Abstract
Simple Summary
The greenhouse whitefly, Trialeurodes vaporariorum is an insect pest of many plant crops including tomato and is especially harmful because it is a vector for a number of plant viral diseases. In this paper, an improved tomato line bred to produce glandular trichomes that exudate the deterrent compounds acylsucroses, which was introgressed from a wild tomato species, was demonstrated to decrease fitness of the insect and showed as a means for controlling the pests and, indirectly, could be an aid to reduce virus transmission to tomato plants.
Abstract
A combination of biological control and host plant resistance would be desirable for optimally controlling the greenhouse whitefly, Trialeurodes vaporariorum in tomato crops. Whitefly settlement preference, oviposition, and survivorship were evaluated on ABL 10-4 and ‘Moneymaker’, two nearly-isogenic tomato lines with, and without, whitefly-resistance traits based on type IV leaf glandular trichomes derived from the tomato wild species Solanum pimpinellifolium, respectively. Significantly reduced preference of T. vaporariorum adult whiteflies for ABL 10-4 leaves was observed. Moreover, T. vaporariorum altered its abaxial–adaxial settling performance on leaves of ABL 10-4 plants. A significantly lower tendency to settle on abaxial leaf surface was observed in ABL 10-4 compared to Moneymaker plants. Furthermore, T. vaporariorum deposited fewer eggs and exhibited a significantly reduced egg to adult survivorship in ABL 10-4 than in Moneymaker plants. Therefore, reduced fitness and distorted performance were observed for T. vaporariorum on ABL 10-4 tomato plants supporting that type IV leaf glandular trichomes might protect them from this pest and, indirectly, from the viruses it transmits.
Keywords: glandular trichomes, tomato, Trialeurodes vaporariorum, whitefly-resistance
1. Introduction
The greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hemiptera, Aleyrodidae), is a major insect pest of global importance in greenhouse-grown vegetables and ornamental cash crops. It is associated with damage to plants during feeding on plant phloem and by excreting honeydew that covers leaf foliage reducing transpiration and favoring the development of sooty mold, which reduces photosynthetic activity and crop quality [1,2,3]. However, the most serious damage caused by the greenhouse whitefly is the transmission of a number of viral diseases in different crop species [4]. In tomato plants (Solanum lycopersicum L.), T. vaporariorum is vector of plant viruses of the genera Crinivirus and Torradovirus (families Closteroviridae and Secoviridae, respectively) [4,5,6,7,8]. Among them, the crinivirus tomato chlorosis virus (ToCV) is a major emerging virus worldwide [4,9]. The direct and indirect damages caused by T. vaporariorum can then result in significant tomato yield losses [10,11].
Control of T. vaporariorum infestations is primarily dependent on the intensive use of insecticides, which disrupts natural biological control and can lead to the development of resistant insect populations [3,12,13] causing environmental and human health damage. Therefore, there is a need to develop more sustainable alternatives to control T. vaporariorum. Several biological control alternatives have been investigated extensively [14,15,16]. Host plant selection is the first stage of plant colonization by whiteflies and plays a major role in determining the evolution of whitefly populations in the field [17,18]. Plants have developed passive and active defenses to deal with pests by affecting their preference and/or performance [19,20]. Host plant resistance to insects can then be considered an interesting alternative to control insects in the framework of integrated pest management programs [21] and is gaining importance as increasing numbers of insecticides are being banned due to environmental and human health concerns. Host plant resistance to insect vectors is also one of the best strategies to manage circulative vector-borne virus diseases [22]. An advantage can then be gained from host plant resistance as an effective means to control whitefly infestations [23,24] which might help to reduce also incidence of transmitted viruses as demonstrated for the whitefly Bemisia tabaci Gennadius [25,26].
Plant resistance to arthropod herbivores is often mediated by phytochemicals that negatively affect the feeding, growth, or reproduction of the pest [27,28,29,30]. In this sense, wild relatives of tomato have been shown to possess effective means of dealing with several insect pests [28,31]. Resistance to the greenhouse whitefly has been reported in some wild tomato relatives such as Solanum peruvianum L., Solanum habrochaites S. Knapp & D.M Spooner, and Solanum pennellii Correll [24,32,33,34,35,36]. External barriers might be involved in whitefly preference for certain host plants. Factors on the leaf surface such as cuticle features and hairiness are known to influence host-plant selection. Glandular and nonglandular trichomes affect settling and survival of herbivores on host plants [37]. Volatile and non-volatile secondary metabolites are produced by glandular trichomes including acylsugars, terpenoids, phenylpropanoids, and flavonoids [37,38,39]. In Solanum species, type IV and type VI leaf glandular trichomes are associated with high levels of resistance to diverse arthropod species including mites, aphids, and whiteflies [36,40,41,42,43,44]. Early works in this area done by Gentile et al. [45] helped to identify resistance to T. vaporariorum in S. hirsutum (syn. S. habrochaites) and S. pennellii associated to a heavy vesture of sticky glandular exudates on leaves and stems. However, challenging the transference of resistance traits from the latter tomato green-fruited relatives to the cultivated tomato complicated the breeding process. Thus, linkage drag not easily removed by backcrossing results into commercially unfavorable alleles also transferred during this process. In recent times, whitefly resistance traits have been reported in wild tomato relatives with red fruits and more closely related to cultivated tomato that can facilitate their use. Thus, whitefly resistance was found in Solanum galapagense S. Darwin and Peralta and Solanum pimpinellifolium L. [25,31,33,46,47]. Interestingly, the traits found in S. galapagense involved in resistance to whiteflies were associated with the presence of type IV leaf glandular trichomes and the acylsugars they produce [41]. These trichomes and acylsugars, which are not present in cultivated tomatoes, also mediate the resistance of the wild species S. pennellii against many tomato pests [48]. Presence of type IV leaf glandular trichomes and acylsugar production was also reported in the accessions TO-937 of S. pimpinellifolium associated to arthropod resistance [40,49]. Moreover, the latter resistance trait demonstrated its effectiveness after successful introgression into the cultivated tomato providing antixenosis and antibiosis resistance to the B. tabaci whitefly and significant control of the worldwide damaging tomato yellow leaf curl virus (TYLCV) (genus Begomovirus, family Geminiviridae) [25,50].
T. vaporariorum is one of the most destructive whitefly species in field and greenhouse crops worldwide [51]. As the behavior of T. vaporariorum is known to be affected by plant volatile cues [52], here we investigated whether the presence of type IV leaf glandular trichomes and acylsugar secretion traits bred in domesticated tomato from S. pimpinellifolium, can help to deter this whitefly as it does with B. tabaci [25]. Therefore, we specifically assessed in the current study (i) the host-acceptance behavior and oviposition of T. vaporariorum in a tomato inbred line which presents type IV leaf glandular trichomes and acylsugar exudates, and (ii) life-history parameters such as egg and nymphal survival.
2. Materials and Methods
2.1. Tomato Plants and Whitefly Population
Two near-isogenic tomato lines were used in this study, the whitefly- and virus-susceptible tomato cv. Moneymaker and ABL 10-4, an advanced backcross (BC5S2) line. For this BC5S2 line, presence of type IV leaf glandular trichomes and enhanced secretion of acylsucroses were derived from the initial cross S. lycopersicum cv. Moneymaker (without type IV leaf glandular trichomes) x S. pimpinellifolium accession TO-937 (with type IV leaf glandular trichomes, IHSM-CSIC germplasm collection) [44]. TO-937 is an inbred wild-tomato line derived from S. pimpinellifolium material collected by our colleague J. Cuartero at 50 m altitude on the coastal plain of Lambayeque Department, Peru, in 1983. The original accession segregated widely for density of glandular trichomes and was fixed by four consecutive selfing and selection steps. Obtaining the BC5S2 line AB 10-4 involved five cycles of combined recurrent crosses toward Moneymaker and subsequent selfing steps with selection for high type-IV leaf trichome density and acylsucrose production, plus two additional final selfing steps [53].
