Abstract
Multiple medical, lifestyle, and environmental conditions, including smoking and particulate pollution, have been considered as risk factors for COronaVIrus Disease 2019 (COVID-19) susceptibility and severity. Taking into account the high level of toxic metals in both particulate matter (PM2.5) and tobacco smoke, the objective of this review is to discuss recent data on the role of heavy metal exposure in development of respiratory dysfunction, immunotoxicity, and severity of viral diseases in epidemiological and experimental studies, as to demonstrate the potential crossroads between heavy metal exposure and COVID-19 severity risk. The existing data demonstrate that As, Cd, Hg, and Pb exposure is associated with respiratory dysfunction and respiratory diseases (COPD, bronchitis). These observations corroborate laboratory findings on the role of heavy metal exposure in impaired mucociliary clearance, reduced barrier function, airway inflammation, oxidative stress, and apoptosis. The association between heavy metal exposure and severity of viral diseases, including influenza and respiratory syncytial virus has been also demonstrated. The latter may be considered a consequence of adverse effects of metal exposure on adaptive immunity. Therefore, reduction of toxic metal exposure may be considered as a potential tool for reducing susceptibility and severity of viral diseases affecting the respiratory system, including COVID-19.
Keywords: Arsenic, Mercury, Cadmium, Lead, Immunity
Graphical abstract
1. Introduction
CoronaVirus Disease 2019 (COVID-19) is an infectious disease caused by coronavirus SARS-CoV-2 that first occurred in October and caused a massive outbreak in December 2019 (Frutos et al., 2020). During the last 10 months COVID-19 affected more than 30 million people worldwide, and is considered as a cause of more than 950,000 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). The virus predominantly affects the respiratory system, causing COVID-19 pneumonia (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c), although other systems are also involved especially in severe cases due to vascular dysfunction (Varga et al., 2020) and cytokine storm (Shimabukuro-Vornhagen et al., 2018) resulting in systemic inflammation (Yuki et al., 2020). These features are tightly linked to COVID-19-associated immune dysregulation (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c) characterized by lymphopenia with a concomitant decline in T helper, T suppressor, and regulatory T cells, and leukocytosis, increased neutrophil-to-lymphocyte-ratio (Qin et al., 2020), as well as lymphocyte and NK-cell dysfunction (Vabret et al., 2020). The key symptoms include fever, cough, fatigue, expectoration, and dyspnea, as well as less common gastrointestinal symptoms including diarrhea, nausea and vomiting (Li et al., 2020a). Moreover, clinical course of the disease is highly variable, being characterized by high number of asymptomatic and mild cases, but frequently progressing to pneumonia, acute respiratory distress syndrome (ARDS) and organ dysfunction (Singhal, 2020). However, the particular causes of such clinical heterogeneity are unclear.
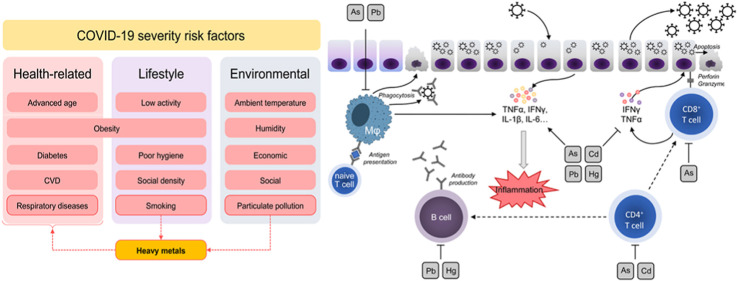
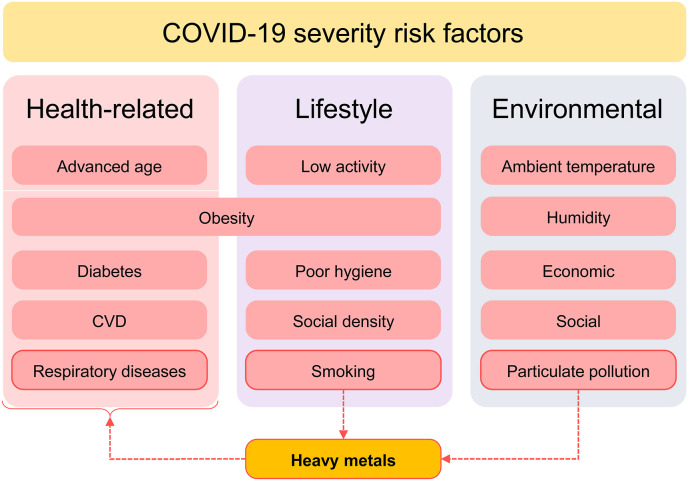
In parallel with the search for therapeutic approaches for the disease (Dong et al., 2020) and assessment of its safety (Javorac et al., 2020), estimation of the potential risk factors is also essential for management of the disease and reducing its risk (Zheng et al., 2020). Multiple medical, lifestyle, and environmental (Tsatsakis et al., 2020) conditions have been considered as risk factors for COVID-19 susceptibility and severity (Fig. 1 ). Given the key role of the respiratory system as a target for COVID-19, respiratory diseases including chronic obstructive pulmonary disease (COPD) (Lippi, Henry, 2020) were shown to be associated with higher disease severity. Obesity (Petrakis et al., 2020), diabetes, cardiovascular diseases (Zheng et al., 2020), and cancer (Dai et al., 2020) were also considered as risk factors for COVID-19 severity. Advanced age is known to be one of the leading factors associated with severity of the disease due to impaired immune response (Wu et al., 2020a). A recent study originating from the UK has demonstrated that lifestyle factors may account up to 51% variability in COVID-19 severity with physical inactivity, obesity, and smoking being the leading ones (Hamer et al., 2020). SARS-CoV-2 is the causal factor contributing to the COVID-19 pamdemic; still environmental factors such as humidity, UV radiation and ambient temperature were shown to affect disease susceptibility and SARS-CoV-2 transmissibility (Ma et al., 2020). Environmental gas and particulate pollution were also shown to affect epidemiological parameters of COVID-19 infection (Yongjian et al., 2020). In view of the role of toxic metals as effector agents in tobacco smoke and particulate pollution toxicity, as well as their respiratory, immunotoxic, and proinflammatory effects, we propose that toxic metal exposure may be also considered as a potential risk factor for COVID-19 severity.
Fig. 1.
The proposed role of heavy metals as a link between risk factors for COVID-19 severity. Both particulate (PM2.5) pollution and smoking are associated with heavy metal exposure that at least partially mediate adverse effects of these factors on the respiratory system. In addition, heavy metal exposure was shown to be associated with higher incidence of obesity, diabetes, and cardiovascular diseases.
Therefore, the objective of the study was to discuss recent data on the role of toxic metal exposure in development of respiratory dysfunction, immunotoxicity, as well as interference of metal toxicity with viral diseases in epidemiological and experimental studies in order to demonstrate the potential crossroads between heavy metal exposure and COVID-19 severity risk. In order to address the objective we have performed a systematic search in the PubMed database using the MeSH terms cadmium, mercury, lead, arsenic, lungs, respiratory function, inflammation, mucociliary clearance; bronchitis, viral diseases, antiviral immunity, immunotoxicity up through August 20, 2020.
1.1. Metal-related risk factors for severe COVID-19
Environmental pollution has been considered to be one of the risk factors for COVID-19 severity and mortality due to impaired respiratory functions, chronic inflammation, and reduced resistance to infections (Conticini et al., 2020; Qu et al., 2020), being supported by indirect data from Northern Italy and China (Martelletti, Martelletti, 2020). Particularly, an increase in PM2.5, PM10, NO2 and O3 concentrations in 120 cities in China has been associated with newly confirmed COVID-19 cases (Zhu et al., 2020). In addition, it has been demonstrated that every 1 μg/m3 increase in PM2.5 level results in an 8% increase in COVID-19 mortality rate (Wu et al., 2020b). However, despite the presence of rather convincing data on the role of environmental pollution as a risk factor for COVID-19 susceptibility, these conclusions should be viewed with caution due to lack of qualitative and quantitative analysis of particulate matter (Contini, Costabile, 2020). It has been also proposed that heavy metals may significantly mediate health hazards of particulate matter (Chen, Lippmann, 2009).
Another potential external factor associated with COVID-19 severity is smoking, although the existing data are highly contradictory. A recent analysis revealed a nearly twofold higher risk of severe COVID-19 (odds ratio (OR) = 1.98; 95% confidence interval (95%CI): 1.29–3.05) (Zhao et al., 2020). However, sensitivity analysis demonstrated that the study by Guan et al. (2020) originating from Wuhan was considered as the main source of heterogeneity, and its exclusion resulted in insignificant association between smoking and COVID-19 severity. Other studies also met with contradictions, indicating both lack of an association (Lippi, Henry, 2020) and a direct association between smoking and COVID-19 severity (Vardavas, Nikitara, 2020). Finally, a systematic review and meta-analysis dated from June 2020 has been demonstrated that current smokers have higher odds for adverse outcome of COVID-19 than non-current smokers, but lower risk in comparison to former smokers (Farsalinos et al., 2020a, Farsalinos et al., 2020b).
Despite significant contradiction in epidemiological data, smoking induced up-regulation of ACE2 receptor (Brake et al., 2020) in addition to lung inflammation, impaired barrier functions, mucus overproduction, and mucociliary clearance (Berlin et al., 2020) that could be mechanistically linked to higher risk of COVID-19. Hazardous effects of tobacco smoke could be attributed to the presence of more than 5000 chemicals, with heavy metals such as cadmium and lead being among the 98 most toxic agents (Talhout et al., 2011).
Taken into account a high level of toxic metals in both particulate matter and tobacco smoke, it is reasonable to propose that metal toxicity may in part underlie the association between PM exposure and COVID-19 severity, although this hypothesis has yet to be confirmed.
1.2. Cadmium
1.2.1. Respiratory dysfunction and diseases
Cadmium (Cd) was shown to underlie a significant part of adverse effects of tobacco smoke exposure through induction of oxidative stress and impaired macrophage functions (Sarigiannis and Salifoglou, 2016; Ganguly et al., 2018). Particularly, it has been demonstrated that urinary Cd levels were associated with lower forced expiratory volume (FEV1) values in current and former smokers, but not in never smokers (Mannino et al., 2004). Cd and lead (Pb) co-exposure resulted in reduced FEV1 and FVC in children living in e-waste recycling area (Zeng et al., 2017). Similar associations between blood Cd and forced vital capacity (FVC), FEV1, FEV1/FVC, peak expiratory force (PEF) were shown in welders (Cetintepe et al., 2019; Torén et al., 2019). Even upon low Cd body burden in exposed workers, urinary Cd levels were associated with higher prevalence of subjective respiratory symptoms despite the lack of significant changes in lung function (Li et al., 2020b).
Several studies have demonstrated the association between Cd exposure markers and respiratory diseases. Cd levels were found to be increased in cell-free bronchoalveolar lavage (BAL) fluid in smokers as compared to non-smokers in a cohort of patients with chronic obstructive pulmonary disease (Sundblad et al., 2016). These findings corroborate observations on higher blood Cd levels in male COPD patients (Oh et al., 2014). It is also noteworthy that in a case-control study conducted in Wuhan, China, urinary Cd levels were inversely associated with FEV1 values (Huang et al., 2016). Cd exposure was also associated with higher risk of wheezing in current smokers in the NHANES 2007–2012 (Yang et al., 2019a). In agreement, hair Cd levels in children were also associated with the number of wheezing episodes even after adjustment for environmental tobacco smoke (Razi et al., 2012).
1.2.2. Mechanisms of respiratory toxicity
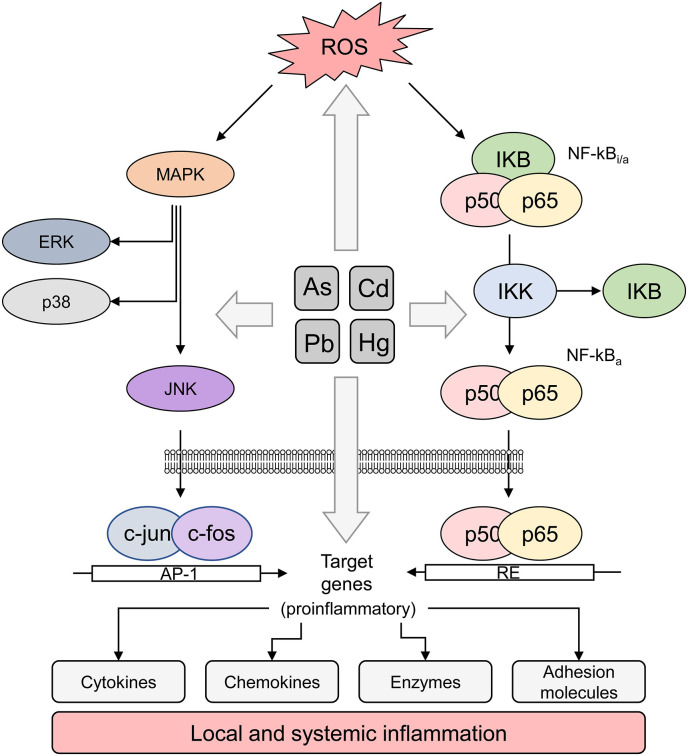
Cd exposure possesses both proinflammatory effects in lung tissue (Fig. 2 ), resulting in higher susceptibility to infectious diseases (Kulas et al., 2019). The mechanisms of Cd toxicity in lung cells may also involve endoplasmic reticulum (ER) stress (Kim et al., 2017) and up-regulation of apoptosis (Heo et al., 2017a, Heo et al., 2017b). Particularly, Cd exposure resulted in activation of proapoptotic signals (Bax, caspase 8) and repression of antiapoptotic Bcl-2 signaling in A549 lung cells (Kiran Kumar et al., 2016). Apoptosis is also known to be involved in the regulation of Cd-induced autophagy in A549 cells (Li et al., 2019).
