Abstract
Background
We aimed to compare and rank the efficacy of different pharmacotherapeutics for patients comorbid with alcohol use disorders and depressive symptoms.
Method
Bayesian network meta‐analysis was performed for three different outcome parameters: alcohol use disorders (AUD) remission rate, percent abstinent days, and scores of depression scales. The surface under the cumulative ranking curves (SUCRA) was used for ranking the efficacy of interventions. Sensitivity analysis and direct pairwise analysis were conducted to validate the main results.
Results
A total of 68 RCTs consisting of 5890 patients were included. Disulfiram could significantly increase the AUD remission rates (OR 5.02, 1.97‐12.95) and the percent abstinent days (MD 17.08, 3.48‐30.93). Disulfiram was associated with the best efficacy in achieving remission (SUCRA 95.1%) and increasing abstinent days (SUCRA 87.6%). Noradrenaline reuptake inhibitor was significantly more efficacious than controls (SMD −2.44, −3.53 to −1.36) and have the first rank (SUCRA 99.0%) in reducing the scores of depression scales. Antiepileptics have relatively higher ranks in efficacy for both AUD and depressive symptoms.
Conclusions
Disulfiram was associated with the best efficacy in achieving abstinence for comorbidity patients. Noradrenaline reuptake inhibitor was demonstrated to be associated with the best efficacy in reducing scores of depression scales. Antiepileptics might be beneficial to both alcohol‐related and depressive symptoms.
Keywords: alcohol use disorders, AUD, depression, depressive symptoms, drug, therapy
The present study conducts a Bayesian network meta‐analysis to compare the efficacy of different pharmacotherapies for patents comorbid with alcohol use disorders and depressive symptoms. Disulfiram was associated with the best efficacy in achieving abstinence, noradrenaline reuptake inhibitor was demonstrated to be the best choice to reduce the scores of depression scales, and antiepileptics might be beneficial to both alcohol‐related and depressive symptoms.

1. INTRODUCTION
Alcohol use disorders (AUD), including alcohol abuse and alcohol dependence, are common psychiatric disorders contribute greatly to the burden of society. 1 Alcohol‐related diseases are one of the leading risk factors of disability and mortality, causing about 3.3 million deaths around the world and taking account for 5.1% of the global burden of disease according to the report of WHO. 2
Depression is a highly prevalent mental illness and is one of the important causes of premature deaths. About 350 million people around the world suffer from depression and nearly one million of them commit suicide every year. 3 Depression will become the second leading cause of global disease burden by 2030. 4
According to the results of epidemiological studies, alcohol use disorders and depression often comorbid with each other. 5 , 6 , 7 It was suggested that suffering from one disease would double the risk of the other. 8 Evidence from longitudinal studies also indicates that there might be a causal link between alcohol use disorders and depression. 9 , 10
The comorbidity of the two diseases can aggravate the condition and worsen the prognosis. On one hand, the existence of alcohol use disorders would prolong the duration of depression, leading to more frequent depressive episodes, and a higher risk of suicide. 11 , 12 On the other hand, lingering depression increases not only the mood‐induced episodes of heavy drinking, but also the risk of relapse during the early abstinence. 13 , 14 Therefore, the treatment and management of both AUD and depression are important public health issues.
Although there are approved medications for AUD and depression alone, the efficacy of these medications in patients comorbid with two diseases is still unclear. Randomized controlled trials (RCTs) of various pharmacotherapy including anti‐craving drugs, 15 , 16 , 17 , 18 , 19 antidepressants, 20 , 21 , 22 , 23 , 24 antiepileptics, 25 , 26 , 27 , 28 , 29 and antipsychotics 30 , 31 , 32 have drawn inconsistent results. Moreover, in clinical practice, it is a challenge in making a decision when facing various available medication options. Therefore, this study aimed at comparing and ranking the efficacy of various pharmaceutic options in treating patients comorbid with alcohol use disorders and depressive symptoms by conducting a Bayesian network meta‐analysis.
2. METHOD
2.1. Search strategy
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement extension for network meta‐analysis. 33 We conducted a comprehensive search in PubMed, Embase, Cochrane CENTRAL, PsycINFO, Cochrane Drugs and Alcohol Group (CDAG), and The Cumulative Index to Nursing and Allied Health Literature (CINAHL) database from inception to January 15, 2020. Construction of search strategy was based on MeSH terms plus free texts. Table S1 presents an example of a search strategy used in PubMed. We also conducted a supplementary search by reviewing the reference lists of related studies and searching related studies in ClinicalTrial.gov.
2.2. Study selection
Randomized clinical trials (RCTs) that compared the efficacy of pharmaceutic interventions with placebo or no‐treatment control or with each other for adults comorbid with alcohol use disorders and depression or depressive symptoms were considered eligible. In the current study, alcohol use disorders should be ascertained based on DSM or ICD‐10 diagnostic criteria. Depression should be ascertained through DSM or ICD‐10 diagnostic criteria. Besides, to fully represent the situation encountered by physicians in clinical practice, study with patients scoring more than the cutoff threshold of valid depression symptoms scales (eg, score more than 7 in the Hamilton Depression Scale) was also considered eligible.
In the current study, outcomes of interest for AUD were AUD remission rate (ie, the percentage of patients who remain consistent abstinence or don't relapse into heavy drinking during the whole treatment session) and percent of abstinent days. Because the measurement of abstinence and no relapse into heavy drinking is less likely to be affected by recall bias than the measurement of alcohol consumption, 34 the outcome of interest for depressive symptoms was the endpoint scores of the depressive symptom scales.
Studies met one or more of the following criteria were excluded: (a) not RCTs (eg, observational studies, reviews, comments, and case reports); (b) participants were mainly adolescents; (c) studies regarded acute alcohol withdrawal; (d) participants comorbid psychosis such as bipolar disorders, schizophrenia, et al.; (e) participants comorbid severe internal disease such as heart failure, liver cirrhosis et al.; (f) interventions belong to the same type; (g) no appropriate outcomes; (h) studies based on the same research; (i) studies compared pharmacotherapy with psychotherapy. Two investigators independently selected the studies, and a third investigator was consulted to resolve controversies.
2.3. Data extraction and quality assessment
We used a pre‐set collection form to extract basic information, intervention characteristics, and outcome data. We preferred to extract the data of treatment sessions. When data of the intention‐to‐treat sample and data of completers were both available, the former was preferable. For those studies with only survival curves available, we used Getdata (version 2.2) to extract the raw data. Two investigators independently conducted the data extraction, and any contradictions were resolved by discussion or by consulting with a third investigator. We used the tool advised by the Cochrane Handbook to assess the risk of bias and evaluate the quality of each included study. 35 The graphics of the summary of the overall risk of bias and the study‐level risk of bias were conducted using Review Manager Software (version 5.3).
2.4. Statistical analysis
We conducted the network meta‐analysis based on the Bayesian method that is more flexible in modeling and more accurate in pooling the results than the traditional frequency theory method. 36 , 37 Network plot was portrayed to present the comparison network of interventions and controls. Nodes represented interventions/controls, and lines represented direct comparisons. The size of nodes represents the size of included sample, and the width of lines represents the number of included trails. The value of deviance information criterion (DIC) was used to determine whether to use a random model or a fixed model. The smaller the DIC value, the better the model fit. The Markov‐chain Monte Carlo simulation (MCMC) method was applied. The number of simulation chains is four, and the number of tuning iteration and simulation iterations was 50 000 and 200 000, respectively. Brooks‐Gelman‐Rubin method was used to assess the iterative simulation. 38 The surface under cumulative ranking curves (SUCRA) values were used for hierarchically ranking the efficacy of interventions. The more a SUCRA value is approached to 100%, the more the corresponding intervention is likely to achieve the best treatment efficacy. 39 , 40 Odds ratios (ORs) were used for binary data (ie, AUD remission rate), and mean differences (MDs) were used for continuous data. Standard mean differences (SMDs) were applied for the situation where different measurement tools were applied among included studies. The node‐splitting analysis was used to assess the local inconsistency, a P‐value > .05 indicated no significant inconsistency between the direct pairwise results and the indirect results. 41 A consistency model is preferred if there is no significant inconsistency indicated. Publication bias was assessed using funnel plots.
