Abstract

A highly enantioselective and diastereoselective total synthesis of the diterpenoid (−)-mitrephorone A is presented. Key to the synthesis are stereocontrolled 1,4-semihydrogenation of a 1,3-diene to a tetrasubstituted double bond, enzyme-catalyzed malonate desymmetrization, and highly diastereoselective nitrile oxide cycloaddition. The streamlined strategy is a considerable improvement to those reported earlier in terms of diastereo- and enantioselectivity. For the first time, the combination of modern Pd-cross-coupling with Cr-catalyzed reduction allows for rapid access to tetrasubstituted olefins with full stereocontrol.
Introduction
(−)-Mitrephorone A (1) is a trachylobane natural product characterized by a pentacyclic carbon skeleton, which includes a tricyclo[3.2.1.02,7]octane (Scheme 1).1,2 The carbon framework of 1 encompasses at the core a unique fully substituted oxetane. Its five contiguous stereocenters along with its complex caged structure render 1 a formidable target for stereoselective synthesis.3,4 In addition to synthetic challenges, (−)-mitrephorone A (1) exhibits cytostatic activity against a number of bacterial and fungal pathogens as well as cytotoxicity against selected cancer cell lines (MCF-7, H460, SF-268).1,2,5
Scheme 1. (−)-Mitrephorone A (1) and Retrosynthetic Analysis.

We have previously reported the first and enantioselective total synthesis of (−)-mitrephorone A (1), which lacked diastereocontrol.3 Herein, we report a new route for a highly enantio- and diastereoselective synthesis of 1. Our retrosynthetic analysis involved disconnection of (−)-mitrephorone A (1) to 2 (Scheme 1). The 1,3-relationship of ketone and tertiary alcohol in 2 is a partial retron for a nitrile oxide cycloaddition strategy via isoxazoline 3.6 We wondered whether the stereogenic center α to the nitrile oxide in 4 would lead to control over facial selectivity in an intramolecular dipolar cycloaddition reaction (4 → 3). A key requirement of this approach is the stereoselective synthesis of the tetrasubstituted olefin embedded in 4. Although traditional approaches to such an olefin might feature condensation reactions of the corresponding tricyclic ketone, we envisioned a different strategy involving diene 5. At the outset, it was not clear how we would control the configuration of a tetrasubstituted olefin. The approach we describe reveals an effective solution to the synthesis of tetrasubstituted olefins, enabling diastereoselective functionalization of chiral tricyclo[3.2.1.02,7]octanes and related structures. More broadly, the new retrosynthetic plan outlined in Scheme 1 leads to novel stereodefined strategies to the caged structure of (−)-mitrephorone A (1) and other trachylobanes.
The successful use of olefin 4 in our synthesis requires control over both facial selectivity and olefin geometry. To address the former, we conceived of an approach involving an intramolecular annulation reaction. The strategic design of precursors such as 9 includes a resident stereogenic center (*) along the connecting backbone as a stereochemical controlling feature (Scheme 2). In addressing the latter, it is important to note that addition reactions to tetrasubstituted olefin 9 set two stereocenters, and olefin geometry dictates their relative configuration ((E)-9 → 10 vs (Z)-9 → 11).
Scheme 2. Stereochemical Considerations Associated with Olefin Functionalization.
Results and Discussion
Olefin Synthesis
There are numerous methods available for ketone olefination en route to 4 (Scheme 1). In our initial studies, we tested several approaches for olefin synthesis from tricyclo[3.2.1.02,7]octanone 12 (Scheme 3).7 The tetrasubstituted alkenes we envisioned synthesizing feature two structural elements that render their stereoselective synthesis challenging: (1) They include an allylic quaternary center, which can reduce reactivity of olefin precursors, and (2) methyl and methylenes at one end of the olefin can be sterically challenging to differentiate when controlling olefin geometry. Initial attempts employing Wittig and Horner–Wadsworth–Emmons olefinations8 as well as McMurry couplings9 did not afford any tetrasubstituted olefins. Claisen rearrangements have been previously employed in the stereoselective synthesis of tetrasubstituted alkenes.10 To this end, we transformed hydroxyketone 12 (95% ee) into allylic acetate 15 in three steps and 40% yield as 1.2:1 mixture of diastereomers (Scheme 3). Ireland–Claisen rearrangement was induced by treatment of 15 with LiHMDS, TBSCl, and HMPA,11 and after treatment with methyl iodide and potassium carbonate, ester 16 was obtained in 87% yield as a 2.3:1 mixture of diastereomers. After reduction using DIBAL-H, the two double bond isomers were separated and assigned via 2D NOESY NMR experiments (see the Supporting Information (SI) for details). Further attempts to optimize the diastereoselectivity in the Ireland–Claisen reaction by employing different silyl groups did not lead to improvement. As we opted for a highly stereoselective synthesis, we subsequently envisioned different routes to the tetrasubstituted olefin not involving nucleophilic addition to tricyclic ketones.
Scheme 3. Synthesis of Tetrasubstituted Olefin via Ireland–Claisen Rearrangement.
