Abstract
Paramagnetic relaxation enhancement (PRE) is the current strategy of choice for enhancing magnetic resonance imaging (MRI) contrast and for accelerating MRI acquisition schemes. Yet, debates regarding lanthanides’ biocompatibility and PRE-effect on MRI signal quantification have raised the need for alternative strategies for relaxation enhancement. Herein, we show an approach for shortening the spin–lattice relaxation time (T1) of fluoride-based nanocrystals (NCs) that are used for in vivo 19F-MRI, by inducing crystal defects in their solid-crystal core. By utilizing a phosphate-based rather than a carboxylate-based capping ligand for the synthesis of CaF2 NCs, we were able to induce grain boundary defects in the NC lattice. The obtained defects led to a 10-fold shorter T1 of the NCs’ fluorides. Such paramagnetic-free relaxation enhancement of CaF2 NCs, gained without affecting either their size or their colloidal characteristics, improved 4-fold the obtained 19F-MRI signal-to-noise ratio, allowing their use, in vivo, with enhanced hotspot MRI sensitivity.
Keywords: 19F-MRI, nanocrystals, crystal engineering, relaxation enhancement, in vivo MRI, crystal defects
The ability to design and control the physical, chemical, electrical, optical, and magnetic properties of small-sized molecular solids has greatly advanced the field of nanocrystal (NC) engineering1−4 contributing to the development of nanomedicine.5 Among their various applications in nanomedicine, NCs are widely used as imaging agents in optical6 and photoacoustic imaging,7 computed tomography (CT),8 and magnetic resonance imaging (MRI),9 and the ability to engineer them in a desired manner has led to enhanced performance. Crystal engineering has been used, for example, to alter quantum dots’ size,10 shape,11 and fluorescent properties.12 Gold NCs have been engineered to have controllable sizes13 and shapes14 as a means to enhance their delivery and performance in both CT and photoacoustic imaging.15 For MRI applications, metal oxide NCs have been designed to have a multimetal core for enhanced sensitivity,16 manganese-oxide core for positive contrast,17 micrometer-size for single-cell visualization,18 or extremely small-size for T1 contrast enhancement.19
Nanosized inorganic fluoride (specifically, CaF2)20 NCs have been recently designed and implemented as imaging tracers benefiting from the advantageous background-free 19F-MRI.21−25 Their small size (<10 nm) and their inorganic solid core make them a unique category of 19F-MRI tracers, distinct from the extensively developed and frequently used perfluorocarbon (PFC) nanoemulsions,22,23 with the potential to be further developed for applications where small-sized NCs and tunable morphologies are essential.26,27 However, one of the main limitations of CaF2 NCs as 19F-MRI agents is their long spin–lattice relaxation time T1 (>10 s), which prolongs the time of data acquisition when signal averaging is needed for an improved signal-to-noise ratio (SNR). One potential strategy for efficient shortening of the T1 of the fluorine-19 content is to induce paramagnetic relaxation enhancement (PRE), which was efficiently demonstrated for large-sized PFC nanoemulsions,28−31 resulting in several-fold enhanced sensitivity of 19F-MRI. Nevertheless, alternatives for paramagnetic dopants need to be considered, not only to address recently raised concerns of lanthanide biocompatibility32,33 but also to allow robust quantification of the 19F-tracer distribution from the 19F-MR signal, as such quantification has been shown to be far from straightforward in solid-materials in the presence of dopants with a strong PRE effect.34 Herein, we propose an alternative to the commonly used PRE approach and show that synthetic induction of crystal defects in small-sized CaF2 NCs significantly shortens the T1 relaxation time of the fluoride within the NC, allowing improved in vivo19F-MRI sensitivity.
The area of surface chemistry bridges the gap between NCs’ fabrication and their properties and has been exploited for strategizing synthetic routes. One approach is to utilize the binding affinities of ligands to the surface of the NCs and their precursors in order to manipulate the morphology of nanomaterials.35 Based on the rationale that Ca2+ (a CaF2 precursor) binds more strongly to phosphate groups than to carboxylate groups,36 CaF2 NCs were synthesized with two different ligands, namely, oleic acid (OA, Figure 1a) and oleyl phosphate (OP, Figure 1b). Note that except for their headgroup, OA and OP share identical organic tails, allowing a similar outer coating, essential for their colloidal stability. Indeed, both OA-CaF2 and OP-CaF2 fabrications result in a similar small core size (8.3 ± 0.8 nm and 8.0 ± 1.3 nm, respectively, Figure 1a–c) and comparable colloidal diameter (10.5 ± 3.0 nm and 10.4 ± 2.9 nm, respectively, Figure 1d).
Figure 1.
