Abstract
Simple Summary
New cancer treatments have prolonged the lives for patients with metastatic melanoma in clinical trials. However, patients in clinical trials often presents with a better prognosis than other patients. It is therefore important to examine the effect of the new treatments in a real-life setting. We found that survival for patients with metastatic melanoma in a nation-wide setting were prolonged and a higher proportion of these patients were alive and without active disease 3 years after they started treatment.
Abstract
Approval of immune checkpoint-inhibitors (ICIs) and BRAF-inhibitors has revolutionized the treatment of metastatic melanoma. Although these drugs have improved overall survival (OS) in clinical trials, real-world evidence for improved long-term survival is still scarce. Clinical data were extracted from the Danish Metastatic Melanoma database. This nation-wide cohort contains data on all patients who received systemic treatment for metastatic melanoma between 2008 and 2016. Ipilimumab, the first approved ICI, was implemented as standard-of-care in Denmark in 2012. Hence, patients were divided in a pre-ICI (2008–2011) and an ICI (2012–2016) era. Patients were defined as long-term survivors if they were alive 3 years after initiation of systemic therapy. Data from 1754 patients were retrieved. Patients treated in the ICI era had an improved median OS (11.3 months, 95% confidence interval (CI) 10.3–12.3) compared with those in the pre-ICI era (median OS 8.3 months, 95% CI 7.4–9.5, p < 0.0001). A higher proportion of long-term survivors was observed in the ICI era (survivors >3 years increased from 13% to 26% and survivors >5 years increased from 9% to 21%; both p < 0.0001). For long-term survivors, known prognostic factors were equally distributed between the two periods, except that long-term survivors in the pre-ICI era were younger. For long-term survivors, 70% were without progression in the ICI era compared with 43% in the pre-ICI era (p < 0.0001). For all patients, the proportion without progression increased from 5% to 18% between the pre-ICI and the ICI era (p < 0.0001), respectively. Implementation of ICI has led to a significant increase in progression-free, long-term survival for real-life patients with metastatic melanoma.
Keywords: long-term survival, nation-wide, real-life, metastatic melanoma, immune checkpoint-inhibitor
1. Introduction
The implementation of immune checkpoint-inhibitors (ICIs) and BRAF (v-raf murine sarcoma viral oncogene homolog B1)/MEK (mitogen-activated protein kinase)-inhibitors (BRAFi/MEKi) over the last decade has revolutionized the standard-of-care of metastatic melanoma [1,2,3,4,5,6,7,8]. Metastatic melanoma has gone through a paradigm shift from a life-threatening situation with scarce and ineffective treatment options to a disease with potent treatment options with the ability to induce prolonged survival.
The CTLA-4 (cytotoxic T lymphocyte-associated antigen 4) inhibitor ipilimumab was the first ICI to be approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2011 after showing an improvement in median overall survival (OS) of 4 months [1,2], and was primarily implemented in Denmark in 2012. After approval by regulatory authorities, the BRAFi vemurafenib was approved as monotherapy in 2011 and more widely used in Denmark during 2012, while combination therapy with the BRAFi dabrafenib and the MEKi trametinib was introduced in 2013. Treatment with anti-programmed death 1 (PD1)-inhibitor was approved and implemented in routine clinical practice in Denmark in 2016.
The approval and implementation of new drugs are followed by an increasing request for real-world evidence to support regulatory decision-making, as support tools in clinical practice, safety surveillance, generating hypotheses for further clinical testing, as well as evaluating implementation of health care services. Evidence generated from real-world data complements evidence from clinical trials in highly selected patients to a broader patient population, providing information that cannot be obtained through a clinical trial. Previous studies have shown that real-life patient cohorts may differ significantly from patients eligible for clinical trials, and evidence of benefits in real-life patients is still limited [9,10]. Therefore, in a Danish consecutively treated, nation-wide cohort of metastatic melanoma patients spanning almost a decade, we aimed to analyze OS with emphasis on long-term survivors in the pre-ICI and ICI era.
