Abstract
Simple Summary
Cytokine-induced killer (CIK) cells are a heterogeneous population of polyclonal T effector cells expanded ex vivo. Here, we updated our last review published in 2012 and provided a synopsis of current 15 clinical studies, including 382 patients with renal cell carcinoma (RCC) enrolled in CIK cell immunotherapy. CIK cells exhibited promising synergistic anti-tumor effects when combined with conventional therapies and showed mild adverse effects in patients with RCC. Preclinical researches also identified potential molecular targets that augmented CIK cell cytotoxicity against renal carcinoma cells. In future, large randomized clinical trials should be organized to further evaluate the clinical efficacy and optimize the treatment modality of CIK cells in RCC.
Abstract
There is growing interest in cytokine-induced killer (CIK) cells on the integrated therapy of patients with RCC, especially those in the late stage or refractory to conventional chemotherapy and radiotherapy. In this review, a total of 15 clinical studies including 681 patients enrolled in CIK cell immunotherapy were outlined. Three-hundred-and-eighty-two patients with RCC were treated with CIK cells alone or in combination with DC vaccination, targeted agents sunitinib or sorafenib, and the PD-1 inhibitor pembrolizumab. Significantly improved 3-year overall survival rate was reported in four trials, whereas remarkably longer median progression-free survival was observed in three studies. Adverse reactions were mild and usually controllable fever and fatigue. Besides, preclinical research progresses were reviewed to increase our understanding about the underlying mechanisms of CIK cell cytotoxicity and identify potential targets to enhance their anti-tumor activity. These studies suggest that CIK cell-based immunotherapy has potential clinical benefits with a good safety profile and could become a promising approach in the combined therapies of RCC patients. However, further large-scale studies are required to evaluate the clinical efficacy of CIK cells and more efforts should be performed to identify the optimal CIK cell-based therapeutic regimen for RCC patients.
Keywords: cytokine-induced killer cells, clinical study, renal cell carcinoma, immunotherapy, preclinical research
1. Introduction
Renal cell carcinoma (RCC), among the 10 most frequently diagnosed cancers, acts as one of the most lethal urological malignancies worldwide [1]. The highest age-standardized incidence rate is in North America (11.7 per 100,000), with Western Europe ranking the second (9.8 per 100,000) [1]. Lots of patients with renal masses remain asymptomatic until the late stages and over 60% of RCCs are detected incidentally with abdominal imaging performed for other reasons [2]. Although most detected lesions are small tumors at the time of diagnosis, locally advanced disease continues to be diagnosed in a notable proportion of patients, with distant metastases present in up to 17% of patients [3].
Surgery is the only curative treatment for localized RCC, but metastatic RCC (mRCC) is usually refractory to conventional therapies [4]. For most patients with mRCC, systemic therapy, including targeted therapy and immunotherapy, is necessarily required [2]. There are several targeted drugs that have been approved for the treatment of mRCC. They mainly consist of inhibitors targeting vascular endothelial growth factor (VEGF) and its receptors (bevacizumab, sunitinib, sorafenib, pazopanib, axitinib, tivozanib and cabozantinib) and mammalian target of rapamycin (mTOR) (everolimus and temsirolimus) [5,6]. Until targeted therapies were introduced in 2006, the first-line treatment strategy for mRCC was immunotherapies based on interleukin-2 (IL-2) combined with interferon-α (IFN-α) [7]. In 2018, the CheckMate 214 study reported superiority of the programmed cell death-1(PD-1) inhibitor nivolumab and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor ipilimumab over sunitinib in intermediate- and poor-risk patients. A significantly higher overall survival (OS) and objective response rates were achieved with nivolumab plus ipilimumab than with sunitinib [8]. These findings resulted in an updated recommendation of the first-line management for mRCC patients.
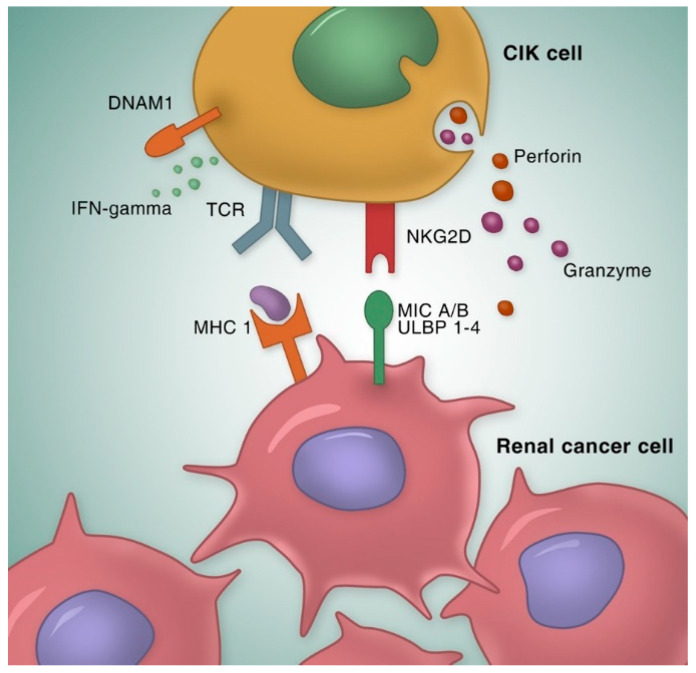
In the past decades, there has been growing interest in adoptive cell-based immunotherapy as a therapeutic option in patients with RCC [9,10,11]. Cytokine-induced killer (CIK) cells, one of the main adoptive cell-based strategies, are a heterogeneous cell population comprising the main effector subset of double positive CD3 and CD56 cells. Schmidt-Wolf et al. first reported the generation of CIK cells ex vivo from peripheral blood mononuclear cells (PBMCs) in 1991, by adding IFN-γ on day 0 and monoclonal antibody against CD3 (anti-CD3 mAb), human recombinant IL-2 and IL-1 on the next day. An increased cytotoxicity of CIK cells against lymphoma was observed in a SCID mouse/human lymphoma model [12]. CIK cells exert a potent major histocompatibility complex (MHC)-unrestricted cytotoxicity against both hematological and solid malignancies with CD3+CD56+ cells as the main effectors. The natural killer group 2 member D (NKG2D) receptor appears to play the most important role in tumor recognition by CIK cells (Figure 1) [13]. NKG2D, a member of the c-type lectin-activating receptor family, recognizes the MHC-class I-like molecules, MICA and MICB and members of the ULBP family (ULPB1-4) that are restrictedly expressed on malignant tissues and mediates subsequently granzyme release [14]. It is also indicated that NK-like structures, including DNAX accessory molecule-1 (DNAM-1) and NKp30 are expressed on CIK cells and involved in the recognition and killing of tumor targets [15]. Furthermore, CIK cells showed reduced allo-reactive potential and minimal graft-versus-host disease (GVHD) activity in several in vitro and in vivo models, compared with conventional T cells [16,17]. This advantage makes CIK cells an appealing and promising alternative to classic donor lymphocyte infusion (DLI) after hematopoietic stem cell trans-plantation (HSCT).
