Abstract
We recently reported that transplantation of autologous bone marrow mononuclear cells (BM-MNCs) may be an effective and promising therapy to treat refractory diabetic sensorimotor polyneuropathy (DSPN) in patients with type 2 diabetes mellitus (T2DM). This study was designed to investigate the potential mechanisms of BM-MNCs therapy, which recruited 60 patients with DSPN, 30 T2DM patients without complications, and 30 healthy control participants. All clinical parameters, the levels of inflammatory markers, and growth factors in the three groups were compared. Patients in DSPN group had higher level of tumor necrosis factor-α (TNF-α) (DSPN vs control, 412.90 ± 64.58 vs 374.81 ± 63.18 pg/mL, P < 0.01) and lower level of vascular endothelial growth factor (VEGF) (DSPN vs control, 140.93 ± 24.78 vs 157.39 ± 25.11 pg/mL, P < 0.01) than those in control group. DSPN group had the highest level of soluble intercellular adhesion molecule-1 (sICAM-1) among three groups (DSPN and DM vs control, 1477.56 ± 228.00 and 1342.17 ± 237.54 vs 1308.00 ± 200.94 ng/mL, P < 0.05). The level of nerve growth factor in the DSPN group was slightly lower than that in the DM group (DSPN vs DM, 3509.11 ± 438.39 vs 3734.87 ± 647.50 pg/mL, P < 0.05). All patients with DSPN received one intramuscular injection of BM-MNCs and clinical follow-ups after the therapy for 2 days, 1, 4, 12, 24, and 48 weeks. Neuropathic symptoms of foot pain, numbness, and weakness were significantly improved within 4 weeks after BM-MNCs injection. Patients with DSPN were divided into the responder (n = 35) and nonresponder groups (n = 19) based on the improvement of nerve conduction velocity at 12 weeks post-transplantation. Compared with nonresponders, responders were younger (57.3 ± 5.2 vs 62.0 ± 4.8, P < 0.01), had a shorter history of diabetes (7.1 ± 2.7 vs 11.2 ± 5.4 years, P < 0.01), and had higher numbers of mobilized CD34+ cells (17.61 ± 2.64 vs 14.79 ± 1.62 ×105/L, P < 0.01) and BM-MNCs (12.05 ± 2.16 vs 9.84 ± 1.53 ×108/L, P < 0.01). The levels of TNF-α and sICAM-1 decreased just after BM-MNCs injection in both groups and slowly reverted to baseline levels. The duration of the downtrend of TNF-α and sICAM-1 in the responder group lasted longer than that in the nonresponder group. Serum level of VEGF in the responder group increased immediately after BM-MNC therapy and reached the highest point after the injection for 12 weeks. On the other hand, VEGF levels in the nonresponder group only increased slightly. Binary logistic regression was performed to evaluate the corresponding prognostic factors for BM-MNCs treatment. The number of applied CD34+ cells and the duration of diabetes were the independent predictors of responding to BM-MNCs therapy. No adverse event associated with the treatment was observed during follow-up observations. These results indicated that BM-MNCs transplantation is an effective and promising therapeutic strategy to treat refractory DSPN. The immune regulation and paracrine function of BM-MNCs may contribute to the improvement of DSPN.
Trial registration:
Chinese Clinical Trial Registry, ChiCTR-TRC-12002570.
Keywords: bone marrow mononuclear cells, diabetic peripheral neuropathy, autologous transplantation, clinical trial
Introduction
Diabetic peripheral neuropathy (DPN) is the most common complication of Type 2 Diabetes Mellitus (T2DM), affecting approximately half of the long-standing T2DM patients1. Several different patterns of neuropathy can present in individuals with diabetes. Of these, the most common is diabetic sensorimotor polyneuropathy (DSPN), which is characterized by the symmetrical loss of sensation in the lower limbs, resulting in increased risks of traumatic injuries or chronic pain perceiving a significant reduction of quality of life and high treatment costs2. However, novel strategies of treating DPN are limited to intensive glucose control and pain alleviating, which have a marginal therapeutic effect in patients with the progressive stage3. With poor prognostic outcomes and high mortality, “No-treatment-options” patients are often suffered by recurrent or incurable foot ulcerations and gangrenous infections, eventually leading to limb amputation.
The pathogenic mechanisms of DPN are multifactorial. It is speculated that diabetic neuropathy is secondary to the deficiency of local growth factors, causing a reduction of vasa vasorum and neuronal damage in diabetic peripheral nerves4. A number of inflammatory mediators are also involved in the progression of DPN5. Stem cell-based therapies aiming at enhancing neovascularization and ameliorating inflammatory cytokines are emerging as a novel treatment strategy for patients with DSPN who are not eligible for traditional drug therapy. Recently, bone marrow-derived stem cell-based therapies have been applied as a promising strategy for experimental diabetic neuropathy in animal models because of their multipotency to inhibit inflammation and promote angiogenesis and neurotrophy6,7.
In our previous study, bone marrow-derived mononuclear cells (BM-MNCs) have been applied in 168 patients with refractory DSPN, demonstrating that BM-MNCs transplantation is an effective and safe therapeutic strategy8. Through regular follow-up and continuous monitoring of clinical indicators, the present study aimed to evaluate the potential mechanisms associated with the therapeutic benefits of BM-MNCs therapy in patients with DSPN.
Materials and Methods
Ethical Approval
This clinical study was undertaken at the Central Hospital of Wuhan, Hubei Province, China. All participants were briefly informed about this study. Informed consent was obtained during the study enrollment. This study was approved by the Ethics Committee of the Central Hospital of Wuhan, Wuhan, China.
Study Design and Participants
In this single-center clinical study, we investigated the clinical effect of BM-MNCs transplantation in 60 patients with refractory DSPN recruited from March 2014 to December 2017. The detailed inclusion and exclusion criteria were listed below. All enrolled patients received the autologous BM-MNCs therapy and followed-up after transplantation for 2 days, 1, 4, 12, 24, and 48 weeks.