Sensitivity analyses were conducted by only including studies that had a treatment session no less than 8 weeks and among studies involved participants with at least moderate depressive symptoms (assessed by valid scales) or with a diagnosis of depression (ascertained by standard criteria) to corroborate the main results.
We also performed direct pairwise analysis by comparing the efficacy of the top five therapeutics (ascertained through SUCRA value) with their corresponding lower‐ranked therapeutics for both AUD and depression outcome parameters. Moreover, since previous traditional meta‐analysis drew inconsistent results on whether antidepressants confer any benefit to improving depressive symptoms compared to controls. 42 , 43 Therefore, we compared the efficacy of non‐antidepressant and antidepressant with controls in improving depressive symptoms, respectively. In this case, heterogeneity of pairwise analysis was assessed with I 2 statistics, in which an I 2 less than 50% indicated no significant heterogeneity.
Statistical analyses of Bayesian network meta‐analysis were conducted using the R software (version 3.6.2) and OpenBUGS software (version 3.2.3). Network plots, cumulative probabilities ranking plots, and pairwise analysis were conducted in Stata software (version 15.2).
3. RESULTS
We identified 6790 hits through searching databases, of which 4203 were removed after reviewing the titles and abstracts. Among the 179 studies included for full‐text reading, 117 of which were further excluded for various reasons. With five additional studies found from the references lists and one study found from ClinicalTrials.gov, eventually, 68 studies were included in the current analysis. A flow chart of studies selection was shown in Figure 1.
FIGURE 1.
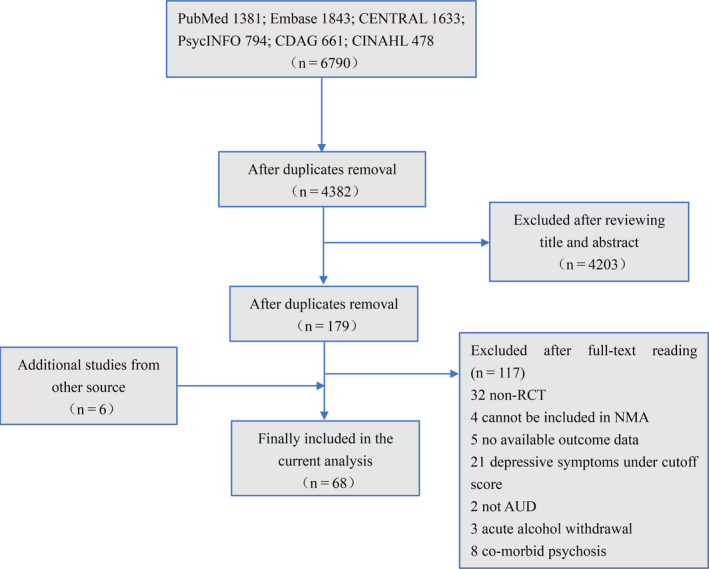
Flow chart of studies selection procedure and reasons for exclusion from the network meta‐analysis
A large part of the included studies were two‐arm trials (85.3%), and the rest of them were three‐arm (11.8%) or four‐arm (3%) trials. We subdivided the included interventions into 18 categories: acamprosate, baclofen, disulfiram, naltrexone, buspirone, bromocriptine, lithium, memantine, antiepileptics (included carbamazepine, topiramate, and tiagabine here), antipsychotics (included aripiprazole and olanzapine here), selective serotonin reuptake inhibitor (SSRI, included citalopram, escitalopram, fluoxetine, paroxetine, and sertraline here), noradrenaline reuptake inhibitor (NRI, included venlafaxine and viloxazine here), serotonin receptor antagonist/reuptake inhibitor (SARI, included nefazodone and trazodone here), tricyclic antidepressants (TCA, included amitriptyline, imipramine, and desipramine here); noradrenaline and specific serotonin antagonist (NaSSA, included mirtazapine here); naltrexone plus disulfiram, and naltrexone plus SSRI. The 68 included RCTs ranged from 8 to 492 in sample sizes and totally consisting of 5891 cases. These studies published from 1976 to 2018 and were conducted in different countries, but most of them were in developed countries. The characteristics of the included studies were summarized in Table S2. The random effect model is adopted in this study for a smaller DIC value compared to the fixed effect model.
3.1. Effect of pharmacotherapies on the AUD remission rate
For AUD remission rates, 44 trials 15 , 16 , 17 , 18 , 19 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 consisting of 4334 cases were included in analysis. Comparisons between 16 different pharmacotherapeutic options and controls were portrayed in a network plot (Figure 2A). Among the 25 direct pairwise comparisons, 11 of which regarded the comparison between different pharmacotherapeutics options. A large proportion of included studies regarded the comparison between baclofen, naltrexone, SSRI, and controls. Disulfiram (OR 5.02, 1.97‐12.95), antiepileptics (OR 2.55, 1.26‐5.22), and naltrexone plus SSRI (OR 2.24, 1.15‐4.50) were significantly better than controls in increasing the AUD remission rate (Figure 3A). Disulfiram was significantly more efficient than acamprosate, antipsychotics, bromocriptine, lithium, naltrexone, and SSRI in achieving AUD remission (Figure 4A). Besides, based on the results of cumulative probabilities ranking (Figures 5 and 6), disulfiram possessed the best rank in the efficacy of achieving AUD remission (SUCRA 95.1%), followed by antiepileptics (SUCRA 77.9%), naltrexone plus disulfiram (SUCRA 73.3%), and naltrexone plus SSRI (SUCRA 72.6%). No significant publication bias was indicated by visually inspecting the funnel plot (Figure S1A). According to the node‐splitting analysis, there was no significant inconsistency detected between the results of the direct and indirect comparison among the 11 closed intervention loops (Figure S2A). The potential scale reduction factor (PSRF) was equal to one, indicating that the number of iterative simulations is enough to reach a good convergency.
FIGURE 2.
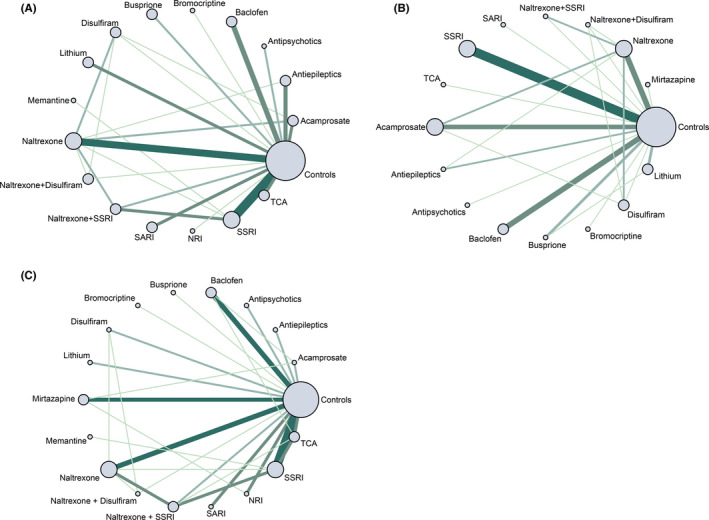
A, network plot for AUD remission; B, network plot for percent abstinent days; C, network plot for reduction of scores of validated depression scales. The size of nodes represents the relative size of included sample; the width of line represents the number of included trails. NRI, noradrenaline reuptake inhibitor; SARI, serotonin receptor antagonist/reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants
FIGURE 3.