Figure 1.
Mechanisms of CIK cell anti-tumor activity. The majority of CIK cell cytotoxicity is exerted through the interaction between NKG2D and its ligand MIC A/B and ULPB 1–4, resulting in granule exocytosis and secretion of cytokines like IFN-γ. NK-like structures, such as DNAM-1, is also involved in the recognition and killing of tumor targets. Besides, CIK cells can eradicate tumors in an MHC-restricted manner by TCR engagement, which is the so-called “dual-functional capability”. CIK cell, cytokine induced killer cells; NKG2D, natural killer group 2 member D; MIC A/B, MHC class I polypeptide-related sequence A/B; ULPB 1–4, UL16 binding protein 1–4. DNAM-1, DNAX accessory molecule-1; TCR, T-cell receptor; MHC 1, major histocompatibility complex 1; IFN-γ, interferon-γ.
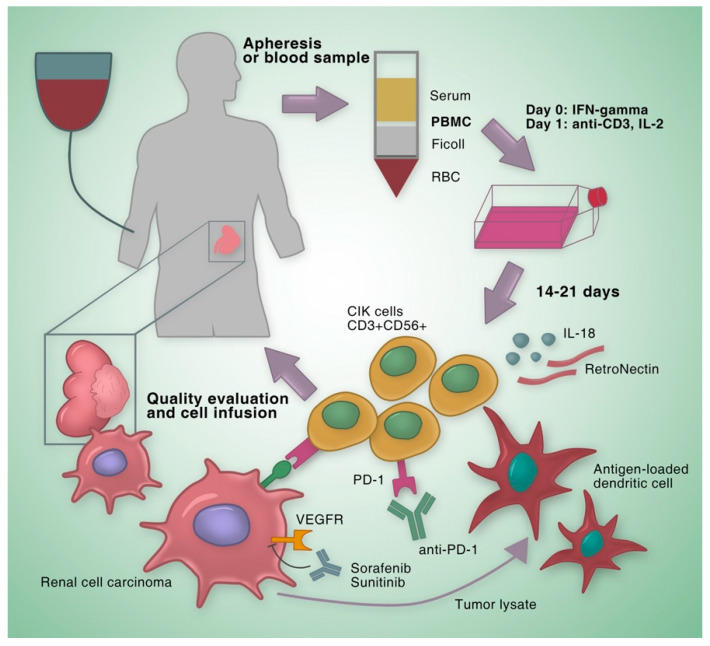
In our last review published in 2012, we summarized eight clinical studies utilizing CIK cells for the treatment of RCC [18]. These studies showed that the combination of CIK cell therapy and standard therapy was superior to standard therapy alone. Since 2012, more efforts have been put to optimize the anti-tumor potency and treatment regimen of CIK cell-based therapies. In the following sections, we will provide an update on our former review by outlining new clinical results of RCC (Figure 2) and presenting progress of adoptive immunotherapy strategies based on CIK cells.
Figure 2.
Strategies for improving clinical curative effects of CIK cells. PBMCs are isolated by density gradient centrifugation and expended with IFN-γ, anti-CD3 and IL-2. After 14–21 days, CIK cells are infused into the patient. The anti-tumor activity of CIK cells can be enhanced by adding cytokines like IL-18 or RetroNectin during expansion. DCs have high ability to present tumor antigens and compensate the lack of tumor antigen specificity of CIK cells. Targeted agents such as sunitinib and sorafenib or immune checkpoint inhibitor anti-PD-1 antibodies also have a synergistic effect with CIK cells. CIK cell, cytokine-induced killer cell; PBMC, peripheral blood mononuclear cell; IFN-γ, interferon-γ; IL-2, interleukin-2; IL-18, interleukin-18; VEGFR, vascular endothelial growth factor receptor; PD-1, programmed cell death-1; DC, dendritic cell; RBC, red blood cell.
2. Clinical Studies on CIK Cells for the Treatment of RCC
A total of 15 clinical studies were reviewed here. Six-hundred-and-eight-one patients representing 55.4% of the total with distinct tumor entities were enrolled in CIK cell immunotherapy. Three-hundred-and-eighty-two patients with RCC were treated with CIK cells alone or in combination with DC vaccination, targeted agents sunitinib or sorafenib, and the PD-1 inhibitor pembrolizumab.
2.1. Clinical Studies on Autologous CIK cells
Twenty patients with unilateral, locally advanced (TNM stage I or II) RCC who had undergone radical nephrectomy were recruited into a study [19]. The patients were randomly divided into two groups, a CIK cell treatment group and a control group. In the CIK cell group, patients received 1–5 × 109 autologous CIK cells intravenously per infusion and 2–10 × 109 CIK cells in total for two consecutive days. Patients were also injected with 20 mg/day thymopentin intramuscularly before CIK cell transfusion and 1 mU rhIL-2 subcutaneously for 10 days after transfusion.
Response Evaluation Criteria in Solid Tumors (RECIST) was used to assess the clinical response every 2 months. In the CIK cell treatment group, a complete response (CR) was achieved in six patients, a partial response (PR) was observed in two patients and stable disease (SD) was remained in two patients. Meanwhile, in the control group, a CR was achieved in five patients, SD remained in three patients, and progressive disease (PD) was observed in two patients. The median progression-free survival (PFS) in the CIK cell group was 32.2 months, which was significantly longer than that of 21.6 months in the control group. However, no statistically significant differences in OS were observed between two groups. All side effects were transient or controllable, including mild arthralgia, laryngeal edema, fatigue, and low-grade fever during lymphocyte infusion or during the early stages of rhIL-2 treatment. Grade 3 or greater adverse events were not observed.
In a retrospective study based on autologous CIK cells, 40 patients with solid tumors were enrolled, including 10 patients with RCC after surgery and chemotherapy [20]. Percentages of CD3+, CD8+ and CD3+CD56+ lymphocyte subsets in patients’ peripheral blood were all significantly increased after CIK cell therapy. The 6-month OS rate was 70.0%, 1-year OS rate was 60.0% and 3-year was 57.5%. A favorable safety profile of CIK cells was observed in this study without severe adverse effect. Only two patients had fever and poor appetite and these symptoms could be relieved by simple treatments.
2.2. Clinical Studies on CIK Cells with Improvement of Culture Methods
Liu et al. reported a case applying modified autologous CIK (mCIK) cells for pleural metastasis of collecting duct carcinoma (CDC) [21]. CDC is a rare but highly aggressive RCC arising from the principal cells of the collecting duct. During the generation of mCIK cells, PBMCs were first primed with anti-CD3 antibody, and a mixture of IL-2, IL-18 and IFN-γ was subsequently added after 3 h. This generation method was distinct from the traditional way in which PBMCs are firstly cultured with IFN-γ, followed 24 h later by the addition of anti-CD3 antibody and IL-2. In every cycle, the patient received intrapleural infusion with modified CIK cells at a dose of 10 × 107 once a day for 5 days. After three cycles of therapy, this patient showed relieved cough, dyspnea, chest distress, thoracalgia, and clearer and less pleural fluid. The only adverse reaction was fever after intrapleural infusion and the patient recovered 2 days later.