Plantlets of ABL 10-4 and tomato cv. Moneymaker were individually sown in pots (30 cm diameter) containing a mixture of 50% soil (54% sand, 24% silt, and 22% clay), 30% horticultural substrate, 15% coconut–fiber substrate and 5% litonite (loaded zeolite). Until used, plants were grown in an insect-proof glasshouse with temperature control (22 to 27 °C day and 17 to 20 °C night) and supplied weekly with nutrient solution. Experiments were conducted taking into account the plant growth stage because significant difference in acylsucrose production between Moneymaker and an advanced backcross lines with type IV leaf glandular trichomes was only achieved after the ten-leaf stage [25].
For the experiments, non-viruliferous T. vaporariorum whiteflies were reared on melon (Cucumis melo L. ‘ANC42’, IHSM-CSIC germplasm collection) plants within wooden cages covered with insect-proof nets, in an insect-proof glasshouse with temperature control (22 to 27 °C day and 17 to 20 °C night) and supplemental light when needed. The initial whitefly colony originated from T. vaporariorum field individuals collected in Malaga (Spain).
2.2. Trichome Observation and Acylsucrose Accumulation Quantification
Type IV trichomes are located on both the adaxial and abaxial sides of the leaf but, in a BC3S2 line also derived from TO-937, they are far more abundant on the abaxial than on the adaxial leaf surface [54]. Type IV trichome density of ABL 10-4 and Moneymaker was measured following the indications by Alba et al. [40]. Previous analysis of TO-937 and the derived S. lycopersicum introgression lines indicated that these produced sucrose esters [40]. Epicuticular leaf acylsucroses were then extracted and de-esterified using the method described by Goffreda et al. [55], and the resulting free-sugar moiety was quantified spectrophotometrically using a hexokinase-based glucose assay. In short, aliquots of acylsucroses were concentrated by evaporation, re-dissolved in methanol and saponified adding 0.04N NaOH. Free sucrose was hydrolyzed to glucose and fructose by adding invertase (Sigma-Aldrich, St. Louis, MO, USA, ref. I9274), and then phosphorylated by adenosinetriphosphate (ATP, Sigma-Aldrich, St. Louis, MO, USA, ref. A26209) in the reaction catalyzed by hexokinase (Sigma-Aldrich, St. Louis, MO, USA, ref. H6389). The resulting glucose-6-phosphate was oxidized to 6-phosphogluconate in the presence of nicotinamide adenine dinucleotide phosphate (NADP, Sigma-Aldrich, St. Louis, MO, USA, ref. N5755) in a reaction catalyzed by glucose-6-phosphate dehydrogenase (Sigma-Aldrich, St. Louis, MO, USA, ref. G6378). The absorbance at 340 nm was recorded, and sucrose quantities were determined by using a sucrose standard curve in the range of 0.15–5.8 mM, and expressed as nmol of sucrose esters per cm2 of leaf area. To normalize the data and stabilize the variance, trichome and acylsucrose measurements were Log (x + 1) transformed prior to analysis. Statistical differences between the means of trichome IV density and acylsucrose production in the two genotypes were analysed by one-way ANOVA and the Fisher’s least significant difference (LSD) test by using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA).
2.3. T. vaporariorum Settling Preference
Whitefly settling behavior of adult individuals of T. vaporariorum was assessed on the adaxial and the abaxial leaflet surfaces of ABL 10-4 and the whitefly-susceptible cv. Moneymaker tomato plants under non-choice conditions following the experimental design described by Rodriguez-Lopez et al. [25]. Detached leaflets from Moneymaker and ABL 10-4 tomato plants at ten-leaf growth stage were used. Six leaflets of one genotype were placed forming a circle in an independent methacrylate box (22 × 22 × 7 cm). Each leaflet petiolule was inserted in a plastic dish (2 cm diameter × 1 cm high) filled with nutrient solution (0.25 g/L of Nutrichem 60, Miller Chemical, Hanover, PA, USA) to maintain leaflet turgor. Leaflets were placed abaxial surface down and at an angle with the horizontal (so that both leaflets surfaces were freely accessible to whiteflies), with their tips directed to the center of the circle formed by leaflets. Thirty adult whiteflies (five whiteflies per leaflet tested, without sex distinction) were released in the center of the circle after a short cold treatment (10 min at 4 °C) to facilitate handling. Each methacrylate box was then covered with a methacrylate lid with an opening covered with muslin for ventilation. The boxes were placed in a growth chamber (26 °C day and 22 °C night, 70% relative humidity, with a 16-h photoperiod at 250 mol·s−1·m−2 photosynthetically active radiation). The number of whiteflies that settled on both surfaces of leaflet was counted at 0.5, 1, 2, 4, 8, 24, and 48 h after release. Each experiment was replicated 12 times. The mean number of whiteflies per leaflet and leaflet surface on each genotype were calculated. Settling preferences were statistically analyzed by Generalized Linear Models (GzLM) with Log as the link function and Poisson as the probability distribution, and the means of the two genotypes at each time point were compared by the least-squares (LS) means test by using the IBM SPSS Statistics package.
2.4. Oviposition of T. vaporariorum
A non-choice test was conducted to compare the oviposition rates of T. vaporariorum on leaves of the two near-isogenic tomato lines cv. Moneymaker and ABL 10-4. Five adult females of T. vaporariorum were selected from the rearing colonies with the help of a stereomicroscope (40×) and placed into a leaf-clip cage (five whiteflies per clip-cage). Previously, whiteflies received a cold treatment (at 4 °C for 10 min) to reduce their activity and facilitate handling for sex distinction. The leaf-clip cages were attached to the abaxial leaflet surface of the fourth newest leaf from the growing point of each test plant (at ten-leaf growth stage). The number of eggs deposited was counted after 24 h using a manual lens (20×) and the mean number of eggs per female was calculated. The experiment included fifteen replicates for the whitefly-susceptible tomato cv. Moneymaker and twenty replicates for ABL 10-4. The adaxial leaf side was not assayed because in our previous observations when developing the inbred lines, whitefly oviposition was never observed to occur on Moneymaker or the improved advanced-backcross lines. Data were analyzed by GzLM with Log as the link function and Poisson as the underlying distribution, and means from the two genotypes were compared by the LS means test by using the IBM SPSS Statistics package.
2.5. Survival of Immature T. vaporariorum
Five adult females of T. vaporariorum were confined into leaf-clip cages as described above. The leaf-clip cages were then attached to the abaxial leaflet surface of the fourth newest leaf from the growing point of each test plant (at ten-leaf growth stage) of Moneymaker and ABL 10-4. The adult whiteflies were removed after 24 h and the remaining founder eggs and nymphs were monitored over the following 33 days until nymphs developed to L4 stage [56] in the whitefly-susceptible Moneymaker cultivar. Analysis of survival time was estimated by the Kaplan–Meier estimator of the survivorship function [57]. Comparison of survivorship functions was made using the generalized Wilcoxon test as described by Hosmer and Lemeshow [58].
