Abstract
Antibody drug conjugates (ADCs) normally compose of a humanized antibody and small molecular drug via a chemical linker. After decades of preclinical and clinical studies, a series of ADCs have been widely used for treating specific tumor types in the clinic such as brentuximab vedotin (Adcetris®) for relapsed Hodgkin's lymphoma and systemic anaplastic large cell lymphoma, gemtuzumab ozogamicin (Mylotarg®) for acute myeloid leukemia, ado-trastuzumab emtansine (Kadcyla®) for HER2-positive metastatic breast cancer, inotuzumab ozogamicin (Besponsa®) and most recently polatuzumab vedotin-piiq (Polivy®) for B cell malignancies. More than eighty ADCs have been investigated in different clinical stages from approximately six hundred clinical trials to date. This review summarizes the key elements of ADCs and highlights recent advances of ADCs, as well as important lessons learned from clinical data, and future directions.
Key words: Antibody drug conjugates, Antibody, Cytotoxic agents, Linker, Clinical application
Graphical abstract
Antibody drug conjugates (ADCs), normally composed of a humanized antibody and small molecular drugs via chemical linkers, represent a rapidly growing field for cancer therapy. In this review, we provide an overview of ADCs in preclinical and clinical development, as well as future directions of ADCs.
1. Introduction
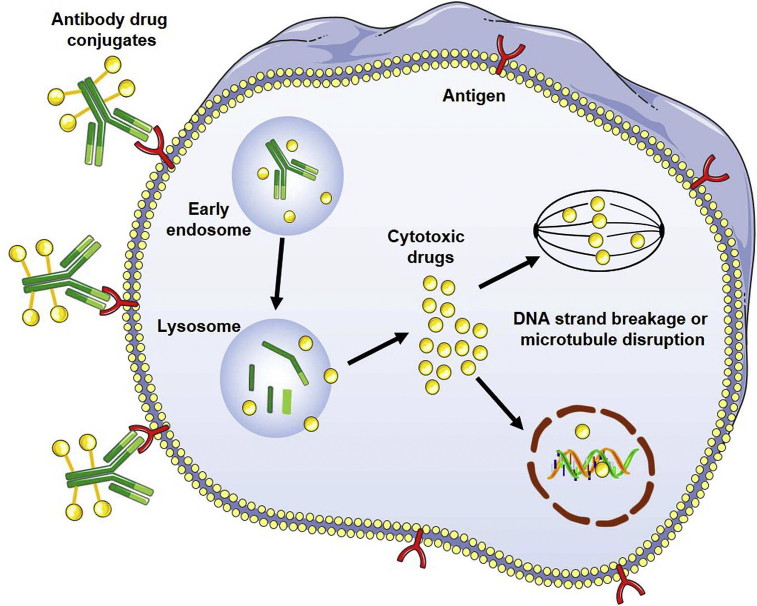
Chemotherapy is one of the major treatment options for cancer therapy1. Although a number of chemotherapy drugs have been widely used in the clinic, serious hurdles still remain such as adverse effects and drug resistance2. Extensive efforts have been made to increase the efficacy of cytotoxic drugs, such as combining different chemotherapeutic drugs and using highly potent agents such as auristatin and maytansine3, 4, 5. However, systemic toxicity and narrow therapeutic window limit their clinical use3. The advances of monoclonal antibody provide opportunities to use their specific binding property for targeted drug delivery6. Based on this concept, antibody drug conjugates (ADCs) are designed and developed through conjugation of antibodies and cytotoxic drugs in the past decades7. As illustrated in Fig. 1, ADCs selectively bind to the receptors of tumor cells8. After that, the receptor–ADC complex is usually internalized through the endocytosis pathway. The linker is cleaved, and cytotoxic drugs are released. Consequently, these drugs induce cytotoxic effects through various mechanisms of action such as binding to the minor groove of deoxyribonucleic acid (DNA) or interacting with tubulin8.
Figure 1.
Illustration of the action mechanism of antibody drug conjugates (ADCs).
In the first-generation ADCs such as BR96–doxorubicin and KS1/4–methotrexate, chemotherapy drugs are usually conjugated to murine antibodies via a non-cleavable linker9,10. However, these ADCs are generally less potent than free drugs11. Then, researchers developed gemtuzumab ozogamicin (GO) with improved efficacy, because GO is consisted of a potent calicheamicin derivative and a humanized antibody to reduce immunogenicity12. Yet, GO has several disadvantages, including an unstable linker, a high percentage of unconjugated antibody, poor CMC (chemistry, manufacturing, and control) properties, as well as high toxicity9,13. Limitations of the first-generation ADCs lead to the development of the second-generation ADCs. The monoclonal antibody (mAb) technology has been established with high tumor cell targeting9. Furthermore, many potent chemotherapy drugs have been discovered9,14. Therefore, compared with the first-generation ADCs, the second-generation ADCs showed better CMC characteristics9. For instance, brentuximab vedotin, ado-trastuzumab emtansine, and inotuzumab ozogamicin are typical second-generation ADCs on the market10. Drawbacks of the second-generation ADCs include off-target toxicity, fast clearance, and competition with unconjugated antibodies15. The lessons learned from the previous ADCs expedite the development of the third-generation ADCs. Site-specific conjugation has been created in the design of ADCs, which could result in homogeneous ADCs with drug–antibody ratio (DAR) of two or 4, as well as improved pharmacokinetics13.
In this review article, we will discuss the key elements of ADCs, overview their preclinical and clinical development, as well as future directions of ADCs.
2. Key elements in antibody drug conjugates
2.1. Antigen selection
The selection of an appropriate antigen is one of the major challenges in the development of ADCs. Three aspects should be considered in antigen selection. (i) High-level expression in tumors while low-level expression in healthy tissues. For example, ado-trastuzumab emtansine targets human epidermal growth factor receptor 2 (HER2), whose expression reaches the level of 2 × 106 in tumor cells compared with 2 × 104 in healthy cells16. (ii) Target antigens express on the tumor cell surface, so that they can be accessible to the antibody13. (iii) The rate of internalization and route of intracellular trafficking13,17,18. It is worth mentioning that non-internalized ADCs can also display therapeutic effects through a strong “bystander effect”, that is, a membrane-permeable drugs are able to induce cell death to the neighboring cells19.
2.2. Antibody selection
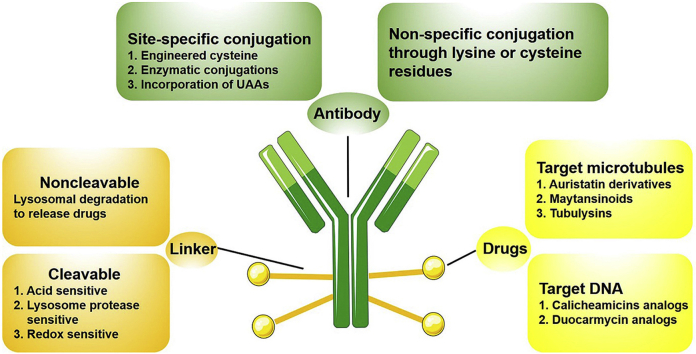
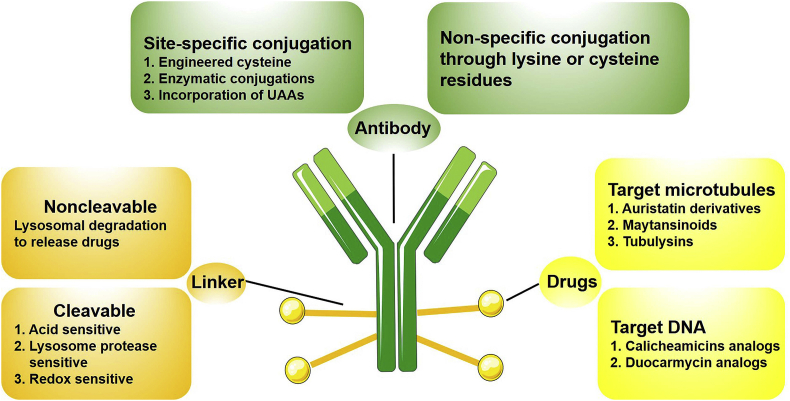
ADCs are composed of three parts, including antibody, drug and linker. To design effective ADCs, all three components are essential and important (Fig. 220).
