Abstract
Background
Despite numerous studies and meta-analyses the prognostic effect of cardiac rehabilitation is still under debate. This update of the Cardiac Rehabilitation Outcome Study (CROS II) provides a contemporary and practice focused approach including only cardiac rehabilitation interventions based on published standards and core components to evaluate cardiac rehabilitation delivery and effectiveness in improving patient prognosis.
Design
A systematic review and meta-analysis.
Methods
Randomised controlled trials and retrospective and prospective controlled cohort studies evaluating patients after acute coronary syndrome, coronary artery bypass grafting or mixed populations with coronary artery disease published until September 2018 were included.
Results
Based on CROS inclusion criteria out of 7096 abstracts six additional studies including 8671 patients were identified (two randomised controlled trials, two retrospective controlled cohort studies, two prospective controlled cohort studies). In total, 31 studies including 228,337 patients were available for this meta-analysis (three randomised controlled trials, nine prospective controlled cohort studies, 19 retrospective controlled cohort studies; 50,653 patients after acute coronary syndrome 14,583, after coronary artery bypass grafting 163,101, mixed coronary artery disease populations; follow-up periods ranging from 9 months to 14 years). Heterogeneity in design, cardiac rehabilitation delivery, biometrical assessment and potential confounders was considerable. Controlled cohort studies showed a significantly reduced total mortality (primary endpoint) after cardiac rehabilitation participation in patients after acute coronary syndrome (prospective controlled cohort studies: hazard ratio (HR) 0.37, 95% confidence interval (CI) 0.20–0.69; retrospective controlled cohort studies HR 0.64, 95% CI 0.53–0.76; prospective controlled cohort studies odds ratio 0.20, 95% CI 0.08–0.48), but the single randomised controlled trial fulfilling the CROS inclusion criteria showed neutral results. Cardiac rehabilitation participation was also associated with reduced total mortality in patients after coronary artery bypass grafting (retrospective controlled cohort studies HR 0.62, 95% CI 0.54–0.70, one single randomised controlled trial without fatal events), and in mixed coronary artery disease populations (retrospective controlled cohort studies HR 0.52, 95% CI 0.36–0.77; two out of 10 controlled cohort studies with neutral results).
Conclusion
CROS II confirms the effectiveness of cardiac rehabilitation participation after acute coronary syndrome and after coronary artery bypass grafting in actual clinical practice by reducing total mortality under the conditions of current evidence-based coronary artery disease treatment. The data of CROS II, however, underscore the urgent need to define internationally accepted minimal standards for cardiac rehabilitation delivery as well as for scientific evaluation.
Keywords: Cardiac rehabilitation, cardiac rehabilitation delivery, acute coronary syndrome, coronary bypass grafting, coronary artery disease, mortality
Introduction
Within the past 25 years, cardiovascular morbidity and mortality after acute coronary syndromes (ACSs) have shown a remarkable decrease which is associated with the implementation of acute coronary revascularisations as well as the application of effective acute and long-term pharmacotherapy.1 Supporting these results from the United States1 the French FAST-MI registry revealed a mortality reduction 6 months after ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) from 17.2% to 5.3% and 6.3%, respectively.2 Moreover, on the basis of the SWEDEHEART registry a marked improvement of 2 years’ survival was found, but strictly associated with the use of acute coronary interventions and evidence-based long-term secondary prevention.3 Accordingly, current evidence-based treatment modalities of ACS and coronary artery disease (CAD) do have a large impact on the acute and long-term success of care delivered to these patients. Against this background, the effects of special treatment modalities such as cardiac rehabilitation (CR) need to be re-evaluated in the light of their added short and long-term clinical and prognostic benefit. The Cardiac Rehabilitation Outcome Study (CROS) aimed to evaluate the prognostic effect of CR after ACS and coronary artery bypass grafting (CABG) in the modern era of cardiovascular treatment modalities. On the basis of predominantly controlled observational studies including a large number of patients, CROS confirmed a beneficial effect of CR (i.e. reduced all-cause mortality after ACS and after CABG).4 However, with CROS it became apparent that minimal requirements for CR delivery (based on published standards and core components)5–8 had to be fulfilled to reach effectiveness. These minimal requirements have been addressed by other recent meta-analyses9–13 with a focus on the volume and intensity of exercise sessions and treatment of cardiovascular risk factors during CR. Not meeting these minimal requirements may explain in part the negative results of some recent studies and meta-analyses.14–16
Against this background, the aim of this CROS update was to re-evaluate the results of CROS I critically in the light of newly published CR studies meeting the strict CROS inclusion criteria. Moreover, the aim of this update was to elucidate further the CR effect on secondary and non-fatal clinical endpoints representing a heterogeneous field in clinical CR research. By evaluating controlled observational studies the CROS data finally reflect everyday clinical care thereby allowing an estimation of how guideline standards are actually translated into clinical practice.
