Abstract
Biological repair of cartilage lesions remains a significant clinical challenge. A wide variety of methods involving mesenchymal stem cells (MSCs) have been introduced. Because of the limitation of the results, most of the treatment methods have not yet been approved by the Food and Drug Administration (FDA). However, bone marrow aspirate concentrate (BMAC) and human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) implantation were approved by Korea FDA. The aim of this study was to evaluate clinical and magnetic resonance imaging (MRI) outcomes after two different types of MSCs implantation in knee osteoarthritis. Fifty-two patients (52 knees) who underwent cartilage repair surgery using the BMAC (25 knees) and hUCB-MSCs (27 knees) were retrospectively evaluated for 2 years after surgery. Clinical outcomes were evaluated according to the score of visual analogue scale (VAS), the International Knee Documentation Committee (IKDC) subjective, and the Knee Injury and Osteoarthritis Outcome Score (KOOS). Cartilage repair was assessed according to the modified Magnetic Resonance Observation of Cartilage Repair Tissue (M-MOCART) score and the International Cartilage Repair Society (ICRS) cartilage repair scoring system. At 2-year follow-up, clinical outcomes including VAS, IKDC, and KOOS significantly improved (P < 0.05) in both groups; however, there were no differences between two groups. There was no significant difference in M-MOCART [1-year (P = 0.261), 2-year (P = 0.351)] and ICRS repair score (P = 0.655) between two groups. Both groups showed satisfactory clinical and MRI outcomes. Implantation of MSCs from BMAC or hUCB-MSCs is safe and effective for repairing cartilage lesion. However, large cases and a well-controlled prospective design with long-term follow-up studies are needed.
Keywords: cartilage repair, articular cartilage injury, MSC, BMAC, hUCB-MSCs
Introduction
Articular cartilage is known to have low potential for healing, and damage from degeneration or trauma can lead to focal cartilage lesions and subsequent osteoarthritis1. Cartilage lesion remains a challenging area in orthopedics, despite various treatment methods having been attempted. The technique of microfracture has shown good short-term clinical results within 2 years; however long-term data for larger and more aged patients have suggested inferior results2,3. In general, the resulting reparative fibrocartilage that develops after microfracture has inferior biomechanical properties and may undergo degenerative changes. Autologous chondrocyte implantation has produced successful hyaline cartilage repair and improved functional outcomes at long-term follow-up4. Despite these satisfactory results, limitations of this technique have been suggested as follows: (1) requirement for two-stage procedures, (2) donor site morbidity, and (3) reduced chondrogenic potential in aged patients.
As the cell-based tissue engineering techniques have improved, various cartilage treatment methods using stem cells [including mesenchymal stem cells (MSCs)] have been proposed5,6. Nevertheless, because of a lack of consistency in studies in terms of treatment methods, number of cells, incubation methods, and paucity of high-quality controlled study, no clear standardized treatment methods have been established6–8. Therefore, most stem cell treatment methods (e.g., adipose cell-derived MSCs or adipose cell source-derived stromal vascular fraction cells) have not yet been approved by the US Food and Drug Administration (FDA). However, bone marrow aspirate concentrate (BMAC) can be prepared in a standardized manner with FDA and Korea FDA (KFDA) approval. Human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) have been developed as a drug product (Cartistem®, Medipost Inc., Sungnam, Gyeonggi-do, South Korea) with the approval of KFDA and can be used in Korea, and a clinical trial is underway in the United States6.
BMAC, containing MSCs, used with hyaluronic acid based scaffold has generated satisfactory clinical outcomes and successful cartilage repair9,10. It is fairly easy to perform bone marrow aspiration from the iliac crest through mini-incision without position change. Subsequently, BMAC is generated using commercialized machines. This method can overcome the limitations of cell-based two-stage procedures, promising one-stage option for cartilage repair procedure with chondrogenic potential and easy control of MSCs. Furthermore, the risk of disease transmission is low. Nevertheless, BMAC contains only a small fraction of MSCs. In general, we can extract about 1.9 × 107 mononuclear cells of approximately 6 ml capacity after aspirating 60 ml of bone marrow9. The number of collected MSCs may vary depending on the patient’s age and general condition11. MSCs from elderly patients have lower differentiation levels and chondrogenicity in vitro 12. For these reasons, satisfactory results may not be obtained. Despite limitations in terms of stem cell counts and ability of differentiation, BMAC contains not only stem cells but also hematopoietic stem cells, platelets, growth factors, and cytokines6,9,13. These components can have substantial paracrine effect14,15.
hUCB-MSCs were first reported by Erices et al16. They have high proliferation rates and can be easily induced to differentiate into chondrocytes. Despite, still under debate on chondrogenic potential of MSC source17–19, hUCB-MSCs have low immunogenicity, and therefore are well suited to allogenic transplantation20. This method has the advantage of low donor site morbidity, no need for preparation procedures, and uniform cell count and quality. Furthermore, it allows implantation of a specific amount of MSCs, regardless of the size of the lesion. Nevertheless, there are risks of contamination or damage because of mistakes in manufacturing or storage prior to use. One study reported satisfactory outcome with 7-year follow-up after hUCB-MSCs transplantation21.