3. Results
3.1. ABL 10-4 Accumulates Type IV Glandular Trichomes and Acylsucroses in Leaves
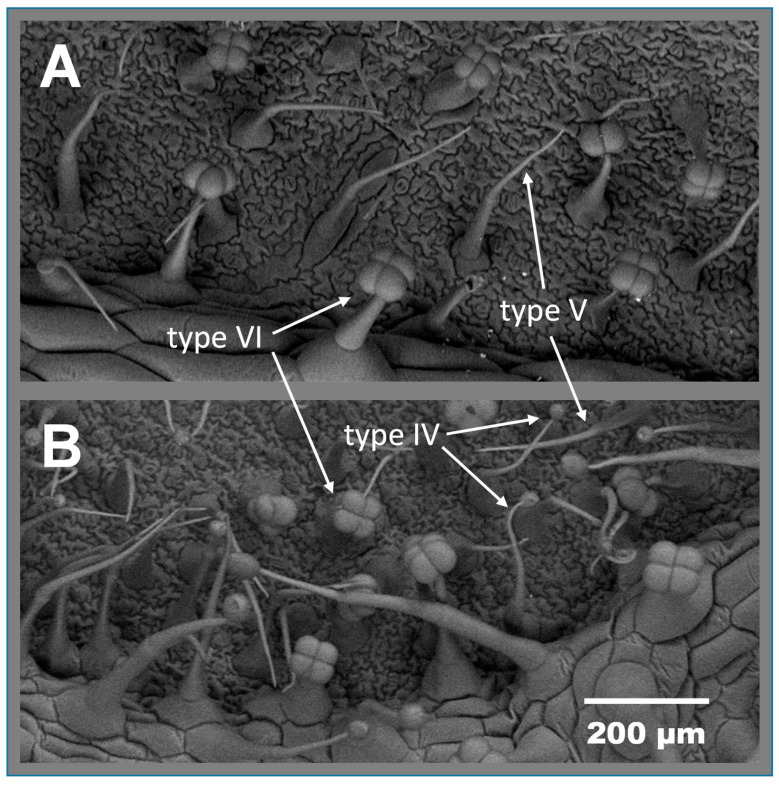
Successful introgression of acylsucrose-producing type IV leaf glandular trichomes in Moneymaker background was achieved in the advanced backcross line (BC5S2) ABL 10-4 (Figure 1B) while no such glandular trichomes are present in the background genotype (Moneymaker) plants (Figure 1A).
Figure 1.
Scanning electron micrographs of abaxial leaf surfaces from (A) ‘Moneymaker’ and (B) the acylsucrose-producer breeding line ABL 10-4. Type IV glandular trichomes are only present in ABL 10-4 while nonglandular type V and glandular type VI trichomes are present in the two genotypes.
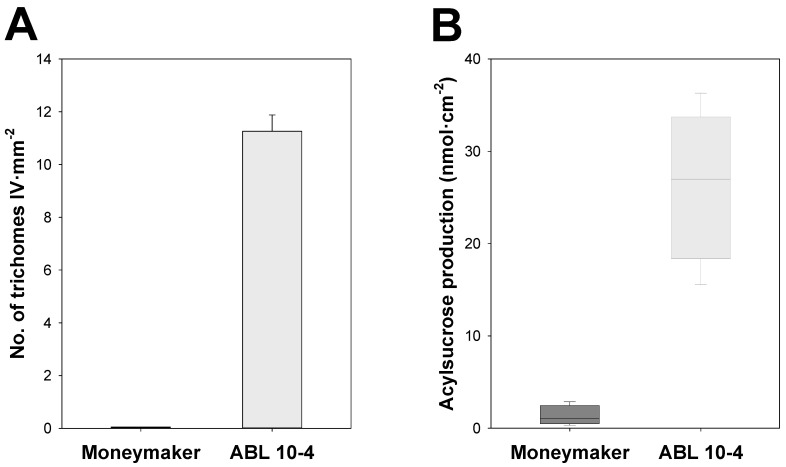
As a result, tomato cv. Moneymaker and its near-isogenic line ABL 10-4 strongly differed for type IV leaf glandular trichome-associated traits. In ABL 10-4, type IV leaf glandular trichomes density was around 11 trichomes per mm2 on the abaxial leaflet surface (Figure 2A). Consequently, a significantly enhanced secretion of acylsucroses was detected in ABL 10-4 compared to Moneymaker plants (p = 0.001, LSD test) (Figure 2B).
Figure 2.
Type IV leaf trichome density and production of acylsucrose in Moneymaker and ABL 10-4 near-isogenic tomato lines. (A) Bar graph showing type IV leaf glandular trichome density on abaxial leaflet surfaces of Moneymaker and ABL 10-4 plants at the ten-leaf growth stage. (B) Box-and-whisker plots showing acylsucrose accumulation on leaflets of Moneymaker and ABL 10-4 plants at the ten-leaf growth stage; the box represents the interquartile range, the horizontal line in the box shows the value of the median, and bars mark the 10th and 90th percentiles.
3.2. T. vaporariorum Repellence in the Genotype with Type IV Glandular Trichomes
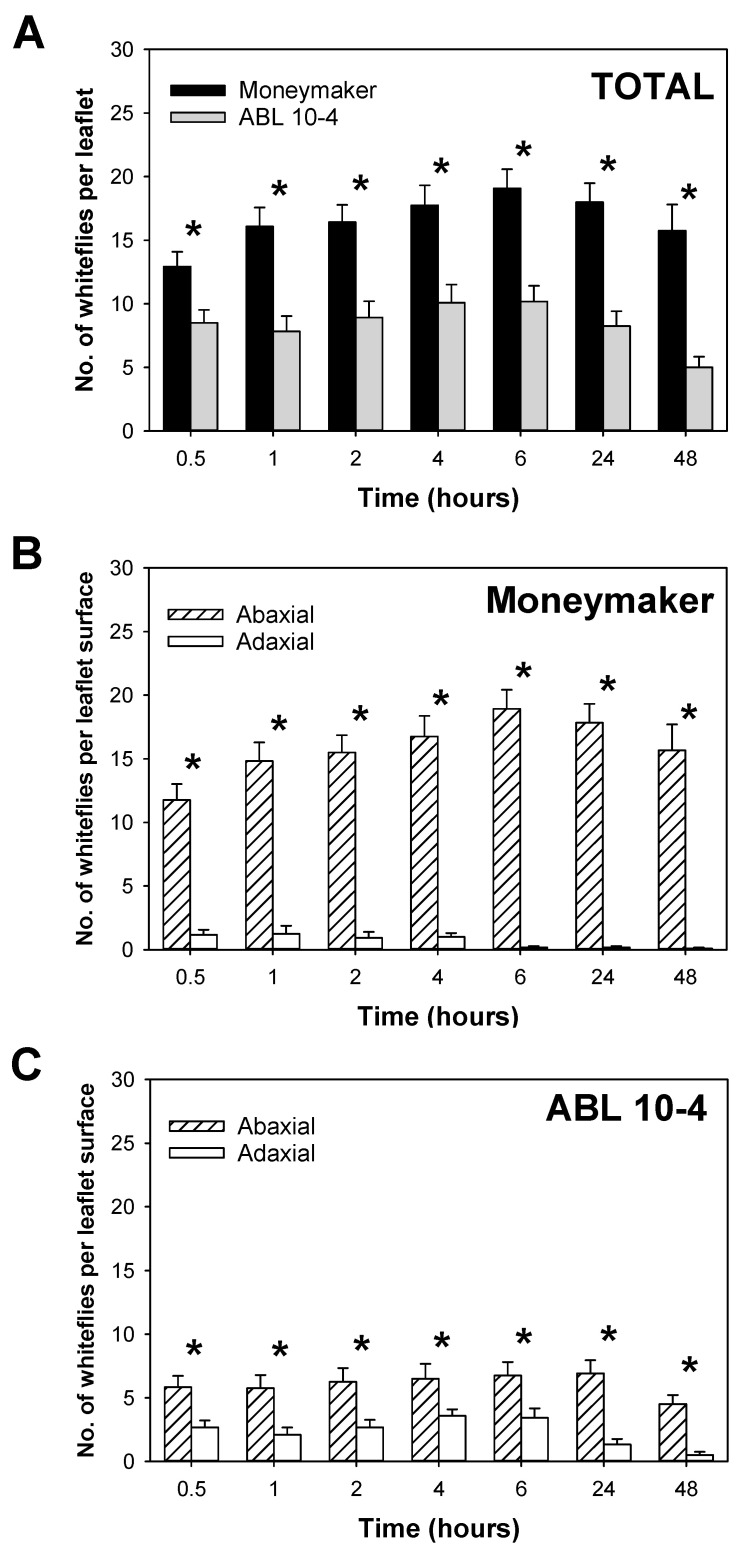
In the repellence experiments, no significant number of dead whiteflies was detected in any treatment. Under the non-choice conditions tested, a significantly (p = 0.001, LS means tests) lower settling preference (antixenosis) of T. vaporariorum was observed at all time points on the leaflets of the acylsucrose-producing genotype ABL 10-4 when compared to Moneymaker (Figure 3A). When counted separately, significantly higher mean number of whiteflies settled on Moneymaker than on ABL 10-4 for the abaxial leaf side while the opposite was observed for the adaxial surface (data shown in Figure 3B,C, statistically analysed as in Figure 3A). Regarding leaf sides comparisons in each plant line, a significantly (p = 0.001, LS means test) higher mean number of whiteflies was counted on the abaxial than on the adaxial side of Moneymaker leaflets since early times after release (Figure 3B). Moreover, significantly higher (p = 0.001, LS means test), but less pronounced differences in the mean number of whiteflies that settled on abaxial than on adaxial leaflet surfaces at every time evaluated were observed in ABL 10-4 (Figure 3C). Taken together, results indicated that T. vaporariorum exhibits a modified settling performance on ABL 10-4 plants; the abaxial side of leaves which is the ideal settling site for this whitefly species, was not so clearly preferred by the insect when exposed to leaflets from the type IV-bearing plant genotype.