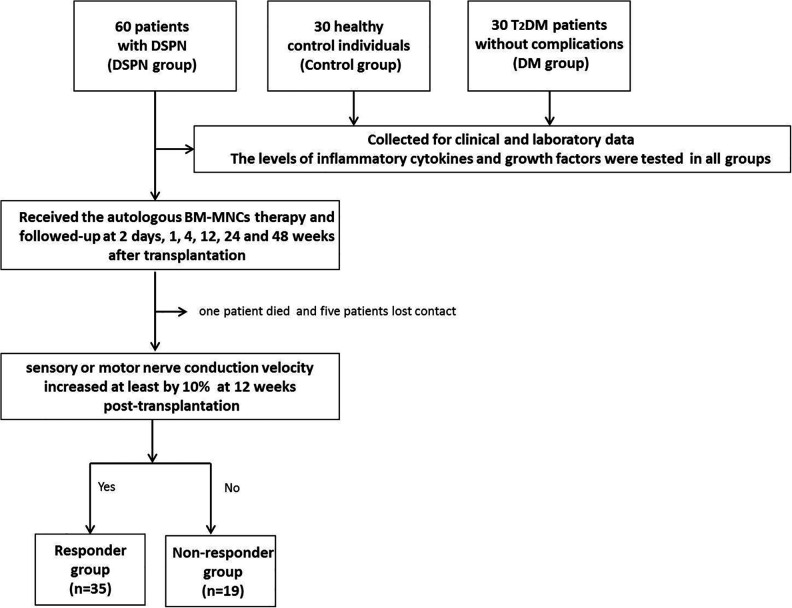
This clinical study also included 30 healthy control individuals (control group) and 30 T2DM patients without complications (DM group). Age, sex, and body mass index (BMI) in all three groups were comparable (all P > 0.05). The flow chart is shown in Fig. 1.
Figure 1.
The flow chart of the study. A schematic overview illustrated participant enrolment in the cohort study. Briefly, a total of 60 patients with DSPN, 30 healthy control participants, and 30 T2DM patients without complications were included. All patients with DSPN received the autologous BM-MNCs therapy and followed-up after transplantation for 2 days, 1, 4, 12, 24, and 48 weeks. During the follow-up period, one patient died and five patients lost contact and did not show in the follow-ups due to various reasons. Then they were divided into the responder (n = 35) and nonresponder groups (n = 19) based on the improvement of nerve conduction velocity (NCV) at 12 weeks post-transplantation.
Inclusion Criteria of DSPN Group
Age at 40–70 years.
T2DM patients defined by the 2013 American Diabetes Association (ADA) standards9.
DSPN was defined as the presence of an abnormality of nerve conduction and a symptom or a sign of distal symmetric polyneuropathy10.
Refractory DSPN, defined as no significant relief of the neuropathic symptoms or signs when combining conventional drug therapies for at least 1 year, which include the antioxidant (α-lipoic acid), aldose reductase inhibitors, and transketolase activators (thiamines and allithiamines).
Exclusion Criteria of DSPN Group
Severe hepatic and renal dysfunctions.
Hypercoagulable states or with hematological diseases.
Foot ulcers and limb deformity.
Pregnancy.
Evidence of malignancy during the last 5 years.
Life expectancy less than 6 months.
Preparation of BM-MNCs
The procedures used for the BM-MNCs treatment have been described in detail previously8. Briefly, patients were subcutaneously injected 5 ug/kg/day recombinant human granulocyte colony-stimulating factor (G-CSF; Qilu Pharmaceutical, China) for three consecutive days to mobilize the stem cells in the bone marrow. After G-CSF mobilization, approximately 200–300 ml bone marrow was harvested from the posterior superior iliac crest under anesthetic conditions in a sterile surgical environment.
The preparation of BM-MNCs was processed in the laminar flow laboratory. Mononuclear stem cells were isolated by Ficoll–Hypaque density-gradient centrifugation. Then, the mononuclear cell layer was harvested and washed three times with normal saline and resuspended in 50 ml normal saline. The total concentration of CD34+ cells in the cell suspension comprising mononuclear cells was calculated using flow cytometry.
Transplantation Procedures
The prepared BM-MNCs suspensions were injected intramuscularly to both thighs and legs of DSPN patients (50 sites, 2 cm × 2 cm in intervals, 1–1.5 cm in depth, 1 ml BM-MNCs per site) under continuous monitoring of vital parameters in a sterile surgical environment. Patients were followed-up for at least 24 h in the intensive care unit after the injection.
Clinical Assessment Before and After Transplantation
The visit schedule for the DSPN group is shown in Supplemental Table 1. Clinical and laboratory data were collected before BM-MNCs administration and at every follow-up visit in the DSPN group. All patients received similar ordinary treatment throughout the course of this clinical study, including intensive control of blood glucose, blood pressure, and blood lipids. Smoking cessation was encouraged during the study. Attention was paid during the follow-up visits specifically to any potential adverse effects due to the transplantation.
Measurement of nerve conduction studies (NCS; Viking Quest, Nicolet Biomedical Inc, WI, USA) was performed by the same experienced technician who was blinded to the patients’ clinical information according to validated standards. Routine NCS measurements were performed pretransplantation and at 12 and 48 weeks post-transplantation. The observation items of NCS included sensory NCV (sNCV), sensory nerve action potential in superficial peroneal and sural nerves, motor NCV (mNCV), and compound muscle action potential in peroneal and posterior tibial nerves.
Neurological evaluations were performed with Toronto Clinical Scoring System (TCSS) before the therapy and at 4, 12, 24, and 48 weeks post-transplantation, including neuropathic symptom (foot pain, numbness, tingling, weakness, ataxia, and upper limb symptoms), lower limb reflex, and sensory tests (pinprick, temperature, light touch, vibration, and position sensation). The maximum TCSS score is 19.
Blood samples of all the subjects in the study were collected to test the levels of inflammatory markers and growth factors at baseline and at each post-transplantation follow-up visit in the DSPN group. Venous blood samples were collected from the subjects after a 12-h overnight fast. The serum was separated and stored at −70 °C. Commercially available enzyme immunoassay (enzyme-linked immunosorbent assay) kits were used to measure the inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor-alpha [TNF-α], IL-10, soluble intercellular adhesion molecule-1 [sICAM-1]) and growth factors (vascular endothelial growth factor [VEGF] and nerve growth factor [NGF]) according to the manufacturer’s instruction. All kits were provided by Quantikine, R&D Systems.