Currently, most reported studies of cartilage repair treatments using the stem cells compared results with those of microfracture10,22. Alternatively, the effects of cartilage regeneration were assessed by comparing the pre- and postoperative status23,24. Because of the limitations of the microfracture technique, it is not appropriate to compare it with the effects of cartilage regeneration using stem cells. To our knowledge, there have been no published comparisons of the effect of various stem cells for cartilage repair. Therefore, the aim of this study is to investigate which approved cell therapy method is more effective for cartilage repair procedure.
Materials and Methods
Subject
We conducted a retrospective review of the patient to whom underwent cartilage repair surgery using the BMAC and hUCB-MSCs between March 2012 and October 2017. In Korea, the approved indication of hUCB-MSCs (Cartistem®, Medipost Inc.) includes cartilage-defective patient with the International Cartilage Repair Society (ICRS) grade IV lesions, regardless of age or size. BMAC is only approved for patients aged between 15 and 50 years, ICRS grade III to IV, and lesion size 2–10 cm2. In this retrospective study, because the indications of the two treatment methods are slightly different, inclusion criteria were set as follows to reduce the selection bias: (1) focal cartilage defects with persistent symptoms of knee joint pain or functional disability, despite a minimum 3 months of conservative treatment; (2) age above 15 years; (3) Kellgren-Lawrence (K-L) grade ≤2; (4) lesion size 2– 10 cm2; (5) ICRS grade IV; and (6) minimum 2-year follow-up. In addition, the BMAC group was only for age between 15 and 50 years. Exclusion criteria were as follows: (1) K-L grade ≥3, (2) tricompartment OA, (3) infectious or inflammatory arthropathy, (4) rheumatoid disease of cancer patients, and (5) less than 2-year follow-up. Coexisting knee pathologies such as meniscal tears, meniscus deficiencies, ligament insufficiencies and tibiofemoral axis malalignment were treated during the same surgical procedure.
Finally, total of 52 patients (52 knees, BMAC: 25 knees, BMAC group and hUCB-MSCs: 27 knees, hUCB group) were enrolled in this retrospective study. Detailed demographic data are summarized in Table 1. All patients provided informed consent prior to treatment. This study protocol was approved by the INHA University Hospital Institutional Review Board (INHA 2019-02-003).
Table 1.
Demographic Data of BMAC and hUCB Group.
| BMAC group | hUCB group | P-value | |||
|---|---|---|---|---|---|
| Number of patients | 25 | 27 | |||
| Age (years) | 39.64 ± 9.83 | 53.93 ± 8.6 | <0.001 | ||
| Gender (M:F) | 13:12 | 11:16 | 0.41 | ||
| Lesion size (cm2) | 4.33 ± 1.66 | 4.77 ± 1.81 | 0.358 | ||
| BMI | 26.19 ± 3.74 | 26.38 ± 3.54 | 0.924 | ||
| Number of lesions | Single lesion | Multiple lesions | Single lesion | Multiple lesions | |
| 19 | 5 | 16 | 4 | ||
| Concomitant surgery | 15 | 19 | |||
| Menisectomy | 5 | 11 | |||
| Meniscus repair | 2 | 0 | |||
| HTO | 1 | 2 | |||
| HTO + menisectomy | 4 | 5 | |||
| HTO + meniscus repair | 0 | 1 | |||
| MAT | 2 | 0 | |||
| ACLR | 1 | 0 | |||
ACLR: anterior cruciate ligament reconstruction; BMAC: bone marrow aspirate concentration; BMI: body mass index; hUCB: human umbilical cord blood; HTO: high tibial osteotomy; MAT: meniscus allograft transplantation.
Surgical Technique
BMAC Group
All the procedures were performed under spinal anesthesia. An arthroscopic examination was performed using a standard anterolateral and anteromedial portal in the lithotomy position. After complete inspection of the joints and assessment of the cartilage defects, marrow aspiration was performed at the ipsilateral iliac crest using an aspiration kit under routine sterile preparation and draping. Sixty milliliters of bone marrow was collected, then centrifuged using a commercially available BMAC system (SmartPReP2®, Harvest Technologies, Plymouth, MA, USA). Coexisting knee pathologies such as meniscus tear (menisectomy or meniscus repair), meniscus deficiency (meniscus allograft transplantation), tibiofemoral axial malalignment (open wedge high tibial osteotomy), and ligamentous insufficiency (anterior cruciate ligament reconstruction) were treated during the same surgery (Table 1). After evaluation and management of coexisting pathologies, a mini-arthrotomy through an incision of approximately 3–4 cm in length was made through the anterolateral or anteromedial portal according to location of the lesion. The chondral defect lesion was debrided and prepared using curettes. Subsequently, small sized holes were made using microfracture awl to the depth about 3–5 mm for the containment of MSCs graft. In the space between the microfracture holes, a 2-mm diameter drill bit was used to create several small holes. After drilling, irrigation was performed to wash out debris. Finally, the lesion area was dried prior to implantation. The defect size was measured and the HA membrane (Hyalofast®, Anika Therapeutics Inc., Bedford, MA, USA) templated according to the size. Subsequently, centrifuged BMAC was added to HA membrane, then implanted on the prepared lesion. After applying the BMAC + HA scaffold, we added fibrin glue (Greenplast®, Greencross, Seoul, Korea) to create more stable fixation of the lesion.
hUCB Group
The same procedure was done with BMAC group up to the procedure of managing coexisting pathology and preparing the lesion without marrow aspiration and making containment subchondral hole. Instead of microfracture awl, 5-mm diameter drill bit was used to perform to the depth about 3–5 mm for the containment of MSCs graft. After preparing the lesion, commercially available hUCB-MSCs [Cartistem®, Medipost Inc., composite of hUCB-MSCs 0.5 x 107/ml and freeze drying sodium hyaluronate (HA)] were mixed according to the manufacturer’s instructions. After mixing hUCB-MSCs and HA, it becomes a type of gel21. Then, the hUCB-MSCs and HA mixture was implanted into the prepared lesions from the base to the surface slowly to avoid any defects.
