Abstract
The need for monitoring tools to better control the ongoing coronavirus disease (COVID-19) pandemic is extremely urgent and the contamination of water resources by excreted viral particles poses alarming questions to be answered. As a first step to overcome technical limitations in monitoring SARS-CoV-2 along the water cycle, we assessed the analytical performance of a dead end hollow fiber ultrafiltration coupled to different options for secondary concentrations to concentrate viral particles from large volume of spiked tap water, seawater and surface water together with two quantitative RT-qPCR detection kits. Spiking the porcine epidemic diarrhea virus (PEDV), an enveloped virus surrogate for SARS-CoV-2, together with the mengovirus, we demonstrated that PEG-precipitation and SENS-kit better recovered PEDV (13.10 ± 0.66%) from tap water, while centrifugal filtration resulted the best option to recover mengovirus regardless of the detection kit used. No statistical significant differences were found when comparing high (10,000 ×g) and low (3500 ×g) centrifugation speeds for the secondary PEG- based concentration of spiked seawater, while considerable inhibition was observed for both viruses detected by NoInh-kit assay. Similarly, the co-concentration of PCR inhibitors and viral particles was observed in surface waters detected with either SENS-kit or NoInh-kit and RNA dilution was needed to achieve acceptable recoveries at the expenses of the overall sensitivity of the method. These methodologies represent suitable options to investigate SARS-CoV-2 occurrence in different water resources and allow to conduct on site sampling of large volume of water.
Keywords: Coronavirus, Tap water, Surface water, Seawater, Concentration, RT-qPCR
Graphical abstract
1. Introduction
The access to safe and clean water is a universal human right (United Nations, 2010), that has been further questioned by the ongoing coronavirus disease (COVID-19) pandemic. While severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19, is mainly a respiratory pathogen, the detection of virus particles in stool supports the hypothesis that fecal-oral transmission may occur (Yeo et al., 2020). Despite this conjecture has not been elucidated yet, wastewater-based epidemiology (WBE) has been implemented worldwide for tracking the pandemic within a given community and for gaining preparedness for future SARS-CoV-2 local outbreaks (Bivins et al., 2020; Farkas et al., 2020; Haramoto et al., 2020; La Rosa et al., 2020; Medema et al., 2020; Randazzo et al., 2020a; Westhaus et al., 2021; WHO, 2020a). Special interests have been also given to the presence of SARS-CoV-2 in effluent wastewater and recreational waters such as river water and seawater to assess public health risks (Cahill and Morris, 2020; Guerrero-Latorre et al., 2020; Liu et al., 2020). A colossal number of laboratories have been involved in wastewater monitoring programs worldwide, being the lack of standard methods the main bottleneck for implementing WBE nation- and world-wide. In this sense, it has been imperative to assess the analytical performances of concentration methods for SARS-CoV-2 in different types of water, as protocols validated for common viral human pathogens such as enteric viruses may not succeed in well-recovering enveloped viruses (Ahmed et al., 2020; Randazzo et al., 2020b). To a larger extent, the controversial debate on the fate of SARS-CoV-2 along the water cycle brought to light the need for the development of robust methods for concentrating enveloped viruses from large volume of water in order to investigate natural water resources such as tap, reclaimed, surface, drinking and sea-waters. In fact, current methods used to concentrate viruses from wastewater are not feasible for larger volumes because of (i) the low viral titers; (ii) the co-concentration of PCR inhibitors (e.g., salt); (iii) the presence of suspended solids, and (iv) the logistics and costs of delivering water samples to laboratories. However, whether the existing methods already validated for concentrating enteric viruses from large volumes are also suitable for enveloped viruses, and therefore used to investigate SARS-CoV-2 contamination in water resources, is unknown.
2. Methods
We assessed the analytical performance of a Dead End Hollow Fiber Ultrafiltration (DEUF) concentration and two quantitative RT-qPCR detection kits with the final aim of developing a tool of interest for studying the potential SARS-CoV-2 contamination of different types of water.
To this end, we concentrated tap, surface and seawaters spiked with porcine epidemic diarrhea virus (PEDV, strain CV777), an enveloped virus member of the Coronaviridae family, and mengovirus (CECT 100000, strain vMC0), a non-enveloped member of the Picornaviridae, used as process controls to evaluate the procedures for concentrating large volume of water. PEDV and mengovirus viral stocks were obtained from Vero and HeLa cells culture infected suspensions, respectively (Puente et al., 2020).