Outcome Assessment of BM-MNCs Transplantation
The primary endpoint of the study was the improvement of NCV. Responder to BM-MNCs therapy was defined as the sensory or motor NCV increased at least by 10% at 12 weeks post-transplantation. Patients without an obvious change of NCV were considered nonresponders.
Statistical Analysis
Measurement data were expressed as mean ± standard deviation (SD) for continuous variables, interquartile ranges for non-normal data, and in percentages for discrete variables. Group differences for a continuous variable were tested by independent t-test or the Mann–Whitney U test. Dichotomous variables were analyzed with Fisher’s exact test. Multiple group comparisons were performed by analysis of variance. The comparison between baseline and each follow-up visit measurements was performed by employing the paired t-test. Subsequently, multiple binary logistic regression analysis was used to study predictors of clinical benefit after BM-MNCs transplantation. Statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Baseline Characteristics of the Entire Study Cohort
The baseline characteristics of the entire study cohort are shown in Table 1. All groups were matched for age, gender, and BMI. The history of smoking and alcohol consumption was similar among the three groups. Compared with those without diabetic complications, patients with diabetic neuropathy had a significantly longer duration of diabetes and significantly higher levels of fasting plasma glucose and glycated hemoglobin (P < 0.001). Systolic and diastolic blood pressure values were lower in the control group but similar in the two diabetic groups (P < 0.05). In the aspect of lipid metabolism, the level of triglyceride was lower in the control group compared with the other two diabetic groups, and the level of high-density lipoprotein was higher in the control group than DSPN group (P < 0.05). Compared with the control group, a higher proportion of patients in the DM group and DSPN group had coronary heart disease and experienced stroke (P < 0.05). As expected, the treatments with medications of antihypertension, lipid-lowering, and antiplatelet were more common in two diabetic groups. Regarding the hypoglycemic therapies used in the two diabetic groups, the use of insulin was marginally higher in the DSPN group compared with the DM group. No difference in the remaining diabetic medications was observed.
Table 1.
Clinical Characteristics of Studied Subjects.
| Study groups | DM | Control | DSPN | Responders | Nonresponders |
|---|---|---|---|---|---|
| N | 30 | 30 | 60 | 35 | 19 |
| Gender (F/M) | 16/14 | 15/15 | 29/31 | 16/19 | 12/7 |
| Age(years)a | 57.6 ± 4.8 | 58.2 ± 4.6 | 59.3 ± 5.6 | 57.3 ± 5.2 | 62.0 ± 4.8 |
| Diabetic duration (years)a | 1.7 ± 1.6 | / | 9.8 ± 5.1 | 7.1 ± 2.7 | 11.2 ± 5.4 |
| Smoking (%) | 15 (50.0%) | 13 (43.3%) | 30 (50.0%) | 17 (48.6%) | 8 (42.1%) |
| Alcohol consumption (%) | 7 (23.3%) | 7 (23.3%) | 16 (26.7%) | 12 (34.3%) | 2 (10.5%) |
| BMI (kg/m2) | 23.74 ± 2.81 | 23.43 ± 2.31 | 23.20 ± 2.45 | 22.75 ± 2.32 | 23.3 ± 2.09 |
| SBP (mmHg)b | 128.2 ± 15.9 | 115.0 ± 14.6 | 130.5 ± 13.0 | 130.8 ± 13.8 | 130.8 ± 12.6 |
| DBP (mmHg)c | 75.9 ± 10.6 | 71.7 ± 10.4 | 77.0 ± 10.3 | 78.1 ± 11.1 | 75.3 ± 9.3 |
| FPG (mmol/L)d | 7.78 ± 2.43 | 4.66 ± 0.42 | 10.54 ± 3.41 | 10.50 ± 3.06 | 10.47 ± 4.31 |
| HbA1c (%)d | 7.55 ± 1.76 | 5.01 ± 0.28 | 9.98 ± 2.52 | 9.61 ± 2.53 | 10.54 ± 2.74 |
| TG (mmol/L)e | 1.78 ± 1.15 | 1.26 ± 0.70 | 1.73 ± 0.80 | 1.78 ± 0.88 | 1.67 ± 0.75 |
| TC (mmol/L) | 4.38 ± 0.99 | 4.61 ± 0.76 | 4.59 ± 1.31 | 4.50 ± 1.38 | 4.72 ± 1.35 |
| HDL (mmol/L)f | 1.16 ± 0.39 | 1.28 ± 0.30 | 1.09 ± 0.25 | 1.12 ± 0.22 | 1.08 ± 0.33 |
| LDL (mmol/L) | 2.49 ± 0.78 | 2.57 ± 0.57 | 2.79 ± 1.02 | 2.93 ± 1.11 | 2.46 ± 0.91 |
| Scr (umol/L) | 64.24 ± 12.92 | 66.68 ± 16.79 | 71.89 ± 19.96 | 68.95 ± 20.61 | 77.10 ± 20.34 |
| Albnmin (g/L) | 41.99 ± 2.82 | 42.42 ± 1.58 | 41.21 ± 3.13 | 40.97 ± 3.00 | 41.55 ± 3.72 |
| History of stroke (%)f | 5 (16.7%) | 0 (0.0%) | 14 (23.3%) | 7 (20.0%) | 7 (36.8%) |
| History of CAD (%)g | 5 (16.7%) | 0 (0.0%) | 26 (43.3%) | 12 (34.3%) | 11 (57.9%) |
| Hypertension medication (%)h | 7 (23.3%) | 0 (0.0%) | 29 (48.3%) | 19 (54.3%) | 7 (36.8%) |
| Lipid-lowering medication (%)h | 8 (26.7%) | 0 (0.0%) | 33 (55.0%) | 19 (54.3%) | 11 (57.9%) |
