Abstract
The COVID-19 pandemic has rapidly changed delivery of cancer care. Many nonurgent surgeries are delayed to preserve hospital resources, and patient visits to health care settings are limited to reduce exposure to SARS-CoV-2. Providers must carefully weigh risks and benefits of delivering immunosuppressive therapy during the pandemic. For breast cancer, a key difference is increased use of neoadjuvant systemic therapy due to deferral of many breast surgeries during the pandemic. In some cases, this necessitates increased use of genomic tumor profiling on core biopsy specimens to guide neoadjuvant therapy decisions. Breast cancer treatment during the pandemic requires multidisciplinary input and varies according to stage, tumor biology, comorbidities, age, patient preferences, and available hospital resources. We present here the Johns Hopkins Women’s Malignancies Program approach to breast cancer management during the COVID-19 pandemic. We include algorithms based on tumor biology and extent of disease that guide management decisions during the pandemic. These algorithms emphasize medical oncology treatment decisions and demonstrate how we have operationalized the general treatment recommendations during the pandemic proposed by national groups, such as the COVID-19 Pandemic Breast Cancer Consortium. Our recommendations can be adapted by other institutions and medical oncology practices in accordance with local conditions and resources. Guidelines such as these will be important as we continue to balance treatment of breast cancer against risk of SARS-CoV-2 exposure and infection until approval of a vaccine.
INTRODUCTION
On March 11, 2020, the World Health Organization declared a pandemic in the setting of > 100,000 cases of a new respiratory illness, coronavirus disease 2019 (COVID-19), caused by infection with a novel coronavirus, SARS-CoV-2.1 Data regarding COVID-19 and cancer are limited, but early reports suggest individuals with cancer, especially those who receive systemic anticancer therapy within 14 days of COVID-19 diagnosis, are more likely to develop severe disease.2-7 Furthermore, individuals with metastatic cancer are more likely to require admission for intensive care, undergo mechanical ventilation, and die as a result of COVID-19.6 Data also implicate health care settings as a source for SARS-CoV-2 transmission, a finding concerning to patients with cancer who frequent cancer centers.8
Many countries have implemented strategies to avoid surges of COVID-19 cases, conserve resources, and protect vulnerable populations from infection.9 Cancer centers have rapidly changed models of care by delaying nonurgent surgeries, increasing home-based therapies, and expanding telemedicine.10,11 Numerous organizations and institutions have issued general and disease-specific guidelines for cancer care.12-17 Although COVID-19 cases have already peaked in some locations, they are increasing in others, and secondary surges are anticipated, suggesting that changes in cancer care will not be short lived.18
For patients with breast cancer, preliminary management recommendations have been proposed by the COVID-19 Pandemic Breast Cancer Consortium.15 These tiered guidelines prioritize surgery, radiation, and systemic therapy interventions by urgency. As in nonpandemic circumstances, treatment decisions must consider stage and tumor biology within the context of comorbidities and individual patient goals.19 Acknowledging the uncertainties of cancer outcomes and SARS-CoV-2 infection risk associated with treating breast cancer, we present here the John Hopkins Women’s Malignancies Program approach to breast cancer management during the pandemic. These stage- and subtype-specific algorithms, endorsed by our multidisciplinary team and patient advocates, represent our strategy to apply available evidence to optimize breast cancer management during this time.
EARLY STAGE BREAST CANCER
Ductal Carcinoma In Situ
In accordance with recommendations from the American College of Surgeons and COVID-19 Pandemic Breast Cancer Consortium, we recommend deferring surgery for ductal carcinoma in situ (DCIS) during the pandemic in the absence of microinvasion or of high suspicion of invasive cancer.13,15 For newly diagnosed estrogen receptor (ER)–negative DCIS, we defer intervention for up to 3-6 months and until after the peak of the pandemic, when surgical supplies are more available. For ER-positive DCIS, we recommend a telemedicine consultation with medical oncology and neoadjuvant endocrine therapy (ET) for up to 6 months. We prefer an aromatase inhibitor (AI) for postmenopausal women20 and tamoxifen for premenopausal women.21 For individuals who previously underwent breast-conserving surgery (BCS) for DCIS, we consider delaying or omitting radiation for those who are ER positive and are able to initiate ET.22-24 Omission of radiation is an option for good-risk disease (low to intermediate grade, < 2.5 cm, surgical margins ≥ 3 mm).25 These recommendations are summarized in Appendix Table A1 (online only).
Early-Stage Invasive Breast Cancer (Stages I-III)
We recommend early multidisciplinary evaluation for most individuals with newly diagnosed clinical stage I-III invasive breast cancer. At Johns Hopkins, surgery is currently available for patients with early-stage invasive breast cancer completing neoadjuvant systemic therapy and for select patients with newly diagnosed invasive breast cancer who desire up-front surgery and/or who are not appropriate candidates for neoadjuvant systemic therapy. Because availability of surgery is fluid, based on resources within the institution and COVID-19 incidence in the community, our surgeons use tiered criteria to prioritize patients for surgery. When possible, we recommend BCS. Contralateral procedures and reconstruction with an expander or immediate implant are currently available on a case-by-case basis after assessment of COVID-19 risk and comorbidity. Limitations in available surgeries may necessitate a staged approach, with BCS of the affected breast performed first and additional surgery after the pandemic. If the desired reconstruction procedure is not immediately available, we consider neoadjuvant systemic therapy to allow deferral of mastectomy.
Triple-negative breast cancer.
