Abstract
Pancreatic cancer is characterized by a hypoxic tumor microenvironment, which is primarily caused by massive fibrosis with pancreatic stellate cells (PSCs) as a main component. Our previous studies have shown that resveratrol can significantly inhibit pancreatic cancer. However, whether resveratrol can inhibit hypoxia-induced cancer development remains unclear. The objective of this study was to explore whether PSCs and hypoxia synergistically mediate aggressiveness in pancreatic cancer and detect the potential pleiotropic protective effects of resveratrol on hypoxia-induced pancreatic cancer progression. Human PSCs were treated with vehicle or resveratrol under normoxic or hypoxic conditions (3% O2), and PSC activation was assessed by immunofluorescence staining. SiRNA was used to silence hypoxia-inducible factor 1 (HIF-1) expression. The invasive capacity of Panc-1 and Mia Paca-2 cells cocultured with conditioned medium from PSCs was assessed by Transwell assays. To examine tumor formation kinetics, KPC (LSL-KrasG12D/+, Trp53fl/+, and Pdx1-Cre) mice were sacrificed at different time points. To investigate the antitumor effects of resveratrol in vivo, 8-wk-old KPC mice were divided into two groups and treated daily with or without 50 mg/kg resveratrol. Our data indicate that hypoxia induces PSC activation via HIF-1 and that the interleukin 6, vascular endothelial growth factor A, and stromal cell-derived factor 1 derived from activated PSCs promote both invasion and the epithelial–mesenchymal transition and inhibit apoptosis in pancreatic cancer cells. However, resveratrol inhibits hypoxia-induced PSC activation, blocks the interplay between PSCs and pancreatic cancer cells, and suppresses the malignant progression of pancreatic cancer and stromal desmoplasia in a KPC mouse model. Our data highlight that activated PSCs and intratumoral hypoxia are essential targets for novel strategies to prevent tumor–microenvironment interactions. Furthermore, the polyphenolic compound resveratrol effectively ameliorates the malignant progression of pancreatic ductal adenocarcinoma.
Keywords: resveratrol, hypoxia, PSCs, stromal desmoplasia, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDAC), which is one of the most lethal malignant tumors of the digestive system, has a dismal prognosis, with a 5-year survival rate of less than 7%; PDAC is projected to be the second leading cause of cancer-related death by 20301. Metastasis is partially responsible for the exceedingly poor clinical prognosis of patients with PDAC2. Nevertheless, the molecular mechanisms underlying the aggressiveness of PDAC remain unclear.
PDAC exhibits the distinct pathological feature of a profuse desmoplastic stroma comprising activated fibroblasts, leukocytes, and extracellular matrix (ECM)3. Fibroblasts in the setting of desmoplastic stroma are currently recognized as activated pancreatic stellate cells (PSCs), which are characterized by proliferation and produce an abundance of collagens, laminin, and fibronectin4. Consequently, the tumor microenvironment (TME) in PDAC exhibits enhanced stiffness, increased hyaluronic acid content, and elevated hydrostatic pressure, resulting in intratumoral hypoperfusion and hypoxia, which may inhibit effective drug delivery into the tumor5.
Intratumoral hypoxia resulting from impaired perfusion in tumor progression facilitates genomic instability, the activation of the unfolded protein response, angiogenesis, inflammation, fibrosis, immunosuppression, a switch to anaerobic metabolism, the epithelial–mesenchymal transition (EMT), metastasis, resistance to chemotherapy and immunotherapy, and the introduction of the cancer stem cell phenotype6. Moreover, in PDAC, intratumoral hypoxia can further activate PSCs and promote aggressiveness. Under conditions of hypoperfusion and hypoxia, PSCs not only participate in fibrosis but also secrete high levels of cytokines and chemotactic factors, such as interleukin 6 (IL-6), vascular endothelial growth factor (VEGF), and stromal cell-derived factor 1 (SDF-1), further supporting tumor growth, invasion, and metastasis7. Hypoperfusion leads to the nutrient-poor microenvironment of pancreatic cancer, which can induce autophagy in PSCs, thus providing access to paracrine factors such as alanine and IL-6 to fuel the malignant progression of PDAC8,9.
Therefore, hypoperfusion, hypoxia, and PSC activation operate in a vicious circle, and disrupting this vicious circle may be a promising strategy to treat pancreatic cancer. Many questions regarding the effective targets remain unanswered, and the mechanisms underlying the maintenance of the anoxic and fibrotic TME during disease progression have not been found and elucidated to date.
Resveratrol (trans-3,4′,5-trihydroxystilbene, RSV) is a polyphenolic compound found naturally in a variety of plants, primarily grapes and Polygonum cuspidatum 10. Numerous studies have shown that RSV possesses various properties that benefit human health, such as antifibrotic, antioxidant, antiaging, and anti-inflammatory activities and biological properties, and that it displays protective effects in cardiac diseases and metabolic disorders11. Previous studies have demonstrated that RSV plays a significant role in repressing the proliferation of pancreatic cancer cells by targeting the PI-3K/protein kinase B (Akt)/nuclear factor-kappa B (NF-κB)12, Hedgehog13, and AMPK/YAP signaling pathways14,15. However, the molecular mechanisms underlying the antifibrotic effects of RSV and whether RSV can inhibit hypoxia-induced PSC activation, block the interplay between PSCs and pancreatic cancer cells, and suppress stromal desmoplasia in vivo remain unclear.
In this study, we investigated the role of hypoxia-induced PSC activation and examined the potential protective effects of RSV on hypoxia-driven pancreatic cancer progression. We discovered that RSV inhibited hypoxia-induced PSC activation, pancreatic cancer cell invasion, and apoptosis by inhibiting hypoxia-inducible factor 1α (HIF-1α) expression. Furthermore, our in vivo studies revealed that the administration of RSV to LSL-KrasG12D/+, Trp53fl/+, and Pdx1-Cre (KPC) mice by gastric perfusion could significantly suppress VEGF-A, SDF-1, IL-6, alpha-smooth muscle actin (α-SMA), and HIF-1α expression and the desmoplastic reaction.
Materials and Methods
All experimental protocols were approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China.
Materials
Antibodies used in this study were purchased from the following sources: anti-HIF-1α (Bioworld, Minneapolis, MN, USA), anti-E-cadherin (Cell Signaling Technology, Danvers, MA, USA), anti-N-cadherin (Cell Signaling Technology), anti-vimentin (Cell Signaling Technology), anti-BCL2 (Abcam, Cambridge, MA, USA), anti-cleaved caspase-3 (Abcam), anti-α-SMA (Sigma-Aldrich, St Louis, MO, USA), and anti-β-actin (Sigma-Aldrich). RSV was purchased from Sigma.
Cell Culture
The human PSCs were isolated from normal pancreatic tissue obtained during liver transplantation surgery from patients. Moreover, the PSCs were isolated and cultured by the methods described in previous studies16 as explained elsewhere. Normal pancreatic tissues were obtained from the Department of Hepatobiliary Surgery at the First Affiliated Hospital of Xi’an Jiaotong University. Cellular morphology, oil red O staining of intracellular fat droplets, and immunofluorescence staining of α-SMA were used to identify the purity of the PSCs as described in previous studies17. The human pancreatic cancer cell lines Panc-1 and Mia Paca-2 were purchased from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS). Panc-1 and Mia Paca-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) (HyClone, Logan, UT, USA) at 37°C with 5% CO2 and 95% air. The media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 μg/ml ampicillin, and 100 μg/ml streptomycin.