Fig. 2.
A simplified scheme depicting proinflammatory mechanisms of heavy metals. Briefly, heavy metal exposure results in increased ROS production and oxidative stress that latter may underlie heavy metal-induced activation of proinflammatory pathways NF-kB and MAPK with subsequent expression of target proinflammatory genes including cytokines, chemokines, enzymes, and adhesion molecules (Metryka et al., 2018; Pollard et al., 2019; Hossein-Khannazer et al., 2020; Hu et al., 2020).
Cd exposure was shown to induce peribronchiolar fibrosis and lung remodeling due to stimulation of vimentin phosphorylation (Li et al., 2017a, Li et al., 2017b). In addition, SMAD-dependent myofibroblast differentiation from lung fibroblast may also underlie Cd-associated pulmonary fibrosis (Hu et al., 2017). Correspondingly, low-dose Cd exposure aggravated polyhexamethylene guanidine-induced lung fibrosis through up-regulation of inflammatory and fibrotic mediators (Kim et al., 2018). Cd exposure in the form of Cd nanoparticles was shown to induce citrullination of proteins (cytokeratins I and II) in lung epithelial cells (Hutchinson et al., 2018).
Cd exposure also results in impaired mucociliary clearance and aberrant mucin production associated with oxidative stress, inflammation, and activation of matrix metalloproteinases in a human airway tissue in vitro model (Xiong et al., 2019). In addition, CdCl2 is also capable of decreasing barrier function of bronchial epithelial cells through altered expression of tight junction proteins zonula occludens-1 (ZO-1) and occluding (Cao et al., 2015).
One of the potential mechanisms of lung damage in response to Cd exposure may include antagonistic relationships between the latter and zinc (Zn) ions (Xu et al., 2017a, Xu et al., 2017b, Xu et al., 2017c; Knoell et al., 2020) that is involved in respiratory protection (Skalny et al., 2020).
1.2.3. Viral diseases, antiviral immunity, and immunotoxicity
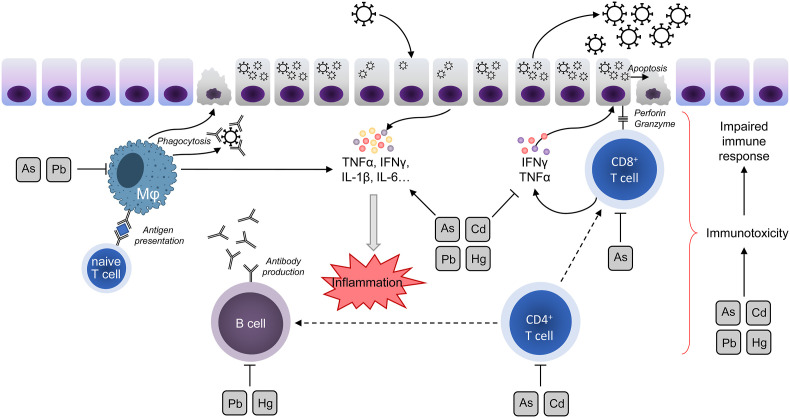
In an experimental study, oral Cd pretreatment was accompanied by significantly increased titers of respiratory syncytial virus (RSV) in lung tissues and severe lung damage due to aggravation of inflammation, oxidative stress, and mitochondrial dysfunction (Go et al., 2018). Correspondingly, Cd exposure potentiated inflammatory lung damage in a murine model of H1N1 infection (Chandler et al., 2019). In addition, Cd exposure promoted influenza virus replication in MCDK cells in a dose-dependent manner (Checconi et al., 2013). Several epidemiological (Krueger, Wade, 2016) and laboratory (Seth et al., 2003) studies also indicated the association between Cd exposure and non-airborne viral diseases, that may be generally attributable to immunotoxic effect of Cd and its negative impact on antiviral immunity (Fig. 3 ).
Fig. 3.
The proposed impact of heavy metals on antiviral immunity. As, Cd, Hg, and Pb were shown to be toxic for both T and B lymphocytes, as well as macrophages, affecting its proliferation and further functioning. Taken together with the negative impact on IFNγ production and proinflammatory activity, heavy metals may significantly contribute to excessive inflammatory and impaired immune response.
Cd toxicity is associated with altered hematopoietic stem and progenitor cells differentiation causing a shift to myelopoiesis from lymphopoiesis (Zhang et al., 2016). In addition, Cd affects T cell subsets characterized by reduction of T-helper (CD4+) cells and induction of cytotoxic T cells (CD8+), being indicative of metal immunotoxicity, and resulting in down-regulation of interferon-γ (IFN-γ) and interleukin-2 (IL-2) production (Pathak, Khandelwal, 2008). Developmental Cd exposure was shown to affect immune system maturation through alteration of DN1 and DN2 thymocyte ratio, also resulting in reduced natural killer (NK)-cell and granulocyte content in spleen accompanied by increased CD4+ and CD8+ T cells, as well as CD45R/B220+ B cells count (Holásková et al., 2012). More recently, a study conducted in male Sprague-Dawley rats demonstrated that subchronic exposure to Cd (32 ppm cadmium chloride in drinking water for 10 weeks) led to a slight increase in the relative weight of the spleen and in the regulatory T cells number. Cd-exposed animals also showed a significant increase in the production of IFN-γ and IL-10, suggesting an impact on immune cell function and cellularity, which may stimulate inflammatory responses (Turley et al., 2019). Generally, these findings are indirectly in agreement with the observation on the inverse association between Cd exposure and T lymphocyte (Zeng et al., 2020) and memory T cell levels in children (Nygaard et al., 2017).
The immunotoxic effect of Cd exposure is also associated with development of aberrant inflammatory response due to altered cytokine expression profile (Hossein-Khannazer et al., 2020). Earlier data have demonstrated that adult female zebrafish exposed to 1 mg L−1 Cd for 24 or 96 h led to an increase in the levels of tumor necrosis factor-α (TNF-α) in the brain, liver and ovary, as well as increase in mRNA levels of nuclear factor erythroid 2–related factor 2 (Nrf2) and nuclear factor-κB (NF-κB) in the liver and ovary in the first evaluation time (Zheng et al., 2016). On the other hand, zebrafish embryos exposed for 15 weeks to 5 μg/L Cd presented a decrease of nitric oxide (NO) and iNOS levels in the liver and spleen, accompanied by decreased transcriptional levels of IL-6, IL-10, IL-1β and TNF-α in liver. These findings indicate an immunosuppressive activity of Cd that was followed by a compensatory effect observed due to an increase in the mRNA levels of these cytokines in the spleen (Guo et al., 2017).
In vitro, exposure to various concentrations of Cd caused decreased viability of RAW 264.7 cell line, murine macrophage-derived cell. Increase in the production of reactive oxygen and nitrogen species was observed in response to activating stimulus by Salmonella enterica Serovar Enteritidis via pattern-recognition receptors (PRRs). When exposed to CdCl2, proinflammatory cytokines IL-12 and chemokine (C-X-C motif) ligand 1 (CXCL1) were highly upregulated whereas IL-6 and IL-10 were suppressed, indicating an important effect of cadmium on the macrophage functions modulation in innate immune response to infection (Riemschneider et al., 2015). Cd exposure may also sustain systemic inflammatory reaction through modulation of gut microbiota and subsequent increase in LPS levels (Tinkov et al., 2018b).
1.3. Mercury
1.3.1. Respiratory dysfunction and diseases
Although respiratory dysfunction is known to be the characteristic feature of acute mercury (Hg) vapor inhalation (Smiechowicz et al., 2017), data on the relationship between environmental Hg pollution and pulmonary function are rather insufficient. Previous studies revealed a significant inverse association between hair Hg and respiratory function in subjects from artisanal and small-scale gold mining areas (Pateda et al., 2018). High serum Hg levels (third tertile) were associated with increased odds of obstructive lung disease (OR = 3.62; 95%CI: 1.29–10.18) in a dusty area (Heo et al., 2017a, Heo et al., 2017b). The results of a follow-up analysis involving 4350 Korean children demonstrated that blood Hg is associated with higher odds of asthma and bronchi hyperresponsiveness (Kim et al., 2015). However, further data show contradictory results, underlining the importance of investigation of the association between prenatal and lifetime Hg exposure and asthma (Heinrich et al., 2017). Serum mercury as well as lead levels were found to be higher in children with recurrent wheezing (Razi et al., 2011). Blood Hg levels were also associated with increased exhaled nitric oxide levels, being considered as a marker of airway inflammation (Min, Min, 2013). It is also notable that high maternal toenail Hg content was associated with increased risk of lower respiratory infections, but not upper respiratory tract infections in infants (Emeny et al., 2019).
1.3.2. Mechanisms of respiratory toxicity
Both methylmercury (CH3Hg) (Yu et al., 2015) and HgCl2 (Park, Park, 2007) were shown to be cytotoxic for A549 cells through induction of oxidative stress and apoptosis. The latter may occur due to Hg-induced modulation of Bax, p53, and Bcl 2 expression (Ali et al., 2018). Similar effect was observed in alveolar type II epithelial cell exposed to mercury (Lu et al., 2010). Correspondingly, both Hg and Cd exposure were shown to induce oxidative stress accompanied by heat shock protein 70 (Hsp 70) expression, although Cd was relatively more toxic as compared to Hg (Han et al., 2007). ER stress may be also considered as the potential mechanism of lung toxicity of Hg (Jagannathan et al., 2017). It is also notable that inhalation of elementary mercury vapor significantly up-regulated proinflammatory cytokine expression in lung tissue (Liu et al., 2003).
Although direct data on the impact of Hg on airway epithelium permeability are insufficient, it has been demonstrated that Hg exposure affects expression of tight junction proteins in colonic epithelial cells (Vazquez et al., 2014).
Morphological analysis demonstrated that Hg exposure may induce inflammatory response characterized by inflammatory cells infiltration and altered extracellular matrix (Naidoo et al., 2019) through proinflammatory effect of the metal (Fig. 2).
1.3.3. Viral diseases, antiviral immunity, and immunotoxicity
Several epidemiological and experimental studies have demonstrated the association between Hg exposure and airborne viral infections. The association between mercury exposure and measles antibodies was found to be significant only in children with low folate and high methylmalonic acid (MMA) values, whereas in other children the relationship was found to be inverse (Gallagher et al., 2011). Similarly, associations between Hg and rubeola antibody levels were affected by folate, MMA, and homocysteine levels (Gallagher et al., 2013).
Experimental studies have clearly demonstrated that Hg exposure may modulate manifestation of airborne viral infections. In particular, pretreatment with inorganic Hg significantly aggravated coxsackievirus B3 (CVB3) infection-induced myocarditis characterized by sever macrophage infiltration and proinflammatory cytokine overproduction, whereas posttreatment did not possess similar effect in mice (Nyland et al., 2012; Penta et al., 2015b). It is also notable that coxsackievirus B3 (CB3) infection in mice is accompanied by a twofold increase in brain Hg content, whereas Se levels were found to be elevated only by 36% (Ilbäck et al., 2005). Taken together with a later observed decrease in intestinal, serum, and hepatic Hg levels (Ilbäck et al., 2008), these findings are indicative of redistribution of Hg into brain providing an additional link between viral diseases and neurotoxicity. The observations on positive association between body Hg burden and parenterally-transmitted HIV infection (Emokpae, Mbonu, 2018) also support indirectly the impact of Hg on antiviral immunity.
In view of the role of bats as carriers for SARS-CoV-2 (Docea et al., 2020), it is also noteworthy that high Hg levels in bats are associated with impaired immunity and higher susceptibility to infectious agents (Becker et al., 2017). It is notable that the role of environmental, including heavy metals, and/or pollution may play a significant role in increased susceptibility of host organisms to viral zoonotic infections was also earlier proposed by Rotshild (2015).
The observed interference between Hg toxicity and viral infections may be mediated by immunotoxic effect of the metal (Gardner, Nyland, 2016) (Fig. 3). Particularly, it has been demonstrated that ethylmercury (thiomersal) and methylmercury, but not HgCl2 possessed cytotoxic effect in a human T cell line (Jurkat) (Guzzi et al., 2012).