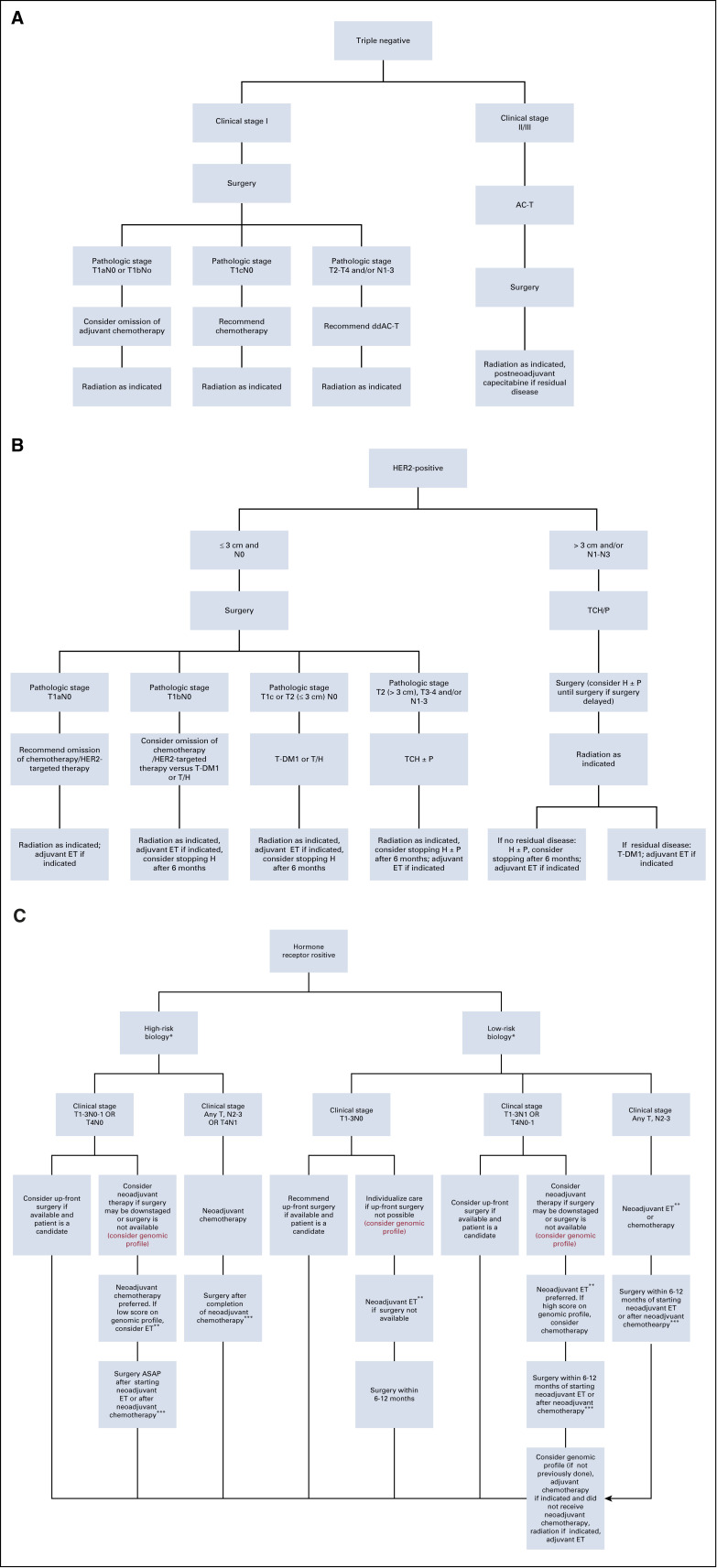
Figure 1A and Appendix Table A2 (online only) summarize our approach to newly diagnosed early-stage triple-negative breast cancer (TNBC). If available, we recommend up-front surgery for clinical T1N0 TNBC and do not recommend adjuvant chemotherapy for pathologic small (T1a-b), node-negative disease. For those with pathologic T1a-bN0 TNBC who desire adjuvant chemotherapy and for those with more advanced pathologic stage after up-front surgery, we initiate adjuvant chemotherapy within the standard time frame. Whenever possible, we administer non–anthracycline-containing regimens, such as docetaxel/cyclophosphamide (TC) for adjuvant treatment of pathologic stage T1cN0 TNBC, as the added benefit of an anthracycline-containing regimen is small in this population, and TC requires fewer clinic visits. For pathologic T2-4 and/or N1-3 TNBC, we recommend adjuvant dose-dense doxorubicin/cyclophosphamide followed by weekly or dose-dense paclitaxel (AC-T).26-29
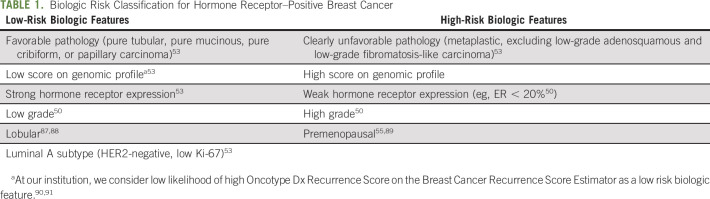
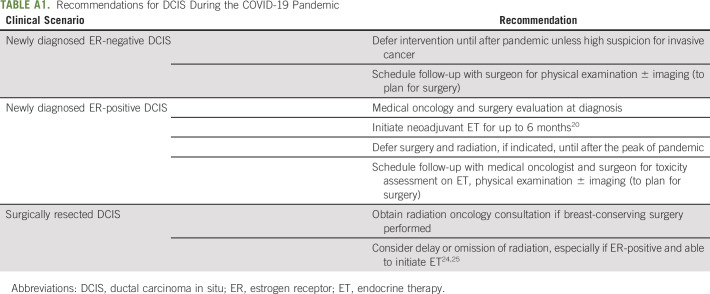
Fig 1.
(A) Johns Hopkins recommended approach to multidisciplinary care for stage I-III triple-negative invasive breast cancer during the COVID-19 pandemic. (B) Johns Hopkins recommended approach to multidisciplinary care for stage I-III HER2-positive invasive breast cancer during the COVID-19 pandemic. (C) Johns Hopkins recommended approach to multidisciplinary care for stage I-III hormone receptor–positive invasive breast cancer during the COVID-19 pandemic. (*) See Table 1 for definitions of low- and high-risk biology. For patients with biologic risk features that are neither clearly high nor low risk, genomic profile may be performed on the core biopsy specimen to guide classification. In cases in which biologic risk features are neither clearly high nor low risk and genomic profiling is not performed, we recommend following lowbiologic-risk arm. (**) The preferred neoadjuvant regimen for postmenopausal women is aromatase inhibitor (AI). The preferrred neoadjuvant regimen for premenopausal women is ovarian function suppression with tamoxifen followed by transition to AI once estradiol is suppressed. (***) If surgery is not available after completion of planned course of neoadjuvant chemotherapy, may initiate neoadjuvant endocrine therapy (ET) until surgery is available. AC-T, doxorubicin and cyclophosphamide followed by paclitaxel; ASAP, as soon as possible; ddAC-T, dose-dense AC-T; H, trastuzumab; P, pertuzumab; T, paclitaxel; TCH, docetaxel/carboplatin/trastuzumab; T-DM1, ado-trastuzumab emtansine.
For clinical prognostic stage II-III TNBC, we recommend neoadjuvant AC-T. Although addition of carboplatin to neoadjuvant AC-T increases likelihood of pathologic complete response, we do not typically include carboplatin, as there is no definite survival benefit and hematologic toxicity increases.30 We likewise have reservations regarding neoadjuvant immunotherapy, especially during the pandemic, because of associated adverse events.31 On completion of neoadjuvant chemotherapy (NACT), surgery should be performed within 4-6 weeks.32 If surgery is delayed because of the pandemic, we do not recommend additional chemotherapy; these patients should be prioritized for surgery when available. If residual disease is identified at surgery after NACT, we consider postneoadjuvant capecitabine as per routine.33
After completion of (neo)adjuvant chemotherapy and surgery, we refer individuals with stage I-III TNBC to radiation oncology per usual criteria. Despite the risk of SARS-CoV-2 exposure with daily radiation visits, we do not recommend delaying radiation for TNBC because of risk of locoregional recurrence.24,34,35
Human epidermal growth factor receptor 2–positive breast cancer.
