Abstract
The nonobese diabetic (NOD) mouse model of type 1 diabetes (T1D) was discovered by coincidence in the 1980s and has since been widely used in the investigation of T1D and diabetic complications. The current in vivo study was originally designed to prospectively assess whether hyperglycemia onset is associated with physical destruction or functional impairment of beta cells under inflammatory insult during T1D progression in diabetes-prone female NOD mice. Prediabetic 16- to 20-wk-old NOD mice were transplanted with green fluorescent protein (GFP)-expressing reporter islets in the anterior chamber of the eye (ACE) that were monitored longitudinally, in addition to glycemia, with and without immune modulation using anti-CD3 monoclonal antibody therapy. However, there was an early and vigorous immune reaction against the GFP-expressing beta cells that lead to their premature destruction independent of autoimmune T1D development in progressor mice that eventually became hyperglycemic. This immune reaction also occurred in nonprogressor NOD recipients. These findings showed a previously unknown reaction of NOD mice to GFP that prevented achieving the original goals of this study but highlighted a new feature of the NOD mice that should be considered when designing experiments using this model in T1D research.
Keywords: autologous transplantation, cell survival, graft survival, islet transplant, diabetes
Introduction
The nonobese diabetic (NOD) mice originated in the 1980s when researchers in Japan were trying to develop a strain of mice prone to cataract, but instead the mice spontaneously developed diabetes at a young age similar to juvenile diabetes in humans, more commonly referred to as type 1 diabetes (T1D)1. It is now established that, like in human T1D, diabetes in the NOD mice results from the autoimmune destruction of the insulin-producing beta cells in the islets of Langerhans (i.e., endocrine pancreas), which leads to insulin insufficiency and increased blood sugar levels (hyperglycemia). Therefore, insulin replacement therapy is required in diabetic NOD mice and T1D patients alike to avoid serious and potentially life-threatening complications. Chronic hyperglycemia can lead to micro- and macro-vascular pathologies that cause serious health problems such as cardiovascular disease, neuropathy (nerve damage), nephropathy (kidney damage), retinopathy (retina damage), and other eye complications. Consequently, diabetic complications in patients with uncontrolled diabetes can lead to serious morbidities such as blindness, poor blood flow to extremities leading to amputations, bacterial and fungal infections of the skin, and even pregnancy problems for the mother and fetus2–11. Many of these diabetic complications are also exhibited by NOD mice. Thus, the NOD mice provide a good model for human T1D and its complications12–17.
NOD mice have been extensively used in research to investigate the immunopathology of T1D and associated diabetic complications, and to develop treatments against them16–21. Several immune mechanisms of the anti-islet autoimmunity have been elaborated in studies using NOD mice. Such studies have provided new opportunities for therapeutic intervention to prevent or reverse T1D22,23. Some of these studies have even led to clinical trials, such as those with the anti-CD3 blocking antibody originally developed in the NOD mice24–26. While various therapeutic approaches to prevent or reverse T1D have been successful in the NOD mice, to date, however, there are no approved immunotherapies to prevent or treat T1D in humans27,28; this is likely due to inherent differences between rodents and man that contribute to the discrepancy between the preclinical and clinical outcomes29–36. Consistently, although the advantages of using the NOD mouse model for diabetes research far outweigh the disadvantages, the fundamental differences between the species should not be overlooked when interpreting experimental findings for potential translation to human application. For example, NOD mice have different diabetes incidence rates between males and females (20% to 30% and 60% to 80%, respectively) and they exhibit more evident peri-insulitis and immune cell infiltration within pancreatic islets (insulitis) than found in human islets of patients with T1D18,37. Also, even though there are similarities, not all the beta cell antigens (autoantigens) that are recognized by the human immune system are identical to those in the NOD mouse38. Moreover, while both NOD mice and human T1D patients can have disturbances in salivary gland function and both can develop xerostomia (dry mouth), enamel hypomineralization, and dental cavities39, the associated alterations in the animal behavior, such as food intake because of oral discomfort, could influence significantly the interpretation of experimental findings if not carefully accounted for in experimental studies. Hence, these and other experimental variables can potentially contribute to the discrepancy between the preclinical and clinical outcomes.
While the NOD mouse model has been and will likely continue to be the workhorses in T1D research for mechanistic studies and treatment discovery efforts, the various inherent species differences between the NOD mice and humans with T1D should be carefully considered during interpretation of preclinical findings, and their casual extrapolation to the clinical setting should be avoided without proper validation. The current study reports on a novel observation that should also be considered when designing experiments with NOD mice. We present evidence of immune attack against syngeneic NOD islets expressing the green fluorescent protein (GFP) when transplanted in late prediabetic female NOD mice. This early and vigorous immune attack occurred independent from the autoimmunity against beta cells that eventually lead later to overt diabetes in the NOD recipients. Notably, there have been prior reports on the potential antigenicity of genetic markers40,41; however, no such evidence has existed in the context of pancreatic islet cells or the NOD mice.