Notably, Weigand et al. (2015) showed that exposure of T and B cell lines (EL4 T cells and WEHI-231 B cells) to low doses of mercury (II) chloride (HgCl2) during 12 or 96 h induced, in the absence of antigenic stimulation, increased levels of signaling molecules, such as ERK1/2, PKCα and p38MAPK, indicating inappropriate activation of these cells. MeHg exposure in Steller sea lion was shown to alter T and B cell proliferation due to aberrant expression of regulatory cytokines including INF-γ, IL-6, IL-10, and TNF-α (Levin et al., 2020). Increased susceptibility to fungal infections was also associated with reduced number of splenic CD3+CD4+ lymphocytes, as well as Th1 and Th17 cells (Batista-Duharte et al., 2018).
In utero exposure of BALB/c mice to HgCl2 by subcutaneous injection was able to induce a significant upregulation of arginase, IFN-γ, STAT1, vitronectin, and TNF Superfamily Member 1 (TNFSF18) in male offspring. These changes, being more pronounced in males, may modulate the baseline immune response and thus reflect risks in adulthood health (Penta et al., 2015a). At the same time, another study demonstrated that MeHg exposure may result in suppression of interferon-γ (IFN-γ) production in peripheral blood mononuclear cells (De Vos et al., 2007). In a more recent study, male C57BL/6 mice exposed subcutaneously to 25 mg/kg/day of methylmercury chloride presented, after seven days, increased expression of TNF-α -related genes in samples of different tissues, including liver, kidney and brain. The same study which included mouse neural cell line (C17.2 cells) also clarified that NF-κB may participate as a transcription factor in the TNF-α induction (Iwai-Shimada et al., 2016), being indicative of the impact of Hg exposure on systemic inflammation (Maqbool et al., 2017). In addition, Hg-induced inflammation is tightly interrelated with autoimmunity (Pollard et al., 2019) that may also impair physiological immune and inflammatory reactions.
1.4. Lead
1.4.1. Respiratory dysfunction and diseases
Several studies have demonstrated an inverse association between environmental lead exposure levels and impaired respiratory function. Specifically, blood lead Pb levels were shown in Polish schoolchildren to have a negative effect on lung vital capacity (VC) and FVC (Little et al., 2017). Living in an e-waste emission area resulted in a significant increase in blood Pb levels that were inversely associated with FEV1 in children (Zeng et al., 2017). It has been also demonstrated that Pb exposure assessed by urinary metal levels is associated with reduced lung function (FEV1) especially as a result of interaction between Pb exposure and antioxidant (NQO1, NAD(P)H quinone dehydrogenase) gene polymorphisms (Wei et al., 2020). Moreover, of all heavy metals studied (Hg, Cd, Pb) only blood Pb levels were inversely and adversely associated with pulmonary function assessed by FEV1 and FVC (Pan et al., 2020).
Pb exposure is known to play a causal role in respiratory disease (Boskabady et al., 2018). Heavy metals including lead were found to be higher in patients with COPD, being interrelated with pulmonary dysfunction and antioxidant activity (Gogoi et al., 2019). Correspondingly, soil and water lead distribution was shown to be associated with patterns of respiratory disease burden in Iran (Ghias, Mohammadzadeh, 2016).
1.4.2. Mechanisms of respiratory toxicity
Lead was found to be one of the metals primarily accounting for PM toxicity in A549 lung cells (Yuan et al., 2019) due to multiple mechanisms. Briefly, intratracheal instillation of Pb nanoparticles was also shown to induce oxidative stress (Lu et al., 2015) and inflammation resulting in lung damage (Li et al., 2013). It is also notable that lung inflammation and fibrosis in Pb-exposed rats were accompanied by a dose-dependent decrease of lung magnesium (Mg) content (Attafi et al., 2018).
Both oxidative stress and inflammation were linked to apoptotic death of lung cells in a model of Pb-induced lung injury in rats, whereas these effects were ameliorated by AMPK/Nrf2 activation (Lu et al., 2018). In particular, Pb exposure induced apoptosis in A549 cells through up-regulation of p53, bax, caspase 3 and 9 and down-regulation of bcl2 mRNA expression (Ahamed et al., 2019). Pb-induced mitochondrial dysfunction may also underlie proapoptotic signaling in the exposed lung cells (Alarifi et al., 2017). Activation of NF-κB and aryl hydrocarbon receptor (AhR) pathways has been considered as the potential mechanism underlying Pb-induced inflammation and toxicity in A549 lung cells (Attafi et al., 2020) (Fig. 2).
Pb-induced lung toxicity is responsible for lung remodeling. In particular, detailed studies by Kaczyńska et al. (2011, 2013) demonstrated that 5-week lead exposure resulted in infiltration (Kaczyńska et al., 2011), alveolar fibrosis, emphysema, and degeneration of type II pneumocytes (Kaczyńska et al., 2013).
1.4.3. Viral diseases, antiviral immunity and immunotoxicity
Several studies demonstrated a relationship between Pb exposure and susceptibility to viral infections, corroborating its role in suppression of antiviral immunity (Rashed, 2011; Emokpae, Mbonu, 2018; Sahin et al., 2019), although no association between Pb and airborne viral infections exists.
Pb is a known toxicant to many organs, however, its mechanisms of action on the immune system are not well-established (Fenga et al., 2017) (Fig. 3). Briefly, lead exposure was shown to inhibit lymphocyte proliferation along with impaired IL-2 and calmodulin production (Li et al., 2012). In addition, Pb exposure was shown to alter Th1/Th2 ratio in chicken blood lymphocytes (Fu et al., 2019). Lead inhalation in sensitized guinea pigs was shown to alter IFN-γ/IL-4 ratio, being indicative of reduction in Th1/Th2 balance (Boskabady et al., 2012). Pb2+ was also shown to be toxic to both B-lymphocytes and especially macrophages, also resulting in reduced IFN-γ production and causing aberrant immune response to viral and bacterial pathogens (Han et al., 2020). These findings corroborate the observed inverse correlation between blood lead levels and T cell count in Pb-exposed subjects (Mishra et al., 2010). However, Pb exposure due to habitation in e-waste area is directly associated with increased number of memory T cells (Cao et al., 2018). Exposure of chicken neutrophils to Pb2+ resulted in a significant increase in IL-1β, 1R, 4, 8, 10, and 12β levels, whereas IL-2 and IFN-γ were down-regulated (Xing et al., 2018).
Early-life Pb exposure was shown to inhibit stimulated thymocyte and splenocyte proliferation also resulting in a dose-dependent decrease in IL-2, IFN-γ, IL-4, IL-10, and TNF-α secretion (Ajouaoi et al., 2019). In addition, Pb2+-induced thymocyte apoptosis is considered as the potential mechanism of lead immunotoxicity (Nishizaki et al., 2003).
Dvorožňáková et al. (2016) evaluated the effects of Hg, Cd and Pb in the immune response using a parasite-infection model. As regards Pb, BALB/c male mice exposed during 35 days and subsequently infected with Ascaris suum on the 21st day presented increase in the levels of IL-5 and IL-10 cytokine production, but TNF-α and IFN-γ production was suppressed. In this study, continuous intoxication with Pb caused susceptibility to the parasite infection.
1.5. Arsenic
1.5.1. Respiratory dysfunction and diseases
Arsenic (As) exposure is known to cause a wide spectrum of malignant and non-malignant respiratory diseases (Ramsey, 2015). However, even subtoxic doses of arsenic may significantly affect respiratory functions. Particularly, a recent meta-analysis demonstrated that arsenic exposure is associated with restrictive impairments in lung function including reductions in forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) (Sanchez et al., 2018). Another study has demonstrated that As-associated FVC reduction is observed on males (Khan et al., 2020). In addition, overweight and obesity were shown to aggravate As-induced lung dysfunction (Nardone et al., 2017). Moreover, impaired lung function in As-exposed children (Ahmed et al., 2017) and adults (Wang et al., 2020; Shih et al., 2020) was also associated with lung inflammation. Spirometric abnormalities in As-exposed children were also associated with higher matrix metalloproteinase-9 (MMP-9) level in sputum (Olivas-Calderón et al., 2015).
Early-life and lifetime As exposure were shown to be associated with reduced respiratory function, as well as higher incidence of respiratory diseases including chronic cough and chronic bronchitis (Steinmaus et al., 2016), even after adjustment for smoking status (Powers et al., 2018). As exposure was also associated with reversible lung obstruction (Siddique et al., 2020).
Doubling of maternal urinary As levels was associated with increased incidence of infections, respiratory symptoms, diarrhea, and fever resulting in a doctor visit or medication use in first-year children (Farzan et al., 2016), with the strongest relationship observed for lower respiratory tract infections (Farzan et al., 2013). Specifically, increased urinary As concentrations (≥6 μg/L) were associated with a more than twofold increase in pneumonia risk (odds ratio (OR) 95% confidence interval (CI) = 1.88 (1.01, 3.53)) in Bangladeshi children (George et al., 2015).
1.5.2. Mechanisms of respiratory toxicity
As exposure has been shown to increase airway inflammation, reactivity, and remodeling characterized by peribronchial thickening that may be related to NLRP3 inflammasome activation (Surolia et al., 2020). As-induced lung damage was also shown to be associated with apoptotic cell death due to oxidative and endoplasmic reticulum stress (Gu et al., 2016), as well as mitochondrial dysfunction (Mahalanobish et al., 2019). Both inflammatory and prooxidant effects of As exposure in lungs may be related to As-induced up-regulation of NF-kB and MAPK activity, as well as alteration of Nrf2 signaling, respectively (Li et al., 2017a, Li et al., 2017b) (Fig. 2). Particularly, As is capable of up-regulation of IKKα/β and NF-κB p65/50 mRNA and protein expression in parallel with a decrease in IκBα levels in lungs (Hu et al., 2019). It is noteworthy that low-dose As exposure was shown to increase autophagy and inflammation in murine lungs, whereas high-dose As aggravated inflammatory response with weak autophagy activation (Zhao et al., 2019).
Prenatal As exposure was shown to impair expression of genes involved in lung morphogenesis (Sox 2), mucus production (Clca3, Muc5b), and ciliary function (Ttc21a) (Zosky et al., 2014). Low-dose As exposure also resulted in partially reversible repression of MUC5AC and MUC5B at mRNA and protein levels through retinoic acid signaling pathway (Liu et al., 2020). TGF-β/Smad pathway signaling may be also involved in As-induced fibrosis (Dai et al., 2019).
As exposure was also shown to impair lung epithelial barrier function by causing aberrant localization and expression of tight junction proteins claudin and occludin, as well as impairing occludin phosphorylation (Sherwood et al., 2013). These data are in agreement with the results of a more recent study demonstrating increased respiratory epithelial permeability and alveolar epithelial type 1 cell injury (Henderson et al., 2017). As-induced decrease in lysozyme secretion was also observed in human bronchial epithelial cells increasing susceptibility to infectious agents (Goodale et al., 2017).
Chowdhury, T., Roymahapatra, G., & Mandal, S. M. (2020). In Silico Identification of a Potent Arsenic Based Approved Drug Darinaparsin against SARS-CoV-2: Inhibitor of RNA dependent RNA polymerase (RdRp) and Necessary Proteases.
1.5.3. Viral diseases, antiviral immunity and immunotoxicity
In contrast to other heavy metals, the role of As in viral diseases is dual. On the one hand, chronic As exposure is known to be tightly associated with the incidence of viral infections due to its immunotoxic effect, whereas on the other, As compounds are used as the potential antiviral agent also inhibiting SARS-CoV-2 viral proteins as assessed by in silico docking analysis (Chowdhury et al., 2020). At the same time, arsenic exposure was shown to be associated with increased H1N1 influenza virus load, as well as reduction in FEV1 in a primarily adult population (Liao et al., 2011). In turn, the relationship between As body burden and non-airborne hepatitis A (Cardenas et al., 2016), B (Cardenas et al., 2018; Zhang et al., 2018), and E (Heaney et al., 2015) viral infections may indirectly reflect negative impact of As on antiviral immunity.
It is also notable that As toxicity may underlie impaired antiviral response in the organism (Fig. 3). Particularly, it has been demonstrated that urinary As levels were found to be inversely associated with post-immunization mumps–specific IgG levels (Raqib et al., 2017).
Experimental data corroborate clinical findings. For example, in chicks, As exposure significantly increased susceptibility to Newcastle disease virus resulting in higher virus titers and impaired antibody responses to T-dependent antigen (Sattar et al., 2016). These data generally are in agreement with the earlier observation of impaired antiviral response and higher pulmonary virus titer in a model of H1N1 influenza infection in C57BL/6J mice (Kozul et al., 2009). Moreover, chronic arsenite exposure significantly increased susceptibility to H1N1 influenza infection through sialic acid binding as well as decreased efficiency of oseltamivir (Amouzougan et al., 2020). In addition, prenatal As exposure significantly aggravated influenza A-induced pulmonary inflammation (Ramsey et al., 2013).
An interesting study by Benyamin et al. (2006) demonstrated that coxsackievirus B3 infection in Balb/c mice results in reduced serum, liver, spleen, heart, pancreas, and kidney As levels along with clinical manifestation of the disease (Benyamin et al., 2006).