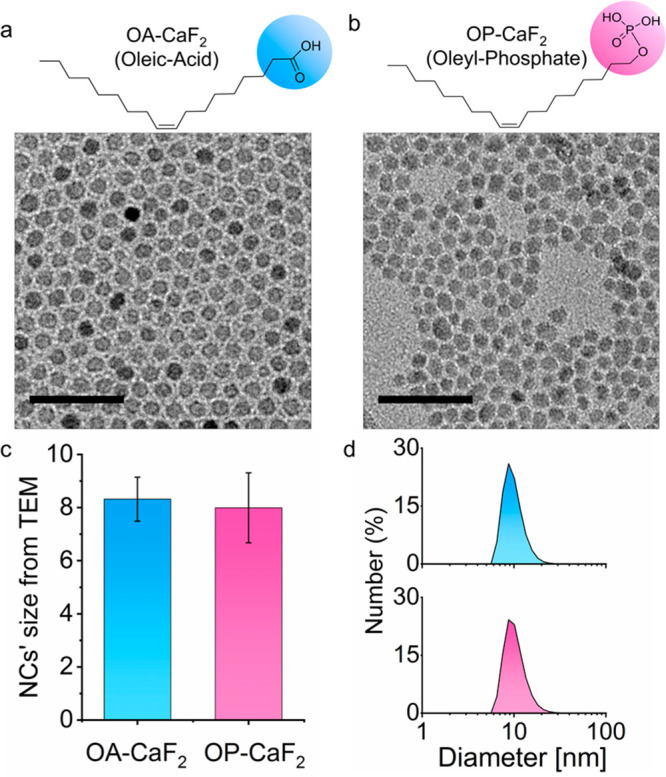
Characterization of OA-CaF2 and OP-CaF2 NCs: The molecular structures of the oleic acid (OA, light blue) and the oleyl phosphate (OP, pink) ligands used to synthesize OA-CaF2 (a) and OP-CaF2 (b), respectively, and representative TEM images of the NCs (scale bar 50 nm). (c) Average diameter of OA-CaF2 (8.3 ± 0.8 nm) and OP-CaF2 NCs (8.0 ± 1.3 nm) as obtained from the TEM images. (d) The colloidal diameter of dispersed OA-CaF2 (10.5 ± 3.0 nm) and OP-CaF2 NCs (10.4 ± 2.9 nm) as obtained from DLS measurements.
High-resolution TEM (HR-TEM) analysis of the crystal structure of OP-CaF2 and OA-CaF2 at the atomic level revealed a remarkable difference in the crystal architecture of the two types of NCs (Figure 2a and Figure 2b). While OA-CaF2 NCs exhibited a well-ordered, highly crystalline lattice (Figure 2a), OP-CaF2 NCs featured clear crystal defects, i.e., grain boundaries (Figure 2b). This observation was further validated by powder X-ray diffraction (XRD) measurements of dried samples of OA-CaF2 (Figure 2c) and OP-CaF2 (Figure 2d) NCs, showing wider XRD patterns for the disordered OP-CaF2 NCs, as demonstrated for other inorganic materials.37 An additional indication for the polycrystallinity of OP-CaF2 NCs was obtained using Raman spectroscopy (Figure S1); both line broadening and Raman shifts were observed for OP-CaF2 NCs (as compared to powders of commercial CaF2 and OA-CaF2), which can be assigned to a smaller grain size within the polycrystalline material. We attribute the crystallographic differences between OP-CaF2 and OA-CaF2 NCs to different growth paths mediated by the nature of the surface ligands present during the synthesis.38,39
Figure 2.
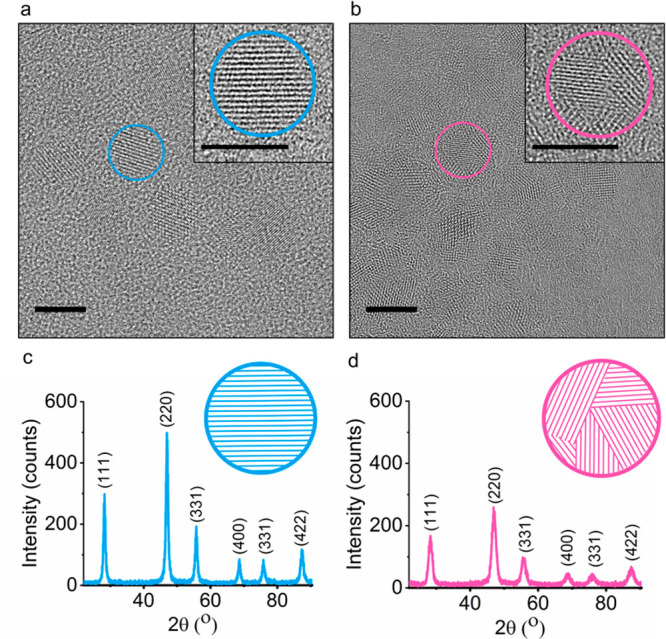
Characterization of OA crystalline features of OA-CaF2 and OP-CaF2 NCs. (a, b) HR-TEM image (scale bar 10 nm, in inset 5 nm) of (a) OA-CaF2 and (b) OP-CaF2 NCs. (c, d) Powder XRD patterns of dry samples of (c) OA-CaF2 and (d) OP-CaF2 NCs.
