Abstract
Autologous platelet-rich plasma (au-PRP) has been widely used for the management of refractory chronic wounds. However, patients with diabetic lower extremity ulcers (DLEUs) usually have complicated clinical conditions, and the utility of au-PRP is limited. In this study, the feasibility, effectiveness, and safety of allogeneic platelet-rich plasma (al-PRP) and au-PRP were investigated and compared in the treatment of DLEUs. A total of 75 in-patients with type 2 diabetes were assigned to the al-PRP group (n = 20), au-PRP group (n = 25), and conventional wound therapeutic (CWT) group (n = 30) matched by the ankle brachial index and ulcer size from December 2015 to August 2018. Based on metabolic and nutritional regulation, infective control, and topical wound management, al-PRP, au-PRP, and CWT were administered to each group, respectively. Evaluation of treatment outcomes was determined by the parameters of wound healing and adverse reactions. The therapeutic times and average concentration of platelets were not significantly different between the au-PRP and al-PRP groups. The wound healing times of the al-PRP group (56.9 ± 29.22 d) and au-PRP group (55.6 ± 33.8 d) were significantly shorter than those of the CWT group (88.0 ± 43.4 d) (P < 0.01), but there was no significant difference between the groups with PRP treatment. Although there was no significant difference in the daily healing area among all groups (P > 0.05), the trend of the healing rate in the al-PRP group (16.77 ± 12.85 mm2), au-PRP group (14.31 ± 18.28 mm2), and CWT group (9.90 ± 8.51 mm2) gradually decreased. No obvious adverse reactions (fever, edema, pain, skin itching, rash, or other sensory abnormalities) were observed in either the au-PRP or the al-PRP groups. Both al-PRP and au-PRP could effectively and safely promote wound healing in patients with DLEUs. Alternatively, al-PRP could be used for DLEUs as an off-the-shelf solution when au-PRP is limited.
Registration number of clinical trials:
ChiCTR1900021317
Keywords: allogeneic platelet-rich plasma, autologous platelet-rich plasma, diabetic lower extremity ulcers, diabetic foot ulcers
Introduction
Lower extremity ulcers affect millions of people, resulting in a significant public health problem and economic burden1. Diabetes is one of the most common causes of lower extremity ulcers2. Diabetic lower extremity ulcers (DLEUs) are prone to being chronic and refractory with a high risk of amputation3,4. Therefore, exploring new technologies to accelerate wound healing is the key to reducing disability and mortality. A systematic review performed by the International Working Group on the Diabetic Foot to evaluate the effect of cell therapy, including platelets, stem cells, and growth factors, indicated that platelet-rich plasma (PRP) has a promising clinical outcome in wound healing5.
PRP is a platelet concentrate separated from whole blood by centrifugation. PRP has been widely applied in the fields of tissue engineering and regenerative medicine6,7. The theoretical benefit of PRP has suggested a positive influence on the migration, proliferation, and angiogenesis in vitro, as well as in animal and human studies6,8,9. Based on the source of whole blood, PRP is commonly prepared as autologous platelet-rich plasma (au-PRP) and allogeneic platelet-rich plasma (al-PRP). In the fields of veterinary medicine and human medicine, au-PRP could be considered an effective adjunctive therapy in promoting healing of skin wounds in animals10,11. Similarly, au-PRP has been verified as a feasibility and safety treatment for venous leg ulcers12,13. In addition, PRP was also used for regenerative medicine in other medical fields, including orthopedics, ophthalmology, and dentistry14–20. In particular, au-PRP can enhance healing in the treatment of diabetic-associated foot ulcers21,22. Our previous clinical and basic studies suggest that au-PRP not only plays a role in stimulating soft tissue regeneration but also shows biological antibacterial properties in the treatment of diabetic foot ulcers with severe infection23,24. However, patients with DLEUs usually have severe complications and comorbidities. Furthermore, the protocol for au-PRP preparation is variable, and the therapeutic outcomes lack reproducibility25. Because of the complicated clinical conditions, the application of au-PRP for patients with DLEUs is limited. Whether al-PRP can have reliable and safe therapeutic effects compared with au-PRP is worthy of investigation. According to a literature review, only a few pilot or case studies of al-PRP have been used to promote tissue regeneration with negligible immunogenicity and great healing effectiveness26,27. The application of al-PRP for DLEU treatment has been less investigated. Additionally, no study has been conducted to compare the safety and effectiveness of al-PRP over au-PRP.
Therefore, to overcome the clinical limitations of au-PRP treatment, this study aims to explore the feasibility, effectiveness, and safety of al-PRP for application in the treatment of DLEUs.
Materials and Methods
Participants
A total of 75 inpatients with DLEUs were enrolled in a diabetes setting from December 2015 to August 2018. Patients were assigned to the al-PRP group (n = 20), au-PRP group (n = 25), or conventional wound treatment (CTW) group (n = 30) matched by the ankle brachial index (ABI) and ulcer size according to the wishes and permits of the patients. The inclusion criteria were as follows: (1) inpatients aged 30 to 90 y, (2) a definite diagnosis of type 2 diabetes mellitus based on the 1999 diabetes diagnosis standard of the World Health Organization, (3) patients with mild to moderate lower limb ischemia (ABI larger than 0.5), and (4) patients with at least one skin ulcer under the knee joint. The exclusion criteria included diabetic ketoacidosis or a hyperosmolar hyperglycemic state; uncontrolled systemic or local infection; dry or moist gangrene; severe coronary, cerebral, and/or renal vascular diseases; malignant tumors; pregnant or breast-feeding women; and hematological system diseases. This study was approved by the Ethical Committee Board of Southwest Hospital and complied with the recommendations of the Declaration of Helsinki. Written informed consent was obtained from all patients.