Another study investigated the efficacy of RetroNectin-activated autologous CIK cells (R-CIK) combined with conventional therapies (chemotherapy and radiotherapy) in patients with metastatic brain tumors [22]. RetroNectin is a 63 kD fragment of recombinant human fibronectin (also called CH-296) that promotes colocalization of virions and target cells to enhance the efficiency of retroviral mediated gene transduction [23]. RetroNectin-activated CIK cells were infused into the participants (1 patient with RCC, 14 patients with non-small cell lung cancer, 4 patients with breast cancer, and 1 patient with thyroid cancer). The OS rate in patients with all tumor entities was 21.4%. The disease control rate was 78.6%. The study also showed that the median PFS of patients receiving R-CIK was 7.7 months and the median OS was 12.6 months. The one patient with RCC achieved a CR without severe toxicities. OS of this patient was 14 months and PFS was 8 months. The results show RetroNectin could be a potential activator to augment the activity of CIK cells.
A study exclusively including patients with metastatic RCC was performed to explore whether the level of the underlying immune inhibitory factor myeloid-derived suppressor cells (MDSCs) was associated with the prognosis of patients receiving CIK cell therapy [24]. Autologous CIK cells were also activated with RetroNectin during generation. Twenty-nine patients received about 5 × 109 CIK cells and 2 million IU IL-2 per day during the following 5 days in one cycle. The treatment schedule for the study consisted of at least 4 and at most 16 cycles in total. Of all evaluable patients, 4 exhibited a PR, 18 remained SD, and 7 showed PD, without CR observed. Moreover, the 1-year survival was 82.8% (24/29). Peripheral blood MDSCs were elevated in almost all RCC patients compared with healthy volunteers and decreased after CIK cell infusion. Also, patients with a relatively low proportion of MDSCs exhibited significantly prolonged survival, which indicated that MDSCs might serve as a potential marker for the prognosis of RCC patients receiving CIK cell treatment.
2.3. Clinical Studies on CIK Cells Combined with Dendritic Cell Vaccination
Dendritic cell (DC)-based vaccines represent a promising therapy that induces anti-tumor immune by initiating an antigen-specific T lymphocyte response [25]. DCs have high ability to present tumor antigens and compensate the lack of tumor antigen specificity of CIK cells. In a randomized controlled trial of 137 patients with postoperative localized and locally advanced RCC, 46 patients were treated with tumor lysate-pulsed DCs and autologous CIK cells, while 45 patients did not receive any postoperative adjuvant therapy [26]. After five intravenous infusions of tumor lysate-pulsed DC-CIK cells (3 × 108 per infusion), an increase of the CD4+/CD8+ ratio and a decrease of CD4+CD25high cells were observed. The metastasis and recurrence rates were significantly decreased, whereas the overall survival rates were significantly increased in the DC-CIK group. The achieved results showed that tumor lysate-pulsed DC-CIK cells could act as a promising postoperative adjuvant immunotherapy by preventing recurrence or metastasis.
In another study involving 121 patients with primary tumors (7 patients with RCC), patients received 1 × 107 DCs for vaccination once a week for 6 weeks and 1 × 109 autologous CIK cells six times within 4 days [27]. The delayed-type hypersensitivity skin tests detected a positive cell-mediated cytotoxicity response with the rate of 76.9%. Most patients achieved improvements in the quality of life (QoL). The treatment was safe and well tolerated and all side effects were mild and controllable.
Wang et al. reported a clinical trial evaluating the efficacy and safety of genetically modified DCs in combination with autologous CIK cells (gmDC-CIK) on patients with RCC [28]. Twenty-eight patients diagnosed with advanced clear cell RCC (ccRCC) (stageIIIB-IV) were enrolled in this study. On day 6 of cell preparation, DCs were pulsed with RNA encoding antigen muc-1 and survivin. In one cycle, patients received four subcutaneous injections of 2–5 × 107 gmDCs on day 7, 9, 11, and 13 and intravenous infusion of 2–5 × 1010 CIK cells on day 11 and 13. The objective response rate (ORR) was 39% and a disease control rate (DCR) was 75%. DCR, but not ORR, was significantly related with cycles of treatment. Only one patient developed grade 1 fever and no other clinically significant side effect was observed.
In another clinical trial, 60 operable RCC patients were randomized to the autologous tumor lysate-pulsed DC-CIK (Ag-DC-CIK) cell treatment group or the control group, and 62 inoperable patients were randomly assigned to the CIK cell treatment group or control group [29]. In operable RCC patients, Ag-DC-CIK cell treatment significantly increased the 3-year disease free survival (DFS) and decreased the recurrence after surgery. Meanwhile, inoperable patients after CIK treatment achieved obviously longer 3-year OS and PFS than patients in the control group. The CD4+/CD8+ T cell ratio in peripheral blood increased after CIK cell treatment, and especially further increased in the autologous tumor lysate-pulsed DC-CIK treatment group. No grade 3 or 4 adverse reaction was observed during the cell transfusion.
Similarly, a study based on DC-CIK cell therapy recruited a total of 410 RCC patients after radical or partial nephrectomy [30]. One-hundred-and-fifty-four patients were included in the DC-CIK cell treatment group, while 256 patients were included in the IFN-α therapy control group. 2–5 × 106 DCs per day were intradermally injected in the inguinal regions bilaterally and intravenous infusion of 3–6 × 109 autologous CIK cells were performed once a day. A 6-day treatment was considered as one course, and the second course started after 1-month interval, for a total of three courses. The 3- and 5-year OS rates in the DC-CIK group (96% and 96%, respectively) were significantly more increased than that in the IFN-α group (83% and 74%, respectively). In addition, a statistically higher PFS was also observed in the DC-CIK group. The patients in the DC-CIK group exhibited good tolerance with just transient low-grade fever and fatigue.
A recent study compared the immune response induced by DC-CIK cell treatment and DC activated cytotoxic T cell (DC-ACT) treatment [31]. One-hundred-and-twelve patients with solid tumors (six with RCC) were assigned to the DC-CIK treatment group and 116 patients (six with RCC) were assigned to the DC-ACT group. In every infusion, patients received (1–3) × 107 DC cells and (1–2) × 109 autologous CIK cells. Each treatment consisted of two DC subcutaneous and three CIK intravenous infusions. Immune cells, such as CD3+ HLA-DR+ T cells and NK cells, and cytokines, like IL-2, IL-6 were significantly increased in DC-CIK group, whereas total CD3+ T cells, CD8+ T cells, CD3+ HLA-DR+ cells and IL-12 were remarkably increased in DC-ACT group. DC-ACT therapy decreased the ratio of IL-4/IFN-γ, IL-4/IL-12 and IL-6/IL-12 when compared with DC-CIK therapy. The results showed DC-ACT therapy promotes immune response towards Th1 cytokine profile, and DC-CIK and DC-ACT therapy may eradicate tumors through different pathways.