Materials and Methods
Animals
All studies were performed under approved protocols by the University of Miami’s Institutional Animal Care and Use Committee. Female NOD mice (NOD/ShiLtJ; stock # 001976) were purchased from Jackson Lab (Bar Harbor, ME, USA). Transgenic NOD mice expressing GFP under the control of mouse insulin I promoter (NODMIPGFP) were a generous donation from M. Hara42. Mice were housed during the studies in micro-isolated cages with free access to autoclaved/irradiated food and water under the supervision of the University of Miami’s Department of Veterinary Resources.
Islet Isolation and Transplantation into the Anterior Chamber of the Eye
Pancreatic islets were obtained by enzymatic digestion of donor pancreata from NODMIPGFP mice42 followed by purification on density gradients using protocols standardized at the Diabetes Research Institute Pre-Clinical Cell Processing and Translational Models Core, as previously described in detail43. After overnight culture, the isolated islets were implanted in the anterior chamber of the eye (ACE; 20 to 40 islet equivalents (IEQs) in one eye) of fully anesthetized NOD recipient mice, as previously described in detail44.
Monitoring Survival of Islets Transplanted in the ACE
Survival of the ACE-transplanted NODMIPGFP islets was assessed by quantitative volumetric measurements of the individual islets based on their backscatter (reflection) of a 633-nm laser and the GFP fluorescence within each islet as a measure of the beta cell mass, as previously described in detail19,21,45. In brief, islets (engrafted on top of the iris) were mapped in digital images of the eye acquired during the first week after transplantation and the same islets were revisited during the longitudinal imaging sessions during the progression of the autoimmune T1D. The islet survival was documented by direct visualization and assessment of their structural integrity in high-resolution digital images and by confocal micrographs acquired in the backscatter/reflection mode. Three-dimensional (3D) confocal micrographs of the individual ACE-transplanted islets in each mouse were acquired using 10× or 20× water immersion objectives in z-stacks spanning the full height of each islet. The quantitative analysis of the individual islet volumes was performed in 3D using Volocity software (PerkinElmer, Waltham, MA, USA), as previously described in detail45.
Diabetes Monitoring
Monitoring of diabetes development in the female NOD mice based on glucose in urine (glucosuria) or blood (glycemia) was started at 7 to 8 wk of age prior to the islet transplantation and was continued thereafter throughout the experiments until euthanasia/necropsy. Glucosuria measurements were initially performed two to three times a week by urine strips (Diastix) and positive glycosuria was confirmed by repeated glycemia measurements from the tail vein using portable OneTouchUltra2 glucometers (Lifescan, MILPITAS, CA, USA)46. As routinely done, overt diabetes (hyperglycemia) onset was defined as nonfasting blood sugar values ≥250 mg/dl (13.88 mmol/l) in three consecutive readings. Diabetes monitoring in established diabetic mice was continued by glycemia measurements.
Treatment
Purified low-endotoxin grade anti-mouse CD3∊ monoclonal antibody (clone 145-2C11) was obtained from Leinco Technologies, Inc. (St Louis, MO, USA) and administered to NOD mice at 50 µg/day intraperitoneally for five consecutive days22,25,26. Treatment was initiated in each mouse as degranulation of the corresponding ACE-transplanted NODMIPGFP islets became evident, as we recently described in detail19. The treatment initiation time ranged from 8 and 22 d after the islet transplantation in the ACE (i.e., at postoperative day [POD]8 to POD22).
Immunostaining
Immunostaining was performed on sections of eyes with ACE-transplanted islets. The eyes were procured from the euthanized recipient NOD mice long after the onset of diabetes/hyperglycemia. Eyes bearing ACE-islet grafts were fixed in 4% paraformaldehyde and cryosectioned at 14-µm-thick sections after freezing in Tissue-Tek Optimal Cutting Temperature compound (VWR, Radnor, PA, USA). The following primary antibodies were used at the indicated dilutions: guinea pig anti-insulin (1:1,000; Dako, Carpinteria, CA, USA), mouse anti-glucagon (1:500; Sigma-Aldrich, St Louis, MO, USA), and rabbit anti-somatostatin (1:500; Novus Biologicals, Littleton, CO, USA). Secondary antibodies (Invitrogen, Carlsbad, CA, USA) against the corresponding host species of each primary antibody and conjugated to Alexa488, Alexa568, or Alexa647 were used at 1:200 dilution.
Data Analysis
Data were analyzed in GraphPad Prism version 8.3.1 (GraphPad Software, La Jolla, CA, USA) and were plotted as means ± SEM unless stated otherwise. Comparisons of median times in the Kaplan–Meier curves of islet destruction or diabetes-free survival were performed using the log-rank (Mantel–Cox) test; P values <0.05 were considered significant.