Nonetheless, several studies demonstrate potential antiviral activity of As compounds. Particularly, As2O3 exposure significantly inhibits growth of human T-cell leukemia virus-infected T-cell lines through induction of apoptosis and cell cycle arrest (Ishitsuka et al., 2000). Similarly, As administration was also shown to inhibit Epstein-Barr Virus replication and stimulates cell death in EBV-positive cells (Yin et al., 2017). Due to the observed effects of As exposure on viral agents of leukemia, As and IFN-α is widely used as an antileukemic agent (Hachiman et al., 2018). At the same time, As2O3 was also shown to reduce susceptibility to Simian immunodeficiency virus after provirus reactivation in macaques (Yang et al., 2019b). Earlier studies have also demonstrated an inhibitory effect of As2O3 on HCV replication (Hwang et al., 2004). Moreover, the results of in silico docking analysis demonstrated that certain arsenicals were capable of inhibiting SARS-CoV-2 RNA dependent RNA polymerase thus decreasing viral replication (Chowdhury et al., 2020), although in vitro and in vivo data are highly required to support this hypothesis.
Generally, the observed difference in As activity is expected to be related to the mode of exposure, where long-term As exposure affects immunity and increases susceptibility to viral agents, whereas acute arsenic exposure may possess antiviral effect due to cytotoxic effect on virus-infected cell.
Among its adverse outcomes, epidemiological, in vitro and in vivo evidences indicate that arsenic is an immunotoxic compound, acting as both immune system suppressor and stimulator mechanisms (Ferrario et al., 2016). As was shown to impair proliferation, differentiation, and activation of macrophages, dendritic cells, and T-cells (Bellamri et al., 2018), especially T regulatory cells (Haque et al., 2017). Particularly, male BALB/c mice exposed to 0.038, 0.38 and 3.8 ppm sodium arsenite for 7, 15 and 30 days through oral gavage showed a significant dose-dependent increase in the ThPOK expression levels in thymus, suggesting an impairment in the regulation of CD4+ T cells differentiation. In the spleen, As increased the number of CD4+ T cells and promoted their differentiation into Treg cells, however, a low secretion of IFN-γ, TNF-α, IL-12, IL-4, IL-5 and IL-10 cytokines was observed in splenocytes (Gera et al., 2017). Activation of NF-kB and downstream signaling was shown to induce As-associated immune suppression (Choudhury et al., 2016).
As2O3 exposure resulted in a significant Nrf2-independent inhibition of both mRNA and protein production of IFNγ, IL-2, and GM-CSF in splenocytes (VanDenBerg et al., 2017). It has been also demonstrated that As2O3 inhibited IFN-α secretion in plasmacytoid dendritic cells (Ye et al., 2020).
Moreover, another study with lymphocytes isolated from the blood of healthy people, showed that exposure to As at a concentration range of 0.05–50 μM for 12 h was able to induce apoptosis mainly through enhancement of intracellular calcium import which causes oxidative stress. Moreover, cellular proteolysis, activation of caspase-3 and lipid peroxidation with 2, 4 or 6 h of exposure, and stimulation of cytokines (IL-2, INF-γ and TNF- α) production after an exposure of 24 h was also associated with As-induced toxicity in the isolated lymphocytes (Zarei et al., 2019). Overall, these findings indicate that ROS generation followed by inflammation play a crucial role in the cytotoxicity. The alteration of cytokine levels found after As exposure can reflect a commitment of lymphocytes, which in turn impairs immune system in fighting against infection (Zarei et al., 2019). In parallel with impaired lymphocyte functioning, As toxicity is also associated with chronic inflammation and reduced phagocytic receptors—Fcγ and complement receptors (CR) (Prasad, Sinha, 2017). Correspondingly, As exposure was shown to affect innate immunity factors in children (Parvez et al., 2019).
1.6. Toxic metals in internal risk factors for COVID-19 severity
In parallel with respiratory dysfunction, certain diseases are also considered as the most significant co-morbidity risk factors for COVID-19 severity, including obesity, diabetes, cardiovascular diseases (hypertension), and cancer. These diseases may also provide an additional link between COVID-19 severity and heavy metal toxicity. Earlier data demonstrate that obesity and diabetes may be associated with Hg (Tinkov et al., 2015; Roy et al., 2017), Сd (Satarug et al., 2010; Tinkov et al., 2017), Pb (Park et al., 2017; Leff et al., 2017), and As (Farkhondeh et al., 2019). Although these associations are rather contradictory and the underlying mechanisms are still unclear, it is proposed that the role of metals and endocrine disruptors may significantly contribute to metabolic disorders. Heavy metal pollution was also demonstrated to be significantly associated with cardiovascular morbidity predominantly through interference with atherogenesis (Solenkova et al., 2014). Particularly, Cd (Tellez-Plaza et al., 2013; Tinkov et al., 2018a), Hg (Houston, 2011; Genchi et al., 2017), Pb (Poręba et al., 2011; Xu et al., 2017a, Xu et al., 2017b, Xu et al., 2017c), and As (Moon et al., 2017; Navas-Acien et al., 2019) exposure levels were directly associated with prevalence of a wide range of cardiovascular diseases including atherosclerosis, coronary heart disease, hypertension, myocardial infarction, stroke, etc. Finally, As and Cd are considered as Group I carcinogens by International Agency for Research on Cancer (IARC).
2. Conclusion
Taken together, the existing data demonstrate that As, Cd, Hg, and Pb exposure is associated with respiratory dysfunction (reduced FVC, FEV1) and respiratory diseases (COPD, bronchitis). These observations corroborate laboratory findings on the role of heavy metal exposure in impaired mucociliary clearance, reduced barrier function, airway inflammation, oxidative stress, and apoptosis. Both clinical and laboratory studies have shown the association between heavy metal exposure and severity of viral diseases, including influenza and respiratory syncytial virus. The latter may be considered a consequence of adverse effects of metal exposure on adaptive immunity (Table 1 ).
Table 1.
A brief summary of the effects of cadmium, mercury, lead, and arsenic on lung dysfunction, mechanisms of lung toxicity, and immunopathology.
| Variable | Patterns | References |
|---|---|---|
| Cadmium (Cd) | ||
| Respiratory function | ↓ FEV1 (current and former smokers) | Mannino et al., 2004 |
| ↓ FEV1, FVC (children) | Zeng et al., 2017 | |
| ↓ FVC, FEV1, FEV1/FVC, PEF (welders) |
Cetintepe et al., 2019; Torén et al., 2019 |
|
| Respiratory diseases | Chronic obstructive pulmonary disease | Oh et al., 2014; Sundblad et al., 2016 |
| Wheezing |
Razi et al., 2012; Yang et al., 2019a |
|
| Lung toxicity mechanisms | Endoplasmic reticulum stress | Kim et al., 2017 |
| Apoptosis | Heo et al., 2017a, Heo et al., 2017b,Kiran Kumar et al., 2016 Li et al., 2019 | |
| Fibrosis | Li et al., 2017a, Li et al., 2017b,Hu et al., 2017,Kim et al., 2018 | |
| ↓ impaired mucociliary clearance | Xiong et al., 2019 | |
| ↓ barrier function and tight junction proteins |
Cao et al., 2015 |
|
| Respiratory viral infections | Respiratory syncytial virus (mice) | Go et al., 2018 |
| H1N1 infection (mice) | Chandler et al., 2019 | |
| influenza (MCDK cells) |
Checconi et al., 2013 |
|
| Immune cell effects | ↓ T-helper (CD4+); ↑ cytotoxic T cells (CD8+) (mice) | Pathak, Khandelwal, 2008 |
| ↓ splenic NK-cells; ↓ granulocytes (mice) | Holásková et al., 2012 | |
| ↑ increased CD4+; CD8+ T cells; CD45R/B220+ B cells (mice) | Holásková et al., 2012 | |
| ↓ T lymphocyte (children) | Zeng et al., 2020 | |
| ↓ memory T cell (children) |
Nygaard et al., 2017 |
|
| Cytokine response | ↓ IFN-γ; IL-2 (mice) | Pathak, Khandelwal, 2008 |
| ↑ IFN-γ; IL-10 (rats) (activated T cells) | Turley et al. 2019 | |
| ↑ TNF-α; Nrf2; NF-κB (zebrafish) | Zheng et al. 2016 | |
| ↓ IL-6; IL-10; IL-1β; TNF-α (zebrafish) | Guo et al. 2017 | |
| ↑ IL-1β; CXCL1; ↓ IL-6; IL-10 (RAW 264.7 macrophages) |
Riemschneider et al. 2015 |
|
| Mercury (Hg) | ||
| Respiratory function |
↓FVC, FEV1 (artisanal and small-scale gold mining area) |
Pateda et al., 2018 |
| Respiratory diseases | COPD (dusty area) | Heo et al., 2017a, Heo et al., 2017b |
| Asthma and bronchi hyperresponsiveness (children) | Kim et al., 2015 | |
| Recurrent wheezing (children) | Razi et al., 2011 | |
| Lower respiratory infections (infants) |
Emeny et al., 2019 |
|
| Lung toxicity mechanisms | Oxidative stress | Yu et al., 2015; Park, Park, 2007; Han et al., 2007 |
| Apoptosis | Yu et al., 2015; Park, Park, 2007; Ali et al., 2018; Lu et al., 2010 | |
| Endoplasmic reticulum stress | Jagannathan et al., 2017 | |
| Inflammation |
Liu et al., 2003,Naidoo et al., 2019 |
|
| Respiratory viral infections | Measles (children) | Gallagher et al., 2011 |
| Rubeola (children) | Gallagher et al., 2013 | |
| Coxsackievirus b3 (mice) | Nyland et al., 2012; Penta et al., 2015b | |
| Coxsackievirus b3 (mice) |
Ilbäck et al., 2005,Ilbäck et al., 2008 |
|
| Immune cell effects | ↓T cell (Jurkat cell line) | Guzzi et al., 2012 |
| ↑ T cell and ↓ B cell proliferation (Steller sea lion) | Levin et al., 2020 | |
| ↓CD3+CD4+; Th1; Th17 cells (mice) |
Batista-Duharte et al., 2018 |
|
| Cytokine response | ↓ INF-γ, IL-6, IL-10, and TNF-α (Steller sea lion) | Levin et al., 2020 |
| ↓ interferon-γ (IFN-γ) | De Vos et al., 2007 | |
| ↑ TNF-α -related gene expression (mice) | Iwai-Shimada et al. 2016 | |
| ↑ NF-κB (C17.2 cells) | Iwai-Shimada et al. 2016 | |
| Lead (Pb) | ||
| Respiratory function | ↓VC; FVC Polish schoolchildren | Little et al., 2017 |
| FEV1 children e-waste emission area | Zeng et al., 2017 | |
| FEV1 | Wei et al., 2020 | |
| FEV1 and FVC |
Pan et al., 2020 |
|
| Respiratory diseases | COPD | Gogoi et al., 2019 |
| Total |
Ghias, Mohammadzadeh, 2016 |
|
| Lung toxicity mechanisms | Oxidative stress | Lu et al., 2015 |
| Inflammation | Kaczyńska et al., 2011; Li et al., 2013; Attafi et al., 2018 | |
| Fibrosis | Kaczyńska et al., 2013; Attafi et al., 2018 | |
| Apoptosis | Alarifi et al., 2017; Lu et al., 2018; Ahamed et al., 2019 | |
| Mitochondrial dysfunction |
Alarifi et al., 2017 |
|
| Respiratory viral infections |
- |
- |
| Immune cell effects | ↓ lymphocyte proliferation (mice) | Li et al., 2012 |
| ↓ T cell (Pb-exposed subjects) | Mishra et al., 2010 | |
| Altered Th1/Th2 ratio (chicken) | Fu et al., 2019 | |
| ↑ memory T cells (children, e-waste-recycling area) |
Cao et al., 2018 |
|
| Cytokine response | ↓ IFN-γ (Macrophage and B-cell lines) | Han et al., 2020 |
| ↑ NF-κB, AhR (A549 lung cells) | Attafi et al., 2020 | |
| ↑ IL-1β, 1R, 4, 8, 10, 12β; ↓IL-2, IFN-γ (chicken) | Xing et al., 2018 | |
| ↓ IL-2, IFN-γ, IL-4, IL-10, TNF-α (mice) | Ajouaoi et al., 2019 | |
| ↑ IL-5, IL-10; ↓TNF-α, IFN-γ (mice) | Dvorožňáková et al., 2016 | |
| Arsenic (As) | ||
| Respiratory function | ↓ FEV1, FVC (meta-analysis) | Sanchez et al., 2018 |
| ↓ FVC (early life exposed subjects) | Khan et al., 2020 | |
| ↓ FVC; ↑ cough, shortness of breath (adults, Northern Chile) | Nardone et al., 2017 | |
| ↓ FEV1, FVC (As-exposed children) | Olivas-Calderón et al., 2015; Ahmed et al., 2017 | |
| ↓ FEV1, FEV1/FVC, FEF75 (coal-burning areas) |
Wang et al., 2020 |
|
| Respiratory diseases | Chronic bronchitis | Steinmaus et al., 2016; Powers et al., 2018 |
| Reversible lung obstruction | Siddique et al., 2020 | |
| Lower respiratory tract infections | Farzan et al., 2013 | |
| Pneumonia |
George et al., 2015 |
|
| Lung toxicity mechanisms | Inflammation | Surolia et al., 2020; Zhao et al., 2019; Hu et al., 2019 |
| Altered lung morphogenesis, ciliary function | Zosky et al., 2014 | |
| Fibrosis | Dai et al., 2019 | |
| Altered barrier function and tight junction proteins | Sherwood et al., 2013 | |
| Impaired respiratory epithelial permeability |
Henderson et al., 2017 |
|
| Respiratory viral infections | H1N1 influenza (adults) | Liao et al., 2011 |
| Newcastle disease virus (chicks) | Sattar et al., 2016 | |
| H1N1 influenza (mice) | Kozul et al., 2009; Amouzougan et al., 2020 | |
| Influenza A (mice) | Ramsey et al., 2013 | |
| Coxsackievirus B3 (mice) |
Benyamin et al., 2006 |
|
| Immune cell effects | ↓ human T-cell growth | Ishitsuka et al., 2000 |
| ↓ macrophages, dendritic cells, and T-cells | Bellamri et al., 2018 | |
| ↓ T regulatory cells | Haque et al., 2017 | |
| ↑ CD4+ T cells and Treg differentiation |
Gera et al. 2017 |
|
| Cytokine response | ↓ IFN-γ, TNF-α, IL-12, IL-4, IL-5 and IL-10 (splenocytes mice) | Gera et al. 2017 |
| ↑ NF-kB, MAPK | Choudhury et al., 2016; Li et al., 2017a, Li et al., 2017b; Hu et al., 2019 | |
| ↓ IFNγ, IL-2, GM-CSF (splenocytes mice) | VanDenBerg et al., 2017 | |
| ↓ IFN-α (plasmacytoid dendritic cells) | Ye et al., 2020 | |
| ↑ IL-2, INF-γ, TNF- α (human lymphocytes) | Zarei et al. 2019 | |
AhR - Aryl hydrocarbon receptor; COPD - Chronic obstructive pulmonary disease; CXCL1 - chemokine (C-X-C motif) ligand 1; FEF75 - forced expiratory flow at 75% of FVC; FEV1 - Forced Expiratory Volume in one second; FVC - Forced Vital Capacity; IFN-γ - interferon-γ; IL – interleukin; MAPK - Mitogen-activated protein kinase; NF-κB – nuclear factor κB; Nrf2 - Nuclear factor erythroid 2-related factor 2; PEF - Peak expiratory flow; TNF-α - Tumor necrosis factor-α; VC – vital capacity
Although direct data linking heavy metal exposure and COVID-19 risk and/or severity are lacking, reduction in heavy metal emissions may significantly reduce lung immunopathology and inflammation, both of which are known to increase the risk of respiratory viral and bacterial diseases. In addition, avoiding heavy metal exposure at the individual level through the use of respirator masks or portable air filters, especially in highly-polluted environments including metropoles, could also contribute to reduction of risk of infection and its severity. The use of zinc, selenium, or other functional antagonists of heavy metals may also prevent adverse effects of heavy metals on respiratory system and immunity. At the same time, both epidemiological and laboratory studies are urgently required to characterize the direct association between heavy metal exposure and COVID-19 risk and pathogenetic mechanisms.