The Preparation of PRP
A modified procedure was performed for au-PRP preparation based on a procedure reported by Li et al28. A total of 50 to 100 ml (based on the wound sizes) of peripheral venous whole blood was aspirated into a sterilized centrifuge tube with acid citrate dextrose solution B anticoagulants on the same day of treatment. Immediately, the erythrocyte concentrate was discarded after centrifugation with a freezing centrifuge (Beckman, Life Sciences, IN, USA) at 600 rpm for 15 min. The remaining plasma was further centrifuged at 1,135 g for 7 min to separate the PRP from platelet-poor plasma” in the section of “preparation of PRP. The number of platelets was calculated with an automatic blood cell analyzer (Sysmex XE-2100; Nigale, Chengdu, China).
For the preparation of al-PRP, an ABO- and Rh-matched platelet concentrate for patient treatment was ordered from the blood bank and delivered to the laboratory on the same day of treatment. The preparation process of al-PRP was the same as that of au-PRP. Before the preparation of PRP, infectious markers were examined in order to exclude hepatitis B virus, hepatitis C virus, and human immunodeficiency virus. Before treatment with PRP, 1,000 IU bovine thrombin was mixed with 1 ml of 10% calcium gluconate injection at a concentration of 1,000 IU/ml; PRP was then activated with the mixed solution described above at a proper proportion of 10:1 (V/V).
Study Design, Procedures, and Follow-up
The study was designed as a prospective, case-controlled cohort, and single-center clinical trial for the purpose of comparing the effectiveness of topical al-PRP or au-PRP application versus standard treatment on DLEUs. All participants received systemic therapies and standard topical wound care. Clinical management included controlling blood sugar, blood pressure, and blood lipids; systemic antibiotic therapy depended on drug sensitivity test of wound drainage or tissue; nutritional support (enough calories, hemoglobin concentration more than 90 g/l, and serum albumin level more than 30 g/l); and nerve-trophic and circulation-improving therapies (mecobalamin 0.5 mg tid and aspirin 100 mg qd if it is necessary) with same procedures in all participants. Local treatments were administrated with same procedures for all patients, which include thoroughly removed nonviable tissue with surgical debridement, drainage with topical dressing, or negative pressure wound therapy. Systemic and local therapies were continued until the observational period ended. Participants in the conventional wound therapeutic (CWT) group were administered the systemic and topical wound therapies described above. When the wound beds were clean without obvious necrotic tissue and purulent secretion, topical administration of al-PRP or au-PRP was performed for participants in the au-PRP group (Fig. 1 ) or au-PRP group (Fig. 2), respectively. If there was no specific discomfort after application, the dressing was changed after 5 d. Generally, if the wound area reduction rate was less than 80% or the remaining wound areas were larger than 1 cm2 within 2 wk after PRP application, PRP treatments were performed28.
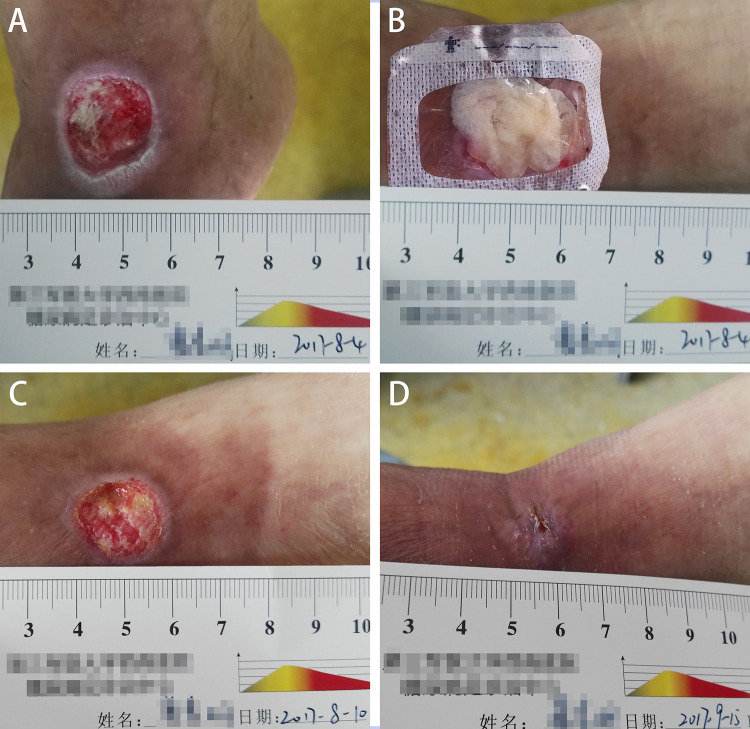
Fig. 1.
A 67-year-old man with diabetes mellitus had a nonhealing ulcer in the front of his left foot at the ankle joint level (A). After debridement, the al-PRP was administrated immediately (B). Six days after application of al-PRP (C). Six weeks after the treatment, the wound was complete closure (D). One application of al-PRP was performed for this patient. al-PRP: allogeneic platelet-rich plasma.
