Abstract
This study investigated ticks and tick-borne microorganisms of small ruminants from five districts of the Federally Administered Tribal Area (FATA) of Pakistan. Morphological (n = 104) and molecular (n = 54) characterization of the ticks revealed the presence of six ixodid ticks: Rhipicephalus (Rh.) haemaphysaloides, Rh. microplus, Rh. turanicus, Haemaphysalis (Hs.) punctata, Hs. sulcata and Hyalomma anatolicum. Phylogenetic analyses of nucleotide sequence data for two mitochondrial (16S and cytochrome c oxidase 1) and one nuclear (second internal transcribed spacer) DNA regions provided strong support for the grouping of the six tick species identified in this study. Microfluidic real-time PCR, employing multiple pre-validated nuclear and mitochondrial genetic markers, detected 11 potential pathogens and endosymbionts in 72.2% of the ticks (n = 54) tested. Rickettsia (R.) massiliae was the most common pathogen found (42.6% of ticks) followed by Theileria spp. (33.3%), Anaplasma (A.) ovis and R. slovaca (25.9% each). Anaplasma centrale, A. marginale, Ehrlichia spp., R. aeschlimannii, R. conorii and endosymbionts (Francisella- and Coxiella-like) were detected at much lower rates (1.9–22.2%) in ticks. Ticks from goats (83.9%) carried significantly higher microorganisms than those from sheep (56.5%). This study demonstrates that ticks of small ruminants from the FATA are carrying multiple microorganisms of veterinary and medical health significance and provides the basis for future investigations of ticks and tick-borne diseases of animals and humans in this and neighboring regions.
Keywords: tick, Anaplasma, goat, Haemaphysalis, microfluidic real-time PCR, Rhipicephalus, Rickettsia, sheep, Theileria, Pakistan
1. Introduction
Ticks are obligate blood-feeding ectoparasites of animals and humans that are distributed globally [1]. They can affect their hosts either directly by causing tick-associated stress, irritation, allergy, anemia, weight loss and paralysis or indirectly by transmitting numerous pathogenic microorganisms including bacteria, fungi, protozoa, rickettsiae, spirochetes and/or viruses [1,2,3]. In production animals such as cattle, buffaloes, goats and sheep, tick-borne protozoal (babesiosis and theileriosis) and rickettsial (anaplasmosis and cowdriosis) diseases cause major health problems as well as production and economic losses mainly in subtropical and tropical regions [1].
Pakistan is a subtropical country where the majority of the rural population is dependent upon livestock including small ruminants for their food and livelihood (Pakistani goat and sheep population: 31.2 million Ovis aries; 78.2 m Capra hircus), particularly in the Federally Administered Tribal Area (FATA) of the north-western part of the country [4,5]. The FATA is located near the Pak-Afghan border and represents one of the least-developed regions in Pakistan due to political unrest and prolonged military crises over the last 50 years. These areas consist of seven tribal agencies (districts) and six frontier regions, which have been recently merged [6]. Nomadic pastoralism is a common practice in this region and > 70% of the human population derives their livelihood from livestock farming [7]. Due to the poor infrastructure, limited resources and inadequate access to veterinary services, tick-borne diseases (TBDs) of humans and animals have a major impact in this region [7].
Although a number of studies have assessed the prevalence of ticks and tick-borne diseases (TTBDs) of small ruminants in different areas of Pakistan [8,9,10,11,12,13,14,15,16,17,18,19], there is a paucity of information from the FATA. Moreover, no study has yet investigated the presence, prevalence and diversity of tick-borne pathogens (TBPs) of small ruminants in this region. Recently, Khan et al. [20] assessed tick burdens on small ruminants in the FATA using morphological methods and found three main genera of ixodid ticks (Haemaphysalis, Hyalomma and Rhipicephalus) but these ticks and associated TBPs were not further characterized in detail using molecular tools.
Specific identification is pivotal for understanding the epidemiology of, and developing effective control strategies for, TTBDs [21]. However, morphological methods do not allow the identification of immature, engorged or damaged tick specimens [22,23]. By contrast, molecular methods provide an alternative and complementary approach for the identification of ticks [24], which employ the characterization of genetic markers such as the mitochondrial cytochrome c oxidase subunit I (cox1) and 16S ribosomal RNA genes [25,26]. As ticks usually harbor and transmit commensals and numerous pathogens, some of which can be of public health significance (e.g., Coxiella burnetii and Crimean-Congo hemorrhagic fever virus) [27,28,29], it is important to detect these microorganisms in ticks to ascertain their prevalence. However, conventional diagnostic methods such as microscopic examination of thin and thick smears usually detect few target pathogens or microorganisms and have a lower sensitivity and specificity than molecular approaches [30]. Therefore, testing ticks as well as their animal hosts using polymerase chain reaction (PCR) based methods for the detection of TBPs and/or commensals provides distinct advantages over conventional detection methods. Recently, a micro-chip-based microfluidic real-time PCR technique was developed to detect and differentiate up to 96 microorganisms per tick in a single PCR procedure [31]. This method has been proven to be well-suited for rapid and large-scale epidemiological and surveillance studies [28,31,32,33,34,35,36] and is anticipated to be an invaluable tool for the detection of microorganisms in ticks from regions such as the FATA.
In the present study, we employed both conventional and PCR-based tools to investigate the diversity of tick taxa from small ruminants and their associated TBPs and/or commensals in the FATA of Pakistan.
2. Materials and Methods
2.1. Study Area and Tick Samples
The FATA represents seven tribal districts in the north-western part of Pakistan (Figure 1). This region has a monsoonal climatic zone in the East and a Mediterranean one in the west such that seasons and rainfall can vary markedly across regions. In agriculture, livestock production is one of the main sources of subsistence for two-thirds of the population. The estimated population of small ruminants in the FATA is ~5.5 million [37].
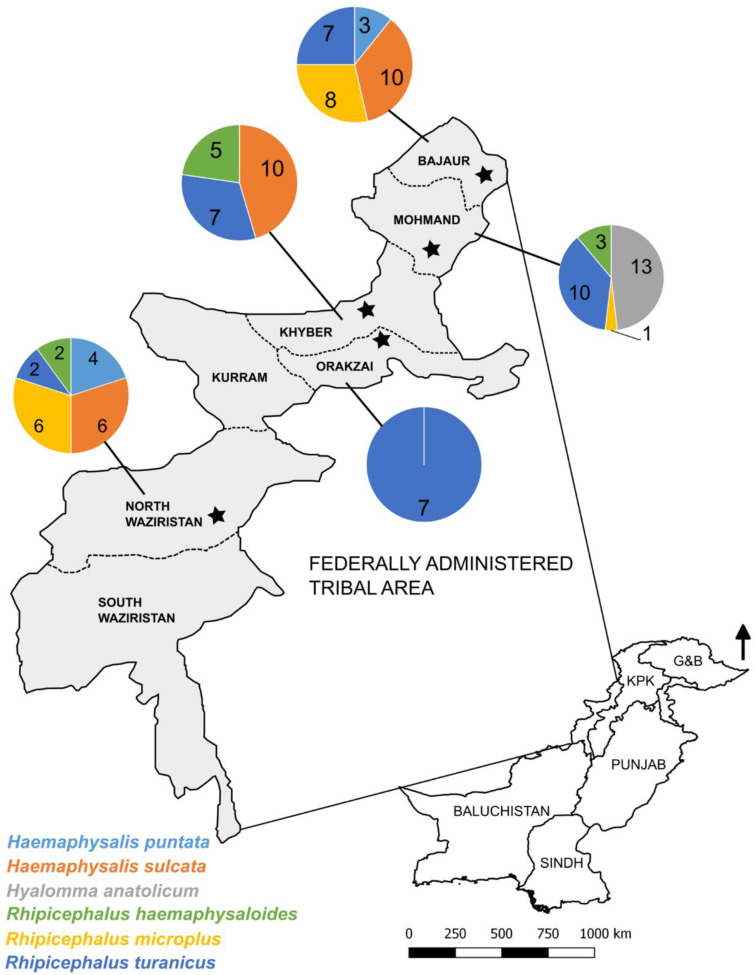
Figure 1.
Map of Pakistan (bottom right) and the Federally Administered Tribal Area (FATA) (grey-colored areas on top left) showing the tribal districts (starred) included in this study. Different colors in each pie chart indicate the distribution of tick species identified from each district.
A convenience sampling method was used to collect 104 hard ticks from sheep (n = 23) and goats (n = 31) from the five tribal districts of Bajaur, Khyber, Mohmand, North Waziristan and Orakzai (Figure 1; Table 1). Ticks were collected using tweezers from various body parts of the animals including ears, neck and the perineal region. Following tick collection, the specimens from individual animals were fixed in 70% ethanol in separate Eppendorf tubes, labelled and stored at an ambient temperature. The collection of ticks from the animals was approved by the Animal Ethics Committee of the Abdul Wali Khan University.
Table 1.