It was previously shown that crystallographic defects, induced by mechanical stress of large-size CaF2 crystals, may facilitate element mobility and enhance dipolar interaction, which could induce T1 shortening40,41 without the use of paramagnetic elements. Encouraged by these studies and with the vision of using CaF2 NCs as nanosized tracers for 19F-MRI applications, we studied the effect of their crystal properties on their 19F-NMR characteristics using a liquid-state high-resolution 19F-NMR setup. Notably, both dispersed OA-CaF2 and OP-CaF2 NCs produced similar 19F-NMR spectra (Figure S2), with a typical CaF2 peak at −109 ppm. Interestingly and importantly for their use as nanotracers in 19F-MRI applications, we found a dramatic difference in the T1 values of the 19F fluoride signal in the colloidal CaF2 NCs (Figure S3a,b), a result of the pronounced grain boundary defects in OP-CaF2 NCs (Figure 2b). This 10-fold reduction in T1 should allow significant improvement in the sensitivity of 19F-MRI studies when using OP-CaF2 compared to OA-CaF2 NCs. Note that the short T2 that is characteristic to nanofluorides was similar in both fabrications (Figure S3c,d); nevertheless, such limitation could be overcome by using an MRI scheme such as ultrashort TE (UTE) or zero TE (ZTE), found to be applicable to both nanofluorides20 and paramagnetic PFCs.29
Next, we assessed whether the significant T1 relaxation enhancement observed for polycrystalline nanofluorides (OP-CaF2) as compared to crystalline nanofluorides (OA-CaF2) in organic solvents could be translatable to improved 19F-MRI sensitivity in vivo. For that purpose, both fabrications were transferred from an organic solvent (cyclohexane) to an aqueous solution by incorporating phospholipids (PLs) into the hydrophobic tails of their capping ligands and stabilizing these colloids with cholesterol content and polyethylene-glycol-modified phospholipids (Figure 3a and Figure S4). The resultant colloids endowed both PL-OA-CaF2 and PL-OP-CaF2 NCs with water solubility and colloidal stability characteristics suitable for in vivo19F-MRI tracers (Figure S5). The stability of the water dispersed NCs in aqueous media was studied for 40 days by both DLS measurements and high-resolution 19F-NMR spectroscopy (Figure S7), showing their long-term stability and resistance to degradation when stored for future uses. It is important to mention here that the 10-fold difference in the T1 values of the two types of nanofluorides was preserved for water-dispersed NCs, with 11 ± 0.2 s for PL-OA-CaF2 and 1 ± 0.2 s for PL-OP-CaF2 NCs (Figure 3d and Figure S6).
Figure 3.
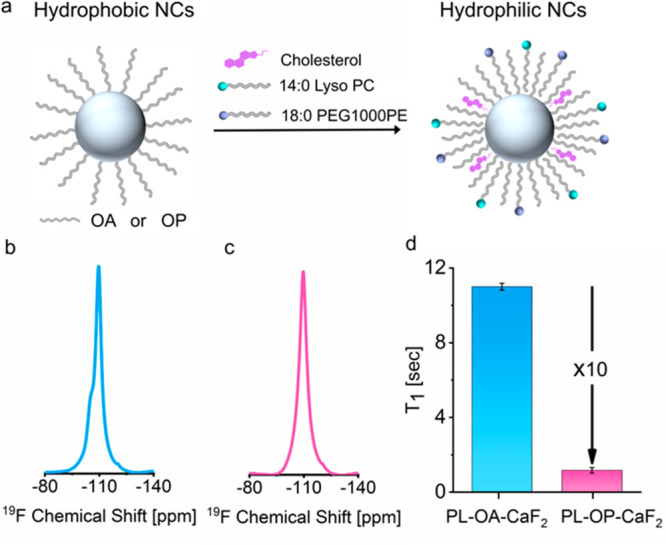
High-resolution 19F-NMR properties of CaF2 NCs in water. (a) Schematic representation of phase transfer (from organic solvent to water) via ligand incorporation of phospholipids (molecular structures in Figure S4). 19F-NMR spectra of (b) PL-OA-CaF2 (light-blue) and (c) PL-OP-CaF2 (pink) dispersed in water. (d) 19F-T1-relaxation times for PL-OA-CaF2 (11.0 ± 0.2 s) and PL-OP-CaF2 NCs (1.2 ± 0.2 s).