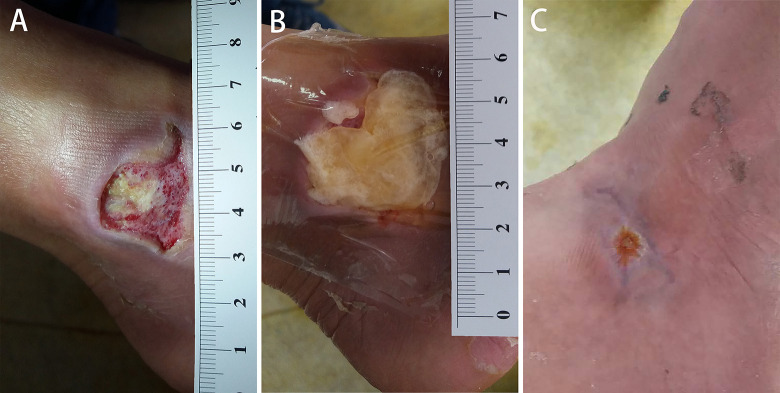
Fig. 2.
A 61-year-old man presented with a diabetic ulcer on the lateral side of right foot at the ankle joint level (A). After debridement, the au-PRP was performed immediately (B). Five weeks after the first application of au-PRP, complete epithelization was achieved. One application was performed for this patient. au-PRP: autologous platelet-rich plasma
All the participants were followed until wound closure was complete or until surgical operation, even amputation. The feasibility, curative effect, and safety of al-PRP and al-PRP were evaluated and compared according to the wound healing time, the average daily healing area and adverse reactions (infection, itching, redness, pain, rash, etc.).
Statistical Methods
The experimental data were processed with SPSS Version 22.0 statistical software (IBM, Armonk, NY, USA). Continuous variables are described as the means ± standard deviations. For the data that satisfied the variables with normal distribution, comparisons of the two groups were made with t-tests, comparisons of multiple groups were performed with one-way analysis of variance, and further pairwise comparisons were performed using Bonferroni tests. For variables that did not meet the normal distribution, comparisons of multiple groups were performed using the Kruskal–Wallis test, and pairwise comparisons using Steel–Dwass test. The enumeration data were described using ratios or rates. Fisher’s exact test was used for comparisons between groups. A test level of P < 0.05 was considered statistically significant.
Results
The characteristics of all participants are shown in Table 1. There were 15 subjects with diabetic foot ulcers and 5 with lower leg ulcers in the al-PRP group, there were 21 subjects with diabetic foot ulcers and 4 with lower leg ulcers in the au-PRP group, and there were 26 subjects with diabetic foot ulcers and 4 with lower leg ulcers in the CWT group. All inpatients completed follow-up in the study. Two participants underwent skin grafting after consultation by a surgeon in the au-PRP group.
Table 1.
Comparison of Clinical Characteristics.
| Variable | al-PRP group (n = 20) | au-PRP group (n = 25) | CWT group (n = 30) | P-value |
|---|---|---|---|---|
| Sex (male/female) | 15/5 | 13/12 | 21/9 | |
| Age, y | 66.3 ± 14.3 | 64.6 ± 11.0 | 66.1 ± 12.1 | 0.71F |
| Diabetes, months | 112 ± 77.5 | 122 ± 92.5 | 140 ± 96.4 | 0.53H |
| Smoking history (%) | 8 (40.0) | 9 (36.0) | 16 (53.3) | 0.42 |
| Hypertension (%) | 8 (40.0) | 15 (60.0) | 20 (66.7) | 0.19 |
| Heart disease (%) | 2 (10.0) | 2 (8.0) | 6 (20.0) | 0.42 |
| Cerebral infarction (%) | 3 (15.0) | 1 (4.0) | 8 (26.7) | 0.09 |
| Hyperlipidemia (%) | 2 (10.0) | 11 (44.0) | 9 (30.0) | 0.08 |
| DN (%) | 18 (90.0) | 22 (88.0) | 28 (93.3) | 0.90 |
| DPV (%) | 17 (85.0) | 24 (96.0) | 29 (96.7) | 0.30 |
| DKD (%) | 8 (40.0) | 7 (28.0) | 18 (60.0) | 0.12 |
| DR (%) | 2 (10.0) | 2 (8.0) | 7 (23.3) | 0.28 |
al-PRP: allogeneic platelet-rich plasma; au-PRP: autologous platelet-rich plasma; CWT: conventional wound therapeutic; DKD: diabetic kidney disease; DN: diabetic neuropathy; DPV: diabetic peripheral vascular disease; DR: diabetic retinopathy.
F: analysis of variance; H: rank sum test (Kruskal–Wallis test); The enumeration data: Fisher’s exact test.
Comparison of Demographic Characteristics
No significant differences were observed in sex, age, diabetes duration, smoking history, etc. among the three groups (P > 0.05). There were no significant differences in complications of hypertension, heart disease, cerebral infarction, hyperlipidemia, diabetic neuropathy, diabetic peripheral vascular disease, diabetic kidney disease, neuropathy, or diabetic retinopathy (P > 0.05).