After implantation, the knee was then ranged carefully through flexion and extension in order to check the stability of the overlying composite of BMAC or hUCB-MSCs materials. The wound was closed and a long leg splint was applied. All the procedures were performed by the MK Kim. After the procedure, the knee was immobilized for 1 week with an extension knee splint. Continuous passive range of motion exercise was recommended at 1 week after surgery, and weight bearing was restricted for 6 weeks. Partial weight bearing was permitted using a crutch at 6 weeks after surgery, and full weight bearing was allowed at 12 weeks postoperatively.
Clinical Evaluation
To assess pain and the functional disability of the knees, we used the visual analogue scale (VAS) pain score (0 = no pain, 10 = worst pain), the International Knee Documentation Committee (IKDC) subjective score, and the Knee Injury and Osteoarthritis Outcome Score (KOOS)25 preoperatively, 6 months, 1 year, and 2 years after surgery.
Magnetic Resonance Imaging Evaluation
Magnetic resonance imaging (MRI) was performed at preoperative, 1 year, and 2 years after surgery. Preoperative and follow-up MRI was performed using a 3.0 T MRI scanner, Discovery MR750W® (GE Healthcare, Chicago, IL, USA) with a dedicated eight channel knee coil. The following sequences were utilized: (a) proton density (PD) spectral presaturation with inversion recovery (SPIR) transversal image [repetition time/echo time (TR/TE), 3,794/28 ms]: field of view (FOV), 160 × 160 mm; matrix, 480 × 288; slice thickness (SL), 3.0 mm with 0.33 mm gap; (b) PD SPIR coronal image (TR/TE, 3,513/28.2 ms): FOV, 160 × 160 mm; matrix, 480 × 288; SL, 3.0 mm with 0.3 mm gap; (c) T2 SPIR sagittal image (TR/TE, 2,002/71.3 ms): FOV, 160 × 160 mm; matrix, 288 × 256; SL, 0.7 mm with 0.3 mm gap; and (d) turbo spin echo T1-weighted sagittal image (TR/TE, 461/15.8 ms): FOV, 160 × 160; matrix, 480 × 288; SL, 3.0 mm with 0.33 mm gap.
The MRI images were analyzed using the modified Magnetic Resonance Observation of Cartilage Repair Tissue (M-MOCART) score. Although MRI is unable to accurately determine the status of the cartilage repair26, the M-MOCART (0 = worst cartilage status, 100 = best articular cartilage status) score highly correlated with clinical outcome24,27. The M-MOCART score was classified as excellent if the score was 80 or more and poor outcome when the score was below 50. To avoid bias, a musculoskeletal-trained radiologist who did not participate in the care of patients and was blinded to this study evaluated the MRI images.
Second-Look Arthroscopy
A second-look arthroscopy was conducted 1 year after first surgery in patients who underwent surgical treatment on the same knee for hardware removal and those who agreed to check repaired cartilage condition during a contralateral knee procedure. During the second-look procedures, the repaired cartilage was inspected and evaluated using the ICRS cartilage repair assessment scoring system (score 0–12) including the degree of defect fill, the degree of graft integration to the adjacent normal articular surface, and the gross appearance of the graft surface28.
Statistical Analysis
The repeated-measures analysis of variance test was used to determine the significance of repeated-measures parameters on continuous scale between two groups for metric parameters. The independent Student’s t-test was also used to compare the metric parameters between two groups. In a prior power analysis, the number of samples required to perform the noninferiority test between the two groups was calculated using the VAS score, IKDC score, and KOOS score as the reference value (α = 0.05, β = 0.2). The noninferiority margin of the number of samples was set based on the established minimal clinical important difference (MCID) of VAS score and IKDC subjective score29. Reference values of sample size [mean difference and standard deviation (SD)] and MCID of KOOS score were set based on the previously performed joint cartilage repair research10,30. At least 20 patients in each group were needed. Results of continuous measurements were presented as mean ± SD, and results on categorical measurements are presented as number. Statistical analysis was conducted using SPSS (Ver 25.0, IBM, Armonk, NY, USA) with significance defined as P < 0.05.
Results
Clinical Outcomes
There was a significant difference in age between the two groups due to differences in age indications (P < 0.0001). There were no differences in terms of gender, lesion size, number of lesions, or body mass index (BMI) (Table 1). There was a difference in concomitant surgeries between the two groups. The average size of the lesion was 4.33 ± 1.66 cm2 in the BMAC group and 4.77 ± 1.81 cm2 in the hUCB group (Table 1).