Results
GFP-expressing Beta Cells in NODMIPGFP Islets Were Attacked When Transplanted in Syngeneic Prediabetic NOD Mice Independent of Autoimmune T1D Development
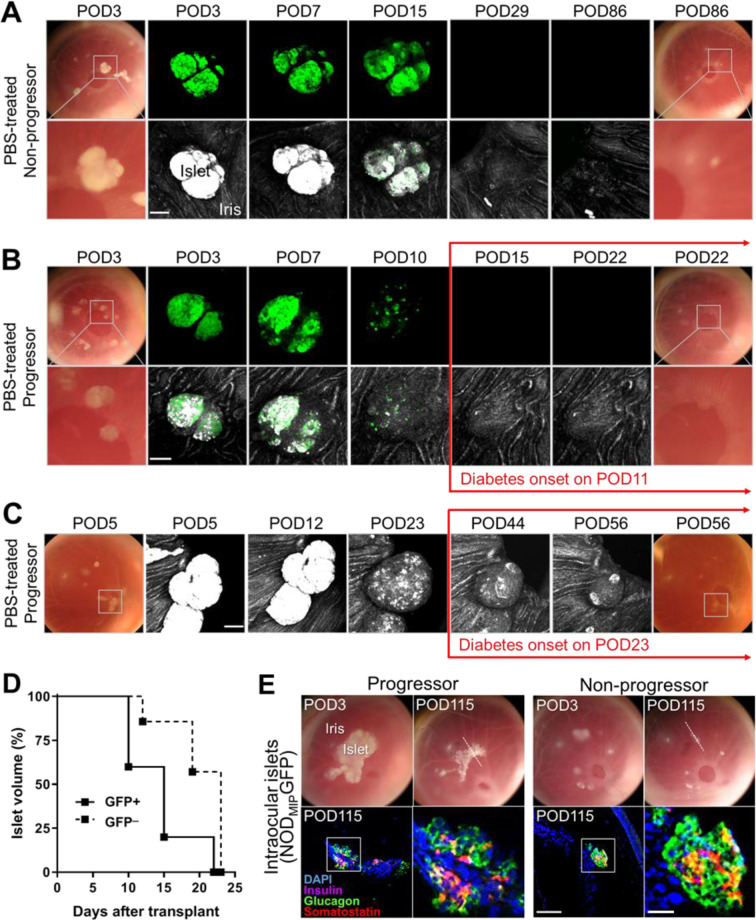
Islets obtained from NODMIPGFP donor mice and transplanted into the ACE of phosphate-buffered saline (PBS)-treated 16- to 20-wk-old late prediabetic female NOD mice were equally attacked and destroyed regardless of whether the recipient mice spontaneously progressed to diabetes/hyperglycemia (i.e., progressors) or remained diabetes-free (i.e., nonprogressors) up to >31 wk of age (Fig. 1A, B). Longitudinal intravital monitoring of the individual ACE-transplanted NODMIPGFP islets revealed quick and consistent destruction of their GFP-expressing beta cells in both progressor and nonprogressor recipients. The destruction kinetics of the GFP-expressing NODMIPGFP islets were similar in the progressors and nonprogressors. In sharp contrast with the GFP-expressing islets (NODMIPGFP), wild-type GFP-negative NOD islets transplanted in the ACE of progressor NOD counterparts (12 to 14 wk old at transplant) were also attacked but with significantly slower kinetics compared to the GFP-expressing beta cells in NODMIPGFP islets in the late prediabetic NOD recipients (Fig. 1C, D). Notably, the destruction of the GFP-negative islets proceeded in conjunction with the progression of autoimmune T1D and it peaked with the onset of diabetes/hyperglycemia (Fig. 1D), as we previously showed19,21. Whereas, the immune attack against the NODMIPGFP islets occurred significantly earlier and it preceded the onset of hyperglycemia in the progressor recipients (Fig. 1B). The immune attack also had comparable kinetics in the nonprogressor recipients where the NODMIPGFP islets were equally destroyed (Fig. 1A). The median survival times in progressor recipients of GFP-positive and GFP-negative islets were, respectively, 15 and 23 d after transplantation (P < 0.05 by log-rank [Mantel–Cox] test). Further immunostaining for the islet hormones insulin, glucagon, and somatostatin in ACE-transplanted NODMIPGFP islets obtained from progressor and nonprogressor recipients after necropsy on POD115 confirmed the absence of insulin-positive cells in remnants of islets that still contained glucagon- and somatostatin-expressing alpha and delta cells, respectively (Fig. 1E).
Figure 1.