CRediT authorship contribution statement
Anatoly V. Skalny: Supervision, Conceptualization. Thania Rios Rossi Lima: Writing - original draft, Data curation, Formal analysis, Investigation, Visualization. Tao Ke: Writing - original draft, Data curation, Formal analysis, Formal analysis, Investigation, Investigation. Ji-Chang Zhou: Writing - original draft, Data curation. Julia Bornhorst: Writing - original draft, Data curation, Formal analysis, Investigation, Visualization. Svetlana I. Alekseenko: Writing - original draft, Data curation, Formal analysis, Investigation. Jan Aaseth: Writing - review & editing, Supervision, Conceptualization. Ourania Anesti: Writing - original draft, Data curation, Formal analysis, Investigation. Dimosthenis A. Sarigiannis: Writing - original draft, Data curation, Formal analysis, Investigation. Aristides Tsatsakis: Writing - review & editing, Supervision, Conceptualization. Michael Aschner: Writing - review & editing, Supervision, Conceptualization. Alexey A. Tinkov: Writing - review & editing, Writing - original draft, Data curation, Formal analysis, Formal analysis, Investigation, Investigation, Conceptualization, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was performed with the support of the Russian Ministry of Science and Higher Education, Project № 0856-2020-00082. MA was supported in part by grants from the National Institute of Environmental Health Sciences (NIEHS) R01ES07331 and R01ES105633. We further thank the German Research Foundation (DFG), DFG Research Unit FOR 2558.
References
- Ahamed M., Akhtar M.J., Alhadlaq H.A. Preventive effect of TiO2 nanoparticles on heavy metal Pb-induced toxicity in human lung epithelial (A549) cells. Toxicol. Vitro. 2019;57:18–27. doi: 10.1016/j.tiv.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Akhtar E., Roy A., von Ehrenstein O.S., Vahter M., Wagatsuma Y., Raqib R. Arsenic exposure alters lung function and airway inflammation in children: a cohort study in rural Bangladesh. Environ. Int. 2017;101:108–116. doi: 10.1016/j.envint.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Ajouaoi S., Bouchmaa N., Idir A., Mernari O., Mouse H.A., Zyad A. Treatment with lead chloride during pregnancy and the postnatal period alters cell proliferation and immune function in Swiss albino mice. Biol. Trace Elem. Res. 2019;1–9 doi: 10.1007/s12011-019-01917-x. [DOI] [PubMed] [Google Scholar]
- Alarifi S., Ali D., Alkahtani S. Involvement of mitochondrial dysfunction in nanosized lead oxide induced cellular damage in human lung alveolar epithelial cells. Toxicol. Environ. Chem. 2017;99(4):680–690. [Google Scholar]
- Ali H.M. Mitigative role of garlic and vitamin E against cytotoxic, genotoxic, and apoptotic effects of lead acetate and mercury chloride on WI-38 cells. Pharmacol. Rep. 2018;70(4):804–811. doi: 10.1016/j.pharep.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Amouzougan E.A., Lira R., Jr., Klimecki W.T. Chronic exposure to arsenite enhances influenza virus infection in cultured cells. J. Appl. Toxicol. 2020;40(4):458–469. doi: 10.1002/jat.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attafi I.M., Korashy H.M., Al Bakheet S.A., Abu Jabal K.A., Belali O.M. Investigation of the impact of Lead exposure on the inorganic and organic compounds profile of lung tissue in a rat model. Faseb. J. 2018;32(1_Suppl. ment) 548-11. [Google Scholar]
- Attafi I.M., Bakheet S.A., Korashy H.M. The role of NF-κB and AhR transcription factors in lead-induced lung toxicity in human lung cancer A549 cells. Toxicol. Mech. Methods. 2020;30(3):197–207. doi: 10.1080/15376516.2019.1687629. [DOI] [PubMed] [Google Scholar]
- Batista-Duharte A., Téllez-Martínez D., Aparecida Jellmayer J., Leandro Portuondo Fuentes D., Campos Polesi M., Martins Baviera A., Zeppone Carlos I. Repeated exposition to mercury (II) chloride enhances susceptibility to S. schenckii sensu stricto infection in mice. J. Fungi. 2018;4(2):64. doi: 10.3390/jof4020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D.J., Chumchal M.M., Bentz A.B., et al. Predictors and immunological correlates of sublethal mercury exposure in vampire bats. R. Soc. Open Sci. 2017;4(4):170073. doi: 10.1098/rsos.170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamri N., Morzadec C., Fardel O., Vernhet L. Arsenic and the immune system. Curr. Opin. Toxicol. 2018;10:60–68. [Google Scholar]
- Benyamin G., Lindh U., Frisk P., Friman G., Ilbäck N.G. Arsenic is decreased in target organs during viral infection in mice. J. Trace Elem. Med. Biol. 2006;20(2):121–126. doi: 10.1016/j.jtemb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Berlin I., Thomas D., Le Faou A.L., Cornuz J. Nicotine Tob. Res; 2020. COVID-19 and Smoking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady M.H., Samarghandian S., Farkhondeh T. Tracheal responsiveness to methacholine and ovalbumin; and lung inflammation in Guinea pigs exposed to inhaled lead after sensitization. Ecotox. Environ. Safe. 2012;86:233–238. doi: 10.1016/j.ecoenv.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Boskabady M., Marefati N., Farkhondeh T., Shakeri F., Farshbaf A., Boskabady M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018;120:404–420. doi: 10.1016/j.envint.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J. Clin. Med. 2020. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Lin H., Muskhelishvili L., Latendresse J., Richter P., Heflich R.H. Tight junction disruption by cadmium in an in vitro human airway tissue model. Respir. Res. 2015;16(1):30. doi: 10.1186/s12931-015-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Xu X., Zhang Y., Zeng Z., Hylkema M.N., Huo X. Increased memory T cell populations in Pb-exposed children from an e-waste-recycling area. Sci. Total Environ. 2018;616:988–995. doi: 10.1016/j.scitotenv.2017.10.220. [DOI] [PubMed] [Google Scholar]
- Cardenas A., Smit E., Bethel J.W., Houseman E.A., Kile M.L. Arsenic exposure and the seroprevalence of total hepatitis A antibodies in the US population: NHANES, 2003–2012. Epidemiol. Infect. 2016;144(8):1641–1651. doi: 10.1017/S0950268815003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A., Smit E., Welch B.M., Bethel J., Kile M.L. Cross sectional association of arsenic and seroprevalence of hepatitis B infection in the United States (NHANES 2003–2014) Environ. Res. 2018;166:570–576. doi: 10.1016/j.envres.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetintepe S.P., Iritas S.B., Gunduzoz M., et al. Relation between lung dysfunction and blood cadmium and lead levels among welders. Expos. Health. 2019;11(1):13–19. [Google Scholar]
- Chandler J.D., Hu X., Ko E.J., et al. Low-dose cadmium potentiates lung inflammatory response to 2009 pandemic H1N1 influenza virus in mice. Environ. Int. 2019;127:720–729. doi: 10.1016/j.envint.2019.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checconi P., Sgarbanti R., Celestino I., et al. The environmental pollutant cadmium promotes influenza virus replication in MDCK cells by altering their redox state. Int. J. Mol. Sci. 2013;14(2):4148–4162. doi: 10.3390/ijms14024148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.C., Lippmann M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal. Toxicol. 2009;21(1):1–31. doi: 10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- Choudhury S., Gupta P., Ghosh S., Mukherjee S., Chakraborty P., Chatterji U., Chattopadhyay S. Arsenic-induced dose-dependent modulation of the NF-κB/IL-6 axis in thymocytes triggers differential immune responses. Toxicology. 2016;357:85–96. doi: 10.1016/j.tox.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Chowdhury T., Roymahapatra G., Mandal S.M. In silico identification of a potent arsenic based approved Drug Darinaparsin against SARS-CoV-2: inhibitor of RNA dependent RNA polymerase (RdRp) and necessary Proteases. 2020. https://chemrxiv.org/articles/In_Silico_Identification_of_a_Potent_Arsenic_Based_Approved_Drug_Darinaparsin_against_SARS-CoV-2_Inhibitor_of_RNA_dependent_RNA_polymerase_RdRp_and_Necessary_Proteases/12200495 available at: [DOI] [PubMed]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;114465 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D., Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere 2020. 2020;11(4):377. doi: 10.3390/atmos11040377. [DOI] [Google Scholar]
- Dai J., Xu M., Zhang X., Niu Q., Hu Y., Li Y., Li S. Bi-directional regulation of TGF-β/Smad pathway by arsenic: a systemic review and meta-analysis of in vivo and in vitro studies. Life Sci. 2019;220:92–105. doi: 10.1016/j.lfs.2019.01.042. [DOI] [PubMed] [Google Scholar]
- Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Canc. Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos G., Abotaga S., Liao Z., Jerschow E., Rosenstreich D. Selective effect of mercury on Th2-type cytokine production in humans. Immunopharmacol. Immunotoxicol. 2007;29(3–4):537–548. doi: 10.1080/08923970701690993. [DOI] [PubMed] [Google Scholar]
- Docea A.O., Tsatsakis A., Albulescu D., et al. A new threat from an old enemy: Re-emergence of coronavirus. Int. J. Mol. Med. 2020;45(6):1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Dvorožňáková E., Dvorožňáková M., Šoltys J. Heavy metal intoxication compromises the host cytokine response in Ascaris suum model infection. Helminthologia. 2016;53(1):14–23. [Google Scholar]
- Emeny R.T., Korrick S.A., Li Z., et al. Prenatal exposure to mercury in relation to infant infections and respiratory symptoms in the New Hampshire Birth Cohort Study. Environ. Res. 2019;171:523–529. doi: 10.1016/j.envres.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emokpae M.A., Mbonu I. Blood levels of some toxic metals in human immunodeficiency virus (HIV) type 1-infection. Ann. Health Res. 2018;4(1):75–81. [Google Scholar]
- Farkhondeh T., Samarghandian S., Azimi‐Nezhad M. The role of arsenic in obesity and diabetes. J. Cell. Physiol. 2019;234(8):12516–12529. doi: 10.1002/jcp.28112. [DOI] [PubMed] [Google Scholar]
- Farsalinos K., Niaura R., Le Houezec J., Barbouni A., Tsatsakis A., Kouretas D., Vantarakis A., Poulas K. Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicology Reports. 2020;7:658–663. doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Barbouni A., Poulas K., Polosa R., Caponnetto P., Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2020;11 doi: 10.1177/2040622320935765. 2040622320935765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan S.F., Korrick S., Li Z., et al. In utero arsenic exposure and infant infection in a United States cohort: a prospective study. Environ. Res. 2013;126:24–30. doi: 10.1016/j.envres.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan S.F., Li Z., Korrick S.A., et al. Infant infections and respiratory symptoms in relation to in utero arsenic exposure in a US cohort. Environ. Health Perspect. 2016;124(6):840–847. doi: 10.1289/ehp.1409282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenga C., Gangemi S., Salvatore V.D., Falzone L., Libra M. Immunological effects of occupational exposure to lead (Review) Mol. Med. Rep. 2017;15:3355–3360. doi: 10.3892/mmr.2017.6381. [DOI] [PubMed] [Google Scholar]
- Ferrario D., Gribaldo L., Hartung T. Arsenic exposure and immunotoxicity: a review including the possible influence of age and sex. Curr Envir Health Rpt. 2016;3:1–12. doi: 10.1007/s40572-016-0082-3. [DOI] [PubMed] [Google Scholar]
- Frutos R., Lopez Roig M., Serra-Cobo J., Devaux C.A. COVID-19: the conjunction of events leading to the coronavirus pandemic and lessons to learn for future threats. Front. Med. 2020;7:223. doi: 10.3389/fmed.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Yang T., Wang W., Xu S. Effect of selenium antagonist lead-induced damage on Th1/Th2 imbalance in the peripheral blood lymphocytes of chickens. Ecotoxicol. Environ. Saf. 2019;175:74–82. doi: 10.1016/j.ecoenv.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Gallagher C.M., Smith D.M., Meliker J.R. Total blood mercury and serum measles antibodies in US children, NHANES 2003–2004. Sci. Total Environ. 2011;410:65–71. doi: 10.1016/j.scitotenv.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Gallagher C.M., Smith D.M., Golightly M.G., Meliker J.R. Total blood mercury and rubella antibody concentrations in US children aged 6–11 years, NHANES 2003–2004. Sci. Total Environ. 2013;442:48–55. doi: 10.1016/j.scitotenv.2012.09.041. [DOI] [PubMed] [Google Scholar]