All water samples used in this study were of blinded origin and collected in April–May 2020. Specifically, a large volume (20L) of tap water (n = 2), seawater (n = 2) and surface water (n = 2) was collected as a simple grab sample and transferred to the laboratory within 6 h to be subsequently processed. All water samples were spiked with 107 PEDV genomic copies (gc) and 108 mengovirus gc and primary concentrated by DEUF as detailed by Cuevas-Ferrando et al. (2020). Different options were evaluated for secondary concentrations depending on the type of water: (i) a polyethylene glycol (PEG) precipitation and a centrifuge filtration with Centricon Plus-70 devices with a 30 kDa cutoff NMWL membrane (Merck Millipore Ltd.) for tap water; (ii) a PEG precipitation at 10,000 ×g or 3500 ×g for seawater; (iii) a PEG precipitation at 3500 ×g for secondary concentrating surface waters. RNA extraction from concentrates was carried out using the NucleoSpin RNA virus kit (Macherey-Nagel GmbH & Co.), including a purification step with Plant RNA Isolation Aid (Ambion). For RNA detection, two commercially available kits were compared. Specifically, One Step PrimeScript™ RT-PCR Kit (Perfect Real Time) (Takara Bio, USA) (referred as SENS-kit) and One Step PrimeScript™ III RT-PCR Kit (Takara Bio, USA) (referred as NoInh-kit) were used. The first kit is claimed to provide a sensitive detection of very small amounts of RNA, while the latter is highly resistant to a wide variety of inhibitory substances. For all assays, undiluted, 10- and 50-fold diluted RNA were tested to check for RT-qPCR inhibitors. Details on RT-qPCR and quantification have been reported by Randazzo et al., 2020a, Randazzo et al., 2020b. The percent virus recovery (r) was calculated as follows:
The effects of the variables considered in this study (concentration method, RT-qPCR kit, virus, dilution) were separately tested for each type of water sample (tap water, seawater, surface water) by the analysis of variance (ANOVA) followed by the Tukey's HSD as post hoc test to obtain homogenous groups. A P value <0.05 was deemed significant.
3. Results
We defined PEDV and mengovirus recovery yields as the performance characteristic for the viral concentration of spiked tap water, seawater and surface water (Fig. 1, Fig. 2, Fig. 3 ). Different modifications for the concentration method specific for each type of water were assessed along with two RT-qPCR quantification assays.
Fig. 1.
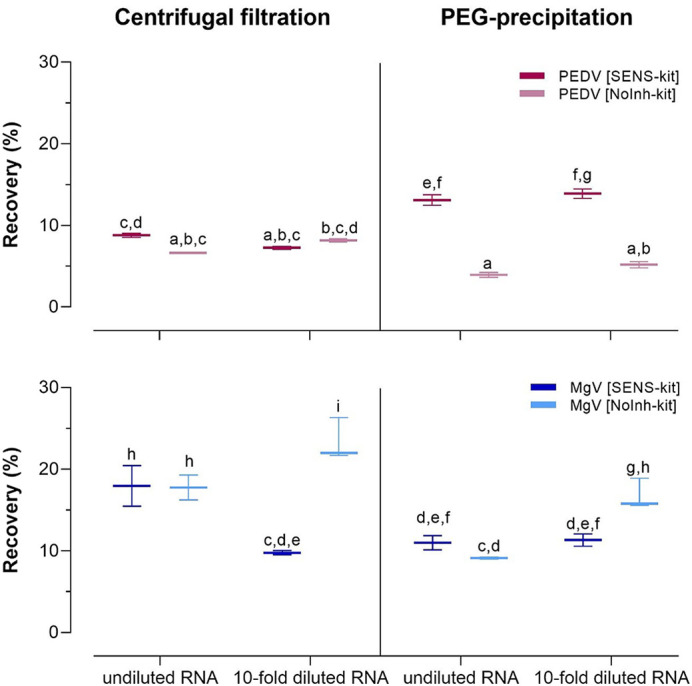
Median recoveries (%) and standard deviations of spiked porcine epidemic diarrhea virus (PEDV) and mengovirus (MgV) in tap water primary concentrated by dead end hollow fiber ultrafiltration followed by a secondary concentration procedure based on a centrifuge filtration or, alternatively, on a polyethylene glycol (PEG) precipitation.