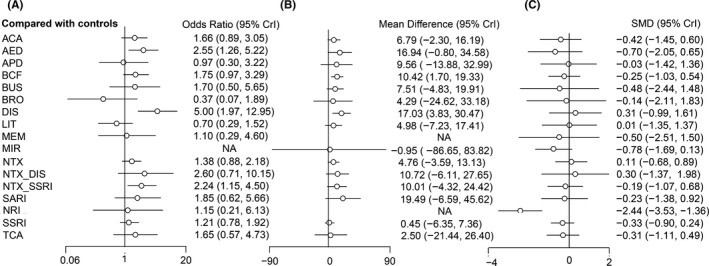
Forest plot of different interventions versus controls for A, AUD remission rate; B, percent abstinent days; C, reduction in scores of depression scales. SMD, standard mean difference. ACA NRI, noradrenaline reuptake inhibitor; acamprosate; AED NRI, noradrenaline reuptake inhibitor; antiepileptics; APD, antipsychotics; BCF, baclofen; BRO, bromocriptine; BUS, buspirone; DIS, disulfiram; LIT, lithium; MEM, memantine; MIR, mirtazapine; NRI, noradrenaline reuptake inhibitor/noradrenaline reuptake inhibitor; NTX, naltrexone; SSRI, selective serotonin reuptake inhibitor; SARI, serotonin receptor antagonist/reuptake inhibitor; TCA, tricyclic antidepressants
FIGURE 4.
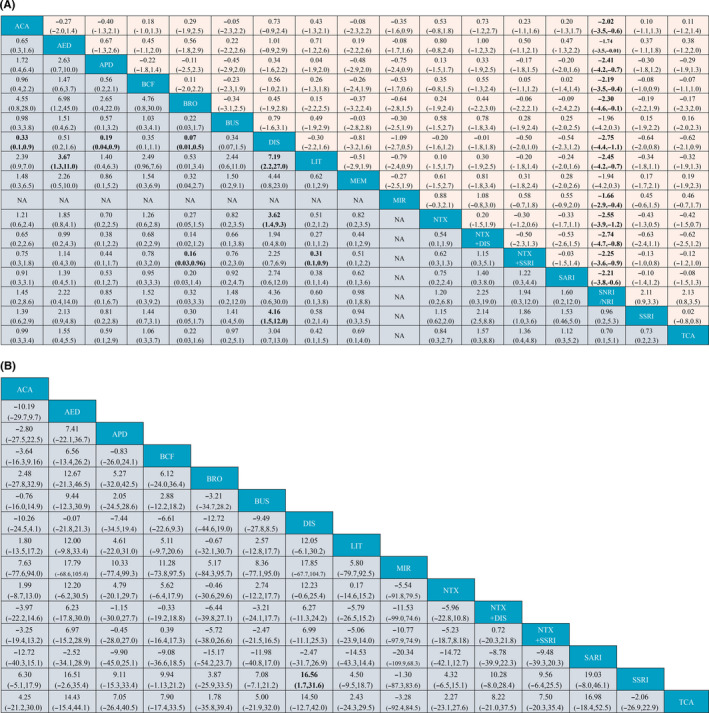
A, League table of comparisons between all interventions. Estimates represent ORs and 95% CrI of the AUD remission rate (purple region) and SMDs and 95% CrI of the reduction in scores of depression scales (orange region), respectively. Each estimate was pooled from the comparison between a column intervention and a row intervention. ACA, acamprosate; AED, antiepileptics; APD, antipsychotics; BCF, baclofen; BRO, bromocriptine; BUS, buspirone; DIS, disulfiram; LIT, lithium; MEM, memantine; MIR, mirtazapine; NRI, noradrenaline reuptake inhibitor/noradrenaline reuptake inhibitor; NTX, naltrexone; SARI, serotonin receptor antagonist/reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants. B, League table of comparisons between all interventions. Estimates represent MDs and 95% CrI of the reduction in scores of depression scales (purple region). Each estimate was pooled from the comparison between a column intervention and a row intervention. ACA, acamprosate; AED, antiepileptics; APD, antipsychotics; BCF, baclofen; BRO, bromocriptine; BUS, buspirone; DIS, disulfiram; LIT, lithium; MEM, memantine; MIR, mirtazapine; NRI, noradrenaline reuptake inhibitor/noradrenaline reuptake inhibitor; NTX, naltrexone; SSRI, selective serotonin reuptake inhibitor; SARI, serotonin receptor antagonist/reuptake inhibitor; TCA, tricyclic antidepressants
FIGURE 5.
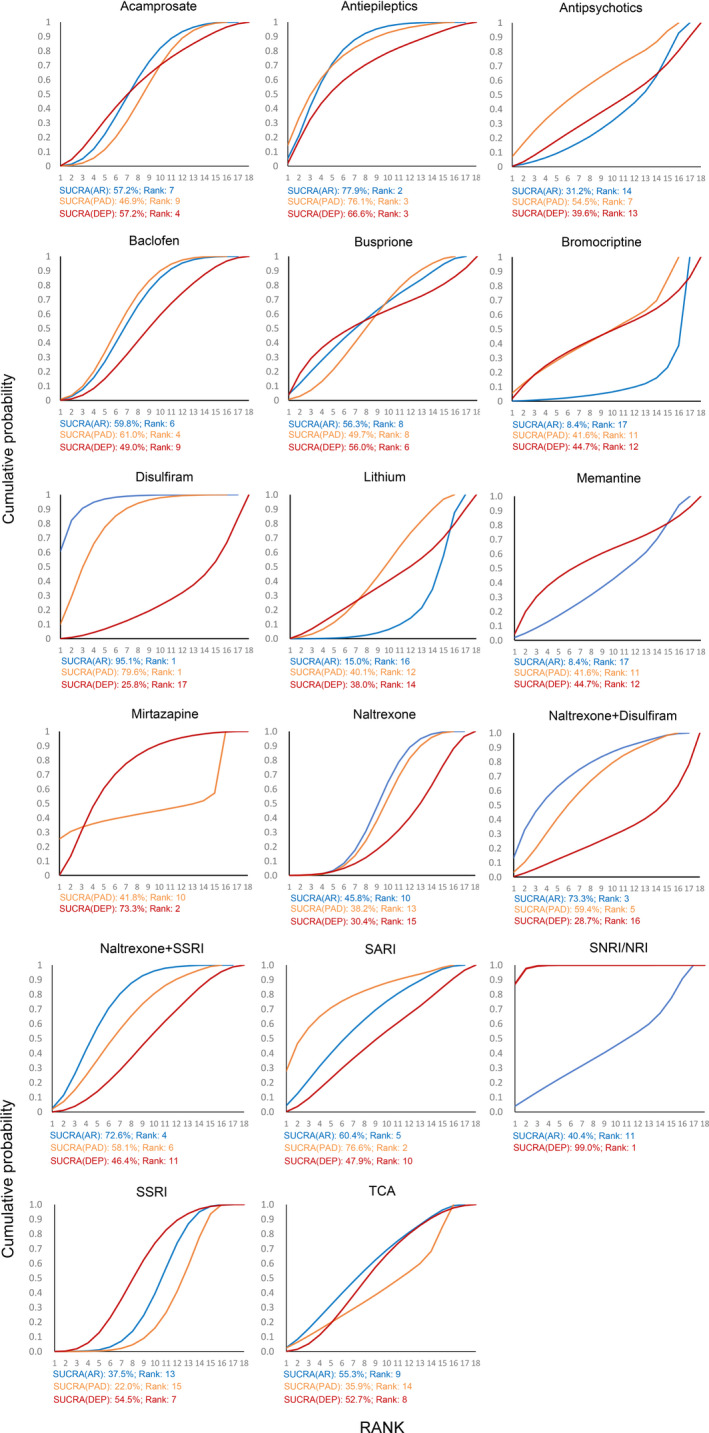
Cumulative probability ranking curve of different interventions for three outcome parameters: AR, AUD remission rate (blue curve); PAD, percent abstinent days (orange curve); DEP, reduction in scores of depression scales (red curve). The more a surface under the cumulative ranking curve (SUCRA) value approached to 100%, the better the corresponding intervention is in efficacy
FIGURE 6.