PSC Activation
The PSCs were cultured to subconfluence under normoxic or hypoxic conditions (3% O2). Fresh serum-free medium was added for 24 h before the conditioned medium (CM) was collected. PSCs were serum-starved for 24 h before being treated for another 24 h with 50 μM RSV or si-HIF-1α under normoxic or hypoxic conditions.
Western Blot Analysis
Total proteins from PSCs, Panc-1 cells, and Mia Paca-2 cells (1 × 106) grown under the experimental conditions described herein were extracted using RIPA lysis buffer (Beyotime, Guangzhou, China). Total proteins from the pancreas of KPC mice were performed as previously described18. A bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA) was used to determine the protein concentrations according to the manufacturer’s instructions. The details of the western blot assay have been previously described19. To assess the expression of the designated proteins, the immunoreactive bands were detected using a chemiluminescence detection system via the peroxidase reaction, and the images of the bands were acquired using a ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA, USA). β-Actin was used as the internal loading control.
Cell Invasion Assay
The effects of CM from PSCs on the invasive capacities of Panc-1 and Mia Paca-2 cells were evaluated by a Matrigel invasion assay. Briefly, Panc-1 cells or Mia Paca-2 cells were serum-starved for 6 to 8 h before being incubated with CM from PSCs for 24 h. Next, the cells (1.5 × 104/ml) were resuspended and gently seeded in the upper compartment of Matrigel-coated Transwell chambers (8-mm pore). The lower compartments were filled with DMEM containing 10% FBS. The next steps were performed following a protocol previously described in detail20. The invaded cancer cells were stained and counted under a microscope (Nikon Instruments Inc.) in 10 randomly selected fields at 200× magnification.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
After the designated intervention was completed, total RNA was extracted by using a Fastgen1000 RNA isolation kit (Fastgen, Shanghai, China) according to the manufacturer’s instructions. Total RNA was subsequently reverse transcribed to cDNA using a PrimeScript RT reagent kit (TaKaRa, Dalian, China). qRT-PCR was performed according to a protocol previously described in detail21. The PCR primer sequences for VEGF-A, IL-6, SDF-1, and GAPDH are shown in supplemental Table S1. The expression level of each target gene was normalized to that of GAPDH. The relative gene expression was calculated by the 2-△△Ct method22.
Enzyme-Linked Immunosorbent Assay (ELISA)
After the cells were cultured in a serum-free medium for 72 h, the CM was collected and centrifuged at 1,500 rpm for 10 min to remove impurities. The supernatants were then frozen in liquid nitrogen until use. Next, the secretion levels of SDF-1 (R&D Systems, Minneapolis, MN, USA), VEGF-A (R&D Systems), and IL-6 (R&D Systems) in the CM from PSCs were detected by ELISAs according to the manufacturer’s protocols.
Immunofluorescence Staining
After completing the indicated treatment, the cells were washed three times with phosphate-buffered saline (PBS) and fixed in 4% formaldehyde for 15 min. Subsequently, the cells were permeabilized with 0.3% Triton X-100, incubated with blocking buffer (5% bovine serum albumin diluted in PBS), and incubated overnight with the appropriate primary antibodies at 4°C. Each slide was washed five times with PBS for 10 min per wash and then incubated for 1 h at room temperature with a Texas Red-conjugated secondary antibody from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Next, the slides were imaged using a fluorescence microscope (Zeiss Instruments confocal microscope) with appropriate excitation and emission spectra at 400× magnification.
Apoptosis Assay
As previously described, apoptosis was determined by flow cytometry using an annexin V-FITC/7-AAD apoptosis detection kit purchased from Becton, Dickinson and Company (BD) (Franklin Lakes, NJ, USA), and the apoptosis rate was analyzed by flow cytometry using a FACSCalibur instrument (BD Biosciences, San Diego, CA, USA)14. The total apoptosis rate was calculated by adding the early apoptotic cells (annexin V-FITC+/7-AAD− populations) and late apoptotic cells (annexin V-FITC+/7-AAD+ populations).
RNA Interference
siRNAs purchased from GenePharma (Shanghai, China) were used to silence HIF-1α for loss-of-function studies. The siRNA sequences against HIF-1α and the negative control sequences are provided in supplemental Table S2. Each siRNA (100 nM) was transfected into PSCs and Panc-1 cells according to the manufacturer’s instructions using Lipofectamine 2000 as the carrier. The knockdown efficiency of the target gene by siRNA was verified by western blot analysis. The siRNA treatment time was 8 h with a serum-free medium for transfection. These cells were used for further experiments 48 h after transfection with serum-containing medium.
In vivo Experiments and Histopathological Analysis
KPC mice, LSL-KrasG12D mice, and Trp53fl/fl mice were purchased from the Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China. The feeding, breeding, and genotypic identification of the KPC (LSL-KrasG12D/+, Trp53fl/+, and Pdx1-Cre) mice were performed as previously described23. To explore tumor formation kinetics, KPC mice were sacrificed at different time points (6, 10, 14, and 18 wk). To investigate the antitumor effects of RSV in vivo, 8-wk-old KPC mice were divided into two groups and treated daily with or without 50 mg/kg RSV and sacrificed at 16 wk based on our previous study24. The methods used for tissue preparation and histopathologic analysis, i.e., hematoxylin and eosin (H&E) staining, immunohistochemical staining, and Masson’s trichrome staining, were performed in accordance with our previously reported methods25. Briefly, standard immunohistochemistry was performed using HIF-1α (Bioworld, St Louis Park, MN, USA), α-SMA (Sigma), VEGF-A, IL-6, and SDF-1 (Abcam) antibodies. Masson’s trichome staining to assess fibrosis was carried out using a kit from Sigma according to the manufacturer’s protocol. The staining results were evaluated by two pathologists blinded to the clinical data, as described by Sinicrope et al.26.
Statistical Analysis
The data presented here are representative of three independent experiments involving at least three replicates per treatment group. Data are presented as the means ± standard deviations. The differences were assessed by Student’s t-test, and P <0.05 was considered statistically significant.