Figure 3.
Trialeurodes vaporariorum settling on leaves of Moneymaker and ABL 10-4 near-isogenic tomato lines in non-choice conditions calculated at different times after the release of five T. vaporariorum adult whiteflies on Moneymaker and ABL 10-4 test leaflets at the center of arena. (A) Total number of whiteflies settling on any of the leaf surfaces of Moneymaker vs. ABL 10-4, (B) number of whiteflies on abaxial vs. adaxial leaf surface of Moneymaker, and (C) on abaxial vs. adaxial leaf surface of ABL 10-4. Asterisks indicate significant differences (p = 0.05) at each time point based on LS means test under generalized linear models (Log link, Poisson distribution) analyses; bars indicate the standard error of the mean.
3.3. Negative Effect of ABL 10-4 on Oviposition and Survival of T. vaporariorum
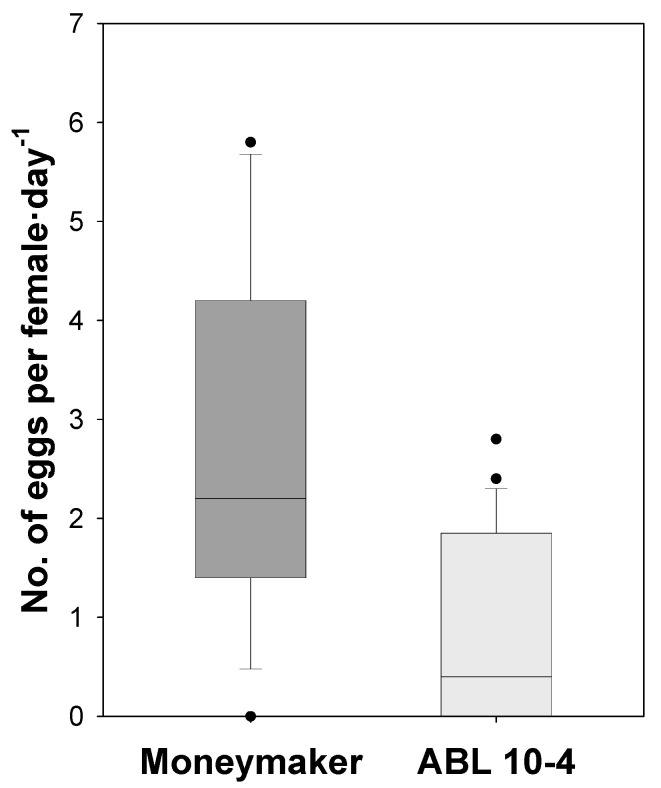
Presence of acylsucrose-producing type IV leaf glandular trichomes in ABL 10-4 not only reduced T. vaporariorum settling but also prevented its oviposition. On ABL 10-4 leaves, T. vaporariorum laid significantly (p = 0.001) lower mean number of eggs per female (0.77 ± 0.20) than on leaves of tomato cv. Moneymaker (2.47 ± 0.46) plants (Figure 4). Therefore, the significantly reduced number of eggs deposited on leaves of ABL 10-4 plants suggested that the presence of type IV acylsucrose-producing trichomes reduced oviposition acceptance of T. vaporariorum.
Figure 4.
Oviposition rates of Trialeurodes vaporariorum on Moneymaker and ABL 10-4 near-isogenic tomato lines. Box-and-whisker plots show the number of eggs per female of T. vaporariorum counted on leaf area of clip-cage (1.5 cm Ø) exposed to whiteflies at 24 h after whitefly release. The box represents the interquartile range, the horizontal line in the box shows the value of the median, bars mark the 10th and 90th percentiles, and dots show outlier values.
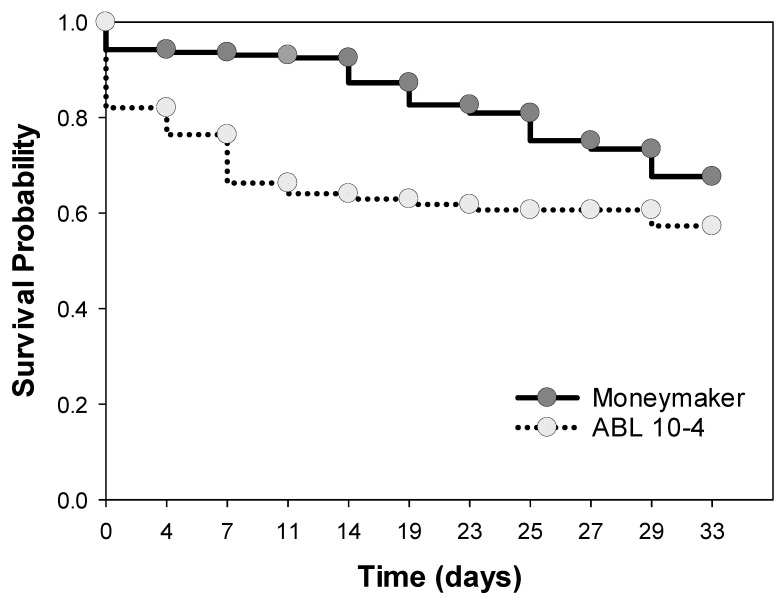
Moreover, a significant negative effect was observed on the survival of T. vaporariorum immature stages on ABL 10-4 compared to Moneymaker. As shown in Figure 5 that represents the survival probability for immature stages of whiteflies from egg (day 1) to L4 nymph (day 33), survival was significantly (p = 0.001; generalized Wilcoxon test) lower on ABL 10-4 than on Moneymaker leaves. Difference in survival probability was highly accentuated at early stages previous to hatching of eggs (about day 11). From day 11 on, the survival probability of T. vaporariorum immature stages on Moneymaker declined to 69% by day 33, whereas in ABL 10-4 was almost constant with a final decline to 59% by day 33. Therefore, the negative effect of the presence of type IV acylsucrose-producing trichomes on immature stages survival is mostly associated with reduced egg survival, rather than on larval survival that, for later stages seemed to be higher on leaves of ABL 10-4.
Figure 5.
Trialeurodes vaporariorum survival probability on plants of Moneymaker and ABL 10-4 near-isogenic tomato lines during a period of 33 days after release. Survival time of T. vaporariorum on leaves of the lines was estimated based on the Kaplan–Meier estimator of the survivorship function [57]. Comparison of survivorship functions was performed by using the test described by Hosmer and Lemeshow (1999).