NODMIPGFP islets are attacked in both progressor and nonprogressor NOD mice independently of autoimmune T1D. (A, B) Representative images (digital photos) and confocal micrographs of NODMIPGFP islets transplanted in the ACE of late prediabetic female NODs (16 to 20 wk old) that either (A) did not progress or (B) progressed to diabetes (hyperglycemia). Islets were clearly visible initially on top of the iris as dense white masses (bottom rows) and corresponding GFP signal (top rows) and became less dense and visible as they were attacked and destroyed. Both progressors and nonprogressors were treated with PBS as control treatment starting on POD8 and PBS intraperitoneal injections were given for five consecutive days (see Methods). The micrographs were acquired in z-stacks (shown as maximum intensity projections). Top rows, on the far left and right, show images of the same eyes at baseline (3 d after transplant; POD3) and at (A) POD86 in the nonprogressor and (B) POD22, which is after diabetes/hyperglycemia onset on POD11. Shown in the middle are longitudinal confocal micrographs of a representative islet pair (in box) in the GFP channel (green) where the GFP-expressing beta cells are clearly visible up to ∼POD15. The bottom rows show, on the far left and right, zoomed images of the boxed islet pair at baseline and POD22 and POD86 in progressors and nonprogressors, respectively, and longitudinal confocal micrographs of the same islet pair in the reflection (backscatter; middle) channel where the islets (white/bright gray) are initially visible on top of the iris surface (gray). (C) Longitudinal (POD5 to POD56) images and confocal micrographs of representative ACE-transplanted wild-type NOD (GFP_) islets in PBS-treated progressor counterparts (16 to 20 wk old at transplant) before and after diabetes/hyperglycemia onset on POD23. Scale bars = 100 µm. (D) Kaplan−Meier curves showing the destruction kinetics of individual NODMIPGFP (GFP+) islets (n = 5 islets in two mice) and wild-type (GFP_) islets (n = 7 islets in two mice) in wild-type prediabetic female NOD recipients that progressed to hyperglycemia/diabetes. (E) Longitudinal images (top) of the same eyes of progressor and nonprogressor NOD recipients of ACE-transplanted NODMIPGFP islets on 3 d after transplantation (POD3) and on POD115. White dotted line in POD115 images shows the location of the cross-section of the eye where immunostaining was performed after necropsy on POD115 (bottom). Shown are fluorescence confocal micrographs of eye sections (corresponding to the location identified by the dotted line on top) that were immunostained for insulin, glucagon, somatostatin, and DAPI nuclear counterstain. Scale bar = 50 µm. Zoomed images on the right correspond to the boxed area in each image on the left. Scale bar = 200 µm. ACE: anterior chamber of the eye; GFP: green fluorescent protein; NOD: nonobese diabetic; PBS: phosphate-buffered saline; POD: postoperative day.
Immune Intervention with Anti-CD3 Antibody Delayed Onset of Diabetes but Not the Destruction of GFP-expressing Beta Cells in NODMIPGFP Islets Transplanted in Pre-Diabetic NOD Mice
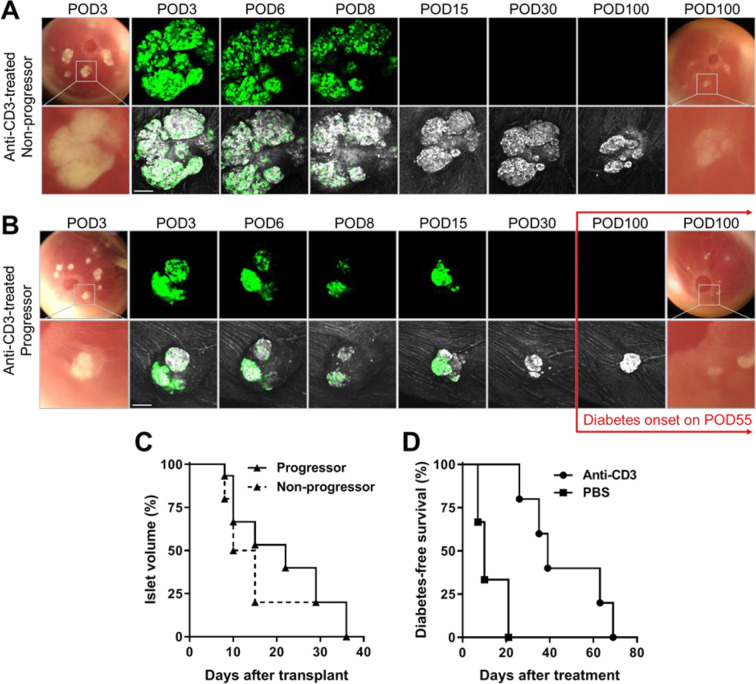
In addition to the above observation in the PBS-treated recipients, ACE-transplanted NODMIPGFP islets were also equally attacked in progressor and nonprogressor female NOD recipients treated with anti-CD3 monoclonal antibody (Fig. 2A, B). Longitudinal direct monitoring of the GFP-expressing beta cells in the ACE showed their quick destruction/disappearance with similar kinetics to those in the PBS-treated counterparts (Fig. 1). The mean time of destruction of the GFP-expressing beta cell was 17.2 ± 6.7 d (±SD). Interestingly, the GFP-negative cells (i.e., non-beta cells detected by laser backscatter/reflection) in the same NODMIPGFP islets remained detectable considerably longer after the complete destruction of their GFP-expressing beta cell counterparts in the anti-CD3-treated progressor and nonprogressor recipients. This persisted well beyond the onset of diabetes/hyperglycemia in the progressors, which was significantly delayed in association with anti-CD3 treatment (Fig. 2A, B, D). Quantitative volume analysis of the GFP-expressing beta cell mass in the corresponding individual NODMIPGFP islets further confirmed similar destruction kinetics in both the anti-CD3-treated progressors and nonprogressors (Fig. 2C). The median time to ≥30% loss in volume (compared to baseline) of the individual NODMIPGFP islets was 22 and 12.5 d after transplant in the progressors and nonprogressors, respectively (P = 0.4284 by log-rank [Mantel–Cox] test). Notably, the time to onset of hyperglycemia/diabetes in progressors that were treated with anti-CD3 antibody was significantly longer compared to the progressor counterparts treated with PBS (Fig. 2D). The corresponding median times of diabetes-free survival post-treatment were 10 and 39 d in the PBS-treated and anti-CD3-treated mice, respectively (P < 0.05 by log-rank [Mantel–Cox] test).