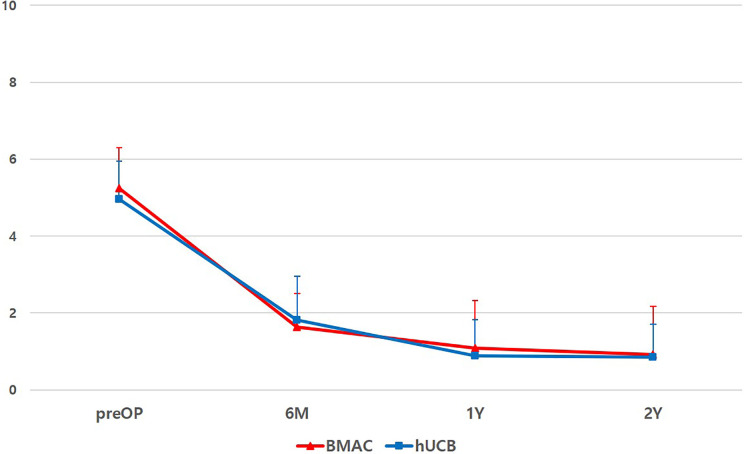
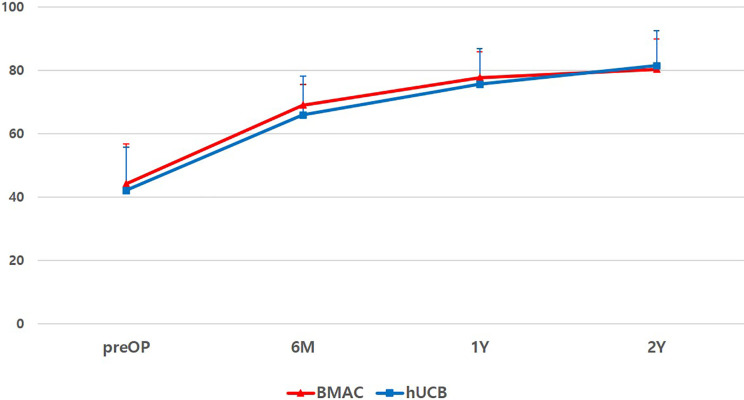
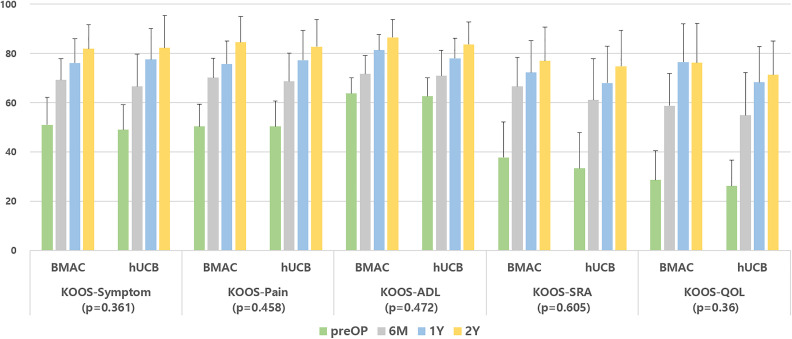
At final follow-up, VAS scores improved from 5.2 ± 1.1 to 0.92 ± 0.98 and from 5.0 ± 1.2 to 0.85 ± 0.86 for the BMAC and hUCB groups, respectively (Fig. 1); IKDC subjective scores improved from 44.17 ± 12.5 to 80.27 ± 9.48 and 42.02 ± 13.63 to 81.35 ± 11.07 for the BMAC and hUCB groups, respectively (Fig. 2). KOOS scores also showed improvement in all categories in both groups (Fig. 3). However, there were no significant differences in terms of VAS (P = 0.369), IKDC subjective (P = 0.434), KOOS scores [symptoms (P = 0.361), pain (P = 0.458), activity of daily life (P = 0.472), sport and recreation (P = 0.605), and quality of life (P = 0.36)] between two groups (Figs. 1 –3). Postoperative intra-articular adhesions occurred in two cases in the BMAC group and three in the hUCB group. There were no infections or conversion to total knee arthroplasty (Table 2).
Figure 1.
Flow of VAS pain score during 2-year follow-up of both groups. There was no significant difference (P = 0.369) between two groups. BMAC: bone marrow aspirate concentrate; hUCB: human umbilical cord blood; preOP: preoperative; VAS: visual analogue scale; 6 M: postoperative 6 month; 1Y: postoperative 1 year; and 2Y: postoperative 2 year.
Figure 2.
Flow of IKDC subjective score during 2-year follow-up of both groups. There was no significant difference (P = 0.434). BMAC: bone marrow aspirate concentrate; hUCB: human umbilical cord blood; IKDC: International Knee Documentation Committee; preOP: preoperative; 6 M: postoperative 6 month; 1Y: postoperative 1 year; and 2Y: postoperative 2 year.
Figure 3.
Flow of KOOS score during 2-year follow-up of both groups. There was no significant difference in all subtypes of KOOS score [symptoms (P = 0.361), pain (P = 0.458), ADL (P = 0.472), SRA (P = 0.605), and QOL (P = 0.36)] between two groups. ADL: activity of daily life; BMAC: bone marrow aspirate concentrate; hUCB: human umbilical cord blood; KOOS: The Knee Injury and Osteoarthritis Outcome Score; preOP: preoperative; QOL: quality of life; SRA: sport and recreation; 6 M: postoperative 6 month; 1Y: postoperative 1 year; and 2Y: postoperative 2 year.
Table 2.
