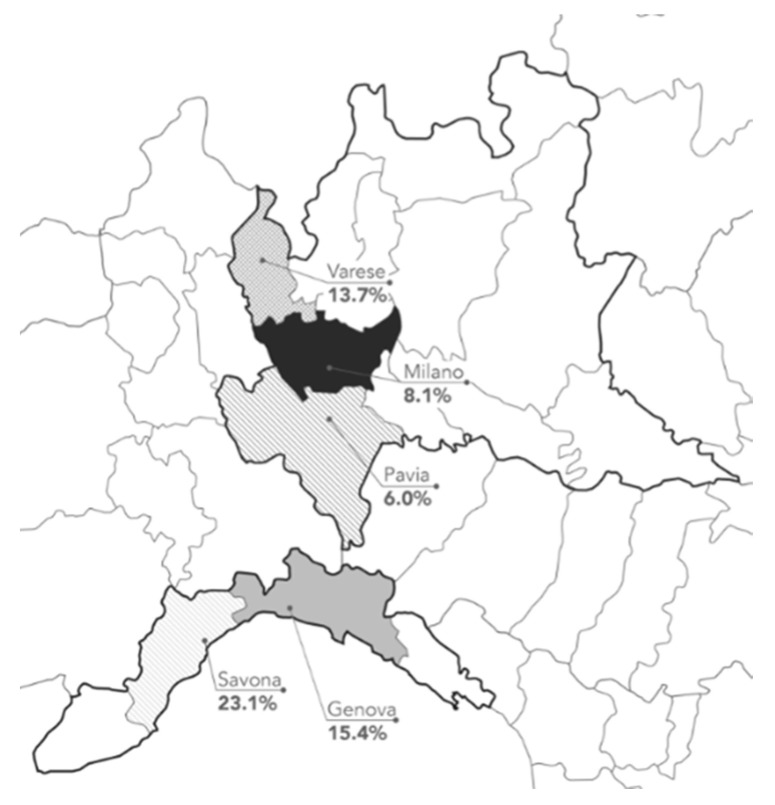
Of the 3609 individuals included in the study population, 398 tested anti-SARS-CoV-2 positive [11.0% (CI 10.0%–12.1%)]. Seroprevalence was higher among women vs. men (12.5% vs. 9.2%, p = 0.002) and varied with age. The rate was highest among adults aged >55 years (13.2%), followed by adults aged 18–35 years (11.9%). As for geographical distribution, the highest prevalence of anti-Sars-COV-2 positivity was reported in the administrative departments of Savona (Figure 1). Table 2 shows estimated prevalence according to the three different laboratories.
Figure 1.
Serologically-confirmed cases of SARS-CoV-2 in the general Italian population from 1 March to 30 April 2020. Red and blue lines represent the boarders of the Lombardia and Liguria regions, respectively. Percentages show the number of positive samples per number tested in each administrative department.
Table 2.
Prevalence of SARS-CoV-2 IgM and IgG antibodies according to the three different laboratories.
| n (%) | Sars-CoV-2 IgG+ or IgM (95% Confidence Interval) |
|
|---|---|---|
| Medical Center | 1885 (52.2) | 11.5% (10.1%–13.0%) |
| Istituto Diagnostico Varelli | 1180 (32.7) | 8.0% (6.5%–9.7%) |
| Casa della salute di Genova | 544 (15.1) | 16.2% (13.2%–19.5%) |
3.3. Factors Associated with Anti-Sars-CoV-2 Antibodies Positivity
Several factors showed an association with anti-SARS-CoV-2 antibodies positivity with univariable analysis (Table 3). The variables that showed a p-value < 0.10 were also included in the GEE model (Table 4). The model showed that the main risk factors associated to SARS-CoV-2 seroprevalence were the following: occupational exposure to the virus (OR = 2.36; 95% CI 1.59–3.50, p = 0.001), living in a long-term care facility (OR = 4.53; 95% CI 3.19–6.45, p = 0.001), and reporting previous symptoms of influenza-like illness (OR = 4.86; 95% CI 3.75–6.30, p = 0.001) or loss of sense of smell or taste (OR = 41.00; 95% CI 18.94–88.71, p = 0.001).
Table 3.