Figure 2.
Anti-CD3 antibody treatment delayed autoimmune T1D onset but not the destruction of GFP-expressing beta cells. (A, B) Representative images and confocal micrographs of NODMIPGFP islets transplanted in the ACE of late prediabetic female NOD mice that either (A) remained diabetes-free (nonprogressor) or (B) progressed to diabetes/hyperglycemia (progressor). Islets were clearly visible initially on top of the iris as dense white masses (bottom rows) and corresponding GFP signal (top rows) and became less dense and visible as they were attacked and destroyed. Both progressors and nonprogressors were treated with anti-CD3 mAb and treatment was started on (A) POD10 and (B) POD8 and was maintained for five consecutive days19,22,26 (see Methods for details). Confocal micrographs were acquired as z-stacks and are shown as maximum intensity projections. Scale bars = 100 µm. (C) Kaplan–Meier curves showing the destruction kinetics of NODMIPGFP NOD islets in the progressors (n = 15 islets in three mice) and nonprogressors (n = 15 islets in two mice). (D) Kaplan–Meier curves showing diabetes-free survival of progressors that received ACE-transplanted NODMIPGFP islets (GFP+) and were treated with PBS (n = 3 mice) or anti-CD3 mAb (n = 5 mice). ACE: anterior chamber of the eye; GFP: green fluorescent protein; mAb: monoclonal antibody; NOD: nonobese diabetic; PBS: phosphate-buffered saline; POD: postoperative day.
Longitudinal In Vivo Analysis of ACE-transplanted NODMIPGFP Islets Revealed Considerable Changes in Their Volume and Granularity During the Anti-GFP Immune Attack and Before Onset of Diabetes
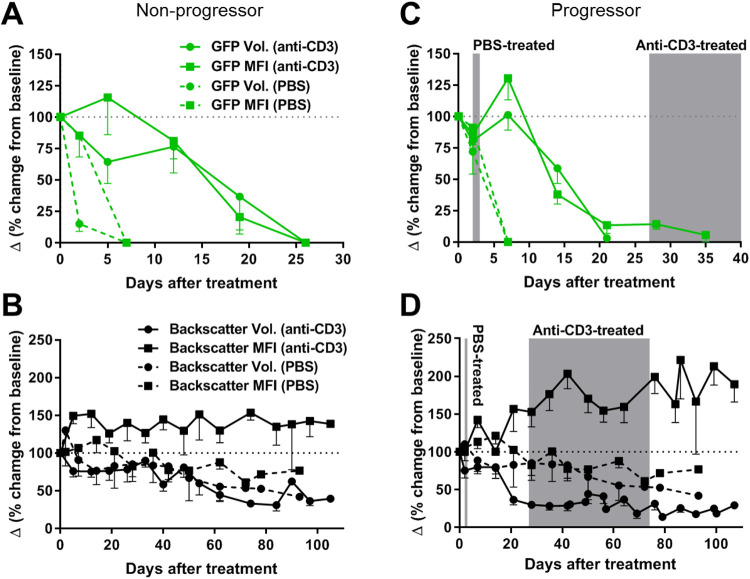
Longitudinal quantitative analyses of the volume and granularity of the individual ACE-transplanted NODMIPGFP islets further showed the progressive deterioration in their structural integrity and morphology consequent to immune attack against the GFP-expressing beta cells. The overall volume of the individual NODMIPGFP islets (measured by backscatter) and the beta cell mass within them (measured by GFP volume) decreased progressively relative to baseline in both the progressor and nonprogressor recipients and despite whether they were treated with PBS (dashed lines) or anti-CD3 (solid lines) (Fig. 3A–D). The analysis further confirmed that destruction of the GFP-expressing beta cells in progressors and nonprogressors occurred within days of transplantation (Fig. 3A, C) and that it preceded the onset of diabetes/hyperglycemia in the progressors regardless of treatment (Fig. 3C). However, there was evident tendency toward slowing down their destruction in association with the anti-CD3 treatment in both progressors and nonprogressors (solid lines), as was evidenced in the longitudinal measurements of the GFP volume (green roubd symbols and the corresponding median fluorescence intensity (MFI) values (green square symbols) (Fig. 3A, C). There was also evident increase in the MFI of the backscatter signal (black square symbols) in the individual islets or their remnants in association with the anti-CD3 treatment compared to PBS, and this was observed in both the progressor and nonprogressor recipients (Fig. 3B, D).
Figure 3.