Results
RSV Inhibits Hypoxia-Induced PSC Activation
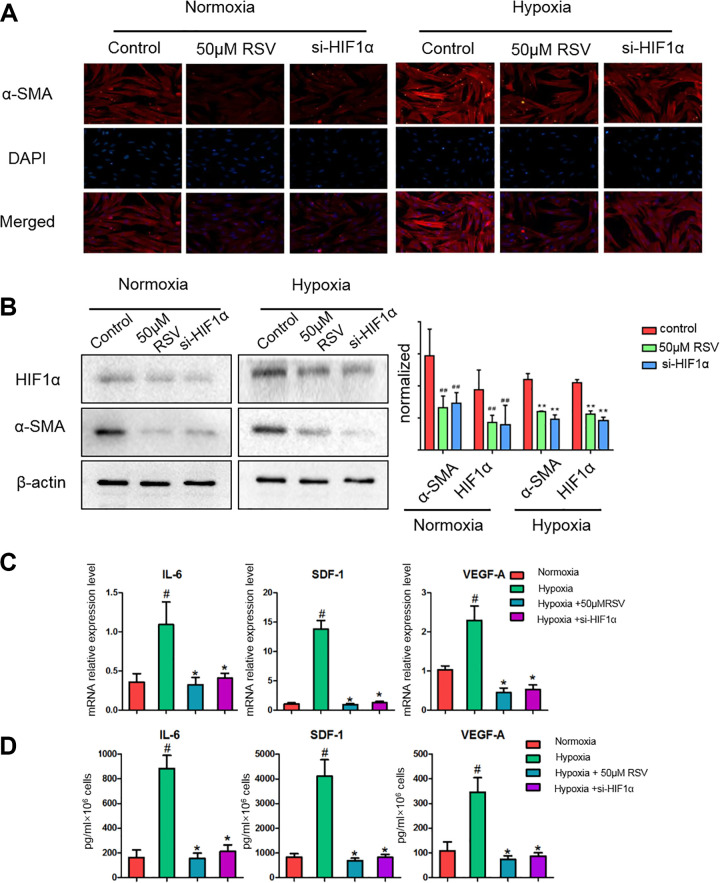
PSCs are important components of the TME. Consistent with the results obtained in previous studies7, compared with normoxia, hypoxia increased PSC activation, with an increase in the expression of α-SMA, and activated the expression of HIF-1α (Fig. 1A). However, the treatment with RSV (50 μM) and the downregulation of HIF-1α expression with siRNA decreased PSC activation as shown by the α-SMA expression (Fig. 1A, B). The underlying mechanism may be that RSV inhibited HIF-1α accumulation under exposure to hypoxia.
Fig. 1.
Resveratrol inhibits hypoxia-induced PSC activation and abolishes the secretion of IL-6, SDF-1, and VEGF-A in hypoxia-induced activated PSCs. (A) PSCs were treated for 24 h with 50 μM resveratrol or si-HIF-1α under normoxic or hypoxic conditions and serum starved for an additional 24 h. α-SMA expression in PSCs seeded on glass coverslips was analyzed by immunofluorescence microscopy. (B) Subconfluent PSCs were treated for 24 h with 50 μM resveratrol or si-HIF-1α under normoxic or hypoxic conditions and serum starved for another 24 h. The cells were lysed, and the expression levels of α-SMA, HIF-1α, and β-actin were detected by western blotting. PSCs were exposed to hypoxic conditions for 24 h in the presence or absence of 50 μM resveratrol or si-HIF-1α; the cells were then serum starved for an additional 24 h. (C) MRNA expression levels of IL-6, SDF-1, and VEGF-A were evaluated by quantitative real-time polymerase chain reaction as described in the Materials and Methods section. (D) The secretion of IL-6, SDF-1, and VEGF-A in PSC cultured medium was determined by enzyme-linked immunosorbent assay as described in the Materials and Methods section. #P < 0.05; ##P < 0.01 vs the untreated group under normoxic conditions. *P < 0.05; **P < 0.01 vs the untreated group under hypoxic conditions. All data represent at least three independent experiments. HIF-1: hypoxia-inducible factor 1; IL-6: interleukin 6; PSC: pancreatic stellate cell; RSV: resveratrol; SDF-1: stromal cell-derived factor 1; α-SMA: alpha-smooth muscle actin; VEGF-A: vascular endothelial growth factor A.
RSV Abolishes the Secretion of IL-6, SDF-1, and VEGF-A in Hypoxia-Activated PSCs
Previous studies have shown that the activated stroma produces large amounts of IL-6, SDF-1, and VEGF-A, significantly increasing the invasive capacity of the surrounding pancreatic cancer cells7. To determine whether hypoxia-activated PSCs overexpress these soluble growth factors and cytokines, we used qRT-PCR to detect IL-6, SDF-1, and VEGF-A expression, which showed that PSCs cultured under hypoxic conditions exhibited higher levels of IL-6, VEGF-A, and SDF-1 transcription and secretion (Fig. 1C). In addition, PSCs cultured under hypoxic conditions secreted greater levels of IL-6, VEGF-A, and SDF-1 in the supernatant (Fig. 1D). These factors are known to participate by modulating the response of tumor cells to activated PSCs. However, the treatment with RSV and si-HIF-1α eliminated the hypoxia-induced overexpression of these factors (Fig. 1C, D).
RSV Suppresses Pancreatic Cancer Cell Invasion and EMT Induced by Hypoxia
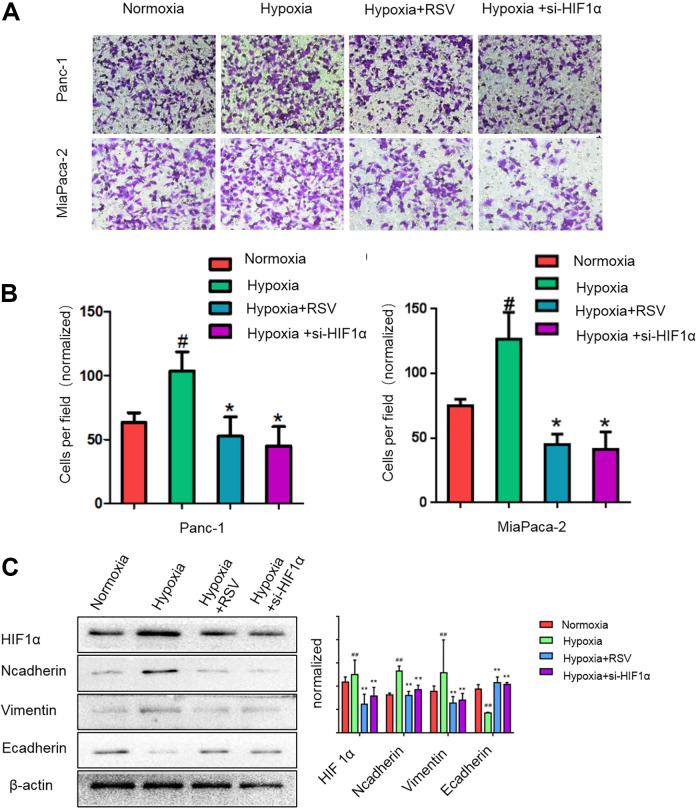
We treated pancreatic cancer cells (Panc-1 and Mia Paca-2) under hypoxic conditions with RSV or si-HIF-1α and assessed their capacity to undergo the EMT and invade through a reconstituted Matrigel barrier. Hypoxia slightly promoted the invasiveness of pancreatic cancer cells (Fig. 2A, B). In addition, hypoxia significantly reduced the E-cadherin level and increased the vimentin level in the pancreatic cancer cells (Fig. 2C), indicating that hypoxia can promote the EMT in pancreatic cancer cells. However, both RSV and si-HIF-1α reduced the hypoxia-enhanced EMT and invasion capacity of pancreatic cancer cells (Fig. 2A, C). The expression changes of EMT in Mia Paca-2 cells were consistent with Panc-1 cells (supplemental Fig. S1A). These findings indicate that pancreatic cancer cells are sensitive to hypoxic conditions, which heighten the capacity of these cells to promote pancreatic cancer invasiveness. Thus, RSV can suppress hypoxia-induced pancreatic cancer cell EMT and invasion.