Reagents and conditions: (a) TBSCl, imidazole, DMAP, CH2Cl2, r.t., 73%; (b) isopropenylmagnesium bromide (14), LaCl3·2LiCl, THF, then 13, 0 °C to r.t., 86%, dr = 6:5; (c) PhNMe2, AcCl, 50 °C, 64%; (d) LiHMDS, TBSCl, HMPA, THF, −78 °C to r.t., then 1 M HCl; (e) MeI, K2CO3, DMF, r.t., 87% over two steps, dr = 2.3:1; (f) DIBAL-H, PhMe, −78 °C to r.t., 59% trans-17, 27% cis-17.
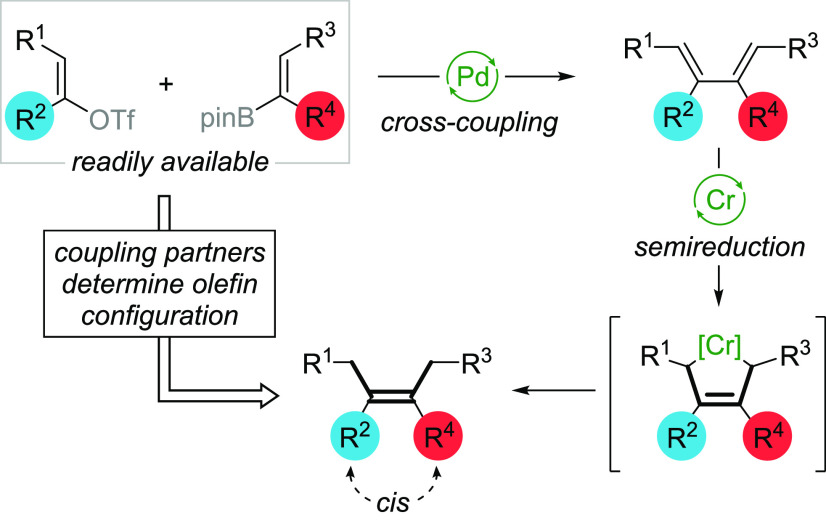
Stereocontrolled preparation of tetrasubstituted olefins has been a longstanding challenge in organic synthesis.8,12 A conceptually new route to tetrasubstituted olefin 4 would require stereoselective reduction of the diene in 5 in a catalyst-controlled process (Scheme 1). 1,3-Dienes may be conveniently accessed via palladium-catalyzed sp2–sp2 cross-coupling. Transition-metal-catalyzed semireductions of dienes by Cr and Ru catalysts have been reported to proceed via the s-cis η4 complex/metallacyclopentene, delivering single olefin isomers (Scheme 4).13 The configuration of the tetrasubstituted olefin would be controlled by the structure of the coupling partners and the reduction mechanism. While the first reports on diene semihydrogenation date back to the 1960s, there is only one report, by Shibasaki, for the synthesis of a tetrasubstituted olefin (trialkyl-substituted acrylonitrile) in a complex structure via semihydrogenation of a 1,3-diene.13b This report predates coupling chemistry, and numerous steps were required to access the 1,3-diene. The combination of modern sp2–sp2 cross-coupling reactions and 1,4-semihydrogenation would allow for rapid access to a tetrasubstituted olefin in a stereodefined manner (Scheme 4).
Scheme 4. Conceptual Approach to Tetrasubstituted Olefin Synthesis via Cross-Coupling and 1,4-Semihydrogenation.
To study this approach, we prepared a series of dienes 20 via Suzuki cross-coupling14 of vinyl boronates 18 with vinyl triflates 19 and subjected them to semihydrogenation conditions (Table 1).15 We selected [Cr(CO)3(η6-MeOBz)] as the catalyst for the 1,4-semihydrogenation over Cp*Ru-based catalysts as very high yields and stereoselectivities have been reported for the application of this catalyst and it is readily accessible in one step from commercially available Cr(CO)6 and MeOBz.13d We focused on tetrasubstituted olefins featuring the same stereochemical challenges as encountered in the natural product. As the design of the cross-coupling partners determines the olefin geometry, varying the coupling partners allows for the selective preparation of both olefin isomers. Accordingly, both olefin isomers 21a and 21b as well as 21c and 21d could be prepared in high stereoselectivities and yields from the corresponding vinyl triflates and boronates. For 21c and 21d, the olefin geometries were confirmed by 2D NOESY NMR experiments (see SI for details). The ketone in 21e was well tolerated under cross-coupling and hydrogenation conditions (97% and 95% yield, respectively). Also, styrenes 21f and 21g could be prepared selectively. Next, we turned our attention to the synthesis of tetrasubstituted olefins on the tricyclo[3.2.1.02,7]octane scaffold. In a first attempt, a symmetric isopropylidene substituent could be installed successfully (21h). During the cross-coupling reaction, most of the silyl ether was cleaved and the alcohol was reprotected prior to hydrogenation. Employing more complex vinyl boronates, 21i and 21j were synthesized stereoselectively. Changing the protecting group to pivalate was well tolerated under both cross-coupling and hydrogenation conditions, and 21k was obtained in 96% over two steps. All hydrogenation reactions were initially performed using 20 mol% catalyst. This led to incomplete conversion for 21e, 21g, 21j, and 21k. Increasing the catalyst loading to 50 mol% ensured full conversion for these substrates. All tetrasubstituted olefins 21 were obtained in >20:1 dr. In most cases, only traces of regioisomeric olefins (<5%) were observed. For 21c, decreasing the catalyst loading to 5 mol% led to 54% conversion after 18 h. After the reaction time was increased to 42 h, full conversion and 89% yield was observed showing that the catalyst is still active after the standard reaction time. Decreasing the H2 pressure to 55 bar completely shut down the reaction. Notably, variation of the concentration between 7 and 50 mM and scaling-up the reaction to 2.3 mmol for 21j had no effect on yield or stereoselectivity.