Comparison of Laboratory Examinations
No significant differences were observed in glycosylated hemoglobin, white blood cell count, C-reactive protein, procalcitonin, ankle brachial index, transcutaneous oxygen partial pressure, etc. among the three groups (P > 0.05) (Table 2).
Table 2.
Comparison of Laboratory Parameters.
| Variable | al-PRP group | au-PRP group | CWT group | P-value |
|---|---|---|---|---|
| HBA1c (%) | 7.46 ± 1.21 | 7.82 ± 2.45 | 8.47 ± 2.74 | 0.68H |
| WBC (1012) | 6.11 ± 1.58 | 6.24 ± 1.42 | 7.49 ± 3.35 | 0.08F |
| CRP (mg/l) | 15.5 ± 26.2 | 3.09 ± 3.54 | 9.71 ± 14.0 | 0.15H |
| PCT (pg/ml) | 0.06 ± 0.045 | 0.03 ± 0.02 | 0.11 ± 0.20 | 0.16H |
| ABI (left) | 0.90 ± 0.22 | 0.93 ± 0.18 | 0.93 ± 0.19 | 0.85H |
| ABI (right) | 0.93 ± 0.21 | 0.92 ± 0.21 | 0.90 ± 0.24 | 0.84H |
| TCPO2 standing (mmHg) | 49.1 ± 27.5 | 46.2 ± 22.2 | 54.5 ± 18.2 | 0.48F |
ABI: ankle brachial index; al-PRP: allogeneic platelet-rich plasma; au-PRP: autologous platelet-rich plasma; CRP: C-reactive protein; CWT: conventional wound therapeutic; HBA1c: glycated hemoglobin; PCT: procalcitonin; TCPO2: transcutaneous oxygen partial pressure; WBC: white blood cell.
F: analysis of variance; H: rank sum test (Kruskal–Wallis test); Count data: chi-square test.
Comparison of the Number and Concentration of Platelets
After preparation, the concentration of enriched platelets in the al-PRP group and the au-PRP group reached (1042.9 ± 180.3) × 109/l and (939.3 ± 237.4) ×109/l, respectively, but no significant difference was found between the two groups. There was also no significant difference in the average number of PRP treatments between the two groups (Table 3).
Table 3.
Comparison of the Average Number of PRP Treatments and Concentration of Platelet Therapy.
| al-PRP group | au-PRP group | P-value | |
|---|---|---|---|
| Initial platelet concentration | (166.2 ± 54.8) × 109/l | (159.6 ± 48.6) × 109/l | 0.26T |
| Average PRP concentration | (1042.9 ± 180.3) × 109/l | (939.3 ± 237.4) × 109/l | 0.11T |
| Times of PRP treatment | 1.85 ± 1.46 | 2.24 ± 1.45 | 0.18H |
al-PRP: allogeneic platelet-rich plasma; au-PRP: autologous platelet-rich plasma; PRP: platelet-rich plasma.
H: rank sum test (Kruskal–Wallis test); Count data: chi-square test; T: Student’s test.
Comparison of Wound Healing Among All Groups
There was no significant difference between the ulcer area and the number of ulcers among all groups (P > 0.05). In terms of the healing time, the healing time of ulcers in the al-PRP group (56.9 ± 29.22 d) and the au-PRP group (55.6 ± 33.8 d) was significantly shorter than that of ulcers in the CTW group (88.0 ± 43.4 d) (P < 0.01), while there was no significant difference between the au-PRP group and the al-PRP group (P > 0.05). Although there was no significant difference in the daily healing area among all groups (P > 0.05), the trend of the healing rate in the al-PRP group (16.77 ± 12.85 mm2), au-PRP group (14.31 ± 18.28 mm2), and CWT group (9.90 ± 8.51 mm2) gradually decreased (Table 4).
Table 4.
Comparison of Wound Healing in All Groups.
| Parameters | al-PRP group | au-PRP group | CWT group | P-value |
|---|---|---|---|---|
| Ulcer area (cm2) | 8.43 ± 6.93 | 5.64 ± 3.91 | 9.73 ± 9.72 | 0.19H |
| Ulcer number | 1.10 ± 0.45 | 1.24 ± 0.44 | 1.40 ± 0.72 | 0.12H |
| Ulcer healing time (d) | 56.9 ± 29.22 | 55.6 ± 33.8 | 88.0 ± 43.4*# | 0.002H |
| Healing area/day (mm2) | 16.77 ± 12.85 | 14.31 ± 18.28 | 9.90 ± 8.509 | 0.12H |
al-PRP: allogeneic platelet-rich plasma; au-PRP: autologous platelet-rich plasma; CWT: conventional wound therapeutic.
H: rank sum test (Kruskal–Wallis test).
* Compared to al-PRP group, P < 0.05; #Compared to al-PRP group, P < 0.05.
Adverse Event Observation
No patient was lost to follow-up or quit in the study. Among the 45 subjects who were treated with either al-PRP or au-PRP intervention for DLEUs, no local or systemic fever; skin redness, edema, itching, rash or burning; or other sensory abnormalities occurred.