Fig. 2.
Resveratrol suppresses the pancreatic cancer cell epithelial–mesenchymal transition and invasion induced by hypoxia. (A, B) Panc-1 or Mia Paca-2 cells were incubated under normoxic conditions for 24 h or under hypoxic condition for 24 h in the presence of 50 μM resveratrol or si-HIF-1α. The cells were then seeded into a Matrigel-coated invasion chamber under normal or hypoxic conditions for 24 h. The invaded cells were quantified by counting the number of cells in 10 random fields at 200× magnification. (C) Subconfluent pancreatic cancer cells (Panc-1) were exposed to normoxia for 24 h or hypoxia for 24 h in the presence of 50 μM resveratrol or si-HIF-1α. HIF-1α, N-cadherin, vimentin, and E-cadherin expression levels in Panc-1 cells were analyzed by western blotting. #P < 0.05; ##P < 0.01 comparing the hypoxia group with the control group. *P < 0.05; **P < 0.01 comparing the hypoxia+RSV or hypoxia+si-HIF-1α group with the hypoxia group. All data represent at least three independent experiments. HIF-1α: hypoxia-inducible factor 1; RSV: resveratrol.
RSV Suppresses Pancreatic Cancer Cell Invasion and EMT Induced by CM from PSCs
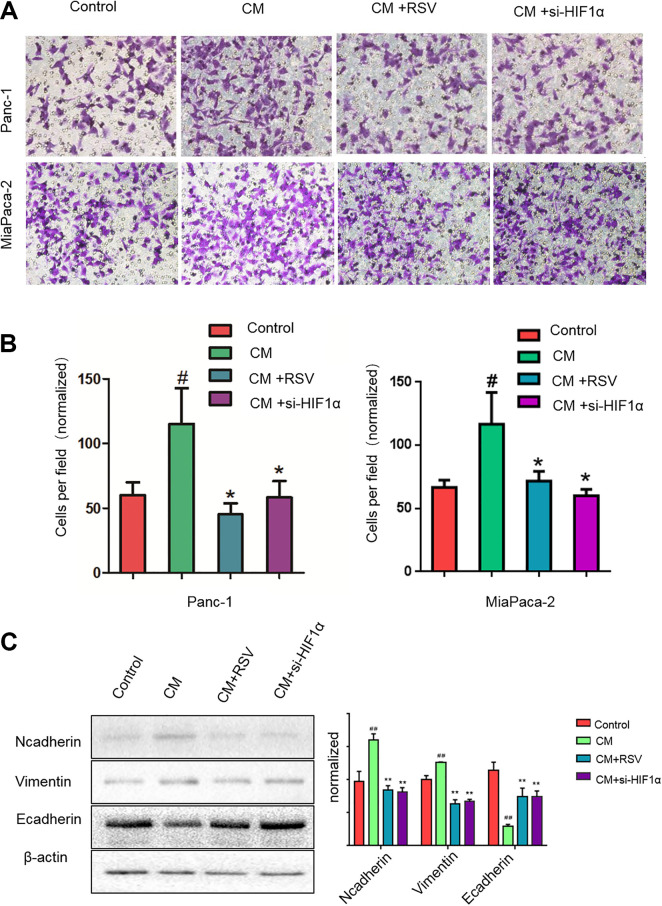
Compared with the PSCs cultured under normoxic conditions, the PSCs cultured under hypoxic conditions showed higher levels of IL-6, VEGF-A, and SDF-1 transcription and secretion; these factors are known to modulate the responses of tumor cells to activated PSCs. Thus, we analyzed whether media from PSCs cultured under hypoxic conditions could facilitate the metastatic potential of cancer cells. We treated pancreatic cancer cells (Panc-1 and Mia Paca-2) with conditioned media from PSCs activated by hypoxic conditions in the presence of RSV or si-HIF-1α and assessed their capacity to undergo the EMT and invade through a reconstituted Matrigel barrier. Exposing PSCs to hypoxic conditions during activation improved their capacity to impact pancreatic cancer cell motility and increased invasiveness (Fig. 3A, B). Additionally, the CM from the PSCs vitally increased the vimentin level and reduced the E-cadherin level in pancreatic cancer cells under hypoxia (Fig. 3C), indicating that CM from PSCs could promote the EMT in pancreatic cancer cells. Moreover, the key role of PSC activation was confirmed via the si-HIF-1α treatment to eliminate the effect of hypoxia and activated PSCs on invasiveness; RSV had an effect similar to that of si-HIF-1 and reduced the pancreatic cancer cell invasion and EMT induced by CM from activated PSCs. We detected the same expression level changes of EMT in Mia Paca-2 cells by western blot (supplemental Fig. S1B). These results indicate that PSCs are sensitive to hypoxia, which heightens the invasiveness of pancreatic cancer.
Fig. 3.
Resveratrol suppresses the pancreatic cancer cell invasion and epithelial–mesenchymal transition induced by CM from PSCs. CM were from PSCs activated by hypoxia. (A, B) Panc-1 or Mia Paca-2 cells were cultured under normoxic conditions with CM from PSCs in the presence or absence of 50 μM resveratrol or si-HIF-1α for 24 h. The cells were seeded into a Matrigel-coated invasion chamber under normoxic conditions for 24 h. The invaded cells were quantified by counting the number of cells at a 200× magnification in 10 random fields. (C) Subconfluent Panc-1 cells were treated under normoxic conditions with CM from PSCs treated with or without 50 μM resveratrol or si-HIF-1α for 24 h. Then, the cells were lysed, and the N-cadherin, vimentin, and E-cadherin expression levels in the Panc-1 cells were analyzed by western blotting. #P < 0.05; ##P < 0.01 comparing the CM group with the control group. *P < 0.05; **P < 0.01 comparing the CM+RSV or CM+si-HIF-1α group with the CM group. All data represent at least three independent experiments. CM: conditioned medium; HIF-1α: hypoxia-inducible factor 1; PSC: pancreatic stellate cell; RSV: resveratrol.