- Ganguly K., Levänen B., Palmberg L., Åkesson A., Lindén A. Cadmium in tobacco smokers: a neglected link to lung disease? Eur. Respir. Rev. 2018;27(147) doi: 10.1183/16000617.0122-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R.M., Nyland J.F. Environmental Influences on the Immune System. Springer; Vienna: 2016. Immunotoxic effects of mercury; pp. 273–302. [Google Scholar]
- Genchi G., Sinicropi M.S., Carocci A., Lauria G., Catalano A. Mercury exposure and heart diseases. Int. J. Environ. Res. Publ. Health. 2017;14(1):74. doi: 10.3390/ijerph14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C.M., Brooks W.A., Graziano J.H. Arsenic exposure is associated with pediatric pneumonia in rural Bangladesh: a case control study. Environ. Health. 2015;14(1):83. doi: 10.1186/s12940-015-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera R., Singh V., Mitra S., et al. Arsenic exposure impels CD4 commitment in thymus and suppress T cell cytokine secretion by increasing regulatory T cells. Sci. Rep. 2017;7:7140. doi: 10.1038/s41598-017-07271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghias M., Mohammadzadeh N. Relationship of respiratory diseases and the lead level in tiran & karvan region, Iran. Int. Arch. Health Sci. 2016. 2016;3(3):145–149. [Google Scholar]
- Go Y.M., Hu X., Kim K.H. Environmental cadmium enhances respiratory syncytial virus infection-caused lung injury via mitochondrial metabolic disruption and oxidative stress. Free Radic. Biol. Med. 2018;128:S26–S27. [Google Scholar]
- Gogoi K., Manna P., Dey T., Kalita J., Unni B.G., Ozah D., Baruah P.K. Circulatory heavy metals (cadmium, lead, mercury, and chromium) inversely correlate with plasma GST activity and GSH level in COPD patients and impair NOX4/Nrf2/GCLC/GST signaling pathway in cultured monocytes. Toxicol. Vitro. 2019;54:269–279. doi: 10.1016/j.tiv.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Goodale B.C., Rayack E.J., Stanton B.A. Arsenic alters transcriptional responses to Pseudomonas aeruginosa infection and decreases antimicrobial defense of human airway epithelial cells. Toxicol. Appl. Pharmacol. 2017;331:154–163. doi: 10.1016/j.taap.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Chen C., Jiang X., Zhang Z. ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis induced by resveratrol and arsenic trioxide in A549 cells. Chem. Biol. Interact. 2016;245:100–109. doi: 10.1016/j.cbi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S.-N., Zheng J.-L., Yuan S.-S., Zhu Q.-L., Wu C.-W. Immunosuppressive effects and associated compensatory responses in zebrafish after full life-cycle exposure to environmentally relevant concentrations of cadmium. Aquat. Toxicol. 2017;188:64–71. doi: 10.1016/j.aquatox.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Guzzi G., Pigatto P.D., Spadari F., La Porta C.A. Effect of thimerosal, methylmercury, and mercuric chloride in Jurkat T Cell Line. Interdiscipl. Toxicol. 2012;5(3):159–161. doi: 10.2478/v10102-012-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiman M., Yoshimitsu M., Ezinne C., Kuroki A., Kozako T., Arima N. In vitro effects of arsenic trioxide, interferon α and zidovudine in adult T cell leukemia/lymphoma cells. Oncol. Lett. 2018;16(1):1305–1311. doi: 10.3892/ol.2018.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Kivimäki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.G., Castranova V., Vallyathan V. Comparative cytotoxicity of cadmium and mercury in a human bronchial epithelial cell line (BEAS-2B) and its role in oxidative stress and induction of heat shock protein 70. J. Toxicol. Environ. Health Part A. 2007;70(10):852–860. doi: 10.1080/15287390701212695. [DOI] [PubMed] [Google Scholar]
- Han B., García‐Mendoza D., van den Berg H., van den Brink N.W. Modulatory effects of Pb2+ on virally challenged chicken macrophage (HD‐11) and B‐lymphocyte (DT40) cell lines in vitro. Environ. Toxicol. Chem. 2020;39:1060–1070. doi: 10.1002/etc.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R., Chaudhary A., Sadaf N. Immunomodulatory role of arsenic in regulatory T cells. Endocr. Metab. Immune disord. Drug targets (formerly current Drug targets-immune. Endocrine & Metabolic Disorders) 2017;17(3):176–181. doi: 10.2174/1871530317666170818114454. [DOI] [PubMed] [Google Scholar]
- Heaney C.D., Kmush B., Navas-Acien A., et al. Arsenic exposure and hepatitis E virus infection during pregnancy. Environ. Res. 2015;142:273–280. doi: 10.1016/j.envres.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J., Guo F., Trepka M.J. Brief report: low-level mercury exposure and risk of asthma in school-age children. Epidemiology. 2017;28(1):116–118. doi: 10.1097/EDE.0000000000000576. [DOI] [PubMed] [Google Scholar]
- Henderson M.W., Madenspacher J.H., Whitehead G.S., Thomas S.Y., Aloor J.J., Gowdy K.M., Fessler M.B. Effects of orally ingested arsenic on respiratory epithelial permeability to bacteria and small molecules in mice. Environ. Health Perspect. 2017;125(9) doi: 10.1289/EHP1878. 097024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo H.R., Kim J., Kim J.Y., et al. Evaluation of cadmium cytotoxicity using alveolar epithelial cells derived from human induced pluripotent stem cells. Eur. Respir. J. 2017;50:PA3912. [Google Scholar]
- Heo J., Park H.S., Hong Y., et al. Serum heavy metals and lung function in a chronic obstructive pulmonary disease cohort. J. Toxicol. Environ. Health Sci. 2017;9(1):30–35. [Google Scholar]
- Holásková I., Elliott M., Hanson M.L., Schafer R., Barnett J.B. Prenatal cadmium exposure produces persistent changes to thymus and spleen cell phenotypic repertoire as well as the acquired immune response. Toxicol. Appl. Pharmacol. 2012;265(2):181–189. doi: 10.1016/j.taap.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein-Khannazer N., Azizi G., Eslami S., et al. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2020;42(1):1–8. doi: 10.1080/08923973.2019.1697284. [DOI] [PubMed] [Google Scholar]
- Houston M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011;13(8):621–627. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Fernandes J., Jones D.P., Go Y.M. Cadmium stimulates myofibroblast differentiation and mouse lung fibrosis. Toxicology. 2017;383:50–56. doi: 10.1016/j.tox.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Wei M., Niu Q., et al. Grape seed proanthocyanidin extract alleviates arsenic-induced lung damage through NF-κB signaling. Exp. Biol. Med. 2019;244(3):213–226. doi: 10.1177/1535370219829881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Li J., Lou B., et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules. 2020;10(2):240. doi: 10.3390/biom10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Xie J., Cui X., et al. Association between concentrations of metals in urine and adult asthma: a case-control study in Wuhan, China. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0155818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson D., Müller J., McCarthy J.E., et al. Cadmium nanoparticles citrullinate cytokeratins within lung epithelial cells: cadmium as a potential cause of citrullination in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:441. doi: 10.2147/COPD.S152028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D.R., Tsai Y.C., Lee J.C., et al. Inhibition of hepatitis C virus replication by arsenic trioxide. Antimicrob. Agents Chemother. 2004;48(8):2876–2882. doi: 10.1128/AAC.48.8.2876-2882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilbäck N.G., Lindh U., Minqin R., Friman G., Watt F. Selenium and mercury are redistributed to the brain during viral infection in mice. Biol. Trace Elem. Res. 2005;108(1–3):215–224. doi: 10.1385/BTER:108:1-3:215. [DOI] [PubMed] [Google Scholar]
- Ilbäck N.G., Frisk P., Tallkvist J., Gadhasson I.L., Blomberg J., Friman G. Gastrointestinal uptake of trace elements are changed during the course of a common human viral (Coxsackievirus B3) infection in mice. J. Trace Elem. Med. Biol. 2008;22(2):120–130. doi: 10.1016/j.jtemb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ishitsuka K., Hanada S., Uozumi K., Utsunomiya A., Arima T. Arsenic trioxide and the growth of human T-cell leukemia virus type I infected T-cell lines. Leuk. Lymphoma. 2000;37(5–6):649–655. doi: 10.3109/10428190009058521. [DOI] [PubMed] [Google Scholar]
- Iwai-Shimada M., Takahashi T., Kim M.-S., Fujimura M., Ito H., Toyama T., Naganuma A., Hwang G.-W. Methylmercury induces the expression of TNF-α selectively in the brain of mice. Sci. Rep. 2016;6:38294. doi: 10.1038/srep38294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan L., Jose C.C., Tanwar V.S., Bhattacharya S., Cuddapah S. Identification of a unique gene expression signature in mercury and 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin co-exposed cells. Toxicol. Res. 2017;6(3):312–323. doi: 10.1039/c6tx00432f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javorac D., Grahovac L., Manić L., Stojilković N., Anđelković M., Bulat Z., Đukić-Ćosić D., Curcic M., Djordjevic A.B. An overview of safety assessment of the medicines currently used in the treatment of COVID-19 disease. Food Chem. Toxicol. 2020;144:111639. doi: 10.1016/j.fct.2020.111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczynska K., Walski M., Szereda-Przestaszewska M. Ultrastructural changes in lung tissue after acute lead intoxication in the rat. J. Electron. Microsc. 2011;60(4):289–294. doi: 10.1093/jmicro/dfr035. [DOI] [PubMed] [Google Scholar]
- Kaczyńska K., Walski M., Szereda-Przestaszewska M. Long-term ultrastructural indices of lead intoxication in pulmonary tissue of the rat. Microsc. Microanal. 2013;19(6):1410–1415. doi: 10.1017/S1431927613013305. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Hira-Smith M., Ahmed S.I., et al. Prospective cohort study of respiratory effects at ages 14 to 26 following early life exposure to arsenic in drinking water. Environmental Epidemiology (Philadelphia, Pa.) 2020;4(2) doi: 10.1097/EE9.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.N., Bae S., Park H.Y., Kwon H.J., Hong Y.C. Low-level mercury exposure and risk of asthma in school-age children. Epidemiology. 2015;26(5):733–739. doi: 10.1097/EDE.0000000000000351. [DOI] [PubMed] [Google Scholar]
- Kim J., Song H., Heo H.R., et al. Cadmium-induced ER stress and inflammation are mediated through C/EBP–DDIT3 signaling in human bronchial epithelial cells. Exp. Mol. Med. 2017;49(9) doi: 10.1038/emm.2017.125. e372-e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Kim S.H., Jeon D., Kim H.Y., Han J.Y., Kim B., Lee K. Low-dose cadmium exposure exacerbates polyhexamethylene guanidine-induced lung fibrosis in mice. J. Toxicol. Environ. Health Part A. 2018;81(11):384–396. doi: 10.1080/15287394.2018.1451177. [DOI] [PubMed] [Google Scholar]
- Kiran Kumar K.M., Naveen Kumar M., Patil R.H., et al. Cadmium induces oxidative stress and apoptosis in lung epithelial cells. Toxicol. Mech. Methods. 2016;26(9):658–666. doi: 10.1080/15376516.2016.1223240. [DOI] [PubMed] [Google Scholar]
- Knoell D.L., Smith D., Bao S., et al. Imbalance in zinc homeostasis enhances lung Tissue Loss following cigarette smoke exposure. J. Trace Elem. Med. Biol. 2020;126483 doi: 10.1016/j.jtemb.2020.126483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozul C.D., Ely K.H., Enelow R.I., Hamilton J.W. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ. Health Perspect. 2009;117(9):1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger W.S., Wade T.J. Elevated blood lead and cadmium levels associated with chronic infections among non-smokers in a cross-sectional analysis of NHANES data. Environmental Health. 2016;15(1):16. doi: 10.1186/s12940-016-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulas J., Ninkov M., Tucovic D., et al. Subchronic oral cadmium exposure exerts both stimulatory and suppressive effects on pulmonary inflammation/immune reactivity in rats. Biomed. Environ. Sci. 2019;32(7):508–519. doi: 10.3967/bes2019.068. [DOI] [PubMed] [Google Scholar]
- Leff T., Stemmer P., Tyrrell J., Jog R. Diabetes and exposure to environmental lead (Pb) Toxics. 2018;6(3):54. doi: 10.3390/toxics6030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Jasperse L., Desforges J.P., et al. Methyl mercury (MeHg) in vitro exposure alters mitogen-induced lymphocyte proliferation and cytokine expression in Steller sea lion (Eumetopias jubatus) pups. Sci. Total Environ. 2020:138308. doi: 10.1016/j.scitotenv.2020.138308. [DOI] [PubMed] [Google Scholar]
- Li S., Zheng-Yan Z., Guo-Qing L., Su-Jun J., Hong Y. Effects of lead on calmodulin content, proliferation and interleukin-2 in lymphocytes of mice. Toxicol. Environ. Chem. 2012;94(5):958–964. [Google Scholar]