Quantitative analysis of the volume and granularity of ACE-transplanted NODMIPGFP islets and GFP-expressing beta cells. (A, B) Analysis of volume (round symbols) and MFI (square symbols) of (A, C) beta cell mass (based on GFP fluorescence) and (B, D) volume/granularity of NODMIPGFP islets (based on laser backscatter/reflection signal) in (A, B) nonprogressor and (C,D) progressor recipients that were treated as indicated either with PBS (dashed lines) or anti-CD3 mAb (solid lines). Gray areas in C and D indicate the time range of diabetes/hyperglycemia onset for the indicated treatment group (PBS or anti-CD3). All data were normalized to baseline (measured at or before the start of treatment; day 0) and are presented as Δ (delta) measured based on the % change relative to baseline (dotted horizontal lines at 100%). All data are presented as means ± SEM. n = 7 to 21 islets in three PBS-treated mice and five anti-CD3-treated mice. ACE: anterior chamber of the eye; GFP: green fluorescent protein; MFI: median fluorescence intensity; NOD: nonobese diabetic; PBS: phosphate-buffered saline.
Discussion
This study was motivated by the need to provide clarity on whether manifestation of diabetic symptoms in T1D occurs after physical loss of a critical beta cell mass or consequent to functional impairment under inflammatory insult47. Widely accepted estimates mainly based on animal models suggest that ∼70% of the insulin-producing beta cell mass must be physically lost (destroyed) before the onset of hyperglycemia and overt diabetes. However, emerging evidence in the human pancreas also suggests the presence of a relatively large beta cells mass that is “spared” in patients with long-standing T1D48. Therefore, the primary objective of this study was to test the hypothesis whether hyperglycemia/diabetes onset in NOD mice is associated with actual physical destruction of beta cells or an impairment state in their function, and whether this functionally “silent” beta cell mass can be recovered (rescued) with therapeutic manipulation at or before the onset of T1D.
We have previously reported on the unique utility of the in vivo platform of ACE-transplanted islets in the NOD mouse model to investigate the immunobiology of T1D longitudinally19,21. We also demonstrated that syngeneic NOD islets transplanted in the ACE of prediabetic NOD mice are reliable reporters of the progression of autoimmune reactions against beta cells in the pancreas and, thus, could prompt timely therapeutic intervention before onset of hyperglycemia19. Here, we utilized this powerful platform to test the above hypothesis using ACE-transplanted NODMIPGFP islets in prediabetic female NOD mice. We planned to use the GFP signal in the beta cells of the ACE-transplanted NODMIPGFP islets as a surrogate measure of insulin production to assess their functional status and structural integrity during spontaneous progression of autoimmune T1D. This was necessary since measuring insulin levels in the plasma include insulin produced by the pancreas. We did this with and without immunomodulation with anti-CD3 monoclonal antibody treatment since it has been shown effective in preventing or delaying the onset of T1D19,22,25,26,49. However, there was unexpected early and quick immune attack against the GFP-expressing beta cells independent of autoimmune T1D, and this occurred regardless of whether the recipient mice progressed, or not, to overt hyperglycemia/diabetes. Interestingly, this vigorous immune reaction to the GFP was not previously reported in the NODMIPGFP mice, as they had similar kinetics of T1D development and incidence rate compared to wild-type NOD mice42. Additionally, our prior experience with ACE-transplanted islets that expressed cyan fluorescent protein (CFP) did not show a similar immune reactivity against the CFP-expressing beta cells in NOD recipients21. While prior reports on GFP immunogenicity exist in other mouse strains, particularly in the context of tumor cells transduced/transfected to express GFP40,41, no prior reports on this phenomenon existed in islet cells that are either transduced to express GFP or derived from transgenic NOD mice, until now. Therefore, the acute immune response against the GFP-expressing beta cells in the transgenic mouse NODMIPGFP islets that we observed here was surprising. Although a similar phenomenon has been described in rats with GFP-expressing hepatocytes50, it remains unclear at this point how the immune activation and priming against GFP in the NODMIPGFP islets occurred since it is not clear whether GFP is released from the NODMIPGFP beta cells; is it in the same insulin granules or segregated in discrete granules or cellular compartments? does it remain cytosolic or is it released with insulin or independently, if any? It is also unclear whether direct or indirect antigen presentation events by antigen-presenting cells were involved, and whether antigen presentation on the surface of the beta cells or antigen shedding and/or capture of soluble GFP was involved. Finally, we do not know whether the age (16 to 20 wk) of the prediabetic female NOD recipients played a role. While further studies are needed to clarify these questions, it is important to note based on the current findings that immune reactivity against GFP-expressing pancreatic islets can occur independent of anti-islet autoimmunity in the NOD mouse model of human T1D.
In summary, diabetic NOD mice share several disease traits of human T1D and, yet, many therapies that have been successful in preventing or treating T1D in NOD mice have not translated as well in human clinical trials. This discrepancy between preclinical findings and clinical outcomes remains a major challenge, and T1D is currently the only autoimmune condition without an approved immunotherapy27,28. While the reasons for this may be complex, the structural and functional differences between rodent and human islets may be an important factor30,34,36,51,52. In conclusion, mouse models have in past years contributed significantly to our understanding of various aspects of human diseases and are likely to continue to do so in the future. However, the unexpected findings reported on here will hopefully help in guiding further T1D research by avoiding this and other inherent differences of this murine model of human T1D and generating preclinical findings that are more translatable to the clinical setting.
Acknowledgments
Special thanks to the staff of the DRI Pre-Clinical Cell Processing and Translational Models Core and to K. Johnson the DRI Histology Core for outstanding technical assistance.