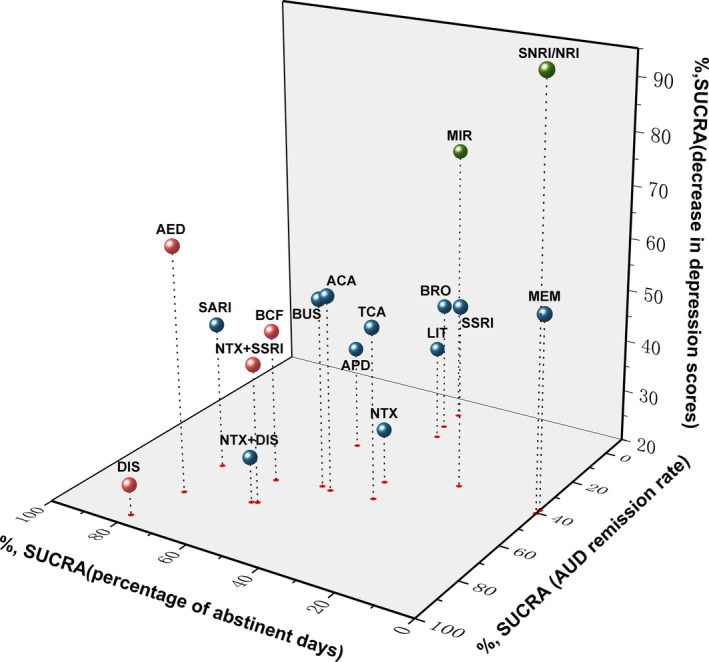
Three‐dimension scatters plot for SUCRA value for three different parameter outcomes. The more a SUCRA value approached to 100%, the better the corresponding intervention is in efficacy. Red colored represented an intervention was significantly more efficacious in the treatment of alcohol‐related symptoms compared to controls. Green colored represented an intervention was significantly more efficacious in reducing the scores of depression scales. ACA, acamprosate; AED, antiepileptics; APD, antipsychotics; BCF, baclofen; BUS, buspirone; BRO, bromocriptine; DIS, disulfiram; LIT, lithium; MEM, memantine; MIR, mirtazapine; NRI, noradrenaline reuptake inhibitor; NTX, naltrexone; SARI, serotonin receptor antagonist/reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants
In sensitivity analysis, after excluding 11 studies in which participants had only mild depressive symptoms, results indicated that disulfiram (OR 4.33, 1.14 to 17.51) and naltrexone plus SSRI (OR 2.55, 1.24 to 5.37) remained significantly better than controls in increasing the AUD remission rate (Table S3). Disulfiram still ranked the first according to the SUCRA value (87.6%). We also conducted a sensitivity analysis by only including studies with treatment session ≥ 8 weeks, and results were similar to the main results (Table S4).
In pairwise analysis used for validation, the relatively higher‐ranked interventions, disulfiram, epileptics, and naltrexone plus SSRI could significantly increase the AUD remission rate compared to their corresponding lower‐ranked interventions, which corroborated the robustness of the main results (Table S5A).
3.2. Effect of pharmacotherapies on the percent abstinent days
As for percent abstinent days, a total of 39 studies 15 , 16 , 17 , 18 , 19 , 21 , 23 , 24 , 25 , 26 , 30 , 44 , 45 , 46 , 48 , 49 , 51 , 52 , 53 , 55 , 56 , 58 , 59 , 60 , 61 , 62 , 65 , 67 , 68 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 consisting of 4050 cases and 15 different interventions were included in the analysis (Figure 2B). Among the 23 direct pairwise comparisons, 8 of which regarded the comparison between different pharmacotherapeutics options. A large part of included studies regarded the comparison between acamprosate, baclofen, naltrexone, SSRI, and controls. Disulfiram (MD 17.03, 3.83‐30.47) and baclofen (MD 10.42, 1.70‐19.33) were significantly associated with higher percentages of abstinent days during the treatment sessions (Figure 3B). Results of the comparisons between all interventions were shown in Figure 4B. Concerning the cumulative probabilities ranking (Figures 5 and 6), disulfiram had the largest SUCRA value (79.6%) in terms of increasing the abstinent days, followed by SARI (SUCRA 76.6%), antiepileptics (SUCRA 76.1%), and baclofen (SUCRA 61.0%). No significant publication bias was indicated by visually inspecting the funnel plot (Figure S1B). In the node‐splitting analysis (Figure S2B), inconsistency was detected in the comparison between disulfiram‐acamprosate (P = .03) and between disulfiram‐controls (P = .02). However, there was no significant inconsistency detected in the other seven closed loops. Besides, the DIC values of the consistency model (161.9) and the inconsistency model (160.5) were similar, and the p‐value of inconsistency model was 0.87. All of these indicated that the integral consistency was moderate. As for model convergency, the PSRF value was 1, indicating a good convergency.
In sensitivity analysis where only studies with participants having at least moderate depressive symptoms or having depression diagnosis were included, results from 28 studies showed that no intervention revealed significantly better efficacy than controls in increasing the abstinent days (Table S3). Sensitivity analysis was also conducted by excluding two studies 44 , 59 with treatment sessions less than 8 weeks. Results showed that only disulfiram was significantly better than controls in increasing the percentages of abstinent days (MD 17.08, 3.48 to 30.93) (Table S4).
In pairwise analysis used for validation, interventions with higher SUCRA (disulfiram, SARI, antiepileptics, baclofen, and naltrexone plus disulfiram) revealed better efficacy on increasing the percent abstinent days compared to their corresponding lower‐ranked interventions (Table S5B).
3.3. Effect of pharmacotherapies on the scores of depression scales
A total of 47 studies 15 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 27 , 28 , 30 , 31 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 58 , 60 , 61 , 63 , 65 , 69 , 70 , 72 , 73 , 76 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 consisting of 3835 cases and 17 interventions were included in the analysis (Figure 2C). Among the 29 direct pairwise comparisons, 13 of which regarded the comparison between different pharmacotherapeutics options. A large part of included studies regarded the comparison between baclofen, naltrexone, SSRI, and controls. According to the network meta‐analysis, only NRI was significantly better than controls in reducing the endpoint scores of depression scale (SMD −2.44, −3.53 to −1.36, Figure 3C). Besides, NRI was significantly more efficient in improving depressive symptoms than other interventions except for memantine and buspirone (Figure 4A). Results of cumulative probabilities ranking (Figures 5 and 6) indicated that NRI was associated with the lowest scores of depression scales at the end of studies (SUCRA 99.0%), followed by mirtazapine (SUCRA 73.3%), antiepileptics (SUCRA 66.6%), and acamprosate (SUCRA 57.2%). Publication bias might exist since asymmetry was noticed by visually inspecting the funnel plot (Figure S1C). In the node‐splitting analysis, no significant inconsistency was detected (Figure S2C).
In sensitivity analysis, after excluding nine studies 15 , 47 , 50 , 52 , 53 , 54 , 72 , 91 , 94 in which participants had only mild depressive symptoms, in addition to NRI (SMD −3.60, −4.91 to −2.29), mirtazapine was also demonstrated to be better than controls in reducing the scores of depression scales (SMD −0.99, −1.91 to −0.07) (Table S3). Results of ranking probabilities were similar to the main analysis with NRI (SUCRA 99.9%) still the first rank followed by mirtazapine (SUCRA 76.0%). We also conducted a sensitivity analysis of 39 studies that had treatment sessions no less than 8 weeks. Results showed that NRI still had the highest SUCRA (99.9%) and significantly better efficacy than controls (SMD −3.96, −5.33 to −2.59) (Table S4).
In the direct meta‐analysis, our findings indicated that compared to controls, pharmacotherapy could significantly reduce the scores of depression scales with a quite modest effect size (SMD −0.25, −0.41 to −0.09). However, only the subgroup of antidepressants (SMD −0.43, −0.68 to −0.17) revealed a significant effect size while thenon‐antidepressants subgroup (SMD −0.10, −0.31 to 0.11) did not (Figure S3A). Results remained similar after excluding one study 20 that contributed to the heterogeneity (Figure S3B). In pairwise analysis compared the efficacy of higher‐ranked interventions to the lower ones, interestedly, NRI revealed no significant differences in reducing the scores of depression scales. We further divided NRI into viloxazine (selective noradrenaline reuptake inhibitor) and venlafaxine (serotonin and noradrenaline reuptake inhibitor), results indicated that only subgroup of viloxazine showed a benefit to reducing the scores of depression scales (Table S5C).
3.4. Quality of studies
As for quality assessment, 28 trials (41.2%) mentioned the detail about random sequence generation. 55 studies (80.9%) applied blinding methods but only 27 trials (39.7%) described allocation concealment. A large part of the included studies had a low risk of attrition bias. Overall, included studies were considered to be low to moderate in quality. The overall and study‐level risk of bias were summarized in Figure S4.