Figure 2.
Rational design of ADCs components20.
Among them, antibodies with a molecular weight of around 150 kDa are a major component of ADCs21. Besides target specificity, antibodies also need to bind with suitable affinity, thereby increasing accumulation and retention in tumor sites3. Most ADCs have binding affinities with KD values ranging from 0.1 to 1.0 nmol/L. Of note, previous studies reported that if the binding affinity is too high, the delivery of the antibody in solid tumors may be affected, which is called the binding-site barrier22,23.
Most antibodies used in the clinic are selected from human immunoglobulin G (IgG), about 150 kDa consisted of two heavy and two light chains24,25. Nowadays, there are many ongoing studies for the use of antibody derived from IgGs26. Generally, antibody derivatives can be classified as antigen-binding fragments (Fab), single-chain variable fragments (scFv) and variable domains (VHH, also named as nanobodies)24,26. Fab and scFv include both the heavy and light domain of the parental IgGs, and retain the size and affinity of the area binding the antigen24. Because of the smaller size compared with regular IgGs, they show improved pharmacokinetics for tumor penetration27. The nanobodies do not have CH1 domain but possess a long complementary determining region 3 (CDR3)26, which displays high stability because of their resistance to denaturing factors28. Moreover, the nanobodies are smaller than the filtration size of kidney, thereby they are excreted through the kidneys with a higher clearance and relatively lower toxicity28, 29, 30. For example, Ploegh and co-workers conjugated a nanobody with oligoglycine-modified cytotoxic payloads, which exhibited higher specificity and lower cytotoxicity towards tumor cells compared with traditional ADCs31.
2.3. Chemotherapy drugs
Several criteria are important for choosing suitable chemotherapy drugs. First, these drugs display high cytotoxicity to tumor cells (normally half maximal inhibitory concentration (IC50) in the nanomolar and picomolar range)5,13,17. Because only nearly 2% of the injected ADCs will distribute into tumors after intravenous administration, thereby resulting in low intracellular concentrations32. Second, these drugs have a functional group or can be derived to be conjugated with the antibody. Third, these drugs are stable in physiological conditions33. Therefore, a relatively small number of cytotoxic drug families are used in current clinical trials. Most of them are derivatives of auristatins or maytansine, which are both microtubule inhibitors34,35. Others are DNA damaging drugs, including (i) drugs inducing double-strand DNA break (e.g., calicheamicin)36 (ii) drugs alkylating DNA (e.g., duocarmycin)37; and (iii) drugs crosslinking with DNA (e.g., pyrrolobenzodiazepine dimers)38. Several representative cytotoxic drugs are discussed in this section.
2.3.1. Auristatin
Auristatin derivatives, including monomethyl analogs monomethyl auristatin E/F (MMAE and MMAF), are the largest class of ADCs in clinical development13. MMAE and MMAF are both derived from dolastatin 10, which is isolated from sea hare39,40. Dolastatin 10 is highly toxic to both tumors and healthy tissues, which leads to its failure in clinical trials41. However, its derivatives MMAE and MMAF are presently used as cytotoxic drugs in ADCs. MMAE can permeate cell membranes, thereby displaying the bystander effect. In contrast, MMAF is more hydrophilic and cannot permeate cell membranes. The lack of bystander effect makes MMAF derived ADCs less efficient compared with MMAE derived ADCs. Meanwhile, MMAF derived ADCs are relatively less toxic42. In 2015, the U.S. Food and Drug Administration (FDA) approved brentuximab vedotin, a MMAE conjugate, to treat Hodgkin lymphoma and anaplastic large cell lymphoma16. In 2019, another MMAE derived ADC, polatuzumab vedotin-piiq, was approved to treat relapsed or refractory diffuse large B-cell lymphoma97.
2.3.2. Maytansinoids
Other kinds of ADCs in clinical development are derivatives of maytansine (maytansinoids, DMs). Maytansine is a natural product isolated from African shrub Maytenus ovatus, whose mechanism is to disrupt microtubule polymerization43. Meanwhile, maytansine is one of the first cytotoxic drugs that have a picomolar IC50 value to tumor cells44. However, due to its systemic toxicity, maytansines also failed in clinical trials45. Incorporation of maytansine derivatives into ADCs significantly improved its therapeutic index46. In 2013, ado-trastuzumab emtansine, a DM1 derived ADC, was approved by the FDA to treat HER-2-positive metastatic breast cancer. Additionally, two more maytansine derivatives, DM1 and DM4-based ADCs are presently in clinical trials47.
2.3.3. Calicheamicins
Calicheamicins that are isolated from the actinomycete Micromonospora echinospora can induce DNA double-strand cleavage through binding to the minor groove of DNA48. Calicheamicins were among the first DNA damaging drugs incorporated in ADCs, but their narrow therapeutic windows and serious side effects limited their clinical applications49. These shortcomings have now been largely overcome because of the advances of ADCs technologies especially the linker chemistry and the optimization of dosing approaches. Two of the five ADCs on market, gemtuzumab ozogamicin (GO) and inotuzumab ozogamicin (InO), are calicheamicin derived ADCs. GO is the first ADC drug on market to treat acute myeloid leukemia. However, GO was withdrawn from the US and European markets because of its adverse effects7. After dose fractionation, in which patients receive three doses of 3 mg/m2 GO instead of one dose of 9 mg/m2 GO, the FDA re-approved GO in 201750. In addition, InO was approved by FDA in 2017 to treat B cell acute lymphoblastic leukemia and other B cell malignancies51.
2.4. Linkers
An effective linker needs to be stable during circulation because the release of drugs in the blood stream will affect ADCs’ pharmacokinetics, thus leading to toxicity and lower therapeutic index48,52. Once the ADCs are internalized into tumor cells, the linker needs to be cleaved, rapidly releasing drugs7,53. One critical factor that should be taken into consideration is the DAR. Too few drug molecules on each antibody result in decreased efficacy, while excessive DAR will lead to poor pharmacokinetics of ADCs because of higher hydrophobicity and lower solubility14,47. It has been reported that the DAR of most clinical trials ADCs are in the range of 2.0–4.014.
Linkers used in typical ADCs can be divided into noncleavable and cleavable linkers4. Noncleavable linkers usually rely on the lysosomal degradation to release the cytotoxic drugs which are attached to the linker and an amino acid residue of the antibody54,55. For example, brentuximab vedotin was designed to use a noncleavable linker (succinimidyl trans-4-(maleimidylmethyl) cyclohexane-1-carboxylate, SMCC) to crosslink the maytansinoid to the HER2 antibody56.
The structure of cleavable linkers includes a position of cleavage between the antibody and the drug3. Usually, based on the cleavage mechanisms, cleavable linkers can be classified into three groups. (i) Acid sensitive, such as hydrazone linkers, that are cleaved in the lysosome because of low pH environment. For example, the hydrazone linker is used in both GO and InO. In addition, although acid cleavable linkers are designed for maintaining stability during circulation and release drugs in the acidic environment, it has been reported that acid cleavable linkers could be associated with nonspecific release of the drugs57. (ii) Lysosomal protease sensitive, such as valine–alanine and valine–citrulline peptide linkers, that are designed to release drugs after cleavage by intracellular proteases. For example, cathepsin B, a lysosomal protease, cleaves the dipeptide bond in the tumor cells58. In addition, a cathepsin B-sensitive dipeptide linkage (valine–citrulline) is used in brentuximab vedotin. (iii) Redox sensitive, such as disulfide linkers, that takes advantage of higher glutathione concentration in tumor microenvironment4,7,55. Optimizing the steric hindrance of disulfide bridges can decrease premature drug release13. For example, this method is applied in the case of anetumab ravtansine and coltuximab ravtansine using a disulfide linker N-hydroxysuccinimidyl-4-(2-pyridyldithio) butanoate (SPDB) to crosslink DM4.