Details of ticks of small ruminants from the FATA of Pakistan used in this study. GenBank accession numbers are also provided for unique 16S, cox1 and ITS-2 sequences.
| Tribal Districts (Coordinates) | Host (Number) |
Tick Species (Based on Microscopy) |
No. of Ticks | GenBank Accession Numbers (Only Unique Sequences) |
||
|---|---|---|---|---|---|---|
| 16S | cox1 | ITS-2 | ||||
| Bajaur (34.856902° N, 71.429936° E) |
Sheep (n = 5) | Haemaphysalis sulcata | 4 | MT799946 | MT800319 | - |
| Rhipicephalus microplus | 6 | MT799951 | MT800322 | - | ||
| Rhipicephalus turanicus | 3 | - | - | - | ||
| Goat (n = 7) | Haemaphysalis punctata | 3 | MT799944 | MT800318 | - | |
| Hs. sulcata | 6 | MT799946 | MT800319 | - | ||
| MT799947 | MT800320 | - | ||||
| Rh. microplus | 2 | - | - | - | ||
| Rh. turanicus | 4 | - | - | - | ||
| Khyber (33.940548° N, 71.049777° E) |
Sheep (n = 5) | Hs. sulcata | 5 | - | - | - |
| Rh. turanicus | 3 | - | - | - | ||
| Goat (n = 7) | Hs. sulcata | 5 | MT799948 | MT800320 | - | |
| Rh. turanicus | 4 | MT799955 | MT800313 | - | ||
| Rhipicephalus haemaphysaloides | 5 | MT799956 | MT800316 | MT818227 | ||
| MT799956 | MT800315 | MT818227 | ||||
| Mohmand (34.535595° N, 71.287421° E) |
Sheep (n = 6) | Hyalomma anatolicum | 6 | MT799950 | MT800311 | MT818222 |
| Rh. turanicus | 5 | MT799954 | MT800312 | MT818226 | ||
| MT799954 | MT800314 | MT818226 | ||||
| Goat (n = 8) | Hy. anatolicum | 7 | MT799950 | MT800311 | MT818222 | |
| Rh. microplus | 1 | - | - | - | ||
| Rh. turanicus | 5 | - | - | - | ||
| Rh. haemaphysaloides | 3 | MT799956 | - | - | ||
| Orakzai (33.667137° N, 70.95468° E) |
Sheep (n = 2) | Rh. turanicus | 4 | - | - | - |
| Goat (n = 2) | Rh. turanicus | 3 | - | - | - | |
| North Waziristan (32.320237° N, 69.859741° E) |
Sheep (n = 5) | Hs. sulcata | 6 | MT799949 | MT800321 | - |
| Rh. microplus | 2 | MT799952 | MT800323 | MT818223 | ||
| Goat (n = 7) | Hs. punctata | 4 | - | - | - | |
| Rh. microplus | 4 | MT799953 | MT800322 | MT818223 | ||
| Rh. turanicus | 2 | - | - | - | ||
| Rh. haemaphysaloides | 2 | MT799956 | MT800317 | MT818227 | ||
| Total | 54 | 104 | ||||
2.2. Morphological Identification of Ticks and DNA Extraction
Using a dissecting microscope (Olympus SZ40, Olympus Corporation, Tokyo, Japan), individual ticks were morphologically identified to species employing dichotomous keys [23,38,39]. Subsequently, DNA was extracted from 54 individual ticks representing each host species in each tribal district. Briefly, following rehydration, each tick was cut in half longitudinally. One half of each tick was washed three times in 15 mL of H20 and then diced finely with a sterile scalpel blade. DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol, except that the proteinase K digestion step was for 24–48 h at 56 °C. The DNA concentration of each sample was estimated using a Nanodrop ND1000.
2.3. Molecular Characterization of Ticks
Two mitochondrial loci (cox1 and 16S) were amplified separately from each individual DNA sample representing each tick specimen using previously published primers [25,40] (see Supplementary Materials Table S1) by conventional PCR in a thermal cycler (T100, BioRad). Additionally, a region of the second internal transcribed spacer (ITS-2) of nuclear ribosomal DNA was amplified using published primers to provide further differentiation of Hyalomma or Rhipicephalus species [41] (see Supplementary Materials Table S1). All PCRs were carried out in a volume of 25 µL containing 3.12 mM of each deoxynucleotide triphosphate (dNTP), 6.25 pmol of each primer and 10 mM Tris-HCl (pH 8.4), 50 mM KCl, MgCl2 at 75 mM (16S reactions), 100 mM (cox1 reactions) or 150 mM (ITS-2 reactions) and 0.6 U of GoTaq flexi DNA polymerase (Promega, Madison, WI, USA). Known positive (Rh. sanguineus DNA, Xng) and negative (milli-Q H2O) controls were included in each PCR run. Aliquots (5 µL) of individual amplicons were examined on 1.5% (w/v) agarose gels stained with GelRed (Biotium, Fremont, CA, USA) and then photographed using a GelDoc system (BioRad, Hercules, CA, USA).
For each locus, amplicons were purified using a Favorgen Gel/PCR purification mini kit (Favorgen, Ping-Tung, Taiwan) and DNA concentration was measured using a spectrophotometer (ND-1000, NanoDrop, Wilmington, DE, USA). Aliquots (5 µL) of individual amplicons were subjected to automated and bidirectional (Sanger) sequencing using the same primers (individually) as employed in the primary PCR (Supplementary Materials Table S1).
2.4. Microfluidic PCR-Based Detection of Tick-Borne Microorganisms
Individual DNA samples from individual ticks (n = 54) were subjected to microfluidic amplification of microorganisms using a 48.48 dynamics array in a Bio-Mark™ real-time PCR system (Fluidigm, CA, USA) as described previously [31]. Target microorganisms (and Gene/DNA markers) were Anaplasma (A.) species (spp.) (16S), A. marginale (msp1), A. platys (groEL), A. phagocytophilum (msp2), A. ovis (msp4), A. centrale (groEL), A. bovis (groEL), Apicomplexa spp. (18S), Babesia (B.) microti (CCTeta), B. canis (18S), B. ovis (18S), B. bovis (CCTeta), B. caballi (rap1), B. bigemina (18S), B. divergens (hsp70), B. vulpes (cox1), Bartonella (Ba.) spp. (ssrA), Ba. henselae (pap31), Borrelia (Bo.) spp. (23S), Bo. burgdorferi s.s. (rpoB), Bo. garinii (rpoB), Bo. afzelii (fla), Bo. valaisiana (ospA), Bo. lusitaniae (rpoB), Bo. spielmanii (fla), Bo. bissettii (rpoB), Bo. miyamotoi (glpQ), Bo. mayonii (fla), Bo. bavariensis (pyrG), Candidatus Neorickettsia mikurensis (groEL), Coxiella (C.) spp. (IS1111 and icd), Ehrlichia (E.) spp. (16S), E. canis (gltA), Francisella spp. (tul4 and fopA), Hepatozoon spp. (18S), Rickettsia (R.) spp. (gltA), R. conorii (ITS), R. slovaca (ITS), R. massiliae (ITS), R. helvetica (ITS), R. aeschlimannii (ITS), R. felis (orfB) and Theileria (Th.) spp. (18S) (see Supplementary Materials Table S2). A no-DNA (negative) control and a DNA-extraction-control to ensure the efficient amplification of ITS-2 and 16S from tick DNA were included in the run on each chip; also included were the DNA of Escherichia (Es.) coli (EDL933 strain) as a microorganism spike-control to ensure efficient amplification/detection using Es. coli (eae)-specific primers/probes in solution (see Supplementary Materials Table S2). The PCR results were validated (only when genus-level detection was achieved) using conventional PCR targeting in the 18S rRNA region [42] (see Supplementary Materials Table S1) and the resultant amplicons were sequenced as described in Section 2.3.
2.5. Sequence and Phylogenetic Analyses
The sequences of cox1, 16S and ITS-2 (ticks) and 18S (TBPs) obtained were examined for quality and then assembled using the denovo assembly function in Geneious Prime 2019.0.4 (http://www.geneious.com). Duplicate sequences were removed using the “find duplicates” function in Geneious and sequences unique to each locus were aligned using MUSCLE v.3.8.31 [43] within MEGA 7.0 [44]. The BLASTn function of the National Centre for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to match the identities of individual sequences; sequence identity matrices were established using BioEdit [45]. After verifying that all cox1 sequences had open reading frames, all unique nucleotide sequences were deposited into the GenBank database (Table 1). Reference sequences for individual loci (representing ticks or TBPs) were obtained from GenBank and aligned. Sequences obtained for ticks were trimmed to consensus lengths of 409 (16S), 549 (cox1) and 293 (ITS-2) bp; sequences obtained for TBPs were aligned over 478 bp (18S).