4. DISCUSSION
The current Bayesian network meta‐analysis included 5891 comorbidity patients across 68 studies. Our findings indicated that disulfiram, antiepileptics, baclofen, and naltrexone plus SSRI were significantly associated with higher AUD remission rates and (or) higher percent abstinent days during the treatment sessions when compared to controls. Among them, disulfiram possessed the best rank in efficacy. As for the outcome parameter for depressive symptoms, noradrenaline reuptake inhibitor (NRI) and mirtazapine exhibited statistical significance over controls in reducing the scores of depression. And NRI might be hierarchically the best in achieving the most pronounced reduction. However, the results should be interpreted cautiously since the significance of the effect size was limited to only selective noradrenaline reuptake inhibitor but not serotonin and noradrenaline reuptake inhibitor (SNRI) and potential publication might exist. Although not approved for the treatment of AUD or mono‐depression, antiepileptics might be a beneficial therapeutic option to both AUD‐related and depressive symptoms in comorbidity patients.
Our findings were consistent with some opinions of a recent systematic review 95 which also concerned the treatment of patients comorbid with alcohol use disorders and depression. However, our study provided more information because we compared the efficacy of different interventions quantitatively using Bayesian network meta‐analysis and drew the results for different outcome parameters.
As one of the earliest approval medications for alcoholism, in our study, disulfiram was demonstrated to be the most efficacious pharmacotherapy in maintaining remission and increasing abstinence days for comorbidity patients. The results remained significant after taking into account the length of treatment sessions and the severity of depression, which corroborating the robustness of our findings. Although inconsistency was detected in comparison between controls, acamprosate, and disulfiram for percent abstinent days, which might be accounted for by the limited number of included studies, the benefit of disulfiram on achieving abstinence was supported by main results and pairwise analysis. Findings from a recent open‐label trial including 41 AUD patients were partly consistent with ours. It found that disulfiram plus lorazepam could significantly increase the percent abstinent days and reduce depressive symptoms. 96 However, in our study, disulfiram showed no benefit to depressive symptoms compared to controls, indicating the need for combination with medication specifically targeting depressive symptoms. By extendedly blocking the acetaldehyde dehydrogenase, disulfiram could trigger uncomfortable "Antabuse" reactions such as tachycardia, flushing, and nausea after drinking, which gradually established the aversion to alcohol and finally achieve the aim of quitting drinking. In addition to the peripheral effect, disulfiram has also been shown to modulate the transmission of dopamine in central nervous systems that plays a pivotal role in reward circuit and substance dependence. 97 , 98
Another anti‐craving medication, the gamma‐aminobutyric acid (GABA)B receptor agonist baclofen, exhibited statistical significance over controls and had a relatively higher rank of efficacy in increasing the percent abstinent days. However, the significance of effect size disappeared when the analysis was conducted only among studies with longer treatment phase and with more severe depression patients, which prevented broad interpretations. Other anti‐craving medication, including acamprosate and naltrexone, conferred no significant benefit to both AUD and depressive symptoms for comorbidity patients compared to controls, which is in line with the results from a recent systematic review. 95 Another meta‐analysis also concluded that, in comparison with placebo, the number needed to treat (NNT) for acamprosate to prevent a return to any drinking and NNT for naltrexone to prevent a return to heavy drinking were both 12, 99 indicating their limited pharmaceutic effect on achieving abstinence.
Antidepressants were supposed to be a pivotal part of treatment for patients with dual diagnosis since treatment targeted only alcohol‐related symptoms was insufficient to achieve complete remission of depression. 100 However, previous meta‐analysis focus on the efficacy of antidepressants drew inconsistent results. Meta‐analysis performed by Foulds et al 101 involved 11 studies concluded that the pooled effect size of change scores of depression scales was significant (SMD 0.25, 0.06‐0.44) only among studies concerned independent depression and was insignificant across all studies. A more recent meta‐analysis 102 included 14 studies indicated that antidepressants were significantly better than placebo on reducing the endpoint scores of interviewer‐rated depression scales (SMD −0.27, −0.49 to −0.04). Our findings were consistent with the latter one and also indicated that non‐antidepressants revealed no benefit to depressive symptoms. Since antidepressants included various types and with quite different mechanisms and efficacy, thus, one of the innovations of our studies was to divide antidepressants into several subtypes, which was more meaningful for clinical practice. Besides, we included a wide spectrum of depressive disorders, including depressive symptoms measured by validated interviewer‐rated or self‐rated scales, and depression ascertained by standard diagnostic criteria, to fully represent the situation encountered by clinicians. According to our findings, noradrenaline reuptake inhibitor was hierarchically the best in reducing the depression scores. When the severity of depression was taken into account, in addition to NRI, another antidepressant‐mirtazapine that also targets at noradrenergic systems also revealed significantly better efficacy than controls in improving depressive symptoms. Notably, in direct pairwise analysis, the statistical significance of the result of NRI was contributed only to viloxazine (selective noradrenaline reuptake inhibitor) but not to venlafaxine (serotonin and noradrenaline reuptake inhibitor, SNRI). Since there was only one study regarding viloxazine included in our analysis, the network meta‐analysis result of NRI should be interpreted with caution and more future studies are warrant. In all, antidepressants, especially NRI and mirtazapine, might be the promising pharmacotherapy for depressive symptoms for comorbidity patients. However, both NRI and mirtazapine showed no significant efficacy on alcohol‐related symptoms compared to controls, which indicated combination with medication specifically target at AUD symptoms (eg, disulfiram) was needed.
Interestedly, although not approved for the treatment of alcohol use disorders and mono‐depression, antiepileptics showed exciting potential and prospects in treatment both alcohol‐related and depressive symptoms according to our findings. Antiepileptics included in this analysis, topiramate and tiagabine, could modulate GABAergic systems in the central amygdala, which was a region proved to be involved in the emotion regulation and alcohol intake. 103 , 104 Besides, these antiepileptics could enhance the inhibitory function of GABA, antagonize the excitatory of the glutamate system, and inhibits dopamine release, which further modulated the reward systems and addictive behaviors. 105 However, two of the included studies regarded antiepileptics were open‐label controlled studies, 27 , 28 and the total number was scarce, so the results should be interpreted with caution and more evidence for validation is warranted.
Several limitations existed in our studies. Firstly, due to the heterogeneity of tolerability data across studies, no attempt was made to compare the tolerability of different therapeutic options in the current network meta‐analysis. However, since the higher‐ranked therapeutic options are approved medication for the treatment of AUD or depression, we considered the tolerability acceptable. Besides, there were other alternatives with relatively higher ranks could be chosen based on the patients’ condition. Secondly, the NMA results and the cumulative probabilities ranking may be influenced by the limited numbers of included studies in some intervention groups which might also contribute to the local inconsistency detected in the analysis. Although the direct pairwise analysis was conducted to validate the main results, cautions still be needed when interpreted the results and more evidence is warranted. Finally, our analysis focused only on pharmacotherapy but not on psychotherapy, which might partly explain the weak benefit of pharmacotherapy showed to depressive symptoms in the current analysis. However, since psychotherapy was not contradicted with drug therapy and safe, we assumed that it was always beneficial or not harmful when used adjunctively to pharmacotherapy.
5. CONCLUSION
Our Bayesian network meta‐analysis indicated that disulfiram was demonstrated to be associated with the best efficacy in achieving remission and increasing abstinent days for patients who comorbid alcohol use disorders (AUD) and depressive symptoms. Noradrenaline reuptake inhibitors (NRI) might hieratically be the best in efficacy for depressive symptoms. Besides, antiepileptics might be beneficial to both alcohol‐related and depressive symptoms. However, more evidence is warranted to corroborate the findings above.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Jiande Li and Ying Peng involved in conceptualization; Jiande Li, Hongxuan Wang, and Ying Peng involved in data search and extraction; Jiande Li and Hongxuan Wang involved in data analysis and software; Jiande Li, Mei Li, Qingyu Shen, and Xiangpen Li interpreted the results; Jiande Li and Hongxuan Wang involved in software; Jiande Li wrote the original draft; Xiaoming Rong and Ying Peng wrote and edited the article.