The Outcome of MRI (M-MOCART Score), Second-Look Arthroscopy, and Complications of Both Treatment Groups.
| BMAC group (N = 25) | hUCB group (N = 27) | P-value | |
|---|---|---|---|
| 1Y M-MOCART score | 65.4 ± 13.46 | 69.63 ± 13.37 | 0.261 |
| 2Y M-MOCART score | 70.20 ± 13.58 | 73.7 ± 13.2 | 0.351 |
| 1Y 2nd look ICRS repair grade score |
N = 12 | N = 16 | 0.655 |
| 9.42 ± 1.83 | 9.75 ± 2.05 | ||
| Complication | |||
| Adhesion | 2 | 3 | |
| Infection | 0 | 0 | |
| TKA conversion | 0 | 0 |
BMAC: bone marrow aspirate concentration; hUCB: human umbilical cord blood; ICRS: The International Cartilage Repair Society; M-MOCART: modified Magnetic Resonance Observation of Cartilage Repair Tissue; MRI: magnetic resonance imaging; TKA: total knee arthroplasty.
MRI Findings
At 1-year MRI, there were four patients in the BMAC group and eight patients in the hUCB group with greater than 80 points on M-MOCART score. By contrast, three patients in BMAC and two patients in hUCB had less than 50 points. At 2-year follow-up, there were seven patients in the BMAC group and 10 patients in the hUCB group with better than 80 points of M-MOCART score. By contrast, two patients in the BMAC and one patient in hUCB had less than 50 points. For both year’s M-MOCART scores, there were no significant differences between the groups. Results of M-MOCART score are summarized in Table 2.
Second-Look Arthroscopy
Second-look arthroscopy was performed in 12 patients in the BMAC group (Fig. 4) and 16 patients in the hUCB group (Fig. 5) with a mean follow-up of 13.2 months. Eleven patients (91.6%) of BMAC group and 14 patients (87.5%) of hUCB group were assessed as normal (ICRS repair score = 12) or nearly normal (ICRS repair score 8–11) condition repair28,31. There was no significant difference between the groups (P = 0.655) (Table 2).
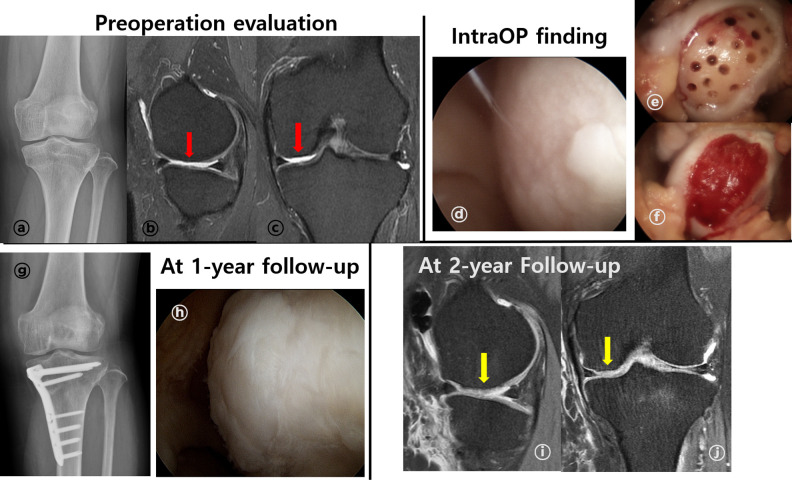
Figure 4.
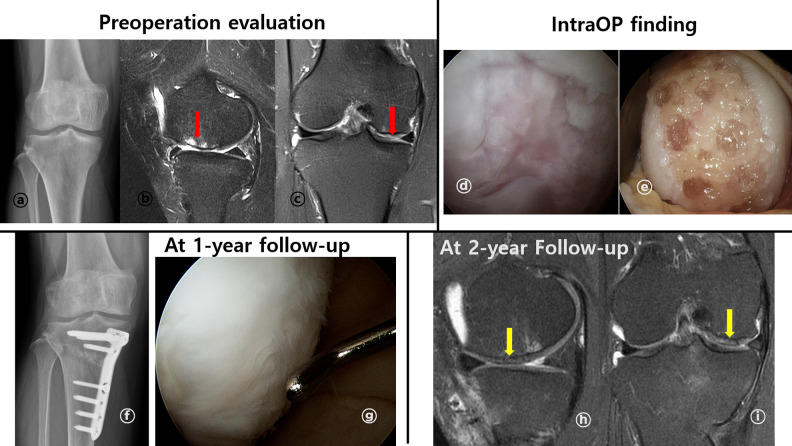
A representative case of cartilage repair using BMAC (female/47-year-old). (A) Standing anteroposterior knee radiography showed varus alignment of left knee (HKA axis: varus 7°). (B and C) Preoperative MRI exam showed ICRS grade IV lesion (red arrow) through T2 proton density fat suppression (T2 PD FS) at MFC through sagittal (B), coronal view (C). (D, E, F) Intraoperative arthroscopic findings. A 4.5 cm2 sized ICRS IV lesion was observed at MFC through arthroscope (D). After preparation for implantation (E), BMAC + HA scaffold implantation was performed with additional fibrin glue fixation (F). (G, H) After 1-year follow-up, standing anteroposterior radiography showed corrected varus alignment (HKA axis: valgus 0.8°) (G). During second-look arthroscopy evaluation, repaired cartilage had some overgrowth, but were well integrated to surrounding tissues and smoothed surface (H). (I, J) At 2-year follow-up, repaired cartilage (yellow arrow) completely filled defect, showed smooth surface and well integrated to around tissue at T2 PD FS MRI images. There was no definite subchondral edema at MRI exam sagittal (I), and coronal (J) view. BMAC: bone marrow aspirate concentrate; HKA: Hip-Knee-Ankle; ICRS: The International Cartilage Repair Society; MFC: medial femoral condyle; and MRI: magnetic resonance imaging.