Table 1. Synthesis of Tetrasubstituted Olefins via Cross-Coupling and Semihydrogenation.


20 mol% catalyst was used.
50 mol% catalyst was used.
5 mol% catalyst was used for 42 h.
During the cross-coupling, most of the silyl ether was cleaved and was reformed using TBSCl, imidazole and DMAP (10–20 mol%) in CH2Cl2 at r.t. The yields refer to combined yields over two steps.
We continued our efforts toward the synthesis of (−)-mitrephorone A (1) using 21j, which was prepared from vinyl boronate 18e and vinyl triflate 19d (Scheme 5). For malonate desymmetrization, we turned to the application of biocatalysis for the stereoselective monohydrolysis of α,α-disubstituted malonate 21j. Subjecting malonate 21j to pig liver esterase (PLE) in a mixture of aqueous phosphate buffer and DMSO (10:1) did not lead to any conversion of starting material.16 In contrast, after silyl ether cleavage with TBAF, the corresponding malonic acid monoester was obtained in 20:1 dr under the same reaction conditions. Reprotection of the hydroxy group with TBSCl and chemoselective reduction of the carboxylic acid to the corresponding alcohol (ClCO2Me followed by NaBH4) afforded alcohol 23 in 68% from malonate 22.17 Oxime 24 was obtained via oxidation with DMP and treatment with hydroxylamine hydrochloride in 58% yield over two steps. Subjecting 24 to PhI(OAc)2 led to its oxidation to the corresponding nitrile oxide,18 which underwent cycloaddition to give isoxazoline 25 in 64% yield as a single diastereomer as determined by analysis of the 1H NMR spectrum. The relative configuration was established by 1D NOE NMR experiments (see SI for details). It is worth noting that the cycloaddition sets two challenging stereocenters, namely the vicinal tertiary ether and quaternary center concomitant with 6-membered ring formation. Notably, when a 1:1 mixture of diastereomers of oxime 24 (epimeric at C4) was subjected to the reaction conditions, two diastereomers were obtained that have the same relative configuration at C4, C9, and C10 as determined by X-ray crystallography (see SI for details). This clearly shows that the facial selectivity in the dipolar cycloaddition is fully controlled by the α stereocenter of the nitrile oxide.
Scheme 5. Synthesis of Isoxazoline 26 via Nitrile Oxide Cycloaddition.

Reagents and conditions: (a) Pd(PPh3)4 (3 mol%), NaHCO3, DME–H2O (9:1), 80 °C, then HCl, MeOH, r.t., 66%; (b) TBSCl, imidazole, DMAP (20 mol%), CH2Cl2, r.t., 94%; (c) H2 (70 bar), [Cr(CO)3(η6-MeOBz)] (50 mol%), acetone, 120 °C, 97%; (d) TBAF, THF, r.t., 91%; (e) pig liver esterase (PLE), aq NaOH, 0.1 M pH 7 sodium phosphate buffer–DMSO (10:1), r.t., dr = 20:1; (f) TBSCl, imidazole, DMAP, CH2Cl2, r.t.; K2CO3, MeOH–THF–H2O (20:10:3), r.t.; (g) ClCO2Me, Et3N, THF, 0 °C to r.t.; NaBH4, MeOH, 0 °C, 68% over three steps; (h) DMP, t-BuOH, CH2Cl2, r.t., 71%; (i) H2NOH·HCl, EtOH–pyr (8:1), r.t., 82%; (j) PhI(OAc)2, MeOH, 0 °C; PhMe, Δ, 64%; (k) TBAF, THF, 60 °C, 99%; (l) DMP, t-BuOH, CH2Cl2, r.t., 86%; (m) MeLi, THF–Et2O (3:1), −78 °C; (n) DMP, t-BuOH, CH2Cl2, r.t., 94% over two steps.
Examination of putative transition states as shown for I and II in Figure 1 proves instructive. Transition state I, incorporating a 1,3-diaxial interaction between an ester and a methyl group, is energetically favored over transition state II, in which a 1,3-dimethyl axial interaction is present (∼2.8 vs ∼3.7 kcal/mol).19 This is consistent with the formation of a single diastereomer as observed by 1H NMR spectroscopy.
Figure 1.
Putative transition states for nitrile oxide cycloaddition.
A common problem in nitrile oxide cycloaddition is dimerization of the nirile oxide, and it has been shown that cycloreversion can be induced by heating.20 However, we did not observe any dimer, and all byproducts were highly polar baseline compounds, which could not be characterized.