| Antiplatelet agents (%)f | 5 (16.7%) | 0 (0.0%) | 14 (23.3%) | 7 (20.0%) | 6 (31.6%) |
| Diabetic medications (%) | |||||
| Insulin (%) | 14 (46.7%) | – | 41 (68.3%) | 22 (62.9%) | 15 (78.9%) |
| Sulfonylureas (%) | 2 (6.7%) | – | 12 (20.0%) | 8 (22.9%) | 3 (15.8%) |
| Metformin (%) | 22 (73.3%) | – | 39 (65.0%) | 23 (65.7%) | 12 (63.2%) |
| Alpha-glucosidase inhibitors (%) | 15 (50.0%) | – | 36 (60.0%) | 20 (57.1%) | 11 (57.9%) |
| Pioglitazone (%) | 5 (16.7%) | – | 13 (21.7%) | 6 (17.1%) | 5 (26.3%) |
| Glinides (%) | 1 (3.3%) | – | 3 (5.0%) | 2 (5.7%) | 1 (5.3%) |
| DPP-4 inhibitors (%) | 9 (30.0%) | – | 19 (31.7%) | 13 (37.1%) | 5 (26.3%) |
| Biomarkers of cytokines | |||||
| IL-6 (pg/mL) | 71.26 ± 12.21 | 70.13 ± 11.17 | 74.61 ± 10.28 | 75.30 ± 10.54 | 73.66 ± 11.26 |
| IL-10 (pg/mL) | 653.83 ± 92.99 | 645.02 ± 79.41 | 630.81 ± 110.89 | 637.26 ± 106.05 | 590.90 ± 106.89 |
| TNF-α (pg/mL)f | 402.31 ± 55.47 | 374.81 ± 63.18 | 412.90 ± 64.58 | 416.34 ± 57.89 | 401.99 ± 74.34 |
| sCIAM-1 (ng/mL)g | 1342.17 ± 237.54 | 1308.00 ± 200.94 | 1477.56 ± 228.00 | 1501.21 ± 249.15 | 1439.96 ± 188.28 |
| NGF (pg/mL)i | 3734.87 ± 647.50 | 3771.08 ± 655.86 | 3509.11 ± 438.39 | 3578.58 ± 499.20 | 3435.49 ± 345.35 |
| VEGF (pg/mL)f | 149.62 ± 26.30 | 157.39 ± 25.11 | 140.93 ± 24.78 | 143.21 ± 25.39 | 134.83 ± 21.65 |
| CD34+ cells ×105/La | / | / | 16.33 ± 2.71 | 17.61 ± 2.64 | 14.79 ± 1.62 |
| Mononuclear cells ×108/La | / | / | 10.99 ± 2.27 | 12.05 ± 2.16 | 9.84 ± 1.53 |
Data are expressed as mean ± SD or number (percentage).
BMI, body mass index; CAD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; DPP-4: dipeptidylpeptidse 4; DSPN, diabetic sensorimotor polyneuropathy; FPG, fasting plasma glucose; HDL, high density lipoprotein; HbA1c, glycosylated hemoglobin; ICAM, intercellular adhesion molecule; IL, interleukin; LDL, low density lipoprotein; NGF, nerve growth factor; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
a Responder versus nonresponder, P < 0.01.
b DSPN versus control, P < 0.0001; DM versus control, P < 0.001.
c DSPN versus control, P < 0.05.
d DSPN versus control, P < 0.0001; DM versus control, P < 0.0001; DSPN versus DM, P < 0.0001.
e DSPN versus control, P < 0.01; DM versus control, P < 0.05.
f DSPN versus control, P < 0.01.
g DSPN versus control, P < 0.001; DSPN versus DM, P < 0.05.
h DSPN versus control, P < 0.0001; DM versus control, P < 0.05; DSPN versus DM, P < 0.05.
i DSPN versus DM, P < 0.05.
The measurements of growth factors and inflammatory markers are shown in Table 1. The levels of TNF-α and VEGF in the DSPN group were respectively higher and lower than that in the control group (P < 0.05). DSPN group had a higher level of sICAM-1 than the other two groups (P < 0.05) and a lower level of NGF than the DM group (P < 0.05).
Clinical Efficacy of Transplantation
During the follow-up period, one patient died (36 weeks after BM-MNCs transplantation) because of myocardial infarction, and five patients lost contact and did not show in the follow-ups due to various reasons. These patients were excluded from further data analysis.
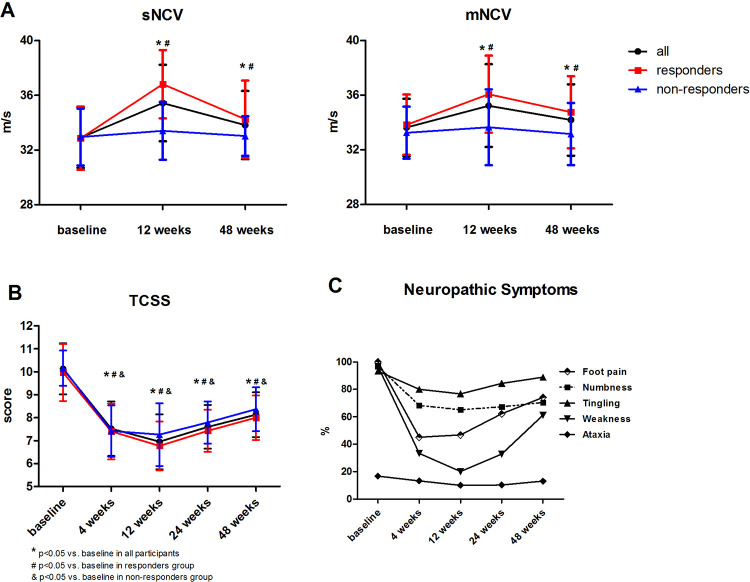
The primary endpoint of the study was reached in 35 of 54 patients with DSPN (64.8%). NCS revealed that both sNCV and mNCV notably improved at 12 weeks after transplantation (Fig. 2A, Supplemental Table 2). sNCV in responder and nonresponder groups increased by 12% and 1.5%, respectively, whereas the increased levels of mNCV in the two groups were 6.6% and 1.2%, respectively. Both groups showed persistent improvement in neuropathic symptoms and signs, which can be reflected from the decline of TCSS (Fig. 2B, Supplemental Table 3). Neuropathic symptoms of foot pain, numbness, and weakness were significantly improved within 4 weeks after BM-MNCs injection and remained remission till 12 weeks. The symptom of weakness improved the most significantly at 24 weeks post-therapy (Fig. 2C, Supplemental Table 4).
Figure 2.
Neurological examinations before and after BM-MNCs therapy. (A) Both sensory and motor never conduction velocity notably improved at 12 weeks after transplantation. (B) The TCSS score decreased after BM-MNCs therapy and remained this downtrend to 48 weeks. (C) The change of neuropathic symptoms before and after BM-MNCs therapy. sNCV, sensory nerve conductive velocity; mNCV, motor nerve conductive velocity; TCSS, Toronto Clinical Scoring System.