In order to quantify the improvement in 19F-MRI sensitivity upon T1 shortening, a phantom composed of two tubes with the same 19F concentration, one containing water-dispersed PL-OP-CaF2 NCs and the other containing water-dispersed PL-OA-CaF2 NCs, was studied (Figure 4a). Indeed, a four times higher SNR was obtained in 19F-MRI for the tube containing the PL-OP-CaF2 as compared to that of PL-OA-CaF2 NCs (Figure 4b,c), acquired with a UTE sequence to detect the 19F-MR signal of the fluorides in the NCs.20 The 10-fold shorter T1 of the fluorides in PL-OP-CaF2 allowed us to shorten dramatically the repetition time (TR) and, thus, to increase the number of signal averages for a given time of acquisition or to shorten the total scan time for a given number of signal averages. Note that in order to obtain a comparable 19F-MRI SNR from the crystalline PL-OA-CaF2 NCs, a much longer TR was needed, and consequently, a more than 1 h acquisition time to allow the same number of signal averages would be required (compared to the 6.5 min needed for TR = 4.2 ms, Figure 4b,c). In order to obtain a comparable SNR of the two types of NCs using a single-scan acquisition, a ten-time longer acquisition was needed for PL-OA-CaF2 NCs as compared to that required for PL-OP-CaF2 NCs (Figure S8).
Figure 4.
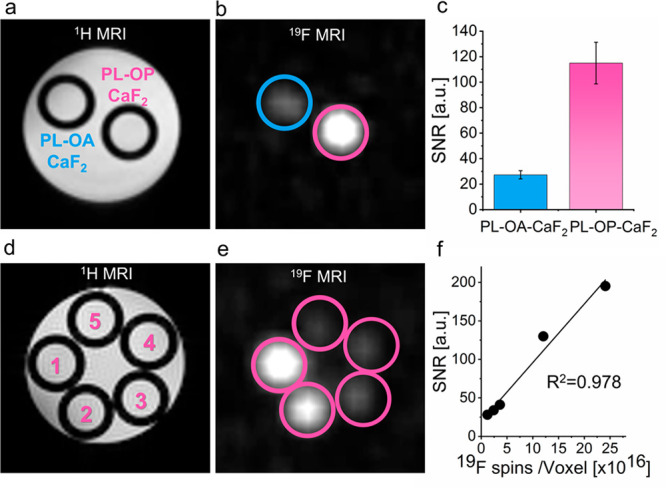
19F-MRI of CaF2 NCs. (a) 1H-MRI and (b) 19F-MRI of a phantom composed of two tubes containing either PL-OA-CaF2 or PL-OP-CaF2 NCs. For 19F-MRI data, a 3D-UTE sequence was used. (c) Calculated SNR values for of PL-OA-CaF2 (27 ± 3) and of PL-OP-CaF2 (115 ± 16) as measured from the 19F-MRI in b. SNR and (d) 1H-MRI and (e) 19F-MRI of a phantom composed of five tubes containing different concentrations of PL-OP-CaF2 (i.e., total 19F): 100, 50, 15, 10, and 5 mM (1–5, respectively, in d). (f) SNR as a function of 19F atoms per voxel (4 mm3), as obtained from the data in e.
To quantify the improved sensitivity in 19F-MRI experiments and to examine the detectability level of PL-OP-CaF2 NCs, a series of tubes containing a range of concentrations was prepared and studied (Figure 4d–f). Notably, by shortening the T1 values of the nanofluorides by one order of magnitude, we were able to detect low 19F concentrations down to 5 mM, 10 times lower than the detectability level of the highly crystalline CaF2.20 For example, an SNR of 28 in the 19F-MRI of the studied phantom was obtained with a 5 mM 19F-concentration (equivalent to 1.2 × 1016 fluorine spins) with a voxel size of 4 mm3, a level of 19F-MRI detectability comparable to that of the commonly used PFC nanoemulsions.42 The very long T2 values of PFCs, however, allows us to acquire their 19F-MRI data using multi-echo-based schemes (i.e., RARE or FSE) and thus provide them with essential improved sensitivity for a given time of data acquisition when directly compared to PL-OP-CaF2 NCs (Figure S9 and Table S1). Nevertheless, it is important to mention that while the short T2 limitation of PL-OP-CaF2 could be overcome by using a UTE-MRI scheme, such sequences are still in their infancy. Therefore, we expect that more advanced UTE protocols that allow multi-echo readouts43 and those based on compressed sensing44 should further improve the SNR/time-unit of 19F-MRI data that is based on nanofluorides even at their given short T2 values.