Figure 5.
A representative case of cartilage repair using hUCB-MSCs (female/53-year-old). (A) Standing anteroposterior knee radiography showed varus alignment of left knee (HKA axis: varus 6.8°), (B and C) preoperative MRI exam showed ICRS IV lesion (red arrow) at MFC through T2 proton density fat suppression (T2 PD FS) sagittal (B), and coronal view (C). (D and E) Intraoperative arthroscopic findings. A 3.9 cm2 sized ICRS grade IV lesion was observed at MFC through arthroscope (D). After preparation for implantation, hUCB-MSCs implantation was performed (E). (F and G) After 1-year follow-up, standing anteroposterior radiography showed corrected varus alignment (HKA axis: valgus 1.5°) (F). At second-look arthroscopy evaluation, repaired cartilage were well growth and integrated to surrounding tissues and smoothed surface as almost normal cartilage (G). (H and I) At 2-year follow-up, repaired cartilage (yellow arrow) completely filled defect, showed smooth surface and well integrated to around tissue at T2 PD FS MRI images. hUCB-MSCs: human umbilical cord blood derived mesenchymal stem cells; HKA: Hip-Knee-Ankle; ICRS: The International Cartilage Repair Society; MFC: medial femoral condyle; and MRI: magnetic resonance imaging.
Subgroup Analysis Based on Age
When compared the results of the age-based cartilage repair regardless of the treatment method, there were no significant differences between younger than 45 years (consist of BMAC: 15 cases and hUCB: 2 cases) and elder than 45 years (consist of BMAC: 10 cases and hUCB: 25 cases) in MRI outcome [1Y-MOCART score (P = 0.849), 2Y-MOCART score (P = 0.83)] and ICRS repair score (P = 0.926). Pre-operative and 2-year follow-up, VAS pain score were also no significant difference (Table 3).
Table 3.
Subgroup Study Based on Age Regardless of the Cell Implantation Method.
| Young age group (≤45) (N = 17) |
Elderly group (>45) (N = 35) |
P-Value | |
|---|---|---|---|
| Age | 33.71 ± 9.29 | 53.54 ± 5.5 | <0.001 |
| 1Y M-MOCART score | 67.06 ± 14.48 | 67.86 ± 13.13 | 0.849 |
| 2Y M-MOCART score | 71.47 ± 12.09 | 72.29 ± 14.11 | 0.830 |
| 1Y ICRS repair score | 9.67 ± 1.63 | 9.59 ± 2.04 | 0.926 |
| Pre-OP VAS score | 5.18 ± 1.07 | 5.06 ± 0.99 | 0.703 |
| 2Y F/U VAS score | 0.94 ± 1.48 | 0.86 ± 0.81 | 0.829 |
ICRS: The International Cartilage Repair Society; M-MOCART: modified Magnetic Resonance Observation of Cartilage Repair Tissue; Pre-OP: preoperative; and VAS: visual analogue scale pain score.
Discussion
The most important finding of our study is that cartilage repair using both BMAC and hUCB-MSCs had satisfactory results and there was no difference from the individual findings of previously published studies10,21,30. The hUCB group showed slightly better results in the M-MOCART score and ICRS repair score; however, these were not statistically significant and there were no significant differences in clinical outcome. To assess cartilage repair status, arthroscopic evaluation is the most accurate method; however, there are limitations for most patients because of the invasive procedure. In this study, only 50% (22 patients/44 patients) could be performed. The use of MRI as an indirect method has been shown to be related to the clinical score26,27,32,33. In this study, we evaluated the degree of cartilage repair status by MRI in patients who did not undergo second-look surgery based on these results. To our knowledge, this was the first study to compare the clinical and MRI outcomes using two types of approved MSCs for cartilage repair.
Two of the patients who underwent BMAC had poor results. One of them was an elite sport coach and one was an active amateur athlete. One patient in the hUCB group, a hard working labor, also showed poor results. Commonly, high physical demand may produce poor results after surgery. In this study, it was difficult to analyze because of the small cases. In a previous study, still under debate, the results suggested that age, physical demand, BMI, and concomitant knee pathology are risk factors for the failure of cartilage repair surgery9,24. Therefore, further studies with larger sample size are necessary to identify the risk factors and prognostic factors with more precision.
The demographics of this retrospective study revealed differences between the two groups of subjects, especially in terms of age and concomitant surgery, when treating only patients for whom there were approved indications. Many previous studies have reported that surgical correction of concomitant knee pathology plays a key role in cartilage repair procedure. While biologic and graft failures will occur, the majority of failures were attributed to untreated background factors such as malalignment, meniscal deficiency, and instability34,35. Although the accompanying knee pathologies were different, concomitant surgery has been attempted to adjust for environment favorable for cartilage repair. We assumed that the environment of the knee joint in both groups was similarly improved.
Generally, cell count and the differential potency of BMAC are reported to decrease with age, and this is the basis for reluctance to use in elderly patients36. In fact, there have been reports suggesting that sufficient numbers of MSCs can be collected from elderly patients37,38. Furthermore, mononuclear stem cells from younger healthy donors have been reported to differ between donors in terms of differentiation potentials11. A recent study have shown that elderly patients underwent successful cartilage repair without significant differences24,31. In the present study, there were no statistical differences between the age groups, regardless of the treatment method, by contrast with the expectation that the cartilage repair effect would be better in younger patients in general.