4. Discussion
A novel approach to global pest management is the use of phytochemicals based on organic chemical compounds that are repellent, with the deterrent effect resulting into reduced insect attractiveness of the plant. Here it was demonstrated that the antixenosis and deterrent traits from S. pimpinellifolium found effective against to B. tabaci [25] were also effective against T. vaporariorum.
The results showed that T. vaporariorum adults were deterred by the type IV leaf glandular trichomes and acylsucroses present on ABL 10-4 leaf surface. The association between insect resistance and the presence and density of type IV leaf glandular trichomes has been reported in S. habrochaites and S. pennellii by several authors [42,55,59,60,61]. Goffreda et al. [42] described that acylsugars in the exudates of type IV trichomes in S. pennellii mediated the resistance to the potato aphid by deterring insect settling and phloem feeding. Moreover, Maluf et al. [48] demonstrated that the foliar acylsugars content was a major component of the resistance to three tomato pests (including the whitefly B. tabaci) in tomato genotypes with type IV leaf glandular trichomes derived from S. pennellii. Foliar acylsugars from type IV leaf glandular trichomes in S. pimpinellifolium TO-937 (the resistance donor of the ABL 10-4 tomato line), were also shown to be sufficient to confer resistance to the two-spotted spider mite Tetranychus urticae Koch (Prostigmata:Tetranychidae) and the whitefly B. tabaci [25,40,44]. Results shown here demonstrated that as for B. tabaci in previously reported studies [54], T. vaporariorum exhibited an altered settling behavior in abaxial and adaxial sides of ABL 10-4 leaves. Most pierce-sucking hemipterans including B. tabaci whiteflies prefer to settle, feed, and oviposit on the abaxial than on the adaxial leaf surface of their host plants soon after landing [43,62,63]. However, a significantly less pronounced preference for the abaxial side was observed for T. vaporariorum, associated with an increased settling on the adaxial side of leaves of ABL 10-4 compared to Moneymaker. These observations demonstrated that the abaxial surface of ABL 10-4 deterred T. vaporariorum settling in contrast to what was observed for leaves of the whitefly-susceptible Moneymaker. Interestingly, in our observations during the preference experiments of this present work and during the previous work at greenhouse cultivations of the plants for selecting the acylsucrose-producing plants, we never detected oviposition on the adaxial leaf sides of either plant genotype. Therefore, ABL 10-4 genotype modifies the innate behavior of T. vaporariorum for settling and feeding on the abaxial surface of tomato leaves. This altered behavior is likely due to the presence of deterrent acylsugars secreted by the type IV leaf glandular trichomes, which are mainly located on the abaxial leaflet surface of the whitefly-resistant genotype [54]. Similar deterrent effect was observed against T. urticae by TO-937 (the type IV leaf glandular trichomes donor of ABL 10-4) which related mostly to abaxial density of type IV leaf glandular trichomes [44]. Therefore, in contrast to that observed in Moneymaker, T. vaporariorum exhibited difficulties settling on abaxial leaflet surface when exposed to ABL 10-4 and an increased number of whiteflies settle on the adaxial side of the leaf. Consequently, the T. vaporariorum tendency to locate preferentially on the abaxial surface of plant leaves, not easily reached by conventional insecticide spraying equipment [13] is altered in ABL 10-4. This altered behavior in the latter tomato genotype might help to increase T. vaporariorum control when using conventional insecticide spraying. Furthermore, when moving to the adaxial leaf surface, T. vaporariorum will become more exposed and then more vulnerable to the action of natural enemies. Better predator activity on adaxial side of leaves was reported, e.g., for Delphastus catalinae, an important coccinellid predator of B. tabaci [64].
Presence of acylsucrose-producing type IV leaf glandular trichomes in ABL 10-4 not only reduced T. vaporariorum settling but also prevented its oviposition. In fact, a similar oviposition reduction effect was also observed for the whitefly B. tabaci in S. pennellii [65] or in the S. pimpinellifolium accession TO-937, the insect-resistance donor of ABL 10-4 [66]. Moreover, presence of acylsugars has been shown to be associated to insect resistance traits in several wild tomato relatives [41,48] also altering oviposition behavior [67,68]. A significant detrimental effect was also observed here for the survival of T. vaporariorum immature stages on ABL 10-4 compared to Moneymaker, mostly associated with egg survival. In this sense, Buta et al. [69] described the isolation and identification of a group of sucrose esters from Nicotiana gossei active against the immature stages of T. vaporariorum when applied topically. Similar to our results, Bas et al. [24] described that the resistance to T. vaporariorum in S. habrochaites f. glabratum is associated to a low pre-adult survival. Our results on immature stages survival as another component of the T. vaporariorum resistance in ABL 10-4 are coincident with previous observations [70,71] that associated the mortality of T. vaporariorum in a number of plant species including tomato to reduced survival of eggs and first developmental stages. Apparently, late instar nymph survival was higher on ABL 10-4 than on Moneymaker plants. This may be due to true better fitness of late instar nymphs in an acylsucrose-rich plant environment but this could also be an artifact caused by biased nymphal populations in the two genotypes. Biased nymphal populations may be produced by adaptation to the acylsucroses of the nymphs that survived the first developmental stages on ABL 10-4 leaves, or by existence of strong bottleneck selection of whitefly genotypes eventually more tolerant to acylsucrose detrimental effects. More precise experiments with nymphs from the different developmental stages individually located on the leaves of the two plant lines would be required to elucidate this aspect.
This present study demonstrates that the insect resistance based on type IV leaf glandular trichomes and acylsucrose secretions derived from S. pimpinellifolium TO-937 and introgressed in a nearly-isogenic BC5S2 tomato line ABL 10-4 can be used as a sustainable control approach to reduce the fitness of the greenhouse whitefly T. vaporariorum on this crop. Transfer of phytochemical-mediated resistance to cultivated tomato species as a means to control multiple pests could significantly reduce the use of agrochemical sprays [28,29,31,68,72]. Previous studies on control of whiteflies based on acylsugar secretions bred from the wild tomato S. pennellii [68,73,74] resulted in sticky plant materials whose use might interfere with the effectiveness of natural enemies used in biological control programs [75,76]. No such unwanted stickiness was observed in the tomato line ABL 10-4 (unpublished observations) that might interfere with biological control of pests. In a previous work we reported that acylsucrose-producing type IV leaf glandular trichomes from S. pimpinellifolium resulted in a reduced preference and affected the feeding behavior of the whitefly B. tabaci that were effective to reduce the spread of TYLCV [25], a virus transmitted by this whitefly. The results of the current study for T. vaporariorum together with the previous results regarding B. tabaci, suggest that the tomato isogenic lines derived from S. pimpinellifolium that exhibit type IV leaf glandular trichomes and acylsucrose secretion might be a promising tool for resistance to either B. tabaci and T. vaporariorum whiteflies in tomato breeding programs. Conventional plant breeding could then contribute to the control of these whiteflies and the viruses they transmit in tomato. Nevertheless, as the effect of vector-resistance on virus spread might depend on a number of factors like, among others, the length of the acquisition, retention, inoculation and latency periods of the virus, the possible existence of non-linear relationship between number of vectors and virus transmission, and the potential enhanced mobility of vector insects in the repellent plants that could eventually increase virus spread, further investigation will be needed to evaluate the possible benefits of using the described T. vaporariorum-resistance as a mean to control the viruses it transmits such as criniviruses or torradoviruses [6].
5. Conclusions
This study demonstrates that the insect resistance based on type IV leaf glandular trichomes and acylsucrose secretions introgressed from a wild tomato into the cultivated species reduces the fitness of the greenhouse whitefly T. vaporariorum. Improved plant materials producing acylsucroses can be useful for sustainable control of this vector insect of several viral diseases affecting tomato crop.