2.4. Clinical Studies on CIK Cells Combined with Targeted Agents
Yang et al. reported a case who was a 58-year-old male diagnosed with stage III clear cell RCC without metastasis [32]. After operation, this patient received combinatorial therapy consisting of autologous CIK cells and sorafenib because of the suspicious nodule identified in his left lung. The sorafenib dose was 400 mg twice a day and CIK cell infusion was conducted approximately every 2 weeks. However, adverse side effects, including grade 3 diarrhea, grade 3 fatigue and grade 2 hand-foot syndrome, were apparent and exceeded the patient’s tolerance. When sorafenib dose was decreased to 200 mg twice a day with CIK cell immunotherapy continued, the adverse effects became mild and well tolerated.
The nodule remained stable for approximately 6 months and then gradually decreased. Suspect metastases again emerged in the mediastinum and lung 6 months later, but the progress rate of these diseases was very slow. The patient maintained a high quality of life throughout the entire treatment process, with a KPS of 90 and greatly extended OS. These results suggest a potential combinatorial regimen consisting of sorafenib and CIK cells for metastatic RCC patients and indicate the safety of CIK cell-based immunotherapy.
Mai et al. retrospectively analyzed the efficacy of sunitinib/sorafenib in combination with autologous DC-CIK cells in 34 metastatic RCC patients after radical nephrectomy [33]. Fifteen patients in group 1 were treated with sunitinib/sorafenib monotherapy, while 19 patients in group 2 were treated with sunitinib/sorafenib in combination with DC-CIK cells. The median PFS and 3-year OS was significantly higher in group 2 and the cases of PD and deaths were less in group 2 than that in group 1. In addition, the 3-year OS was higher in sunitinib + DC-CIK group than that in sorafenib + DC-CIK group.
2.5. Clinical Studies on CIK Cells Combined with Immune Checkpoint Inhibitors
A phase I study aimed to assess the safety and clinical activity of the PD-1 blockade pembrolizumab in combination with autologous DC-CIK cells [34]. Thirty-seven patients with various solid tumor entities, including 8 with RCC, were enrolled in this study. Patients received more than eight cycles of intravenous infusion of pembrolizumab-activated autologous DC-CIK cells. The median OS and PFS were 270 and 162 days, respectively at the time of report. In RCC patients, objective responses (CR or PR) were observed in two of the eight patients, and the overall disease control rate in RCC patients was 75.0%. Treatment-related adverse reactions were observed in 20/31 patients. Two patients with bladder cancer and hepatocellular carcinoma, respectively, exhibited grade 3 or 4 toxicities, including fever and chills. All adverse reactions were reversible or controllable.
The anti-tumor activity of pembrolizumab-activated DC-CIK cells was then confirmed ex vivo. Autologous renal carcinoma cells were collected from one patient with PR and another with SD after treatment. Expression of PD-L1 was upregulated by DC-CIK cells and after being cocultured with pembrolizumab; the cytotoxicity and IFN-γ secretion level of DC-CIK cells were obviously increased.
Wang et al. reported an RCC case treated with the anti-PD-1 mAb pembrolizumab combined with CIK cell transfer [35]. This patient is an 80-year-old male with multiple metastases after partial nephrectomy. The tumor biopsy from this patient showed moderate CD3+ T cell infiltration, but no PD-1 or PD-L1 expression. After receiving four cycles of pembrolizumab combined with eight cycles of autologous CIK cell transfer, he achieved a CR. This patient developed gingivitis after the first cycle of pembrolizumab and pneumonia after the second cycle of pembrolizumab, for which he received systematic antibiotic treatment. No other severe adverse effects were observed. These findings showed the anti-tumor potency and safety of the PD-1 inhibitor in combination with CIK cell therapy on RCC.
The study design, clinical responses, adverse effects and conclusions of all clinical studies discussed above are outlined in Table 1.
Table 1.
Clinical studies applying CIK cells for the treatment of RCC.
| Study | Number of Patients | Therapeutic Approach | Clinical Response | Adverse Event | Conclusion |
|---|---|---|---|---|---|
| Liu et al. [21] | 1 (1 RCC) | Modified auto-CIKs | Symptomatic improvement (relieved cough, dyspnea, chest distress and thoracalgia) and reduction of pleural fluid | Fever (~38 °C) but recovered 2 days later | The patient achieved partial success |
| Zhan et al. [26] | 137 (137 RCC) | Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: IFN-α Group 3: no adjuvant therapy |
Increased CD4+/CD8+ ratio, decreased CD4+CD25high cells and significantly higher 3- and 5-year OS rates | Controllable fever and fatigue in DC-CIK group | Postoperative immunotherapy with tumor lysate-pulsed DC-CIK cells may prevent recurrence/metastasis and increase OS rates |
| Zhang et al. [20] | 40 (10 RCC) | Auto-CIKs | 6-month, 1-year and 3-year OS rates were 70.0, 60.0 and 57.5%, respectively; median OS was 34.9 months; 6 recurrence, 12 metastasis and 15 deaths in all patients (2 recurrence, 5 metastasis and 3 deaths in RCC) | Controllable fever and poor appetite | CIK cell therapy can be an effective adjuvant instrument of the routine anti-tumor treatment |
| Cui et al. [27] | 121 (7 RCC) | Tumor lysate-pulsed DCs + auto-CIKs | Improvements in the physical strength, appetite and sleeping status | Controllable fever, insomnia, anorexia, joint soreness and skin rashes | Tumor lysate-pulsed DC-CIK cells were safe without serious adverse side-effects |
| Zhang et al. [19] | 20 (20 RCC) | Group 1: auto-CIKs Group 2: control |
Significantly longer median PFS; 6 CR, 4 SD in CIK group and 5 CR, 3 SD, 2 PD in control group | Mild arthralgia, laryngeal edema, fatigue, and low-grade fever | CIK cell treatment could prolong survival in patients with RCC after radical nephrectomy |
| Wang et al. [28] | 28 (28 RCC) | GmDCs + auto-CIKs | 4 CR, 7 PR, 10 SD, 6 PD and 1 death | Flu-like symptoms with fever | DCR was significantly related with cycles of treatment |
| Wang et al. [24] | 29 (29 RCC) | Auto-CIKs + IL-2 | 4 PR, 18 SD and 7 PD; 1-year survival was 82.8%; median PFS and OS were 7.7 months and 12.6 months, respectively | Grade 1 and 2 fever and grade 2 diarrhea | Auto-CIKs can induce regression of RCC; MDSCs can serve as a potential marker for the prognosis of patients receiving a CIK-based therapy |
| Li et al. [22] | 20 (1 RCC) | RetroNectin-activated auto-CIKs + conventional therapies | 1 CR, 5 PR, 9 SD and 5 PD; median PFS and OS were 7.7 months and 12.6 months, respectively (1 CR with an OS of 14 months and PFS of 8 months in RCC) | Fever | RetroNectin-activated CIKs combined with conventional therapies could improve the prognosis of metastatic brain tumor patients |