Moreover, and very importantly, the fact that PL-OP-CaF2 NCs and PFC-based emulsions differ in size by one order of magnitude (i.e., ∼10 nm for CaF2 and ∼100 nm for PFCs, Figure S10a) show that each of the nanoformulations could be used and may be more applicable for different approaches due to their expected different biodistribution, clearance profiles, and accessibility to a desired target. Showing that the chemical shift in the 19F-NMR of PFC-based nanoemulsions (−91 ppm for VS1000) differs from that of CaF2 NCs (−109 ppm) by almost 20 ppm demonstrates the potential of using the two nanofabrications in future “multicolor” 19F-MRI studies (Figure S10b–h). Thus, capitalizing on this multiplexing feature, given their very different hydrodynamic diameter (Figure S10a), may open new opportunities to combine these nanoformulations for noninvasive multiplexed imaging, for example, in studies where the size of the imaging agent is essential.
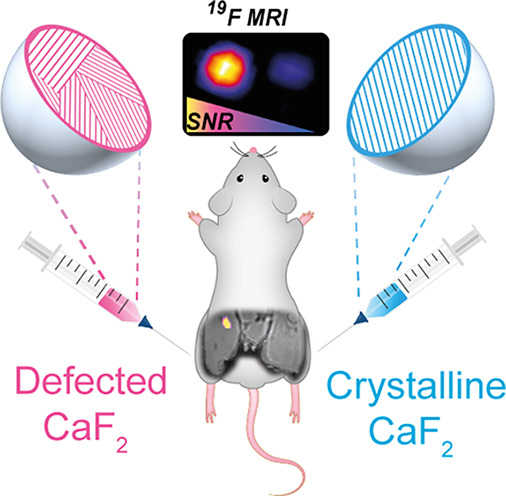
Finally, to evaluate the potential of PL-OP-CaF2 NCs as imaging tracers for in vivo 19F-MRI applications and to determine the gain in sensitivity, noninvasively, in a live intact subject, both designed NCs were intramuscularly injected into mouse legs (Figure 5a) after determining their cytotoxicity profile using three different cell-based assays, namely, (i) CCK-8 assay (Figure S11), (ii) MTT assay (Figure S12), and (iii) LDH-cytotoxicity assay (Figure S13). A localized 19F-NMR spectrum of each leg showed a comparable intensity of the CaF2 peak (−109 ppm; Figure 5b,c and Figure S14) when the acquisition parameters were adjusted to the T1 properties of each formulation, indicating the comparable CaF2 concentration in the two injection sites. Significantly, although the same fluoride content was confirmed for both injections, a notable 19F-MRI signal was picked up only in the leg injected with PL-OP-CaF2 NCs (Figure 5d,e), which could be displayed as a “hotspot” map overlaid on anatomical high-resolution 1H-MRI (Figure 5f). These results demonstrate that, while avoiding the use of paramagnetic elements and without introducing the PRE-effect for shortening T1 values, we were able to extensively enhance the longitudinal relaxation rates of small-sized fluoride-NCs to improve 19F-MRI performances.
Figure 5.
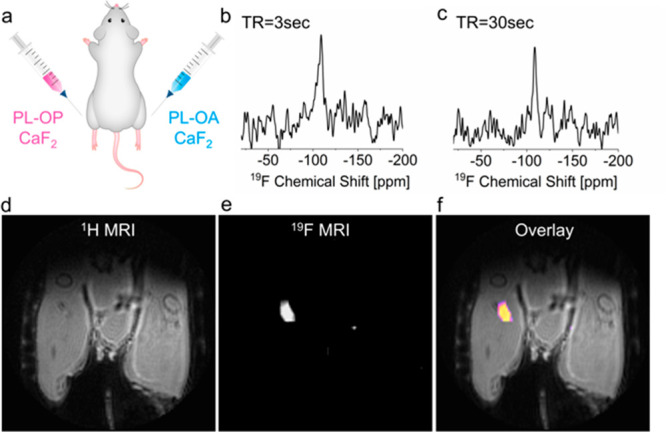
In vivo 19F-NMR and 19F-MRI of injected PL-OA-CaF2 and PL-OP-CaF2 NCs. (a) Scheme of the injection setup. (b) 19F-ISIS spectra acquired from the right leg (PL-OP- CaF2 injection) using TR = 3 s and (c) from the left leg (PL-OA-CaF2 injection) using TR = 30 s. (d) 1H-MRI, (e) 19F-MRI (acquired with a 3D-UTE sequence), and (f) 19F-MRI shown as a pseudocolor map overlaid on the anatomical 1H-MR image of a live mouse.