Discussion
Patients with DLEUs have difficulty healing due to the loss of wound healing-related growth factors and inflammatory imbalance in the topical wound bed29,30. Novel biological therapies may regulate and reverse the possible mechanism of wound imbalance31,32. au-PRP-released growth factors and antibacterial peptides can increase antibacterial effects and promote diabetic wound repair, as well as relieve wound pain23,24,33–35. A systematic review and meta-analysis involving 431 participants with diabetic ulcers in eight randomized controlled trails indicated a significantly increased ratio of complete ulcer healing and reduced areas of ulcers under the treatment of au-PRP36. Although the therapeutic application of au-PRP in the past 20 y has been demonstrated to be a safe and effective treatment, most patients with diabetic wounds are prone to be elderly and have a long diabetes duration, malnutrition, infection, thrombocytopenia, hypovolemia, anemia, skin problems, and immune dysfunction as well as take antiplatelet drugs37–39. Treating patients with these conditions is difficult, especially when using au-PRP or skin grafting. According to a review of previous literature, al-PRP has been successfully applied for wound repair in animal studies40–42 and human clinical studies26,43,44. To the best of our knowledge, this is the first study to verify the feasibility, effectiveness, and safety of al-PRP in the treatment of DLEUs to replace its autologous counterpart.
In this study, al-PRP and au-PRP could be enriched by the separation of autologous platelets and banked allogeneic platelets. Most importantly, al-PRP showed no significant difference in the concentration of platelets with au-PRP in order to reach an similarly effective concentration of approximately six times above baseline. Previous studies indicated that a satisfactory result would be achieved with a proper concentration of PRP approximately five times above baseline10,45,46.
An important point to realize here is that increased rotation force can result in a higher platelet concentration; however, forces that are too high may induce an early activation of platelets, resulting in the loss of growth factors and therapeutic efficiency of PRP39.
Since al-PRP can be prepared well and in an adequate quantity by centrifugation with banked platelets, there is also the opportunity to use al-PRP for the promotion of wound healing, even in diabetic patients with poor physical and mental conditions, due to the difficulties of au-PRP preparation and skin grafting. In particular, the repetitive collection of whole blood may cause an additional health burden to patients because of red blood cell damage and white blood cell loss, which may worsen their illness and delay wound healing. Thus, al-PRP from well-characterized donors has been considered an off-the-shelf solution because it can avoid harvesting large quantities of whole blood and can fully utilize precious blood resources47. Additionally, because of the great variability of issues induced by different protocols, au-PRP can be compromised, leading to reduced clinical efficacy25. al-PRP was prepared by a blood component separator with whole blood from a healthy donor stored in a blood bank. Therefore, it could be considered a biological therapeutic product using a standard protocol of preparation. The remaining blood components could be fully used in the clinic.
This result suggested that both au-PRP and al-PRP could accelerate ulcer healing on the basis of conventional wound treatment in the present study. However, there was no significant difference in the healing time between the au-PRP and al-PRP groups, suggesting that al-PRP was not superior to au-PRP. The possible influence on effectiveness induced by the total amount of platelets was excluded because the treatment times of PRP and the average platelet concentration were not significantly different. The results of al-PRP and au-PRP were consistent with those of previous studies on diabetic foot ulcers28,44,48. A comparative study that assessed the efficacy and safety of platelets from ABO and rhesus-matched blood bank samples for diabetic foot ulcers revealed a significant improvement in the healing of the al-PRP group at 12 wk44. In another study of 10 patients with aggressive, life-threatening, and refractory ulcers, a combination treatment of al-PRP, fibrin glue, and collagen matrix also had a satisfying outcome48. Similarly, a randomized clinical trial of au-PRP was performed by Ran et al. to examine the safety and effectiveness, and the topical application of au-PRP facilitated wound healing of diabetic chronic refractory cutaneous ulcers based on standard treatment28. In another study, compared to local antiseptic dressings, au-PRP was more effective in terms of the healing rate and prevention of infection in clean diabetic ulcers49. This is the first study to confirm that al-PRP has an equivalent effectiveness and safety to accelerate wound healing of DLEUs. Furthermore, the present study was supported by a basic study of cartilage culture, in which the results suggested that allogeneic freeze-dried platelet lysate presented equivalent effects compared to frozen autologous platelet lysate50.
For both au- and al-PRP, their role depends on the high concentration of various growth factors produced by the degranulation of concentrated platelets, including fibroblast growth factor, transforming growth factor, insulin-like growth factor, vascular endothelial growth factor, and platelet-derived growth factor23,39. These growth factors are released after activation by thrombin or calcium chloride and play an important role in tissue repair and regeneration. On the other hand, PRP can inhibit the growth of Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus faecalis for up to 2 h of incubation through chemokine (C-C motif) ligand-3, chemokine (C-C motif) ligand-5, and chemokine (C-X-C motif) ligand-151. CaCl2-activated PRP exhibited antimicrobial activity against Methicillin-resistant S. aureus infection to improve the infected wound healing re-epithelization and granulation tissue formation in dogs52.
In terms of clinical practice, PRP provides a “biological antibacterial agent” to treat diabetic skin ulcers with severe or multidrug-resistant infection23,53–55. A previous pilot study that compared the use of al-PRP with the current best practice approach of chronic wound dressing protocols for pressure sores reported that there was no significant difference in volume reduction between the two groups. However, the rate of granulation tissue proliferation in an al-PRP-treated group was significantly faster than that in the control group during the first 2 wk of treatment, suggesting that al-PRP treatment plays an important role in granulation tissue proliferation in the early stage of initiation26. For the speed of healing area per day, there was a reduced trend but no significant difference between treatment with al-PRP and au-PRP. After carefully reviewing the treatment, two patients underwent skin grafting according to the suggestion of the surgeon in the au-PRP group. Therefore, the healing times of these two patients were shortened, resulting in an increase in the healing rate.