2. Results
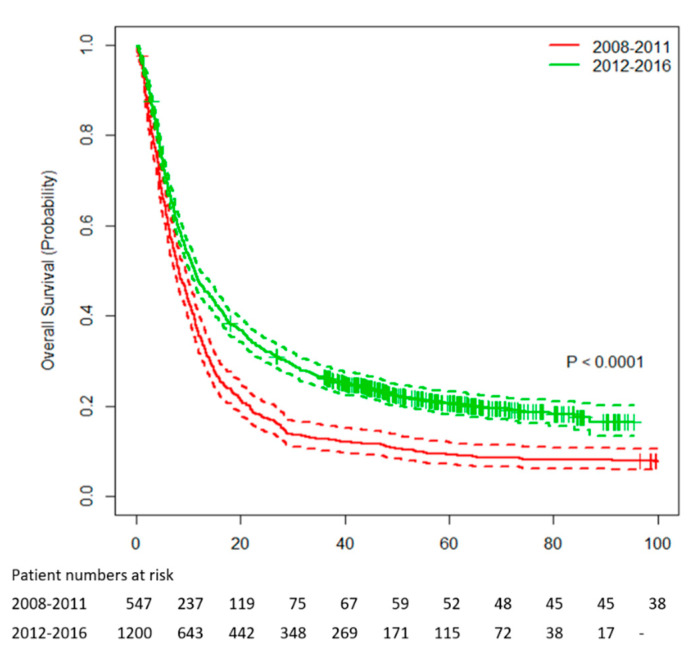
A total of 1202 patients received systemic treatment in the ICI era from 2012 to 2016 compared with 552 patients in the pre-ICI era from 2008 to 2011. Median OS was significantly higher for patients starting first-line treatment in the ICI era with 11.3 months (95% confidence interval (CI) 10.3–12.3) compared with 8.3 months (95% CI 7.4–9.5, p < 0.0001) in the pre-ICI era (Figure 1). The corresponding 1-, 2-, 3-, 4-, and 5-year survival rates were 48%, 33%, 26%, 23%, and 21% in the ICI era compared with 36%, 18%, 13%, 11%, and 9% in the pre-ICI era, respectively. Median follow-up was 62.1 (95% CI 59.6–66.0) months.
Figure 1.
Overall survival for patients with metastatic melanoma between 2008–2011 and 2012–2016.
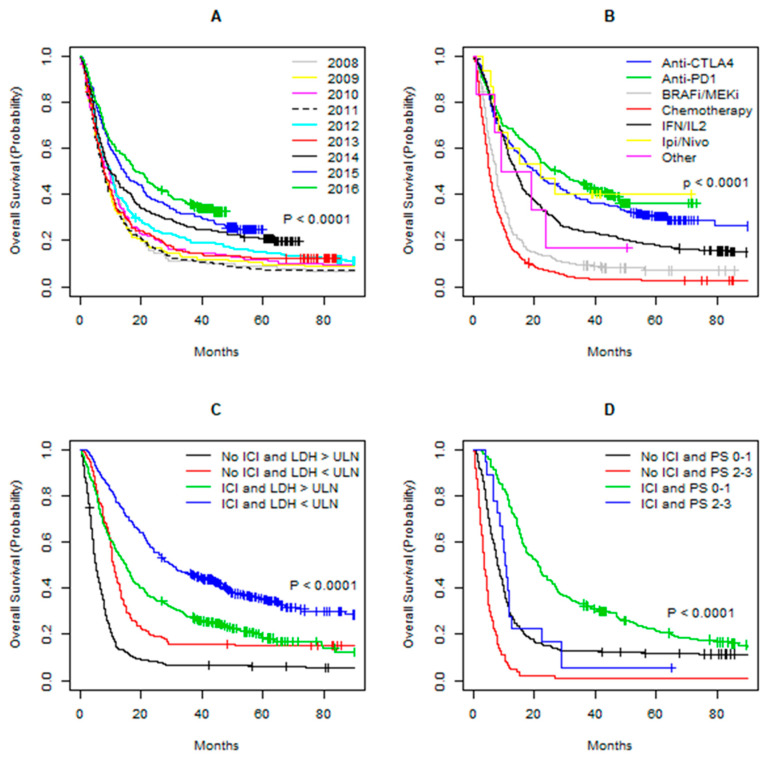
In the ICI era, there was a higher proportion of patients with poor prognostic factors such as poor performance status (PS) and elevated LDH, while a higher proportion of patients with liver metastases was observed in the pre-ICI era (Table 1a). On the other hand, there were no differences in disease stage distribution or presence of CNS metastases between the two periods. In the pre-ICI era, 51% received high-dose interleukin-2/interferon-alpha (IL2/IFN) and 46% received chemotherapy as first-line treatment, while only a small fraction of patients received BRAFi/MEKi, anti-CTLA-4, or other first-line treatment primarily in clinical trials. First-line treatment distribution in the ICI era was more diverse as 26% received anti-CTLA-4, 20% received chemotherapy, 20% received BRAFi/MEKi, 12% received high-dose IL2/IFN, and 21% received a treatment regimen containing anti-PD1 antibodies. Survival according to subgroups is shown in Figure 2A–D.
Table 1.