- Li Q., Hu X., Bai Y., et al. The oxidative damage and inflammatory response induced by lead sulfide nanoparticles in rat lung. Food Chem. Toxicol. 2013;60:213–217. doi: 10.1016/j.fct.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Li F.J., Surolia R., Li H., et al. Translational Research in Acute Lung Injury and Pulmonary Fibrosis: low-dose cadmium exposure induces peribronchiolar fibrosis through site-specific phosphorylation of vimentin. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313(1):L80. doi: 10.1152/ajplung.00087.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhao L., Zhang Y., et al. Imbalanced immune responses involving inflammatory molecules and immune-related pathways in the lung of acute and subchronic arsenic-exposed mice. Environ. Res. 2017;159:381–393. doi: 10.1016/j.envres.2017.08.036. [DOI] [PubMed] [Google Scholar]
- Li L.Q., Huang T., Wang Y.Q., et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J. Med. Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Li X., Xu J., Qi N., Zhang Z. Unchanged pulmonary function and increased prevalence of subjective respiratory symptoms in non-smoking workers with low cadmium body burden. Biol. Trace Elem. Res. 2020;194(1):84–88. doi: 10.1007/s12011-019-01756-w. [DOI] [PubMed] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(20):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.M., Chio C.P., Cheng Y.H., Hsieh N.H., Chen W.Y., Chen S.C. Quantitative links between arsenic exposure and influenza A (H1N1) infection‐associated lung function exacerbations risk. Risk Anal.: Int. J. 2011;31(8):1281–1294. doi: 10.1111/j.1539-6924.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur. J. Intern. Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir. Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little B.B., Ignasiak Z., Sławinska T., Posłuszny P., Malina R.M., Wiegman D.L. Blood lead levels, pulmonary function and agility in Polish schoolchildren. Ann. Hum. Biol. 2017;44(8):723–728. doi: 10.1080/03014460.2017.1387284. [DOI] [PubMed] [Google Scholar]
- Liu J., Lei D., Waalkes M.P., Beliles R.P., Morgan D.L. Genomic analysis of the rat lung following elemental mercury vapor exposure. Toxicol. Sci. 2003;74(1):174–181. doi: 10.1093/toxsci/kfg091. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu F., Liang W., Zhu L., Lantz R.C., Zhu J., Chen Y. Arsenic represses airway epithelial mucin expression by affecting retinoic acid signaling pathway. Toxicol. Appl. Pharmacol. 2020:114959. doi: 10.1016/j.taap.2020.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T.H., Chen C.H., Lee M.J., et al. Methylmercury chloride induces alveolar type II epithelial cell damage through an oxidative stress-related mitochondrial cell death pathway. Toxicol. Lett. 2010;194(3):70–78. doi: 10.1016/j.toxlet.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Lu C.F., Yuan X.Y., Li L.Z., Zhou W., Zhao J., Wang Y.M., Peng S.Q. Combined exposure to nano-silica and lead induced potentiation of oxidative stress and DNA damage in human lung epithelial cells. Ecotoxicol. Environ. Saf. 2015;122:537–544. doi: 10.1016/j.ecoenv.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Lu J., Jiang H., Liu B., et al. Grape seed procyanidin extract protects against Pb-induced lung toxicity by activating the AMPK/Nrf2/p62 signaling axis. Food Chem. Toxicol. 2018;116:59–69. doi: 10.1016/j.fct.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., et al. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalanobish S., Saha S., Dutta S., Sil P.C. Mangiferin alleviates arsenic induced oxidative lung injury via upregulation of the Nrf2-HO1 axis. Food Chem. Toxicol. 2019;126:41–55. doi: 10.1016/j.fct.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Mannino D.M., Holguin F., Greves H.M., Savage-Brown A., Stock A.L., Jones R.L. Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. Thorax. 2004;59(3):194–198. doi: 10.1136/thorax.2003.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool F., Niaz K., Hassan F.I., Khan F., Abdollahi M. Immunotoxicity of mercury: pathological and toxicological effects. J. Environ. Sci. Health Part C. 2017;35(1):29–46. doi: 10.1080/10590501.2016.1278299. [DOI] [PubMed] [Google Scholar]
- Martelletti L., Martelletti P. Air pollution and the novel Covid-19 disease: a putative disease risk factor. SN Comprehensive Clinical Medicine. 2020:1–5. doi: 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metryka E., Chibowska K., Gutowska I., et al. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci. 2018;19(6):1813. doi: 10.3390/ijms19061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.Y., Min K.B. Association between total blood mercury and exhaled nitric oxide in US adults. Nitric Oxide. 2013;29:53–58. doi: 10.1016/j.niox.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Mishra K.P., Rani R., Yadav V.S., Naik S. Effect of lead exposure on lymphocyte subsets and activation markers. Immunopharmacol. Immunotoxicol. 2010;32(3):446–449. doi: 10.3109/08923970903503668. [DOI] [PubMed] [Google Scholar]
- Moon K.A., Oberoi S., Barchowsky A., et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 2017;46(6):1924–1939. doi: 10.1093/ije/dyx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo S.V.K., Bester M.J., Arbi S., Venter C., Dhanraj P., Oberholzer H.M. Oral exposure to cadmium and mercury alone and in combination causes damage to the lung tissue of Sprague-Dawley rats. Environ. Toxicol. Pharmacol. 2019;69:86–94. doi: 10.1016/j.etap.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Nardone A., Ferreccio C., Acevedo J., et al. The impact of BMI on non-malignant respiratory symptoms and lung function in arsenic exposed adults of Northern Chile. Environ. Res. 2017;158:710–719. doi: 10.1016/j.envres.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A., Sanchez T.R., Mann K., Jones M.R. Arsenic exposure and cardiovascular disease: evidence needed to inform the dose-response at low levels. Curr. Epidemiol. Rep. 2019;6(2):81–92. [Google Scholar]
- Nishizaki Y., Nakao H., Umebayashi C., Iwase K., Tatsuishi T., Satoh M., Oyama Y. Increase in number of annexin V-positive living cells of rat thymocytes by intracellular Pb2+ Environ. Toxicol. Pharmacol. 2003;15(1):45–51. doi: 10.1016/j.etap.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Nygaard U.C., Li Z., Palys T., et al. Cord blood T cell subpopulations and associations with maternal cadmium and arsenic exposures. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0179606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyland J.F., Fairweather D., Shirley D.L., Davis S.E., Rose N.R., Silbergeld E.K. Low-dose inorganic mercury increases severity and frequency of chronic coxsackievirus-induced autoimmune myocarditis in mice. Toxicol. Sci. 2012;125(1):134–143. doi: 10.1093/toxsci/kfr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C.M., Oh I.H., Lee J.K., Park Y.H., Choe B.K., Yoon T.Y., Choi J.M. Blood cadmium levels are associated with a decline in lung function in males. Environ. Res. 2014;132:119–125. doi: 10.1016/j.envres.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Olivas-Calderón E., Recio-Vega R., Gandolfi A.J., et al. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol. Appl. Pharmacol. 2015;287(2):161–167. doi: 10.1016/j.taap.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Guo Y., Xiang H., Hui Y., Ju H., Xu S., Li L. Effects of lead, mercury, and cadmium co-exposure on children's pulmonary function. Biol. Trace Elem. Res. 2020;194(1):115–120. doi: 10.1007/s12011-019-01772-w. [DOI] [PubMed] [Google Scholar]
- Park E.J., Park K. Induction of reactive oxygen species and apoptosis in BEAS-2B cells by mercuric chloride. Toxicol. Vitro. 2007;21(5):789–794. doi: 10.1016/j.tiv.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Park S.S., Skaar D.A., Jirtle R.L., Hoyo C. Epigenetics, obesity and early-life cadmium or lead exposure. Epigenomics. 2017;9(1):57–75. doi: 10.2217/epi-2016-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F., Akhtar E., Khan L., et al. Exposure to low-dose arsenic in early life alters innate immune function in children. J. Immunot. 2019;16(1):201–209. doi: 10.1080/1547691X.2019.1657993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateda S.M., Sakakibara M., Sera K. Lung function assessment as an early biomonitor of mercury-induced health disorders in artisanal and small-scale gold mining areas in Indonesia. Int. J. Environ. Res. Publ. Health. 2018;15(11):2480. doi: 10.3390/ijerph15112480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N., Khandelwal S. Impact of cadmium in T lymphocyte subsets and cytokine expression: differential regulation by oxidative stress and apoptosis. Biometals. 2008;21(2):179–187. doi: 10.1007/s10534-007-9106-7. [DOI] [PubMed] [Google Scholar]
- Penta K.L., Altomare D., Shirley D.L., Nyland J.F. Female immune system is protected from effects of prenatal exposure to mercury. AIMS Environmental Science. 2015;2(3):448–463. [Google Scholar]
- Penta K.L., Fairweather D., Shirley D.L., Rose N.R., Silbergeld E.K., Nyland J.F. Low-dose mercury heightens early innate response to coxsackievirus infection in female mice. Inflamm. Res. 2015;64(1):31–40. doi: 10.1007/s00011-014-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., Kouretas D., Spandidos D., Tsatsakis A. Obesity-a risk factor for increased COVID-19 prevalence, severity and lethality. Mol. Med. Rep. 2020;22:9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard K.M., Cauvi D.M., Toomey C.B., Hultman P., Kono D.H. Mercury-induced inflammation and autoimmunity. Biochim. Biophys. Acta Gen. Subj. 2019;1863(12):129299. doi: 10.1016/j.bbagen.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poręba R., Gać P., Poręba M., Andrzejak R. Environmental and occupational exposure to lead as a potential risk factor for cardiovascular disease. Environ. Toxicol. Pharmacol. 2011;31(2):267–277. doi: 10.1016/j.etap.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Powers M., Sanchez T.R., Grau-Perez M., et al. Low-to-Moderate arsenic exposure and respiratory health in American Indian communities. Ann. Am. Thorac. Soc. 2018;15(Suppl. 2):S128–S129. [Google Scholar]
- Prasad P., Sinha D. Low-level arsenic causes chronic inflammation and suppresses expression of phagocytic receptors. Environ. Sci. Pollut. Res. 2017;24(12):11708–11721. doi: 10.1007/s11356-017-8744-8. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ. Sci. Technol. Mar. 2020;23 doi: 10.1021/acs.est.0c01102. acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Ramsey K. Handbook of Arsenic Toxicology. Academic Press; 2015. Arsenic and respiratory disease; pp. 335–347. [Google Scholar]
- Ramsey K.A., Foong R.E., Sly P.D., Larcombe A.N., Zosky G.R. Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environ. Health Perspect. 2013;121(10):1187–1193. doi: 10.1289/ehp.1306748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Ahmed S., Ahsan K.B., et al. Humoral immunity in arsenic-exposed children in rural Bangladesh: total immunoglobulins and vaccine-specific antibodies. Environ. Health Perspect. 2017;125(6) doi: 10.1289/EHP318. 067006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed M.N. The role of trace elements on hepatitis virus infections: a review. J. Trace Elem. Med. Biol. 2011;25(3):181–187. doi: 10.1016/j.jtemb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Razi C.H., Akin O., Harmanci K., Akin B., Renda R. Serum heavy metal and antioxidant element levels of children with recurrent wheezing. Allergol. Immunopathol. 2011;39(2):85–89. doi: 10.1016/j.aller.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Razi C.H., Akın K.O., Harmancı K., et al. Relationship between hair cadmium levels, indoor ETS exposure and wheezing frequency in children. Allergol. Immunopathol. 2012;40(1):51–59. doi: 10.1016/j.aller.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Riemschneider S., Herzberg M., Lehmann J. Subtoxic doses of cadmium modulate inflammatory properties of murine RAW 264.7 macrophages. Biomed. Res. Int. 2015. 2015:295303. doi: 10.1155/2015/295303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshild E.V. Ecology of Ebola fever in terms of natural disease model. Trace Elem Med. 2015;16:3–11. [Google Scholar]
- Roy C., Tremblay P.Y., Ayotte P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ. Res. 2017;156:747–760. doi: 10.1016/j.envres.2017.04.038. [DOI] [PubMed] [Google Scholar]