| Zheng et al. [30] | 410 (410 RCC) | Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: IFN-α |
Significantly increased 3-, 5-year OS rates and PFS in DC-CIK group | Transient low-grade fever and fatigue | Adjuvant DC-CIK treatment after surgery prolonged PFS and reduced mortality |
| Zhao et al. [29] | 122 (122 RCC) | In operable patients: Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: control in inoperable patients: Group 1: auto-CIKs Group 2: control |
Significantly higher 3-year DFS and decreased risk of post-operative disease progression and relapse in operable patients; Significantly higher 3-year OS rate, median OS and PFS in inoperable patients | Flu-like symptoms such as fever and fatigue | DC-CIK cells might be more efficient and personalized for the patients with tumor resection, and CIK cells could improve the prognosis for inoperable patients |
| Yang et al. [32] | 1 (1 RCC) | Auto-CIKs + sorafenib | Metastasis remained stable | No serious adverse reactions | CIK cells combined with sorafenib could result in a synergistic effect |
| Mai et al. [33] | 34 (34 RCC) | Group 1: sunitinib/sorafenib monotherapy Group 2: DCs + auto-CIKs + sunitinib/sorafenib |
Significantly higher median PFS and 3-year OS rate; more SD and less PD and death | No serious adverse reactions in group 2; bone marrow suppression, oral ulcer, fatigue, and hand-foot syndrome | Sunitinib/sorafenib combined with CIK cells could significantly prolong the median PFS and 3-year OS |
| Wang et al. [35] | 2 (1 RCC) | Auto-CIKs + pembrolizumab | 1 CR (RCC) and 1 near-CR | No serious adverse reactions associated with CIK cells | Pembrolizumab in combination with CIK cells led to potent anti-tumor activity in RCC; CD3+ T cell infiltration in baseline tumor biopsies is a potential predictive biomarker |
| Chen et al. [34] | 37 (8 RCC) | DCs + auto-CIKs + pembrolizumab | 2 CR, 5 PR, 13 SD and 11 PD (1 CR, 1 PR, 4 SD, 2 PD in RCC); median OS and PFS were 270 and 162 days | All treatment-related adverse reactions were reversible or controllable; grade 3 or 4 toxicities, including fever and chills, were observed in two patients | Pembrolizumab-activated autologous DC-CIK cells were safe and effective in advanced solid tumors |
| Li et al. [31] | 228 (12 RCC) | Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: tumor lysate-pulsed DCs + cytotoxic T cells |
Elevated percentage of CD3+ HLA-DR+ T cells, NK cells and cytokines such as IL-2, IL-6 in group 1; Elevated total CD3+ T cells, CD8+ T cells, CD3+ HLA-DR+ cells and IL-12 in group 2 | -- | DCs combined with cytotoxic T cells have more dominance to induce Th1 cytokine response instead of skewing toward the Th2 cytokine profile |
Auto-CIKs: autologous CIK cells; DCs: dendritic cells; IFN-α: interferon-α; OS: overall survival; PFS: progression-free survival; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; gmDCs: genetically modified dendritic cells; DFS: disease-free survival; DCR: disease control rate; IL-2: interleukin-2; MDSCs: myeloid-derived suppressor cells.
3. Preclinical Researches to Improve the Anti-Tumoral Activity of CIK Cells
Ever-growing clinical trials have been conducted with CIK cells in cancer patients, owing to the relative easiness of CIK cell preparation ex vivo, low toxicity and GVHD activity, and their encouraging cytolytic activities against various tumor cells [15]. However, the clinical benefits of CIK cells reported in some studies are still limited and fall for inability of exerting a sustained and prolonged anti-tumor response. Researchers are exploring novel methods to modify CIK cell-based immunotherapies for a higher efficacy and more specific recognition against RCC. These preclinical researches conducted with CIK cells are concluded in Table 2.
Table 2.
Summary of preclinical researches conducted with CIK cells on RCC.
| Study | Method | Conclusion |
|---|---|---|
| Finke et al. [37] | CIK cells transfected with IL-7 gene | Improved proliferation rate and increased TNF-α production of transfected CIK cells; significantly higher cytotoxic activity against the RCC cell line |
| Hillebrand et al. [38] | DCs transduced with adenoviruses carrying human CD40L (Ad-hCD40L) + CIK cells | Co-culture of Ad-hCD40L DCs with CIK cells led to a significant stimulation of tumor-specific CIK cells, with increased proliferation and cytotoxicity |
| Sievers et al. [40] | Telomerase peptide pulsed DCs + CIK cells | Significantly increased cytotoxic activity against RCC cell lines and autologous, telomerase positive primary cell cultures |
| Zhang et al. [41] | An anti-TIGIT functional antibody + CIK cells | CIK cells with TIGIT blocked indicated increased proliferation, higher cytotoxicity against tumor cells expressing CD155 and higher expression of IFN-γ, IL-6, and TNF-α |
| Setiawan et al. [42] | Peptide P60+ CIK cells | CIK cells combined with P60 resulted in a significant decrease in the viability of renal and pancreatic cancer cell lines |
| Dehno et al. [43] | CIK cells treated with nivolumab and ipilimumab | CIK cells treated with nivolumab and ipilimumab had no remarkable effect on the viability of RCC cells; the combination of nivolumab and ipilimumab significantly increased the proliferation and IFN-γ secretion of CIK cells |
IL-7: interleukin-7; TNF-α: tumor necrosis factor-α; DCs: dendritic cells; TIGIT: T cell Ig and ITIM domain; IFN-γ: interferon-γ; IL-6: interleukin-6.
Genetically modified CIK cells are of potential therapeutic value in the treatment of RCC. IL-7 is a 25kDa cytokine, which induced the proliferation of mature CD4+ and CD8+ T cells and also CIK cells [36]. Finke et al. used the adenovirus-enhanced CD3 receptor-mediated gene transfer for transfection with the IL-7 gene [37]. CIK cells transfected with IL-7 gene showed more IL-7 expression and a higher proliferation rate as compared with non-transfected cells. Expression of IL-7 also increased the production of tumor necrosis factor-α (TNF-α) by CIK cells and improved the cytotoxicity against RCC, malignant melanoma and colon carcinoma cell lines, indicating CIK cells transfected with an IL-7 gene expression construct may be valuable for adoptive immunotherapy on RCC. In another study, DCs were transduced with adenoviruses carrying human CD40L (Ad-hCD40L) to stimulate the anti-tumor activity of CIK cells [38]. CD40L is the ligand of CD40, a member of the tumor necrosis factor receptor family and is essential in antigen-presenting cells (APCs) activation and cytotoxic T lymphocytes (CTLs) generation [39]. DCs transduced with Ad-hCD40L had high expression of soluble CD40L and membrane-bound CD40L and showed a T-helper cell type 1 shift of expressed cytokines/chemokines. In addition, Ad-hCD40L transduction of DCs also significantly stimulated the proliferation of CIK cells and their cytotoxicity against RCC cell lines. This observation shows the immunomodulatory potential of genetically modified DCs for the CIK cell-based immunotherapy.