Baseline characteristics between 2008–2011 and 2012–2016.
| a. All Patients | b. Long-Term Survivors a | |||||
|---|---|---|---|---|---|---|
| Variable | 2008–2011 | 2012–2016 | p Value b | 2008–2011 | 2012–2016 | p Value b |
| Total number of patients | 552 | 1202 | 70 (13) | 313 (26) | <0.0001 | |
| Emigrated (%) | 3 (<1) | 2 (<1) | 0 | 0 | ||
| Age | <0.0001 | 0.0007 | ||||
| <70 years | 410 (74) | 739 (62) | 60 (86) | 203 (65) | ||
| ≥70 years | 141 (26) | 463 (39) | 10 (14) | 110 (35) | ||
| Male gender (%) | 336 (61) | 687 (57) | 0.1428 | 36 (51) | 116 (55) | 0.659 |
| Melanoma type | 0.1443 | 0.1267 | ||||
| Cutaneous | 420 (76) | 928 (77) | 59 (84) | 257 (82) | ||
| Mucosal | 18 (3) | 52 (4) | 0 (0) | 15 (5) | ||
| Ocular | 30 (6) | 81 (7) | 0 (0) | 6 (2) | ||
| Unknown primary | 82 (15) | 139 (12) | 11 (16) | 35 (11) | ||
| NA | 2 | 2 | 0 | 0 | ||
| PS (%) | <0.0001 | 0.9151 | ||||
| 0–1 | 275 (92) | 325 (76) | 61 (98) | 71 (99) | ||
| 2–3 | 25 (8) | 102 (24) | 1 (2) | 1 (1) | ||
| NA | 252 | 775 | 8 | 241 | ||
| BRAF status (%) | c | c | ||||
| Wildtype | 546 (50) | 162 (53) | ||||
| Any mutation | 551 (50) | 142 (47) | ||||
| NA | 105 | 8 | ||||
| LDH (%) | 0.0243 | 0.736 | ||||
| Below ULN | 153 (54) | 431 (46) | 38 (63) | 187 (66) | ||
| Above ULN | 133 (47) | 508 (54) | 22 (37) | 94 (34) | ||
| NA | 266 | 263 | 10 | 28 | ||
| Stage (%) | 0.2682 | 0.9146 | ||||
| M1a | 65 (12) | 173 (15) | 20 (29) | 89 (29) | ||
| M1b | 81 (15) | 145 (12) | 16 (23) | 59 (19) | ||
| M1c | 294 (54) | 624 (53) | 29 (41) | 134 (44) | ||
| M1d | 107 (20) | 226 (19) | 5 (7) | 25 (8) | ||
| NA | 5 | 34 | 0 | 5 | ||
| Metastatic sites | ||||||
| CNS (%) | 107 (20) | 226 (19) | 0.7312 | 5 (7) | 25 (8) | 0.8121 |
| NA | 5 | 4 | 0 | 0 | ||
| Bone (%) | 120 (22) | 293 (25) | 0.1278 | 11 (16) | 41 (13) | 0.6055 |
| NA | 5 | 45 | 0 | 6 | ||
| Liver (%) | 216 (40) | 388 (34) | 0.0165 | 10 (14) | 61 (20) | 0.2809 |
| NA | 5 | 45 | 0 | 6 | ||
| Lung (%) | 299 (55) | 580 (50) | 0.0805 | 28 (40) | 135 (44) | 0.5448 |
| NA | 5 | 45 | 0 | 6 | ||
| First line treatment (%) | <0.0001 | <0.0001 | ||||
| IL2/IFN | 283 (51) | 138 (12) | 61 (87) | 40 (13) | ||
| Chemotherapy | 255 (46) | 241 (20) | 7 (10) | 11 (4) | ||
| Anti-PD1 | 0 (0) | 252 (21) | 0 (0) | 111 (36) | ||
| Anti-CTLA-4 | 7 (1) | 306 (26) | 1 (1) | 116 (37) | ||
| BRAFi/MEKi | 4 (1) | 239 (20) | 1 (1) | 22 (7) | ||
| ICI (blinded) | 0 | 3 (<1) | 0 (0) | 2 (1) | ||
| Dabratram/Trametinib/Pembrolizumab | 0 | 5 (<1) | 0 (0) | 4 (1) | ||
| Ipilimumab/Nivolumab | 0 (0) | 15 (1) | 0 (0) | 6 (2) | ||
| Other d | 3 (1) | 3 (<1) | 0 (0) | 1 (<1) | ||
| Anti-CTLA-4 during 1.-7. line of therapy | <0.0001 | 0.4287 | ||||
| Yes | 131 (24) | 561 (47) | 39 (57) | 193 (62) | ||
| No | 419 (76) | 639 (53) | 30 (44) | 120 (38) | ||
| NA | 2 | 3 | 1 | 0 | ||
| Anti-PD1 during 1.-7. line of therapy | <0.0001 | <0.0001 | ||||
| Yes | 18 (3) | 529 (44) | 16 (24) | 241 (77) | ||
| No | 529 (97) | 669 (56) | 52 (77) | 72 (23) | ||
| NA | 5 | 4 | 2 | 0 | ||
| BRAFi/MEKi during 1.-7. line of therapy | <0.0001 | 0.5326 | ||||
| Yes | 46 (8) | 434 (36) | 19 (28) | 75 (24) | ||
| No | 503 (92) | 767 (64) | 50 (73) | 238 (76) | ||
| NA | 3 | 1 | 1 | 0 | ||
| Any anti-CTLA-4 or anti-PD1 during 1.-7. line of therapy | <0.0001 | <0.0001 | ||||
| Yes | 133 (24) | 790 (66) | 41 (59) | 290 (93) | ||
| No | 417 (76) | 411 (34) | 28 (41) | 23 (7) | ||
| NA | 2 | 1 | 1 | 0 | ||
| Best objective response (%) | <0.0001 | 0.5224 | ||||
| CR | 23 (8) | 105 (11) | 22 (35) | 97 (33) | ||
| PR | 47 (16) | 242 (26) | 18 (29) | 81 (28) | ||
| SD | 108 (36) | 237 (25) | 17 (27) | 66 (23) | ||
| PD | 126 (41) | 365 (39) | 6 (10) | 49 (17) | ||
| NA | 248 | 253 | 7 | 20 | ||
| Treatment lines received (%) | 0.0004 | 0.0054 | ||||