Prevalence of Sars-CoV-2 IgM and IgG antibodies and univariate analysis of factors potentially associated with infection (n = 3609).
| Characteristics | Tested | SARS-CoV-2 IgG+ or IgM+ |
Univariate Analysis | ||
|---|---|---|---|---|---|
| N | n (%) | OR | 95% CI | p-Value | |
| Sex | |||||
| Female | 2007 | 251 (12.5) | 1.36 | 1.12–1.65 | 0.002 |
| Male | 1602 | 147 (9.2) | Ref | Ref | Ref |
| Age group (Years) | |||||
| 18–35 | 556 | 66 (11.9) | 1.10 | 0.83–1.46 | 0.50 |
| 36–45 | 631 | 45 (7.1) | 0.57 | 0.41–0.79 | 0.001 |
| 46–55 | 929 | 90 (9.7) | 0.82 | 0.64–1.05 | 0.24 |
| >55 | 1493 | 197 (13.2) | 1.44 | 1.17–1.78 | 0.001 |
| Living in a long-term care facility | |||||
| No | 3402 | 312 (9.2) | Ref | Ref | Ref |
| Yes | 207 | 86 (41.5) | 7.56 | 5.58–10.23 | 0.001 |
| Occupational exposure | |||||
| No | 3430 | 363 (10.6) | Ref | Ref | Ref |
| Yes | 178 | 35 (19.7) | 2.60 | 1.76–3.88 | 0.001 |
| Private Exposure | |||||
| No | 3469 | 376 (10.8) | Ref | Ref | Ref |
| Yes | 140 | 21 (15.0) | 1.45 | 0.90–2.36 | 0.12 |
| Occurrence of Symptoms in the previous month | |||||
| No symptoms | 3147 | 226 (7.2) | Ref | Ref | Ref |
| Influenza-like illness | 427 | 427 (34.2) | 6.71 | 5.27–8.54 | 0.001 |
| Loss of sense or taste | 35 | 26 (74.3) | 37.33 | 17.28–80.64 | 0.001 |
CI Confidence Interval; OR Odd ratio; Ref Reference.
Table 4.
Results of the generalized estimating equations model of risk factors associated with SARS-CoV-2 seroprevalence.
| Characteristics | OR | 95% CI | p-Value |
|---|---|---|---|
| Male sex | 0.79 | 0.63–1.01 | 0.06 |
| Age 36–45 | 1.40 | 0.99–1.93 | 0.06 |
| Age > 55 | 1.17 | 0.88–1.55 | 0.27 |
| Living in a long-term care facility | 4.53 | 3.19–6.45 | 0.001 |
| Occupational exposure | 2.36 | 1.59–3.50 | 0.001 |
| Prior history of influenza-like illness | 4.86 | 3.75–6.30 | 0.001 |
| Prior history of loss of sense or taste | 41.00 | 18.94–88.71 | 0.001 |
CI Confidence Interval; OR Odd ratio.
4. Discussion
In the present observational study performed on a large sample of subject in northern Italy, we found the following: (1) the overall seroprevalence of anti-SARS-CoV-2 antibodies (IgG and/or IgM) was 11.0%; (2) occupational exposure to the virus, long-term care facility residency, as well as previous symptoms of influenza-like illness or loss of sense of smell or taste were independently associated with anti-SARS-CoV-2 positivity.
To the best of our knowledge, this is one of the first reports that attempts to describe the prevalence of coronavirus disease and to evaluate the potential circulation of SARS-CoV-2 in North Italy. The findings of our study showed that in a definite geographical area of Italy, approximately 630,000 people might have developed antibodies (11.0% of 5,784,974 inhabitants). This figure is significantly higher than the number of molecular-confirmed SARS-CoV-2 infections (~32,600 cases in the five administrative departments) reported by the Protezione Civile and the Italian National Institute of Health as of 30 April 2020 [2]. The high observed seroprevalence is consistent with recent studies (Table 5) performed in other heavily affected areas of Europe: 9.7% in Geneva, Switzerland [10] and 10.0% in Madrid, Spain [11,12].
Table 5.
Summary of articles published in the literature reporting data regarding prevalence of SARS-CoV-2 antibodies in the general population.