3. Site-specific conjugation
As previously described, a suitable DAR is important to the design of ADCs. Site-specific conjugation can produce a consistent generation of relatively homogeneous ADCs products without altering the antigen binding affinity. Three strategies are mainly used for site-specific conjugation on the antibody: (i) engineered cysteines59, 60, 61; (ii) enzymatic conjugations62; and (iii) incorporation of unnatural amino acids63, 64, 65.
3.1. Engineered cysteine
The thiol group in the cysteine side chain can be used for site-specific modification, because of its high nucleophilicity. Companies such as Genentech, Seattle Genetics, Pfizer have developed different ADCs with engineered cysteines60,66. These ADCs have a uniform DAR of 2 or 4. Furthermore, ADCs constructed by this method showed encouraging in vivo results, including higher efficacy and better toleration compared with conventional ADCs67. For example, vadastuximab talirine, which consists of anti-CD33 antibodies with engineered cysteines and pyrrolobenzodiazepine (PBD) dimer through a cleavable dipeptide linker (valine-alanine), is the first ADC with site-specific conjugation38.
3.2. Enzymatic conjugations
Several enzymes such as the bacterial derived formyl glycine generating enzyme (FGE), transglutaminases, glycotransferases, and sortases have been used for conjugating the antibodies68. The reaction sites of antibodies are designed to react specifically to the corresponding functional groups. Therefore, the enzymatic conjugation method leads to site-specific conjugation and homogeneous DARs. For example, SMARTag® is a technology that uses FGE. FGE can insert the antibody after a sequence of specific amino acid is recognized. Then, the cysteine is converted into formylglycine69. Finally, the engineered antibody can selectively react with aldehyde-specific drugs via the reaction based on the hydrazino-Pictet–Spengler ligation70.
3.3. Incorporation of unnatural amino acid
Incorporation of unnatural amino acids (UAAs) with bioorthogonal groups are also used on site-specific conjugation. The most common method of UAAs incorporation is to engineer transfer RNA (tRNA) synthetases and recognize UAAs, thus resulting the genetic coding of the UAAs71. For instance, Tian et al.72 reported a site-specific ADC using UAAs. Compared to traditional cysteine conjugated ADCs, this ADC may possess better selectivity and efficacy both in vitro and in vivo72. Yet, the UAAs-based methodology needs special techniques and reagents for preparation and manufacturing59.
4. Preclinical development of antibody drug conjugates
In current clinical trials, calicheamicins, auristatin and maytansinoid are the most commonly used cytotoxic drugs in ADCs. Meanwhile, several other types of drugs are in the stage of preclinical development, such as microtubule inhibitors73, anthracyclines74 and amatoxins75.
4.1. Microtubule inhibitors
The approval of ADCs based on auristatin and maytansinoid accelerates the development of new microtubule inhibitors drugs. Tubulysins, a series of peptidic compounds, are representative examples. Tubulysins are originally isolated from myxobacteria and show potent inhibition through tubulin polymerization76. Among different types of tubulysins, tubulysin D is the most effective one with cytotoxic activity in the range of picomolar in various tumor cell lines77. In 2014, Cohen et al.78 developed ADCs that are consisted of trastuzumab and the stable tubulysin analogs Tub-OH or Tub-OMOM. Both 131I-labeled and unlabeled versions of the tubulysins are conjugated to the surface lysines through a noncleavable N-hydroxysuccinimide linker. These ADCs showed favorable therapeutic effects both in vitro and in vivo78.
4.2. Anthracyclines
Recent studies on anthracyclines such as nemorubicin and its major metabolite, PNU-159682, indicate that these agents might overcome the limitations of doxorubicin such as drug resistance and cardiac toxicity74. Furthermore, compared with doxorubicin, PNU-159682 showed three orders of magnitude more cytotoxic activity against different tumor cell lines including doxorubicin resistant cells79. These features are associated with tight and stable bindings of PNU-159682 to DNA80. Yu et al.74 conjugated PNU-159682 to anti-CD22 antibody through a maleimidocaproyl-valine-citrulline-p-aminobenzoyloxycarbonyl (mc-vc-PAB) and diethylamine linker. This ADC is 2–20-fold more effective than pinatuzumab vedotin in vitro and displays therapeutic effects in four types of xenograft tumors. Furthermore, it may overcome the drug resistance induced by the p-glycoprotein81.
4.3. Amatoxins
Amatoxins are a class of peptide toxins. α-Amanitin, a representative example was originally isolated from the amanita phalloides mushroom and was found to be an inhibitor of the eukaryotic RNA polymerase II75, inducing transcriptional arrest and leading to tumor cells death82. Moldenhauer et al.83 conjugated α-amanitin to chiHEA125 (a chimerized anti-human epithelial cell adhesion molecule monoclonal antibody) via a glutarate linker. This ADC has a picomolar IC50 value in Colo205 and MCF-7 tumor cells83. Moreover, it also displayed tumor inhibition in a BxPc-3 pancreatic xenograft model83.
5. Antibody drug conjugates in clinical trials
ADCs have become an important class of anti-cancer drugs, with a dramatically increasing number of ADCs in clinical studies for treating hematologic malignancies and solid tumors over the past 5 years13,33. Table 1 lists the approved ADCs. Four of these ADCs are designed to treat hematologic malignancies, in which the target antigens are more accessible for circulating ADCs compared to solid tumors84. Table 2 lists the ADCs presently in phase II or phase III clinical studies. A mass of ADCs are in phase I clinical trials, which are not listed here. The clinical results of ADCs that are approved or in phase III clinical trials are further discussed in this section.
Table 1.
Marketed antibody drug conjugates (ADCs).
| ADC | Target antigen | Linker | Cytotoxin | Developer | Indication(s) | Phase |
|---|---|---|---|---|---|---|
| Gemtuzumab ozogamicin | CD33 | Cleavable hydrazone | Calicheamicin | Pfizer | Acute myeloid leukemia | FDA approved in 2000; withdrawn in 2010; reapproved in 2017 |
| Brentuximab vedotin | CD30 | Cleavable dipeptide | MMAE | Seattle Genetics/Takeda | Hodgkin lymphoma, systemic anaplastic large cell lymphoma | FDA accelerated approval in 2011; full approval in 2015 |
| o-Trastuzumab emtansine | HER2 | Noncleavable (SMCC) | DM1 | Genentech/Roche | HER2-positive breast cancer | FDA approved in 2013 |
| Inotuzumab ozogamicin | CD22 | Cleavable hydrazone | Calicheamicin | Pfizer | Acute lymphoblastic leukemia | FDA approved in 2017 |
| Polatuzumab vedotin-piiq | CD79b | Cleavable dipeptide | MMAE | Genentech/Roche | Relapsed or refractory diffuse large B-cell lymphoma | FDA accelerated approval in 2019 |
Table 2.
Antibody drug conjugates (ADCs) in phase III and phase II development.