Letters denote homogeneous groups according to the analysis of variance (ANOVA) and Tukey's HSD post hoc test (p < 0.05).
Fig. 2.
Median recoveries (%) and standard deviations of spiked porcine epidemic diarrhea virus (PEDV) and mengovirus (MgV) in seawater primary concentrated by dead end hollow fiber ultrafiltration followed by a secondary concentration procedure based on polyethylene glycol (PEG) precipitation using a high- (10,000 ×g) or low- (3500 ×g) speed centrifugation.
Letters denote homogeneous groups according to the analysis of variance (ANOVA) and Tukey's HSD post hoc test (p < 0.05).
Fig. 3.
Median recoveries (%) and standard deviations of spiked porcine epidemic diarrhea virus (PEDV) and mengovirus (MgV) in surface water primary concentrated by dead end hollow fiber ultrafiltration followed by a secondary concentration procedure based on polyethylene glycol (PEG) precipitation using a low- (3500 ×g) speed centrifugation.
#, negative.
Letters denote homogeneous groups according to the analysis of variance (ANOVA) and Tukey's HSD post hoc test (p < 0.05).
In tap water, significant differences were observed between centrifugal filtration and PEG precipitation, but not between the SENS-kit and the NoInh-kit. PEDV showed higher recoveries when secondary concentrated by PEG-precipitation and detected by SENS-kit (13.10 ± 0.66%), while lower recoveries of 3.94 ± 0.28% were yielded by NoInh-kit. On the contrary, mengovirus was better recovered with centrifugal filtration regardless of the detection kit used, being the recovery rates of 17.95 ± 2.50% for SENS-kit and 17.76 ± 1.52 for NoInh-kit (Fig. 1). As it could be expected, no significant PCR inhibitions were detected for both PEDV and mengovirus by using either SENS-kit or NoInh-kit in concentrated tap water.
The options evaluated for the secondary concentration of spiked seawater samples showed no statistical significant differences between the centrifugation speeds (10,000 ×g and 3500 ×g), while considerable inhibition was observed for both viruses detected by NoInh-kit assay. Specifically, centrifugation at high (10,000 ×g) and low (3500 ×g) speed recovered 3.36 ± 0.10% and 2.98 ± 0.05% of PEDV, and 10.19 ± 0.19% and 9.45 ± 0.12% of mengovirus, respectively, detecting undiluted RNA with SENS-kit (Fig. 2). On the contrary, when viral detection was carried out by NoInh-kit on undiluted RNAs, recoveries of 0.76 ± 0.00% and 0.84 ± 0.03% for PEDV, and 0.81 ± 0.07% and 1.52 ± 0.17% for mengovirus were yielded at high and low speed, respectively. This indicates the presence of PCR inhibitions that was confirmed by the higher recovery rates achieved by diluting the RNAs by 10-fold and 50-fold (Fig. 2).
Similarly, the co-concentration of inhibitors and viral particles was observed in surface waters detected either with SENS-kit or NoInh-kit. The recovery rates of undiluted RNA resulted as low as 0.82 ± 0.06% and 0.51 ± 0.12% for PEDV and 0.29 ± 0.05% and 0.22 ± 0.02% for mengovirus with SENS-kit or NoInh-kit, respectively. Again, by diluting viral RNA by 10-fold and 50-fold, the recoveries rates resulted higher than 2.89% in all cases (Fig. 3).
4. Discussion
SARS-CoV-2 has been detected in effluent waters from wastewater treatments plants (Randazzo et al., 2020b), and in surface water polluted with wastewater (Guerrero-Latorre et al., 2020; Rimoldi et al., 2020), highlighting the need for protocols to non-sewage testing (Cahill and Morris, 2020; WHO, 2020a).
The present study reports the analytical performances of several modifications of a DEUF method to concentrate viruses from large volumes of tap water, seawater and surface waters of interest for studying the potential contamination of water resources by SARS-CoV-2. Until recently, studies to assess the efficiency of concentration methods in water matrices mostly involved nonenveloped virus, such as human enteric viruses (reviewed by Bofill-Mas and Rusiñol, 2020; Haramoto et al., 2018; Ikner et al., 2012; Matrajt et al., 2018), even the need to investigate enveloped viruses along the water cycle was already raised following SARS, MERS, Ebola and avian influenzas outbreaks (Wigginton et al., 2015). This farseeing call for validated analytical tools lays its reason on the structural and biochemical differences between nonenveloped and enveloped viruses questioning that methods developed for the former would not fit for concentrating the latter. Interestingly, PEG precipitation has been applied as a secondary concentration step to recover enveloped viruses from large volume of water (reviewed by Bofill-Mas and Rusiñol, 2020), and an optimized procedure based on glass wool primary concentration detected naturally occurring alphacoronavirus in surface water in Saudi Arabia (Blanco et al., 2019).