Methods
This review was conducted and reported according to the PRISMA statement (preferred reporting items for systematic reviews and meta-analyses), and the MOOSE statement (meta-analysis of observational studies in epidemiology).17,18 The core methods used were essentially unchanged compared to the 2016 publication. The study protocol was prospectively published in PROSPERO (CRD42014007084).19
Study eligibility criteria
Randomised controlled trials (RCTs) as well as prospective controlled cohort studies (pCCS) and retrospective controlled cohort studies (rCCS) of multi-component CR versus usual care, with a follow-up period of at least 6 months, were investigated. We included men and women of all ages after hospitalisation for ACS or CABG, respectively. In addition, we included studies enrolling mixed populations of patients after ACS and/or after CABG as a basic requirement, as well as patients with chronic stable CAD with or without elective percutaneous coronary intervention (PCI). Patient enrolment had to be carried out by 1995 or later. The primary endpoint was total mortality. Secondary endpoints mainly included non-fatal cardiovascular events, hospital readmissions and mixed endpoints. The detailed study selection criteria were presented previously (see Supplementary Material (SM), Table SM 1).4
Search methods and identification of studies
For the previous review4 highly sensitive search strategies were developed to identify two types of studies: RCT and CCS regardless of the studies’ current status (published, unpublished, finished or ongoing). A detailed description of the elaboration of the search strategy is available in the previous review.4
For this update, we restricted our search to the following four databases: PubMed, Embase, Cochrane Central Register of Controlled Trials and the World Health Organization’s International Clinical Trials Registry Platform (ICTRP). Databases which did not contribute studies for inclusion in the previous review were no longer deployed. The search informing this update comprised the period 23 December 2015 to 4 September 2018. No language restrictions were applied. Details of all search strategies are documented in Supplementary Material (see Table SM 2). In addition to searching electronic databases, the references of recent systematic reviews were screened.
Study selection
The titles and abstracts of all references were independently evaluated by at least two members of the reference selection board (AS, CHD, BR). Abstracts of potential interest were re-evaluated and selected for full text evaluation (FTE) and structured study evaluation (SSE), respectively, consented within the whole board. FTE for assessing main inclusion criteria and SSE with quality assessment was performed and consented within an extended reference selection board (AS, CHD, BR, PD) including a biometrician (KJ). The primary reasons for study exclusion are given in Table SM 4.
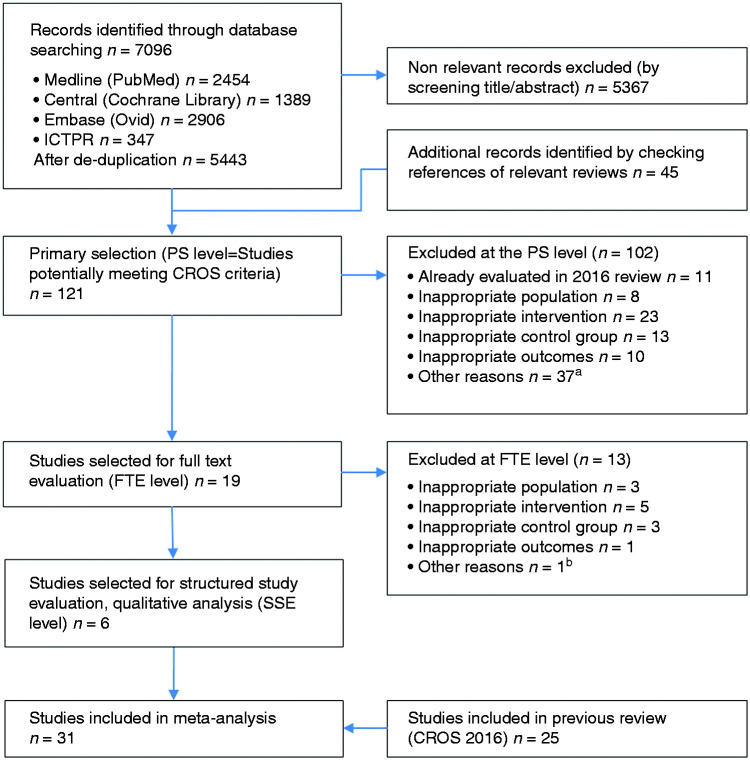
For the meta-analysis, the studies resulting from the SSE process of the current update were merged with the selected studies from the 2016 publication. The study selection process is outlined in Figure 1.