| Author | Country; Area | Number of Participants | Prevalence of Anti-SARS-CoV-2 Antibodies |
|---|---|---|---|
| Petersen M.S. [13] | Faroe Islands; Nationwide study | 1075 | 0.6% |
| Biggs H. [14] | U.S.; two metropolitan Atlanta counties | 696 | 2.5% |
| Menachemi N. [15] | U.S; Indiana | 3658 | 2.79% |
| Fischer B. [16] | Germany; three federal states | 3186 | 0.91% |
| Pollan M. [11] | Spain; Nationwide study | 61,075 | 5.0% |
| Havers F. [17] | U.S; 10 sites | 16,025 | From 1.0% (San Francisco) to 6.9% (New York City) |
| Amorim Filho L. [18] | Brazil; Rio de Janeiro | 2857 | 4.0% |
| Percivalle E. [19] | Italy; Lodi area | 390 | 23.0% |
| Soriano V. [12] | Spain, Madrid | 674 | 13.8% |
| Stringhini S. [10] | Switzerland, Geneve | 2766 | 9.7% |
| Sood N. [20] | U.S., Los Angeles | 1702 | 4.3% |
Living in a long-term care facility was the strongest predictors of SARS-CoV-2 infection and was reported by 21.6% of anti-SARS-CoV-2-positive participants (n = 86/398). This connection was not unexpected [21,22,23], since long-term care facilities often have limited or no infection control programs [24,25] and are usually congregative settings where elderly people have greater exposure to infected patients in the case of respiratory outbreaks [26,27,28]. Therefore, our results emphasized the importance of implementing strategic bundles for infections prevention in long-term care facilities [29]. In this regard, educational interventions on healthcare providers’ knowledge, as well as active surveillance of suspected cases and implementation of barrier precautions, were shown to play a vital role in limiting the spread of other respiratory outbreaks [26,27,28].
Reporting an occupational exposure to the virus also emerged as an independent factor associated with SARS-CoV-2 infection and was reported by 8.7% of anti-SARS-CoV-2-positive participants (n = 35/398). However, approximately two-thirds of anti-SARS-CoV-2-positive participants did not report any apparent risk depicting the widespread circulation of the virus in the Italian community, where it has become endemic.
As for clinical symptoms, we found that the prevalence of SARS-CoV-2 antibodies depends on the type of clinical manifestation reported by the patient, being particularly high in people who reported loss of smell or taste [30,31]. Interestingly, 8.6% of participants (n = 277/3224) who did not report any symptoms presented antibodies positivity. This finding suggests that non-apparent infection is relatively common in a healthy, active population, thus supporting the hypothesis that, as is true for other coronavirus infections [32], SARS-CoV-2 infection might also be asymptomatic or pauci-symptomatic and resolves spontaneously without any complications in many cases.
In our opinion, the findings of our study could have several implications for pandemic management. Because the real number of patients with SARS-CoV-2 infection is significantly higher than the PCR-confirmed cases, stringent lockdown strategies might possibly be re-implemented only when the intensive care units’ capacities to handle emergencies are overwhelmed. Since a large proportion of patients with SARS-CoV-2 infection are asymptomatic, contract tracing methods to limit the spread of the infection could be particularly challenging. Thus, screening strategies beyond a symptoms-driven approach will be necessary for Italy (e.g., use of mobile applications) to identify enough infected persons to reach SARS-CoV-2 elimination targets [33]; our data could also be useful for vaccine design and implementation.
There are several limitations that should be discussed. Firstly, we do did have any information regarding previous SARS-CoV-2 molecular testing among those patients who tested positive. Accordingly, we cannot provide valuable estimates of antibody prevalence in people positive and negative in PCR testing. Secondly, we analyzed serum samples from patients who voluntarily decided to be tested. Therefore, the clinical characteristics of the sample might differ from those of the general Italian population. Thirdly, geographical prevalence of anti-SARS-CoV-2 antibodies might have been influenced by the type of serological tests used. However, the diagnostic performances of each test are similar to each other; in addition, the highest percentage of infected patients in the Liguria region agrees with recent evidence, suggesting the presence of anti-SARS-CoV-2 antibodies among blood donors from Savona and Genova since December 2019 (unpublished data reported by the Ligurian regional health authority ALISA). Fourthly, all tests we used are non-FDA approved and are yet to be validated. Therefore, prevalence estimates could change once new information on the accuracy of tests are available. Fifthly, the interpretation of the test is still under discussion, because even patients with confirmed SARS-CoV-2 infections have low or non-detectable antibodies titles several weeks after acute infection [34]. Lastly, based on the specificities of testing kits, we cannot exclude that some participants had false positive results due to past or present infection with other viruses, including non-SARS-CoV-2 coronavirus strains [35]. In addition, antibody response may be impaired in elderly, immuno-compromised or immunosuppressed participants, and may produce false negative serology test results [36].