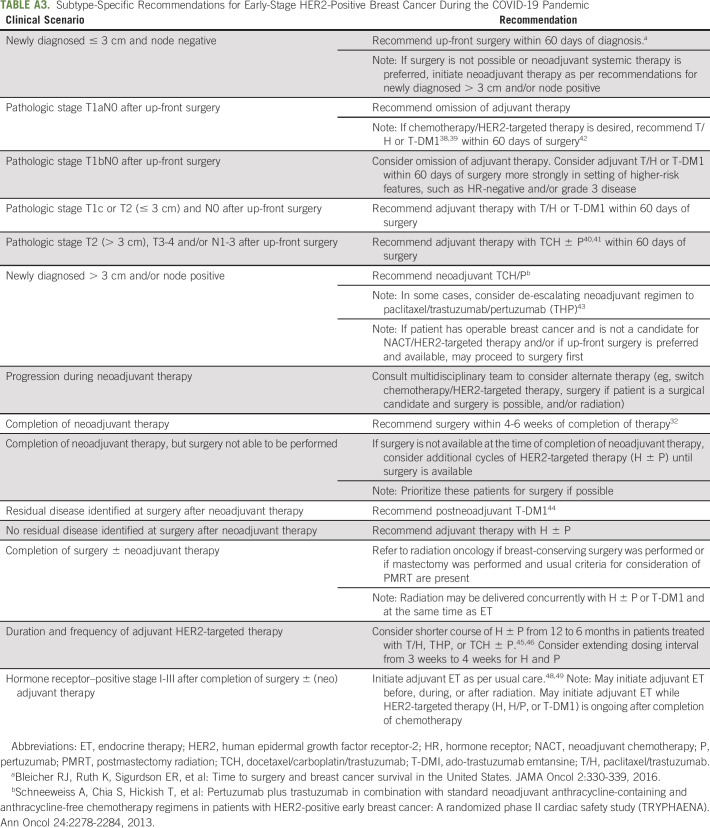
Figure 1B and Appendix Table A3 (online only) summarize our approach to newly diagnosed early-stage human epidermal growth factor receptor 2 (HER2)–positive breast cancer during the pandemic. Although the COVID-19 Pandemic Breast Cancer Consortium suggests up-front surgery only for T1N0 HER2-positive breast cancer, we also favor up-front surgery for small T2 (≤ 3 cm) N0 disease, with the goal of de-escalating adjuvant systemic therapy (and the attendant risks of visits to the cancer center and immunosuppression) if early stage is confirmed pathologically. If surgery reveals pathologic T1aN0 disease, we do not recommend adjuvant chemotherapy/HER2-targeted therapy, given low recurrence risk.36,37 For pathologic stage T1bN0 HER2-positive breast cancer, we discuss pros and cons of adjuvant chemotherapy/HER2-targeted therapy but consider it most strongly for individuals with hormone receptor (HR)-negative and/or grade 3 disease. For individuals with pathologic T1bN0 disease who opt for adjuvant chemotherapy/HER2-targeted therapy and for those with pathologic T1cN0 or small T2N0 (≤ 3 cm) disease, we recommend paclitaxel/trastuzumab (T/H) or trastuzumab emtansine (T-DM1). Although toxicity profiles differ, both adjuvant T/H and T-DM1 are associated with favorable disease-free survival and minimal hematologic toxicity.38,39 A potential advantage of adjuvant T-DM1 over T/H during the pandemic is the every-3-week dosing interval. For individuals found to have more extensive disease after up-front surgery, we recommend standard adjuvant chemotherapy/HER2-targeted therapy with docetaxel/carboplatin/trastuzumab (TCH) ± pertuzumab (P).40,41 As per routine, we initiate adjuvant systemic therapy within 60 days of surgery for HER2-positive breast cancer.42
In general, we recommend NACT/HER2-targeted therapy for those with tumor size > 3 cm and/or clinically positive axillary lymph node(s). Our preferred neoadjuvant regimen is TCH ± P, but in select circumstances we consider de-escalation with paclitaxel/trastuzumab/pertuzumab.43
After completion of NACT/HER2-targeted therapy, surgery should be performed within 4-6 weeks.32 If surgery is not available, we recommend additional cycles of HER2-targeted therapy (H ± P) until surgery is available. If residual disease is identified at surgery, we treat with standard postneoadjuvant T-DM1.44 If there is no residual disease at surgery, we administer adjuvant H ± P. To limit clinic visits during the pandemic, we consider shortening HER2-targeted therapy with H ± P from 12 to 6 months and extending the dosing interval from 3 weeks to 4 weeks.45,46 Although there is interest in subcutaneous trastuzumab, there are challenges with implementation, including requirement for administration by a health care provider and insurance coverage.
After completion of primary therapy for HER2-positive early breast cancer, we refer to radiation oncology per standard guidelines.47 As is the case for TNBC, we do not recommend delaying radiation for HER2-positive breast cancer because of risk of locoregional recurrence.24,34 After completion of primary therapy, we initiate adjuvant ET per usual care for individuals with HR-positive and HER2-positive breast cancer.48,49
HR-positive breast cancer.
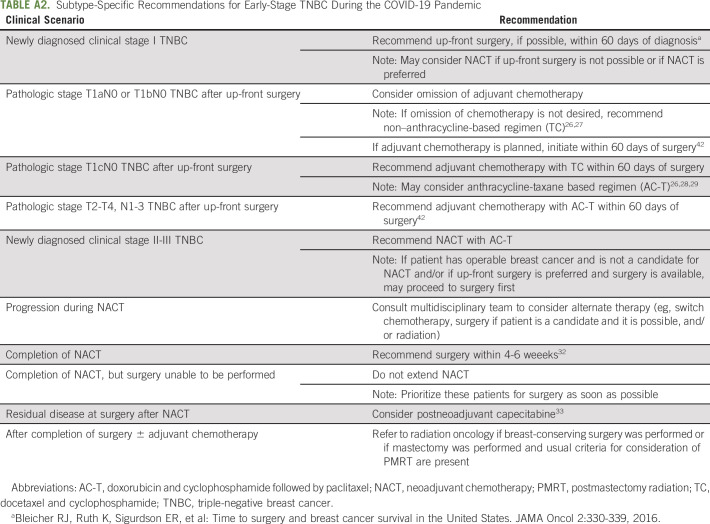
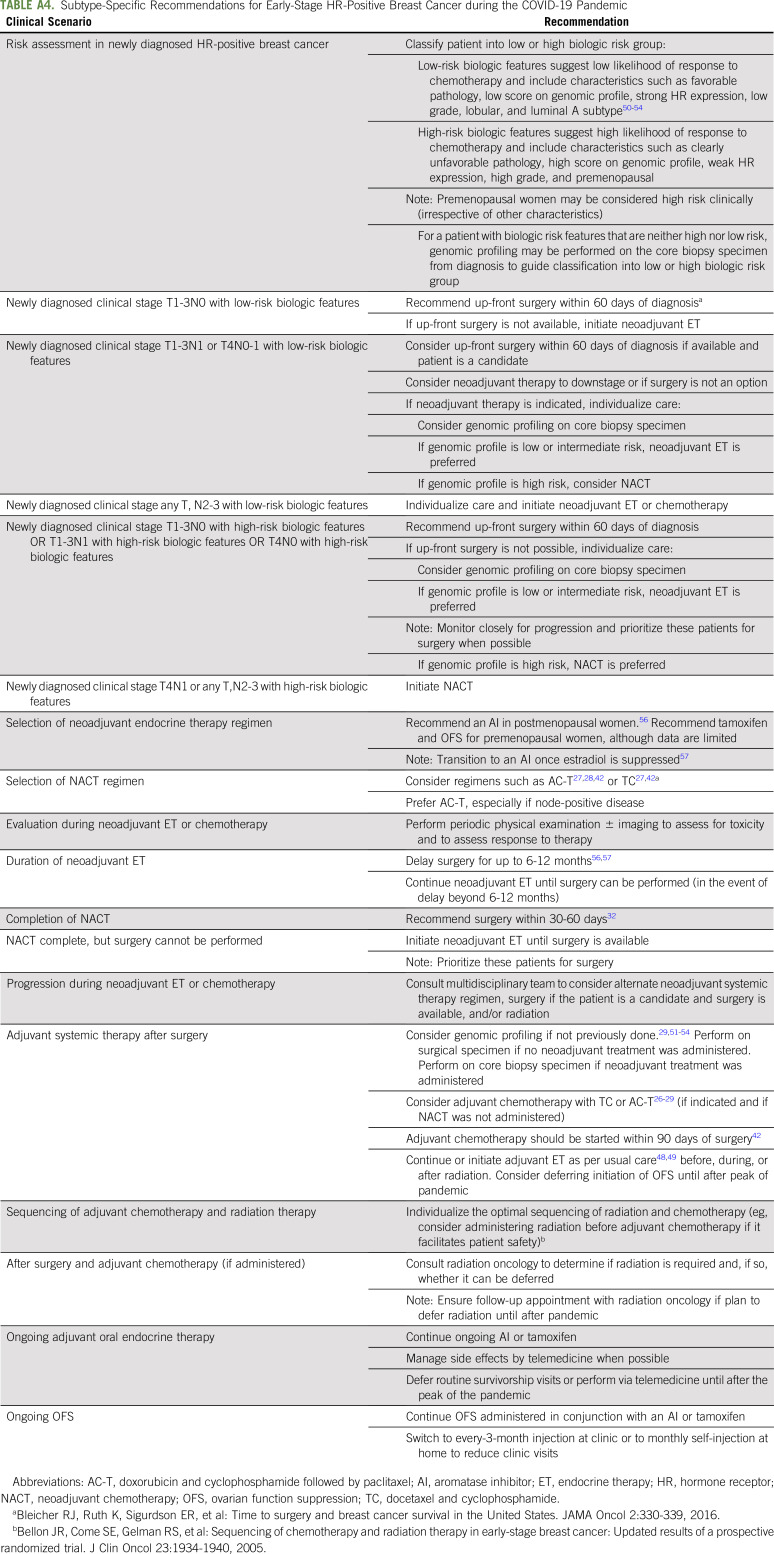