In summary, we propose here a paramagnetic-free approach for T1-relaxation enhancement as an alternative to the extensively used PRE effect, avoiding the need for paramagnetic elements in MRI studies. We demonstrate that inducing defects in small-sized nanofluorides allows us to shorten the T1 of their fluoride content by 10-fold, resulting in a 4-fold increase in the SNR of 19F-MRI studies at a given scan time. While PRE has been at the core of many MRI studies for many decades,45 allowing researchers to shorten both transverse46 and longitudinal47 relaxation times for enhanced image contrast, it has been exploited also to shorten the T1 values of fluorinated materials29 for improved SNR in 19F-MRI studies. Our demonstration that controlling the synthetic conditions of fluoride-based NCs and engineering crystal defects (specifically, grain boundaries) to shorten the T1 of nanofluorides, which together with their manifested in vivo capabilities, offers a novel strategy for fabricating paramagnetic-free nanotracers for in vivo 19F MRI studies. While there is still a scope for shortening the T1 of nanoflurides, the presented approach for nanocrystalline-defects relaxation enhancement (NDRE) should be further developed by using other strategies to rationalize architecture-relaxation relationships in NCs that are proposed as imaging nanotracers for “hotspot” MRI, even beyond nanofluorides.
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 677715).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.nanolett.0c02549.
Experimental methods and supplementary figures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Talapin D. V.; Lee J. S.; Kovalenko M. V.; Shevchenko E. V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110 (1), 389–458. 10.1021/cr900137k. [DOI] [PubMed] [Google Scholar]
- Yin Y.; Alivisatos A. P. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 2005, 437 (7059), 664–70. 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- De Trizio L.; Manna L. Forging Colloidal Nanostructures via Cation Exchange Reactions. Chem. Rev. 2016, 116 (18), 10852–87. 10.1021/acs.chemrev.5b00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer-Jungemann A.; Feliu N.; Bakaimi I.; Hamaly M.; Alkilany A.; Chakraborty I.; Masood A.; Casula M. F.; Kostopoulou A.; Oh E.; Susumu K.; Stewart M. H.; Medintz I. L.; Stratakis E.; Parak W. J.; Kanaras A. G. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119 (8), 4819–4880. 10.1021/acs.chemrev.8b00733. [DOI] [PubMed] [Google Scholar]
- Blanco E.; Shen H.; Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33 (9), 941–51. 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X.; Pinaud F. F.; Bentolila L. A.; Tsay J. M.; Doose S.; Li J. J.; Sundaresan G.; Wu A. M.; Gambhir S. S.; Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307 (5709), 538–44. 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta I.; Dichiarante V.; Pigliacelli C.; Cavallo G.; Terraneo G.; Bombelli F. B.; Metrangolo P.; Resnati G. 19F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem. Rev. 2015, 115 (2), 1106–1129. 10.1021/cr500286d. [DOI] [PubMed] [Google Scholar]
- Popovtzer R.; Agrawal A.; Kotov N. A.; Popovtzer A.; Balter J.; Carey T. E.; Kopelman R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008, 8 (12), 4593–6. 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh Y. M.; Jun Y. W.; Song H. T.; Kim S.; Choi J. S.; Lee J. H.; Yoon S.; Kim K. S.; Shin J. S.; Suh J. S.; Cheon J. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J. Am. Chem. Soc. 2005, 127 (35), 12387–91. 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- Cademartiri L.; Montanari E.; Calestani G.; Migliori A.; Guagliardi A.; Ozin G. A. Size-dependent extinction coefficients of PbS quantum dots. J. Am. Chem. Soc. 2006, 128 (31), 10337–46. 10.1021/ja063166u. [DOI] [PubMed] [Google Scholar]
- Peng X.; Manna L.; Yang W.; Wickham J.; Scher E.; Kadavanich A.; Alivisatos A. P. Shape control of CdSe nanocrystals. Nature 2000, 404 (6773), 59–61. 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- Xiong H. M.; Shchukin D. G.; Mohwald H.; Xu Y.; Xia Y. Y. Sonochemical synthesis of highly luminescent zinc oxide nanoparticles doped with magnesium(II). Angew. Chem., Int. Ed. 2009, 48 (15), 2727–31. 10.1002/anie.200805590. [DOI] [PubMed] [Google Scholar]