RSV Reverses the Inhibitory Effects on the Apoptosis of Pancreatic Cancer Cells Induced by CM from Hypoxia-Activated PSCs
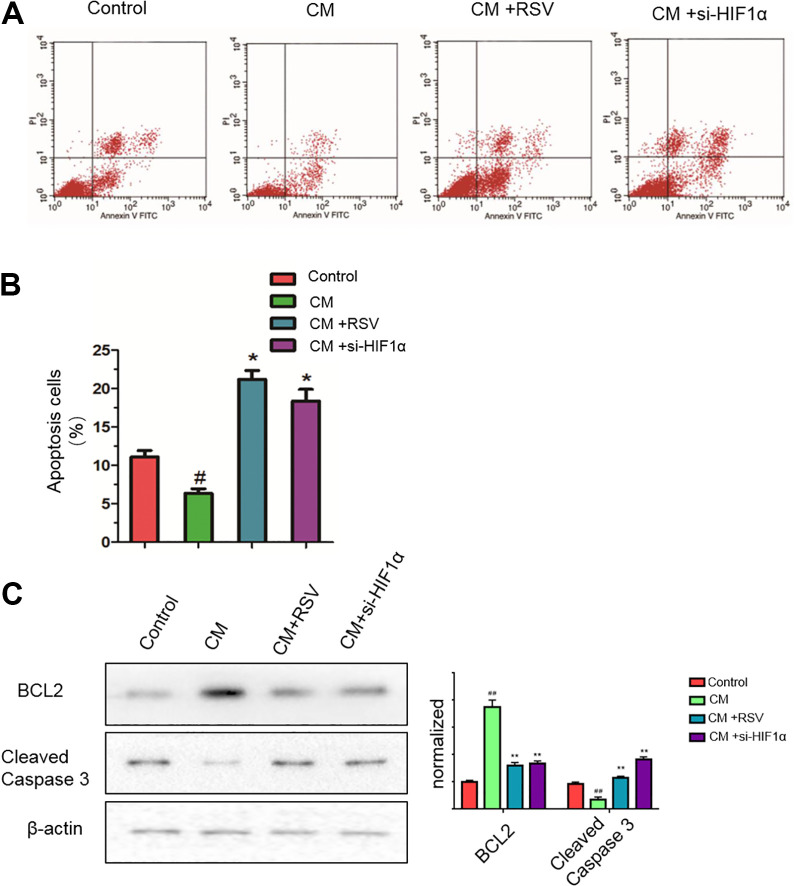
In addition, we tested the effects of RSV on pancreatic cancer cell apoptosis induced by CM from PSCs. As shown in Fig. 4A, B, CM from activated PSCs slightly reduced the number of apoptotic cells compared with that in the untreated controls. However, the treatment of PSCs with RSV or si-HIF-1 significantly increased the number of apoptotic CM-exposed Panc-1 cells. Consistent with these results, western blotting demonstrated that RSV and si-HIF-1 reduced the level of the antiapoptotic protein Bcl-2 and increased the levels of cleaved caspase-3, a marker of cell apoptosis (Fig. 4C). These results clearly demonstrate that RSV can reverse the inhibitory effects on apoptosis in pancreatic cancer cells induced by conditioned media from hypoxia-activated PSCs.
Fig. 4.
Resveratrol reverses the inhibitory effects on apoptosis in pancreatic cancer cells induced by CMs from hypoxia-activated PSCs. CM were from PSCs activated by hypoxia. (A, B) Panc-1 cells were cultured under normoxic conditions with CM from PSCs treated with or without 50 μM resveratrol or si-HIF-1α for 24 h. Next, Panc-1 cell apoptosis was detected by flow cytometry. (C) Subconfluent Panc-1 cells were treated under normoxic conditions with CM from PSCs treated with or without either 50 μM resveratrol or si-HIF-1α for 24 h. Next, the cells were lysed, and the protein levels of the apoptosis markers BCL2 and cleaved caspase-3 in Panc-1 cells were analyzed by western blotting. #P < 0.05; ##P < 0.01comparing the CM group with the control group. *P < 0.05; **P < 0.01comparing the CM+RSV or CM+si-HIF-1α group with the CM group. All data represent at least three independent experiments. CM: conditioned medium; HIF-1α: hypoxia-inducible factor 1; PSC: pancreatic stellate cell; RSV: resveratrol.
KPC Mice Recapitulate the Histopathological Features of PDAC Observed in Human Patients
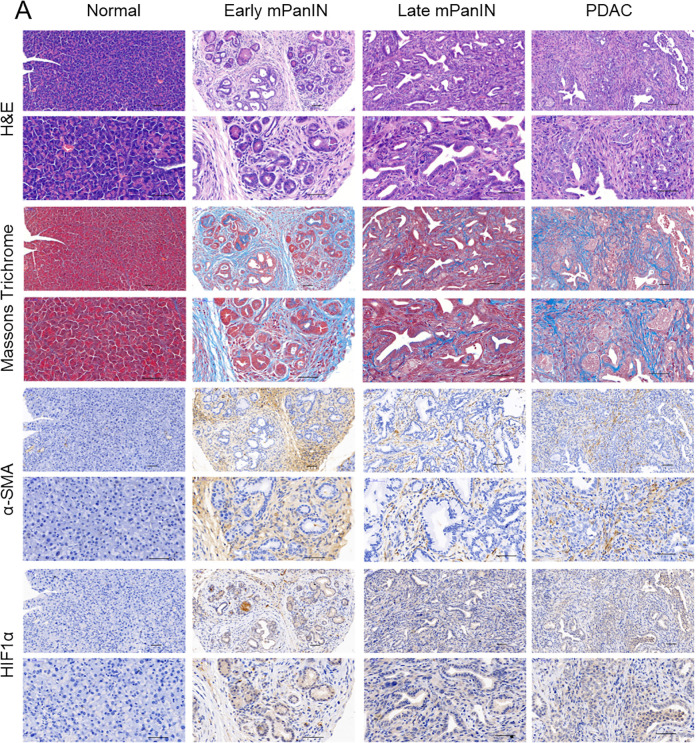
The generation of genetically engineered mouse models of pancreatic cancer that faithfully recapitulate the human disease can elucidate the importance of the TME in disease pathogenesis and the therapeutic response. KPC mice display the spontaneous development of defined histopathologic stages of pancreatic cancer progression that mimics human disease. To observe the dynamic process of stromal desmoplasia and the role of HIF-1α, we generated KPC mice that can recapitulate the dynamic alterations during the development of pancreatic cancer from pancreatic intraepithelial neoplasia (PanIN) to invasive PDAC27. First, we sacrificed KPC mice at the designated time points (6, 10, 14, and 18 wk) to explore tumor formation kinetics and conducted H&E staining to display the alterations in the histopathological structure during the progression from PanIN to invasive PDAC (Fig. 5). Next, Masson’s trichrome staining was performed to assess stromal desmoplasia during PDAC progression (Fig. 5).
Fig. 5.
The KPC mouse model recapitulates the pathological characteristics of human pancreatic cancer. Representative images of H&E staining, Masson’s trichrome staining, and immunohistochemical staining of α-SMA and HIF-1α from normal pancreatic, early mPanIN, late mPanIN, and invasive PDAC tissues. Scale bars = 50 μm. α-SMA: alpha-smooth muscle actin; H&E: hematoxylin and eosin; HIF-1: hypoxia-inducible factor 1; mPanIN: murine pancreatic intraepithelial neoplasia; PDAC: pancreatic ductal adenocarcinoma.
Collagen fiber staining was initiated during early murine PanIN (mPanIN) and increased when the lesions progressed to invasive PDAC (Fig. 5), indicating that the desmoplastic reaction was gradually exacerbated during the progression of PDAC. Along with Masson’s trichrome staining, the α-SMA and HIF-1α-stained specimens were examined and were found to increase from early mPanIN to invasive PDAC (Fig. 5). Collectively, these results faithfully demonstrated the similarity in pancreatic cancer initiation and progression between transgenic mice and human patients.