Treatment decisions for newly diagnosed clinical stage I-III HR-positive breast cancer are complicated, as pandemic conditions sometimes necessitate diversion from commonly used therapeutic paradigms. Figure 1C and Appendix Table A4 (online only) summarize our approach to newly diagnosed early-stage HR-positive breast cancer according to biologic risk. We recommend considering biologic risk features when making treatment decisions for newly diagnosed early-stage HR-positive breast cancer.50-54 Low-risk biologic features suggest low likelihood of response and/or small benefit from (neo)adjuvant chemotherapy, whereas high-risk biologic features suggest the converse (Table 1). We typically consider premenopausal women as clinically high risk (irrespective of other characteristics).55
TABLE 1.
Biologic Risk Classification for Hormone Receptor–Positive Breast Cancer
If available, we prefer up-front surgery for clinical stage T1-3N0 HR-positive breast cancer with low-risk biologic features who are operative candidates.47 If up-front surgery or the desired procedure is not available, neoadjuvant ET can be initiated and surgery delayed for up to 6-12 months.56,57 We also consider up-front surgery for newly diagnosed clinical stage T1-3N1 or T4N0-1 HR-positive breast cancer with low-risk biologic features if surgery is available and the patient is an operative candidate. If up-front surgery is unavailable or not preferred (especially for larger tumors or nodal involvement if neoadjuvant therapy can reduce extent of breast and/or axillary surgery), we consider genomic profiling on the core biopsy specimen. If genomic profiling confirms low risk, we favor neoadjuvant ET for up to 6-12 months; if it indicates high risk, we consider NACT.58-60
We use a slightly different approach for newly diagnosed early-stage HR-positive breast cancer with high-risk biologic features. For clinical stage T1-3N0-1 or T4N0 disease, we favor up-front surgery if the patient is a candidate and the desired surgery is available. As for low-biologic-risk tumors, if up-front surgery is unavailable or not preferred, we suggest neoadjuvant therapy, and genomic profiling may be performed to clarify tumor biology and aid the choice between neoadjuvant ET and NACT. If genomic profiling confirms high risk, we favor NACT. If genomic profiling demonstrates low risk despite the other high-risk biologic features, we discuss pros and cons of neoadjuvant ET and NACT and individualize the approach.
For patients with newly diagnosed clinical N2-3 HR-positive breast cancer, we generally favor neoadjuvant systemic therapy regardless of biologic risk. For those with high-risk biologic features, we recommend NACT, and for those with low-risk biologic features, we individualize the approach after discussing pros and cons of neoadjuvant ET and NACT.
As per usual, we recommend AI over tamoxifen for neoadjuvant ET in postmenopausal women.56 For neoadjuvant ET in premenopausal women, we favor ovarian function suppression (OFS) with tamoxifen followed by transition to AI once estradiol is suppressed; however, data supporting this approach are limited, and careful monitoring is required.57 Surgery can usually be safely deferred for up to 6-12 months in individuals receiving neoadjuvant ET; however, we favor careful monitoring and surgery as soon as possible in high biologic risk.56,57 If surgery is delayed beyond 6-12 months, neoadjuvant ET should be continued until surgery is available.
For selection of NACT in early HR-positive breast cancer, we favor AC-T, especially for node-positive disease.26,28,29 On completion of NACT, surgery should be performed within 30-60 days.32 If surgery cannot be performed within that time frame, we recommend neoadjuvant ET until surgery is available, but such patients should be prioritized for surgery.