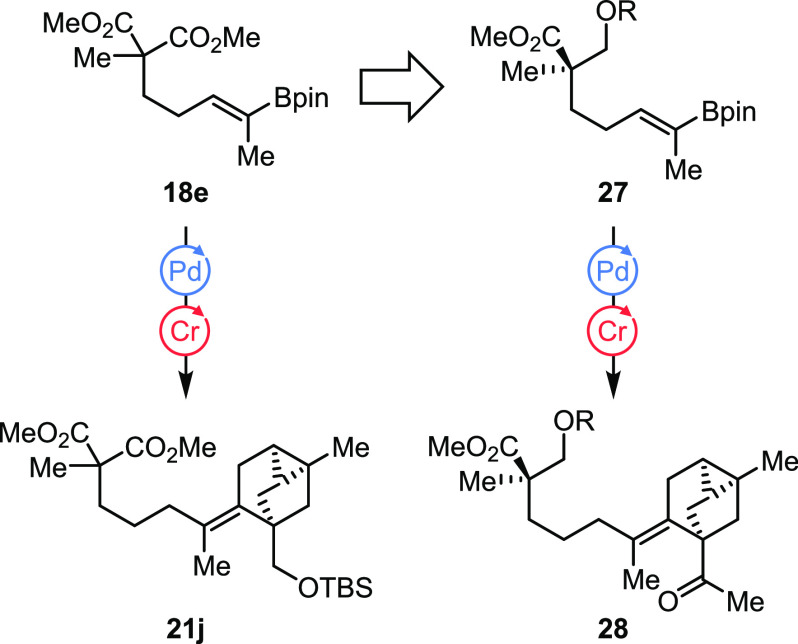
Despite being scalable and relatively high yielding (7.6% over 16 steps from 12), we aimed to further optimize the route toward isoxazoline 26 with respect to the following points: (1) Achiral vinyl boronate 18e could be replaced by a chiral, enantioenriched analogue 27, which would render the synthesis more convergent (Scheme 6). It is important to note that the dr of coupling product 28 will depend on the ee of vinyl boronate 27. (2) The protecting group strategy is suboptimal: The TBS ether in vinyl triflate 19d is cleaved during cross-coupling and was reprotected for hydrogenation but enzymatic desymmetrization only proceeded with the free alcohol. So again a sequence of deprotection, desymmetrization, and reprotection had to be carried out. Transformation of the alcohol to the corresponding methyl ketone prior to cross-coupling may improve the synthesis.
Scheme 6. Envisioned Optimization of the Route.
Malonate Desymmetrization Studies
For the asymmetric synthesis of enantioenriched vinyl boronate 27, we wanted to further explore the stereoselective monohydrolysis of α,α-disubstituted malonates. Previous studies on desymmetrization of 2-methyl-2-alkylmalonates 29 have shown that the length of R has a strong effect on the enantioselectivity in the hydrolysis to give 30.21 Accordingly, we prepared a series of malonates 29a–f, which vary in length and nature of side chain and, after enzymatic transformation, could all be elaborated to 7 (Table 2).22,23 Dimethyl malonates 29 may be conveniently prepared by alkylation reactions.24 For rapid determination of enantioselectivities in our study, we developed a quick assay involving coupling of acids 30 with (S)-phenylethanamine to the corresponding diastereomeric amides.25 To benchmark the method, we repeated the pig liver esterase-mediated hydrolysis of TBS-protected 2-methyl-2-hydroxymethylmalonate 29a, which has been previously reported by Keese.16 The enantiomeric excess we observed for the formation of 30a was in full agreement with Keese’s result (95% ee). When we subjected vinyl boronate 29b to the enzymatic step, low yield (9%) and modest enantioselectivity (75% ee) were observed. Consequently, we examined the enzymatic reaction with 29c and 29d, which furnished products in 74% and 97% yield, respectively, albeit in low enantiomeric excess, 20% and 23% ee, respectively. Examination of silyl ethers 29e and 29f revealed that the former afforded 30e in 67% yield and the highest enantiomeric excess, namely >95% ee. Interestingly, no reaction was observed for the analogous TBDPS-protected substrate 29f.26 As a consequence of its high yield and enantiomeric excess, 30e was selected for further studies. Based on previous investigations, the absolute configuration of carboxylic acid 30e was tentatively assigned as (R).16,21
Table 2. Enzymatic Desymmetrization of Malonates 29a.
Synthesis of Vinyl Boronate 33
Enantiopure27,28 malonic acid monoester 30e was transformed into silyl ether 31 via chemoselective reduction of the carboxylic acid to the corresponding alcohol (ClCO2Me followed by NaBH4),17 and TBDPS protection in 61% yield from 29e (Scheme 7). Selective cleavage of the TBS-ether in 31 with PPTS in ethanol,29 subsequent oxidation with DMP, and Wittig methylenation of the resulting aldehyde afforded olefin 32 in 75% yield over three steps. Vinyl boronate 33 was prepared via cross-metathesis of 32 with isopropenylboronic acid pinacol ester (18d) in 49% yield.23
Scheme 7. Synthesis of Vinyl Boronate 33.
Reagents and conditions: (a) pig liver esterase (PLE), aq NaOH, 0.1 M pH 7 sodium phosphate buffer–DMSO (10:1), r.t.; (b) ClCO2Me, Et3N, THF, 0 °C to r.t.; NaBH4, MeOH, 0 °C, 64% from 29e; (c) TBDPSCl, imidazole, DMAP (20 mol%), CH2Cl2, r.t., 95%; (d) PPTS (20 mol%), EtOH, r.t.; (e) DMP, t-BuOH, CH2Cl2, r.t., 87% over two steps; (f) MePPh3Br, KOt-Bu, THF, r.t., 86%; (g) isopropenylboronic acid pinacol ester (18d), Grubbs second-generation catalyst (10 mol%), CH2Cl2, 50 °C, 49%.