Moreover, to assess the sensitivity of RT-qPCR assays to inhibitors, we compared two quantitative detection kits: one claimed to be optimized for low RNA amounts (SENS-kit) and a second specified to be highly resistant to a wide variety of inhibitory substances (NoInh-kit). Despite the use of a contaminants/inhibitors removal product before RNA extraction (Plant RNA Isolation Aid), we observed a different sensitivity of RT-qPCR assays to co-concentrated inhibitory substances, being the SENS-kit less prone to such limiting factor. Nucleic acid dilution is a well-known approach to evaluate the presence of inhibitors in complex matrices (ISO 15216-1:2017; McKee et al., 2015), however, the sensitivity of the assay decreases according to the dilution factor applied. In our study, we had to dilute up to 50-fold the RNA concentrated from surface water and seawater samples to overcome PCR inhibition effects. This resulted in exceeding the detection limit of the assay in some cases (Fig. 3). Recent studies aimed to detect SARS-CoV-2 in river water reported none to minimal inhibitors carryover in samples concentrated from 1 to 5 l (Guerrero-Latorre et al., 2020; Haramoto et al., 2020; Rimoldi et al., 2020).
Despite the approaches applied to evaluate possible RT-qPCR inhibitions (e.g., RNA dilution, internal or external amplification controls), the concentration methods used and the nature of water sampled, the feasibility of a given method finally relies on its sensitivity. This latter is mostly correlated to the volume of the concentrated sample but also to the co-concentration of inhibitors. These factors could explain the reason of the divergent proneness to inhibition found in our study, in which large volumes of water samples (20l) were concentrated.
Assessing a secondary concentration method for tap water, we found that PEG-precipitation resulted the best option for concentrating PEDV, an enveloped virus suggested as SARS-CoV-2 surrogate, while the centrifugal filtration was observed to better recover mengovirus, a non-enveloped virus included in the ISO 15216-1:2017 as process control to detect human enteric viruses. In contrast, high and low centrifugation speeds did not significantly differ in recovering both spiked viruses from seawater. These findings are of importance because of the shortage of provision of the centrifugal filtration units currently occurring in European market, which are linked to the current pandemic situation. Similarly, by using either high or low centrifugation speeds, a larger number of laboratories could be involved in seawater monitoring programmes, even those equipped with simple bench centrifuges.
In general, data on water reservoirs contaminated by human enteric viruses are limited (Haramoto et al., 2018), and to date, no evidences of SARS-CoV-2 occurrence in natural water resources have been reported. In line, WHO and CDC agree in defining the risk associated with contracting SARS-CoV-2 via water sources as low (CDC, 2020; WHO, 2020b). However, in the midst of the current pandemic, chances of SARS-CoV-2 transmission routes cannot be excluded, especially in densely populated areas with poor sanitization systems or when overflows occur (Bhowmick et al., 2020; Heller et al., 2020).
Further research is required for monitoring the potential SARS-CoV-2 contamination of downstream waters used for irrigation or recreational purposes, as well as drinking water resources in settings with limited availability of water, sanitation and hygiene (Street et al., 2020).
To this end, large volume of water has to be sampled and concentration methods need to be validated by either using SARS-CoV-2 spiked or naturally contaminated waters, along with the determination of the limit of detection. Meanwhile, the assessed methodologies represent suitable options to investigate SARS-CoV-2 occurrence in different water resources and allow to conduct on site sampling of large volume of water.
CRediT authorship contribution statement
ECF and APC: Investigation, Editing and Reviewing; WR: data analyses and first draft writing; AA, SG, WR and GS: Conceptualization, Supervision, Writing, Editing and Reviewing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank Prof. A. Carvajal from University of Leon for kindly providing PEDV CV777 strain and Agustin Garrido Fernandez for his support in the concentration procedures.
We acknowledge the GAMASER, Global Omnium S.L. and SEPRONA for arranging water sampling.