- Chhour P.; Kim J.; Benardo B.; Tovar A.; Mian S.; Litt H. I.; Ferrari V. A.; Cormode D. P. Effect of Gold Nanoparticle Size and Coating on Labeling Monocytes for CT Tracking. Bioconjugate Chem. 2017, 28 (1), 260–269. 10.1021/acs.bioconjchem.6b00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.; Bao L.; Zhang C.; Lin J.; Luo T.; Yang D.; He M.; Li Z.; Gao G.; Gao B.; Fu S.; Cui D. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials 2011, 32 (36), 9796–809. 10.1016/j.biomaterials.2011.08.086. [DOI] [PubMed] [Google Scholar]
- Chen Y. S.; Zhao Y.; Yoon S. J.; Gambhir S. S.; Emelianov S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14 (5), 465–472. 10.1038/s41565-019-0392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H.; Huh Y. M.; Jun Y. W.; Seo J. W.; Jang J. T.; Song H. T.; Kim S.; Cho E. J.; Yoon H. G.; Suh J. S.; Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13 (1), 95–9. 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- Kim T.; Momin E.; Choi J.; Yuan K.; Zaidi H.; Kim J.; Park M.; Lee N.; McMahon M. T.; Quinones-Hinojosa A.; Bulte J. W.; Hyeon T.; Gilad A. A. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. J. Am. Chem. Soc. 2011, 133 (9), 2955–61. 10.1021/ja1084095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E. M.; Skrtic S.; Sharer K.; Hill J. M.; Dunbar C. E.; Koretsky A. P. MRI detection of single particles for cellular imaging. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (30), 10901–6. 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. H.; Lee N.; Kim H.; An K.; Park Y. I.; Choi Y.; Shin K.; Lee Y.; Kwon S. G.; Na H. B.; Park J. G.; Ahn T. Y.; Kim Y. W.; Moon W. K.; Choi S. H.; Hyeon T. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133 (32), 12624–31. 10.1021/ja203340u. [DOI] [PubMed] [Google Scholar]
- Ashur I.; Allouche-Arnon H.; Bar-Shir A. Calcium Fluoride Nanocrystals: Tracers for In Vivo 19F Magnetic Resonance Imaging. Angew. Chem., Int. Ed. 2018, 57 (25), 7478–7482. 10.1002/anie.201800838. [DOI] [PubMed] [Google Scholar]
- Ahrens E. T.; Flores R.; Xu H.; Morel P. A. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 2005, 23 (8), 983–7. 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- Flogel U.; Ding Z.; Hardung H.; Jander S.; Reichmann G.; Jacoby C.; Schubert R.; Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 2008, 118 (2), 140–8. 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta I.; Mastropietro A.; Cordiglieri C.; Gazzera L.; Baggi F.; Baselli G.; Bruzzone M. G.; Zucca I.; Cavallo G.; Terraneo G.; Baldelli Bombelli F.; Metrangolo P.; Resnati G. A superfluorinated molecular probe for highly sensitive in vivo(19)F-MRI. J. Am. Chem. Soc. 2014, 136 (24), 8524–7. 10.1021/ja503270n. [DOI] [PubMed] [Google Scholar]
- Thurecht K. J.; Blakey I.; Peng H.; Squires O.; Hsu S.; Alexander C.; Whittaker A. K. Functional hyperbranched polymers: toward targeted in vivo 19F magnetic resonance imaging using designed macromolecules. J. Am. Chem. Soc. 2010, 132 (15), 5336–7. 10.1021/ja100252y. [DOI] [PubMed] [Google Scholar]
- Nakamura T.; Sugihara F.; Matsushita H.; Yoshioka Y.; Mizukami S.; Kikuchi K. Mesoporous silica nanoparticles for (19)F magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6 (3), 1986–1990. 10.1039/C4SC03549F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral H.; Matsumoto Y.; Mizuno K.; Chen Q.; Murakami M.; Kimura M.; Terada Y.; Kano M. R.; Miyazono K.; Uesaka M.; Nishiyama N.; Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6 (12), 815–23. 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- Betzer O.; Shilo M.; Opochinsky R.; Barnoy E.; Motiei M.; Okun E.; Yadid G.; Popovtzer R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: an in vivo study. Nanomedicine 2017, 12 (13), 1533–1546. 10.2217/nnm-2017-0022. [DOI] [PubMed] [Google Scholar]
- de Vries A.; Moonen R.; Yildirim M.; Langereis S.; Lamerichs R.; Pikkemaat J. A.; Baroni S.; Terreno E.; Nicolay K.; Strijkers G. J.; Grull H. Relaxometric studies of gadolinium-functionalized perfluorocarbon nanoparticles for MR imaging. Contrast Media Mol. Imaging 2014, 9 (1), 83–91. 10.1002/cmmi.1541. [DOI] [PubMed] [Google Scholar]