For individuals with HR-positive breast cancer who have up-front surgery, we recommend genomic profiling on the surgical specimen per usual indications if not previously performed.29,51-54 If indicated, we offer adjuvant chemotherapy with TC or AC-T as per routine care.26-29 We favor omitting chemotherapy if the expected benefit is small, even in limited node-positive disease. For individuals receiving neoadjuvant ET followed by surgery with residual disease, we consider genomic profiling on the core biopsy specimen if not already performed. We recommend adjuvant chemotherapy if high risk, although clear selection criteria in this scenario are unavailable. We consider delaying adjuvant chemotherapy for HR-positive breast cancer for up to 90 days after surgery, with the hope that risk of COVID-19 will decrease before initiation; however, we recommend caution delaying care, given uncertainties about the time course of the pandemic.42
After completion of surgery with or without adjuvant chemotherapy for HR-positive early breast cancer, we refer to radiation oncology per usual criteria. Radiation can be deferred for several months in select patients with low-risk HR-positive breast cancer receiving adjuvant ET.24,61 On the basis of low local recurrence risk in women > 65-70 years of age with small, N0, HR-positive breast cancer after BCS in the setting of adjuvant ET, radiation may be omitted.24,62,63 Adjuvant ET should be offered per standard care, although we consider deferring initiation of OFS until after the pandemic in appropriate individuals. To minimize clinic visits, we offer individuals already receiving monthly OFS the options of monthly home self-administration or injections in clinic every 3 months.64
METASTATIC BREAST CANCER
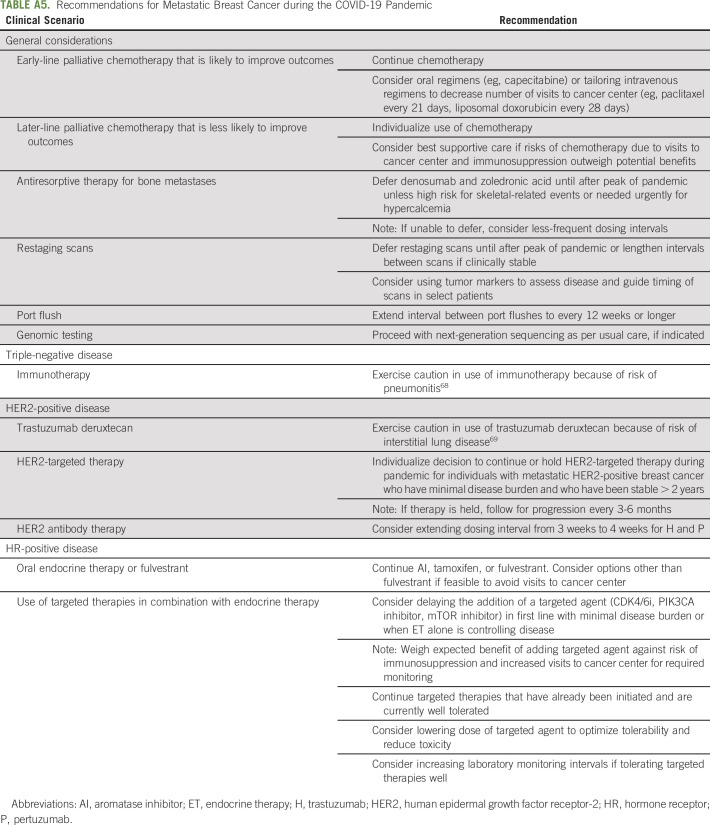
In general, we agree with recommendations for metastatic breast cancer (MBC) management proposed by the COVID-19 Pandemic Breast Cancer Consortium.15 We recommend that patients receiving early-line palliative systemic therapy that is likely to improve outcomes continue therapy, but risks and benefits of later-line therapy must be considered carefully. As per routine, we assess tumor genomics with next-generation sequencing when indicated.47 For HER2-positive MBC with minimal disease burden and an extended period of stability, we consider holding therapy with surveillance for progression every 3-6 months.65,66 To decrease frequent visits for those receiving H ± P, we offer extending the dosing interval from 3 weeks to 4 weeks, especially if receiving other treatments every 4 weeks.67
We advise caution in the use of therapies with high risk of pulmonary toxicity, such as immunotherapy for metastatic TNBC68 or trastuzumab deruxtecan for HER2-positive MBC.69 For HR-positive MBC, we generally continue ET and targeted therapies that are well tolerated. However, we weigh risks and benefits of administering targeted agents with ET for newly diagnosed or progressing HR-positive MBC and for elderly individuals with comorbidities, because of potential toxicities.
With the exception of patients with high risk for skeletal-related events or symptomatic hypercalcemia, we defer or extend dosing intervals for denosumab and zoledronic acid in individuals with bone metastases until after the peak of the pandemic.70,71 Although consideration of oral bisphosphonates for bone metastases is appealing, we are unaware of data to support this. Last, in individuals with MBC who are clinically stable, we recommend delaying routine restaging scans, monitoring tumor markers, and lengthening intervals between laboratory studies if safe (Appendix Table A5, online only).
GENERAL CONSIDERATIONS
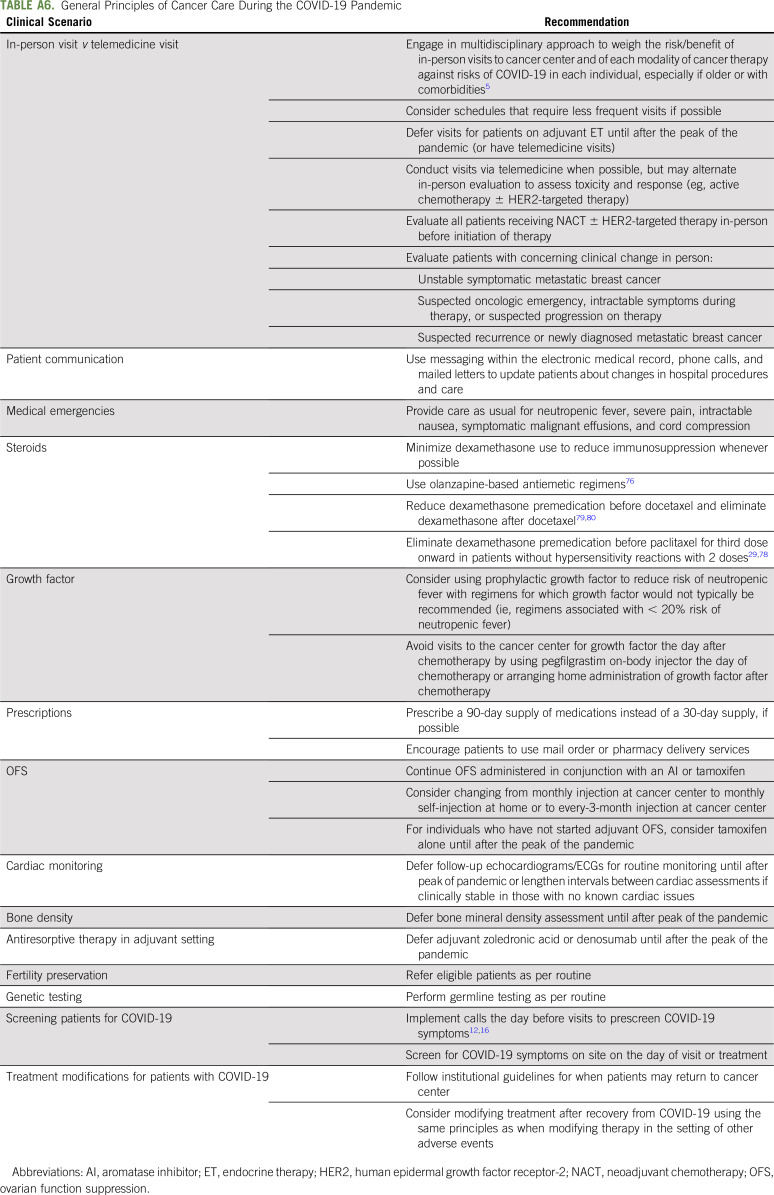
Despite risk of exposure to SARS-CoV-2 for patients and providers, we recommend in-person clinic visits in the setting of suspected oncologic emergencies, progression, recurrence, new diagnoses, and unstable or symptomatic MBC. In the neoadjuvant setting, we recommend baseline in-person evaluation followed by alternating in-person and telemedicine visits. In the metastatic setting, we favor intermittent in-person evaluations to assess disease and toxicities.
