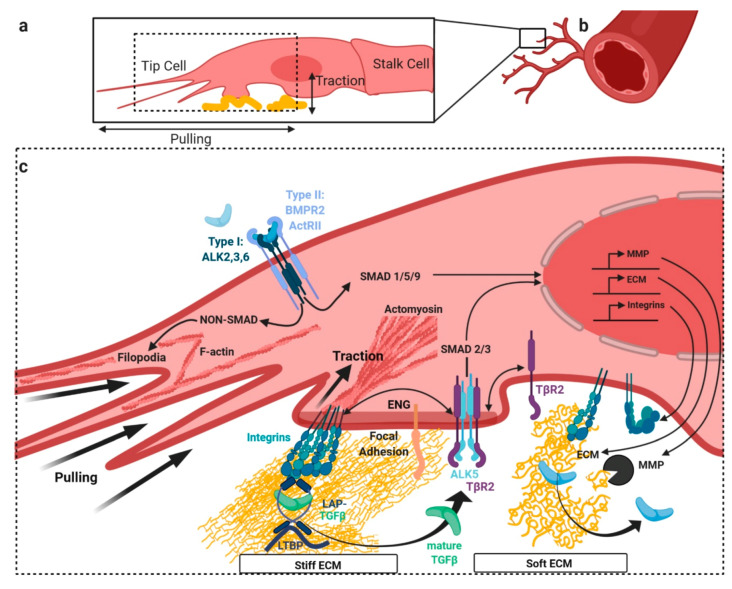
Figure 3.
Crosstalk with the TGFβ/BMP SMAD pathway at focal adhesions (FA). In angiogenesis tip-cells at the sprouting front (a) emerging from pre-existing blood vessels (b) display enhanced FA-ECM contacts and filamentous (F-) actin driven filopodia (c). Exemplified at the sprouting front (lower caption), actin reorganization is in part orchestrated by non-SMAD signaling. FAs influence mechanical properties of the ECM, including stiffness. These contacts are mainly mediated by integrins, providing the cell with inside-out signaling properties. Integrins can directly bind ECM molecules outside the cell and are indirectly bound to the intracellular contractile actomyosin cytoskeleton. Endoglin (ENG) interacts with both, integrins and signaling receptors. TGFβ in its latent form (LAP-TGFβ) is bound to ECM filaments via LTBP. Viscoelastic properties of ECM directly influence integrin-mediated TGFβ retrieval from LAP-TGFβ-LTBP complexes. Integrins release mature TGFβ from the latent complex by exerting pulling forces, through the contraction of the actomyosin cytoskeleton. Stiff ECM allows more efficient release of mature ligand than soft ECM. In a feedback-loop, SMAD signaling in turn regulates the expression of ECM genes, integrins, and MMPs, which support the softening and the release of ligands by degrading the ECM. Abbreviations: ENG—endoglin, LAP—latency associated peptide, ECM—extracellular matrix, LTBP—latent TGFβ- binding protein, and MMPs—matrix metalloproteinases.