ETHICAL STATEMENT
The current study does not require an ethical statement.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by The National Key Research and Development Program of China (2018YFC1314400 and 2018YFC1314401).
Li J, Wang H, Li M, et al. Efficacy of pharmacotherapeutics for patients comorbid with alcohol use disorders and depressive symptoms—A bayesian network meta‐analysis. CNS Neurosci Ther. 2020;26:1185–1197. 10.1111/cns.13437
REFERENCES
- 1. Connor JP, Haber PS, Hall WD. Alcohol use disorders. Lancet. 2016;387(10022):988‐998. 10.1016/S0140-6736(15)00122-1 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global status report on alcohol and health. 2018.
- 3. World Health Organization . Depression and other common mental disorders. Global Health Estimates. 2017.
- 4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chou SP, Lee HK, Cho MJ, Park JI, Dawson DA, Grant BF. Alcohol use disorders, nicotine dependence, and co‐occurring mood and anxiety disorders in the United States and South Korea‐a cross‐national comparison. Alcohol Clin Exp Res. 2012;36(4):654‐662. 10.1111/j.1530-0277.2011.01639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM‐IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67(2):247‐257. 10.4088/jcp.v67n0211 [DOI] [PubMed] [Google Scholar]
- 7. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097‐1106. 10.1001/archpsyc.62.10.1097 [DOI] [PubMed] [Google Scholar]
- 8. Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106(5):906‐914. 10.1111/j.1360-0443.2010.03351.x [DOI] [PubMed] [Google Scholar]
- 9. Li J, Wang H, Li M, et al. Effect of alcohol use disorders and alcohol intake on the risk of subsequent depressive symptoms: a systematic review and meta‐analysis of cohort studies. Addiction. 2020;115(7):1224‐1243. [DOI] [PubMed] [Google Scholar]
- 10. Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Arch Gen Psychiatry. 2009;66(3):260‐266. 10.1001/archgenpsychiatry.2008.543 [DOI] [PubMed] [Google Scholar]
- 11. Carton L, Pignon B, Baguet A, et al. Influence of comorbid alcohol use disorders on the clinical patterns of major depressive disorder: a general population‐based study. Drug Alcohol Depend. 2018;187:40‐47. 10.1016/j.drugalcdep.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 12. Hawton K, Casanas ICC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1–3):17‐28. 10.1016/j.jad.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 13. Greenfield SF, Weiss RD, Muenz LR, et al. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55(3):259‐265. [DOI] [PubMed] [Google Scholar]
- 14. Suter M, Strik W, Moggi F. Depressive symptoms as a predictor of alcohol relapse after residential treatment programs for alcohol use disorder. J Subst Abuse Treat. 2011;41(3):225‐232. 10.1016/j.jsat.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 15. Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176(11):530‐534. [DOI] [PubMed] [Google Scholar]
- 16. Pettinati HM, Oslin DW, Kampman KM, et al. A double‐blind, placebo‐controlled trial combining sertraline and naltrexone for treating co‐occurring depression and alcohol dependence. Am J Psychiatry. 2010;167(6):668‐675. 10.1176/appi.ajp.2009.08060852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laaksonen E, Koski‐Jannes A, Salaspuro M, Ahtinen H, Alho H. A randomized, multicentre, open‐label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2008;43(1):53‐61. 10.1093/alcalc/agm136 [DOI] [PubMed] [Google Scholar]
- 18. Petrakis I, Ralevski E, Nich C, et al. Naltrexone and disulfiram in patients with alcohol dependence and current depression. J Clin Psychopharmacol. 2007;27(2):160‐165. 10.1097/jcp.0b13e3180337fcb [DOI] [PubMed] [Google Scholar]
- 19. Beraha EM, Salemink E, Goudriaan AE, et al. Efficacy and safety of high‐dose baclofen for the treatment of alcohol dependence: a multicentre, randomised, double‐blind controlled trial. Eur Neuropsychopharmacol. 2016;26(12):1950‐1959. 10.1016/j.euroneuro.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 20. Altamura AC, Mauri MC, Girardi T, Panetta B. Alcoholism and depression: a placebo controlled study with viloxazine. Int J Clin Pharmacol Res. 1990;10(5):293‐298. [PubMed] [Google Scholar]
- 21. Cornelius JR, Salloum IM, Ehler JG, et al. Double‐blind fluoxetine in depressed alcoholic smokers. Psychopharmacol Bull. 1997;33(1):165‐170. [PubMed] [Google Scholar]
- 22. Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double‐blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21(2):143‐153. 10.1097/00004714-200104000-00005 [DOI] [PubMed] [Google Scholar]
- 23. Hernandez‐Avila CA, Modesto‐Lowe V, Feinn R, Kranzler HR. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcohol Clin Exp Res. 2004;28(3):433‐440. [DOI] [PubMed] [Google Scholar]
- 24. Moak DH, Anton RF, Latham PK, Voronin KE, Waid RL, Durazo‐Arvizu R. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo‐controlled trial. J Clin Psychopharmacol. 2003;23(6):553‐562. 10.1097/01.jcp.0000095346.32154.41 [DOI] [PubMed] [Google Scholar]
- 25. Baltieri DA, Daro FR, Ribeiro PL, de Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103(12):2035‐2044. 10.1111/j.1360-0443.2008.02355.x [DOI] [PubMed] [Google Scholar]
- 26. Batki SL, Pennington DL, Lasher B, et al. Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. Alcohol Clin Exp Res. 2014;38(8):2169‐2177. 10.1111/acer.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paparrigopoulos T, Tzavellas E, Karaiskos D, Kourlaba G, Liappas I. Treatment of alcohol dependence with low‐dose topiramate: an open‐label controlled study. BMC Psychiatry. 2011;11: 10.1186/1471-244X-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paparrigopoulos T, Tzavellas E, Karaiskos D, Malitas P, Liappas I. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375‐1380. 10.1177/0269881109103799 [DOI] [PubMed] [Google Scholar]
- 29. Mueller TI, Stout RL, Rudden S, et al. A double‐blind, placebo‐controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcohol Clin Exp Res. 1997;21(1):86‐92. 10.1111/j.1530-0277.1997.tb03733.x [DOI] [PubMed] [Google Scholar]
- 30. Guardia J, Segura L, Gonzalvo B, et al. A double‐blind, placebo‐controlled study of olanzapine in the treatment of alcohol‐dependence disorder. Alcoholism: Clin Exp Res. 2004;28(5):736‐745. 10.1097/01.ALC.0000125352.06688.F7 [DOI] [PubMed] [Google Scholar]
- 31. Han DH, Kim SM, Choi JE, Min KJ, Renshaw PF. Adjunctive aripiprazole therapy with escitalopram in patients with co‐morbid major depressive disorder and alcohol dependence: clinical and neuroimaging evidence. J Psychopharmacol. 2013;27(3):282‐291. 10.1177/0269881112472563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaw GK, Majumdar SK, Waller S, MacGarvie J, Dunn G. Tiapride in the long‐term management of alcoholics of anxious or depressive temperament. Br J Psychiatry. 1987;150:164‐168. 10.1192/bjp.150.2.164 [DOI] [PubMed] [Google Scholar]
- 33. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777‐784. 10.7326/m14-2385 [DOI] [PubMed] [Google Scholar]
- 34. Gao J, Cao J, Guo T, Xiao Y. Association between alcoholic interventions and abstinence rates for alcohol use disorders: a meta‐analysis. Medicine (Baltimore). 2018;97(50):e13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta‐analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):956‐964. 10.1111/j.1524-4733.2008.00347.x [DOI] [PubMed] [Google Scholar]
- 37. Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279‐301. 10.1177/0962280207080643 [DOI] [PubMed] [Google Scholar]
- 38. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Computational Graphical Statistics. 1998;7(4):434. [Google Scholar]
- 39. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105‐3124. 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 40. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med. 2010;29(7–8):932‐944. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 42. Foulds JA, Sellman JD, Mulder RT. Antidepressant therapy for depressed patients with an alcohol use disorder. Aust N Z J Psychiatry. 2016;50(3):199‐200. 10.1177/0004867415609427 [DOI] [PubMed] [Google Scholar]
- 43. Agabio R, Trogu E, Pani PP. Antidepressants for the treatment of people with co‐occurring depression and alcohol dependence. Cochrane Database Systematic Rev. 2018;4(4):CD008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake a preliminary double‐blind randomized controlled study. Alcohol Alcoholism. 2002;37(5):504‐508. [DOI] [PubMed] [Google Scholar]