Phylogenetic analyses were performed on individual 16S, cox1 and ITS-2 sequence datasets using the Bayesian Inference (BI), Neighbor Joining (NJ) and Maximum Likelihood (ML) methods. The BI was conducted using the MrBayes plugin within Geneious [46] whereas NJ and ML analyses were performed using MEGA. The best-fit evolutionary models were estimated separately for individual sequence alignments (Tamura 3-parameter model [47] with gamma-distribution for 16S, Tamura-Nei model [48] with a proportion of invariable sites and gamma distribution for cox1 and Tamura 3-parameter model [47] with a proportion of invariable sites for ITS-2) using the Bayesian information criteria in jModelTest v.3.7 [49]. The nodal support in NJ and ML trees was tested through bootstrap analyses (10,000 replicates). The posterior probabilities of BI analyses were calculated for 2,000,000 generations (ngen = 2,000,000), saving every 100th tree (samplefreq = 100). Phylogenetic trees of ticks were rooted using Argas (Ar.) persicus (16S: KJ13358, cox1: FN394341) and Haemaphysalis (Hs.) longicornis (ITS-2: HQ005301) as outgroups whereas those of TBPs were rooted using B. bigemina (18S: KF112076).
2.6. Statistical Analyses
Results obtained from microfluidic PCR-based analysis/testing were analyzed using GraphPad 5 Prism (GraphPad software Inc. La Jolla, CA, USA). Chi-square and Fisher’s exact tests were used to compare the occurrence of microorganisms in different tick species from different hosts and tribal districts.
3. Results
3.1. Morphological Characterization of Ticks
The morphological examination of ticks (n = 104) revealed that the majority belonged to the genus Rhipicephalus (Rh. haemaphysaloides: n = 10; Rh. turanicus: n = 33; Rh. microplus: n = 15) followed by Haemaphysalis (Hs. sulcata: n = 26; Hs. punctata: n = 7) and Hyalomma (Hy. anatolicum: n = 13) (Table 1). Rhipicephalus turanicus, Rh. microplus, Hs. sulcata and Hy. anatolicum were present on both sheep and goats whereas Hs. punctata and Rh. haemaphysaloides occurred only on goats. Rhipicephalus turanicus was found in all five tribal districts whereas Rh. microplus, Rh. haemaphysaloides and Hs. sulcata were each found in three districts (i.e., Bajaur, Mohmand and North Waziristan; Khyber, Mohmand and North Waziristan; Bajaur, Khyber and North Waziristan, respectively). Haemaphysalis punctata and Hy. anatolicum occurred exclusively in two (Bajaur and North Waziristan) and one (Mohmand) districts, respectively (Table 1).
3.2. Sequence and Phylogenetic Analyses of Ticks
A total of 25 unique sequences of 16S (n = 12) and cox1 (n = 13) were obtained with most sequences representing Rhipicephalus (14) followed by Haemaphysalis (9) and Hyalomma (2). Partial ITS-2 unique sequences (n = 4) were also obtained for Hyalomma (n = 1) and Rhipicephalus (n = 3), which confirmed the identity of closely related species within these two genera. Intraspecific pairwise comparisons revealed the highest nucleotide differences within Rh. haemaphysaloides (cox1: 0.2–7.6%) followed by Hs. sulcata (16S: 0.3–1.5% and cox1: 0.8–1.8%, respectively) and Rh. microplus (0.3–0.5% and 0.2%, respectively) (Supplementary Materials Tables S3–S5).
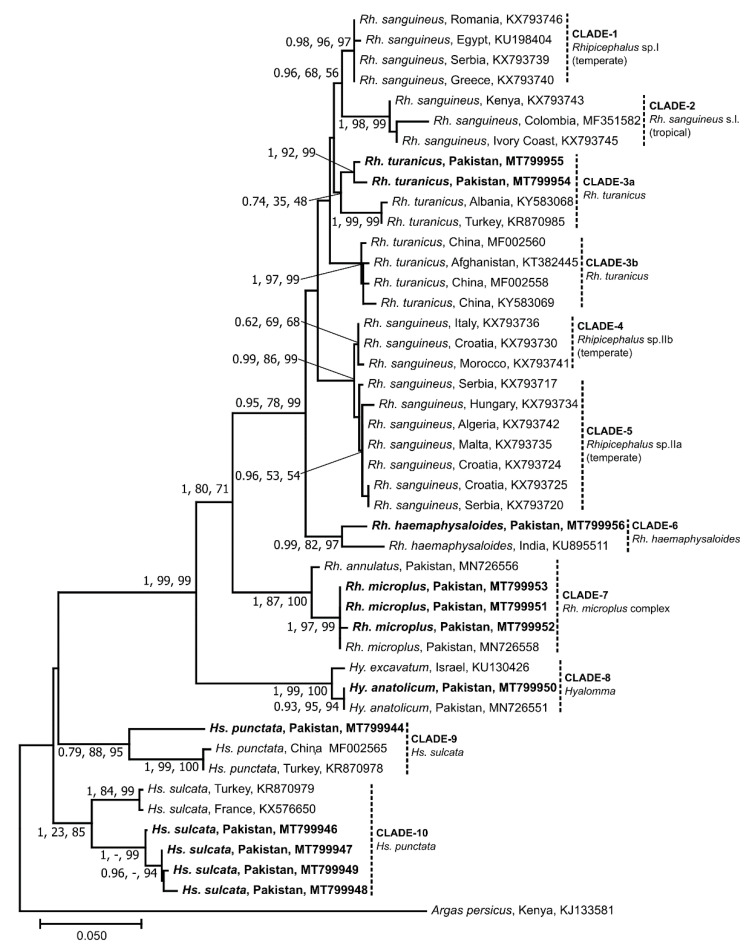
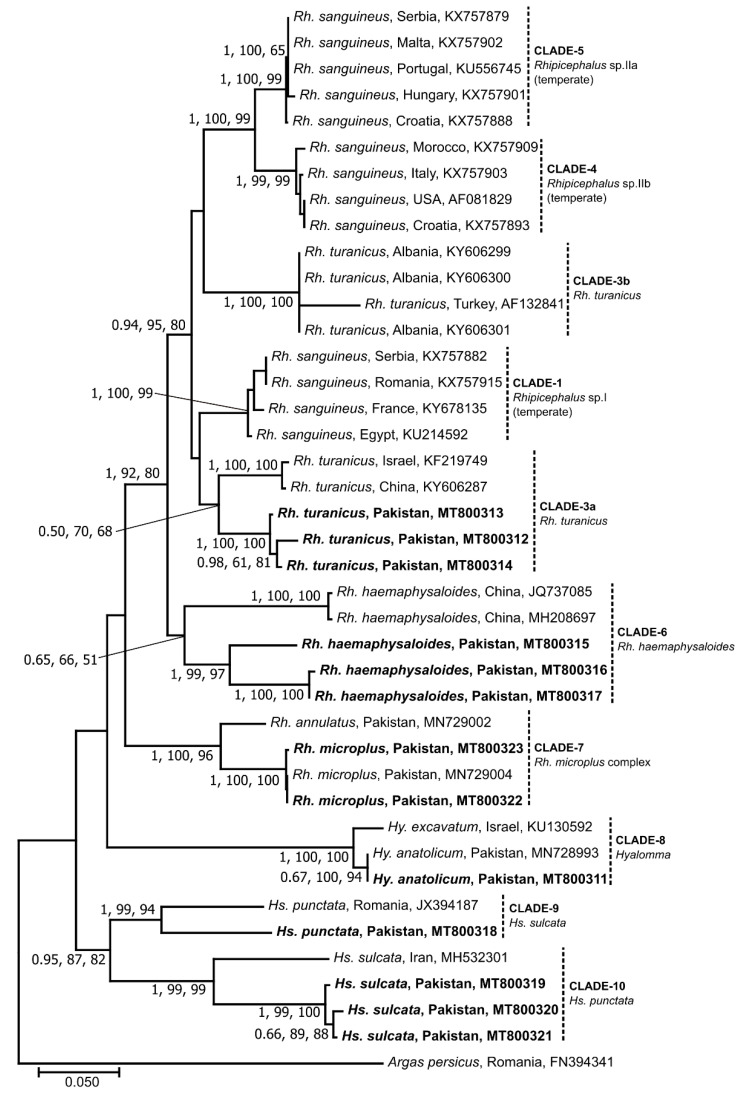
Separate phylogenetic analyses of 16S, cox1 or ITS-2 sequence data sets using the BI, ML and NJ methods produced trees with similar topologies; hence, only NJ trees were presented along with bootstrap and posterior probability (PP) support values for NJ and ML and BI, respectively (Figure 2, Figure 3 and Figure 4). For both 16S and cox1, the groupings were similar with a few minor differences. The sequences determined in this study grouped into six clades (clade-3a, 6–10) (Figure 2 and Figure 3). For 16S and cox1, clade-3a consisted of two (GenBank accession nos. MT799954 and MT799955) and three (GenBank: MT800312–MT800314) sequences, respectively, which grouped together with previously published sequences of Rh. turanicus from Albania, Turkey, China and Israel with a weak nodal support (16S: posterior probabilities for BI = 0.74; bootstrap for ML = 35%; bootstrap for NJ = 48%; cox1: 0.50, 70%, 68%) (Figure 2 and Figure 3). Clade-6 comprised one and three sequences of 16S (MT799956) and cox1 (GenBank: MT800315–MT800317), respectively, which clustered with Rh. haemaphysaloides from India and China with weak to strong statistical support (16S: 0.99, 82%, 97%; cox1: 0.65, 66%, 51%). Within clade-7, three and two sequences of 16S (GenBank: MT799951–MT799953) and cox1 (GenBank: MT800322 and MT800323), respectively, clustered with Rh. microplus sequences from Pakistan with strong nodal support values (16S: 1, 97%, 99%; cox1: 1, 100%, 100%) (Figure 2 and Figure 3). Clade-8 contained one sequence each of 16S (GenBank: MT799950) and cox1 (GenBank: MT800311), which grouped with Hy. anatolicum from Pakistan with weak to strong statistical support (16S: 0.93, 95%, 94%; cox1: 0.67, 100%, 94%) (Figure 2 and Figure 3). Clade-9 also consisted of one sequence each for 16S (GenBank: MT799944) and cox1 (GenBank: MT800318), which grouped with Hs. punctata sequences from China, Turkey and Romania with weak to strong nodal support (16S: 0.79, 88%, 95%; cox1: 1, 99%, 94%). Clade-10 comprised four and three sequences of 16S (GenBank: MT799946–MT799949) and cox1 (GenBank: MT800319–MT800321), respectively, which clustered with Hs. sulcata sequences from France, Turkey and Iran with variable nodal support (16S: 1, 23%, 85%; cox1: 1, 99%, 99%) (Figure 2 and Figure 3).