The characteristics of responder and nonresponder groups before BM-MNCs therapy were listed in Table 1. Compared with that in the nonresponder group, patients in the responder group were younger (P < 0.01) and with a longer duration of diabetes (P < 0.01). BM-MNCs products of the responder group were characterized by a higher CD34+ hematopoietic progenitor cell count (P < 0.001) and a higher number of total BM-MNCs (P < 0.001) than that of non-responders.
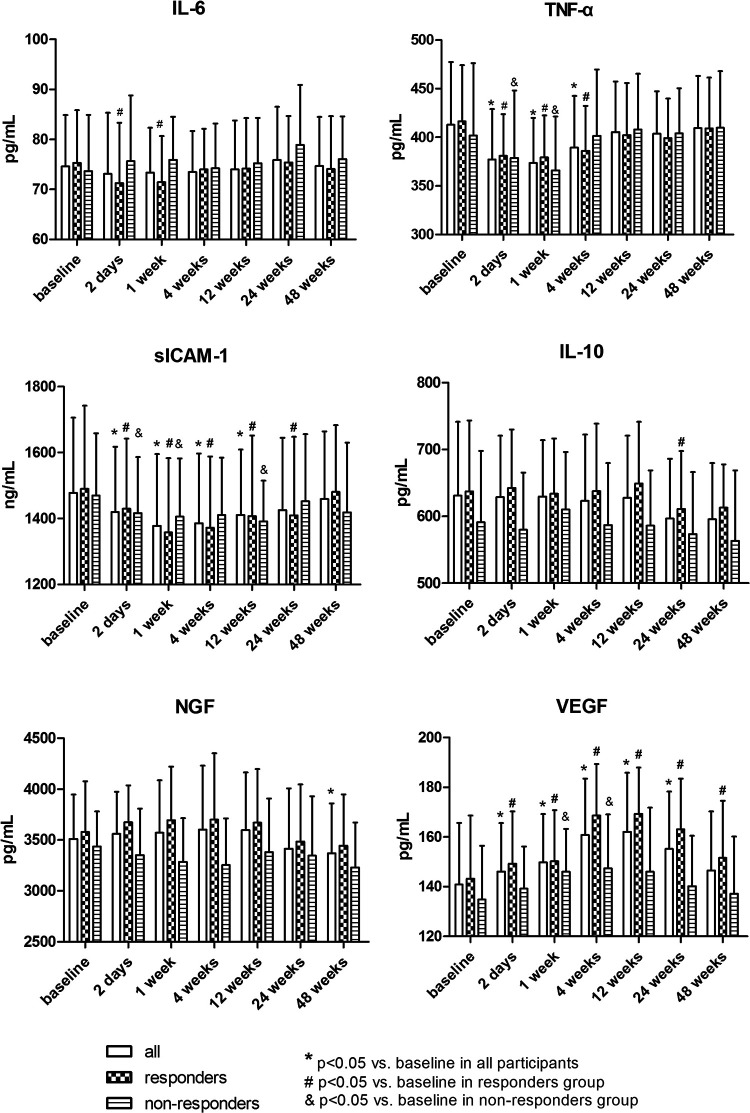
As shown in Fig. 3, the levels of TNF-α and sICAM-1 decreased just after BM-MNCs transplantation in all groups and reverted to the baseline level at 12 weeks and 24 weeks, respectively. This downtrend duration lasted longer in the responder group than in the nonresponder group, suggesting that the immune regulation function of BM-MNCs may play a critical part in the treatment of DSPN. The serum level of VEGF in the responder group was upregulated immediately after BM-MNC therapy, reaching the highest level at 12 weeks, whereas we only viewed a slight increase of VEGF in the nonresponder group. The baseline levels of other cytokines were similar to those after BM-MNCs therapy.
Figure 3.
Growth factors and inflammatory cytokines before and after bone marrow mononuclear cell therapy.
Procedural Safety
No infection, bleeding, or other complications associated with BM-MNCs transplantation were detected. Three patients (5%) experienced short-term episodes of slight pain at the injection sites 2 h after BM-MNCs transplantation, which can be well tolerated with only mild discomfort.
During follow-up, no effect on liver or kidney functions was observed, and no case of rejection or malignancy was detected in all participants.
Prognostic Factors for BM-MNCs Treatment
Prognostic factors are important when deciding on treatment options. We evaluated the clinical outcomes and identified the corresponding prognostic factors for BM-MNCs treatment of patients with DSPN. Binary logistic regression was performed to ascertain the effects of age, duration of diabetes, the number of CD34+ cells, and BM-MNCs on the likelihood of responding to the cell therapies. The binary logistic regression model was statistically significant (×2(4) = 4.596, P = 0.032). The model explained 46.6% (Nagelkerke R 2) of the variance in treatment outcome and correctly classified 79.6% of cases. The number of applied CD34+ cells (P = 0.012, Exp(B) = 1.567, 95% confidence interval (CI) 1.106–2.222) and duration of diabetes (P = 0.025, Exp(B) = 0.760, 95% CI 0.597–0.967) emerged as independent predictors of responding to the cell therapy.
The number of total BM-MNCs positively correlated with an increase of VEGF after 4 weeks (P = 0.023, r = 0.292). A similar but weaker correlation was observed between the absolute number of CD34+ cells and the increase of VEGF after 4 weeks (P = 0.041, r = 0.264) (Fig. 4).
Figure 4.