| 1 | 552 (100) | 1202 (100) | 70 (100) | 313 (100) | ||
| 2 | 206 (37) | 614 (51) | 47 (67) | 193 (62) | ||
| 3 | 79 (14) | 281 (23) | 32 (46) | 96 (31) | ||
| 4 | 30 (5) | 109 (9) | 21 (30) | 46 (15) | ||
| 5 | 20 (4) | 47 (4) | 19 (27) | 29 (9) | ||
| 6 | 5 (1) | 16 (1) | 5 (7) | 12 (4) | ||
| 7 | 0 (0) | 4 (<1) | 0 (0) | 4 (1) | ||
a Overall survival above 3 years; b chi-square-test; c analysis of BRAF mutation status was not performed; d dendritic cell vaccination, T cell therapy, intralesional IL2; PS = performance status; NA = not applicable; IL2/IFN = interleukin-2/interferon-alpha; ICI = immune checkpoint-inhibitor; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; ULN = upper level of normal.
Figure 2.
Kaplan–Meier curves of overall survival (OS) for patients with metastatic melanoma. (A) OS per treatment year. (B) OS per type of first-line treatment. (C) OS for patients with LDH above or below the upper level of normal (ULN) with or without immune checkpoint-inhibitor (ICI) therapy any time during their treatment course. (D) OS for patients in performance status (PS) 0–1 or 2–3 with or without ICI therapy any time during their treatment course. Ipi/Nivo = Ipilimumab/Nivolumab; IL2/IFN = interleukin-2/interferon-alpha.
2.1. Long-Term Survivors
The proportion of patients with survival above 3 years doubled from 13% in the pre-ICI era to 26% in the ICI era (p < 0.0001) (Table 1b). Long-term survivors in the ICI era were older and a higher proportion had received anti-PD1 at any time during their treatment course compared with long-term survivors in the pre-ICI era. There was no difference in disease stage distribution; PS; LDH level; and presence of CNS, liver, lung, or bone metastases between the two periods. It should be noted that almost no patients in PS 2 or above at baseline became long-term survivors in either treatment era, although there was a high amount of missing values. A similar tendency was not observed with elevated LDH level. Fewer long-term survivors needed additional treatment lines in the ICI era, as only 31% and 15% received third and fourth line treatment compared with 46% and 30% in the pre-ICI era (p = 0.0054), respectively. In other terms, a higher proportion of long-term survivors in the ICI era ended their treatment course after the second or the third treatment line compared with the pre-ICI era.
Lower disease stage at baseline was associated with a higher likelihood to become a long-term survivor both in the pre-ICI and the ICI era, as shown in Table 2. Interestingly, a higher proportion of patients with M1c became long-term survivors in the ICI era compared with the pre-ICI era (22% vs. 10%, p < 0.0001). Similarly, only 5% and 9% of patients that presented with liver or lung metastases became long-term survivors in the pre-ICI, which increased to 16% (p < 0.0001) and 23% (p < 0.0001) in the ICI era, respectively. For patients with bone metastases, there was no significant difference between the two periods.
Table 2.
Disease stage and metastatic sites according to survival status between 2008–2011 and 2012–2016.
| 2008–2011 | 2012–2016 | ||||
|---|---|---|---|---|---|
| Pts with OS < 3 Years (%) | Pts with OS ≥ 3 Years (%) | Pts with OS < 3 Years (%) | Pts with OS ≥ 3 Years (%) | p Value a | |
| Melanoma stage | |||||
| M1a | 44 (69) | 20 (31) | 84 (49) | 89 (51) | 0.0056 |
| M1b | 65 (80) | 16 (20) | 85 (59) | 59 (41) | 0.0012 |
| M1c | 264 (90) | 29 (10) | 490 (79) | 134 (22) | <0.0001 |
| M1d | 100 (95) | 5 (5) | 201 (89) | 25 (11) | 0.0632 |
| Metastatic sites | |||||
| Bone | 108 (91) | 11 (9) | 252 (86) | 41 (14) | 0.1883 |
| Liver | 205 (95) | 10 (5) | 327 (84) | 61 (16) | <0.0001 |
| Lung | 269 (91) | 28 (9) | 444 (77) | 135 (23) | <0.0001 |
a Pearson’s chi-square test. Pts = patients; OS = overall survival.