Figure 1.
Study selection flow chart. aOther reasons PS level: reviews, letters, study protocol, only abstract available; bOther reasons FTE level: referral only, no information about CR enrollment and adherence available. ICTRP: International Clinical Trials Registry Platform; PS: primary selection of extracted studies; FTE: full-text evaluation; CR: cardiac rehabilitation; SSE: structured study evaluation and quality analysis according to the checklist of methodological issues on non-randomised studies.20
Study evaluation process
The study evaluation included design, data sources, information on population, interventions, controls, calculation and presentation of outcomes and handling of bias. For RCTs the Cochrane risk of bias table (http://tech.cochrane.org/revman/download), and for the CCSs the checklists of methodological issues on non-randomised studies,20,21 and the Newcastle Ottawa Scale (NOS) were used.22 To facilitate the study evaluation with respect to the management of confounding, age, gender, smoking, diabetes, history of stroke, history of acute myocardial infarction (AMI), reduced left ventricular ejection fraction and acute or early PCI during AMI have been prespecified as potential confounders.
Data extraction
Data extraction was performed by two biometricians independently (KJ, MH), using a standardised extraction form. Disagreements were solved by consensus. We extracted the following information from each eligible article: name of first author, year of publication, study location (country), study design, data source, number of participants, population (ACS, CABG or mixed), inclusion period, inclusion criteria, follow-up time, mean age of participants, proportion of men, intervention characteristics, control characteristics, reported outcomes, information on outcomes, data on outcomes, covariates included in the adjusted models.
Statistical analysis
The analyses were separated with regard to population (AMI, CABG or mixed) and study design (RCT, pCCS and rCCS). For time-to-event outcomes, the hazard ratio (HR) with its 95% confidence interval (CI) was chosen as the effect measure per study. If possible, log HRs and their standard errors were extracted directly, preferably from an adjusted model and matched-group analysis. If they were not reported but adequate univariate analyses were available, an indirect estimation method was used.23,24 In some study publications, instead of HR adjusted odds ratios (ORs) at the end of follow-up or only absolute numbers of events to calculate ORs were reported. HRs and ORs were reported and pooled separately in the present review.25 For dichotomous outcomes, the OR with its 95% CI was used as the effect measure per study. If no event occurred in one or in both arms, a continuity correction of 0.5 per cell was applied. For consistency, we re-calculated the treatment effect to be in the same direction, as necessary, with an HR or OR above 1 indicating a higher risk of CR with respect to each outcome. HRs were combined using the generic inverse variance method. ORs were pooled using the Mantel–Haenszel method or the generic inverse variance method. The latter one was used when at least one study reported an adjusted OR. Random effects models were used to calculate overall effect estimates and CIs because we assumed heterogeneity between the ‘true’ effects of the different CR programmes used in the studies. All results were investigated for statistical heterogeneity by I2 statistics with 0–30% representing no or only small, 30–60% moderate, 50–90% substantial and 75–100% considerable heterogeneity.26 A statistical investigation of potential publication bias based on a test of funnel plot asymmetry could not be done because of too few studies per single meta-analysis.26 Nevertheless, sensitivity analyses for the outcome total mortality have been performed with respect to extracted results of alternative analysis techniques (e.g. independent groups instead of matched groups). There are some deviations from the review protocol published in PROSPERO.19 ORs instead of relative risks were used as effect measure for dichotomous outcomes because in some studies adjusted ORs and no absolute numbers are reported. Furthermore, it was not possible to undertake the planned subgroup analyses due to the small number of studies in each subgroup. R version 3.5.1 (R Foundation for Statistical Computing, 2015) and the R ‘meta’ package version 4.9-2 (developed by Guido Schwarzer) was used for all statistical analyses.