Figure 2.
Phylogenetic relationships of partial 16S rRNA sequences of ticks collected from small ruminants in the FATA of Pakistan. Phylogenies were inferred using Neighbor Joining (NJ, this tree), Maximum Likelihood (ML) and Bayesian Inference (BI) methods. Sequences determined herein are shown in bold. The country of origin and GenBank accession numbers for each sequence are also provided. Nodal support values are presented as a posterior probability for BI (left) followed by bootstrap values for ML and NJ. The scale-bar indicates the number of inferred substitutions per site.
Figure 3.
Phylogenetic relationships of partial cox1 sequences of ticks collected from small ruminants in the FATA of Pakistan. Phylogenies were inferred using Neighbor Joining (NJ, this tree), Maximum Likelihood (ML) and Bayesian Inference (BI) methods. Sequences determined herein are shown in bold. The country of origin and GenBank accession numbers for each sequence are also provided. Nodal support values are presented as a posterior probability for BI (left) followed by bootstrap values for ML and NJ. The scale-bar indicates the number of inferred substitutions per site.
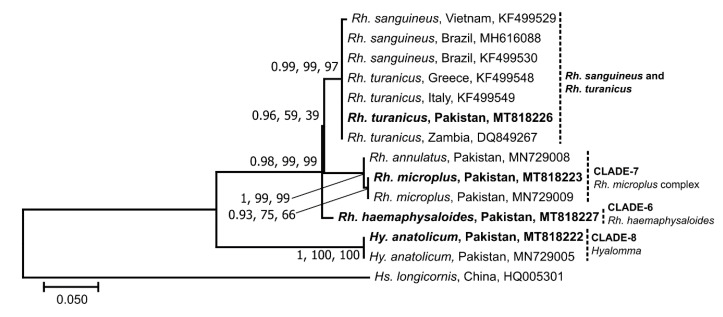
Figure 4.
Phylogenetic relationships of partial sequences of the second internal transcribed spacer of the nuclear ribosomal DNA of Rhipicephalus and Hyalomma ticks collected from small ruminants in the FATA of Pakistan. Phylogenies were inferred using Neighbor Joining (NJ, this tree), Maximum Likelihood (ML) and Bayesian Inference (BI) methods. Sequences determined herein are shown in bold. The country of origin and GenBank accession numbers for each sequence are also provided. Nodal support values are presented as a posterior probability for BI (left) followed by bootstrap values for ML and NJ. The scale-bar indicates the number of inferred substitutions per site.
The relationships of ITS-2 sequences (GenBank: MT818222 and MT818223) were consistent with those obtained using 16S and cox1 data (Figure 4). However, the Rh. turanicus sequence (GenBank: MT818226) clustered with Rh. turanicus and Rh. sanguineus sequences from Zambia, Italy, Brazil and Vietnam with strong nodal support (0.99, 99%, 97%). Moreover, no reference ITS-2 sequence was available for Rh. haemaphysaloides and the sequence of a specimen (GenBank: MT818227), which grouped with Rh. haemaphysaloides in 16S and cox1 phylogenies (Figure 2 and Figure 3). The corresponding ITS-2 sequence grouped outside of the Rhipicephalus group with strong nodal support (1, 99%, 99%) (Figure 4).
3.3. Diversity of Microorganisms in Ticks
DNA of at least one of 11 microorganisms was detected in a total of 39 of 54 (72.2%) ticks tested (Table 2). Rickettsia massiliae was the most commonly detected pathogen (42.6%) followed by Theileria spp. (33.3%), A. ovis and R. slovaca (25.9%), A. centrale (9.3%), Ehrlichia spp., R. conorii and R. aeschlimannii (5.6%) and A. marginale (1.9%) (Table 3). Furthermore, endosymbionts including Francisella-like and Coxiella-like were also detected in 22.2% and 7.4% of ticks, respectively (Table 3).
Table 2.
Occurrence of various microorganisms in six tick species of small ruminants from the FATA of Pakistan.
| Tick Species | District | Host | No. of Ticks Tested | No. of Ticks Infected | Microorganisms Detected |
|---|---|---|---|---|---|
| Haemaphysalis punctata | Bajaur, North Waziristan | Goat | 4 | 3 | Anaplasma ovis, Rickettsia massiliae, Coxiella-like, Theileria spp. |
| Hs. sulcata | Bajaur, Khyber, North Waziristan | Sheep | 8 | 4 | A. marginale, A. ovis, A. centrale, R. slovaca, R. massiliae, Francisella-like, Coxiella-like, Theileria spp. |
| Goat | 6 | 5 | A. centrale, Ehrlichia spp., R. slovaca, R. massiliae, Francisella-like, Coxiella-like, Theileria spp. | ||
| Hyalomma anatolicum | Mohmand | Sheep | 3 | 3 | A. ovis, Ehrlichia spp., R. slovaca, R. massiliae, Francisella-like, Theileria spp. |
| Goat | 3 | 3 | A. ovis, R. massiliae, Francisella-like, Theileria spp. | ||
| Rhipicephalus microplus | Bajaur, Mohmand, North Waziristan | Sheep | 4 | 2 | A. ovis, Francisella-like, R. aeschlimannii, R. massiliae, R. slovaca |
| Goat | 4 | 3 | A. centrale, R. massiliae, Francisella-like | ||
| Rh. turanicus | Bajaur, Khyber, Orakzai, Mohmand, North Waziristan | Sheep | 8 | 4 | A. ovis, R. conorii, R. slovaca, R. massiliae, R. aeschlimannii, Francisella-like, Theileria spp. |
| Goat | 10 | 8 | A. ovis, A. centrale, R. conorii, R. slovaca, R. massiliae, R. aeschlimannii, Francisella-like, Theileria spp. | ||
| Rh. haemaphysaloides | Khyber, Mohmand, North Waziristan | Goat | 4 | 4 | A. ovis, A. centrale, R. slovaca, R. massiliae, Theileria spp. |
| Total | 54 | 39 | |||
Table 3.
Prevalence of microorganisms in ticks of small ruminants in the FATA of Pakistan.