2.2. Progression-Free Long-Term Survival
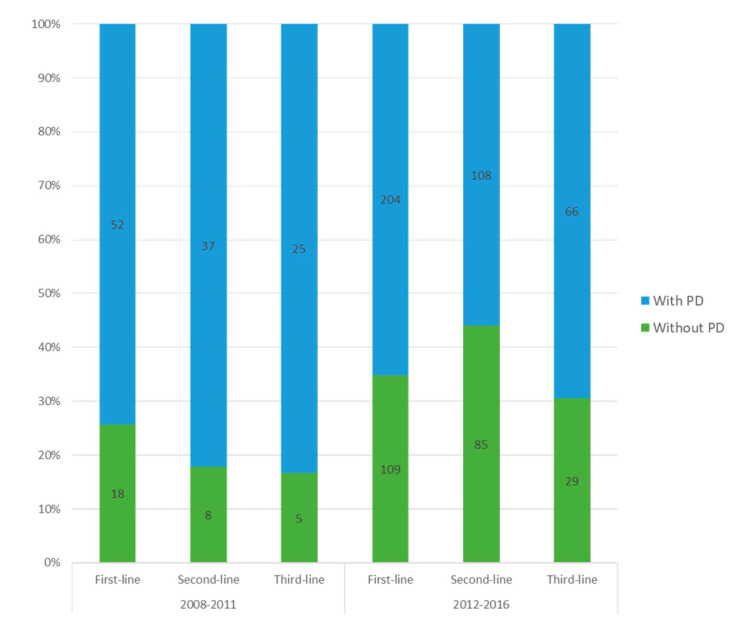
The absolute number of patients that received more than three lines of treatments was low and the following analyses were thus based on assessment of progression at the first to third line of treatment. In the pre-ICI era, the highest proportion of long-term survivors without progression was identified following first-line treatment (26%) and decreased at second-line (18%) and third-line (17%) treatment. In contrast, the proportion of long-term survivors without progression increased from first-line (35%) to second-line (44%) treatment and remained high (31%) at third-line treatment in the ICI era (Figure 3).
Figure 3.
Histogram of long-term survivors with or without progression on first-, second-, and third-line treatment. Number of patients is indicated on the bars; PD = progressive disease.
Long-term survivors without progression were pooled and the proportion increased significantly from 43% in the pre-ICI era to 70% in the ICI era (p < 0.0001). In the pre-ICI era, a third of patients without progression had received any ICI during their treatment course compared with over 90% in the ICI era, though a high proportion of patients progress on ICI in general. For the whole patient cohort, there was a significantly increase in patients without progression from 5% in the pre-ICI era to 18% in the ICI era (p < 0.0001).
2.3. Prognostic Factors for Improved Overall Survival
In univariate analyses, age below 70 years; female gender; PS of 0–1; cutaneous and mucosal melanoma type; normal LDH level; low disease stage; absence of CNS, bone, lung, and liver metastases; and ICI treatment were significantly associated with improved survival (Supplementary Table S1). Owing to the overlap between variables, disease stage and a combined ICI category were chosen together with age, gender, PS, melanoma type, and LDH to be entered in multivariate analysis using multiple imputations. Treatment with BRAFi/MEKi any time during their treatment course was not associated with survival in univariate analysis. Patients with a BRAF mutation treated with a BRAFi/MEKi had a higher proportion of poor prognostic baseline factors such as elevated LDH, poor PS, and higher disease stage compared with BRAF mutated patients who did not receive treatment with a BRAFi/MEKi any time during their treatment course (Supplementary Table S2). Fifty-two percent of patients treated with BRAFi/MEKi received it as their first-line treatment, while the majority of the non-BRAFi/MEKi treated patients received CTLA-4-inhibitor (33%) or IL2/IFN (24%) as their first-line treatment. It should be noted that some patients started their first-line treatment in the pre-ICI era and were tested later in their treatment course (data not shown).
The multivariate analysis confirmed known prognostic factors such as PS of 2 or above, elevated LDH level, and increasing disease stage together with ocular melanoma to be independently associated with decreased OS (Table 3). Treatment with ICI was significantly associated with a 61% (95% CI 0.35–0.44, p < 0.0001) reduction in the risk of death, irrespective of known prognostic risk factors.
Table 3.