- Sahin M., Karayakar F., Koksal A.R., et al. Changes in liver tissue trace element concentrations during hepatitis B viral infection treatment. Biol. Trace Elem. Res. 2019;188(2):245–250. doi: 10.1007/s12011-018-1414-y. [DOI] [PubMed] [Google Scholar]
- Sanchez T.R., Powers M., Perzanowski M., George C.M., Graziano J.H., Navas-Acien A. A meta-analysis of arsenic exposure and lung function: is there evidence of restrictive or obstructive lung disease? Curr. Environ. Health Rep. 2018;5(2):244–254. doi: 10.1007/s40572-018-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarigiannis D.A., Salifoglou A. Research directives toward deciphering adverse outcome pathways induced by environmental metallotoxins. Current Opinion in Chemical Engineering. 2016;13:161–169. [Google Scholar]
- Satarug S., Garrett S.H., Sens M.A., Sens D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar A., Khan A., Hussain H.I., et al. Immunosuppressive effects of arsenic in broiler chicks exposed to Newcastle disease virus. J. Immunot. 2016;13(6):861–869. doi: 10.1080/1547691X.2016.1217105. [DOI] [PubMed] [Google Scholar]
- Seth P., Husain M.M., Gupta P., Schoneboom B.A., Grieder F.B., Mani H., Maheshwari R.K. Early onset of virus infection and up-regulation of cytokines in mice treated with cadmium and manganese. Biometals. 2003;16(2):359–368. doi: 10.1023/a:1020682716212. [DOI] [PubMed] [Google Scholar]
- Sherwood C.L., Liguori A.E., Olsen C.E., Lantz R.C., Burgess J.L., Boitano S. Arsenic compromises conducting airway epithelial barrier properties in primary mouse and immortalized human cell cultures. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0082970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y.H., Argos M., Turyk M.E. Urinary arsenic concentration, airway inflammation, and lung function in the US adult population. Environ. Res. 2019;175:308–315. doi: 10.1016/j.envres.2019.05.031. [DOI] [PubMed] [Google Scholar]
- Shimabukuro-Vornhagen A., Godel P., Subklewe M., Stemmler H.J., Schlosser H.A., Schlaak M., et al. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique A.E., Rahman M., Hossain M.I., et al. Association between chronic arsenic exposure and the characteristic features of asthma. Chemosphere. 2020;246:125790. doi: 10.1016/j.chemosphere.2019.125790. [DOI] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020:1–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny A.V., Rink L., Ajsuvakova O.P., et al. Zinc and respiratory tract infections: perspectives for COVID-19. Int. J. Mol. Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiechowicz J., Skoczynska A., Nieckula-Szwarc A., Kulpa K., Kübler A. vol. 5. SAGE open medical case reports; 2017. Occupational Mercury Vapour Poisoning with a Respiratory Failure, Pneumomediastinum and Severe Quadriparesis. 2050313X17695472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solenkova N.V., Newman J.D., Berger J.S., Thurston G., Hochman J.S., Lamas G.A. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am. Heart J. 2014;168(6):812–822. doi: 10.1016/j.ahj.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C., Ferreccio C., Acevedo J., et al. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol. Appl. Pharmacol. 2016;313:10–15. doi: 10.1016/j.taap.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundblad B.M., Ji J., Levänen B., et al. Extracellular cadmium in the bronchoalveolar space of long-term tobacco smokers with and without COPD and its association with inflammation. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:1005. doi: 10.2147/COPD.S105234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surolia R., Li F.J., Mattapally S., et al. B29. Infection and Immune Interplay in Lung Injury. American Thoracic Society; 2020. Role of PAD2 regulated inflammasome signaling in arsenic induced airway inflammation and remodeling. A2976-A2976. [Google Scholar]
- Talhout R., Schulz T., Florek E., Van Benthem J., Wester P., Opperhuizen A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Publ. Health. 2011;8(2):613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M., Jones M.R., Dominguez-Lucas A., Guallar E., Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atherosclerosis Rep. 2013;15(10):356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov A.A., Ajsuvakova O.P., Skalnaya M.G., et al. Mercury and metabolic syndrome: a review of experimental and clinical observations. Biometals. 2015;28(2):231–254. doi: 10.1007/s10534-015-9823-2. [DOI] [PubMed] [Google Scholar]
- Tinkov A.A., Filippini T., Ajsuvakova O.P., et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017;601:741–755. doi: 10.1016/j.scitotenv.2017.05.224. [DOI] [PubMed] [Google Scholar]
- Tinkov A.A., Filippini T., Ajsuvakova O.P., et al. Cadmium and atherosclerosis: a review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018;162:240–260. doi: 10.1016/j.envres.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Tinkov A.A., Gritsenko V.A., Skalnaya M.G., Cherkasov S.V., Aaseth J., Skalny A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018;235:429–434. doi: 10.1016/j.envpol.2017.12.114. [DOI] [PubMed] [Google Scholar]
- Torén K., Olin A.C., Johnsson Å., et al. The association between cadmium exposure and chronic airflow limitation and emphysema: the Swedish CArdioPulmonary BioImage Study (SCAPIS pilot) Eur. Respir. J. 2019;54(5) doi: 10.1183/13993003.00960-2019. [DOI] [PubMed] [Google Scholar]
- Tsatsakis A., Petrakis D., Nikolouzakis T.K., Docea A.O., Calina D., Vinceti M., Goumenou M., Kostoff R.N., Mamoulakis C., Aschner M., Hernández A.F. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020;141:111418. doi: 10.1016/j.fct.2020.111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley A.E., Zagorski J.W., Kennedy R.C., Freeborn R.A., Bursley J.K., Edwardsd J.R., Rockwell C.E. Chronic low-level cadmium exposure in rats affects cytokine production by activated T cells. Toxicol. Res. 2019;8:227–237. doi: 10.1039/c8tx00194d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDenBerg K.R., Freeborn R.A., Liu S., Kennedy R.C., Zagorski J.W., Rockwell C.E. Inhibition of early T cell cytokine production by arsenic trioxide occurs independently of Nrf2. PloS One. 2017;12(10) doi: 10.1371/journal.pone.0185579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez M., Velez D., Devesa V. In vitro evaluation of inorganic mercury and methylmercury effects on the intestinal epithelium permeability. Food Chem. Toxicol. 2014;74:349–359. doi: 10.1016/j.fct.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang Q., Zou Z., Zheng F., Zhang A. Human arsenic exposure and lung function impairment in coal-burning areas in Guizhou, China. Ecotoxicol. Environ. Safe. 2020;190:110174. doi: 10.1016/j.ecoenv.2020.110174. [DOI] [PubMed] [Google Scholar]
- Wei W., Wu X., Bai Y., et al. Lead exposure and its interactions with oxidative stress polymorphisms on lung function impairment: results from A longitudinal population-based study. Environ. Res. 2020:109645. doi: 10.1016/j.envres.2020.109645. [DOI] [PubMed] [Google Scholar]
- Weigand K.L., Reno J.L., Rowley B.M. Low-level mercury causes inappropriate activation in T and B lymphocytes in the absence of antigen stimulation. J. Ark. Acad. Sci. 2015;69:22. [Google Scholar]
- Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. medRxiv; 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M., Jin X., Wang J., Shi Q., Cai J., Xu S. The antagonistic effect of selenium on lead-induced immune dysfunction via recovery of cytokine and heat shock protein expression in chicken neutrophils. Biol. Trace Elem. Res. 2018;185(1):162–169. doi: 10.1007/s12011-017-1200-2. [DOI] [PubMed] [Google Scholar]
- Xiong R., Wu Q., Trbojevich R., et al. Disease-related responses induced by cadmium in an in vitro human airway tissue model. Toxicol. Lett. 2019;303:16–27. doi: 10.1016/j.toxlet.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Xu C., Shu Y., Fu Z., Hu Y., Mo X. Associations between lead concentrations and cardiovascular risk factors in US adolescents. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-09701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Hu H., Hong Y.A. Body burden of heavy metals among HIV high risk population in USA. Environ. Pollut. 2017;220:1121–1126. doi: 10.1016/j.envpol.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Xu Y.M., Gao Y.M., Wu D.D., et al. Aberrant cytokine secretion and zinc uptake in chronic cadmium‐exposed lung epithelial cells. PROTEOM. Clin. Appl. 2017;11(3–4):1600059. doi: 10.1002/prca.201600059. [DOI] [PubMed] [Google Scholar]
- Yang G., Sun T., Han Y.Y., Rosser F., Forno E., Chen W., Celedón J.C. Serum cadmium and lead, current wheeze, and lung function in a nationwide study of adults in the United States. J. Allergy Clin. Immunol. Pract. 2019;7(8):2653–2660. doi: 10.1016/j.jaip.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Feng F., Li P., et al. Arsenic trioxide impacts viral latency and delays viral rebound after termination of ART in chronically SIV‐infected macaques. Adv. Sci. 2019;6(13):1900319. doi: 10.1002/advs.201900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ricard L., Siblany L., et al. Arsenic trioxide induces regulatory functions of plasmacytoid dendritic cells through interferon-α inhibition. Acta Pharm. Sin. B. 2020;10(6):1061–1072. doi: 10.1016/j.apsb.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q., Sides M., Parsons C.H., Flemington E.K., Lasky J.A. Arsenic trioxide inhibits EBV reactivation and promotes cell death in EBV-positive lymphoma cells. Virol. J. 2017;14(1):121. doi: 10.1186/s12985-017-0784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongjian Z., Jingu X., Fengming H., Liqing C. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Duan J., Li Y., et al. Combined toxicity of amorphous silica nanoparticles and methylmercury to human lung epithelial cells. Ecotoxicol. Environ. Safe. 2015;112:144–152. doi: 10.1016/j.ecoenv.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Wu Y., Ge X., Nie D., Wang M., Zhou H., Chen M. In vitro toxicity evaluation of heavy metals in urban air particulate matter on human lung epithelial cells. Sci. Total Environ. 2019;678:301–308. doi: 10.1016/j.scitotenv.2019.04.431. [DOI] [PubMed] [Google Scholar]
- Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M.H., Pourahmad J., Nassireslami E. Toxicity of arsenic on isolated human lymphocytes: the key role of cytokines and intracellular calcium enhancement in arsenic induced cell death. Main Group Met. Chem. 2019;42:125–134. [Google Scholar]
- Zeng X., Xu X., Boezen H.M., Vonk J.M., Wu W., Huo X. Decreased lung function with mediation of blood parameters linked to e-waste lead and cadmium exposure in preschool children. Environ. Pollut. 2017;230:838–848. doi: 10.1016/j.envpol.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Zeng Q., Zhang W.X., Zheng T.Z., et al. Prenatal and postnatal cadmium exposure and cellular immune responses among pre-school children. Environ. Int. 2020;134:105282. doi: 10.1016/j.envint.2019.105282. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu X., Sun S., et al. Cadmium modulates hematopoietic stem and progenitor cells and skews toward myelopoiesis in mice. Toxicol. Appl. Pharmacol. 2016;313:24–34. doi: 10.1016/j.taap.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Huang J., Feng M., Tong Y.Q., Guan X.H., Jiang H.W., Wei S. Elevated arsenic exposure is associated with an increased risk of chronic hepatitis B virus infection: NHANES (2003–2014) in US adults. Curr. Med. Sci. 2018;38(4):610–617. doi: 10.1007/s11596-018-1921-2. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Su X., Gao Y., Yin H., Wang L., Qiao R., Wang S. Exposure of low‐concentration arsenic‐initiated inflammation and autophagy in rat lungs. J. Biochem. Mol. Toxicol. 2019;33(7) doi: 10.1002/jbt.22334. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of Covid‐19: a systemic review and meta‐analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25889. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.-L., Yuan S.-S., Chang-Wen Lv W-M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio) Aquat. Toxicol. 2016;180:36–44. doi: 10.1016/j.aquatox.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2):16–25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosky G.R., Ramsey K.A., Carter K.W., Bosco A., McKenna K.L., Sly P.D. vol. 16. 2014. In utero arsenic exposure via drinking water alters genes related to lung growth and mucociliary function; p. 29. (TSANZ 2011 Annual Scientific Meetings). [Google Scholar]