Footnotes
Contribution Statement: VRA obtained/analyzed data and drafted the manuscript. BBH-R and AC conducted experiments and proofed the manuscript. MHA conceived and designed the study, conducted experiments, analyzed and interpreted data, and wrote the manuscript. All authors approved the published version of the manuscript.
Ethical approval: All animal studies in the report were approved by the University of Miami’s Institutional Animal Care and Use Committee (IACUC).
Statement of Human and Animal Rights: All animal procedures in this study were conducted in accordance with protocols approved by the University of Miami’s Institutional Animal Care and Use Committee (IACUC protocol # 17-171). No human studies were performed.
Statement of Informed Consent: There are no human subjects in this study and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Duality of Interest: MHA is consultant for Biocrine, an unlisted biotech company that is using the ACE technique as a research tool. All other authors declare no duality of interest associated with their contribution to this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from the Diabetes Research Institute Foundation (DRIF) and grants from the National Institutes of Health (NIH) R56AI130330 (NIAID) and UC4DK116241/K01DK097194/F32DK083226 (NIDDK) to MHA, and the University of Miami's Leadership Alliance Program to BBH-R.
ORCID iD: Midhat H. Abdulreda  https://orcid.org/0000-0002-0146-5876
https://orcid.org/0000-0002-0146-5876
References
- 1. Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29(1):1–13. [DOI] [PubMed] [Google Scholar]
- 2. Cai X, McGinnis JF. Diabetic retinopathy: animal models, therapies, and perspectives. J Diabetes Res. 2016;2016(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–745. [DOI] [PubMed] [Google Scholar]
- 4. Wang N, Guo C, Han P, Li T. Glycated albumin indicates peripheral diabetic neuropathy. Acta Diabetol. 2016;53(6):973–979. [DOI] [PubMed] [Google Scholar]
- 5. Popović S, Canović F, Ilić M, Rafajlovski S, Dimitrijević-Srećović V, Matanović D, Vujović S, Djordjević P, Gostiljac D. Matrix metalloproteinase-9 index as a possible parameter for predicting acute coronary syndrome in diabetics. Vojnosanit Pregl. 2015;72(5):421–426. [DOI] [PubMed] [Google Scholar]
- 6. Della-Morte D, Ricordi C, Guadagni F, Rundek T. Measurement of subclinical carotid atherosclerosis may help in predicting risk for stroke in patients with diabetes. Metab Brain Dis. 2013;28(3):337–339. [DOI] [PubMed] [Google Scholar]
- 7. Seki N, Nishimura M, Matsumoto T, Fukazawa M, Kenmochi T. Relationship between BNP level and renal function in diabetic nephropathy with microalbuminuria. J Diabetes Complications. 2013;27(1):92–97. [DOI] [PubMed] [Google Scholar]
- 8. Grauslund J. Eye complications and markers of morbidity and mortality in long-term type 1 diabetes. Acta Ophthalmol. 2011;89(Thesis 1):1–19. [DOI] [PubMed] [Google Scholar]
- 9. Yudovsky D, Nouvong A, Schomacker K, Pilon L. Assessing diabetic foot ulcer development risk with hyperspectral tissue oximetry. J Biomed Opt. 2011;16(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barinova IV, Krivova IS, Barabanov VM, Savel’ev SV, Petrukhin VA, Burumkulova FF, Shidlovskaia NV. Diabetic fetopathy in gravida with autoimmune hemolytic anemia complicated by steroid diabetes [in Russian]. Arkh Patol. 2010;72(1):39–40. [PubMed] [Google Scholar]
- 11. Ekberg K, Brismar T, Johansson BL, Lindstrom P, Juntti-Berggren L, Norrby A, Berne C, Arnqvist HJ, Bolinder J, Wahren J. C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care. 2007;30(1):71–76. [DOI] [PubMed] [Google Scholar]
- 12. Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7(6):727–738. [DOI] [PubMed] [Google Scholar]
- 13. Leiter EH. The NOD mouse: a model for insulin-dependent diabetes mellitus. Curr. Protoc. Immunol. 2001;Chapter 15:15910–15923. [DOI] [PubMed] [Google Scholar]
- 14. Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat. Rev. Immunol. 2004;4(12):989–997. [DOI] [PubMed] [Google Scholar]
- 15. Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23(2):115–126. [DOI] [PubMed] [Google Scholar]
- 16. Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. [DOI] [PubMed] [Google Scholar]
- 17. Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. [DOI] [PubMed] [Google Scholar]
- 19. Abdulreda MH, Molano RD, Faleo G, Lopez-Cabezas M, Shishido A, Ulissi U, Fotino C, Hernandez LF, Tschiggfrie A, Aldrich VR, Tamayo-Garcia A, et al. In vivo imaging of type 1 diabetes immunopathology using eye-transplanted islets in NOD mice. Diabetologia. 2019;62(7):1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernandez LF, Buchwald P, Abdulreda MH. Effect of Arginse-1 inhibition on the incidence of autoimmune diabetes in NOD mice. Curre Res Diabetes Obes J. 2018;5(3):1–13. [PMC free article] [PubMed] [Google Scholar]