Multivariate analysis with multiple imputations 2008–2016.
| HR a | 95% CI | p Value | |
|---|---|---|---|
| Age | 0.3876 | ||
| <70 years | 1.00 | ||
| ≥70 years | 1.05 | 0.93–1.19 | |
| Gender | 0.0886 | ||
| Female | 1.00 | ||
| Male | 1.10 | 0.98–1.23 | |
| PS | <0.0001 | ||
| 0–1 | 1.00 | ||
| ≥2 | 1.83 | 1.39–2.40 | |
| LDH | <0.0001 | ||
| Below ULN | 1.00 | ||
| Above ULN | 1.91 | 1.77–2.12 | |
| Stage | |||
| M1a | 1.00 | ||
| M1b | 1.27 | 1.01–1.61 | 0.0385 |
| M1c | 1.79 | 1.49–2.16 | <0.0001 |
| M1d | 2.64 | 2.14–3.28 | <0.0001 |
| Melanoma type | |||
| Cutaneous | 1.00 | ||
| Mucosal | 1.20 | 0.90–1.61 | 0.2071 |
| Ocular | 1.57 | 1.18–2.09 | 0.0016 |
| Unknown primary | 1.02 | 0.86–1.21 | 0.8156 |
| Any anti-CTLA-4 or anti-PD1 therapy b | <0.0001 | ||
| No | 1.00 | ||
| Yes | 0.39 | 0.35–0.44 |
a Cox regression analysis, p < 0.05 in univariate analyses as entry; b anytime during their treatment course. HR = hazard ratio; CI = confidence interval; PS = performance status; ULN = upper level of normal.
3. Discussion
In this nation-wide retrospective analysis, we have chosen two eras; that is, 2008–2011 and 2012–2016, to reflect the change in real-life practice as a very limited number of patients received ICI before 2012. We found that median OS improved from 8.3 months in the pre-ICI era to 11.3 months in the ICI era with a corresponding increase in the tail of the curve for real-life patients. Over the years, treatment changed from ineffective chemotherapy and toxic high-dose IL2/IFN to treatments with BRAFi/MEKi and ICI. High-dose IL2/IFN can induce long-term survival for a limited patient population, while patients with poor performance, with LDH more than two times above ULN or elevated neutrophils did not benefit [11,12]. The observed increase in the number of patients treated in 2012–2016 reflects the selection of a wider patient cohort owing to better treatment options with a more tolerable toxicity profile over time supported by a higher proportion of poor prognostic features in the expanded and older patient cohort. However, the age-adjusted incidence rate in malignant melanoma over the last 10 years is increasing in Denmark [13,14] and it is uncertain if this is also followed by a corresponding increase in metastatic melanoma.
There were only minor differences in the distribution of prognostic factors between the two periods for all patients, primarily in favor of the pre-ICI era with better PS and lower LDH levels. Thus, we consider it unlikely that the improved mOS and long-term survival can be explained by these differences in baseline characteristics. It was unexpected that treatment with BRAFi/MEKi did not affect OS, as it has been shown to improve OS in phase 3 trials [3,4]. Use of BRAFi/MEKi in national practice could be biased by patient selection towards patients with symptomatic large tumor burden, rapidly progressive disease, poor prognostic factors, or patients unfit for ICI, as indicated in our data. Moreover, the results could be influenced by BRAFi given as monotherapy in the early ICI era, because MEKi was implemented in Denmark from 2013. It is important to notice that our findings do not mean that BRAFi/MEKi is not an effective treatment. Still, it should be noticed that the findings from clinical trials are not directly referable to real-life patients and selection is essential.
Nonetheless, even with an improvement in mOS to 11.3 months, metastatic melanoma still has a dismal prognosis. However, there were significant further improvements since 2015 as anti-PD1 and combination ICI became standard first-line treatment. Moreover, it is encouraging that a higher proportion of patients obtained long-term survival without progression not only at first-line treatment, but also later treatment lines, and the need for further treatment lines declined over time in the ICI era. These findings might reflect that ipilimumab initially was approved as a second-line treatment and anti-PD1 treatment became available in later lines during the implementation period. Most long-term survivors had limited disease, but also, patients with M1b and M1c disease, and there were even indications of patients with M1d disease, had a higher chance of becoming a long-term survivor in the ICI era. Still, a large proportion of real-life patients do not benefit sufficiently from the new treatment possibilities and we find it concerning that almost no patients in PS 2 become long-term survivors.
Our survival data are in coherence with data from a large hospital-based cancer-registry that covers approximately 70% of all newly diagnosed cancers in the United States, although the percentual coverage of metastatic melanoma is uncertain [15]. Their database contained comprehensive information of pre-systemic treatment with data on prior surgery, comorbidity, insurance status, LDH level, and type of first-line treatment, but not later lines. In their retrospective dataset of more than 15,000 patients with synchronous metastatic melanoma, mOS improved from 8.4 months at pre-approval (of ICI and BRAFi) to 10.2 months at post-approval for patients treated for cutaneous stage 4 melanoma. Their 4-year OS improved from 18% to 23.5%, which is similar to our 4-year OS in the ICI era, but with a lower relative increase than in our nation-wide cohort. Baseline characteristics were only reported for patients receiving ICI, while survival was reported for all patients pre- and post-approval; therefore, it is uncertain if general baseline characteristics are similar between cohorts. Mangana et al. compared survival between treatment types in a retrospective real-life cohort of 395 patients treated at three Swiss hospitals [16]. Median OS for their reference chemotherapy (treatment-naïve) group from 2008 to 2009 was 7.1 months, and comparable to our pre-ICI group, while treatment-naïve patients on targeted therapy or ICI from 2010 to 2014 had an mOS of 14.6 months, which is considerably higher than both the American cohort and our cohort. It should be noted that there was a high proportion of CNS metastases in all patient cohorts, ranging between 33% to 53%, which could be owing to meticulous screening for CNS metastases before the treatment started and not only for symptomatic CNS metastases. In both studies, a description of metastatic sites for all treated patients besides CNS metastases, PS, or subsequent treatment lines received was not reported. On the other hand, both studies had a more detailed histopathological description of the primary tumor compared with ours. These differences may make direct comparison difficult.