| ADC | Target antigen | Linker | Cytotoxin | Developer | Indication(s) | Phase | NCT number |
|---|---|---|---|---|---|---|---|
| Rovalpituzumab tesirine | DLL3 | Cleavable dipeptide | PBD dimer | AbbVie (Stemcentrx) | Small-cell lung cancer | III |
NCT03061812 (ongoing) NCT03033511 (ongoing) |
| Mirvetuximab soravtansine | FOLR1 | Cleavable disulfide | DM4 | ImmunoGen | Ovarian, endometrial, non-small cell lung cancer | III | NCT02631876 (ongoing) |
| Depatuxizumab mafodotin | EGFR | Noncleavable (mc) | MMAF | AbbVie | Glioblastoma and other EGFR-positive tumors | III | NCT02573324 (ongoing) |
| Sacituzumab govitecan | Trop-2 | Acid-labile ester | SN-38 | Immunomedics | Triple-negative breast cancer, urothelial and other cancers | III |
NCT02574455 (ongoing) NCT03901339 (ongoing) |
| Naratuximab emtansine | CD37 | Noncleavable (SMCC) | DM1 | ImmunoGen | Diffuse large B cell lymphoma and follicular lymphoma | II | NCT01534715 (ongoing) |
| Lorvotuzumab mertansine | CD56 | Cleavable disulfide | DM1 | ImmunoGen | Leukemia | II | NCT01237678 (completed) |
| Coltuximab ravtansine | CD19 | Cleavable disulfide | DM4 | ImmunoGen | Diffuse large B cell lymphoma, acute lymphocytic leukaemia | II | NCT01472887 (completed) NCT01440179 (terminated) NCT01470456 (completed) |
| Indatuximab ravtansine | CD138 | Cleavable disulfide | DM4 | Biotest | Multiple myeloma | II |
NCT01638936 (completed) NCT01001442 (completed) |
| Anetumab ravtansine | Mesothelin | Cleavable disulfide | DM4 | Bayer Health Care | Mesothelioma and other solid tumors | II |
NCT03926143 (ongoing) NCT03023722 (ongoing) NCT02839681 (terminated) |
| SAR566658 | CA6 | Cleavable disulfide | DM4 | Sanofi | Triple-negative breast cancer | II | NCT02984683 (completed) |
| Glembatumumab vedotin | gpNMB | Cleavable dipeptide | MMAE | Celldex | Metastatic breast cancer and melanoma | II |
NCT01997333 (completed) NCT02302339 (terminated) |
| PSMA ADC | PSMA | Cleavable dipeptide | MMAE | Progenics/Seattle Genetics |
Prostate cancer | II |
NCT02020135 (completed) NCT01695044 (completed) |
| Pinatuzumab vedotin | CD22 | Cleavable dipeptide | MMAE | Genentech/Roche | Diffuse large B-cell lymphoma, follicular non-Hodgkin lymphoma | II | NCT01691898 (completed) |
| Telisotuzumab vedotin | ABT-700 | Cleavable dipeptide | MMAE | AbbVie/Pierre Fabre | Advanced solid tumors cancer and non-small cell lung cancer | II | NCT02099058 (ongoing) |
| SGN-LIV1A | LIV-1 | Cleavable dipeptide | MMAE | Seattle Genetics | Breast cancer, lung cancer | II |
NCT01042379 (ongoing) NCT03310957 (ongoing) NCT04032704 (ongoing) |
| AGS-16C3F | ENPP3 | Noncleavable (mc) | MMAF | Agensys/Astellas | Renal cell carcinoma | II | NCT02639182 (ongoing) |
5.1. Gemtuzumab ozogamicin
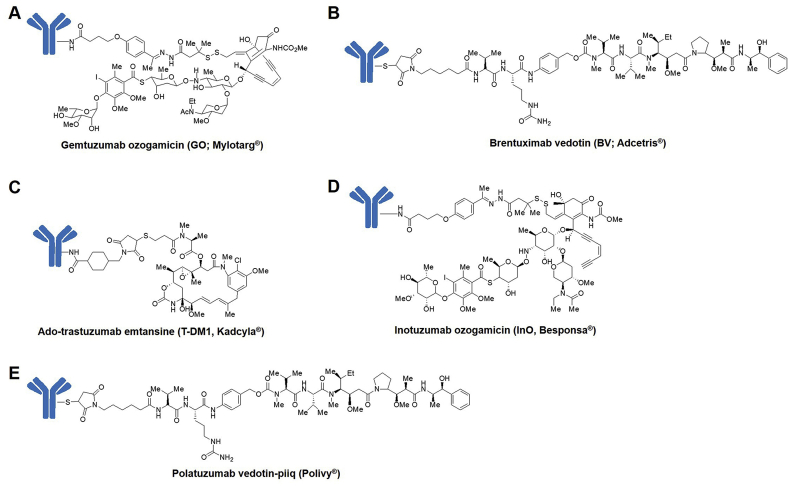
Gemtuzumab ozogamicin (GO; Mylotarg®, Fig. 3A) is the first ADC approved by the FDA85. GO is consisted of a CD33 monoclonal antibody and calicheamicin via a cleavable hydrazone linker85. In 2000, Based on three phase II trials, GO received accelerated approval for treating patients aged 60 and older with CD33-positive acute myeloid leukemia (AML) who are unable to use other cytotoxic chemotherapy8,86. The overall response rates (ORR) of GO were 26%–30% and the side effects contained hepatic veno-occlusive disease and delayed hematopoietic recovery86,87. Meanwhile, one phase III trial (NCT00085709) tested the addition of GO during induction therapy in patients under the age of 61 and no significant benefit of GO was observed88. Moreover, toxic effects were observed including hepatotoxicity, infusion reactions and pulmonary toxicity88. These clinical results lead to Pfizer's voluntary withdrawal of GO in 20107. After dose optimization (patients receive three doses of 3 mg/m2 GO instead of one dose of 9 mg/m2 GO before), the FDA re-approved GO in 201750.
Figure 3.
Structures of (A) gemtuzumab ozogamicin. (B) brentuximab vedotin. (C) ado-trastuzumab emtansine. (D) inotuzumab ozogamicin, and (E) polatuzumab vedotin-piiq.
5.2. Brentuximab vedotin
The second ADC approved by the FDA is brentuximab vedotin (BV; Adcetris®, Fig. 3B)41, which is made by conjugation of MMAE and an anti-CD30 antibody through a protease-cleavable dipeptide linker34. Because of the results of phase II trials, 75% ORR in relapsed Hodgkin's lymphoma89 and 86% ORR in systemic anaplastic large cell lymphoma90, BV received accelerated approval in 2011. Adverse events mainly contained neuropathy, neutropenia, anemia and thrombocytopenia89,90. Among them, neuropathy was the most frequent adverse event which happened in patients treated with BV56. According to the encouraging results of the phase III trial (AETHERA, NCT01100502) that investigated the utilization of BV as consolidation treatment in Hodgkin's lymphoma, BV received the full approval in 201591.
5.3. Ado-trastuzumab emtansine
Ado-trastuzumab emtansine (T-DM1; Kadcyla®, Fig. 3C) is the third ADC on market introduced in 201392. T-DM1 is consisted of maytansinoid DM1 and the anti-HER2 antibody43. T-DM1 received approval according to the phase III trial (EMILIA, NCT00829166)93,94. In T-DM1 arm, the median duration of progression-free survival (PFS) was 9.6 months and in active comparator, the median duration of PFS was 6.4 months (P < 0.001)94. The overall survival (OS), which is 30.9 months versus 25.1 months (P < 0.001), and the ORR, which is 43.6% versus 30.8% (P < 0.001) also supported the T-DM1 over comparator93. Furthermore, the overall rate of adverse events was lower in the T-DM1 arm (40.8%) than in the comparator arm (57.0%), as well as the rate of serious adverse events (15.5% versus 18.0%)93.
5.4. Inotuzumab ozogamicin
Inotuzumab ozogamicin (InO; Besponsa®, Fig. 3D) is the fourth approved ADC drug introduced in 201751. InO is composed of calicheamicin derivative and the anti-CD22 antibody through a cleavable hydrazone linker51. InO received the FDA approval according to the results of the phase III trial (INO-VATE, NCT01564784)95. In this trial, acute lymphocytic leukemia patients were randomized and treated with InO or a defined investigator's choice94. The complete remission was 80.7% in the InO arm vs. 29.4% in the comparator arm (P < 0.001). In the InO arm, the PFS was 5.0 months, while only 1.7 months in the comparator arm (P < 0.001)94,95. Several other phase III studies are currently ongoing including the combination with frontline therapy (NCT03150693) and post-induction chemotherapy (NCT03959085).
5.5. Polatuzumab vedotin-piiq
The most recent ADC on market (in June 2019) is polatuzumab vedotin-piiq (Polivy®, Fig. 3E), prepared by conjugation of MMAE to an anti-CD79b antibody through a protease-cleavable dipeptide linker96. According to the results of the phase Ib/II GO29365 study (NCT02257567), polatuzumab vedotin-piiq received the accelerated approval97. In this trial, large B-cell lymphoma patients were randomized and treated with polatuzumab vedotin plus bendamustine and rituximab (BR) or BR alone97. The complete response rate was 40% in polatuzumab vedotin plus BR arm, compared to 18% in BR alone arm97. Objective response rate was 45% in the polatuzumab vedotin plus BR arm, compared to 18% in the BR alone arm97.