Synthesis of Tetrasubstituted Olefin 37
We subsequently transformed hydroxyketone 12(7) into a suitable building block for cross coupling. Following a short sequence (Comins reagent (34); DMP; MeLi; DMP), 35 was prepared (Scheme 8).30 Cross-coupling of 35 with vinyl boronate 33 afforded 1,3-diene 36 in 65% yield.14 Intermediate 36 was subjected to hydrogenation conditions in the presence of 50 mol% of catalyst [Cr(CO)3(η6-MeOBz)]. Desired tetrasubstituted olefin 37 was obtained in 93% yield as a single olefin isomer.
Scheme 8. Synthesis of Tetrasubstituted Olefin 37.
Reagents and conditions: (a) TMSCl, imidazole, THF, r.t., then KHMDS, −78 °C, then Comins reagent (34), −78 °C, then aq HCl, r.t., 79%; (b) DMP, t-BuOH, CH2Cl2, r.t., 85%; (c) MeLi, THF, −78 °C; (d) DMP, t-BuOH, CH2Cl2, r.t., 52% over two steps; (e) 33, Pd(PPh3)4 (2.5 mol%), NaHCO3, DME–H2O (9:1), 85 °C, 65%; (f) H2 (70 bar), [Cr(CO)3(η6-MeOBz)] (50 mol%), acetone, 120 °C, 93%.
Completion of the Carbon Skeleton via Nitrile Oxide Cycloaddition
Silyl ether 37 was converted into oxime 38 via deprotection with TBAF, oxidation with DMP and oxime formation with hydroxylamine hydrochloride in 62% yield over three steps (Scheme 9).6c Subjecting 38 to the same nitrile oxide cycloaddition conditions as described above afforded isooxazoline 26 in 52% yield as a single diastereomer along with 10% recovered starting material. No side products were observed in significant quantities (>5% yield). Enolization of 26 with LDA or synthesis of the corresponding trimethylsilyl enol ether followed by treatment with BF3·OEt2 did not induce intramolecular addition to the isoxazoline. In contrast, after N-methylation of 26 using Meerwein’s salt (Me3OBF4),31 the intermediate isoxazolinium salt 39 was subjected in situ to TMSOTf and Et3N to induce cyclization. The Mannich-type reaction afforded isoxazolidine 40 in 69% yield and completed the carbon skeleton of the natural product. Treatment of 40 with Zn in AcOH at 50 °C led to N–O bond cleavage with concomitant methylamine elimination to give enone 41 in 65% yield. Various attempts to induce oxa-Michael addition of 41 and either trapping the resulting enolate as the corresponding enol ether (e.g., TBSOTf, 2,6-lutidine or proton sponge) or oxidizing it (IPh2BF4, I2/NaHCO3, NBS/NaHCO3, PhI(OAc)2/KOt-Bu, O2/KOt-Bu, MoOPH/KHMDS) were unsuccessful. Also, oxidative transformation of the enone to the diosphenol proved unfruitful (epoxidation followed by rearrangement or dihydroxylation followed by elimination). At this point, 41 was reduced to the corresponding saturated ketone 42 with H2 and Pd/C in 77% yield.32 Isoxazolidine 40 could also be directly reduced to 42 using H2 and Pd/C in the presence of AcOH at 80 °C in 72% yield.
Scheme 9. Synthesis of 42 via Nitrile Oxide Cycloaddition.

Reagents and conditions: (a) TBAF, THF, r.t.; (b) DMP, t-BuOH, CH2Cl2, r.t., 73% over two steps; (c) H2NOH·HCl, pyr–EtOH (8:1), r.t., 85%; (d) PhI(OAc)2, MeOH, 0 °C; PhMe, Δ, 52%; (e) Me3OBF4, CH2Cl2, r.t., then TMSOTf, Et3N, r.t., 69%; (f) Zn, AcOH, 50 °C, 65%; (g) H2 (1 atm), Pd/C (30 mol%), EtOAc, r.t., 77%; (h) H2 (1 atm), Pd/C, EtOAc–AcOH (5:1), 80 °C, 72%.
α-Oxidation of 42 (O2, KOt-Bu, then PPh3) gave α-hydroxyketone 43 in 72% yield (Scheme 10).33 Treatment with DMP furnished hydroxydiosphenol 44 in 74% yield. It is worth noting that oxidation of 42 to diosphenol 44 was carried out in an efficient sequence with the tertiary alcohol being unprotected. This was not possible in our first route employing an isomeric ketone, which necessitated protection of the tertiary alcohol as its silyl ether.3 Finally, (−)-mitrephorone A (1) was obtained via oxidative cyclization of 44 mediated by Koser’s reagent (PhI(OH)OTs) in the presence of NaHCO3 in 72% yield and >99% ee, which compares favorably with previous total syntheses (88 and 85% ee).3,4a,34,35 Spectroscopic data of the material we obtained is identical with that reported for the natural isolate.2
Scheme 10. Completion of the Synthesis.
Reagents and conditions: (a) KOt-Bu, O2, THF, −78 °C, then PPh3, −78 °C to r.t., 72%; (b) DMP, t-BuOH, CH2Cl2; SiO2, hexane–EtOAc (3:1), 74%; (c) PhI(OH)OTs, NaHCO3, CH2Cl2, 72%.