| Microorganisms | Bajaur | Khyber | Mohmand | Orakzai | North Waziristan | % Prevalence (Proportion) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sheep (n = 5) |
Goat (n = 7) |
Sheep (n = 5) |
Goat (n = 7) |
Sheep (n = 6) |
Goat (n = 8) |
Sheep (n = 2) |
Goat (n = 2) |
Sheep (n = 5) |
Goat (n = 7) |
||
| Anaplasma marginale | - | - | 1 | - | - | - | - | - | - | - | 1.9 (1/54) |
| A. ovis | 1 | - | 1 | 1 | 3 | 4 | 1 | - | 1 | 2 | 25.9 (14/54) |
| A. centrale | - | 1 | 1 | - | - | 1 | - | - | - | 2 | 9.3 (5/54) |
| Ehrlichia spp. | - | - | - | 1 | 2 | - | - | - | - | - | 5.6 (3/54) |
| Rickettsia conorii | - | - | - | 1 | 1 | 1 | - | - | - | - | 5.6 (3/54) |
| R. slovaca | 1 | 2 | - | 3 | 3 | 1 | 1 | 2 | 1 | - | 25.9 (14/54) |
| R. massiliae | 1 | 1 | 2 | 4 | 3 | 4 | 1 | 2 | 1 | 4 | 42.6 (23/54) |
| R. aeschlimannii | 1 | - | - | - | 1 | 1 | - | - | - | - | 5.6 (3/54) |
| Francisella-like | 2 | - | - | 1 | 4 | 3 | 1 | 1 | - | - | 22.2 (12/54) |
| Coxiella-like | - | - | - | 1 | - | - | - | - | 1 | 2 | 7.4 (4/54) |
| Theileria spp. | 1 | 1 | 2 | 1 | 3 | 5 | 1 | 2 | - | 2 | 33.3 (18/54) |
| Total | 7 | 5 | 7 | 13 | 20 | 20 | 5 | 7 | 4 | 12 | |
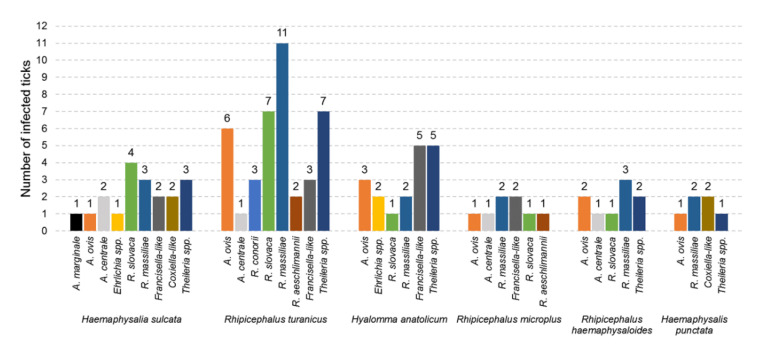
The occurrence of microorganisms varied significantly across different districts (x2 = 61.5, df = 4, P < 0.0001) (Bajaur, 41.7%; Khyber, 75%; Mohmand, 86%; Orakzai, 75%; North Waziristan, 83%) as well as between ticks (P < 0.0001) collected from sheep (56.5%) and goats (83.9%) (Table 2 and Table 3). A significant variation (x2 = 90.33, df = 5, P < 0.0001) was recorded in the prevalence of microbes in different tick species; it was highest in Hy. anatolicum and Rh. haemaphysaloides (100%) followed by Hs. punctata (75%), Rh. turanicus (66.7%), Hs. sulcata (64.3%) and Rh. microplus (62.5%) (Table 2, Figure 5). The most diversity of microbes was detected within Hs. sulcata (9 of 11 microorganisms) followed by Rh. turanicus (8), Hy. anatolicum and Rh. microplus (6), Rh. haemaphysaloides (5) and Hs. punctata (4) (Figure 5).
Figure 5.
Number of ticks infected with microorganisms from small ruminants in the FATA of Pakistan. The species of tick is shown at the bottom while the number of individual species of ticks infected with the microorganism species (x axis) is shown above each bar (y axis) of the chart.
3.4. Co-Occurrence of Microorganisms in Ticks
Of the 39 ticks found positive for microbes, DNA of one or more microorganisms was present in 11 (28.2%) and 28 (71.8%) ticks, respectively (Table 4). DNA of two, three, four, five or seven microorganisms was found in 28.6%, 39.2%, 25%, 3.6% and 3.6% ticks, respectively (Table 4). DNA of six microorganisms (A. ovis, A. centrale, R. slovaca, R. massiliae, Francisella-like and Theileria spp.) was present either as single or a mixed infection whereas DNA of the other five microbes was found only as mixed infections (Table 4). Rh. turanicus ticks were positive for a maximum of seven microorganisms followed by Hs. sulcata, Hy. anatolicum, Rh. haemaphysaloides and Rh. Microplus, which were positive for four and Hs. punctata for three (data not shown).
Table 4.
Single and mixed presence of DNA of microorganisms in ticks of small ruminants in the FATA of Pakistan.
| Microorganisms | Number of Ticks Positive for Single and Mixed Infections | |||||
|---|---|---|---|---|---|---|
| Bajaur (n = 12) |
Khyber (n = 12) |
Mohmand (n = 14) |
Orakzai (n = 4) |
North Waziristan (n = 12) | Total | |
| Single | ||||||
| Anaplasma ovis | - | - | - | - | 1 | 1 |
| A. centrale | - | - | - | - | 1 | 1 |
| Rickettsia slovaca | 1 | 1 | - | - | 1 | 3 |
| R. massiliae | - | 1 | - | - | 2 | 3 |
| Francisella-like | - | - | 1 | - | - | 1 |
| Theileria spp. | 1 | 1 | - | - | - | 2 |
| Double | ||||||
| A. centrale, R. massiliae | - | - | - | - | 1 | 1 |
| A. ovis, Theileria spp. | - | - | 1 | - | 1 | 2 |
| A. ovis, R. massiliae. | - | 1 | - | - | 1 | |
| A. ovis, Coxiella-like | - | - | - | - | 1 | 1 |
| Francisella-like, Theileria spp. | - | - | 1 | - | - | 1 |
| R. massiliae, R. slovaca | - | 1 | - | - | - | 1 |
| R. massiliae, Coxiella-like | - | - | - | - | 1 | 1 |
| Triple | ||||||
| A. ovis, R. massiliae, Theileria spp. | - | 1 | 1 | - | - | 2 |
| A. ovis, R. massiliae, R. slovaca | - | - | 1 | - | - | 1 |
| A. ovis, Francisella-like, Theileria spp. | 1 | - | - | - | - | 1 |
| A. centrale, R. massiliae, R. slovaca | 1 | - | - | - | - | 1 |
| Coxiella-like, Ehrlichia spp., Francisella-like, | - | 1 | - | - | - | 1 |
| Coxiella-like, R. massiliae, Theileria spp. | - | - | - | - | 1 | 1 |
| Ehrlichia spp., Francisella-like, Theileria spp. | - | - | 1 | - | - | 1 |
| Francisella-like, R. massiliae, R. slovaca | - | - | 1 | - | - | 1 |
| R. conorii, R. massiliae, R. slovaca | - | 1 | - | - | - | 1 |
| R. massiliae, R. slovaca, Theileria spp. | - | - | - | 1 | - | 1 |
| Quadruple | ||||||
| A. centrale, A. marginale, R. massiliae, Theileria spp. | - | 1 | - | - | - | 1 |
| A. centrale, A. ovis, R. massiliae, Theileria spp. | - | 1 | - | - | 1 | |
| A. ovis, Ehrlichia spp., Francisella-like, Theileria spp. | - | - | 1 | - | - | 1 |
| A. ovis, R. massiliae, Francisella-like, Theileria spp. | - | - | 1 | - | - | 1 |
| Francisella-like, R. aeschlimannii, R. massiliae, R. slovaca | 1 | - | - | - | - | 1 |
| Francisella-like, R. massiliae, R. slovaca, Theileria spp. | - | - | - | 1 | - | 1 |
| R. aeschlimannii, R. conorii, R. massiliae, R. slovaca | - | 1 | - | - | 1 | |
| Quintuple | ||||||
| A. ovis, Francisella-like, R. massiliae, R. slovaca, Theileria spp. | - | - | - | 1 | - | 1 |
| Septuple | ||||||
| A. ovis, Francisella-like, R. aeschlimannii, R. conorii, R. massiliae, R. slovaca, Theileria spp. | - | - | 1 | - | - | 1 |
| Total | 5 | 9 | 12 | 3 | 10 | 39 |
3.5. Genetic Relationship of Selected Microorganisms
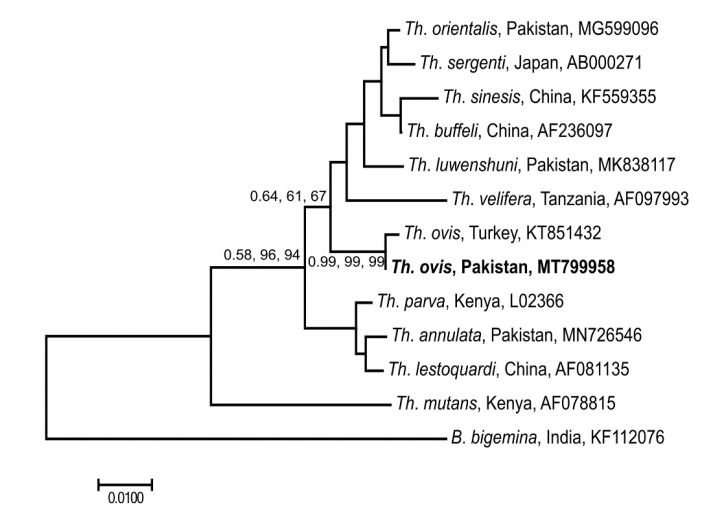
Phylogenetic analyses (using BI, ML and NJ) of the 18S sequence data for piroplasms determined herein (GenBank: MT799958) confirmed the presence of Th. ovis as it grouped with the reference sequence of Th. ovis from Turkey (GenBank: KT851432) with strong nodal support (0.99, 99%, 99%) (Figure 6).
Figure 6.
Phylogenetic relationship of 18S sequences of Theileria spp. from ticks of small ruminants in the FATA of Pakistan. Phylogenies were inferred using Neighbor Joining (NJ, this tree), Maximum Likelihood (ML) and Bayesian Inference (BI) methods. Sequences determined herein are shown in bold. The country of origin and GenBank accession numbers for each sequence are also provided. Nodal support values are presented as a posterior probability for BI (left) followed by bootstrap values for ML and NJ. The scale-bar indicates the number of inferred substitutions per site.