5.6. Rovalpituzumab tesirine
Rovalpituzumab tesirine (Rova-T) is an ADC that utilizes a cleavable dipeptide linker for conjugating PBD dimer to the anti-delta-like protein 3 (DLL3) antibody98. In a phase I trial (NCT01901653), dose escalation test of pharmacokinetics, safety and preliminary efficacy of Rova-T were evaluated in recurrent small cell lung cancer patients99,100. The maximum tolerated dose (MTD) was 0.4 mg/kg, the ORR was 17%, the duration of response (DOR) was 2.89 months, the clinical benefit rate (CBR) was 58%, the PFS was 2.79 months and the OS was 4.76 months99. Until now, two phase III studies about Rova-T are active including the comparison study with topotecan (NCT03061812) and a maintenance treatment for small cell lung cancer (NCT03033511).
5.7. Mirvetuximab soravtansine
Mirvetuximab soravtansine comprises an anti-folate receptor alpha (FRα) antibody conjugating to DM4 through a cleavable disulfide linker101. In a phase I trial, activity and safety of mirvetuximab soravtansine were evaluated in ovarian or peritoneal cancer patients102. The confirmed ORR was 26%, the median PFS was 4.8 months and the median DOR was 19.1 weeks102. Especially, for the patients who received three or fewer prior lines of treatment, the ORR, PFS and DOR were 39%, 6.7 months and 19.6 weeks respectively102. Furthermore, the adverse events including fatigue (30%), nausea (37%), blurred vision (41%) and diarrhea (44%) were mainly grade 1 or 2103. Currently, one phase III study is active to compare with investigator's choice of chemotherapy (NCT02631876).
5.8. Depatuxizumab mafodotin
Depatuxizumab mafodotin is prepared by conjugation of MMAF to an anti-epidermal growth factor receptor (EGFR) antibody through a non-cleavable linker103. In a phase I study (NCT01800695), pharmacokinetics, effect and safety of depatuxizumab mafodotin plus temozolomide were evaluated in patients with glioblastoma multiforme104. The most frequent adverse events were photophobia (35%), fatigue (38%) and blurred vision (63%)104. The 6-month OS rate was 69.1%, the 6-month PFS rate was 25.2% and the ORR was 14.3%104. Based on the encouraging results, a phase II trial (NCT02343406) and a phase III trial (NCT02573324) are ongoing to test the therapeutic effect in newly diagnosed or recurrent glioblastoma.
5.9. Sacituzumab govitecan
Sacituzumab govitecan is consisted of an anti-tumor-associated calcium signal transducer 2 (Trop-2) antibody and the SN-38 via an acid-labile ester linker105. A phase I/II trial (NCT01631552) showed that the median PFS was 5.5 months, the response rate was 33.3%, the CBR was 45.4%, the median DOR was 7.7 months, and the OS was 13.0 months106. Besides, frequent grade ≥3 adverse events included anemia and neutropenia106. Furthermore, two phase III trials are currently active to cure triple-negative breast cancer (NCT02574455) and HR+/HER2– metastatic breast cancer (NCT03901339).
6. Challenges for clinical applications of ADCs
The approval of GO, BV, T-DM1, InO, and polatuzumab vedotin-piiq have boosted the quantity of ADCs in clinical trials. Up to now, more than 80 ADCs were examined in a wide variety of clinical trials107. However, the clinical trials for approximately 55 ADCs have been terminated107. There are many challenges for the clinical applications of ADCs, among which toxic effects are most formidable15,107. The toxicity of ADCs is mainly caused by the chemotherapeutic drugs15. For example, MMAE conjugated drugs induce neutropenia and peripheral neuropathy, MMAF causes ocular toxicities and thrombocytopenia; DM1 is associated with neutropenia, gastrointestinal effects and thrombocytopenia, DM4 mainly causes ocular toxicity; calicheamicin conjugated drugs suggest thrombocytopenia and hepatic dysfunction as frequent toxicity15. There are several approaches to decrease toxic effects. The most practical method is to tune the dosing regimen107. For instance, after a phase III trial with fractionated dosing, the FDA re-approved GO50. Another way to maximize the therapeutic index is to use biomarkers to select the right patient population108,109, monitor response signals in early stage107,110, or guide the combination therapy111,112.
Another challenge is the specificity of antibodies113. Based on the rationale, target antigens need to express high levels in tumors and minimal expression in healthy tissues, thus making the target antigen tumor-specific13. However, most tumor antigens also express in normal tissues, which makes antigens tumor-associated rather than tumor-specific113. For example, the major toxicity of SGN-15 (also known as BR-96 doxorubicin), which is consisted of doxorubicin and anti-Lewis Y antibody, is hemorrhagic gastritis114. The primary cause of hemorrhagic gastritis is the expression of Lewis Y antigen in the gastric mucosa cells114. Another example is the bivatuzumab mertansine, which targets the CD44v6 antigen115. In a phase I trial (NCT02254018), fatal exfoliate of skin toxicity was observed because the target antigen was also expressed in the deep layers of skin115.
Lastly, current preclinical models cannot predict ADCs’ activity in human patients113. Although a large number of ADCs show therapeutic benefits in rodent tumor models, many of them are not effective in the clinic. One reason is the difference between rodent and human antigens116. To solve this challenge, it is essential to comprehensively characterize human antigen and carefully select its corresponding antibody78.
7. Future directions of antibody drug conjugates
ADCs represent a rapidly increasing field in cancer therapy. Various ADCs technologies developed over the past decade have created a large variety of possibilities for designing new ADCs13. For instance, promising antigen targets are uncovered for both solid and hematologic tumors57,117. Plenty of highly potent drugs have been discovered including microtubule inhibitors, anthracyclines, and amatoxins, which may become important complements of auristatins and maytansinoids75, 76, 77,79,80. New generation linkers have been characterized in order to improve the therapeutic window of ADCs13,57,118, 119, 120. Future directions include bispecific ADCs that are designed to increase both potency and selectivity121, 122, 123, or deliver multiple classes of payloads124. Furthermore, the combination strategies are currently explored in many clinical trials, such as combining with checkpoint inhibitors (NCT02605915, NCT01896999, NCT02581631, NCT02684292, and NCT02572167) and traditional chemotherapies (NCT03959085, NCT03187210, NCT01476410, and NCT01771107). Although there remain many obstacles to overcome, the development of new ADCs provides tremendous opportunities for future cancer treatment.
Acknowledgments
This work was supported by the start-up fund from the College of Pharmacy at The Ohio State University.
Author contributions
Pengxuan Zhao wrote the paper. Yuebao Zhang, Wenqing Li, and Christopher Jeanty edited the paper. Guangya Xiang provided constructive advice and edited the paper. Yizhou Dong conceived the review topic and wrote the paper.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Guangya Xiang, Email: youjiti@mails.tjmu.edu.cn.
Yizhou Dong, Email: dong.525@osu.edu.