RSV Inhibits VEGF-A, SDF-1, and IL-6 Expression and Mitigates the Desmoplastic Reaction in KPC Mice
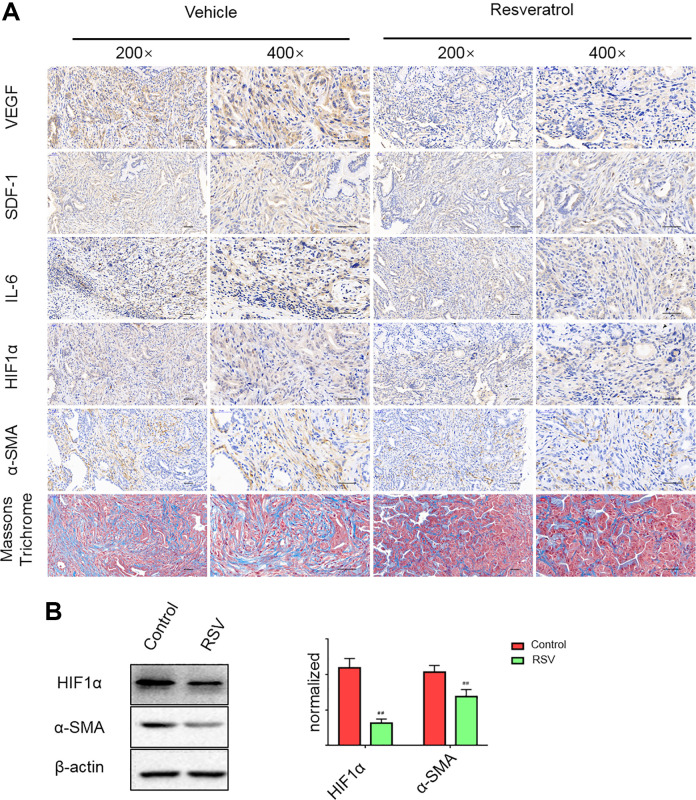
To further explore whether RSV can inhibit VEGF-A, SDF-1, and IL-6 expressions and mitigate the desmoplastic reaction in vivo, we treated 8-wk-old KPC mice daily with vehicle or RSV and sacrificed after 8 wk of treatment. Compared with the vehicle control, RSV effectively inhibited VEGF-A, IL-6, and SDF-1 expressions in vivo and markedly mitigated the desmoplastic reaction (Fig. 6A). The development of stromal desmoplasia during the progression of PDAC is due to PSC activation and the extensive secretion of ECM proteins, which can further exacerbate hypoxia in pancreatic cancer. To further investigate whether the mitigation of stromal desmoplasia by RSV occurred via the repression of PSC activation and the inhibition of HIF-1α expression, we performed immunohistochemical staining and found that RSV greatly decreased α-SMA and HIF-1α expression in KPC mice (Fig. 6A), which is consistent with the results of western blot (Fig. 6B). Collectively, these results showed that RSV can significantly suppress VEGF-A, SDF-1, IL-6, α-SMA, and HIF-1α expression and the desmoplastic reaction in vivo.
Fig. 6.
Resveratrol inhibits VEGF-A, SDF-1, and IL-6 expression and mitigates the desmoplastic reaction in KPC mice. (A) Representative images of Masson’s trichrome staining and immunohistochemical staining for VEGF-A, SDF-1, IL-6, α-SMA, and HIF-1α in the vehicle control and resveratrol intervention groups. Scale bars = 50 μm. (B) Resveratrol inhibited α-SMA and HIF-1 expression in KPC mice with pancreatic cancer was detected by western blot. #P < 0.05; ##P < 0.01 vs the untreated group. HIF-1: hypoxia-inducible factor 1; IL-6: interleukin 6; SDF-1: stromal cell-derived factor 1; α-SMA: alpha-smooth muscle actin; VEGF-A: vascular endothelial growth factor A.
Discussion
The data presented here are consistent with the critical role of certain components of the TME, i.e., hypoxia and PSCs, in the development of pancreatic cancer toward aggressive phenotypes. The results are summarized as follows: (i) stromal reactivity depends on hypoxia; (ii) hypoxia and activated PSCs synergistically promote pancreatic cancer cell invasiveness and EMT and inhibit apoptosis via the activation of HIF-1α; and (iii) RSV can inhibit hypoxia-induced PSC activation, block the interplay between PSCs and pancreatic cancer cells, and suppress VEGF-A, SDF-1, IL-6, α-SMA, and HIF-1α expression and the desmoplastic reaction in vivo.
Cancer-associated fibroblast-like cells (CAFs) in the tumor stroma have been demonstrated to profoundly influence the initiation and progression of human cancer28–30. PDAC is a particularly highly lethal and aggressive disease often characterized by stromal fibrosis or the desmoplastic reaction due to the production of ECM by activated PSCs31. A symbiosis between activated PSCs and pancreatic cancer cells is being increasingly investigated: exposure to pancreatic cancer cell secretions including mitogenic and fibrogenic factors facilitates PSC activation7,32. In turn, activated PSCs produce platelet-derived growth factor, SDF, IL-6, insulin-like growth factor 1, connective tissue growth factor, matrix metalloproteinases, type I collagen, and other factors that mediate effects on cancer progression and chemotherapeutic resistance7,32,33. Therefore, the different stromal frameworks affected by this pathway have significant impacts on the behavior and terminal differentiation of PSCs. These findings are consistent with our observation that Panc-1 cells and Mia Paca-2 cells contacting activated PSCs in the stroma show an obvious increase in the EMT and invasiveness and a decrease in apoptosis. Similarly, in several cancer models, including melanoma, mammary carcinoma, and prostate carcinoma, CAFs have been reported to promote motility and aggressiveness34–36. Additionally, activated stromal prostate fibroblasts induce the EMT and stemness in carcinoma cells, further highlighting the effect of these fibroblasts on metastatic tumor formation37,38.
Solid tumors often endure hypoxic tension, which is mainly caused by abnormal vasculature formation in the rapidly growing tumor mass39. Our data show that hypoxia is the synergistic factor promoting PSC activation as well as eliciting the secretion of key cytokines such as IL-6, VEGF-A, and SDF-1, which exert proangiogenic and proinflammatory effects. Tumor hypoxia is currently considered a pivotal determinant of tumor progression in several cancer models because it is related to de novo angiogenesis, profound alterations in tumor metabolism, and the development of motile behavior40,41. All these events synergistically promote the metastatic spread of invasive cells. Our previous results showed that PSCs are sensitive to mild hypoxia (3% O2)42. In fact, exposure to mild hypoxia causes the dramatic activation of PSCs in a manner dependent on HIF-1α accumulation. Previous studies investigating melanoma have shown that melanoma cells strictly rely on oxidative stress under mild hypoxic conditions and that reactive oxygen species (ROS) scavenging can abolish the effects of hypoxia; the ROS-mediated repression of prolyl hydroxylases and the oxygen-dependent degradation of HIF-1 have been verified in the redox-dependent stabilization of HIF-143. This finding is consistent with those in our previous study showing that under hypoxic conditions, the ROS-dependent activation of HIF-1 induces PSC activation and pancreatic cancer invasion. Gao et al. discovered that the antitumorigenic effect of antioxidants such as N-acetyl cysteine and vitamin C in murine models of Myc-mediated tumorigenesis are indeed dependent on HIF-144, consistent with the core role exerted by ROS in sensing hypoxia. Therefore, we speculated that in our study, hypoxia may induce PSC activation through the ROS-mediated mobilization of HIF-1, thereby leading to the promotion of pancreatic cancer invasion and EMT and the inhibition of pancreatic cancer cell apoptosis by paracrine IL-6, SDF-1, and VEGF-A; conversely, RSV can suppress this process.