For most other scenarios, we recommend care via telemedicine. Although we had not established telemedicine before the pandemic, we were forced to implement it rapidly. Despite steep patient and provider learning curves, telemedicine has proven to be user friendly and compatible with billing, especially with relaxation of state licensing requirements.72,73 Routine survivorship visits can be conducted by telemedicine or deferred until after the pandemic. Most ET adverse effects, including hot flashes, arthralgias, and sexual concerns, can be managed via telemedicine and remote education.
To further limit the risk of SARS-CoV-2 transmission, we recommend extending intervals for routine monitoring (eg, follow-up echocardiograms and ECGs in the absence of known cardiac problems and bone mineral density evaluation) and deferring bone-modifying therapy in the adjuvant setting until after the pandemic. We continue to offer germline testing to eligible candidates, especially if results will affect treatment decisions.74 In addition, we continue to offer fertility preservation to eligible interested young women before systemic therapy.
Regardless of stage, we recommend modifying treatment to reduce immunosuppression and frequent visits if possible. Oral or intravenous regimens with less-frequent dosing and immunosuppression should be used, although there may be trade-offs between these factors. Prophylactic growth factor should be considered with regimens for which it would not typically be recommended.75 When possible, we administer pegfilgrastim on-body injector the day of chemotherapy or arrange home administration of growth factor afterward. We recommend minimizing steroid use to mitigate immunosuppression. To do so, we have implemented olanzapine-based antiemetic regimens for moderate to highly emetogenic chemotherapy.76,77 In addition, we eliminate dexamethasone premedication for weekly paclitaxel after the second dose in the absence of hypersensitivity and use single-dose intravenous dexamethasone before docetaxel instead of multiple oral doses (Appendix Table A6, online only).29,78-80
In conclusion, during the COVID-19 pandemic, delivering breast cancer care necessitates balancing risks associated with delay or pursuit of less-aggressive cancer therapy with risks of COVID-19 exposure and infection in limited-resource environments and with much uncertainty.3,81,82 We are currently only able to test asymptomatic patients with cancer before surgery and interventional radiology procedures. As testing capabilities for COVID-19 expand, testing before delivery of chemotherapy may be considered.
Our experiences treating breast cancer during the pandemic have given us greater appreciation of the modest benefits of toxic therapies and comfort in using genomic platforms to guide neoadjuvant therapy and remote systems of care delivery. However, the pandemic has highlighted weaknesses within our health care system, including disparities, lack of insurance, inadequate supplies, poorly validated diagnostic biomarkers, and inadequate contingency planning. This guideline, although immediately applicable to breast cancer care during the COVID-19 pandemic, may serve as a template for selection and sequencing of breast cancer therapies during future crises.
We eagerly await the achievements of massive global efforts to overcome COVID-19 and ongoing national efforts to collect data in patients with cancer with COVID-19 to characterize determinants of susceptibility and outcomes.83-86 Meanwhile, as hospitals commit resources to fight COVID-19, the oncology community will continue to provide quality care for those who carry the burden of cancer during the pandemic.
Appendix
TABLE A1.
Recommendations for DCIS During the COVID-19 Pandemic
TABLE A2.
Subtype-Specific Recommendations for Early-Stage TNBC During the COVID-19 Pandemic
TABLE A3.
Subtype-Specific Recommendations for Early-Stage HER2-Positive Breast Cancer During the COVID-19 Pandemic
TABLE A4.
Subtype-Specific Recommendations for Early-Stage HR-Positive Breast Cancer during the COVID-19 Pandemic
TABLE A5.
Recommendations for Metastatic Breast Cancer during the COVID-19 Pandemic
TABLE A6.
General Principles of Cancer Care During the COVID-19 Pandemic
SUPPORT
Supported in part by the Centers for Disease Control and Prevention. We would also like to ad a sentence indicating that this work is supported by Sidney Kimmel Comprehensive Cancer Center Core grant P30-CA006973.
The views expressed do not necessarily reflect the official policies of the Department of Health and Human Services, nor does the mention of trade names, commercial practices, or organizations endorsed by the US Government.
AUTHOR CONTRIBUTIONS
Conception and design: Cesar A. Santa-Maria, Rima Couzi, Raquel Nunes, Mary Wilkinson, John H. Fetting, Lisa Jacobs, Elissa D. Thorner, Antonio C. Wolff, Vered Stearns, Karen L. Smith, Jennifer Y. Sheng, Neha Mangini, Haval Norman, Roisin M. Connolly, Evanthia T. Roussos Torres, Jean L. Wright
Provision of study material or patients: Deborah K. Armstrong, Lisa Jacobs, Vered Stearns, Jennifer Sheng, Cesar Santa-maria, Neha Mangini, Haval Norman, Rima Couzi, Raquel Nunes, Mary Wilkinson, Kala Visvanthan, Roisin Connolly, Evanthia Roussos Torres, John Fetting, Jessica Tao, Jean Wright, Antonio Wolff, Karen Smith
Collection and assembly of data: Jennifer Y. Sheng, Cesar A. Santa-Maria, Neha Mangini, Haval Norman, Jessica J. Tao, Antonio C. Wolff, Vered Stearns, Karen L. Smith, Rima Couzi, Raquel Nunes, Mary Wilkinson, Kala Visvanthan, Roisin M. Connolly, Evanthia Roussos Torres, John H. Fetting, Deborah Armstrong, Lisa Jacobs, Jean Wright
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Management of Breast Cancer During the COVID-19 Pandemic: A Stage- and Subtype-Specific Approach
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jennifer Y. Sheng
Travel, Accommodations, Expenses: Nutramax
Cesar A. Santa-Maria
Consulting or Advisory Role: Polyphor, Genomic Health, Halozyme, Bristol Myers Squibb, Athenex
Research Funding: MedImmune, Pfizer, Tesaro (Inst)
Rima Couzi
Stock and Other Ownership Interests: Cellunova, (I)
Consulting or Advisory Role: Precision Biosciences (I)
Research Funding: Pharmacyclics (I)
Patents, Royalties, Other Intellectual Property: Husband receives royalties from Cellunova relating to his inventions that were licensed to the company (I)
Raquel Nunes
Research Funding: bioTheranostics
Uncompensated Relationships: Agendia
Kala Visvanathan
Patents, Royalties, Other Intellectual Property: Licensing of patent with Cepheid (Inst)
Roisin M. Connolly
Research Funding: Genentech/Roche (Inst), Puma Biotechnology (Inst), Novartis (Inst), Merck (Inst), Macrogenics (Inst)
Travel, Accommodations, Expenses: Genentech/Roche
Deborah K. Armstrong
Consulting or Advisory Role: Cue Biopharma, AbbVie, Eisai
Research Funding: Clovis Oncology (Inst), AstraZeneca (Inst), Advaxis (Inst), Syndax (Inst), Pfizer (Inst), Tesaro (Inst), Eisai (Inst)
Other Relationship: AstraZeneca
Jean L. Wright
Employment: Johns Hopkins Hospital
Honoraria: ASTRO
Research Funding: Oncospace
Elissa D. Thorner
Consulting or Advisory Role: Solace
Christine Hodgdon
Honoraria: Roche/Genentech
Consulting or Advisory Role: Roche/Genentech
Travel, Accommodations, Expenses: Lilly
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceutical
Research Funding: Biomarin (Inst), Celldex (Inst)
Patents, Royalties, Other Intellectual Property: Antonio C. Wolff has been named as inventor on 1 or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to Johns Hopkins University (JHU), and participates in a royalty sharing agreement with JHU.