Results
Study characteristics
The study characteristics (design, population, interventions, controls and primary results) of the newly identified studies are presented in Table 1. With respect to the design, only two RCTs (n = 240 patients) fulfilled the CROS criteria increasing the total number of RCTs to three (n = 2053 patients). In addition, two rCCSs (n = 5238 patients) and two pCCSs (n = 3193 patients) were newly identified. Thus, a total of 18 rCCSs (n = 211,334 patients) and nine pCCSs (n = 15,386 patients) were considered for final analysis.
Table 1.
Newly identified studies selected for quantitative analysis; baseline study characteristics and overall results.
| Study Publication year Country | Study design | Population (P): a. Data sources b. Number of included participants (N) c. Index events d. Inclusion period e. Other inclusion criteria and characteristics f. Age (y, mean ± SD or as stated) g. Gender (male, %) | Intervention (I): a. Number (n) b. Structured and multi-component CR (SMC-CR)? c. Start after index event d. Duration (time period and/or total number of CR sessions) e. Frequency (CR exercise sessions per week) f. CR setting | Control (C): a. Number (n) b. Treatment, characteristics | Outcome (O): a. Follow-up period b. Outcomes c. According to the CROS criteria (numbers according to Table 1) d. Other outcomes not predefined by CROS II | Overall results, with respect to endpoints 1–10 as defined by CROS. Definitions are given at the end of the table* | Remarks |
| Espinosa Caliani et al., 2004,48 Spain | pCCS | a. Institutional, Hospital Clínico Universitario Virgen de la Victoria, Málaga, Spain. b. N = 153 c. AMI d. Not stated; after 1995 e. Control group did not accept CR programme f. 49.9 ± 8.4 (CR+) g. 53.5 ± 9.5 (no CR) h. 93.5 | a. n = 113 b. SMC-CR c. Immediately after discharge d. (Phase I) e. 12 weeks (phase II) f. At least 9 mo (phase III) g. n = 3 (24 sessions) + educational talks, dietary and nutritional advice, psychological support (3 mo, phase II). Maintenance phase III until 12 mo h. primary care centre (phase II, III) | a. n = 40 b. CR non-attenders | a. 1 y1 y post AMI b. (10) c. Quality of life, exercise capacity, body mass index | Event rate (%CR+/noCR)) Endpoint 10 (angina, hospitalisation, re-infarction, cardiac insufficiency and/or death): 6.7/6.7 (P = NS) | - Only patients with low-risk MI - CR by patients’ decision - CR supervised by ‘family doctor’ not by cardiologist - CR programme accredited by Cardiology Spanish Society |
| Lee et al., 2016,49 Canada | pCCS | a. Data linkage: ASAN Medical Center-Left MAIN Revascularisation registry (single-centre retrospective database) b. N = 3040 c. Mixed population: patients with unprotected LMCA stenosis >50% with subjective or objective ischaemia; ACS (64.2%), silent ischaemia (8%), stable AP (27.8%) d. 01/01/1995–31/12/2010 e. Patients treated with PCI (37.7%), CABG (49.1%) or medically (13.2%); end of follow-up 31/08/2014 f. 60.8 ± 10.3 (CR+) g. 62.4 ± 10.5 (no CR) h. 76.2 (CR+) i. 72.9 (no CR) | a. n = 596 n = 507 (matched pairs) b. SMC-CR c. Within 3 mo after index hospitalisation (phase II) d. 3 Mo (36 sessions) e. n = 3 f. Outpatient | a. n = 2444 n = 507 (matched pairs) b. CR non-attenders | a. Mdn 7.3 years (IQR, 4.4–10.2 