- Kislukhin A. A.; Xu H.; Adams S. R.; Narsinh K. H.; Tsien R. Y.; Ahrens E. T. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat. Mater. 2016, 15 (6), 662–8. 10.1038/nmat4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi A. H.; Wang C.; Adams S. R.; Zhu W.; Narsinh K.; Xu H.; Gray D. L.; Tsien R. Y.; Ahrens E. T. Fluorous-Soluble Metal Chelate for Sensitive Fluorine-19 Magnetic Resonance Imaging Nanoemulsion Probes. ACS Nano 2019, 13 (1), 143–151. 10.1021/acsnano.8b04881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q.; Li Y.; Bo S.; Yuan Y.; Yang Z.; Chen S.; Zhou X.; Jiang Z. X. Paramagnetic nanoemulsions with unified signals for sensitive (19)F MRI cell tracking. Chem. Commun. 2018, 54 (47), 6000–6003. 10.1039/C8CC02938E. [DOI] [PubMed] [Google Scholar]
- Rogosnitzky M.; Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals 2016, 29 (3), 365–76. 10.1007/s10534-016-9931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. M.; Atanasova I. P.; Blasi F.; Ay I.; Caravan P. A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc. 2015, 137 (49), 15548–57. 10.1021/jacs.5b10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Zhang Q.; Joos J. J.; Smet P. F.; Schmedt auf der Günne J. Blind spheres of paramagnetic dopants in solid state NMR. Phys. Chem. Chem. Phys. 2019, 21 (19), 10185–10194. 10.1039/C9CP00953A. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Dobbie A. R.; Cui K.; Veinot J. G. A convenient method for preparing alkyl-functionalized silicon nanocubes. J. Am. Chem. Soc. 2012, 134 (34), 13958–61. 10.1021/ja3061497. [DOI] [PubMed] [Google Scholar]
- Gebauer D.; Cölfen H.; Verch A.; Antonietti M. The Multiple Roles of Additives in CaCO3 Crystallization: A Quantitative Case Study. Adv. Mater. 2009, 21 (4), 435–439. 10.1002/adma.200801614. [DOI] [Google Scholar]
- Abdellatief M.; Abele M.; Leoni M.; Scardi P. Solid State Nuclear Magnetic Resonance and X-ray Diffraction Line Profile Analysis of heavily deformed fluorite. Thin Solid Films 2013, 530, 44–48. 10.1016/j.tsf.2012.09.020. [DOI] [Google Scholar]
- Pan A.; He B.; Fan X.; Liu Z.; Urban J. J.; Alivisatos A. P.; He L.; Liu Y. Insight into the Ligand-Mediated Synthesis of Colloidal CsPbBr3 Perovskite Nanocrystals: The Role of Organic Acid, Base, and Cesium Precursors. ACS Nano 2016, 10 (8), 7943–54. 10.1021/acsnano.6b03863. [DOI] [PubMed] [Google Scholar]
- Sun S.; Yuan D.; Xu Y.; Wang A.; Deng Z. Ligand-Mediated Synthesis of Shape-Controlled Cesium Lead Halide Perovskite Nanocrystals via Reprecipitation Process at Room Temperature. ACS Nano 2016, 10 (3), 3648–57. 10.1021/acsnano.5b08193. [DOI] [PubMed] [Google Scholar]
- Heitjans P.; Indris S. J. J. o. m. s. Fast diffusion in nanocrystalline ceramics prepared by ball milling. J. Mater. Sci. 2004, 39 (16–17), 5091–5096. 10.1023/B:JMSC.0000039189.17243.72. [DOI] [Google Scholar]
- Ruprecht B.; Wilkening M.; Steuernagel S.; Heitjans P. J. J. o. m. c. Anion diffusivity in highly conductive nanocrystalline BaF2: CaF2 composites prepared by high-energy ball milling. J. Mater. Chem. 2008, 18 (44), 5412–5416. 10.1039/b811453f. [DOI] [Google Scholar]
- Waiczies S.; Millward J. M.; Starke L.; Delgado P. R.; Huelnhagen T.; Prinz C.; Marek D.; Wecker D.; Wissmann R.; Koch S. P.; Boehm-Sturm P.; Waiczies H.; Niendorf T.; Pohlmann A. Enhanced Fluorine-19 MRI Sensitivity using a Cryogenic Radiofrequency Probe: Technical Developments and Ex Vivo Demonstration in a Mouse Model of Neuroinflammation. Sci. Rep. 2017, 7 (1), 9808. 10.1038/s41598-017-09622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbalski P. J.; Cochran A. S.; Akinyi T. G.; Thomen R. P.; Fugate E. M.; Lindquist D. M.; Pratt R. G.; Cleveland Z. I. Preclinical hyperpolarized (129) Xe MRI: ventilation and T2 * mapping in mouse lungs at 7 T using multi-echo flyback UTE.. NMR Biomed. 2020, 33 (7), e4302 10.1002/nbm.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabich H. T.; Benning M.; Sederman A. J.; Holland D. J. Ultrashort echo time (UTE) imaging using gradient pre-equalization and compressed sensing. J. Magn. Reson. 2014, 245, 116–24. 10.1016/j.jmr.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Lauffer R. B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem. Rev. 1987, 87 (5), 901–927. 10.1021/cr00081a003. [DOI] [Google Scholar]
- Na H. B.; Song I. C.; Hyeon T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21 (21), 2133–2148. 10.1002/adma.200802366. [DOI] [Google Scholar]
- Caravan P.; Ellison J. J.; McMurry T. J.; Lauffer R. B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99 (9), 2293–2352. 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