SDF-1 and VEGF-A, which are associated with the chemoattraction between cancer cells and endothelial cells and angiogenesis, and IL-6, which participates in proinflammatory responses, have been found to be transcriptionally controlled by HIF-143. A previous study34 investigating melanoma showed that activated CAF secretes these cytokines following simultaneous exposure to an anoxic environment. These data suggest that activated PSCs exposed to hypoxic conditions play an active role in attracting pancreatic cancer cells to different locations. Active modulators of this chemoattraction include SDF-1, VEGF-A, and IL-6; this observation validates their pleiotropic effects in the progression of pancreatic cancer. Hence, the surrounding stroma, with intratumoral hypoxic areas, might play a role in attracting metastatic pancreatic cancer cells from the original lesions, thus facilitating metastasis.
In recent decades, numerous researchers have reported that RSV is a chemopreventive agent, mediating growth inhibition, apoptosis, and cell-cycle arrest and altering biomarker expression in various human cancer cell lines45,46. Previous studies have shown that RSV exerts antifibrotic effects in pancreatic cancer because it effectively suppresses PSC-enhanced gene expression via the downregulation of the NF-κB and AKT pathways47,48. However, previous studies have not reported the effect of RSV on PSC in hypoxia. Our data show that RSV, a polyphenolic compound, could inhibit α-SMA under normoxia and that the degree was similar to that in hypoxia at first. In addition, we found that the expression of HIF-1α was increased in hypoxic environments from previous studies7,49,50, which caused the elevated expression of SDF-1, VEGF-A, and IL-6. The microenvironment of pancreatic cancer is a hypoxic environment. On the one hand, a normoxia environment is not similar to the pancreatic cancer microenvironment. On the other hand, α-SMA is not expressed highly under normoxia. Although RSV could inhibit α-SMA under normoxia and the degree was similar to that in hypoxia, it makes more sense that α-SMA expression increases after hypoxia-induced PSC activation. The reduction in α-SMA represents a reduction in PSC activation. Moreover, our results indicate that RSV abrogates hypoxia-driven PSC activation and inhibit pancreatic cancer cell EMT and invasion, and reverses the resistance to apoptosis induced by CM from PSCs. Additionally, RSV suppresses the activation of hypoxia-enhanced HIF-1α and abolishes hypoxia-induced SDF-1, VEGF-A, and IL-6 secretion in PSCs. Moreover, RSV significantly suppresses VEGF-A, SDF-1, IL-6, α-SMA, and HIF-1α expression and the desmoplastic reaction in a KPC mouse model. These data indicate that RSV effectively suppresses the interaction between pancreatic cancer cells and PSCs under hypoxic conditions, thus ameliorating stromal fibrosis in pancreatic cancer in vivo. Hence, the results of our study further emphasize the influence of the microenvironment on pancreatic cancer progression by highlighting that activated PSCs and intratumoral hypoxia are critical factors that have potential novel strategies to block tumor–microenvironment interactions. In addition to hypoxia, mediators in the TME, such as VEGF-A, SDF-1, and IL-6, which participate in cancer-related inflammation and angiogenesis, provide targets for innovative diagnostic and therapeutic strategies for metastatic pancreatic cancer; furthermore, the polyphenolic compound RSV can effectively ameliorate the malignant progression of PDAC.
Conclusions
In summary, these data are consistent with the essential roles of some TME components. First, stromal reactivity in pancreatic cancer depends on hypoxia. Second, hypoxia and activated PSCs synergistically promote pancreatic cancer cell invasiveness and EMT and inhibit apoptosis via the activation of HIF-1α. Third, RSV can inhibit hypoxia-induced PSC activation; block the interplay between PSCs and pancreatic cancer cells; suppress VEGF-A, SDF-1, IL-6, α-SMA, and HIF-1α expression; and mitigate the desmoplastic reaction in KPC mice. Collectively, these results suggest that the polyphenolic compound RSV can effectively ameliorate the malignant progression of PDAC.
Supplemental Material
Supplementary_Figure_1 for Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation by Ying Xiao, Tao Qin, Liankang Sun, Weikun Qian, Jie Li, Wanxing Duan, Jianjun Lei, Zheng Wang, Jiguang Ma, Xuqi Li, Qingyong Ma and Qinhong Xu in Cell Transplantation
Supplemental Material, Table_S1 for Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation by Ying Xiao, Tao Qin, Liankang Sun, Weikun Qian, Jie Li, Wanxing Duan, Jianjun Lei, Zheng Wang, Jiguang Ma, Xuqi Li, Qingyong Ma and Qinhong Xu in Cell Transplantation
Supplemental Material, Table_S2 for Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation by Ying Xiao, Tao Qin, Liankang Sun, Weikun Qian, Jie Li, Wanxing Duan, Jianjun Lei, Zheng Wang, Jiguang Ma, Xuqi Li, Qingyong Ma and Qinhong Xu in Cell Transplantation
Footnotes
Ethical Approval: This study was approved by the Ethics Committee of the First Affiliated Hospital of Medical College, Xi’an Jiaotong University, China.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the protocols approved by the Ethics Committee of the First Affiliated Hospital of Medical College, Xi’an Jiaotong University.
Statement of Informed Consent: There were no human subjects included in this work, so informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (No. 81672434, 81702916), Scientific and Technological Development Research Project Foundation of Shaanxi Province(No. 2019SF-157), the Fundamental Research Funds for the Central Universities in Xi’an Jiaotong University(No. xzy012019095) and the First Affiliated Hospital of Xi’an Jiaotong University(No.2017MS-06).