4. Discussion
This study provides the first insight into the molecular diversity of ticks, TBPs and endosymbionts in ticks from small ruminants in the FATA, Pakistan. The occurrence of Hyalomma and Rhipicephalus species characterized in our study is consistent with what has been reported previously from small and large ruminants in selected areas of Pakistan [8,15,50]. However, this study provides the first genetic evidence for Hs. sulcata, Hs. punctata, Rh. haemaphysaloides and Rh. turanicus from Pakistan.
Within the Rh. sanguineus group (clades 1–5), the sequences of Rh. turanicus determined here (clade-3a) clustered with the sequences belonging to the temperate lineage of Rh. sanguineus (clade-1) (Figure 2 and Figure 3) indicating a close similarity between the two species. The NCBI blast results also supported these findings as two of the 16S sequences determined here (GenBank: MT799954 and MT799955) were similar (96–97.2%) to those of Rh. sanguineus (GenBank: KR870984) and Rh. turanicus (GenBank: KR870985) from Turkey (data not shown). Likewise, three cox1 sequences (GenBank: MT800312–MT800314) were similar (90.5–93.9%) to those of Rh. sanguineus (GenBank: MF426015) and Rh. turanicus (GenBank: MN853166) from Portugal and Kazakhstan, respectively (data not shown). The taxonomic classification of Rh. turanicus has been recently studied by Bakkes et al. [51] who refuted the monophyletic nature of Rh. turanicus and proposed a new species, i.e., Rh. africanus n sp. Moreover, the authors also provided evidence of the existence of two lineages corresponding to southern Europe and the Middle East/Asia with differing climates. We also found a similar pattern here as Rh. turanicus sequences (from this study and references) clustered into two distinct clades (3a and 3b) (Figure 2 and Figure 3). These two clades may represent two separate species (cf. [51]). For Rh. haemaphysaloides, the genetic differences (0.2–7.6%) in cox1 sequences inferred here suggest the existence of two distinct lineages or even distinct species as their genetic similarity is < 95%, the value generally considered to be the threshold of conspecificity for these genes in ticks [52,53,54,55,56]. However, due to limited genetic data being available for this tick species, it is challenging to define a threshold for species delineation.
This study also provides the first molecular evidence for Hs. sulcata and Hs. punctata in Pakistan. The molecular-phylogenetic analyses of Haemaphysalis sequences confirmed and supported morphological characterization. However, the NCBI blast results demonstrated considerably large nucleotide differences from previously reported sequences of the same species (Hs. sulcata: 16S; 4.7–5.5%, cox1; 10.4–10.7%, Hs. punctata: 16S; 6.8%, cox1; 10.7%) from Turkey and Iran (Hs. sulcata) and China and Romania (Hs. punctata) (data not shown). These large differences could be partly due to the limited availability of gene sequences of these ticks. Future studies should focus on the morphological and genetic characterization of Haemaphysalis ticks collected from different climatic zones of Pakistan. Furthermore, additional genetic markers such as the elucidation of complete mitochondrial genomes [56,57] would allow the phylogeny of Haemaphysalis and other ticks to be resolved.
In this study, microfluidic real-time PCR-based screening of ticks demonstrated a higher prevalence of microorganisms (72.2%) as well as a higher percentage of ticks (71.8%) testing positive for multiple microorganisms (Table 3 and Table 4). The results were validated by conventional PCR for microorganisms whose specific identification was not achieved. However, genetic characterization was not successful for some microorganisms (such as the Ehrlichia species) due to low cycle threshold (Ct) values. Moreover, this study does not establish the mammalian- or tick-origin of detected microorganisms since ticks were collected while feeding on their hosts (i.e., goats and sheep). In addition, the detection of DNA of multiple microorganisms in several ticks does not imply the co-transmission to their hosts. Furthermore, this study does not provide estimates for the prevalence and distribution of different tick species in small ruminants from the FATA as ticks were collected from a smaller population. However, the prevalence of microorganisms was significantly higher in ticks collected from goats (83.9%) compared with those from sheep (56.5%) as shown in Table 3. Although there have been reports of higher prevalences of ticks [15] and internal parasites [58] in goats compared with sheep, most reports on the prevalence of haemoparasites in small ruminants from Pakistan and elsewhere are contrary to this finding [11,59,60,61,62]. For example, Iqbal et al. [11] reported 32 and a 5% prevalence of piroplasms in sheep and goats from the Punjab and KPK provinces of Pakistan, respectively. Likewise, Azmi et al. [59] and Rjeibi et al. [61] found higher prevalences of Theileria and piroplasms in sheep compared with goats from Palestine and Tunisia, respectively. Further molecular testing would be required to establish such differences, if any, as a smaller number of ticks were tested in the present study.
Francisella-like and Coxiella-like endosymbionts detected in this study are non-pathogenic mutualistic and/or commensal microbes, which play a key role in the tick’s developmental process and pathogen transmission [63,64]. We detected a higher prevalence of DNA of R. massiliae and R. slovaca in ticks in all five districts with a low prevalence of R. aeschlimannii (Bajaur and Mohmand) and R. conorii (Khyber and Mohmand) (see Table 3). All of these rickettsiae belong to the spotted fever group (SFG) and, thus, have zoonotic potential. The significance and risk of infections of SFG rickettsiae are higher in Asian countries where surveillance and diagnostic facilities are limited and new cases of rickettsial infections are increasing [65]. Due to a large influx of livestock and immigrants during the Afghan War, it is possible that new potential pathogens might have been imported into this region [7]. Moreover, most of the families in the FATA are living with their animals and there is a general lack of awareness about TBDs of veterinary and public health importance [7]. Our findings and previous reports of rickettsial species from ticks in Pakistan [28] highlight the need to establish a diagnostic surveillance system for zoonotic rickettsial pathogens in this country.
A higher prevalence of Theileria spp. (33.3%) was also found in ticks from all five districts and the molecular characterization of piroplasms revealed that they belonged to Th. ovis. However, it is not possible to exclude the possibility of another caprine/ovine Theileria species as Th. lestoquardi has been reported from small ruminants in Pakistan such as by Riaz et al. [16]. Anaplasma ovis was detected in 25.9% of all tick species identified herein and it is most frequently associated with anaplasmosis in small ruminants worldwide [64]. However, most of these cases are subclinical infections with a low-grade fever [66]. More recently, a variant of A. ovis has also been associated with human infection in Cyprus [67]. However, due to the lack of a proper diagnostic and surveillance system in Pakistan it is difficult to ascertain the economic losses and zoonotic threat due to this and other TBPs detected in this study.
Finding ticks that carry DNA of up to seven microorganisms of veterinary and medical significance indicates the level of risk associated with tick infestation to animals as well as humans. Previously, a similar level of co-occurrence of endosymbionts (i.e., Francisella-like and Coxiella-like) and pathogens (belonging to Anaplasma, Babesia, Bartonella, Borrelia, Ehrlichia, Hepatozoon, Rickettsia and Theileria genera) in bovine ticks was reported from the Punjab and Sindh provinces of Pakistan [28]. Similarly, microbiome analyses of Dermacentor silvarum and Ixodes persulcatus ticks from China revealed the presence of up to 29 and 373 bacterial genera, respectively, belonging to endosymbionts and pathogens [68,69]. These high levels of co-occurrence of microorganisms in ticks encourage the large-scale implementation of such high-throughput tools in resource-scarce settings where routine surveillance facilities are not accessible.
5. Conclusions
This study provides the first molecular insights into ticks, TBPs and endosymbionts of ticks from small ruminants of the FATA, Pakistan. Findings of this study demonstrate that multiple species of the Rhipicephalus group exist that are not distinguishable based on morphological data alone. Furthermore, ticks of small ruminants from the FATA carry multiple microorganisms of a veterinary and medical health significance. This study highlights the need to explore further tick and TBPs of livestock and wildlife species in this region of the country to guide the development of control measures and extension programs for farmers.
Acknowledgments
We are thankful to the livestock owners for their cooperation. Adil Khan is a thankful recipient of International Research Support Initiative Program from the Higher Education Commission of Pakistan. The authors are also thankful to Abid Ali (Abdul Wali Khan University Mardan, Pakistan) for providing lab space for sample storage.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/9/1428/s1, Table S1: PCR primers and thermocycling conditions used in this study, Table S2: List of primers and probes used in this study for the identification of microorganisms and ticks, Table S3: Pairwise comparisons of 16S nucleotide sequences (aligned over 429 bp) of ticks determined herein. Nucleotide similarity and percentage differences are given above and below the diagonal, respectively, Table S4: Pairwise comparisons of cox1 nucleotide sequences (aligned over 673 bp) of ticks determined herein. Nucleotide similarity and percentage differences are given above and below the diagonal, respectively, Table S5: Pairwise comparisons of ITS-2 nucleotide sequences (aligned over 273 bp) of ticks belonging to Rhipicephalus and Hyalomma genera determined herein. Nucleotide similarity and percentage differences are given above and below the diagonal, respectively.