Telomerase is a ribonucleoprotein enzyme that prevents the end of the chromosome from damage or fusion. Activation of telomerase is detected in nearly all human cancers. Telomerase peptide was added to pulse DCs and induce the tumor-specific lysis of CIK cells [40]. CIK cells had a significant increase in cytotoxic activity against RCC cells after being co-cultured with telomerase peptide pulsed DCs when compared with CIK cells without co-culture, that is 100% versus 41.7% at an effector-to-target (E/T) ratio of 60:1. The activated CIK cells also exerted specific cytotoxicity against autologous, telomerase positive primary cell cultures. Telomerase could, thus, serve as a specific tumor-associated antigen for RCC and allow the generation of antigen specific CIK cells.
T cell Ig and ITIM domain (TIGIT) is a newly identified inhibitory receptor expressed on T and natural killer (NK) cells [44,45]. A study showed that TIGIT was also expressed by CIK cells and interacted with CD155, the human poliovirus receptor expressed in many types of normal and cancer cells [41]. The anti-TIGIT functional antibody blocked TIGIT expression of CIK cells and promoted their proliferation. Cytotoxicity against CD155 positive tumor cells was enhanced with an increased level of cytokines, such as IFN-γ, IL-6, and TNF-α. However, inhibition of DNAX accessory molecule-1 (DNAM-1) reduced the elevated anti-tumor activity and IFN-γ level by TIGIT blockade. The results indicated that TIGIT shared the same ligand, CD155, as DNAM-1 and played an inhibitory role in CIK cell cytotoxicity. This finding provides a new molecular target for the improvement of CIK cell immunotherapy.
Peptide P60 has been shown to inhibit the immunosuppressive functions of regulatory T-cells (T-regs) by targeting towards the transcriptional regulator protein forkhead box P3 (FOXP3) [46]. As T-regs was shown to reduce the effectiveness of CIK cells against tumor cells, P60 was added to improve the cytolytic activity against renal and pancreatic cancer cells [12,42]. No increase in IFN-γ secretion and changes in the distribution of major effector cell populations in CIK cells was detected. However, P60 treatment resulted in a significant decrease in the viability of renal and pancreatic cancer cell lines co-cultured with CIK cells.
Recently, the anti-PD-1 mAb nivolumab and anti-CTLA-4 mAb ipilimumab were investigated if the two immune checkpoint inhibitors could stimulate the cytotoxicity of CIK cells against RCC cell lines [43]. Although nivolumab and ipilimumab combined with CIK cells had no remarkable effect on the viability of tumor cells, combination of the two antibodies significantly increased the proliferation and IFN-γ secretion of CIK cells. This finding indicated the possibility of combining immune checkpoint inhibitors with CIK cells as a potential treatment option for RCC.
4. Conclusions
The clinical studies based on CIK cells have shown big promise in the treatment of RCC patients, including those who are in the late stage or refractory to standard therapies. The MHC-unrestricted anti-tumor activity against a wide range of tumor types is one of the most important characteristics of CIK cells. CIK cells are also featured as their relatively low and easily controllable toxicities. In our previous report, the primary side effects of CIK cell therapy were grade 1–2 toxicities like fever, chills, fatigue, headache, and skin rash [47]. Low-grade fever ranged from 37.5 to 40 °C, was the most common adverse event and usually recovered without or with simple treatments [48]. In addition, CIK cells exhibited a reduced risk of GVHD, making CIK cells an ideal candidate for immunotherapy in both matched and mismatched recipients [49].
Many attempts have been made to optimize the treatment modality of CIK cell therapy on RCC. As the clinical studies described above, CIK cells combined with conventional therapies, DC vaccination, targeted drugs or immune checkpoint blockade could achieve better clinical benefits than the standard treatments alone. One of the most successful clinical studies that should be highlighted is reported by Zhao et al. [29]. In 122 patients with different stages of RCC, CIK cell treatment remarkably increased the 3-year survival no matter if it was in operable patients or in inoperable patients. This study suggested personalized CIK cell-based therapy would help to obtain clinical benefits in patients with different stages. Besides sunitinib and sorafenib, which show promising anti-tumor activity combined with CIK cells, other receptor tyrosine kinases, like c-Met, also play an important role in the growth of RCC. Increased expression of c-Met was observed in all RCC subtypes and associated with poor prognosis in RCC [50]. Balan et al. reported that c-Met inhibited immune cell-mediated killing of RCC cells by increasing the expression of PD-L1 [51]. Therefore, c-Met inhibitors may also exert synergistic anti-tumor effect with CIK cells.
Moreover, a variety of preclinical researches have been developed and explored new target molecules and pathways to augment the efficacy of CIK cells’ anti-tumor activities. The newly identified inhibitory receptor TIGIT serves as a novel and encouraging target to increase the anti-tumor activity of CIK cells. With the elevated secretion of IFN-γ after TIGIT blockade, expression of PD-1 on CIK cells and PD-L1 on both CIK and tumor cells could also be detected to further develop the cytotoxicity profile of CIK cells by immune checkpoint blockade.
Although there is growing interest in CIK cells as an important cellular anti-tumor therapy on RCC, the heterogeneity in study design, clinical protocol and evaluation criteria of clinical studies all make it difficult to assess and compare the clinical efficacy. In 2010, the first international platform for the registration of clinical trials on CIK cells, IRCC, was established to standardize the clinical data collection [52]. The first update took place in 2015 and the second was published recently [47,53]. Further large-scale studies are required to evaluate the clinical efficacy of CIK cells and more efforts should be performed to identify the optimal CIK cell-based therapeutic combinations on RCC.
Acknowledgments
The author Y.Z. would like to acknowledge the support from China Scholarship Council.