Conclusion
We have reported a highly enantioselective and diastereoselective total synthesis of (−)-mitrephorone A (1, >99% ee). The synthesis relies on intramolecular nitrile oxide cycloaddition, which sets two stereocenters, forms one all-carbon ring and introduces an isoxazoline, which serves as a handle for elaboration of the cycloadduct to the natural product. Additional salient features of the synthesis include highly enantioselective pig liver esterase-catalyzed malonate desymmetrization, 1,4-semihydrogenation of a 1,3-diene, and hypervalent iodine-mediated oxidative cyclization to furnish the oxetane. Stereo- and regioselective synthesis of tetrasubstituted double bonds via sp2–sp2 cross-coupling and 1,4-semihydrogenation has no precedence and represents a powerful method for olefin synthesis.
Acknowledgments
We thank Ján Kovacovic for assistance with high-pressure reactions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c09520.
This work was funded by the European Research Council (OLECAT, Grant-ID 833540).
The authors declare no competing financial interest.
Supplementary Material
References
- Fraga B. M. The Trachylobane Diterpenes. Phytochem. Anal. 1994, 5, 49–56. 10.1002/pca.2800050202. [DOI] [Google Scholar]
- Li C.; Lee D.; Graf T. N.; Phifer S. S.; Nakanishi Y.; Burgess J. P.; Riswan S.; Setyowati F. M.; Saribi A. M.; Soejarto D. D.; Farnsworth N. R.; Falkinham J. O. III; Kroll D. J.; Kinghorn A. D.; Wani M. C.; Oberlies N. H. A Hexacyclic ent-Trachylobane Diterpenoid Possessing an Oxetane Ring from Mitrephora glabra. Org. Lett. 2005, 7, 5709–5712. 10.1021/ol052498l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M. J. R.; Schneider M.; Brandstätter M.; Krautwald S.; Carreira E. M. Total Synthesis of (−)-Mitrephorone A. J. Am. Chem. Soc. 2018, 140, 16704–16710. 10.1021/jacs.8b09685. [DOI] [PubMed] [Google Scholar]
- a Wein L. A.; Wurst K.; Angyal P.; Weisheit L.; Magauer T. Synthesis of (−)-Mitrephorone A via a Bioinspired Late Stage C-H Oxidation of (−)-Mitrephorone B. J. Am. Chem. Soc. 2019, 141, 19589–19593. 10.1021/jacs.9b11646. [DOI] [PMC free article] [PubMed] [Google Scholar]; Very recently, Renata and co-workers reported a semisynthetic approach to (−)-mitrephorone A:; b Zhang X.; King-Smith E.; Dong L.-B.; Yang L.-C.; Rudolf J. D.; Shen B.; Renata H. Divergent synthesis of complex diterpenes through a hybrid oxidative approach. Science 2020, 369, 799–806. 10.1126/science.abb8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgoda-Pols J. R.; Freyer A. J.; Killmer L. B.; Porter J. R. Antimicrobial diterpenes from the stem bark of Mitrephora celebica. Fitoterapia 2002, 73, 434–438. 10.1016/S0367-326X(02)00124-7. [DOI] [PubMed] [Google Scholar]
- For a discussion on nitrile oxide cycloaddition followed by reductive cleavage for the synthesis of β-hydroxyketones, see:; a Bode J. W.; Carreira E. M. Stereoselective Syntheses of Epothilones A and B via Directed Nitrile Oxide Cycloaddition. J. Am. Chem. Soc. 2001, 123, 3611–3612. 10.1021/ja0155635. [DOI] [PubMed] [Google Scholar]; b Muri D.; Carreira E. M. Stereoselective Synthesis of Erythronolide A via Nitrile Oxide Cycloadditions and Related Studies. J. Org. Chem. 2009, 74, 8695–8712. 10.1021/jo901817b. [DOI] [PubMed] [Google Scholar]; c Becker N.; Carreira E. M. Hydroxyl-Directed Nitrile Oxide Cycloaddition Reactions with Cyclic Allylic Alcohols. Org. Lett. 2007, 9, 3857–3858. 10.1021/ol7017032. [DOI] [PubMed] [Google Scholar]
- Schneider M.; Richter M. J. R.; Krautwald S.; Carreira E. M. Asymmetric Synthesis of the Tricyclooctane Core of Trachylobane Natural Products and Related Terpenoids. Org. Lett. 2019, 21, 8705–8707. 10.1021/acs.orglett.9b03303. [DOI] [PubMed] [Google Scholar]
- Wittig and Horner–Wadsworth–Emmons olefinations have previously been shown to suffer from low reactivity and stereoselectivity in the formation of tetrasubstituted double bonds. In our studies, we were able to react the tricyclooctanone with methyltriphenylphosphonium bromide or diethyl cyanomethylphosphonate, but the obtained di- or trisubstituted olefins could not be transformed into tetrasubstituted olefins. For a review on the synthesis of tetrasubstituted olefins, including Wittig and Horner–Wadsworth–Emmons olefinations of carbonyls, see:; Flynn A. B.; Ogilvie W. W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745. 10.1021/cr050051k. [DOI] [PubMed] [Google Scholar]