years) b. (1), (2), (4), (5), (8) c. Risk factors’ modification, exercise capacity, QoL, return to work, psychological results | Event rate (% CR+/no CR)) Endpoint 1: 13.3/18.5 Endpoint 2: 10.4/15.5 Endpoint 4: 3.0/6.7 P < 0.001 for all Endpoint 5: 2.0/3.4 P = 0.07 Endpoint 8: 7.3/10.9 P = 0.006 HR (95% CI) after multivariate analysis Endpoint 1: 0.70 (0.49–1.00); P = 0.05 Endpoint 2: 0.69 (0.48–0.97); P = 0.03 Endpoints 4, 5, 8: P = NS HR (95% CI) propensity-matched pairs Endpoint 1: 0.62 (0.43–0.89); P = 0.009 Endpoint 2: 0.54 (0.36–0.80); P = 0.002 Endpoints 4, 5, 8: P = NS | - Participation in CR was defined as attending at least one outpatient CR session (phase II) within 3 mo after index hospitalisation |
| Aronov et al. 201730 Russia | RCT | a. Institutional Moscow Centre of Interventional Cardioangiology. b. N = 36 c. Patients with IHD who had undergone CABG d. Not stated; after 1995 e. – f. 58.6 ± 7.0 (CR+) 55.9 ± 7.0 (no CR) g. 100 | a. n = 18 b. SMC-CR (educational programme + physical training) c. 2–8 weeks after CABG (mean 7.8 ± 1.6 weeks) d. 4 mo e. n = 3 f. monitored (medical supervision) or not-monitored (home based) | a. n = 18 b. CR non-attenders; only educational programme available | a. 1 year b. (1), (6), (8), (10) c. Exercise and echocardiography parameters, lipid levels, QoL, AP attacks, return to work | Event (nr CR+/nr no CR) Endpoint 1: 0/0 Endpoint 6: 1/3 Endpoint 8: 1/1 Endpoint 10 (AP, MI, re-vascularisation, hospitalisation for IHD exacerbation): 2/7 | - Publication in Russian language (translations received from Cochrane Russia and a private agency) - No statistical analyses of the results - CR had educational component only - Contact to author not successful |
| Hautala et al., 2017,33 Finland | RCT | a. EFEX-CARE (Effectiveness of Exercise Cardiac Rehabilitation) database of the Finnish Healthcare setting b. N = 204 c. ACS d. 02/2011–05/2014 e. Exclusion criteria: NYHA ≥III, scheduled or emergency CABG, UA, severe peripheral atherosclerosis, diabetic retinopathy or neuropathy, inability to perform regular home-based exercises (i.e. severe musculoskeletal problems) f. 60 ± 11 (CR+), 62 ± 9 (no CR) g. 73 (CR+), 71 (no CR) | a. n = 109 (drop-out, n = 31) b. SMC-CR c. within 1 week after hospital discharge d. 1 year e. n = 4–5 (1 in hospital session per week and home-based sessions for 6 mo; thereafter home based only) + information, motivation, education, social and vocational support f. Outpatient | a. n = 95 (drop-out, n = 25) b. UC | a. 1 year b. (10) c. Healthcare costs, quality-adjusted life years, cost-effectiveness | Event rate (%CR+/no CR) Endpoint 10 after 1 year: (combination of death, recurrent acute coronary event, or hospitalisation for HF) 4.6/16.8, P = 0.004 | - Centre-based CR under supervision of cardiologists and physiotherapists, all components of SMC-CR were available to most of the patients, no information about psychological support (information provided by the author) |