References
- 1.Miller D.R. A tribute to Sidney Farber–the father of modern chemotherapy. Br J Haematol. 2006;134:20–26. doi: 10.1111/j.1365-2141.2006.06119.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchikama K., An Z. Antibody–drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9:33–46. doi: 10.1007/s13238-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert J.M., Berkenblit A. Antibody–drug conjugates for cancer treatment. Annu Rev Med. 2018;69:191–207. doi: 10.1146/annurev-med-061516-121357. [DOI] [PubMed] [Google Scholar]
- 4.Chari R.V., Miller M.L., Widdison W.C. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 5.Frei E. Combination cancer therapy: presidential address. Cancer Res. 1972;32:2593–2607. [PubMed] [Google Scholar]
- 6.Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 7.Diamantis N., Banerji U. Antibody–drug conjugates—an emerging class of cancer treatment. Br J Cancer. 2016;114:362–367. doi: 10.1038/bjc.2015.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sievers E.L., Senter P.D. Antibody–drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 9.Abdollahpour-Alitappeh M., Lotfinia M., Gharibi T., Mardaneh J., Farhadihosseinabadi B., Larki P. Antibody–drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physiol. 2019;234:5628–5642. doi: 10.1002/jcp.27419. [DOI] [PubMed] [Google Scholar]
- 10.Dosio F., Brusa P., Cattel L. Immunotoxins and anticancer drug conjugate assemblies: the role of the linkage between components. Toxins (Basel) 2011;3:848–883. doi: 10.3390/toxins3070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chari R.V. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Adv Drug Deliv Rev. 1998;31:89–104. doi: 10.1016/s0169-409x(97)00095-1. [DOI] [PubMed] [Google Scholar]
- 12.Sievers E.L., Linenberger M. Mylotarg: antibody-targeted chemotherapy comes of age. Curr Opin Oncol. 2001;13:522–527. doi: 10.1097/00001622-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Beck A., Goetsch L., Dumontet C., Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 14.Sau S., Alsaab H.O., Kashaw S.K., Tatiparti KIyer A.K. Advances in antibody–drug conjugates: a new era of targeted cancer therapy. Drug Discov Today. 2017;22:1547–1556. doi: 10.1016/j.drudis.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody–drug conjugates. mAbs. 2016;8:659–671. doi: 10.1080/19420862.2016.1156829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shefet-Carasso L., Benhar I. Antibody-targeted drugs and drug resistance—challenges and solutions. Drug Resist Updat. 2015;18:36–46. doi: 10.1016/j.drup.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M., Bryan W.J., Miller L.L., Chang A.Y., Gradishar W.J., Kufe D.W. Phase II study of adozelesin in untreated metastatic breast cancer. Anticancer Drugs. 1998;9:779–782. doi: 10.1097/00001813-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lambert J.M. Typical antibody–drug conjugates. In: Olivier K.J., Hurvitz S.A., editors. Antibody–drug conjugates: fundamentals, drug development, and clinical outcomes to target cancer. John Wiley & Sons; 2016. pp. 1–32. [Google Scholar]
- 19.Kovtun Y.V., Audette C.A., Ye Y., Xie H., Ruberti M.F., Phinney S.J. Antibody–drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66:3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 20.García-Alonso S., Ocaña A., Pandiella A. Resistance to antibody–drug conjugates. Cancer Res. 2018;78:2159–2165. doi: 10.1158/0008-5472.CAN-17-3671. [DOI] [PubMed] [Google Scholar]
- 21.Beck A., Haeuw J.F., Wurch T., Goetsch L., Bailly C., Corvaia N. The next generation of antibody–drug conjugates comes of age. Discov Med. 2010;10:329–339. [PubMed] [Google Scholar]
- 22.Adams G.P., Schier R., McCall A.M., Simmons H.H., Horak E.M., Alpaugh R.K. High affinity restricts the localization and tumor penetration of single-chain Fv antibody molecules. Cancer Res. 2001;61:4750–4755. [PubMed] [Google Scholar]
- 23.Thurber G.M., Schmidt M.M., Wittrup K.D. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher D., Helma J., Schneider A.F., Leonhardt H., Hackenberger C.P. Nanobodies: chemical functionalization strategies and intracellular applications. Angew Chem Int Ed. 2018;57:2314–2333. doi: 10.1002/anie.201708459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidarsson G., Dekkers G., Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520–537. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayat S.M., Sahebkar A. Antibody–drug conjugates: smart weapons against cancer. Arch Med Sci. 2019;15:1–6. doi: 10.5114/aoms.2019.83020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holliger P., Hudson P.J. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 28.Hassanzadeh-Ghassabeh G., Devoogdt N., De Pauw P., Vincke C., Muyldermans S. Nanobodies and their potential applications. Nanomedicine. 2013;8:1013–1026. doi: 10.2217/nnm.13.86. [DOI] [PubMed] [Google Scholar]
- 29.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira S., Heukers R., Sornkom J., Kok R.J., van Bergen En Henegouwen P.M. Targeting tumors with nanobodies for cancer imaging and therapy. J Contr Release. 2013;172:607–617. doi: 10.1016/j.jconrel.2013.08.298. [DOI] [PubMed] [Google Scholar]
- 31.Fang T., Duarte J.N., Ling J., Li Z., Guzman J.S., Ploegh H.L. Structurally defined αMHC-II nanobody–drug conjugates: a therapeutic and imaging system for B-cell lymphoma. Angew Chem Int Ed. 2016;55:2416–2420. doi: 10.1002/anie.201509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters C., Brown S. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35 doi: 10.1042/BSR20150089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birrer M.J., Moore K.N., Betella I., Bates R.C. Antibody–drug conjugate-based therapeutics: state of the science. J Nat Cancer Inst. 2019;111:538–549. doi: 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- 34.Doronina S.O., Toki B.E., Torgov M.Y., Mendelsohn B.A., Cerveny C.G., Chace D.F. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 35.Widdison W.C., Wilhelm S.D., Cavanagh E.E., Whiteman K.R., Leece B.A., Kovtun Y. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49:4392–4408. doi: 10.1021/jm060319f. [DOI] [PubMed] [Google Scholar]
- 36.Smith A.L., Nicolaou K. The enediyne antibiotics. J Med Chem. 1996;39:2103–2117. doi: 10.1021/jm9600398. [DOI] [PubMed] [Google Scholar]
- 37.Elgersma R.C., Coumans R.G., Huijbregts T., Menge W.M., Joosten J.A., Spijker H.J. Design, synthesis, and evaluation of linker-duocarmycin payloads: toward selection of HER2-targeting antibody–drug conjugate SYD985. Mol Pharm. 2015;12:1813–1835. doi: 10.1021/mp500781a. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland M.S., Walter R.B., Jeffrey S.C., Burke P.J., Yu C., Kostner H. SGN-CD33A: a novel CD33-targeting antibody–drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- 39.Luesch H., Harrigan G., Goetz G., Horgen F. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr Med Chem. 2002;9:1791–1806. doi: 10.2174/0929867023369051. [DOI] [PubMed] [Google Scholar]
- 40.Pettit G.R., Kamano Y., Herald C.L., Tuinman A.A., Boettner F.E., Kizu H. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J Am Chem Soc. 1987;109:6883–6885. [Google Scholar]
- 41.Senter P.D., Sievers E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 42.Dan N., Setua S., Kashyap V., Khan S., Jaggi M., Yallapu M. Antibody–drug conjugates for cancer therapy: chemistry to clinical implications. Pharmaceuticals. 2018;11:1–22. doi: 10.3390/ph11020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert J.M., Chari R.V.J. Ado-trastuzumab Emtansine (T-DM1): an antibody–drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57:6949–6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 44.Chen H., Lin Z., Arnst K., Miller D., Li W. Tubulin inhibitor-based antibody–drug conjugates for cancer therapy. Molecules. 2017;22:1–28. doi: 10.3390/molecules22081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Issell B.F., Crooke S.T. Maytansine. Cancer Treat Rev. 1978;5:199–207. doi: 10.1016/s0305-7372(78)80014-0. [DOI] [PubMed] [Google Scholar]