Currently, al-PRP has not been widely used in clinical applications; the risk of immune reactions and crossed contamination is the major obstacle. In this study, patients treated with al-PRP showed no obvious local inflammation, allergies, or other adverse reactions, similar to patients treated with au-PRP. We speculate the following possible reasons. First, al-PRP was prepared with ABO- and Rh-matched by a blood bank platelet concentrate, which required a strict process of sterilization and the exclusion of infectious disease. Second, al-PRP was applied locally to the lower extremity ulcers, which may be the reason why an immune response, such as a skin allergy, was not observed. An animal experiment was employed to investigate the immunogenicity, indicating that a severe and chronic immune response was not triggered with an intramuscular injection of al-PRP in rabbits27. Finally, a possible mechanism of “immune exemption” is as follows: al-PRP, as a type of gel, can be degraded and absorbed completely in the topical wound so that little al-PRP enters into the circulatory system and avoids most alloantigens (human leukocyte antigens (HLA) and human platelet antigens (HPA))11. The structure and expression levels of platelet surface antigen after activation may be altered and may reduce immunogenicity56.
The preparation of al-PRP is simple and easy, and the therapeutic effects and safety are certain. al-PRP is a new type of biological therapeutic recommended for patients with the following complicated conditions: (1) platelet deficiency or disease, (2) anemia, (3) malnutrition or cachexia, (4) severe immune dysfunction, (5) bleeding disorders, (6) severe infection or sepsis, (7) undergoing chemotherapy, (8) long-term use of antiplatelet drugs, (9) severe edema with skin grafting difficulty, and (10) withdrawal from biological therapy but refusal of au-PRP. However, there are some limitations in this study. Firstly, this was a proof-of-principle pilot study with a limited number of cases. Secondly, because of ethical reason, the study cannot be designed as a double-blinded trail, which might reduce the argumentation intensity of the data. Finally, a large-scale randomized controlled study should be conducted to further determine the pros and cons of al-PRP in the future.
Conclusion
The use of al-PRP could provide a feasible, effective, and safe biological therapy as an off-the-shelf solution for DLEUs according to the present pilot study. A large-size investigation is needed to further explore the ultimate value of al-PRP when au-PRP is limited.
Footnotes
Ethical Approval: The ethical approval to report this work was obtained from the Ethical Committee Board of Southwest Hospital.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the protocol approved by “the Ethical Committee Board of Southwest Hospital” and complied with the recommendations of the Declaration of Helsinki.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Author Contributions: MH, XG, TL, and YC conducted and collected the study. XJ, ZL, GY, and YF analyzed the data and contributed to the discussion. BC, DGA, and WD wrote the manuscript, contributed to the discussion, reviewed, and edited the manuscript. All authors read and approved the final manuscript. WD took full responsibility for this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Fundamental Research Funds for the Central Universities at Chongqing University (Grant No. 2019CDYGYB020), the Joint Medical Research Programs of Chongqing Science and Technology Bureau and Health Commission Foundation (Grant No.2019MSXM028), Basic Research and Frontier Exploration of Science and Technology Commission by Chongqing Municipality (Grant No. cstc2018jcyjAX0335), and Yuzhong District (Grant No. 20180156) awarded to Dr Wuquan Deng and Dr Xiaoyan Jiang.
ORCID iD: Wuquan Deng  https://orcid.org/0000-0002-5450-9713
https://orcid.org/0000-0002-5450-9713
References
- 1. Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower-extremity ulcers. N Engl J Med. 2017;377(16):1559–1567. [DOI] [PubMed] [Google Scholar]
- 2. Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. Br J Dermatol. 2015;173(2):379–390. [DOI] [PubMed] [Google Scholar]
- 3. Humphries MD, Brunson A, Li CS, Melnikow J, Romano PS. Amputation trends for patients with lower extremity ulcers due to diabetes and peripheral artery disease using statewide data. J Vasc Surg. 2016;64(6):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. [DOI] [PubMed] [Google Scholar]
- 5. Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, Price PE, Jeffcoate WJ. International working group on the diabetic foot. effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(Suppl):154–168. [DOI] [PubMed] [Google Scholar]
- 6. Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24(3):173–182. [DOI] [PubMed] [Google Scholar]
- 7. Lang S, Loibl M, Herrmann M. Platelet-rich plasma in tissue engineering: hype and hope. Eur Surg Res. 2018;59(3-4):265–275. [DOI] [PubMed] [Google Scholar]