Author Contributions
Conceptualization, A.J., A.C.-C., S.M. and R.B.G.; methodology, A.J., A.C.-C., A.G., A.K.; software, A.G., A.J.; validation, A.G., A.K., C.G., L.M.-H.; formal analysis, A.G., A.J.; investigation, A.G., A.K., C.G.; resources, A.J., R.B.G., A.C.-C., S.M.; data curation, A.G., A.J., A.C.-C.; writing—original draft preparation, A.G., A.J.; writing—review and editing, A.G., A.J., A.C.-C., R.B.G., C.G.G.; visualization, A.J., C.G.G., N.N., A.C.-C., S.M.; supervision, A.J., R.B.G., S.N., S.A., S.M.; project administration, A.J., R.B.G., C.G.G., A.C.-C., S.M.; funding acquisition, A.J., R.B.G., S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The visit of A.K. to the University of Melbourne was funded by the Higher Education Commission of Pakistan.
Conflicts of Interest
The authors declare no conflict of interest.
Availability of Data and Material
The datasets supporting the conclusion of this article are included within the article and supplementary material files. Nucleotide sequences reported in this article are available via GenBank.
References
- 1.Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J.F., Magnarelli L.A. Biology of Ticks. Infect. Dis. Clin. North Am. 2008;22:195–215. doi: 10.1016/j.idc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Sonenshine D.E., Roe R.M. Biology of Ticks. 2nd ed. Oxford University Press; Oxford, UK: 2014. [Google Scholar]
- 4.Government of Pakistan, Ministry of Finance, Islamabad Pakistan Economic Survey 2019–2020; pp. 17–41. [(accessed on 22 August 2020)]; Available online: http://www.finance.gov.pk/survey_1920.html.
- 5.Shah S.A.A., Parveen S., Khalil J. Governance challenges in mainstreaming of Federally Administered Tribal Areas into Khyber Pakhtunkhwa. FWU J. Soc. Sci. 2019;13:131–145. [Google Scholar]
- 6.Noor S., Hashmi A.S., Bukhari S.T. Fata Merger with Khyber Pakhtunkhwa: Prospects and Opportunities. ISSRA Papers 2018. [(accessed on 22 August 2020)]; Available online: https://prdb.pk/article/fata-merger-with-khyber-pakhtunkhwa-prospects-and-opportuni-4653.
- 7.Nieto N.C., Khan K., Uhllah G., Teglas M.B. The emergence and maintenance of vector-borne diseases in the Khyber Pakhtunkhwa province, and the Federally Administered Tribal Areas of Pakistan. Front. Physiol. 2012;3:250. doi: 10.3389/fphys.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali A., Khan M.A., Zahid H., Yaseen P.M., Khan M.Q., Nawab J., Rehman Z.U., Ateeq M., Khan S., Ibrahim M. Seasonal dynamics, record of Ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019;10:793. doi: 10.3389/fphys.2019.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain S.I., Kumar G.A. The incidence of ticks (Ixodoidea: Ixodidae) infesting sheep and goat in Sind Province Pakistan. Pak. J. Zool. 1985;17:89–97. [Google Scholar]
- 10.Iqbal A., Siddique F., Mahmood M.S., Shamim A., Zafar T., Rasheed I., Saleem I., Ahmad W. Prevalence and impacts of ectoparasitic fauna infesting goats (Capra hircus) of district Toba Tek Singh, Punjab, Pakistan. Glob. Vet. 2014;12:158–164. [Google Scholar]
- 11.Iqbal F., Khattak R., Ozubek S., Khattak M., Rasul A., Aktas M. Application of the reverse line blot assay for the molecular detection of Theileria and Babesia sp. in sheep and goat blood samples from Pakistan. Iran. J. Parasitol. 2013;8:289–295. [PMC free article] [PubMed] [Google Scholar]
- 12.Irshad N., Qayyum M., Hussain M., Khan M. Prevalence of tick infestation and theileriosis in sheep and goats. Pak. Vet. J. 2010;30:178–180. [Google Scholar]
- 13.Karim S., Budachetri K., Mukherjee N., Williams J., Kausar A., Hassan M.J., Adamson S., Dowd S.E., Apanskevich D., Arijo A., et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017;11:e5681. doi: 10.1371/journal.pntd.0005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M.A., Khan M., Ahmad I., Khan M., Anjum A., Durrani A., Hameed K., Kakar I., Wajid A., Ramazan M. Risk factors assessment and molecular characterization of Theileria in small ruminants of Balochistan. J. Anim. Plant Sci. 2017;27:1190–1196. [Google Scholar]
- 15.Rehman A., Nijhof A.M., Sauter-Louis C., Schauer B., Staubach C., Conraths F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit. Vectors. 2017;10:190. doi: 10.1186/s13071-017-2138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riaz M., Nazir M.M., Tasawar Z., Ahmed A.N., Ayaz M., Akram Q., Lindsay D.S. Molecular epidemiology and prevalence of Theileria lestoquardi and Theileria ovis infection in goats infested with tick vectors from Multan, Pakistan. J. Med. Entomol. 2019;56:844–848. doi: 10.1093/jme/tjy229. [DOI] [PubMed] [Google Scholar]
- 17.Sajid M.S., Iqbal Z., Khan M.N., Muhammad G., Needham G., Khan M.K. Prevalence, associated determinants, and in vivo chemotherapeutic control of hard ticks (Acari: Ixodidae) infesting domestic goats (Capra hircus) of lower Punjab, Pakistan. Parasitol. Res. 2011;108:601–609. doi: 10.1007/s00436-010-2103-8. [DOI] [PubMed] [Google Scholar]
- 18.Shah A., Shah S.R., Rafi M.A., Noorrahim M.S., Mitra A. Identification of the prevalent ticks (Ixodid) in goats and sheep in Peshawar, Pakistan. J. Entomol. Zool. Stud. 2015;3:11–14. [Google Scholar]
- 19.Shahzad W., Haider N., Mansur-ud-Din A., Munir R., Saghar M.S., Mushtaq M.H., Ahmad N., Akbar G., Mehmood F. Prevalence and molecular diagnosis of Babesia ovis and Theileria ovis in Lohi sheep at Livestock Experiment Station (LES), Bahadurnagar, Okara, Pakistan. Iran. J. Parasitol. 2013;8:570–578. [PMC free article] [PubMed] [Google Scholar]
- 20.Khan A., Nasreen N., Niaz S., Shah S.S.A., Mitchell R.D., III, Ayaz S., Naeem H., Khan L., De León A.P. Tick burden and tick species prevalence in small ruminants of different agencies of the Federally Administered Tribal Areas (FATA), Pakistan. Int. J. Acarol. 2019;45:374–380. doi: 10.1080/01647954.2019.1663930. [DOI] [Google Scholar]
- 21.Diarra A.Z., Almeras L., Laroche M., Bérenger J.M., Koné A.K., Bocoum Z., Dabo A., Doumbo O., Raoult D., Parola P. Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Negl. Trop. Dis. 2017;11:e5762. doi: 10.1371/journal.pntd.0005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporale D.A., Rich S.M., Spielman A., Telford S.R., Kocher T. Discriminating between Ixodes ticks by means of mitochondrial DNA sequences. Mol. Phylogenet. Evol. 1995;4:361–365. doi: 10.1006/mpev.1995.1033. [DOI] [PubMed] [Google Scholar]
- 23.Walker A., Bouattour A., Camicas J., Estrada-Peña A., Horak I., Latif A., Pegram R.G., Preston P.M.A. Ticks of Domestic Animals in Africa. A Guide to Identification of Species. Bioscience Reports; London, UK: 2003. [Google Scholar]
- 24.Cuickshank R.H. Molecular markers for the phylogenetics of mites and ticks. Syst. Appl. Acarol. 2002;7:3–14. [Google Scholar]
- 25.Black W.C., Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahler M., Filippova N.A., Morel P.C., Gothe R., Rinder H. Relationships between species of the Rhipicephalus sanguineus group: A molecular approach. J. Parasitol. 1997;83:302. doi: 10.2307/3284460. [DOI] [PubMed] [Google Scholar]
- 27.Duron O., Noël V., McCoy K.D., Bonazzi M., Sidi-Boumedine K., Morel O., Vavre F., Zenner L., Jourdain E., Durand P., et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015;11:e1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghafar A., Cabezas-Cruz A., Galon C., Obregon D., Gasser R.B., Moutailler S., Riaz N. Bovine ticks harbor a diverse array of microorganisms in Pakistan. Parasit. Vectors. 2020;13:1. doi: 10.1186/s13071-019-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasi K.K., Sas M.A., Sauter-Louis C., Von Arnim F., Gethmann J.M., Schulz A., Wernike K., Groschup M.H., Conraths F.J. Epidemiological investigations of Crimean-Congo hemorrhagic fever virus infection in sheep and goats in Balochistan, Pakistan. Ticks Tick Borne Dis. 2020;11:101324. doi: 10.1016/j.ttbdis.2019.101324. [DOI] [PubMed] [Google Scholar]
- 30.Salih D., El Hussein A., Singla L. Diagnostic approaches for tick-borne haemoparasitic diseases in livestock. J. Veter Med. Anim. Health. 2015;7:45–56. doi: 10.5897/jvmah2014.0345. [DOI] [Google Scholar]
- 31.Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Pihl T.P.B., et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabezas-Cruz A., Allain E., Ahmad A.S., Saeed M.A., Rashid M.I., Ashraf K., Yousfi L., Shehzad W., Indjein L., Rodriguez-Valle M., et al. Low genetic diversity of Ehrlichia canis associated with high co-infection rates in Rhipicephalus sanguineus (sl) Parasit. Vectors. 2019;12:12. doi: 10.1186/s13071-018-3194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gondard M., Delannoy S., Pinarello V., Aprelon R., Devillers E., Galon C., Pradel J., Vayssier-Taussat M., Albina E., Moutailler S. Upscaling the surveillance of tick-borne pathogens in the French Caribbean islands. Pathogens. 2020;9:176. doi: 10.3390/pathogens9030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grech-Angelini S., Stachurski F., Vayssier-Taussat M., Devillers E., Casabianca F., Lancelot R., Uilenberg G., Moutailler S. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 2020;67:745–757. doi: 10.1111/tbed.13393. [DOI] [PubMed] [Google Scholar]
- 35.Lejal E., Moutailler S., Šimo L., Vayssier-Taussat M., Pollet T. Tick-borne pathogen detection in midgut and salivary glands of adult Ixodes ricinus. Parasit. Vectors. 2019;12:152. doi: 10.1186/s13071-019-3418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelet L., Joncour G., Devillers E., Torina A., Vayssier-Taussat M., Bonnet S.I., Moutailler S. Tick species, tick-borne pathogens and symbionts in an insular environment off the coast of Western France. Ticks Tick Borne Dis. 2016;7:1109–1115. doi: 10.1016/j.ttbdis.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 37.FATA Secretariat, Government of Pakistan. Food Agriculture Organization of the United Nations Agriculture Policy for FATA: Policy period (2016–2025) [(accessed on 22 August 2020)]; Available online: http://extwprlegs1.fao.org/docs/pdf/pak173416.pdf.