| Doimo et al., 2018,32 Italy | rCCS | a. Patients discharged from two tertiary hospitals b. N = 1280 c. Mixed population; STEMI (n = 378), NSTEMI (n = 265), CABG with or without valve surgery (n = 353) or planned PCI (n = 284) d. 01/01/2009–31/12/2010 e. Non-residents in the region or with severe non-cardiac comorbidities (i.e. end-stage tumours), dementia, or immobilised patients, were excluded from the CR group. 13% of eligible patients did not attend CR f. 68 ± 11 (CR+), 66 ± 12 (no CR) g. 68 (CR+), 75 (no CR) | a. n = 839; STEMI (n = 251), NSTEMI (n = 162), CABG (n = 243), PCI (n = 183) b. SMC-CR c. 89 days (average) d. 5 mo (average) e. 1st part (10 sessions of 45 min of cyclette training 2 times/week for 5 weeks); 2nd part (18 sessions of 45 min of gym training 3 times/week for 6 weeks) supervised by trained nurse and physiotherapist. Other components: Lifestyle counseling at every visit + nutritional advice once/mo + psychological support a. Outpatient | a. n = 441; STEMI (n = 127), NSTEMI (n = 103), CABG (n = 110), PCI (n = 101) b. CR non-attenders receiving all other components of CR | a. Mdn 82 mo (IQR 60 – 89 mo) b. PEP: (9) SEP: (1), (2), (6) c. Effect of CR in various subgroups | Event rate (% CR+/no CR) Endpoint 1: 17/18 (P = 0.861) Endpoint 2: 6/6 (P = 0.623) Endpoint 6: 15/27 (P < 0.001)) Endpoint 9: 18/30 (P < 0.001)) HR (95% CI) Endpoint 9: 0.578 (0.432–0.773); P < 0.001 Event rate, propensity matched analysis (% CR+/no CR) Endpoint 1: 10/19 (p = 0.002) Endpoint 2: 2/7 (P = 0.008) Endpoint 6: 25/11 (P < 0.001)) Endpoint 9: 29/13 (P < 0.001)) | - Group allocation by different hospitals - Multivariable regression model and propensity score matching analysis (covariates: age, sex, hypertension, LVEF, DM, smoking, CKD, dyslipidaemia, previous PCI, previous ACS, BB, ACEi/ARB, statins/ezetimibe) - Statistical analysis does not address cardiovascular mortality adequately - 5-year composite endpoint as primary outcome (hospitalisation for cardiovascular causes and cardiovascular mortality) |
| Sunamura et al., 2018,50 The Netherlands | rCCS | a. Patients from Erasmus Medical Centre (no CR), Rotterdam were propensity score matched with patients from Capri Cardiac Rehabilitation Cater, Rotterdam (CR+) b. N = 3958 c. ACS followed by primary PCI d. 2003–2011 e. Excluded: patients with cardiogenic shock (2.3%) and with early (within 60 d post-PCI) death (5.2%) f. 59.0 ± 9.9 (CR+), 58.8 ± 11.83 (no CR) g. 77 (CR+), 78 (no CR) | a. n = 1159 b. SMC-CR c. Mdn 4–6 weeks d. 12 weeks e. n = 2 (1.5 h group exercise session). Other components: verbal and written instructions on how to deal with exercise, diet, smoking cessation, and stress management. Individual consultations with psychiatrist, psychologist, and social workers was available if necessary. Complete CR if attended at least 75% of the physical programme f. Outpatient | a. n = 1159 b. no CR participants | a. Mdn 10 years 4–12 year (range) b. (1) c. Mortality rates of CR completion vs. non-completion | Cumulative rates (% CR+/no CR) Endpoint 1 at 5 years: 6.4/10.4 Endpoint 1 at 10 years: 14.7/23.5 HR (95% CI) Endpoint 1 at 10 years: (unadjusted) 0.56 (0.43–0.73) (adjusted) 0.61 (0.46–0.81); P < 0.001 | - Propensity score matching analysis 1:1 (covariates: age, sex, STEMI, current smoking, family history of CAD, HTN, hypercholesterolemia, DM, prior MI, prior history of PCI or CABG, proximal LAD lesion, socioeconomic status) |
Descriptive values of metric variables are given in mean or mean plus standard deviation (SD), if applicable. Other calculations are noted in the table.