Many factors such as comorbidity, PS, patient involvement, and/or symptomatic metastases influence the decision of treatment strategy. It is not possible to capture these considerations in a clinical trial and the evaluation of implementation of new treatment strategies in a nation-wide cohort over time is thus essential to evaluate if survival is improved in general, as well as to examine if it is the same real-life patient-type that becomes a long-term survivor. The collaboration and organization of the Danish health care system makes it possible to gather such real-life data on an almost complete national cohort of patients with metastatic melanoma, with the limitation being that missing values for LDH and PS and the lack of BRAF mutation status in the pre-ICI era might bias data.
4. Materials and Methods
4.1. Patient Population
All patients treated consecutively for metastatic melanoma between 1 January 2008 and 31 December 2016 were included in this retrospective study. Records were retrieved from the Danish Metastatic Melanoma Database (DAMMED). The database is nation-wide and includes data on baseline characteristics, disease stage, treatment lines received, response to treatment, reasons for treatment discontinuation, date of progression, and time of death. The database has an estimated coverage of >95% of all metastatic melanoma diagnoses within Denmark. Treatment is centralized at the departments of Oncology at Herlev, Odense, Aalborg, and Aarhus University Hospital.
The study was approved by the Danish Patient Safety Authority (3–3013-2306/1) and the Data Protection Agency (2012–58-0004).
4.2. Treatment and Response
Patients were treated according to best practice at time of referral with chemotherapy (primarily temozolomide), immunotherapy (combined high-dose interleukin-2 and interferon-alpha (IL2/IFN)), immune checkpoint-inhibitors (ipilimumab, pembrolizumab, nivolumab), BRAFi (vemurafenib, dabrafenib, encorafenib) alone or in combination with MEKi (cobimetinib, trametinib, binimetinib), or included in a clinical trial. Patients were analyzed according to treatment type, time-period, and survival below or above 3 years. Ipilimumab was approved in 2011 (EMA and FDA), however, the cut-off between the pre-ICI era (2008–2011) and the ICI era (2012–2016) was chosen as ipilimumab was generally implemented in the clinic in Denmark in 2012.
Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumor (RECIST) guidelines 1.0 [17]. Progression-free survival was defined as the time from initiation of systemic treatment to the date of documented disease progression or censored at last follow-up (31 December 2019). OS was defined as the time from initiation of first-line systemic treatment to death or censored at last follow-up.
4.3. Statistical Analyses
Differences in distributions of baseline characteristics were evaluated with the chi-square test. Median OS was analyzed with the Kaplan–Meier method and compared with the log-rank test. A p-value < 0.05 was considered statistically significant. Median follow-up time was analyzed with the reverse Kaplan–Meier method.
Univariate analysis was performed with the log-rank test and a significance level of 0.05 was chosen as an entry for the multivariate analysis using the Cox proportional hazard model with reported hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). Missing baseline values were imputed using multiple imputations with the chained equation method under the missing-at-random assumption.
Statistical analyses were performed with R [18].
5. Conclusions
The implementation of ICI has led to a significant increase in progression-free, long-term survival for real-life patients with metastatic melanoma.
Acknowledgments
The authors would like to thank Ulrich Heide Koehler (Braintrust consult, Denmark) and Tobias Wirenfeldt Klausen (Center for Cancer Immune Therapy (CCIT-DK), Department of Oncology, Herlev University Hospital, Denmark) for contributing with data extraction, and Tobias Wirenfeldt Klausen is acknowledged for discussion concerning statistical analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/9/2591/s1, Table S1: Univariate Analysis of Patient Characteristics 2008–2016. Table S2: Baseline characteristics for BRAF mutated patients treated with or without BRAFi/MEKi.
Author Contributions
Conceptualization, A.V.S., E.E., M.D., and I.M.S.; formal analysis, A.V.S.; investigation, A.V.S., E.E., L.B., H.S., M.D., and I.M.S.; data curation, E.E., L.B., and H.S.; writing—original draft preparation, A.V.S.; writing—review and editing, A.V.S., E.E., L.B., H.S., M.D., and I.M.S.; visualization, A.V.S., E.E., M.D., and I.M.S.; supervision, I.M.S.; project administration, A.V.S.; funding acquisition, I.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by government funds through the Ministry of Finance, Denmark. The Danish Metastatic Melanoma database received financial support from BMS, Roche, MSD, and Novartis. The funders have no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing; or in the decision to publish the results.