- 38.Barker S.C., Walker A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa. 2014;3816:1–144. doi: 10.11646/zootaxa.3816.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Walker J.B., Keirans J.E., Horak I.G. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World. Cambridge University Press; New York, NY, USA: 2000. [Google Scholar]
- 40.Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 41.Rees D., Dioli M., Kirkendall L.R. Molecules and morphology: Evidence for cryptic hybridization in African Hyalomma (Acari: Ixodidae) Mol. Phylogenet. Evol. 2003;27:131–142. doi: 10.1016/S1055-7903(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 42.Gubbels M.J., De Vos A.P., Van der Weide M., Viseras J., Schouls L.M., De Vries E., Jongejan F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 1999;37:1782–1789. doi: 10.1128/JCM.37.6.1782-1789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 46.Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 47.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- 48.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 49.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghafar A., Gasser R.B., Rashid I., Ghafoor A., Riaz N. Exploring the prevalence and diversity of bovine ticks in five agro-ecological zones of Pakistan using phenetic and genetic tools. Ticks Tick Borne Dis. 2020;11:101472. doi: 10.1016/j.ttbdis.2020.101472. [DOI] [PubMed] [Google Scholar]
- 51.Bakkes D.K., Chitimia-Dobler L., Matloa D., Oosthuysen M., Mumcuoglu K.Y., Mans B.J., Matthee C.A. Integrative taxonomy and species delimitation of Rhipicephalus turanicus (Acari: Ixodida: Ixodidae) Int. J. Parasitol. 2020;50:577–594. doi: 10.1016/j.ijpara.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Bakkes D.K., De Klerk D., Latif A.A., Mans B.J. Integrative taxonomy of Afrotropical Ornithodoros (Ornithodoros) (Acari: Ixodida: Argasidae) Ticks Tick Borne Dis. 2018;9:1006–1037. doi: 10.1016/j.ttbdis.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Chitimia-Dobler L., Langguth J., Pfeffer M., Kattner S., Küpper T., Friese D., Dobler G., Guglielmone A.A., Nava S. Genetic analysis of Rhipicephalus sanguineus sensu lato ticks parasites of dogs in Africa north of the Sahara based on mitochondrial DNA sequences. Vet. Parasitol. 2017;239:1–6. doi: 10.1016/j.vetpar.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Lado P., Nava S., Mendoza-Uribe L., Cáceres A.G., De La Mora J.D., Licona-Enriquez J.D., La Mora D.D.-D., Labruna M.B., Durden L.A., Allerdice M.E., et al. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: Phenotypic plasticity or incipient speciation? Parasit. Vectors. 2018;11:610. doi: 10.1186/s13071-018-3186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L.H., Zhang Y., Wang J.Z., Li X.S., Yin S.Q., Zhu D., Xue J.B., Li S.G. High genetic diversity in hard ticks from a China-Myanmar border county. Parasit. Vectors. 2018;11:469. doi: 10.1186/s13071-018-3048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mans B.J., Featherston J., Kvas M., Pillay K.A., De Klerk D.G., Pienaar R., De Castro M.H., Schwan T.G., Lopez J.E., Teel P., et al. Argasid and ixodid systematics: Implications for soft tick evolution and systematics, with a new argasid species list. Ticks Tick Borne Dis. 2019;10:219–240. doi: 10.1016/j.ttbdis.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Wang T., Zhang S., Pei T., Yu Z., Liu J. Tick mitochondrial genomes: Structural characteristics and phylogenetic implications. Parasit. Vectors. 2019;12:451. doi: 10.1186/s13071-019-3705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorski P., Niznikowski R., Strzelec E., Popielarczyk D., Gajewska A., Wedrychowicz H. Prevalence of protozoan and helminth internal parasite infections in goat and sheep flocks in Poland. Arch. Tierzucht. 2004;47:43–49. [Google Scholar]
- 59.Azmi K., Al-Jawabreh A., Abdeen Z. Molecular Detection of Theileria ovis and Theleiria equi in Livestock from Palestine. Sci. Rep. 2019;9:11557. doi: 10.1038/s41598-019-47965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chochlakis D., Ioannou I., Sharif L., Kokkini S., Hristophi N., Dimitriou T., Tselentis Y., Psaroulaki A. Prevalence of Anaplasma sp. in goats and sheep in Cyprus. Vector Borne Zoonotic Dis. 2009;9:457–463. doi: 10.1089/vbz.2008.0019. [DOI] [PubMed] [Google Scholar]
- 61.Rjeibi M.R., Gharbi M., Mhadhbi M., Mabrouk W., Ayari B., Nasfi I., Jedidi M., Sassi L., Rekik M., Darghouth M.A. Prevalence of piroplasms in small ruminants in North-West Tunisia and the first genetic characterization of Babesia ovis in Africa. Parasite. 2014;21:23. doi: 10.1051/parasite/2014025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben Saïd M., Belkahia H., Alberti A., Zobba R., Bousrih M., Yahiaoui M., Daaloul-Jedidi M., Mamlouk A., Gharbi M., Messadi L. Molecular survey of Anaplasma species in small ruminants reveals the presence of novel strains closely related to A. phagocytophilum in Tunisia. Vector Borne Zoonotic Dis. 2015;15:580–590. doi: 10.1089/vbz.2015.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guizzo M.G., Parizi L.F., Nunes R.D., Schama R., Albano R.M., Tirloni L., Oldiges D.P., Vieira R.P., Oliveira W.H.C., Leite M.D.S., et al. A Coxiella mutualist symbiont is essential to the development of Rhipicephalus microplus. Sci. Rep. 2017;7:17554. doi: 10.1038/s41598-017-17309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macaluso K.R., Sonenshine D.E., Ceraul S.M., Azad A.F. Rickettsial Infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 65.Satjanadumrong J., Robinson M.T., Hughes T., Blacksell S.D. Distribution and ecological drivers of spotted fever group Rickettsia in Asia. EcoHealth. 2019;16:611–626. doi: 10.1007/s10393-019-01409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuttler K.L. Anaplasma infections in wild and domestic ruminants: A review. J. Wildl. Dis. 1984;20:12–20. doi: 10.7589/0090-3558-20.1.12. [DOI] [PubMed] [Google Scholar]
- 67.Chochlakis D., Ioannou I., Tselentis Y., Psaroulaki A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010;16:1031–1032. doi: 10.3201/eid1606.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duan D.Y., Liu G.H., Cheng T.Y. Microbiome analysis of the saliva and midgut from partially or fully engorged female adult Dermacentor silvarum ticks in China. Exp. Appl. Acarol. 2020;80:543–558. doi: 10.1007/s10493-020-00478-2. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X.C., Yang Z.N., Lu B., Ma X.F., Zhang C.X., Xu H.J. The composition and transmission of microbiome in hard tick, Ixodes persulcatus, during blood meal. Ticks Tick Borne Dis. 2014;5:864–870. doi: 10.1016/j.ttbdis.2014.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusion of this article are included within the article and supplementary material files. Nucleotide sequences reported in this article are available via GenBank.