- Casimiro-Garcia A.; Micklatcher M.; Turpin J. A.; Stup T. L.; Watson K.; Buckheit R. W.; Cushman M. Novel Modifications in the Alkenyldiarylmethane (ADAM) Series of Non-Nucleoside Reverse Transcriptase Inhibitors. J. Med. Chem. 1999, 42, 4861–4874. 10.1021/jm990343b. [DOI] [PubMed] [Google Scholar]
- a Wipf P.Claisen Rearrangements. Comprehensive Organic Synthesis; Pergamon Press: Oxford, 1991; Vol. 5, pp 827–873. [Google Scholar]; b Ziegler F. E. The Thermal, Aliphatic Claisen Rearrangement. Chem. Rev. 1988, 88, 1423–1452. 10.1021/cr00090a001. [DOI] [Google Scholar]
- Clark D. A.; Kulkarni A. A.; Kalbarczyk K.; Schertzer B.; Diver S. T. Tandem Enyne Metathesis and Claisen Rearrangement: A Versatile Approach to Conjugated Dienes of Variable Substitution Patterns. J. Am. Chem. Soc. 2006, 128, 15632–15636. 10.1021/ja063132m. [DOI] [PubMed] [Google Scholar]
- Wang J.; Dong Z.; Yang C.; Dong G. Modular and regioselective synthesis of all-carbon tetrasubstituted olefins enabled by an alkenyl Catellani reaction. Nat. Chem. 2019, 11, 1106–1112. 10.1038/s41557-019-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Frankel E. N.; Selke E.; Glass C. A. Homogeneous 1,4-addition of Hydrogen Catalyzed by Tricarbonyl(arene)chromium Complexes. J. Am. Chem. Soc. 1968, 90, 2446–2448. 10.1021/ja01011a058. [DOI] [Google Scholar]; b Sodeoka M.; Ogawa Y.; Kirio Y.; Shibasaki M. Stereocontrolled Synthesis of Exocyclic Olefins Using Arene Tricarbonyl Chromium Complex-Catalyzed Hydrogenation. I. Efficient Synthesis of Carbacyclin and Its Analogs. Chem. Pharm. Bull. 1991, 39, 309–322. 10.1248/cpb.39.309. [DOI] [PubMed] [Google Scholar]; c Steines S.; Englert U.; Drießen-Hölscher B. Stereoselective catalytic hydrogenation of sorbic acid and sorbic alcohol with new Cp*Ru complexes. Chem. Commun. 2000, 217–218. 10.1039/a909355i. [DOI] [Google Scholar]; d Hudecek M.; Toma S. A simple route to (η6-arene)tricarbonyl complexes and a systematic study of different catalysts for complexation of arenes with Cr(CO)6. J. Organomet. Chem. 1991, 406, 147–151. 10.1016/0022-328X(91)83181-3. [DOI] [Google Scholar]
- Su N.; Theorell J. A.; Wink D. J.; Driver T. G. Copper-Catalyzed Formation of α-Alkoxycycloalkenones from N-Tosylhydrazones. Angew. Chem., Int. Ed. 2015, 54, 12942–12946. 10.1002/anie.201505993. [DOI] [PubMed] [Google Scholar]
- Vinyl boronates 18 were prepared via alkyne hydroboration or purchased from commercial sources. Vinyl triflates 19 were prepared from the corresponding ketones (see SI for details).
- Luyten M.; Müller S.; Herzog B.; Keese R. Enzyme-Catalyzed Hydrolysis of Some Functionalized Dimethyl Malonates. Helv. Chim. Acta 1987, 70, 1250–1254. 10.1002/hlca.19870700503. [DOI] [Google Scholar]
- Prantz K.; Mulzer J. Decarboxylative Grob-Type Fragmentations in the Synthesis of Trisubstituted Z Olefins: Application to Peloruside A, Discodermolide, and Epothilone D. Angew. Chem., Int. Ed. 2009, 48, 5030–5033. 10.1002/anie.200901740. [DOI] [PubMed] [Google Scholar]
- Mendelsohn B. A.; Lee S.; Kim S.; Teyssier F.; Aulakh V. S.; Ciufolini M. A. Oxidation of Oximes to Nitrile Oxides with Hypervalent Iodine Reagents. Org. Lett. 2009, 11, 1539–1542. 10.1021/ol900194v. [DOI] [PubMed] [Google Scholar]
- Corey E. J.; Feiner N. F. Computer-Assisted Synthetic Analysis. A Rapid Computer Method for the Semiquantitative Assignment of Conformation of Six-Membered Ring Systems. 2. Assessment of Conformational Energies. J. Org. Chem. 1980, 45, 765–780. 10.1021/jo01293a002. [DOI] [Google Scholar]
- Curran D. P.; Fenk C. J. Thermolysis of Bis[2-[(trimethylsilyl)oxy]prop-2-yl]furoxan (TOP-furoxan). The First Practical Method for Intermolecular Cycloaddition of an in Situ Generated Nitrile Oide with 1,2-Di- and Trisubstituted Olefins. J. Am. Chem. Soc. 1985, 107, 6023–6028. 10.1021/ja00307a031. [DOI] [Google Scholar]
- a Björkling F.; Boutelje J.; Gatenbeck S.; Hult K.; Norin T.; Szmulik P. Enzyme Catalysed Hydrolysis of Dialkylated Propanedioic Acid Diesters, Chain Length Dependent Reversal of Enantioselectivity. Tetrahedron 1985, 41, 1347–1352. 10.1016/S0040-4020(01)96536-6. [DOI] [Google Scholar]; b Banerjee S.; Wiggins W. J.; Geoghegan J. L.; Anthony C. T.; Woltering E. A.; Masterson D. S. Novel synthesis of various orthogonally protected Cα-methyllysine analogues and biological evaluation of a Vapreotide analogue containing (S)-α-methyllysine. Org. Biomol. Chem. 2013, 11, 6307–6319. 10.1039/c3ob41282b. [DOI] [PubMed] [Google Scholar]