Acknowledgments
The authors are grateful to Rocío Escobar-Bravo for the scanning electron micrographs of tomato leaves.
Author Contributions
Conceptualization, E.M. and R.F.-M.; methodology, M.J.R.-L., E.M., and R.F.-M.; validation, M.J.R.-L., E.M., and R.F.-M.; formal analysis, M.J.R.-L.; investigation, M.J.R.-L.; resources, M.J.R.-L., E.M., and R.F.-M.; data curation, M.J.R.-L.; writing—original draft preparation, M.J.R.-L.; writing—review and editing, E.M., and R.F.-M.; visualization, M.J.R.-L., E.M., and R.F.-M.; supervision, E.M. and R.F.-M.; project administration, E.M. and R.F.-M.; and funding acquisition, E.M. and R.F.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by several grants. R.F.-M. research was supported by grant RTI2018-094277-B-C22/AEI/10.13039/501100011033 and E.M. research by grant PID2019-107657RB-C21/AEI/10.13039/501100011033, funded by the Agencia Estatal de Investigación—Ministerio de Ciencia e Innovación, Spain; E.M. and R.F.-M. research activities were also supported by grant P18-RT-1249 from Consejería de Economía, Conocimiento y Universidad, Junta de Andalucía, Spain. Grants have the assistance from the European Regional Development Fund (ERDF). M.J.R.-L. was the recipient of a FPI (BES-2005-10164) PhD fellowship from Ministerio de Economía y Competitividad, Spain, co-financed by the European Social Fund (ESF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wainaina J.M., De Barro P., Kubatko L., Kehoe M.A., Harvey J., Karanja D., Boykin L.M. Global phylogenetic relationships, population structure and gene flow estimation of Trialeurodes vaporariorum (Greenhouse whitefly) Bull. Entomol. Res. 2017;108:5–13. doi: 10.1017/S0007485317000360. [DOI] [PubMed] [Google Scholar]
- 2.Van Lenteren J.C. Biological control in protected crops: Where do we go? Pestic. Sci. 1992;36:321–327. doi: 10.1002/ps.2780360403. [DOI] [Google Scholar]
- 3.Gorman K., Hewitt F., Denholm I., Devine G.J. New developments in insecticide resistance in the glasshouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest Manag. Sci. 2002;58:123–130. doi: 10.1002/ps.427. [DOI] [PubMed] [Google Scholar]
- 4.Navas-Castillo J., Fiallo-Olive E., Sanchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011;49:219–248. doi: 10.1146/annurev-phyto-072910-095235. [DOI] [PubMed] [Google Scholar]
- 5.Brown J.K., Czosnek H. Whitefly transmission of plant viruses. Adv. Bot. Res. 2002;36:65–100. [Google Scholar]
- 6.Navas-Castillo J., Lopez-Moya J.J., Aranda M.A. Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann. Appl. Biol. 2014;165:155–171. doi: 10.1111/aab.12147. [DOI] [Google Scholar]
- 7.Wintermantel W.M. Emergence of Greenhouse Whitefly (Trialeurodes vaporariorum) Transmitted Criniviruses as threats to Vegetable and Fruit Production in North America. APSnet Feature Story; St. Paul, MN, USA: 2004. [Google Scholar]
- 8.Fiallo-Olive E., Pan L.L., Liu S.S., Navas-Castillo J. Transmission of begomoviruses and other whitefly-borne viruses: Dependence on the vector species. Phytopathology. 2020;110:10–17. doi: 10.1094/PHYTO-07-19-0273-FI. [DOI] [PubMed] [Google Scholar]
- 9.Fiallo-Olive E., Navas-Castillo J. Tomato chlorosis virus, an emergent plant virus still expanding its geographical and host ranges. Mol. Plant Pathol. 2019;20:1307–1320. doi: 10.1111/mpp.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson M.W., Caprio L.C., Coughlin J.A., Tabashnik B.E., Rosenheim J.A., Welter S.C. Effect of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on yield of fresh market tomatoes. J. Econ. Entomol. 1992;85:2370–2376. doi: 10.1093/jee/85.6.2370. [DOI] [Google Scholar]
- 11.Mansilla-Córdova P.J., Bampi D., Rondinel-Mendoza N.V., Melo P.C.T., Lourenção A.L., Rezende J.A.M. Screening tomato genotypes for resistance and tolerance to Tomato chlorosis virus. Plant Pathol. 2018;67:1231–1237. doi: 10.1111/ppa.12826. [DOI] [Google Scholar]
- 12.Omer A.D., Johnson M.W., Tabashnik B.E., Ullman D.E. Association between insecticide use and greenhouse whitefly (Trialeurodes vaporariorum Westwood) resistance to insecticides in Hawaii. Pestic. Sci. 1993;37:253–259. doi: 10.1002/ps.2780370304. [DOI] [Google Scholar]
- 13.Dittrich V., Uk S., Ernst G.H. Whiteflies: Their Bionomics, Pest Status and Management. Intercept Press; Andover, Hants, UK: 1990. Chemical control and insecticide resistance of whiteflies; pp. 263–285. [Google Scholar]
- 14.van Lenteren J.C., van Roermund H.J.W., Sütterlin S. Biological control of greenhouse whitefly (Trialeurodes vaporariorum) with the parasitoid Encarsia formosa: How does It work? Biol. Control. 1996;6:1–10. doi: 10.1006/bcon.1996.0001. [DOI] [Google Scholar]
- 15.Quesada-Moraga E., Maranhao E.A.A., Valverde-Garcia P., Santiago-Alvarez C. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements, and toxicogenic activity. Biol. Control. 2006;36:274–287. doi: 10.1016/j.biocontrol.2005.09.022. [DOI] [Google Scholar]
- 16.Meekes E.T.M., Fransen J.J., van Lenteren J.C. Pathogenicity of Aschersonia spp. against whiteflies Bemisia argentifolii and Trialeurodes vaporariorum. J. Invertebr. Pathol. 2002;81:1–11. doi: 10.1016/S0022-2011(02)00150-7. [DOI] [PubMed] [Google Scholar]
- 17.Byrne D.N., Bellows T.S.J. Whitefly Biology. Annu. Rev. Entomol. 1991;36:431–457. doi: 10.1146/annurev.en.36.010191.002243. [DOI] [Google Scholar]
- 18.Coombe P.E. Visual behaviour of the greenhouse whitefly, Trialeurodes vaporariorum. Physiol. Entomol. 1982;7:243–251. doi: 10.1111/j.1365-3032.1982.tb00297.x. [DOI] [Google Scholar]
- 19.Broekgaarden C., Snoeren T.A.L., Dicke M., Vosman B. Exploiting natural variation to identify insect-resistance genes. Plant Biotechnol. J. 2011;9:819–825. doi: 10.1111/j.1467-7652.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 20.Walling L.L. Advances in Botanical Research. Volume 51. Academic Press; Cambridge, MA, USA: 2009. Adaptive defense responses to pathogens and insects; pp. 551–612. [Google Scholar]
- 21.Fraser R.S.S. Integrated pest and disease management in protected crops. Outlook Agric. 1992;21:169–175. doi: 10.1177/003072709202100304. [DOI] [Google Scholar]
- 22.Fereres A., Moreno A. Integrated Control Measures against Viruses and Their Vectors. Caister Academic Press; Wymondham, UK: 2011. pp. 237–261. [Google Scholar]
- 23.Nombela G., Beitia F., Muniz M. Variation in tomato host response to Bemisia tabaci (Hemiptera: Aleyrodidae) in relation to acyl sugar content and presence of the nematode and potato aphid resistance gene Mi. Bull. Entomol. Res. 2000;90:161–167. doi: 10.1017/S0007485300000274. [DOI] [PubMed] [Google Scholar]