- 46.Lambert J.M. Drug-conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol. 2013;76:248–262. doi: 10.1111/bcp.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez H.L., Cardarelli P.M., Deshpande S., Gangwar S., Schroeder G.M., Vite G.D. Antibody–drug conjugates: current status and future directions. Drug Discov Today. 2014;19:869–881. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Shor B., Gerber H.P., Sapra P. Preclinical and clinical development of inotuzumab–ozogamicin in hematological malignancies. Mol Immunol. 2015;67:107–116. doi: 10.1016/j.molimm.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Damle N.K., Frost P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr Opin Pharmacol. 2003;3:386–390. doi: 10.1016/s1471-4892(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 50.Jen E.Y., Ko C.W., Lee J.E., Del Valle P.L., Aydanian A., Jewell C. FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res. 2018;24:3242–3246. doi: 10.1158/1078-0432.CCR-17-3179. [DOI] [PubMed] [Google Scholar]
- 51.Lamb Y.N. Inotuzumab ozogamicin: first global approval. Drugs. 2017;77:1603–1610. doi: 10.1007/s40265-017-0802-5. [DOI] [PubMed] [Google Scholar]
- 52.Hughes B. Antibody–drug conjugates for cancer: poised to deliver?. Nat Rev Drug Discov. 2010;9:665–667. doi: 10.1038/nrd3270. [DOI] [PubMed] [Google Scholar]
- 53.Teicher B.A., Chari R.V. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:6389–6397. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 54.Doronina S.O., Mendelsohn B.A., Bovee T.D., Cerveny C.G., Alley S.C., Meyer D.L. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–124. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 55.Erickson H.K., Park P.U., Widdison W.C., Kovtun Y.V., Garrett L.M., Hoffman K. Antibody–maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66:4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- 56.Younes A., Bartlett N.L., Leonard J.P., Kennedy D.A., Lynch C.M., Sievers E.L. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 57.Senter P.D. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13:235–244. doi: 10.1016/j.cbpa.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Mason S.D., Joyce J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chudasama V., Maruani A., Caddick S. Recent advances in the construction of antibody–drug conjugates. Nat Chem. 2016;8:114–119. doi: 10.1038/nchem.2415. [DOI] [PubMed] [Google Scholar]
- 60.Junutula J.R., Raab H., Clark S., Bhakta S., Leipold D.D., Weir S. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 61.Lyons A., King D.J., Owens R.J., Yarranton G.T., Millican A., Whittle N.R. Site-specific attachment to recombinant antibodies via introduced surface cysteine residues. Protein Eng. 1990;3:703–708. doi: 10.1093/protein/3.8.703. [DOI] [PubMed] [Google Scholar]
- 62.Sunbul M., Yin J. Site specific protein labeling by enzymatic posttranslational modification. Org Biomol Chem. 2009;7:3361–3371. doi: 10.1039/b908687k. [DOI] [PubMed] [Google Scholar]
- 63.Axup J.Y., Bajjuri K.M., Ritland M., Hutchins B.M., Kim C.H., Kazane S.A. Synthesis of site-specific antibody–drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109:16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabuka D., Rush J.S., Gregory W.D., Wu P., Bertozzi C.R. Site-specific chemical protein conjugation using genetically encoded aldehyde tags. Nat Protoc. 2012;7:1052–1067. doi: 10.1038/nprot.2012.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young T.S., Ahmad I., Yin J.A., Schultz P.G. An enhanced system for unnatural amino acid mutagenesis in E. coli. J Mol Biol. 2010;395:361–374. doi: 10.1016/j.jmb.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 66.Jeffrey S.C., Burke P.J., Lyon R.P., Meyer D.W., Sussman D., Anderson M. A potent anti-CD70 antibody–drug conjugate combining a dimeric pyrrolobenzodiazepine drug with site-specific conjugation technology. Bioconjug Chem. 2013;24:1256–1263. doi: 10.1021/bc400217g. [DOI] [PubMed] [Google Scholar]
- 67.Shen B.Q., Xu K., Liu L., Raab H., Bhakta S., Kenrick M. Conjugation site modulates the in vivo stability and therapeutic activity of antibody–drug conjugates. Nat Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 68.Schumacher D., Hackenberger C.P., Leonhardt H., Helma J. Current status: site-specific antibody drug conjugates. J Clin Immunol. 2016;36:100–107. doi: 10.1007/s10875-016-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albers A.E., Garofalo A.W., Drake P.M., Kudirka R., de Hart G.W., Barfield R.M. Exploring the effects of linker composition on site-specifically modified antibody–drug conjugates. Eur J Med Chem. 2014;88:3–9. doi: 10.1016/j.ejmech.2014.08.062. [DOI] [PubMed] [Google Scholar]
- 70.Drake P.M., Albers A.E., Baker J., Banas S., Barfield R.M., Bhat A.S. Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes. Bioconjug Chem. 2014;25:1331–1341. doi: 10.1021/bc500189z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kline T., Steiner A.R., Penta K., Sato A.K., Hallam T.J., Yin G. Methods to make homogenous antibody drug conjugates. Pharm Res. 2015;32:3480–3493. doi: 10.1007/s11095-014-1596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian F., Lu Y., Manibusan A., Sellers A., Tran H., Sun Y. A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci U S A. 2014;111:1766–1771. doi: 10.1073/pnas.1321237111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray B.C., Peterson M.T., Fecik R.A. Chemistry and biology of tubulysins: antimitotic tetrapeptides with activity against drug resistant cancers. Nat Prod Rep. 2015;32:654–662. doi: 10.1039/c4np00036f. [DOI] [PubMed] [Google Scholar]
- 74.Yu S.F., Zheng B., Go M., Lau J., Spencer S., Raab H. A novel anti-CD22 anthracycline-based antibody–drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21:3298–3306. doi: 10.1158/1078-0432.CCR-14-2035. [DOI] [PubMed] [Google Scholar]
- 75.Vetter J. Toxins of amanita phalloides. Toxicon. 1998;36:13–24. doi: 10.1016/s0041-0101(97)00074-3. [DOI] [PubMed] [Google Scholar]
- 76.Sasse F., Sieinmetz H., Heil J., Hoefle G., Reichenbach H. Tubulysins, new cytostatic peptides from myxobacteria acting on microtubuli. J Antibiot. 2000;53:879–885. doi: 10.7164/antibiotics.53.879. [DOI] [PubMed] [Google Scholar]
- 77.Patterson A.W., Peltier H.M., Sasse F., Ellman J.A. Design, synthesis, and biological properties of highly potent tubulysin D analogues. Chemistry. 2007;13:9534–9541. doi: 10.1002/chem.200701057. [DOI] [PubMed] [Google Scholar]
- 78.Cohen R., Vugts D.J., Visser G.W., Stigter-van Walsum M., Bolijn M., Spiga M. Development of novel ADCs: conjugation of tubulysin analogues to trastuzumab monitored by dual radiolabeling. Cancer Res. 2014;74:5700–5710. doi: 10.1158/0008-5472.CAN-14-1141. [DOI] [PubMed] [Google Scholar]
- 79.Quintieri L., Geroni C., Fantin M., Battaglia R., Rosato A., Speed W. Formation and antitumor activity of PNU-159682, a major metabolite of nemorubicin in human liver microsomes. Clin Cancer Res. 2005;11:1608–1617. doi: 10.1158/1078-0432.CCR-04-1845. [DOI] [PubMed] [Google Scholar]
- 80.Mazzini S., Scaglioni L., Mondelli R., Caruso M., Sirtori F.R. The interaction of nemorubicin metabolite PNU-159682 with DNA fragments d (CGTACG) 2, d (CGATCG) 2 and d (CGCGCG) 2 shows a strong but reversible binding to G:C base pairs. Bioorg Med Chem. 2012;20:6979–6988. doi: 10.1016/j.bmc.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 81.Pfeifer M., Zheng B., Erdmann T., Koeppen H., McCord R., Grau M. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia. 2015;29:1578–1586. doi: 10.1038/leu.2015.48. [DOI] [PubMed] [Google Scholar]
- 82.Han A.Q., Olson W.C. Next-generation antibody–drug conjugate technologies. In: Olivier K.J., Hurvitz S.A., editors. Antibody–drug conjugates: fundamentals, drug development, and clinical outcomes to target cancer. John Wiley & Sons; 2016. pp. 473–503. [Google Scholar]
- 83.Moldenhauer G., Salnikov A.V., Lüttgau S., Herr I., Anderl J., Faulstich H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J Natl Cancer Inst. 2012;104:622–634. doi: 10.1093/jnci/djs140. [DOI] [PubMed] [Google Scholar]