Author Contributions
Conceptualization, Y.Z. and I.G.H.S.-W.; writing—original draft preparation, Y.Z.; writing—review and editing, J.E., M.R. and I.G.H.S.-W.; supervision and project administration, I.G.H.S.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This review article received no funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Capitanio U., Bensalah K., Bex A., Boorjian S.A., Bray F., Coleman J., Gore J.L., Sun M., Wood C., Russo P. Epidemiology of renal cell carcinoma. Eur. Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungberg B., Albiges L., Abu-Ghanem Y., Bensalah K., Dabestani S., Fernández-Pello S., Giles R.H., Hofmann F., Hora M., Kuczyk M.A. European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur. Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Capitanio U., Montorsi F. Renal cancer. Lancet. 2016;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh J.J., Purdue M.P., Signoretti S., Swanton C., Albiges L., Schmidinger M., Heng D.Y., Larkin J., Ficarra V. Renal cell carcinoma. Nat. Rev. Dis. Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppin C., Kollmannsberger C., Le L., Porzsolt F., Wilt T.J. Targeted therapy for advanced renal cell cancer (RCC): A Cochrane systematic review of published randomised trials. Bju Int. 2011;108:1556–1563. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 6.Hudes G.R. Targeting mTOR in renal cell carcinoma. Cancer. 2009;115:2313–2320. doi: 10.1002/cncr.24239. [DOI] [PubMed] [Google Scholar]
- 7.Vuky J., Motzer R.J. Cytokine therapy in renal cell cancer. Urol. Oncol. Semin. Orig. Investig. 2000;5:249–257. doi: 10.1016/S1078-1439(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 8.Motzer R.J., Tannir N.M., McDermott D.F., Frontera O.A., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houot R., Schultz L.M., Marabelle A., Kohrt H. T-cell–based immunotherapy: Adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015;3:1115–1122. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]
- 10.Kim J.S., Chung I.S., Lim S.H., Park Y., Park M.J., Kim J.Y., Kim Y.G., Hong J.T., Kim Y., Han S.-B. Preclinical and clinical studies on cytokine-induced killer cells for the treatment of renal cell carcinoma. Arch. Pharm. Res. 2014;37:559–566. doi: 10.1007/s12272-014-0381-x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Wolf I., Finke S., Trojaneck B., Denkena A., Lefterova P., Schwella N., Heuft H., Prange G., Korte M., Takeya M. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br. J. Cancer. 1999;81:1009–1016. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Wolf I., Negrin R.S., Kiem H.-P., Blume K.G., Weissman I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X., Zhu A., Cai X., Jia Z., Han W., Ma L., Zhou M., Qian K., Cen L., Chen B. Role of NKG2D in cytokine-induced killer cells against multiple myeloma cells. Cancer Biol. 2012;13:623–629. doi: 10.4161/cbt.19850. [DOI] [PubMed] [Google Scholar]
- 14.Diefenbach A., Jamieson A.M., Liu S.D., Shastri N., Raulet D.H. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 15.Introna M. CIK as therapeutic agents against tumors. J. Autoimmun. 2017;85:32–44. doi: 10.1016/j.jaut.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Baker J., Verneris M.R., Ito M., Shizuru J.A., Negrin R.S. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon γ production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.V97.10.2923. [DOI] [PubMed] [Google Scholar]
- 17.Verneris M.R., Ito M., Baker J., Arshi A., Negrin R.S., Shizuru J.A. Engineering hematopoietic grafts: Purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol. Blood Marrow Transpl. 2001;7:532–542. doi: 10.1016/S1083-8791(01)70014-6. [DOI] [PubMed] [Google Scholar]
- 18.Jäkel C.E., Hauser S., Rogenhofer S., Müller S.C., Brossart P., Schmidt-Wolf I.G. Clinical studies applying cytokine-induced killer cells for the treatment of renal cell carcinoma. Clin. Dev. Immunol. 2012;2012:1–7. doi: 10.1155/2012/473245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Wang J., Wang Y., Lu X.-C., Fan H., Liu Y., Zhang Y., Feng K.-C., Zhang W.-Y., Chen M.-X. Autologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomy. Clin. Dev. Immunol. 2013;2013:1–12. doi: 10.1155/2013/195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Zhu L., Wei J., Liu L., Yin Y., Gu Y., Shu Y. The effects of cytokine-induced killer cells for the treatment of patients with solid tumors: A clinical retrospective study. J. Cancer Res. Clin. Oncol. 2012;138:1057–1062. doi: 10.1007/s00432-012-1179-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Sui J., Zhang Z., Ren X., Luan L., Yang Q., Gu S., Wank R., Laumbacher B., Song X. Inhibition of pleural metastasis of collecting duct carcinoma of the kidney by modified cytokine-induced killer cells: A case report and review of the literature. Oncol. Lett. 2010;1:955–958. doi: 10.3892/ol.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Wang Y., Zhao L., Xu L., Zhang Y., Mai L., Gao Q. Efficacy of RetroNectin-activated cytokine-induced killer cell therapy in metastatic brain tumor patients. Oncol. Res. Treat. 2015;38:160–165. doi: 10.1159/000380890. [DOI] [PubMed] [Google Scholar]
- 23.Kimizuku F., Taguchi Y., Ohdate Y., Kawase Y., Shimojo T., Hashino K., Kato I., Sekiguchi K., Titani K. Production and characterization of functional domains of human fibronectin expressed in Escherichia coli. J. Biochem. 1991;110:284–291. doi: 10.1093/oxfordjournals.jbchem.a123572. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Zhang Y., Liu Y., Wang L., Zhao L., Yang T., He C., Song Y., Gao Q. Association of myeloid-derived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. J. Immunother. 2014;37:43–50. doi: 10.1097/CJI.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 25.Santos P.M., Butterfield L.H. Dendritic cell–based cancer vaccines. J. Immunol. 2018;200:443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan H., Xin G., Pu X., Li W., Li Z., Zhou X., Qiu J. A randomized controlled trial of postoperative tumor lysate-pulsed dendritic cells and cytokine-induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma. Chin. Med. J. 2012;125:3771–3777. [PubMed] [Google Scholar]
- 27.Cui Y., Yang X., Zhu W., Li J., Wu X., Pang Y. Immune response, clinical outcome and safety of dendritic cell vaccine in combination with cytokine-induced killer cell therapy in cancer patients. Oncol. Lett. 2013;6:537–541. doi: 10.3892/ol.2013.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Zhang B., Gao H., Ding G., Wu Q., Zhang J., Liao L., Chen H. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer. 2014;14:251. doi: 10.1186/1471-2407-14-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X., Zhang Z., Li H., Huang J., Yang S., Xie T., Huang L., Yue D., Xu L., Wang L. Cytokine induced killer cell-based immunotherapies in patients with different stages of renal cell carcinoma. Cancer Lett. 2015;362:192–198. doi: 10.1016/j.canlet.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 30.Zheng K., Tan J.-M., Wu W.-Z., Qiu Y.-M., Zhang H., Xu T.-Z., Sun X.-H., Zhuo W.-L., Wang D., Zhang J.-P. Adjuvant dendritic cells vaccine combined with cytokine-induced-killer cell therapy after renal cell carcinoma surgery. J. BUON. 2015;20:505–513. [PubMed] [Google Scholar]