Funding
The study was funded by grants from CSIC (202070E101), Generalitat Valenciana (Covid_19-SCI), and MICIU co-founded by AEI/FEDER, UE (AGL2017-82909). EC-F is recipient of a predoctoral contract from the MICINN, Call 2018. WR is holder of the APOSTD/2018/150 postdoctoral contract from Generalitat Valenciana.
Editor: Damia Barcelo
References
- ISO 15216-1 . Method for Quantification. vol. 15216. 2017. Microbiology of food and animal feed—horizontal method for determination of hepatitis a virus and norovirus in food using real-time RT-PCR—Part 1. [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A.…Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Science of the Total Environment. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick G.D., Dhar D., Nath D., Ghangrekar M.M., Banerjee R., Das S., Chatterjee J. Coronavirus disease 2019 (COVID-19) outbreak: some serious consequences with urban and rural water cycle. Npj Clean Water. 2020;3(1):32. doi: 10.1038/s41545-020-0079-1. [DOI] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F.…Bibby K. Wastewater-Based Epidemiology: Global Collaborative to Maximize Contributions in the Fight against COVID-19. Environmental Science and Technology. 2020 doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Blanco A., Abid I., Al-Otaibi N., Pérez-Rodríguez F.J., Fuentes C., Guix S.…Bosch A. Glass Wool Concentration Optimization for the Detection of Enveloped and Non-enveloped Waterborne Viruses. Food and Environmental Virology. 2019;11(2):184–192. doi: 10.1007/s12560-019-09378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Mas S., Rusiñol M. Recent trends on methods for the concentration of viruses from water samples. Current Opinion in Environmental Science & Health. 2020;16:7–13. doi: 10.1016/j.coesh.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill N., Morris D. Recreational waters – a potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020;740:140122. doi: 10.1016/j.scitotenv.2020.140122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Water. 2020. https://www.cdc.gov/coronavirus/2019-ncov/faq.html#Water Retrieved from.
- Cuevas-Ferrando E., Randazzo W., Pérez-Cataluña A., Sánchez G. HEV occurrence in waste and drinking water treatment plants. Front. Microbiol. 2020;10 doi: 10.3389/fmicb.2019.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas K., Hillary L.S., Malham S.K., McDonald J.E., Jones D.L. Wastewater and public health: the potential of wastewater surveillance for monitoring COVID-19. Current Opinion in Environmental Science and Health. 2020;17:14–20. doi: 10.1016/j.coesh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikner L.A., Gerba C.P., Bright K.R. Concentration and recovery of viruses from water: a comprehensive review. Food and Environmental Virology. 2012;4(2):41–67. doi: 10.1007/s12560-012-9080-2. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Thompson J.R., Carducci A., Bi X. Potential secondary transmission of SARS-CoV-2 via wastewater. Sci. Total Environ. 2020;749:142358. doi: 10.1016/j.scitotenv.2020.142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrajt G., Naughton B., Bandyopadhyay A.S., Meschke J.S. A review of the most commonly used methods for sample collection in environmental surveillance of poliovirus. Clin. Infect. Dis. 2018;67(suppl_1):S90–S97. doi: 10.1093/cid/ciy638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.M., Spear S.F., Pierson T.W. The effect of dilution and the use of a post-extraction nucleic acid purification column on the accuracy, precision, and inhibition of environmental DNA samples. Biol. Conserv. 2015;183:70–76. doi: 10.1016/j.biocon.2014.11.031. [DOI] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environmental Science & Technology Letters. 2020 doi: 10.1021/acs.estlett.0c00357. acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Puente H., Randazzo W., Falcó I., Carvajal A., Sánchez G. Rapid selective detection of potentially infectious porcine epidemic diarrhea coronavirus exposed to heat treatments using viability RT-qPCR. Front. Microbiol. 2020;11:1911. doi: 10.3389/fmicb.2020.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D.…Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Science of the Total Environment. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for Covid-19: an African perspective. Sci. Total Environ. 2020;743:140719. doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . 2010. The Right to Water. Resolution a/RES/64/292. General Comment No. 15. [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M.…Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – Suitability for COVID-19 surveillance and potential transmission risks. Science of The Total Environment. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Status of Environmental Surveillance for SARS-CoV-2 Virus. [Google Scholar]
- WHO . 2020. Water, Sanitation, Hygiene and Waste Management for COVID-19. [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environmental Science: Water Research and Technology. 2015;1(6):735–746. doi: 10.1039/c5ew00125k. [DOI] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterology and Hepatology. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]