- Hesse M. J.; Butts C. P.; Willis C. L.; Aggarwal V. K. Diastereodivergent Synthesis of Trisubstituted Alkenes through Protodeboronation of Allylic Boronic Esters: Application to the Synthesis of the Californian Red Scale Beetle Pheromone. Angew. Chem., Int. Ed. 2012, 51, 12444–12448. 10.1002/anie.201207312. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K.; Grubbs R. H. Formal Vinyl C-H Activation and Allylic Oxidation by Olefin Metathesis. Angew. Chem., Int. Ed. 2002, 41, 3171–3174. . [DOI] [PubMed] [Google Scholar]
- Craig D.; Funai K.; Gore S. J.; Kang A.; Mayweg A. V. W. Transannular Claisen rearrangement reactions for the synthesis of vinylcyclobutanes: formal synthesis of (±)-grandisol. Org. Biomol. Chem. 2011, 9, 8000–8002. 10.1039/c1ob06619f. [DOI] [PubMed] [Google Scholar]
- For all malonates 29, racemic hydrolysis with sodium hydroxide followed by coupling with (S)-phenylethanamine gave the corresponding amides in 1:1 dr, thus showcasing the usability of this method to determine the enantiomeric excess of the intermediate carboxylic acids. No remaining intermediate carboxylic acid was observed in 1H NMR analysis of unpurified ester amides.
- None of the investigated malonates 29 is well soluble in the phosphate buffer. The absence of reactivity for 29f may be attributed to (1) lack of solubility or (2) poor fit into the enzyme pocket. The aqueous conditions required for the enzymatic resolution along with accessibility to the lipase active site result in dependences on substrate structure that are subtle.
- The assay used for determination of enantiomeric excesses in Table 2 has its limitations for ee’s > 90% because the dr’s of the ester amides cannot be accurately determined by 1H NMR analysis. Therefore, carboxylic acid 30e was reduced to the corresponding alcohol followed by benzoylation. The enantiomeric excess was determined by chiral HPLC (>99% ee, see SI for details).
- The enantiomeric excess of 30e (>99% ee) is considered as enantiopure.
- Prakash C.; Saleh S.; Blair I. A. Selective De-Protection of Silyl Ethers. Tetrahedron Lett. 1989, 30, 19–22. 10.1016/S0040-4039(01)80311-7. [DOI] [Google Scholar]
- Cruz A. C. F.; Miller N. D.; Willis M. C. Intramolecular Palladium-Catalyzed Direct Arylation of Alkenyl Triflates. Org. Lett. 2007, 9, 4391–4393. 10.1021/ol702044z. [DOI] [PubMed] [Google Scholar]
- Henneböhle M.; Le Roy P.-Y.; Hein M.; Ehrler R.; Jäger V. Isoxazolinium Salts in Asymmetric Synthesis. 1. Stereoselective Reduction Induced by a 3′-Alkoxy Stereocentre. A new Approach to Polyfunctionalized β-Amino Acids. Z. Naturforsch., B: J. Chem. Sci. 2004, 59b, 451–467. 10.1515/znb-2004-0414. [DOI] [Google Scholar]
- The configuration of the newly formed stereocenter was assigned based on a ball-and-stick model of enone 41 and the directive effect of the nearby hydroxy group. For the latter, see:; Thompson H. W. Stereochemical Control of Reductions. The Directive Effect of Carbomethoxy vs. Hydroxymethyl groups in Catalytic Hydrogenation. J. Org. Chem. 1971, 36, 2577–2580. 10.1021/jo00817a001. [DOI] [Google Scholar]
- Paquette L. A.; Hofferberth J. E. Effect of 9,10-Cyclic Acetal Stereochemistry on Feasible Operation of the α-Ketol Rearrangement in Highly Functionalized Paclitaxel (Taxol) Precursors. J. Org. Chem. 2003, 68, 2266–2275. 10.1021/jo020627v. [DOI] [PubMed] [Google Scholar]
- For a discussion on potential mechanisms involving oxygen- and carbon-bound hypervalent iodine species, see:; Arava S.; Kumar J. N.; Maksymenko S.; Iron M. A.; Parida K. N.; Fristrup P.; Szpilman A. M. Enolonium Species-Umpoled Enolates. Angew. Chem., Int. Ed. 2017, 56, 2599–2603. 10.1002/anie.201610274. [DOI] [PubMed] [Google Scholar]
- In their recent synthesis of Preuisolactone A, Trauner and co-workers used a similar reaction, in which they formed a γ-lactone via oxidative cyclization of a carboxylic acid and a diosphenol.; Novak A. J. E.; Grigglestone C. E.; Trauner D. A Biomimetic Synthesis Elucidates the Origin of Preuisolactone A. J. Am. Chem. Soc. 2019, 141, 15515–15518. 10.1021/jacs.9b08892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.