In the case of hUCB, the same number and capacity of cells could always be transplanted, irrespective of age. However, as mentioned above, BMAC has different cell counts, differentiation potentials, cytokines, and growth factors depending on the age and individual patient’s conditions. Nevertheless, there were no significant differences between the two groups. In this regard, the “medical signaling effect” proposed by Caplan and Correa39 has attracted attention. Even after implantation of MSCs, the implanted MSCs disappeared after 6–8 weeks, and the cells were replaced with host cells40–42. Therefore, in the cartilage repair procedure, the importance of signaling with the surrounding environment, i.e., paracrine effect, is emphasized more than stem cell count. According to this concept, MSCs, rather than participating in tissue formation, play the role of a site-regulated “drugstore” in vivo by releasing trophic and immunomodulatory factors31,39,40. Therefore, we believe that even BMAC with a low fraction of stem cell content can achieve effective cartilage repair. Many studies are needed to determine the role of cell count, cytokine, growth factor concentration, and knee joint environment in induction of cartilage repair6,9,11,43. Considering the results to date, it would be better to choose the cartilage repair procedure by considering the patient’s individual characteristics. BMAC may be preferred for young, physically healthy people, whereas hUCB-MSCs would be preferred for large sized lesion, old age, or fear for donor site morbidity.
The present study has some limitations. First, it is a retrospective study design, with a small sample size, short-term follow-up period, and high risk of bias due attributable to differences in age and coexisting knee pathology between the two treatment groups. Therefore, there is a need for large sample sizes in well-designed prospective randomized trial. Second, biopsy evaluation was not performed. Many studies reported that repaired cartilage was composed of hyaline like cartilage30,44,45. However, as Gobbi et al.31 found, although the ICRS repair score was high, it may not be possible to show precise results because there were cases where the actual biopsy showed a mixed (hyaline + fibrocartilage) repair. In this case, we could not guarantee the long-term clinical results. Furthermore, the second-look arthroscopy was performed at 1 year after surgery; therefore, we could not determine whether the repaired cartilage changed after the first year. Also, second-look arthroscopy was conducted only with a small patient in both groups. There is a high risk of selection bias to evaluate outcomes. Third, there are a variety of factors that can affect the results of cartilage repair, including age, BMI, coexisting knee lesions, and physical demand, all of which may limit the interpretation of the results. Fourth, the use of fibrin glue in the BMAC group may have affected the outcomes. According to a literature review, the fibrinogen concentration can affect cell proliferation and migration46,47.
Despite these limitations, our present study has some merits. To our knowledge, this was the first study to compare the clinical and MRI outcomes using BMAC and hUCB-MSCs for cartilage repair. This can help surgeons determine what kind of cartilage repair method is better for their patients. We also compared the results of the age-based cartilage repair regardless of the treatment method. In this way, we were able to suggest what additional research would be needed to determine effective cartilage repair in the elderly while supporting existing research results.
Conclusion
The results of our study are encouraging and show that implantation of MSCs from BMAC or hUCB-MSCs for cartilage repair in knee osteoarthritis is safe and effective for repairing cartilage lesion in each indication. Therefore, if applied with proper patient indication, both methods could be expected satisfactory outcome. However, due to differences in patient groups, the effectiveness of the two methods could not be accurately compared. Thus, large cases and a well-controlled prospective design with long-term follow-up studies are needed to set proper indication.
Footnotes
Ethical Approval: All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol of this study was approved by the Inha University Hospital Institutional Review Board (approval number: INHAUH 2019-02-003).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Korea Food and Drug Administration (KFDA) guideline for cartilage repairing procedure. And the protocol of this study was approved by the Inha University Hospital Institutional Review Board (approval number: INHAUH 2019-02-003).
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Inha University Hospital Research Grant. The funding body had no influence in the design of the study and collection, analysis, and interpretation of data and in writing the article.