- 8. Etulain J, Mena HA, Meiss RP, Frechtel G, Gutt S, Negrotto S, Schattner M. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep. 2018;8(1):1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tambella AM, Martin S, Cantalamessa A, Serri E, Attili AR. Platelet-rich plasma and other hemocomponents in veterinary regenerative medicine. Wounds. 2018;30(11):329–336. [PubMed] [Google Scholar]
- 10. Tambella AM, Attili AR, Dupré G, Cantalamessa A, Martin S, Cuteri V, Marcazzan S, Del Fabbro M. Platelet-rich plasma to treat experimentally-induced skin wounds in animals: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0191093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tambella AM, Attili AR, Dini F, Palumbo PA, Vullo C, Serri E, Scrollavezza P, Dupré G. Autologous platelet gel to treat chronic decubital ulcers: a randomized, blind controlled clinical trial in dogs. Vet Surg. 2014;43(6):726–733. [DOI] [PubMed] [Google Scholar]
- 12. Burgos AN, Lobato I, Hernández I, Sebastian KS, Rodríguez B, March AG, Perez Salvador A, Arce V, Garcia-Alvarez A, Gomez-Fernandez MC, Grandes G, et al. Autologous platelet-rich plasma in the treatment of venous leg ulcers in primary care: a randomised controlled, pilot study. J Wound Care. 2018;27(Sup 6):S20–S24. [DOI] [PubMed] [Google Scholar]
- 13. Moneib HA, Youssef SS, Aly DG, Rizk MA, Abdelhakeem YI. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: a comparative study. J Cosmet Dermatol. 2018;17(3):495–501. [DOI] [PubMed] [Google Scholar]
- 14. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. [DOI] [PubMed] [Google Scholar]
- 15. Faillace V, Tambella AM, Fratini M, Paggi E, Dini F, Laus F. Use of autologous platelet-rich plasma for a delayed consolidation of a tibial fracture in a young donkey. J Vet Med Sci. 2017;79(3):618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcazzan S, Taschieri S, Weinstein RL, Del Fabbro M. Efficacy of platelet concentrates in bone healing: a systematic review on animal studies - part b: large-size animal models. Platelets. 2018;29(4):338–346. [DOI] [PubMed] [Google Scholar]
- 17. Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: a prospective randomized controlled study. Orthopade. 2019;48(3):239–247. [DOI] [PubMed] [Google Scholar]
- 18. Alio JL, Rodriguez AE, Arriba D, Gisbert S, Abdelghany AA. Treatment with platelet-rich plasma of surgically related dormant corneal ulcers. Eur J Ophthalmol. 2018;28(5):515–520. [DOI] [PubMed] [Google Scholar]
- 19. Del Fabbro M, Bucchi C, Lolato A, Corbella S, Testori T, Taschieri S. Healing of postextraction sockets preserved with autologous platelet concentrates. a systematic review and meta-analysis. J Oral Maxillofac Surg. 2017;75(8):1601–1615. [DOI] [PubMed] [Google Scholar]
- 20. Bhujbal R, Malik AN, Kumar N, Kv S, Parkar IM, Mb J. Comparative evaluation of platelet rich plasma in socket healing and bone regeneration after surgical removal of impacted mandibular third molars. J Dent Res Dent Clin Dent Prospects. 2018;12(3):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen. 2015;23(5):638–643. [DOI] [PubMed] [Google Scholar]
- 22. Martinez ZMJ, Martí CAJ, Solà I, Expósito JA. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016. ;(5):CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deng W, Boey J, Chen B, Byun S, Lew E, Liang Z, Armstrong DG. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care. 2016;25(7):39–397. [DOI] [PubMed] [Google Scholar]
- 24. Li T, Ma Y, Wang M, Wang T, Wei J, Ren R, He M, Wang G, Boey J, Armstrong DG, Deng W, et al. Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect Drug Resist. 2019;12:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anitua E, Prado R. Addressing reproducibility in stem cell and PRP therapies. Trends Biotechnol. 2019;37(4):340–344. [DOI] [PubMed] [Google Scholar]
- 26. Scevola S, Nicoletti G, Brenta F, Isernia P, Maestri M, Faga A. Allogenic platelet gel in the treatment of pressure sores: a pilot study. Int Wound J. 2010;7(3):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang ZY, Huang AW, Fan JJ, Wei K, et al. The potential use of allogeneic platelet-rich plasma for large bone defect treatment: immunogenicity and defect healing efficacy. Cell Transplant. 2013;22(1):175–187. [DOI] [PubMed] [Google Scholar]
- 28. Li L, Chen D, Wang C, Yuan N, Wang Y, He L, Yang Y, Chen L, Liu G, Li X, Ran X. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: a prospective, randomized clinical trial. Wound Repair Regen. 2015;23(4):495–505. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Li Y, Li H, Zhu B, Wang L, Guo B. GDF11 improves angiogenic function of EPCs in diabetic limb ischemia. Diabetes. 2018;67(10):2084–2095. [DOI] [PubMed] [Google Scholar]
- 30. Xu F, Othman B, Lim J, Batres A, Ponugoti B, Zhang C. Foxo1 inhibits diabetic mucosal wound healing but enhances healing of normoglycemic wounds. Diabetes. 2015;64(1):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y, Ma Y, Li N. Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res Ther. 2018;9(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Everts PA, Brown Mahoney C, Hoffmann JJ, Schönberger JP, Box HA, van Zundert A, Knape JT. Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors. 2006;24(3):165–171. [DOI] [PubMed] [Google Scholar]