- 31.Li C., Zhu D., Zhao Y., Guo Q., Sun W., Li L., Gao D., Zhao P. Dendritic Cells Therapy with Cytokine-Induced Killer Cells and Activated Cytotoxic T Cells Attenuated Th2 Bias Immune Response. Immunol. Investig. 2019;49:522–534. doi: 10.1080/08820139.2019.1696360. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Lin H., Zhao L., Song Y., Gao Q. Combination of sorafenib and cytokine-induced killer cells in metastatic renal cell carcinoma: A potential regimen. Immunotherapy. 2017;9:629–635. doi: 10.2217/imt-2016-0133. [DOI] [PubMed] [Google Scholar]
- 33.Mai H.-X., Mei G.-H., Zhao F.-L., Li B.-T., Tang Y.-Y., Zhang B., Xu X.-J., Chen L.-J. Retrospective analysis on the efficacy of sunitinib/sorafenib in combination with dendritic cells-cytokine-induced killer in metastasis renal cell carcinoma after radical nephrectomy. J. Cancer Res. 2018;14:427. doi: 10.4103/0973-1482.180609. [DOI] [PubMed] [Google Scholar]
- 34.Chen C.-L., Pan Q.-Z., Weng D.-S., Xie C.-M., Zhao J.-J., Chen M.-S., Peng R.-Q., Li D.-D., Wang Y., Tang Y. Safety and activity of PD-1 blockade-activated DC-CIK cells in patients with advanced solid tumors. Oncoimmunology. 2018;7:e1417721. doi: 10.1080/2162402X.2017.1417721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Liu X., Till B., Sun M., Li X., Gao Q. Combination of cytokine-induced killer cells and programmed cell death-1 blockade works synergistically to enhance therapeutic efficacy in metastatic renal cell carcinoma and non-small cell lung cancer. Front. Immunol. 2018;9:1513. doi: 10.3389/fimmu.2018.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoll B., Lefterova P., Csipai M., Finke S., Trojaneck B., Ebert O., Micka B., Roigk K., Fehlinger M., Schmidt-Wolf G.D. Generation of cytokine-induced killer cells using exogenous interleukin-2,-7 or-12. Cancer Immunol. Immunother. 1998;47:221–226. doi: 10.1007/s002620050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finke S., Trojaneck B., Lefterova P., Csipai M., Wagner E., Kircheis R., Neubauer A., Huhn D., Wittig B., Schmidt-Wolf I. Increase of proliferation rate and enhancement of antitumor cytotoxicity of expanded human CD3+ CD56+ immunologic effector cells by receptor-mediated transfection with the interleukin-7 gene. Gene Ther. 1998;5:31–39. doi: 10.1038/sj.gt.3300560. [DOI] [PubMed] [Google Scholar]
- 38.Hillebrand R.M., Vogt A., Strassburg C.P., Gonzalez-Carmona M.A., Schmidt-Wolf I.G. Immune Check Point CD40–CD40L Activates Dendritic and Effector Cells Against Human Renal Carcinoma Cells. Anticancer Res. 2019;39:4643–4652. doi: 10.21873/anticanres.13645. [DOI] [PubMed] [Google Scholar]
- 39.French R.R., Chan H.C., Tutt A.L., Glennie M.J. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 40.Sievers E., Albers P., Schmidt-Wolf I.G., MÄRTEN A. Telomerase pulsed dendritic cells for immunotherapy for renal cell carcinoma. J. Urol. 2004;171:114–119. doi: 10.1097/01.ju.0000094803.60928.d7. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B., Zhao W., Li H., Chen Y., Tian H., Li L., Zhang L., Gao C., Zheng J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016;65:305–314. doi: 10.1007/s00262-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setiawan M.F., Rudan O., Vogt A., Gonzalez-Carmona M.A., Langhans B., Schmidt-Wolf R., Garofano F., Strassburg C.P., Lasarte J.J., Casares N. FOXP3 Inhibitory Peptide P60 Increases Efficacy of Cytokine-induced Killer Cells Against Renal and Pancreatic Cancer Cells. Anticancer Res. 2019;39:5369–5374. doi: 10.21873/anticanres.13730. [DOI] [PubMed] [Google Scholar]
- 43.Dehno M.N., Li Y., Weiher H., Schmidt-Wolf I.G. Increase in Efficacy of Checkpoint Inhibition by Cytokine-Induced-Killer Cells as a Combination Immunotherapy for Renal Cancer. Int. J. Mol. Sci. 2020;21:3078. doi: 10.3390/ijms21093078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X., Harden K., Gonzalez L.C., Francesco M., Chiang E., Irving B., Tom I., Ivelja S., Refino C.J., Clark H. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10:48. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 45.Stanietsky N., Simic H., Arapovic J., Toporik A., Levy O., Novik A., Levine Z., Beiman M., Dassa L., Achdout H. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casares N., Rudilla F., Arribillaga L., Llopiz D., Riezu-Boj J.I., Lozano T., López-Sagaseta J., Guembe L., Sarobe P., Prieto J. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J. Immunol. 2010;185:5150–5159. doi: 10.4049/jimmunol.1001114. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Schmidt-Wolf I.G. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020 doi: 10.1002/jcp.29827. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Xia L., Zhang Y., Wang Y., Lu X., Shi F., Liu Y., Chen M., Feng K., Zhang W. Analysis of adverse events following the treatment of autologous cytokine-induced killer cells for adoptive immunotherapy in malignant tumour sufferers. Expert Opin. Biol. 2015;15:481–493. doi: 10.1517/14712598.2015.988134. [DOI] [PubMed] [Google Scholar]
- 49.Introna M., Lussana F., Algarotti A., Gotti E., Valgardsdottir R., Micò C., Grassi A., Pavoni C., Ferrari M.L., Delaini F. Phase II study of sequential infusion of donor lymphocyte infusion and cytokine-induced killer cells for patients relapsed after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2017;23:2070–2078. doi: 10.1016/j.bbmt.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Gibney G., Aziz S., Camp R., Conrad P., Schwartz B., Chen C., Kelly W., Kluger H. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann. Oncol. 2013;24:343–349. doi: 10.1093/annonc/mds463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balan M., Teran E.M.Y., Waaga-Gasser A.M., Gasser M., Choueiri T.K., Freeman G., Pal S. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J. Biol. Chem. 2015;290:8110–8120. doi: 10.1074/jbc.M114.612689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hontscha C., Borck Y., Zhou H., Messmer D., Schmidt-Wolf I. Clinical trials on CIK cells: First report of the international registry on CIK cells (IRCC) J. Cancer Res. Clin. Oncol. 2011;137:305–310. doi: 10.1007/s00432-010-0887-7. [DOI] [PubMed] [Google Scholar]
- 53.Schmeel L.C., Schmeel F.C., Coch C., Schmidt-Wolf I.G. Cytokine-induced killer (CIK) cells in cancer immunotherapy: Report of the international registry on CIK cells (IRCC) J. Cancer Res. Clin. Oncol. 2015;141:839–849. doi: 10.1007/s00432-014-1864-3. [DOI] [PubMed] [Google Scholar]