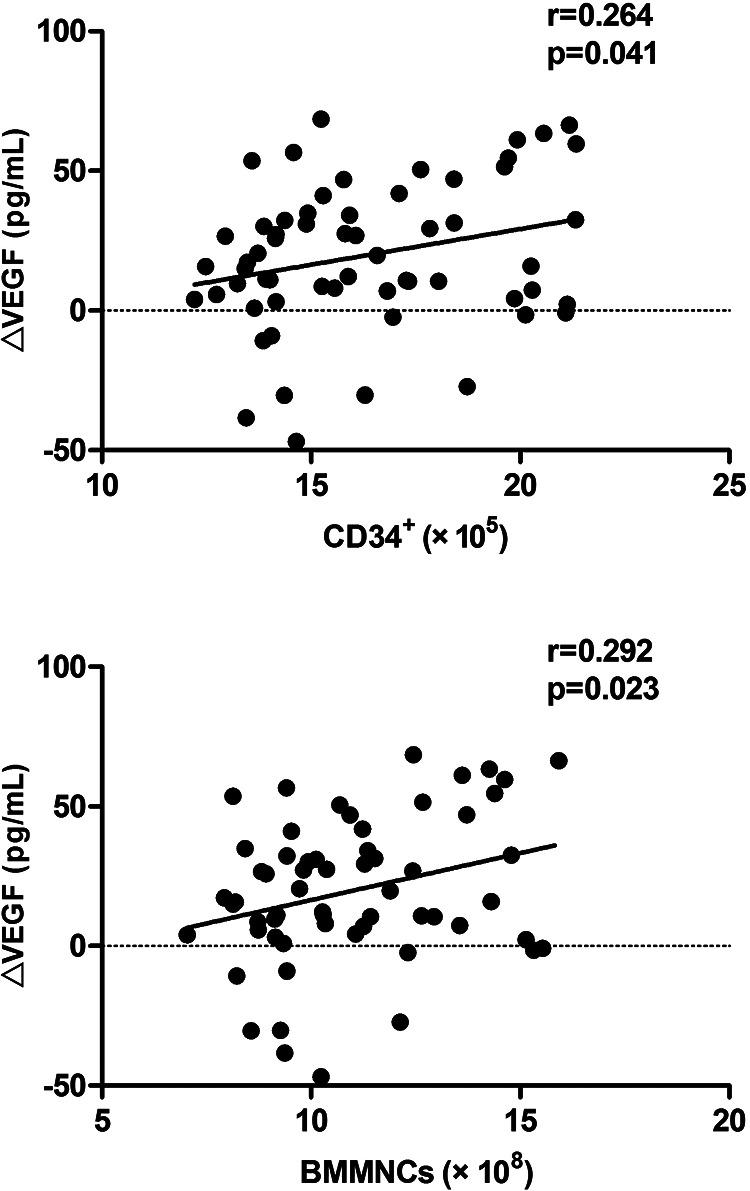
The correlation between an increase of VEGF at 4 weeks post-transplantation and the numbers of CD34+ cells and BM-MNCs. BM-MNC, bone marrow mononuclear cell; VEGF, vascular endothelial growth factor.
Discussion
Almost 50% of long-standing T2DM patients are affected by DPN11. and up to 20% will experience peripheral chronic neuropathic pain12, resulting in a dramatically decreased quality of life. Currently, no curable therapy is available for patients with the progressive stage of DPN. Intensive glucose control is a recommended strategy in ADA guidelines, which may prevent the progression of DPN. However, it cannot cure already established nerve injuries. For patients who experienced abnormal neuropathic symptoms took the maximum dose of symptomatic treatment, the relief is usually incomplete. As a result, the management of effective therapy for refractory DPN is urgently needed.
Our previous clinical study showed that autologous transplantation of BM-MNCs effectively improved neuropathic symptoms and restored the peripheral nerve functions, which demonstrated BM-MNCs transplantation was an effective and safe treatment for refractory DSPN. In this follow-up study, first, we compared the concentrations of inflammatory cytokines and growth factors in healthy individuals, diabetes mellitus patients without complications, and DSPN patients. Our present study showed that patients with DSPN had relatively higher levels of TNF-α, sICAM-1, and lower levels of VEGF and NGF when compared with healthy control participates and diabetic patients without complications, which indicate that the association between various levels of inflammatory cytokines and growth factors may participate in the development and progression of DSPN.
Known pathologies reported in DSPN included axonal atrophy, demyelination, nerve fiber loss, and blunted regeneration of nerves. In addition to these pathogeneses, several studies have reported the link between subclinical inflammation and diabetic neuropathy13,14. Diabetic TNF-α−/− mice show no evidence of abnormal nerve function. A single injection of infliximab to suppress the level of TNF-α in diabetic TNF-α+/+ mice ameliorates the electrophysiological and biochemical deficits15. The involvement of TNF-α in chronic inflammation regulation and immune response may result in various nerve damages. As one of the cell adhesion molecules, sICAM-1 reflects a low-grade vascular inflammation and may be associated with the development of a diabetic complication16. The level of sICAM-1 is higher in patients with DSPN compared with those without complications17. The levels of sICAM-1 are associated with NCV and vibration perception thresholds, suggesting a vascular involvement in the development of DSPN18,19. VEGF induces angiogenesis by stimulating the proliferation and migration of endothelial cells in ischemic tissues, improving tissue ischemia20. Both VEGF and NGF promote neural regeneration and survival of neurons21. An increase in inflammatory markers and a decrease in growth factors were in mutual effect with each other and in synergistic effect with the development and progression of DSPN.
These findings suggest that a therapeutic method targeting both inflammation regulation and improvement of growth factors may be an effective treatment of DSPN. Recently, the transplantation of BM-MNCs has attracted a great deal of attention as a possible therapeutic approach for various clinical targets, including ischemic heart disease22,23 and peripheral arterial disease24. Various animal studies have shown the beneficial effects of using BM-MNCs in treating the experimental DPN model7. Intramuscular injection of BM-MNCs preferentially targets to peripheral nerves, especially around vasa nervorum, and increases expression of angiogenic and neurotrophic factors. In Hasegawa’s study, using a neutralizing antibody of VEGF suppresses the therapeutic effect of BM-MNCs, which further confirms the mechanism of BM-MNCs’ paracrine property7. The expression of angiogenic and neurotrophic factors results in the improvement of vasa nervorum functions25. NGF releasing from BM-MNCs improves regeneration of sciatic nerve in adult rats and stimulates the proliferation of Schwann and satellite cells26. The immune-suppressive property of BM-MNCs to ameliorate inflammatory reaction in the peripheral nerve has not been observed in DPN animal models. But in a study including Segment Elevation Myocardial Infarction (STEMI) patients, BM-MNCs transplantation was performed via an intracoronary route. Their results indicated that BM-MNCs transplantation after STEMI affects the balance between proinflammatory and anti-inflammatory cytokines27. Additional studies are required to fully understand the immunomodulation effects of BM-MNCs transplantation in treating DPN.