5. Conclusions
In conclusion, the results of the present study demonstrate that infection rates based on surveillance data considerably underestimated the infection rates during the SARS-CoV-2 virus pandemic in Italy. The seroprevalence was much higher among people living in long-term care facilities or those with occupational exposure. In our opinion, these findings warrant further investigation into SARS-CoV-2 antibody prevalence among the Italian population.
Author Contributions
Conceptualization, M.B. (Marco Berruti) and M.B. (Matteo Bassetti); A.V.; methodology, A.V.; software, A.A.; P.B.; M.B. (Matteo Bassetti); R.C.; G.G.; D.R.G.; formal analysis, A.V. M.B. (Matteo Bassetti); investigation, A.A.; P.B.; M.B. (Matteo Bassetti); R.C.; G.G.; L.B.-L.; M.V.; N.V.; resources, A.A.; P.B.; M.B. (Michele Brignole); R.C.; G.G.; L.B.-L.; M.V.; N.V.; data curation, A.A.; P.B.; M.B. (Michele Brignole).; R.C.; G.G.; L.B.-L.; M.V.; N.V.; A.V.; writing—original draft preparation, A.V.; M.B. (Matteo Bassetti); writing—review and editing, A.V. P.P.; M.B. (Matteo Bassetti); L.T.; L.M.; I.B.; L.B.; C.R.; D.B. visualization, A.S.; supervision, M.B. (Matteo Bassetti); P.P.; funding acquisition A.A.; P.B.; M.B. (Michele Brignole); R.C.; G.G.; L.B.-L.; M.V.; N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Outside the submitted work, M.B. (Matteo Bassetti) has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, Bayer, Basilea, BioMeérieux, Cidara, Gilead, Menarini, MSD, Nabriva, Paratek, Pfizer, Roche, Melinta, Shionogi, Tetraphase, VenatoRx, and Vifor and has received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead, Pfizer, and MSD. Outside the submitted work, D.R.G. reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia and Correvio Italia. The authors declare no conflict of interest.
References
- 1.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 2. [(accessed on 30 April 2020)]; Available online: http://www.salute.gov.it/portale/nuovocoronavirus/
- 3.Bassetti M., Vena A., Giacobbe D.R. The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. Eur. J. Clin. Investig. 2020;50:e13209. doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim G.U., Kim M.J., Ra S.H., Lee J., Bae S., Jung J., Kim S.-H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020;26:948.e1–948.e3. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thursky K., Cordova S.P., Smith D., Kelly H. Working towards a simple case definition for influenza surveillance. J. Clin. Virol. 2003;27:170–179. doi: 10.1016/S1386-6532(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G., Salvagno G.L., Pegoraro M., Militello V., Caloi C., Peretti A., Gaino S., Bassi A., Bovo C., Cascio G.L. Assessment of immune response to SARS-CoV-2 with fully automated MAGLUMI 2019-nCoV IgG and IgM chemiluminescence immunoassays. Clin. Chem. Lab. Med. 2020;58 doi: 10.1515/cclm-2020-0473. [DOI] [PubMed] [Google Scholar]
- 8.Padoan A., Cosma C., Sciacovelli L., Faggian D., Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020;58 doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- 9.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., De Ridder D., Petrovic D., Schrempft S., Marcus K., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M., Sanmartín J.L., Fernández-García A., Cruz I., De Larrea N.F., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soriano V., Meirino R., Corral O., Guallar M.P. SARS-CoV-2 antibodies in adults in Madrid, Spain. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen M.S., Strom M., Christiansen D.H., Fjallsbak J.P., Eliasen E.H., Johansen M., Veyhe A.S., Kristiansen M.F., Gaini S., Møller L.F., et al. Seroprevalence of SARS-CoV-2-Specific Antibodies, Faroe Islands. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2611.202736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggs H.M., Harris J.B., Breakwell L., Dahlgren F.S., Abedi G.R., Szablewski C.M., Drobeniuc J., Bustamante N.D., Almendares O., Schnall A.H., et al. Estimated Community Seroprevalence of SARS-CoV-2 Antibodies—Two Georgia Counties, 28 April–3 May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:965–970. doi: 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menachemi N., Yiannoutsos C.T., Dixon B.E., Duszynski T.J., Fadel W.F., Wools-Kaloustian K.K., Needleman N.U., Box K., Caine V., Norwood C., et al. Population Point Prevalence of SARS-CoV-2 Infection Based on a Statewide Random Sample—Indiana, 25–29 April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:960–964. doi: 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer B., Knabbe C., Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Eurosurveillance. 