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Vered Stearns
Research Funding: AbbVie, Pfizer, MedImmune, Novartis, Puma Biotechnology, Biocept
Other Relationship: Immunomedics
Karen L. Smith
Stock and Other Ownership Interests: AbbVie (I), Abbott Laboratories (I)
Honoraria: AsiM CME
Research Funding: Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2. Desai A, Sachdeva S, Parekh T, et al: COVID-19 and cancer: Lessons from a pooled meta-analysis. JCO Glob Oncol 6:557-559, 2020. [DOI] [PMC free article] [PubMed]
- 3. Cannistra SA, Haffty BG, Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol. 2020;38:2206–2207. doi: 10.1200/JCO.20.00858. [DOI] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1183/13993003.00547-2020. Guan W, Liang W, Zhao Y, et al: Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J 55:2000547, 2020. [DOI] [PMC free article] [PubMed]
- 6. doi: 10.1158/2159-8290.CD-20-0422. Dai M, Liu D, Liu M, et al: Patients with cancer appear more vulnerable to SARS-COV-2: A multi-center study during the COVID-19 outbreak. Cancer Discov 10:783-791, 2020. [DOI] [PMC free article] [PubMed]
- 7. Zhang L, Zhu F, Xie L, et al: Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan. Ann Oncol . . [epub ahead of print on March 26, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. doi: 10.1001/jamaoncol.2020.0980. Yu J, Ouyang W, Chua MLK, et al: SARS-CoV-2 transmission in patients with cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol . [epub ahead of print on March 25, 2020] [DOI] [PMC free article] [PubMed]
- 9. Bedford J, Enria D, Giesecke J, et al. COVID-19: Towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. doi: 10.1016/S1470-2045(20)30201-1. Burki TK: Cancer care in the time of COVID-19. Lancet Oncol 21:628, 2020. [DOI] [PMC free article] [PubMed]
- 11. Lambertini M, Toss A, Passaro A, et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: Young oncologists’ perspective. ESMO Open. 2020;5:e000759. doi: 10.1136/esmoopen-2020-000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.6004/jnccn.2020.7560. Ueda M, Martins R, Hendrie PC, et al: Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J Natl Compr Cancer Netw . [epub ahead of print on March 20, 2020] [DOI] [PubMed] [Google Scholar]
- 13. American College of Surgeons: COVID-19 guidelines for triage of breast cancer patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/breast-cancer.
- 14. American Society of Clinical Oncology: COVID-19 Provider & Practice Information. https://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19.
- 15. doi: 10.1007/s10549-020-05644-z. Dietz JR, Moran MS, Isakoff SJ, et al: Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat 181:487-497, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.6004/jnccn.2020.7572. Cinar P, Kubal T, Freifeld A, et al: Safety at the time of the COVID-19 pandemic: How to keep our oncology patients and healthcare workers safe. J Natl Compr Cancer Netw . [epub ahead of print on April 15, 2020] [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1016/j.breast.2020.04.006. Curigliano G, Cardoso MJ, Poortmans P, et al: Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast 52:8-16, 2020. [DOI] [PMC free article] [PubMed]
- 18. Johns Hopkins Coronavirus Resource Center: COVID-19 map. https://coronavirus.jhu.edu/map.html.
- 19. Selby P, Popescu R, Lawler M, et al. The value and future developments of multidisciplinary team cancer care. Am Soc Clin Oncol Educ Book. 2019;39:332–340. doi: 10.1200/EDBK_236857. [DOI] [PubMed] [Google Scholar]
- 20. Hwang ES, Hyslop T, Hendrix LH, et al. Phase II single-arm study of preoperative letrozole for estrogen receptor-positive postmenopausal ductal carcinoma in situ: CALGB 40903 (Alliance) J Clin Oncol. 2020;38:1284–1292. doi: 10.1200/JCO.19.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cartlidge CW, Johns N, Hackney RJ, et al: Neoadjuvant endocrine therapy for ER+ DCIS can lead to disease regression and allows BCS in up to a third of patients with disease >40mm at diagnosis. Cancer Res 79, 2019 (suppl 4; abstr P4-15-04) [Google Scholar]
- 22. Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:849–856. doi: 10.1016/S0140-6736(15)01168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staley H, McCallum I, Bruce J. Postoperative tamoxifen for ductal carcinoma in situ. Cochrane Database Syst Rev. 2012;10:CD007847. doi: 10.1002/14651858.CD007847.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. doi: 10.1016/j.adro.2020.03.014. Wright JL, Alcorn S, McNutt T, et al: An integrated program in a pandemic: Johns Hopkins Radiation Oncology Department. Adv Radiat Oncol (in press) [DOI] [PMC free article] [PubMed]
- 25. McCormick B, Winter K, Hudis C, et al. RTOG 9804: A prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;33:709–715. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology) J Clin Oncol. 2017;35:2647–2655. doi: 10.1200/JCO.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 28. Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 29. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 32. Sanford RA, Lei X, Barcenas CH, et al. Impact of time from completion of neoadjuvant chemotherapy to surgery on survival outcomes in breast cancer patients. Ann Surg Oncol. 2016;23:1515–1521. doi: 10.1245/s10434-015-5020-3. [DOI] [PubMed] [Google Scholar]
- 33. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant Capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 34. Braunstein LZ, Taghian AG, Niemierko A, et al. Breast-cancer subtype, age, and lymph node status as predictors of local recurrence following breast-conserving therapy. Breast Cancer Res Treat. 2017;161:173–179. doi: 10.1007/s10549-016-4031-5. [DOI] [PubMed] [Google Scholar]
- 35. Coles CE, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fehrenbacher L, Capra AM, Quesenberry CP, Jr, et al. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: A cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–2158. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 37. Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: A multi-institutional study. J Clin Oncol. 2014;32:2142–2150. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tolaney SM, Trippa L, Barry W, et al: TBCRC 033: A randomized phase II study of adjuvant trastuzumab emtansine (T-DM1) vs paclitaxel (T) in combination with trastuzumab (H) for stage I HER2-positive breast cancer (BC) (ATEMPT). Cancer Res 80, 2020 (suppl 4; abstr GS1-05-GS1) [Google Scholar]
- 40. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gagliato D de M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32:735–744. doi: 10.1200/JCO.2013.49.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nitz UA, Gluz O, Christgen M, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): Final analysis of the WSG-ADAPT HER2+/HR- phase II trial: Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann Oncol. 2017;28:2768–2772. doi: 10.1093/annonc/mdx494. [DOI] [PubMed] [Google Scholar]
- 44. von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 45. Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393:2591–2598. doi: 10.1016/S0140-6736(19)30653-1. [DOI] [PubMed] [Google Scholar]