- 84.Sharkey R.M., Goldenberg D.M. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev. 2008;60:1407–1420. doi: 10.1016/j.addr.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bross P.F., Beitz J., Chen G., Chen X.H., Duffy E., Kieffer L. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 86.Sievers E.L., Larson R.A., Stadtmauer E.A., Estey E., Löwenberg B., Dombret H. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 87.Giles F.J., Kantarjian H.M., Kornblau S.M., Thomas D.A., Garcia-Manero G., Waddelow T.A. Mylotarg™ (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer. 2001;92:406–413. doi: 10.1002/1097-0142(20010715)92:2<406::aid-cncr1336>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 88.Petersdorf S.H., Kopecky K.J., Slovak M., Willman C., Nevill T., Brandwein J. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Younes A., Gopal A.K., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pro B., Advani R., Brice P., Bartlett N.L., Rosenblatt J.D., Illidge T. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 91.Moskowitz C.H., Nademanee A., Masszi T., Agura E., Holowiecki J., Abidi M.H. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 92.Amiri-Kordestani L., Blumenthal G.M., Xu Q.C., Zhang L., Tang S.W., Ha L. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20:4436–4441. doi: 10.1158/1078-0432.CCR-14-0012. [DOI] [PubMed] [Google Scholar]
- 93.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kantarjian H.M., DeAngelo D.J., Stelljes M., Martinelli G., Liedtke M., Stock W. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DiJoseph J.F., Armellino D.C., Boghaert E.R., Khandke K., Dougher M.M., Sridharan L. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103:1807–1814. doi: 10.1182/blood-2003-07-2466. [DOI] [PubMed] [Google Scholar]
- 96.Deeks E.D. Polatuzumab vedotin: first global approval. Drugs. 2019;79:1467–1475. doi: 10.1007/s40265-019-01175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sehn L., Flowers C., McMillan A., Morschhauser F., Salles G., Felizzi F. Estimation of long-term survival with polatuzumab vedotin plus bendamustine and rituximab for patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) Hematol Oncol. 2019;37:257–258. [Google Scholar]
- 98.Saunders L.R., Bankovich A.J., Anderson W.C., Aujay M.A., Bheddah S., Black K. A DLL3-targeted antibody–drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7:1–14. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pietanza M., Spigel D., Bauer T., Ready N., Glisson B., Morgensztern D. 7LBA Safety, activity, and response durability assessment of single agent rovalpituzumab tesirine, a delta-like protein 3 (DLL3)-targeted antibody drug conjugate (ADC), in small cell lung cancer (SCLC) Eur J Cancer. 2015;51:712. [Google Scholar]
- 100.Rudin C.M., Pietanza M.C., Bauer T.M., Spigel D.R., Ready N., Morgensztern D. Safety and efficacy of single-agent rovalpituzumab tesirine (SC16LD6.5), a delta-like protein 3 (DLL3)-targeted antibody–drug conjugate (ADC) in recurrent or refractory small cell lung cancer (SCLC) J Clin Oncol. 2016;34:8505. [Google Scholar]
- 101.Ab O., Whiteman K.R., Bartle L.M., Sun X., Singh R., Tavares D. IMGN853, a folate receptor-α (FRα)–targeting antibody–drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors. Mol Cancer Ther. 2015;14:1605–1613. doi: 10.1158/1535-7163.MCT-14-1095. [DOI] [PubMed] [Google Scholar]
- 102.Moore K.N., Martin L.P., O'Malley D.M., Matulonis U.A., Konner J.A., Perez R.P. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha–targeting antibody–drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol. 2017;35:1112–1118. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Den Bent M.J., Gan H.K., Lassman A.B., Kumthekar P., Merrell R., Butowski N. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80:1209–1217. doi: 10.1007/s00280-017-3451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lassman A.B., Van Den Bent M.J., Gan H.K., Reardon D.A., Kumthekar P., Butowski N. Safety and efficacy of depatuxizumab mafodotin+temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial. Neuro Oncol. 2018;21:106–114. doi: 10.1093/neuonc/noy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldenberg D.M., Cardillo T.M., Govindan S.V., Rossi E.A., Sharkey R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody–drug conjugate (ADC) Oncotarget. 2015;6:22496–22512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bardia A., Mayer I.A., Vahdat L.T., Tolaney S.M., Isakoff S.J., Diamond J.R. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 107.Coats S., Williams M., Kebble B., Dixit R., Tseng L., Yao N.S. Antibody drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res. 2019;25:5441–5448. doi: 10.1158/1078-0432.CCR-19-0272. [DOI] [PubMed] [Google Scholar]
- 108.Bergstralh D.T., Sekelsky J. Interstrand crosslink repair: can XPF-ERCC1 be let off the hook?. Trends Genet. 2008;24:70–76. doi: 10.1016/j.tig.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Deans A.J., West S.C. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raja R., Kuziora M., Brohawn P.Z., Higgs B.W., Gupta A., Dennis P.A. Early reduction in ctDNA predicts survival in patients with lung and bladder cancer treated with durvalumab. Clin Cancer Res. 2018;24:6212–6222. doi: 10.1158/1078-0432.CCR-18-0386. [DOI] [PubMed] [Google Scholar]
- 111.Emens L., Esteva F., Beresford M. Results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC) Cancer Res. 2018;79 PD3-1. [Google Scholar]
- 112.Matulonis U., Moore K., Martin L., Vergote I., Castro C., Gilbert L. 949P mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody–drug conjugate (ADC), with pembrolizumab in platinum-resistant ovarian cancer (PROC): initial results of an expansion cohort from FORWARD II, a phase Ib study. Ann Oncol. 2018;29:339. [Google Scholar]
- 113.Tolcher A. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol: Off J Eur Soc Med Oncol. 2016;27:2168–2172. doi: 10.1093/annonc/mdw424. [DOI] [PubMed] [Google Scholar]
- 114.Saleh M.N., Sugarman S., Murray J., Ostroff J.B., Healey D., Jones D. Phase I trial of the anti-Lewis Y drug immunoconjugate BR96–doxorubicin in patients with Lewis Y-expressing epithelial tumors. J Clin Oncol. 2000;18:2282–2292. doi: 10.1200/JCO.2000.18.11.2282. [DOI] [PubMed] [Google Scholar]
- 115.Tijink B.M., Buter J., De Bree R., Giaccone G., Lang M.S., Staab A. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12:6064–6072. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- 116.Mukherjee A., Waters A.K., Babic I., Nurmemmedov E., Glassy M.C., Kesari S. Antibody drug conjugates: progress, pitfalls, and promises. Hum Antibodies. 2019;27:53–62. doi: 10.3233/HAB-180348. [DOI] [PubMed] [Google Scholar]
- 117.Alley S.C., Okeley N.M., Senter P.D. Antibody–drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 118.Kovtun Y.V., Audette C.A., Mayo M.F., Jones G.E., Doherty H., Maloney E.K. Antibody–maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70:2528–2537. doi: 10.1158/0008-5472.CAN-09-3546. [DOI] [PubMed] [Google Scholar]
- 119.Lyon R.P., Bovee T.D., Doronina S.O., Burke P.J., Hunter J.H., Neff-LaFord H.D. Reducing hydrophobicity of homogeneous antibody–drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol. 2015;33:733–735. doi: 10.1038/nbt.3212. [DOI] [PubMed] [Google Scholar]
- 120.Widdison W.C., Ponte J.F., Coccia J.A., Lanieri L., Setiady Y., Dong L. Development of anilino–maytansinoid ADCs that efficiently release cytotoxic metabolites in cancer cells and induce high levels of bystander killing. Bioconjug Chem. 2015;26:2261–2278. doi: 10.1021/acs.bioconjchem.5b00430. [DOI] [PubMed] [Google Scholar]
- 121.Sheng W., Shang Y., Li L., Zhen Y. An EGFR/CD13 bispecific fusion protein and its enediyne-energized analog show potent antitumor activity. Anti Cancer Drugs. 2014;25:82–91. doi: 10.1097/CAD.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 122.Thornlow D.N., Cox E.C., Walker J.A., Sorkin M., Plesset J.B., DeLisa M.P. Dual site-specific antibody conjugates for sequential and orthogonal cargo release. Bioconjug Chem. 2019;30:1702–1710. doi: 10.1021/acs.bioconjchem.9b00244. [DOI] [PubMed] [Google Scholar]
- 123.Walker J.A., Bohn J.J., Ledesma F., Sorkin M.R., Kabaria S.R., Thornlow D.N. Substrate design enables heterobifunctional, dual “click” antibody modification via microbial transglutaminase. Bioconjug Chem. 2019;30:2452–2457. doi: 10.1021/acs.bioconjchem.9b00522. [DOI] [PubMed] [Google Scholar]
- 124.Maruani A., Smith M.E., Miranda E., Chester K.A., Chudasama V., Caddick S. A plug-and-play approach to antibody-based therapeutics via a chemoselective dual click strategy. Nat Commun. 2015;6:1–9. doi: 10.1038/ncomms7645. [DOI] [PMC free article] [PubMed] [Google Scholar]