- 24.Bas N., Mollema C., Lindhout P. Resistance in Lycopersicon hirsutum f. glabratum to the greenhouse whitefly (Trialeurodes vaporariorum) increases with plant age. Euphytica. 1992;64:189–195. doi: 10.1007/BF00046048. [DOI] [Google Scholar]
- 25.Rodriguez-Lopez M.J., Garzo E., Bonani J.P., Fereres A., Fernandez-Muñoz R., Moriones E. Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of Tomato yellow leaf curl virus. Phytopathology. 2011;101:1191–1201. doi: 10.1094/PHYTO-01-11-0028. [DOI] [PubMed] [Google Scholar]
- 26.Yao Q., Peng Z., Tong H., Yang F., Xing G., Wang L., Zheng J., Zhang Y., Su Q. Tomato plant flavonoids increase whitefly resistance and reduce spread of tomato yellow leaf curl virus. J. Econ. Entomol. 2019;112:2790–2796. doi: 10.1093/jee/toz199. [DOI] [PubMed] [Google Scholar]
- 27.Walling L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- 28.Bleeker P.M., Mirabella R., Diergaarde P.J., VanDoorn A., Tissier A., Kant M.R., Prins M., de Vos M., Haring M.A., Schuurink R.C. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA. 2012;109:20124–20129. doi: 10.1073/pnas.1208756109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanDoorn A., de Vos M. Resistance to sap-sucking insects in modern-day agriculture. Front. Plant Sci. 2013;4:222. doi: 10.3389/fpls.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glas J.J., Schimmel B.C.J., Alba J.M., Escobar-Bravo R., Schuurink R.C., Kant M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012;13:17077–17103. doi: 10.3390/ijms131217077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vosman B., van’t Westende W.P.C., Henken B., van Eekelen H.D.L.M., de Vos R.C.H., Voorrips R.E. Broad spectrum insect resistance and metabolites in close relatives of the cultivated tomato. Euphytica. 2018;214:46. doi: 10.1007/s10681-018-2124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muigai S.G., Bassett M.J., Schuster D.J., Scott J.W. Greenhouse and field screening of wild Lycopersicon germplasm for resistance to the whitefly Bemisia argentifolii. Phytoparasitica. 2003;31:27–38. doi: 10.1007/BF02979764. [DOI] [Google Scholar]
- 33.Lucatti A.F., Alvarez A.E., Machado E.G., Gilardón E. Resistance of tomato genotypes to the greenhouse whitefly Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae) Neotrop. Entomol. 2010;39:792–798. doi: 10.1590/S1519-566X2010000500019. [DOI] [PubMed] [Google Scholar]
- 34.Romanow L.R., de Ponti O.M.B., Mollema C. Resistance in tomato to the greenhouse whitefly: Analysis of population dynamics. Entomol. Exp. Appl. 1991;60:247–259. doi: 10.1111/j.1570-7458.1991.tb01545.x. [DOI] [Google Scholar]
- 35.De Ponti O.M.B., Mollema C. Emerging breeding strategies for insect resistance. In: Stalker H.T., Murphy J.P., editors. Plant Breeding in the 1990s. CAB International; Wallingford, UK: 1992. pp. 323–346. [Google Scholar]
- 36.Maliepaard C., Bas N., van Heusden S., Kos J., Pet G., Verkerk R., Vrieunk R., Zabel P., Lindhout P. Mapping of QTLs for glandular trichome densities and Trialeurodes vaporariorum (greenhouse whitefly) resistance in an F2 from Lycopersicon esculentum × Lycopersicon hirsutum F. glabratum. Heredity. 1995;75:425–433. doi: 10.1038/hdy.1995.155. [DOI] [Google Scholar]
- 37.Wagner G.J., Wang E., Shepherd R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004;93:3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicke M., Baldwin I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Bleeker P.M., Diergaarde P.J., Ament K., Guerra J., Weidner M., Schutz S., de Both M.T.J., Haring M.A., Schuurink R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009;151:925–935. doi: 10.1104/pp.109.142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alba J.M., Montserrat M., Fernandez-Munoz R. Resistance to the two-spotted spider mite (Tetranychus urticae) by acylsucroses of wild tomato (Solanum pimpinellifolium) trichomes studied in a recombinant inbred line population. Exp. Appl. Acarol. 2009;47:35–47. doi: 10.1007/s10493-008-9192-4. [DOI] [PubMed] [Google Scholar]
- 41.Vosman B., Kashaninia A., van’t Westende W., Meijer-Dekens F., van Eekelen H., Visser R.G.F., de Vos R.C.H., Voorrips R.E. QTL mapping of insect resistance components of Solanum galapagense. Theor. Appl. Genet. 2018;132:531–541. doi: 10.1007/s00122-018-3239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goffreda J.C., Mutschler M.A., Tingey W.M. Feeding behavior of potato aphid affected by glandular trichomes of wild tomato. Entomol. Exp. Appl. 1988;48:101–107. doi: 10.1111/j.1570-7458.1988.tb01152.x. [DOI] [Google Scholar]
- 43.Oriani M.A.d.G., Vendramim J.D. Influence of trichomes on attractiveness and ovipositional preference of Bemisia tabaci (Genn.) B biotype (Hemiptera: Aleyrodidae) on tomato genotypes. Neotrop. Entomol. 2010;39:1002–1007. doi: 10.1590/S1519-566X2010000600024. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Munoz R., Salinas M., Alvarez M., Cuartero J. Inheritance of resistance to two-spotted spider mite and glandular leaf trichomes in wild tomato Lycopersicon pimpinellifolium (Jusl.) Mill. J. Am. Soc. Hortic. Sci. 2003;128:188–195. doi: 10.21273/JASHS.128.2.0188. [DOI] [Google Scholar]
- 45.Gentile A.G., Stoner A.K., Webb R.E. Resistance in Lycopersicon and Solanum to greenhouse whiteflies. J. Econ. Entomol. 1968;61:1355–1357. doi: 10.1093/jee/61.5.1355. [DOI] [Google Scholar]
- 46.Firdaus S., van Heusden A.W., Hidayati N., Supena E.D.J., Visser R.G.F., Vosman B. Resistance to Bemisia tabaci in tomato wild relatives. Euphytica. 2012;187:31–45. doi: 10.1007/s10681-012-0704-2. [DOI] [Google Scholar]
- 47.McDaniel T., Tosh C.R., Gatehouse A.M.R., George D., Robson M., Brogan B. Novel resistance mechanisms of a wild tomato against the glasshouse whitefly. Agron. Sustain. Dev. 2016;36:14. doi: 10.1007/s13593-016-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maluf W.R., Maciel G.M., Gomes L.A.A., Cardoso M.d.G., Goncalves L.D., da Silva E.C., Knapp M. Broad-spectrum arthropod resistance in hybrids between high- and low-acylsugar tomato lines. Crop Sci. 2010;50:439–450. doi: 10.2135/cropsci2009.01.0045. [DOI] [Google Scholar]
- 49.Silva K.F.A.S., Michereff-Filho M., Fonseca M.E.N., Silva-Filho J.G., Texeira A.C.A., Moita A.W., Torres J.B., Fernandez-Muñoz R., Boiteux L.S. Resistance to Bemisia tabaci biotype B of Solanum pimpinellifolium is associated with higher densities of type IV glandular trichomes and acylsugar accumulation. Entomol. Exp. Appl. 2014;151:218–230. doi: 10.1111/eea.12189. [DOI] [Google Scholar]
- 50.Rojas M.R., Macedo M.A., Maliano M.R., Soto-Aguilar M., Souza J.O., Briddon R.W., Kenyon L., Bustamante R.F.R., Zerbini F.M., Adkins S., et al. World management of geminiviruses. Annu. Rev. Phytopathol. 2018;56:637–677. doi: 10.1146/annurev-phyto-080615-100327. [DOI] [PubMed] [Google Scholar]