2020;25:2001285. doi: 10.2807/1560-7917.ES.2020.25.28.2001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.-F., Gibbons A., et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, 23 March–12 May 2020. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- 18.Amorim Filho L., Szwarcwald C.L., Mateos S.O.G., Leon A., Medronho R.A., Veloso V.G., Lopes J.I.F., De Moraes Sobrino Porto L.C., Chieppe A., Werneck G.L. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev. Saude Publica. 2020;54:69. doi: 10.11606/s1518-8787.2020054002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percivalle E., Cambie G., Cassaniti I., Nepita E.V., Maserati R., Ferrari A., Di Martino R., Isernia P., Mojoli F., Bruno R., et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Eurosurveillance. 2020;25:2001031. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood N., Simon P., Ebner P., Eichner D., Reynolds J., Bendavid E., Bhattacharya J. Seroprevalence of SARS-CoV-2-Specific Antibodies Among Adults in Los Angeles County, California, on 10–11 April 2020. JAMA. 2020;323:2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L.F., Seetharaman S. Preventing the Spread of COVID-19 to Nursing Homes: Experience from a Singapore Geriatric Centre. J. Am. Geriatr. Soc. 2020;68:942. doi: 10.1111/jgs.16447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrams H.R., Loomer L., Gandhi A. Grabowski DCCharacteristics of, U.S. Nursing Homes with COVID-19 Cases. J. Am. Geriatr. Soc. 2020;68 doi: 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., Lewis J., Baer A., Kawakami V., Lukoff M.D., et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone P.W., Herzig C.T., Pogorzelska-Maziarz M., Carter E., Bjarnadottir R.I., Semeraro P.K., Cohen C.C., Travers J., Schweon S. Understanding infection prevention and control in nursing homes: A qualitative study. Geriatr. Nurs. 2015;36:267–272. doi: 10.1016/j.gerinurse.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzig C.T., Stone P.W., Castle N., Pogorzelska-Maziarz M., Larson E.L., Dick A.W. Infection Prevention and Control Programs in US Nursing Homes: Results of a National Survey. J. Am. Med. Dir. Assoc. 2016;17:85–88. doi: 10.1016/j.jamda.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pena S.A., Davis S.S., Lu X., Sakthivel S.K.K., Peret T.C.T., Rose E.B., Smelser C., Schneider E., Stone N.D., Watson J. Severe Respiratory Illness Associated with Human Metapneumovirus in Nursing Home, New Mexico, USA. Emerg. Infect. Dis. 2019;25:383–384. doi: 10.3201/eid2502.181298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ursic T., Miksic N.G., Lusa L., Strle F., Petrovec M. Viral respiratory infections in a nursing home: A six-month prospective study. BMC Infect. Dis. 2016;16:637. doi: 10.1186/s12879-016-1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seynaeve D., Augusseau-Riviere B., Couturier P., Morel-Baccard C., Landelle C., Bosson J.L., Gavazzi G., Mallaret M.-R. Outbreak of Human Metapneumovirus in a Nursing Home: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2020;21:104–109.e1. doi: 10.1016/j.jamda.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montoya A., Cassone M., Mody L. Infections in Nursing Homes: Epidemiology and Prevention Programs. Clin. Geriatr. Med. 2016;32:585–607. doi: 10.1016/j.cger.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 30.De Maria A., Varese P., Dentone C., Barisione E., Bassetti M. High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients. J. Med. Virol. 2020 doi: 10.1002/jmv.25995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butowt R., Bilinska K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020;11:1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 32.Al-Tawfiq J.A. Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19) Travel Med. Infect. Dis. 2020;35:101608. doi: 10.1016/j.tmaid.2020.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacobucci G. Sixty seconds on the contact tracing app. BMJ. 2020;369:m1818. doi: 10.1136/bmj.m1818. [DOI] [PubMed] [Google Scholar]
- 34.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., Ling Y., Zhang Y., Xun J., Lu L., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. :2020. doi: 10.2139/ssrn.3566211. [DOI] [Google Scholar]
- 35.Fang B., Meng Q.H. The laboratory’s role in combating COVID-19. Crit. Rev. Clin. Lab. Sci. 2020;57:400–414. doi: 10.1080/10408363.2020.1776675. [DOI] [PubMed] [Google Scholar]
- 36.Ghaffari A., Meurant R., Ardakani A. COVID-19 Serological Tests: How Well Do They Actually Perform? Diagnostics. 2020;10:453. doi: 10.3390/diagnostics10070453. [DOI] [PMC free article] [PubMed] [Google Scholar]