- 21. Miska J, Abdulreda MH, Devarajan P, Lui JB, Suzuki J, Pileggi A, Berggren PO, Chen Z. Real-time immune cell interactions in target tissue during autoimmune-induced damage and graft tolerance. J Exp Med. 2014;211(3):441–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabello-Kindelan C, Mackey S, Sands A, Rodriguez J, Vazquez C, Pugliese A, Bayer AL. Immunomodulation followed by antigen-specific treg infusion controls islet autoimmunity. Diabetes. 2020;69(2):215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anastasia G Daifofotis, Scott Koenig, Lucienne Chatenoud, Kevan C Herold. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol. 2013;149(3):268–278. [DOI] [PubMed] [Google Scholar]
- 25. Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91(1):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158(6):2947–2954. [PubMed] [Google Scholar]
- 27. Donath MY, Hess C, Palmer E. What is the role of autoimmunity in type 1 diabetes? A clinical perspective . Diabetologia. 2014;57(4):653–655. [DOI] [PubMed] [Google Scholar]
- 28. Skyler JS. Hope vs hype: where are we in type 1 diabetes? Diabetologia. 2018;61(3):509–516. [DOI] [PubMed] [Google Scholar]
- 29. Of men, not mice. Nat Med. 2013;19(4):379–379. [DOI] [PubMed] [Google Scholar]
- 30. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Köhler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 2008;7(6):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacques-Silva MC, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman DM, Kenyon NS, Ricordi C, Pileggi A, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A. 2010;107(14):6465–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Braun M, Ramracheya R, Bengtsson M, Clark A, Walker J, Johnson P, Rorsman P. GABA is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59(7):1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011;17(7):888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets. 2009;1(2):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bach J-F. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocrine Reviews. 2018;15(4):516–542. [DOI] [PubMed] [Google Scholar]
- 38. Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets?. Nature Medicine. 1999;5(6):601–604. [DOI] [PubMed] [Google Scholar]
- 39. Yeh C-K, Harris SE, Mohan S, Horn D, Fajardo R, Chun Y-HP, Jorgensen J, MacDougall M, Abboud-Werner S. Hyperglycemia and xerostomia are key determinants of tooth decay in type 1 diabetic mice. Laboratory Investigation. 2012;92(6):868–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, Harmon JW, Sun Z. Cellular GFP toxicity and Immunogenicity: potential confounders in in vivo cell tracking experiments. Stem Cell Rev Rep. 2016;12(5):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6(7):1305–1312. [DOI] [PubMed] [Google Scholar]
- 42. Grapov D, Fahrmann J, Hwang J, Poudel A, Jo J, Periwal V, Fiehn O, Hara M. Diabetes associated metabolomic perturbations in NOD mice. Metabolomics. 2015;11(2):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50(9):1983–1991. [DOI] [PubMed] [Google Scholar]
- 44. Abdulreda MH, Caicedo A, Berggren P-O. Transplantation into the anterior chamber of the eye for longitudinal, non-invasive in vivo imaging with single-cell resolution in real-time. J Vis Exp. 2013(73):1–7(e50466). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abdulreda MH, Faleo G, Molano RD, Lopez-Cabezas M, Molina J, Tan Y, Echeverria OA, Zahr-Akrawi E, Rodriguez-Diaz R, Edlund PK, Leibiger I, et al. High-resolution, noninvasive longitudinal live imaging of immune responses. Proc Natl Acad Sci U S A. 2011;108(31):12863–12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, Kenyon NS, Ricordi C, Burkly LC, Inverardi L. Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes. 2001;50(2):270–276. [DOI] [PubMed] [Google Scholar]
- 47. Burke SJ, Stadler K, Lu D, Gleason E, Han A, Donohoe DR, Rogers RC, Hermann GE, Karlstad MD, Collier JJ. IL-1beta reciprocally regulates chemokine and insulin secretion in pancreatic beta-cells via NF-kappaB. Am J Physiol Endocrinol Metab. 2015;309(8):715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pugliese A, Yang M, Kusmarteva I, Heiple T, Vendrame F, Wasserfall C, Rowe P, Moraski JM, Ball S, Jebson L, Schatz DA, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaglia J, Kissler S. Anti-CD3 antibody for the prevention of type 1 diabetes: a story of perseverance. Biochemistry. 2019;58(40):4107–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maeda H, Shigoka M, Wang Y, Fu Y, Wesson RN, Lin Q, Montgomery RA, Enzan H, Sun Z. Disappearance of GFP-positive hepatocytes transplanted into the liver of syngeneic wild-type rats pretreated with retrorsine. PLoS One. 2014;9(5):1–10(e95880). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodriguez-Diaz R, Molano RD, Weitz JR, Abdulreda MH, Berman DM, Leibiger B, Leibiger IB, Kenyon NS, Ricordi C, Pileggi A, Caicedo A, et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 2018;27(3):549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abdulreda MH, Rodriguez-Diaz R, Cabrera O, Caicedo A, Berggren PO. The different faces of the pancreatic islet. Adv Exp Med Biol. 2016;938:11–24. [DOI] [PubMed] [Google Scholar]