ORCID iDs: Tao Qin  https://orcid.org/0000-0002-7674-698X
https://orcid.org/0000-0002-7674-698X
Qin-hong Xu  https://orcid.org/0000-0003-0654-5290
https://orcid.org/0000-0003-0654-5290
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. [DOI] [PubMed] [Google Scholar]
- 3. Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144(6):1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li X, Han L, Li W, Sun H, Wu E, et al. Alpha-Mangostin inhibits hypoxia-driven ROS-induced PSC activation and pancreatic cancer cell invasion. Cancer Lett. 2014;347(1):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Endo S, Nakata K, Ohuchida K, Takesue S, Nakayama H, Abe T, Koikawa K, Okumura T, Sada M, Horioka K, Zheng B, et al. Autophagy is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;152(6):1492–1506. [DOI] [PubMed] [Google Scholar]
- 9. Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, et al. Erratum: pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;540(7631):150. [DOI] [PubMed] [Google Scholar]
- 10. Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50(11):3337–3340. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari Z, Zhang C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: in vivo demonstration with magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2010;299(4):H985–H994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu Q, Duan W, Yu S, Wang F, Wu E. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kappaB pathway. Curr Med Chem. 2013;20(33):4185–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li W, Cao L, Chen X, Lei J, Ma Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol Rep. 2016;35(3):1718–1726. [DOI] [PubMed] [Google Scholar]
- 14. Jiang Z, Chen X, Chen K, Sun L, Gao L, Zhou C, Lei M, Duan W, Wang Z, Ma Q, Ma J. YAP inhibition by resveratrol via activation of AMPK enhances the sensitivity of pancreatic cancer cells to gemcitabine. Nutrients. 2016;8(10):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gemcitabine plus bevacizumab no better than gemcitabine alone in clinical study of 600 patients with pancreatic cancer. Cancer Biol Ther. 2007;6(2):137. [PubMed] [Google Scholar]
- 16. Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115(2):421–432. [DOI] [PubMed] [Google Scholar]
- 17. Duan W, Chen K, Jiang Z, Chen X, Sun L, Li J, Lei J, Xu Q, Ma J, Li X, Han L, et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett. 2017;385:225–233. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi K, Ehata S, Koinuma D, Morishita Y, Soda M, Mano H, Miyazono K. Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene. 2018;37(21):2757–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma J, Duan W, Han S, Lei J, Xu Q, Chen X, Jiang Z, Nan L, Li J, Chen K, Han L, et al. Ginkgolic acid suppresses the development of pancreatic cancer by inhibiting pathways driving lipogenesis. Oncotarget. 2015;6(25):20993–21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma Q, Zhang M, Wang Z, Ma Z, Sha H. The beneficial effect of resveratrol on severe acute pancreatitis. Ann N Y Acad Sci. 2011;1215:96–102. [DOI] [PubMed] [Google Scholar]
- 22. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 23. Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, Zhou C, Gao L, Lei M, Yan B, Cao J, et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer. 2017;16(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian W, Li J, Chen K, Jiang Z, Cheng L, Zhou C, Yan B, Cao J, Ma Q, Duan W. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci. 2018;208:253–261. [DOI] [PubMed] [Google Scholar]
- 25. Chen K, Qian W, Li J, Jiang Z, Cheng L, Yan B, Cao J, Sun L, Zhou C, Lei M, Duan W, et al. Loss of AMPK activation promotes the invasion and metastasis of pancreatic cancer through an HSF1-dependent pathway. Mol Oncol. 2017;11(10):1475–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B . bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55(2):237–241. [PubMed] [Google Scholar]
- 27. Lee JW, Komar CA, Bengsch F, Graham K, Beatty GL. Genetically engineered mouse models of pancreatic cancer: the KPC model (LSL-Kras(G12D/+) ;LSL-Trp53(R172H/+) ;Pdx-1-Cre), its variants, and their application in immuno-oncology drug discovery. Curr Protoc Pharmacol. 2016;73:14–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316(17):2713–2722. [DOI] [PubMed] [Google Scholar]
- 29. Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. EXP Cell Res. 2010;316(8):1324–1331. [DOI] [PubMed] [Google Scholar]
- 30. Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duner S, Lopatko LJ, Ansari D, Gundewar C, Andersson R. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. 2010;10(6):673–681. [DOI] [PubMed] [Google Scholar]
- 32. Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):69–74. [DOI] [PubMed] [Google Scholar]
- 33. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Comito G, Giannoni E, Di Gennaro P, Segura CP, Gerlini G, Chiarugi P. Stromal fibroblasts synergize with hypoxic oxidative stress to enhance melanoma aggressiveness. Cancer Lett. 2012;324(1):31–41. [DOI] [PubMed] [Google Scholar]
- 35. Taddei ML, Giannoni E, Raugei G, Scacco S, Sardanelli AM, Papa S, Chiarugi P. Mitochondrial oxidative stress due to complex i dysfunction promotes fibroblast activation and melanoma cell invasiveness. J Signal Transduct. 2012;2012:684592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31(1-2):195–208. [DOI] [PubMed] [Google Scholar]
- 37. Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–6956. [DOI] [PubMed] [Google Scholar]
- 38. Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14(12):2361–2371. [DOI] [PubMed] [Google Scholar]
- 39. Casillas AL, Toth RK, Sainz AG, Singh N, Desai AA, Kraft AS, Warfel NA. Hypoxia-inducible PIM kinase expression promotes resistance to antiangiogenic agents. Clin Cancer Res. 2018;24(1):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15(4):660–666. [DOI] [PubMed] [Google Scholar]
- 41. Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 2007;26(2):341–352. [DOI] [PubMed] [Google Scholar]
- 42. Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan W, Sun Q, Xu J, Wu Z, Wu E. Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner. Mol Cancer. 2013;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samanta D, Prabhakar NR, Semenza GL. Systems biology of oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2017;9(4):1382–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12(3):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buhrmann C, Shayan P, Popper B, Goel A, Shakibaei M. Sirt1 is required for resveratrol-mediated chemopreventive effects in colorectal cancer cells. Nutrients. 2016;8(3):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu FW, Tsai LL, Yu CH, Chen PN, Chou MY, Yu CC. Impairment of tumor-initiating stem-like property and reversal of epithelial-mesenchymal transdifferentiation in head and neck cancer by resveratrol treatment. Mol Nutr Food Res. 2012;56(8):1247–1258. [DOI] [PubMed] [Google Scholar]
- 47. Lin Z, Zheng LC, Zhang HJ, Tsang SW, Bian ZX. Anti-fibrotic effects of phenolic compounds on pancreatic stellate cells. BMC Complement Altern Med. 2015;15:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsang SW, Zhang H, Lin Z, Mu H, Bian ZX. Anti-fibrotic effect of trans-resveratrol on pancreatic stellate cells. Biomed Pharmacother. 2015;71:91–97. [DOI] [PubMed] [Google Scholar]
- 49. Chen YT, Chen FW, Chang TH, Wang TW, Hsu TP, Chi JY, Hsiao YW, Li CF, Wang JM. Hepatoma-derived growth factor supports the antiapoptosis and profibrosis of pancreatic stellate cells. Cancer Lett. 2019;457:180–190. [DOI] [PubMed] [Google Scholar]
- 50. Yan B, Jiang Z, Cheng L, Chen K, Zhou C, Sun L, Qian W, Li J, Cao J, Xu Q, Ma Q, et al. Paracrine HGF/c-MET enhances the stem cell-like potential and glycolysis of pancreatic cancer cells via activation of YAP/HIF-1alpha. Exp Cell Res. 2018;371(1):63–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figure_1 for Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation by Ying Xiao, Tao Qin, Liankang Sun, Weikun Qian, Jie Li, Wanxing Duan, Jianjun Lei, Zheng Wang, Jiguang Ma, Xuqi Li, Qingyong Ma and Qinhong Xu in Cell Transplantation
Supplemental Material, Table_S1 for Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation by Ying Xiao, Tao Qin, Liankang Sun, Weikun Qian, Jie Li, Wanxing Duan, Jianjun Lei, Zheng Wang, Jiguang Ma, Xuqi Li, Qingyong Ma and Qinhong Xu in Cell Transplantation
Supplemental Material, Table_S2 for Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation by Ying Xiao, Tao Qin, Liankang Sun, Weikun Qian, Jie Li, Wanxing Duan, Jianjun Lei, Zheng Wang, Jiguang Ma, Xuqi Li, Qingyong Ma and Qinhong Xu in Cell Transplantation