To investigate the mechanisms underlying the therapeutic effect of BM-MNC in patients with DSPN, we examined the changes of several growth factors and inflammation cytokines after BM-MNCs transplantation. The serum levels of TNF-α and sICAM-1 decreased at 2 days after BM-MNCs transplantation and this decrement persisted within the next 4 weeks. The level of VEGF moderately increased and reached the maximum at 12 weeks after BM-MNCs transplantation. We divided the DSPN patients into the responder and nonresponder groups based on the improvement of NCV. The degree and duration of trends of VEGF, TNF-α, and sICAM-1 variations are more pronounced in the responder group than the nonresponder group. We are interested to find that IL-10, an anti-inflammatory cytokine, were slightly lower in the nonresponder group than the responder group. Although the difference did not reach statistical significance, it may suggest that IL-10 may be a protective factor for DSPN. It was also reported that IL-10 inhibits apoptosis of Schwann cells induced by advanced glycation end products (AGEs) in vitro 28. Thus, through our study, we speculated that both the paracrine effect of growth factors and immune regulation of BM-MNCs participated in treating DSPN. The anti-inflammatory effect may take effect in the early stage of the treatment, and the paracrine effect started later and lasted for a relatively longer time. In our study, we observed several neuropathic symptoms, such as foot pain, numbness, and weakness, were improved the most significantly at 12 weeks after BM-MNCs therapy. However, most of the neuropathic symptoms reappeared at 48 weeks. The changes in neuropathic symptoms were consistent with the changes in NCV and might be due to the changes in inflammatory cytokines and growth factors. Given that DSPN is a disease progressing over a long time, a single injection of BM-MNCs may not be enough to maintain the nerve function over a long period of time. The administration route and frequency of BM-MNCs need further investigation.
The present study investigated prognostic factors predictive of the therapeutic effect of BM-MNCs. Binary logistic regression revealed that the number of applied CD34+ cells and the duration of diabetes are the independent predictors of responding to BM-MNCs therapy. The absolute number of applied BM-MNCs and CD34+ were positively correlated with the increase of VEGF after 4 weeks of transplantation. It was postulated that the amount of CD34+ cells had a crucial influence on the outcome of BM-MNCs therapy. CD34+, which is broadly accepted as a marker of hematopoietic progenitor cells, also expressed on the surface of endothelial progenitor cells. CD34+ cells have the potential for neovascularization in ischemic tissue to restore the microcirculation and improve tissue perfusion29. Patients with T2DM showed impaired mobilization ability of proangiogenic cells and CD34+ cells after G-CSF stimulation30. In a meta-analysis of the trails using G-CSF to stimulate bone marrow stem cells in treating patients with cardiovascular disease, there is a strong negative correlation between the prevalence of diabetes and the mobilization of CD34+ cells in response to G-CSF31. These reports strongly imply that diabetes may adversely affect the therapeutic potential of autologous BM-MNCs therapy in patients with diabetic neuropathy. Patients with longer diabetic duration may have a worse survival and mobilization potential of bone marrow stem cells. Future clinical studies should consider the timing of bone marrow stem cell therapy and how to improve the mobilization of bone marrow stem cells in patients with diabetes. Several small molecules or growth factors can be used to improve the mobilization ability of stem cells32–34. More efforts are needed to transform these experimental findings into clinical applications in the future.
To our knowledge, this is the first clinical study to explore the clinical efficacy, safety, and possible mechanisms of BM-MNCs therapy for patients with refractory DSPN. Several limitations of our study must be mentioned. First of all, the low number of participates with DSPN and short follow-up duration may decrease the statistical power of the study. The lack of a placebo-treated group may lead to a biased effect. Second, we used the improvement of NCV as the primary endpoint of our study, instead of the invasive method of peripheral nerve and cutaneous biopsies, which cannot prove the neovascularization mechanisms of BM-MNCs directly. Thus, future randomized double-blinded controlled trials with larger sample sizes and longer-term follow-ups using biopsy of histological evaluation as the primary endpoint are needed to assess the therapeutic effect of BM-MNCs transplantation. Third, using unfractionated BM-MNCs in the present study, we cannot find out which type of cells among the overall BM-MNCs is the most responsible for the beneficial effects to relieve the neurological symptoms.
In conclusion, BM-MNCs therapy may become a potential therapeutic option to treat diabetic neuropathy via the mechanism of paracrine and immunoregulation effect.
Supplemental Material
Supplement_materials_0703 for Autologous Bone Marrow Mononuclear Cell Transplantation Therapy Improved Symptoms in Patients with Refractory Diabetic Sensorimotor Polyneuropathy via the Mechanisms of Paracrine and Immunomodulation: A Controlled Study by Wei Wei, Li Li, Lin Deng, Zhong-Jing Wang, Jing-Jian Dong, Xiao-Yu Lyu, Ting Jia, Li Wang, Hong-Xiang Wang, Hong Mao and Shi Zhao in Cell Transplantation
Footnotes
Author Contributions: WW and ZS contributed to the conception and design of the study. MH and ZS contributed to funding acquisition. WW and LL acquired and analyzed the data. WW wrote and revised the manuscript and contributed to the discussion. MH and ZS reviewed and edited the manuscript and contributed to the discussion. DJJ performed the nerve conduction study. WL and WHX performed the preparation of BM-MNCs. DL, WZJ, JT, and LXY performed the procedures of BM-MNCs transplantation. All authors read and approved the final manuscript.
Ethics Approval: This clinical study was approved by our institutional review board.
Statement of Human and Animal Rights: This clinical study was approved by the Ethics Committee of the Central Hospital of Wuhan (Hubei, China, Ethical approval No. 2014-003).
Statement of Informed Consent: Written informed consent was obtained from all of the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/ or authorship of this article: This work was supported by a grant from the Research Fund for projects of Hubei provincial Health Department (No. JX4B56) and the National Natural Science Foundation of China (No. 81370942).