- 45. Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: a controlled study. Alcohol Clin Exp Res. 1998;22(3):573‐579. 10.1111/j.1530-0277.1998.tb04295.x [DOI] [PubMed] [Google Scholar]
- 46. Chick J, Aschauer H, Hornik K. Efficacy of fluvoxamine in preventing relapse in alcohol dependence: a one‐year, double‐blind, placebo‐controlled multicentre study with analysis by typology. Drug Alcohol Depend. 2004;74(1):61‐70. 10.1016/j.drugalcdep.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 47. Ciraulo DA, Barlow DH, Gulliver SB, et al. The effects of venlafaxine and cognitive behavioral therapy alone and combined in the treatment of co‐morbid alcohol use‐anxiety disorders. Behav Res Ther. 2013;51(11):729‐735. 10.1016/j.brat.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dongier M, Vachon L, Schwartz G. Bromocriptine in the treatment of alcohol dependence. Alcohol Clin Exp Res. 1991;15(6):970‐977. 10.1111/j.1530-0277.1991.tb05197.x [DOI] [PubMed] [Google Scholar]
- 49. Dorus W, Ostrow DG, Anton R, et al. Lithium treatment of depressed and nondepressed alcoholics. J Am Med Assoc. 1989;262(12):1646‐1652. 10.1001/jama.262.12.1646 [DOI] [PubMed] [Google Scholar]
- 50. Fawcett J, Clark DC, Aagesen CA. A double‐blind, placebo‐controlled trial of lithium carbonate therapy for alcoholism. Archives General Psychiatry. 1987;44(3):248‐256. [DOI] [PubMed] [Google Scholar]
- 51. Gual A, Balcells M, Torres M, Madrigal M, Diez T, Serrano L. Sertraline for the prevention of relapse in detoxicated alcohol dependent patients with a comorbid depressive disorder: a randomized controlled trial. Alcohol Alcoholism. 2003;38(6):619‐625. 10.1093/alcalc/agg124 [DOI] [PubMed] [Google Scholar]
- 52. Gupta M, Verma P, Rastogi R, Arora S, Elwadhi D. Randomized open‐label trial of baclofen for relapse prevention in alcohol dependence. Am J Drug Alcohol Abuse. 2017;43(3):324‐331. 10.1080/00952990.2016.1240797 [DOI] [PubMed] [Google Scholar]
- 53. Hauser P, Fuller B, Ho SB, Thuras P, Kern S, Dieperink E. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173‐1183. 10.1111/add.13787 [DOI] [PubMed] [Google Scholar]
- 54. Janiri L, Gobbi G, Mannelli P, Pozzi G. Effects of fluoxetine and antidepressant doses on short‐term outcome of detoxified alcoholics. Int Clin Psychopharmacol. 1996;11(2):109‐117. 10.1097/00004850-199611020-00005 [DOI] [PubMed] [Google Scholar]
- 55. Kranzler HR, Burleson JA, Del Boca FK, et al. Buspirone treatment of anxious alcoholics. A placebo‐controlled trial. Arch Gen Psychiatry. 1994;51(9):720‐731. 10.1001/archpsyc.1994.03950090052008 [DOI] [PubMed] [Google Scholar]
- 56. Malec E, Malec T, Gagne MA, Dongier M. Buspirone in the treatment of alcohol dependence: a placebo‐controlled trial. Alcohol Clin Exp Res. 1996;20(2):307‐312. 10.1111/j.1530-0277.1996.tb01644.x [DOI] [PubMed] [Google Scholar]
- 57. Mason BJ, Kocsis JH, Ritvo EC, Cutler RB. A double‐blind, placebo‐controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. J Am Med Assoc. 1996;275(10):761‐767. 10.1001/jama.275.10.761 [DOI] [PubMed] [Google Scholar]
- 58. McGrath PJ, Nunes EV, Stewart JW, et al. Imipramine treatment of alcoholics with primary depression: a placebo‐ controlled clinical trial. Archives General Psychiatry. 1996;53(3):232‐240. [DOI] [PubMed] [Google Scholar]
- 59. Merry J, Reynolds CM, Bailey J, Coppen A. Prophylactic treatment of alcoholism by lithium carbonate. A controlled study. Lancet. 1976;2(7984):481‐482. [DOI] [PubMed] [Google Scholar]
- 60. Morgenstern J, Kuerbis AN, Chen AC, Kahler CW, Bux DA Jr, Kranzler HR. A randomized clinical trial of naltrexone and behavioral therapy for problem drinking men who have sex with men. J Consult Clin Psychol. 2012;80(5):863‐875. 10.1037/a0028615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morley KC, Baillie A, Fraser I, et al. Baclofen in the treatment of alcohol dependence with or without liver disease: Multisite, randomised, double‐blind, placebo‐controlled trial. Br J Psychiatry. 2018;212(6):362‐369. 10.1192/bjp.2018.13 [DOI] [PubMed] [Google Scholar]
- 62. Morley KC, Teesson M, Reid SC, et al. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi‐centre, randomized, double‐blind, placebo‐controlled trial. Addiction. 2006;101(10):1451‐1462. 10.1111/j.1360-0443.2006.01555.x [DOI] [PubMed] [Google Scholar]
- 63. Muhonen LH, Lonnqvist J, Juva K, Alho H. Double‐blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392‐399. [DOI] [PubMed] [Google Scholar]
- 64. Nunes EV, McGrath PJ, Quitkin FM, et al. Imipramine treatment of alcoholism with comorbid depression. Am J Psychiatry. 1993;150(6):963‐965. 10.1176/ajp.150.6.963 [DOI] [PubMed] [Google Scholar]
- 65. OMalley SS, Robin RW, Levenson AL, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non‐natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32(7):1271‐1283. 10.1111/j.1530-0277.2008.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oslin DW. Treatment of late‐life depression complicated by alcohol dependence. Am J Geriatric Psychiatry. 2005;13(6):491‐500. 10.1097/00019442-200506000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism: Clin Exp Res. 2000;24(7):1041‐1049. 10.1111/j.1530-0277.2000.tb04648.x [DOI] [PubMed] [Google Scholar]
- 68. Poldrugo F. Acamprosate treatment in a long‐term community‐based alcohol rehabilitation programme. Addiction. 1997;92(11):1537‐1546. [PubMed] [Google Scholar]
- 69. Roy‐Byrne PP, Pages KP, Russo JE, et al. Nefazodone treatment of major depression in alcohol‐dependent patients: a double‐blind, placebo‐controlled trial. J Clin Psychopharmacol. 2000;20(2):129‐136. 10.1097/00004714-200004000-00003 [DOI] [PubMed] [Google Scholar]
- 70. Stella L, Addolorato G, Rinaldi B, et al. An open randomized study of the treatment of escitalopram alone and combined with gamma‐hydroxybutyric acid and naltrexone in alcoholic patients. Pharmacol Res. 2008;57(4):312‐317. 10.1016/j.phrs.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 71. Tiihonen J, Ryynänen OP, Kauhanen J, Hakola HPA, Salaspuro M. Citalopram in the treatment of alcoholism: a double‐blind placebo‐controlled study. Pharmacopsychiatry. 1996;29(1):27‐29. [DOI] [PubMed] [Google Scholar]
- 72. Le Bon O, Murphy JR, Staner L, et al. Double‐blind, placebo‐controlled study of the efficacy of trazodone in alcohol post‐withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol. 2003;23(4):377‐383. 10.1097/01.jcp.0000085411.08426.d3 [DOI] [PubMed] [Google Scholar]
- 73. Adamson SJ, Sellman JD, Foulds JA, et al. A randomized trial of combined citalopram and naltrexone for nonabstinent outpatients with co‐occurring alcohol dependence and major depression. J Clin Psychopharmacol. 2015;35(2):143‐149. 10.1097/jcp.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 74. Book SW, Thomas SE, Randall PK, Randall CL. Paroxetine reduces social anxiety in individuals with a co‐occurring alcohol use disorder. J Anxiety Disord. 2008;22(2):310‐318. 10.1016/j.janxdis.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co‐occurring alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2005;29(3):395‐401. 10.1097/01.alc.0000156129.98265.57 [DOI] [PubMed] [Google Scholar]
- 76. Cornelius JR, Chung T, Douaihy AB, et al. Mirtazapine in comorbid major depression and an alcohol use disorder: a double‐blind placebo‐controlled pilot trial. Psychiatry Res. 2016;242:326‐330. 10.1016/j.psychres.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics: a double‐blind, placebo‐controlled trial. Archives General Psychiatry. 1997;54(8):700‐705. 10.1001/archpsyc.1997.01830200024004 [DOI] [PubMed] [Google Scholar]
- 78. Fawcett J, Kravitz HM, McGuire M, et al. Pharmacological treatments for alcoholism: revisiting lithium and considering buspirone. Alcohol Clin Exp Res. 2000;24(5):666‐674. 10.1111/j.1530-0277.2000.tb02038.x [DOI] [PubMed] [Google Scholar]
- 79. Kranzler HR, Mueller T, Cornelius J, et al. Sertraline treatment of co‐occurring alcohol dependence and major depression. J Clin Psychopharmacol. 2006;26(1):13‐20. 10.1097/01.jcp.0000194620.61868.35 [DOI] [PubMed] [Google Scholar]
- 80. Ponizovsky AM, Rosca P, Aronovich E, Weizman A, Grinshpoon A. Baclofen as add‐on to standard psychosocial treatment for alcohol dependence: a randomized, double‐blind, placebo‐controlled trial with 1 year follow‐up. J Subst Abuse Treat. 2015;52:24‐30. 10.1016/j.jsat.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 81. Sonne SC, Potter JS, Rosenthal R, Hiott R. The use of acamprosate in individuals with alcohol dependence and comorbid anxiety or depression. Clinicaltrialsgov [wwwclinicaltrialsgov]. 2006;https://clinicaltrials.gov/ct2/show/results/NCT00330174.