- 33. Piccin A, Pierro DAM, Canzian L. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15(4):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fabbro MD, Bortolin M, Taschieri S, Ceci C, Weinstein RL. Antimicrobial properties of platelet-rich preparations. a systematic review of the current pre-clinical evidence. Platelets, 2016;27(4):276–285. [DOI] [PubMed] [Google Scholar]
- 35. Miller JD, Rankin TM, Hua NT, Ontiveros T, Giovinco NA, Mills JL, Armstrong DG. Reduction of pain via platelet-rich plasma in split-thickness skin graft donor sites: a series of matched pairs. Diabet Foot Ankle. 2015;6:24972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu Z, Qu S, Zhang J, Cao X, Wang P, Huang S, Shi F, Dong Y, Wu J, Tang B, Zhu J. Efficacy and Safety of Platelet-rich plasma for patients with diabetic ulcers: a systematic review and meta-analysis. Adv Wound Care (New Rochelle). 2019;8(7):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haughey L, Barbul A. Nutrition and lower extremity ulcers: causality and/or treatment. Int J Low Extrem Wounds. 2017;16(4):238–243. [DOI] [PubMed] [Google Scholar]
- 38. Jones CI. Platelet function and ageing. Mamm Genome. 2016;27(7-8):358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marques LF, Stessuk T, Camargo IC, Sabeh Junior N, dos Santos L, Ribeiro-Paes JT. Platelet-rich plasma (PRP): methodological aspects and clinical applications. Platelets. 2015;26(2):101–113. [DOI] [PubMed] [Google Scholar]
- 40. Rajabi H, Sheikhani Shahin H, Norouzian M. The healing effects of aquatic activities and allogenic injection of platelet-rich plasma (PRP) on injuries of Achilles tendon in experimental rat. World J Plast Surg. 2015;4 (1):66–73. [PMC free article] [PubMed] [Google Scholar]
- 41. Abouelnasr K, Hamed M, Lashen S. Enhancement of abdominal wall defect repair using allogenic platelet-rich plasma with commercial polyester/cotton fabric (Damour) in a canine model. J Vet Med Sci. 2017;79 (7):1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung TH, Baek DS, Kim N, Park JH, Park C. Topical allogeneic platelet-rich plasma treatment for a massive cutaneous lesion induced by disseminated intravascular coagulation in a toy breed dog. Ir Vet J. 2015;68(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smrke D, Gubina B, Rozman P. Allogeneic platelet gel with autologous cancellous bone graft for the treatment of a large bone defect. Eur Surg Res. 2007;39(3):170–174. [DOI] [PubMed] [Google Scholar]
- 44. Jeong SH, Han SK, Kim WK. Treatment of diabetic foot ulcers using a blood bank concentrate. Plast Reconstr Surg 2010;125(3):944–952. [DOI] [PubMed] [Google Scholar]
- 45. Rodriguez IA, Growney Kalaf EA, Bowlin GL, Sell SA. Platelet-rich plasma in bone regeneration: engineering the delivery for improved clinical efficacy. Biomed Res Int. 2014;2014:392398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mazzucco L, Balbo V, Guaschino R. “Reasonable compromise” to define the quality standards of platelet concentrate for non-transfusion use (CPunT). Transfus Apher Sci. 2012;47(2):207–211. [DOI] [PubMed] [Google Scholar]
- 47. Anitua E, Prado R, Orive G. Allogeneic platelet-rich plasma: at the dawn of an off-the-shelf therapy? Trends Biotechnol. 2017;35(2):91–93. [DOI] [PubMed] [Google Scholar]
- 48. Asadi M, Alamdari DH, Rahimi HR, Aliakbarian M, Jangjoo A. Treatment of life-threatening wounds with a combination of allogenic platelet-rich plasma, fibrin glue and collagen matrix, and a literature review. Exp Ther Med. 2014;8(2):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. 2017;38:206–211. [DOI] [PubMed] [Google Scholar]
- 50. Camargo Garbin L, McIlwraith CW, Frisbie DD. Evaluation of allogeneic freeze-dried platelet lysate in cartilage exposed to interleukin 1-β in vitro. BMC Vet Res. 2019;15(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mariani E, Filardo G, Canella V, Berlingeri A, Bielli A, Cattini L, Landini MP, Kon E, Marcacci M, Facchini A. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy. 2014;16(9):1294–1304. [DOI] [PubMed] [Google Scholar]
- 52. Farghali HA, AbdElKader NA, AbuBakr HO, Aljuaydi SH, Khattab MS, Elhelw R, Elhariri M. Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. Sci Rep. 2019;9(1):12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang W, Guo Y, Kuss M, Shi W, Aldrich AL, Untrauer J, Kielian T, Duan B. Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng Part B Rev. 2019;25(3):225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun S, Wang C, Chen D, Cen S, Lv X, Wen X, Liu M, Lu W, Zhao J, Ran X. Combating superbug without antibiotic on a postamputation wound in a patient with diabetic foot. Int J Low Extrem Wounds. 2016;15(1):74–77. [DOI] [PubMed] [Google Scholar]
- 55. Drago L, Bortolin M, Vassena C, Romanò CL, Taschieri S, Del Fabbro M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS One. 2014;9(9):e107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schaller M, Hofmann A, Connor J, Nydegger UE. Filtered platelet concentrates from pooled buffy coats show comparable storage lesions when stored for 9 d at 20-24 degrees C or when supplemented with thromboSol at 2-6 degrees C. Eur J Haematol. 2000;64(6):401–410. [DOI] [PubMed] [Google Scholar]