ORCID iD: Shi Zhao  https://orcid.org/0000-0002-8093-8335
https://orcid.org/0000-0002-8093-8335
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(1):136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Javed S, Alam U, Malik RA. Treating diabetic neuropathy: present strategies and emerging solutions. Rev Diabet Stud. 2015;12(1–2):63–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47(6):1055–1063. [DOI] [PubMed] [Google Scholar]
- 5. Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a therapeutic target for diabetic neuropathies. Curr Diab Rep. 2016;16(3):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim H, Park JS, Choi YJ, Kim MO, Huh YH, Kim SW, Han JW, Lee J, Kim S, Houge MA, Ii M, et al. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27(7):1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasegawa T, Kosaki A, Shimizu K, Matsubara H, Mori Y, Masaki H, Toyoda N, Inoue-Shibata M, Nishikawa M, Iwasaka T. Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Exp Neurol. 2006;199(2):274–280. [DOI] [PubMed] [Google Scholar]
- 8. Mao H, Wei W, Fu XL, Dong JJ, Lyu XY, Jia T, Tang Y, Zhao S. Efficacy of autologous bone marrow mononuclear cell transplantation therapy in patients with refractory diabetic peripheral neuropathy. Chin Med J (Engl). 2019;132(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, Green J, Palumbo P, Perkins BA, Whitehouse F, Jones TL. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36(10):3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8(Suppl 2):S50–S62. [DOI] [PubMed] [Google Scholar]
- 13. Herder C, Lankisch M, Ziegler D, Rathmann W, Koenig W, Illig T, Döring A, Thorand B, Holle R, Giani G, Martin S. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany). Diabetes Care. 2009;2(4):680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herder C, Bongaerts BWC, Rathmann W, Heier M, Kowall B, Koenig W, Thorand B, Roden M, Meisinger C, Ziegler D. Differential association between biomarkers of subclinical inflammation and painful polyneuropathy: results from the KORA F4 study. Diabetes Care. 2015;38(1):91–96. [DOI] [PubMed] [Google Scholar]
- 15. Yamakawa I, Kojima H, Terashima T, Katagi M, Oi J, Urabe H, Sanada M, Kawai H, Chan L, Yasuda H, Maegawa H. Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab. 2011;301(5):E844–E852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blüher M, Unger R, Rassoul F, Richter V, Paschke R. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia. 2002;45(2):210–216. [DOI] [PubMed] [Google Scholar]
- 17. Zakareia FA. Electrophysiological changes, plasma vascular endothelial growth factor, fatty acid synthase, and adhesion molecules in diabetic neuropathy. Neurosciences (Riyadh). 2008;3(4):374–379. [PubMed] [Google Scholar]
- 18. Jude EB, Abbott CA, Young MJ, Anderson SG, Douglas JT, Boulton AJ. The potential role of cell adhesion molecules in the pathogenesis of diabetic neuropathy. Diabetologia. 1998;41(3):330–336. [DOI] [PubMed] [Google Scholar]
- 19. Hussain MJ, Peakman M, Gallati H, Lo SS, Hawa M, Viberti GC, Watkins PJ, Leslie RD, Vergani D. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia. 1996;39(1):60–69. [DOI] [PubMed] [Google Scholar]
- 20. Deguchi T, Hashiguchi T, Horinouchi S, Uto T, Oku H, Kimura K, Makisumi K, Arimura K. Serum VEGF increases in diabetic polyneuropathy, particularly in the neurologically active symptomatic stage. Diabet Med. 2009;26(3):247–252. [DOI] [PubMed] [Google Scholar]
- 21. Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69(4):229–285. [DOI] [PubMed] [Google Scholar]
- 22. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. [DOI] [PubMed] [Google Scholar]
- 23. Regueiro A, Cuadrado-Godia E, Bueno-Betí C, Diaz-Ricart M, Oliveras A, Novella S, Gené GG, Jung C, Subirana I, Ortiz-Pérez JT, Roqué M. Mobilization of endothelial progenitor cells in acute cardiovascular events in the PROCELL study: time-course after acute myocardial infarction and stroke. J Mol Cell Cardiol. 2015;80:146–155. [DOI] [PubMed] [Google Scholar]
- 24. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–435. [DOI] [PubMed] [Google Scholar]
- 25. Han JW, Sin MY, Yoon Y. Cell therapy for diabetic neuropathy using adult stem or progenitor cells. Diabetes Metab J. 2013;37(2):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, Mendez RMB, Mello-Silva JPC, Cabral-da-Silva MC, De Mello FG, de Melo Reis RA, Mendez-Otero R. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159(2):540–549. [DOI] [PubMed] [Google Scholar]
- 27. Alestalo K, Miettinen JA, Vuolteenaho O, Huikuri H, Lehenkari P. Bone marrow mononuclear cell transplantation restores inflammatory balance of cytokines after ST segment elevation myocardial infarction. PLoS One. 2015;10(12):e0145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu SQ, Bao WJ, Men XL, Liu Y, Sun J, Li J, Liu HL, Cai H, Zhang W, Lou J, Peng L. Interleukin-10 protects schwann cells against advanced glycation end products-induced apoptosis via NF-κB suppression. Exp Clin Endocrinol Diabetes. 2020;128(2):89–96. [DOI] [PubMed] [Google Scholar]
- 29. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fadini GP, Albiero M, de Kreutzenberg SV, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2013;36(4):943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fadini GP, Avogaro A. Diabetes impairs mobilization of stem cells for the treatment of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168(2):892–897. [DOI] [PubMed] [Google Scholar]
- 32. Fadini GP, Fiala M, Cappellari R, Danna M, Park S, Poncina N, Menegazzo L, Albiero M, DiPersio J, Stockerl-Goldstein K, Avogaro A. Diabetes limits stem cell mobilization following G-CSF but not plerixafor. Diabetes. 2015;64(8):2969–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu F, Chen D, Sun X, Xie HH, Yuan H, Jia WP. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63(5):1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng Z, Garikipati VNS, Nickoloff E, Wang C, Polhemus DJ, Zhou J, Benedict C, Khan M, Verma SK, Rabinowitz JE, Lefer D. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation. 2016;134(19):1467–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement_materials_0703 for Autologous Bone Marrow Mononuclear Cell Transplantation Therapy Improved Symptoms in Patients with Refractory Diabetic Sensorimotor Polyneuropathy via the Mechanisms of Paracrine and Immunomodulation: A Controlled Study by Wei Wei, Li Li, Lin Deng, Zhong-Jing Wang, Jing-Jian Dong, Xiao-Yu Lyu, Ting Jia, Li Wang, Hong-Xiang Wang, Hong Mao and Shi Zhao in Cell Transplantation