Conflicts of Interest
A.V.S. has no conflict of interest. E.E. received personal fees for lecture from Merck, BMS, and Roche and non-financial support from Merck and BMS. L.B. is on the advisory board/consultancy for Merck MSD, Novartis, Roche, BMS, Bayer, Eisai, and Sanofi-Genzyme. H.S. has received honoraria from MSD, Novartis, BMS, Incyte, and Pierre Fabre; and received a grant from MSD. M.D. has received honoraria for lectures from Roche and Novartis (past two years). I.M.S. is on the advisory board of MSD, Novartis, and Pierre Fabre; received a limited research grant from BMS; received honoraria for lectures from BMS, MSD, Pierre Fabre, Roche, and consultancy for Roche; received clinical trial fees from MSD and Novartis; and is the principal investigator on clinical trials from BMS, MSD, and Novartis of interest.
References
- 1.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A Ascierto P., Del Vecchio M., Robert C., Mackiewicz A., Chiarion-Sileni V., Arance A., Lebbé C., Bastholt L., Hamid O., Rutkowski P., et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611–622. doi: 10.1016/S1470-2045(17)30231-0. [DOI] [PubMed] [Google Scholar]
- 3.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C., Karaszewska B., Schachter J., Rutkowski P., Mackiewicz A., Stroyakovskiy D., Lichinitser M., Dummer R., Grange F., Mortier L., et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 5.Long G., Stroyakovskiy D., Gogas H., Levchenko E., De Braud F.G.M., Larkin J., Garbe C., Jouary T., Hauschild A., Grob J.-J., et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J., Ascierto P.A., Dréno B., Atkinson V., Liszkay G., Maio M., Mandalà M., Demidov L., Stroyakovskiy D., Thomas L., et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 7.Schachter J., Ribas A., Long G., Arance A., Grob J.-J., Mortier L., Daud A.I., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 8.Robert C., Long G., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 9.Donia M., Ellebaek E., Øllegaard T.H., Duval L., Aaby J.B., Hoejberg L., Køhler U.H., Schmidt H., Bastholt L., Svane I.M. The real-world impact of modern treatments on the survival of patients with metastatic melanoma. Eur. J. Cancer. 2019;108:25–32. doi: 10.1016/j.ejca.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Donia M., Kimper-Karl M.L., Høyer K.L., Bastholt L., Schmidt H., Svane I.M. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur. J. Cancer. 2017;74:89–95. doi: 10.1016/j.ejca.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Bastholt L., Svane I.M., Bjerregaard J.K., Herrstedt J., Hróbjartsson A., Schmidt H. High-dose interleukin-2 and interferon as first-line immunotherapy for metastatic melanoma: Long-term follow-up in a large unselected Danish patient cohort. Eur. J. Cancer. 2019;115:61–67. doi: 10.1016/j.ejca.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt H., Suciu S., Punt C.J., Gore M., Kruit W., Patel P., Lienard D., Von Der Maase H., Eggermont A.M.M., Keilholz U. Pretreatment Levels of Peripheral Neutrophils and Leukocytes As Independent Predictors of Overall Survival in Patients With American Joint Committee on Cancer Stage IV Melanoma: Results of the EORTC 18951 Biochemotherapy Trial. J. Clin. Oncol. 2007;25:1562–1569. doi: 10.1200/JCO.2006.09.0274. [DOI] [PubMed] [Google Scholar]
- 13.Helvind N.M., Hölmich L.R., Smith S., Glud M., Andersen K.K., Dalton S.O., Drzewiecki K.T. Incidence of In Situ and Invasive Melanoma in Denmark From 1985 Through 2012: A National Database Study of 24,059 Melanoma Cases. JAMA Dermatol. 2015;151:1087–1095. doi: 10.1001/jamadermatol.2015.1481. [DOI] [PubMed] [Google Scholar]
- 14.Nordcan Database. [(assessed on 8 June 2020)]; Available online: http://www-dep.iarc.fr/NORDCAN/DK/Table1.asp?cancer=310®istry=208&sYear=2007&eYear=2016&sex=1&type=0&eapc=2&age_from=1&age_to=18&submit=Execute.
- 15.Dobry A.S., Zogg C.K., Hodi F.S., Smith T.R., Ott P.A., Iorgulescu B. Management of metastatic melanoma: Improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol. Immunother. 2018;67:1833–1844. doi: 10.1007/s00262-018-2241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangana J., Cheng P.F., Kaufmann C., Amann V.C., Frauchiger A.L., Stögner V., Held U., Von Moos R., Michielin O., Braun R., et al. Multicenter, real-life experience with checkpoint inhibitors and targeted therapy agents in advanced melanoma patients in Switzerland. Melanoma Res. 2017;27:358–368. doi: 10.1097/CMR.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer E., Therasse P., Bogaerts J., Schwartz L., Sargent D.J., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [(assessed on 8 June 2020)]. Available online: http://www.R-project.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.