- 46. Earl HM, Hiller L, Vallier A-L, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 48. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gage MM, Rosman M, Mylander WC, et al. A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Cancer. 2015;15:467–472. doi: 10.1016/j.clbc.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 52. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 53. Goetz MP, Gradishar WJ, Anderson BO, et al. NCCN guidelines insights: Breast cancer, version 3.2018. J Natl Compr Canc Netw. 2019;17:118–126. doi: 10.6004/jnccn.2019.0009. [DOI] [PubMed] [Google Scholar]
- 54. Krop I, Ismaila N, Andre F, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017;35:2838–2847. doi: 10.1200/JCO.2017.74.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chollet-Hinton L, Anders CK, Tse C-K, et al. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: A case-control study. Breast Cancer Res. 2016;18:79. doi: 10.1186/s13058-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): A double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13:345–352. doi: 10.1016/S1470-2045(11)70373-4. [DOI] [PubMed] [Google Scholar]
- 58. Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 59. Yardley DA, Peacock NW, Shastry M, et al. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: Correlation of pathologic complete response with the 21-gene recurrence score. Breast Cancer Res Treat. 2015;154:299–308. doi: 10.1007/s10549-015-3613-y. [DOI] [PubMed] [Google Scholar]
- 60. Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: A multicenter trial. J Surg Oncol. 2017;115:917–923. doi: 10.1002/jso.24610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol. 2009;27:16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 62. Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 63. Kunkler IH, Williams LJ, Jack WJL, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 64. Masuda N, Iwata H, Rai Y, et al. Monthly versus 3-monthly goserelin acetate treatment in pre-menopausal patients with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2011;126:443–451. doi: 10.1007/s10549-010-1332-y. [DOI] [PubMed] [Google Scholar]
- 65. Steenbruggen TG, Bouwer NI, Smorenburg CH, et al. Radiological complete remission in HER2-positive metastatic breast cancer patients: What to do with trastuzumab? Breast Cancer Res Treat. 2019;178:597–605. doi: 10.1007/s10549-019-05427-1. [DOI] [PubMed] [Google Scholar]
- 66. Moilanen T, Mustanoja S, Karihtala P, et al. Retrospective analysis of HER2 therapy interruption in patients responding to the treatment in metastatic HER2+ breast cancer. ESMO Open. 2017;2:e000202. doi: 10.1136/esmoopen-2017-000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu Y-Y, Huang T-C, Tsai T-N, et al. The clinical efficacy and cardiotoxicity of fixed-dose monthly trastuzumab in HER2-positive breast cancer: A single institutional analysis. PLoS One. 2016;11:e0151112. doi: 10.1371/journal.pone.0151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. doi: 10.1056/NEJMoa1809615. Schmid P, Adams S, Rugo HS, et al: Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018. [DOI] [PubMed]
- 69. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Poznak C, Somerfield MR, Barlow WE, et al. Role of bone-modifying agents in metastatic breast cancer: An American Society of Clinical Oncology-Cancer Care Ontario focused guideline update. J Clin Oncol. 2017;35:3978–3986. doi: 10.1200/JCO.2017.75.4614. [DOI] [PubMed] [Google Scholar]
- 71. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA. 2017;317:48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elkaddoum R, Haddad FG, Eid R, et al: Telemedicine for cancer patients during COVID-19 pandemic: Between threats and opportunities. Future Oncol . [epub ahead of print on May 1, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. doi: 10.1016/j.jamcollsurg.2020.04.030. Smith WR, Atala AJ, Terlecki RP, et al: Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic. J Am Coll Surg 10.1016/j.jamcollsurg.2020.04.030 [epub ahead of print on April 30, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Daly MB, Pilarski R, Yurgelun MB, et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw. 2020;18:380–391. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 75. Becker PS, Griffiths EA, Alwan LM, et al. NCCN guidelines insights: Hematopoietic growth factors, version 1.2020. J Natl Compr Canc Netw. 2020;18:12–22. doi: 10.6004/jnccn.2020.0002. [DOI] [PubMed] [Google Scholar]
- 76. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134–142. doi: 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shivaprakash G, Udupa KS, Sarayu V, et al. Olanzapine versus aprepitant for the prophylaxis of chemotherapy-induced nausea and vomiting in breast cancer patients receiving doxorubicin-cyclophosphamide regimen: A prospective, nonrandomized, open-label study. Indian J Pharmacol. 2017;49:451–457. doi: 10.4103/ijp.IJP_846_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Berger MJ, Vargo C, Vincent M, et al. Stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support Care Cancer. 2015;23:2019–2024. doi: 10.1007/s00520-014-2556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chouhan JD, Herrington JD. Single premedication dose of dexamethasone 20 mg IV before docetaxel administration. J Oncol Pharm Pract. 2011;17:155–159. doi: 10.1177/1078155210367950. [DOI] [PubMed] [Google Scholar]
- 80. Kang RY, Yoo KS, Han HJ, et al. Evaluation of the effects and adverse drug reactions of low-dose dexamethasone premedication with weekly docetaxel. Support Care Cancer. 2017;25:429–437. doi: 10.1007/s00520-016-3420-y. [DOI] [PubMed] [Google Scholar]
- 81. doi: 10.7326/M20-1133. Kutikov A, Weinberg DS, Edelman MJ, et al: A war on two fronts: Cancer care in the time of COVID-19. Ann Intern Med 172:756-758, 2020. [DOI] [PMC free article] [PubMed]
- 82. Hanna TP, Evans GA, Booth CM. Cancer, COVID-19 and the precautionary principle: Prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17:268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ong MBH: Lowy: “Our patients are counting on us, and we must not let them down.” Cancer Letter https://cancerletter.com/articles/20200417_1/
- 84. Ong MBH: Doroshow: NCI to accrue patients for COVID-19 longitudinal cohort. Cancer Letter https://cancerletter.com/articles/20200417_2/
- 85. Thompson M, Fu P Jr, West HJ, et al: COVID-19 and Cancer Consortium. https://cancerletter.com/articles/20200320_3/
- 86. Mulcahy N: ASCO announces its own COVID-19 and cancer registry. Medscape http://www.medscape.com/viewarticle/928579.
- 87. doi: 10.1002/cncr.30699. Marmor S, Hui JYC, Huang JL, et al: Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer 123:3015-3021, 2017. [DOI] [PubMed] [Google Scholar]
- 88. doi: 10.1016/S1470-2045(06)71011-7. Katz A, Saad ED, Porter P, et al: Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol 8:55-62, 2007. [DOI] [PubMed] [Google Scholar]
- 89. doi: 10.1053/j.seminoncol.2009.03.001. Anders CK, Johnson R, Litton J, et al: Breast cancer before age 40 years. Semin Oncol 36:237-249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. doi: 10.1200/JCO.2016.67.7195. Kim H-S, Umbricht CB, Illei PB, et al: Optimizing the use of gene expression profiling in early-stage breast cancer. J Clin Oncol 34:4390-4397, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Breast Cancer Recurrence Score Estimator. http://www.breastrecurrenceestimator.onc.jhmi.edu/