ORCID iDs: Dong Jin Ryu  https://orcid.org/0000-0003-2455-5230
https://orcid.org/0000-0003-2455-5230
Myung Ku Kim  https://orcid.org/0000-0002-4171-8831
https://orcid.org/0000-0002-4171-8831
References
- 1. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. [DOI] [PubMed] [Google Scholar]
- 2. Scotti C, Gobbi A, Karnatzikos G, Martin I, Shimomura K, Lane JG, Peretti GM, Nakamura N. Cartilage repair in the inflamed joint: considerations for biological augmentation toward tissue regeneration. Tissue Eng Part B Rev. 2016;22(2):149–159. [DOI] [PubMed] [Google Scholar]
- 3. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986–1996. [DOI] [PubMed] [Google Scholar]
- 4. Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562–567. [DOI] [PubMed] [Google Scholar]
- 5. Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park Y-B, Ha C-W, Rhim JH, Lee H-J. Stem cell therapy for articular cartilage repair: review of the entity of cell populations used and the result of the clinical application of each entity. Am J Sports Med. 2018;46(10):2540–2552. [DOI] [PubMed] [Google Scholar]
- 7. Robinson PG, Murray IR, West CC, Goudie EB, Yong LY, White TO, LaPrade RF. Reporting of mesenchymal stem cell preparation protocols and composition: a systematic review of the clinical orthopaedic literature. Am J Sports Med. 2019;47(4):991–1000. [DOI] [PubMed] [Google Scholar]
- 8. Piuzzi NS, Hussain ZB, Chahla J, Cinque ME, Moatshe G, Mantripragada VP, Muschler GF, LaPrade RF. Variability in the preparation, reporting, and use of bone marrow aspirate concentrate in musculoskeletal disorders: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2018;100(6):517–525. [DOI] [PubMed] [Google Scholar]
- 9. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gobbi A, Whyte GP. One-stage cartilage repair using a hyaluronic acid-based scaffold with activated bone marrow-derived mesenchymal stem cells compared with microfracture: five-year follow-up. Am J Sports Med. 2016;44(11):2846–2854. [DOI] [PubMed] [Google Scholar]
- 11. Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33(6):1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–682. [DOI] [PubMed] [Google Scholar]
- 13. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–1937. [DOI] [PubMed] [Google Scholar]
- 14. Krych AJ, Nawabi DH, Farshad-Amacker NA, Jones KJ, Maak TG, Potter HG, Williams RJ. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91–98. [DOI] [PubMed] [Google Scholar]
- 15. Cotter EJ, Wang KC, Yanke AB, Chubinskaya S. Bone marrow aspirate concentrate for cartilage defects of the knee: from bench to bedside evidence. Cartilage. 2018;9(2):161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. [DOI] [PubMed] [Google Scholar]
- 17. Berg L, Koch T, Heerkens T, Bessonov K, Thomsen P, Betts D. Chondrogenic potential of mesenchymal stromal cells derived from equine bone marrow and umbilical cord blood. Vet Comp Orthop Traumatol. 2009;22(5):363–370. [DOI] [PubMed] [Google Scholar]
- 18. Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, Muneta T, Arikawa-Hirasawa E, Sekiya I, Kaneko K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. 2012;7(9):e45517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. [DOI] [PubMed] [Google Scholar]
- 20. Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26(11):2865–2874. [DOI] [PubMed] [Google Scholar]
- 21. Park Y-B, Ha C-W, Lee C-H, Yoon YC, Park Y-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koh Y-G, Kwon O-R, Kim Y-S, Choi Y-J, Tak D-H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97–109. [DOI] [PubMed] [Google Scholar]
- 23. Koh Y-G, Choi Y-J, Kwon S-K, Kim Y-S, Yeo J-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308–1316. [DOI] [PubMed] [Google Scholar]
- 24. Kim YS, Choi YJ, Lee SW, Kwon OR, Suh DS, Heo DB, Koh YG. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthr Cartil. 2016;24(2):237–245. [DOI] [PubMed] [Google Scholar]
- 25. Hambly K, Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36(9):1695–1704. [DOI] [PubMed] [Google Scholar]
- 26. de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, Saris DBF. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695–1702. [DOI] [PubMed] [Google Scholar]
- 27. Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S, Trattnig S. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38(5):934–942. [DOI] [PubMed] [Google Scholar]
- 28. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85(A Suppl 2):58–69. [DOI] [PubMed] [Google Scholar]
- 29. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function. Arthritis Care Res (Hoboken). 2011;63(0 11): S208–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648–657. [DOI] [PubMed] [Google Scholar]
- 31. Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2494–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guermazi A, Roemer FW, Alizai H, Winalski CS, Welsch G, Brittberg M, Trattnig S. State of the art: MR imaging after knee cartilage repair surgery. Radiology. 2015;277(1):23–43. [DOI] [PubMed] [Google Scholar]
- 33. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41(6):1426–1434. [DOI] [PubMed] [Google Scholar]
- 34. Krych AJ, Hevesi M, Desai VS, Camp CL, Stuart MJ, Saris DBF. Learning from failure in cartilage repair surgery: an analysis of the mode of failure of primary procedures in consecutive cases at a tertiary referral center. Orthop J Sports Med. 2018;6(5):2325967118773041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286(2):188–195. [DOI] [PubMed] [Google Scholar]
- 36. Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthr Cartil. 2010;18(5):705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bodle JC, Teeter SD, Hluck BH, Hardin JW, Bernacki SH, Loboa EG. Age-related effects on the potency of human adipose-derived stem cells: creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Eng Part C Methods. 2014;20(12):972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juneja SC, Viswanathan S, Ganguly M, Veillette C. A simplified method for the aspiration of bone marrow from patients undergoing hip and knee joint replacement for isolating mesenchymal stem cells and In Vitro chondrogenesis. Bone Marrow Res. 2016;2016:3152065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park WS, Ahn SY, Sung SI, Ahn J-Y, Chang YS. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res. 2018;83(1–2):214–222. [DOI] [PubMed] [Google Scholar]
- 41. van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47(5):1634–1643. [DOI] [PubMed] [Google Scholar]
- 42. Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med. 2010;14(9):2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yanke AB, Chubinskaya S. The state of cartilage regeneration: current and future technologies. Curr Rev Musculoskelet Med. 2015;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gigante A, Cecconi S, Calcagno S, Busilacchi A, Enea D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech. 2012;1(2):e175–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Kaps C, Gigante A. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20(6):562–569. [DOI] [PubMed] [Google Scholar]
- 46. Guo H-D, Wang H-J, Tan Y-Z, Wu J-H. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A. 2011;17(1–2):45–58. [DOI] [PubMed] [Google Scholar]
- 47. Lee HH, Haleem AM, Yao V, Li J, Xiao X, Chu CR. Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations. Tissue Eng Part A. 2011;17(15–16):1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]