- 51.Lee D.-H., Nyrop J.P., Sanderson J.P. Effect of host experience of the greenhouse whitefly, Trialeurodes vaporariorum, on trap cropping effectiveness. Entomol. Exp. Appl. 2010;137:193–203. doi: 10.1111/j.1570-7458.2010.01052.x. [DOI] [Google Scholar]
- 52.Darshanee H.L.C., Ren H., Ahmed N., Zhang Z.F., Liu Y.H., Liu T.X. Volatile-mediated attraction of greenhouse whitefly Trialeurodes vaporariorum to tomato and eggplant. Front. Plant Sci. 2017;8:1285. doi: 10.3389/fpls.2017.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Escobar-Bravo R., Alba J.M., Pons C., Granell A., Kant M.R., Moriones E., Fernandez-Muñoz R. A jasmonate-inducible defense trait transferred from wild into cultivated tomato establishes increased whitefly resistance and reduced viral disease Incidence. Front. Plant Sci. 2016;7:1732. doi: 10.3389/fpls.2016.01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Lopez M.J., Garzo E., Bonani J.P., Fernandez-Muñoz R., Moriones E., Fereres A. Acylsucrose-producing tomato plants forces Bemisia tabaci to shift its preferred settling and feeding site. PLoS ONE. 2012;7:e33064. doi: 10.1371/journal.pone.0033064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goffreda J.C., Steffens J.C., Mutschler M.A. Association of epicuticular sugars with aphid resistance in hybrids with wild tomato. J. Am. Soc. Hortic. Sci. 1990;115:161–165. doi: 10.21273/JASHS.115.1.161. [DOI] [Google Scholar]
- 56.Perring T.M., Stansly P.A., Liu T.X., Smith H.A., Andreason S.A. Whiteflies: Biology, ecology, and management. In: Wakil W., Brust G.E., Perring T.M., editors. Sustainable Management of Arthropod Pests of Tomato. Academic Press; San Diego, CA, USA: 2018. [Google Scholar]
- 57.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 58.Hosmer D.W., Lemeshow S. Applied Logistic Regression. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2000. Introduction to the logistic regression model; pp. 1–30. [Google Scholar]
- 59.Snyder J.C., Carter C.D. Leaf trichomes and resistance of Lycopersicum hirsutum and Lycopersicum esculentum to spider mites. J. Am. Soc. Hortic. Sci. 1984;109:837–843. [Google Scholar]
- 60.Goffreda J.C., Szymkowiak E.J., Sussex I.M., Mutschler M.A. Chimeric tomato plants show that aphid resistance and triacylglucose production are epidermal autonomous characters. Plant Cell. 1990;2:643–649. doi: 10.1105/tpc.2.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fery R.L., Kennedy G.G. Genetic analysis of 2-tridecanone concentration, leaf trichome characteristics, and tobacco hornwork resistance in tomato. J. Am. Soc. Hortic. Sci. 1987;112:886–891. [Google Scholar]
- 62.Calabrese E.J., Edwards L.J. Light and gravity in leaf-side selection by green peach aphid, Myzus persicae. Ann. Entomol. Soc. Am. 1976;69:1145–1146. doi: 10.1093/aesa/69.6.1145. [DOI] [Google Scholar]
- 63.Simmons A.M. Settling of crawlers of Bemisia tabaci (Homoptera: Aleyrodidae) on five vegetable hosts. Ann. Entomol. Soc. Am. 2002;95:464–468. doi: 10.1603/0013-8746(2002)095[0464:SOCOBT]2.0.CO;2. [DOI] [Google Scholar]
- 64.Guershon M., Gerling D. Effects of plant and prey characteristics on the predatory behavior of Delphastus catalinae. Entomol. Exp. Appl. 2006;121:15–21. doi: 10.1111/j.1570-8703.2006.00455.x. [DOI] [Google Scholar]
- 65.Liedl B.E., Lawson D.M., White K.K., Shapiro J.A., Cohen D.E., Carson W.G., Trumble J.T., Mutschler M.A. Acylsugars of wild tomato Lycopersicon pennellii alters settling and reduces oviposition of Bemisia argentifolii (Homoptera:Aleyrodidae) J. Econ. Entomol. 1995;88:742–748. doi: 10.1093/jee/88.3.742. [DOI] [Google Scholar]
- 66.Alba J.M. Ph.D. Thesis. Facultad de Ciencias, Universidad de Málaga; Málaga, Spain: 2006. Herencia de los Mecanismos de Resistencia a Arena Roja en Tomate. [Google Scholar]
- 67.Smeda J.R., Schilmiller A.L., Anderson T., Ben-Mahmoud S., Ullman D.E., Chappell T.M., Kessler A., Mutschler M.A. Combination of acylglucose QTL reveals additive and epistatic genetic interactions and impacts insect oviposition and virus infection. Mol. Breed. 2017;38:3. doi: 10.1007/s11032-017-0756-z. [DOI] [Google Scholar]
- 68.Leckie B.M., D’Ambrosio D.A., Chappell T.M., Halitschke R., De Jong D.M., Kessler A., Kennedy G.G., Mutschler M.A. Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS ONE. 2016;11:e0153345. doi: 10.1371/journal.pone.0153345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buta J.G., Lusby W.R., Neal J.W., Waters R.M., Pittarelli G.W. Sucrose esters from Nicotiana gossei active against the greenhouse whitefly Trialeuroides vaporariorum. Phytochemistry. 1993;32:859–864. doi: 10.1016/0031-9422(93)85220-L. [DOI] [Google Scholar]
- 70.Woets J., van Lenteren J.C. The parasite-host relationship between Encarsia formosa (Hymenoptera: Aphelinidae) and Trialeurodes vaporariorum (Homoptera: Aleyrodidae). 6. The influence of the host plant of the greenhouse white fly and its parasite Encarsia formosa. Bull. Srop. 1976;1974:151–164. [Google Scholar]
- 71.van de Merendonk S., van Lenteren J.V. Determination of mortality of greenhouse whitefly Trialeurodes vaporariorum (Westwood) (Homoptera: Aleyrodidae) eggs, larvae and pupae on four host-plant species: Eggplant (Solanum melongena L.), cucumber (Cucumis sativus L.), tomato (Lycopersicum esculentum L.) and paprika (Capsicum annuum L.) Med. Van De Fac. Landbouwwet. Rijksuniv. Gent. 1978;43:421–429. [Google Scholar]
- 72.Mutschler M.A., Steffens J.C., Tingey W. Broad based insect resistance from L. pennellii: Its nature and potential for pest control in tomato. In: Yoder J.I., editor. Molecular Biology of Tomato: Fundamental Advances and Crop Improvement. Technomic publishing Co., Inc.; Lancaster, PA, USA: 1993. pp. 285–290. [Google Scholar]
- 73.Berlinger M.J., Dahan R. Breeding for resistance to virus transmission by whiteflies in tomatoes. Int. J. Trop. Insect Sci. 1987;8:783–784. doi: 10.1017/S1742758400022918. [DOI] [Google Scholar]
- 74.Resende J.T.d., Maluf W.R., Cardoso M.d.G., Gonçalves L.D., Faria M.V., Nascimento I.R.d. Resistance of tomato genotypes to the silverleaf whitefly mediated by acylsugars. Hortic. Bras. 2009;27:345–348. doi: 10.1590/S0102-05362009000300015. [DOI] [Google Scholar]
- 75.De Ponti O.M.B., Romanow L.R., Berlinger M.J. Whitefly plant relationships—Plant resistance. In: Gerling D., editor. Whiteflies: Their Bionomics, Pest Status and Management. Gerling Dan Intercept Press; Andover, UK: 1990. pp. 91–106. [Google Scholar]
- 76.Van Lenteren J.C. A greenhouse without pesticides: Fact or fantasy? Crop Prot. 2000;19:375–384. doi: 10.1016/S0261-2194(00)00038-7. [DOI] [Google Scholar]