- 82. Witte J, Bentley K, Evins AE, et al. A randomized, controlled, pilot study of acamprosate added to escitalopram in adults with major depressive disorder and alcohol use disorder. J Clin Psychopharmacol. 2012;32(6):787‐796. 10.1097/JCP.0b013e3182726764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cornelius J. Treatment of young adults with comorbid AUD/MDD: a pilot medication Trial Clinicaltrialsgov. Mar 2017; https://clinicaltrials.gov/ct2/show/study/NCT02646449.
- 84. Altintoprak AE, Zorlu N, Coskunol H, Akdeniz F, Kitapcioglu G. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co‐morbid depressive disorder: a randomized, double‐blind study. Human Psychopharmacol. 2008;23(4):313‐319. 10.1002/hup.935 [DOI] [PubMed] [Google Scholar]
- 85. Cocchi R. Paroxetine vs amitryptiline depressed in alcoholics. Conference Paper. Eur Neuropsychopharmacol. 1997;7(Suppl. 2):S254 10.1016/S0924-977X(97)00416-1 [DOI] [Google Scholar]
- 86. Goyer PF, Brown GL, Minichiello MD, Major LF. Mood‐altering effects of disulfiram in alcoholics. J Stud Alcohol. 1984;45(3):209‐213. 10.15288/jsa.1984.45.209 [DOI] [PubMed] [Google Scholar]
- 87. Habrat B, Zaloga B. A double‐blind controlled study of the efficacy and acceptability of tianeptine in comparison with fluvoxamine in the treatment of depressed alcoholic patients. Psychiatr Pol. 2006;40(3):579‐597. Skutecznosc i tolerancja tianeptyny w leczeniu zaburzen depresyjnych u pacjentow uzaleznionych od alkoholu. wieloosrodkowe badanie kontrolowane metoda podwojnie slepej proby z uzyciem fluwoksaminy. [PubMed] [Google Scholar]
- 88. Krupitsky EM, Burakov AM, Ivanov VB, et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug Alcohol Dependence. 1993;33(2):157‐163. 10.1016/0376-8716(93)90057-W [DOI] [PubMed] [Google Scholar]
- 89. Liappas J, Paparrigopoulos T, Malitas P, Tzavellas E, Christodoulou G. Mirtazapine improves alcohol detoxification. J Psychopharmacol. 2004;18(1):88‐93. 10.1177/0269881104040241 [DOI] [PubMed] [Google Scholar]
- 90. Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):55‐60. 10.1016/j.pnpbp.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 91. Petrakis IL, Ralevski E, Desai N, et al. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology. 2012;37(4):996‐1004. 10.1038/npp.2011.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Roy A. Placebo‐controlled study of sertraline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998;44(7):633‐637. 10.1016/S0006-3223(97)00509-X [DOI] [PubMed] [Google Scholar]
- 93. Shaw JA, Donley P, Morgan DW, Robinson JA. Treatment of depression in alcoholics. Am J Psychiatry. 1975;132(6):641‐644. 10.1176/ajp.132.6.641 [DOI] [PubMed] [Google Scholar]
- 94. Tollefson GD, Montague‐Clouse JON, Tollefson SL. Treatment of comorbid generalized anxiety in a recently detoxified alcoholic population with a selective serotonergic drug (Buspirone). J Clin Psychopharmacol. 1992;12(1):19–26. [DOI] [PubMed] [Google Scholar]
- 95. Hillemacher T, Frieling H. Pharmacotherapeutic options for co‐morbid depression and alcohol dependence. Expert Opin Pharmacother. 2019;20(5):547‐569. 10.1080/14656566.2018.1561870 [DOI] [PubMed] [Google Scholar]
- 96. Bogenschutz MP, Bhatt S, Bohan J, et al. Coadministration of disulfiram and lorazepam in the treatment of alcohol dependence and co‐occurring anxiety disorder: an open‐label pilot study. Am J Drug Alcohol Abuse. 2016;42(5):490‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Charpeaud T, Geneste J, Schmidt J, Llorca PM, Brousse G. Disulfiram and addiction: reminders and new perspectives of use. Therapies. 2011;66(3):273‐280. Le disulfirame (Esperal(®)) dans le traitement des addictions : rappels et nouvelles perspectives d'utilisation. doi:10.2515/therapie/2011025. [DOI] [PubMed] [Google Scholar]
- 98. Di Ciano P, Manvich DF, Pushparaj A, et al. Effects of disulfiram on choice behavior in a rodent gambling task: association with catecholamine levels. Psychopharmacology. 2018;235(1):23‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kenna GA. Medications acting on the serotonergic system for the treatment of alcohol dependent patients. Curr Pharm Des. 2010;16(19):2126‐2135. 10.2174/138161210791516396 [DOI] [PubMed] [Google Scholar]
- 100. Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36(3):249‐255. 10.1093/alcalc/36.3.249 [DOI] [PubMed] [Google Scholar]
- 101. Foulds JA, Adamson SJ, Boden JM, Williman JA, Mulder RT. Depression in patients with alcohol use disorders: systematic review and meta‐analysis of outcomes for independent and substance‐induced disorders. J Affective Disorders. 2015;185:47‐59. 10.1016/j.jad.2015.06.024 [DOI] [PubMed] [Google Scholar]
- 102. Agabio R, Leggio L. Baclofen in the treatment of patients with alcohol use disorder and other mental health disorders. Frontiers Psychiatry. 2018;9:464 https://www.researchgate.net/publication/327938809_Baclofen_in_the_Treatment_of_Patients_With_Alcohol_Use_Disorder_and_Other_Mental_Health_Disorders [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre‐ and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100(4):2053‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Olmsted CL, Kockler DR. Topiramate for alcohol dependence. Ann Pharmacother. 2008;42(10):1475‐1480. 10.1345/aph.1L157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