Mdn: median; N: number of total population; n: number of subpopulation; mo: month(s); ACEi: angiotensin-converting enzyme inhibitors; (A)MI: (acute) myocardial infarction; AP: angina pectoris; ARB: angiotensin receptor blockers; CABG: coronary artery bypass grafting; BB: beta-blockers; ACEi/ARB CAD: coronary artery disease; CKD: chronic kidney disease; CR: cardiac rehabilitation; DM: diabetes mellitus; HF: heart failure; IHD: ischaemic heart disease; IQR: interquartile range; LAD: left anterior descending coronary artery; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; pCCS: prospective controlled cohort trial; PCI: percutaneous coronary intervention; PEP: primary endpoint; QoL: quality of life; rCCS: retrospective controlled cohort trial; RCT: randomised controlled trial; SEP: secondary endpoint; SMC-CR: structured and multicomponent CR; (N) STEMI: (non) ST-elevation myocardial infarction; UC: usual care including ambulatory supervision by family doctor and/or cardiologist, and may also include advise to exercise at home.
Three new studies enrolled 4315 patients after ACS (total of 15 studies; n = 50,653 patients), one additional study included 36 patients after CABG (total of 10 studies; n = 14,583 patients), while two newly identified studies recruited 4320 patients in ‘mixed populations’ (total of 11 studies; n = 163,101 patients).
The CR setting was ‘outpatient’ in all new studies (total of 27) and the CR duration varied from 12 weeks to 12 months, thereby not changing the range of 3–4 weeks up to 12 months identified in the previous CROS study. Moreover, the previously reported ‘CR intensity’ ranging from two up to more than five exercise sessions per week plus motivation, information, education and psychosocial interventions with variable intensities and combinations remained unchanged.
Notably, the included studies reveal a considerable heterogeneity not only with respect to the predefined study designs (RCT, pCCS, rCCS), and populations (after ACS, after CABG, mixed CAD populations), but also with respect to study endpoints and biometrical evaluation (Tables 2, 3 and 4 and Figure 2). For this reason, the majority of the secondary endpoints predefined by CROS could not be integrated into a meta-analysis (Table 2, Figure 2).
Table 2.
Summary of results.
| Outcome | Population (number of studies) | Design (number of studies) | Events/number of patients (CR) | Events/number of patients (control) | HR (95% CI) | OR (95% CI); pooling method | Heterogeneity: I2; tau2; P value |
|---|---|---|---|---|---|---|---|
| Total mortality | ACS (11) | RCT (1) | 82/903 | 84/910 | 1.01 (0.85–1.21) | NA | |
| pCCS (4) | NO/3519 | NO/2063 | 0.37 (0.20–0.69) | 18%; 0.092; P = 0.30 | |||
| rCCS (4) | NO/12,033 | NO/24,266 | 0.64 (0.53–0.76) | 33%;0.011; P = 0.22 | |||
| rCCS (2) | 109/2901 | 241/1846 | 0.20 (0.08–0.48); MH | 60%; 0.288; P = 0.11 | |||
| CABG (6) | RCT (1) | 0/18 | 0/18 | 1.00 (0.02–53.12); NA | NA | ||
| pCCS (1) | 1/149 | 5/89 | 0.11 (0.01–0.99); NA | NA | |||
| rCCS (4) | NO/5109 | NO/7889 | 0.62 (0.54–0.70) | 0%; 0; P = 0.71 | |||
| Mixed (10) | pCCS (2) | 254/3407 | 398/2939 | 0.66 (0.55–0.79) | 0%; 0; P = 0.72 | ||
| rCCS (5) | NO/2606 | NO/3577 | 0.52 (0.36–0.77) | 84%;0.145; P < 0.01 | |||
| rCCS (3) | 1700/71,674 | 3,806/71,160 | 0.68 (0.34–1.37); NA | 94%; 0.339; P < 0.01 | |||
| Cardiovascular mortality | ACS (2) | pCCS (1) | 18/2505 | 32/1042 | 0.44 (0.24–0.82) | NA | |
| pCCS (1) | 0/37 | 1/37 | 0.32 (0.01–8.23); NA | NA | |||
| CABG (2) | pCCS (1) | 0/18 | 0/18 | 1.00 (0.02–53.12); NA | NA | ||
| rCCS (1) | NO/527 | NO/4747 | 0.64 (0.51–0.81) | NA | |||
| Mixed (3) | pCCS (1) | 37/507 | 75/507 | 0.54 (0.36–0.80) | NA | ||
| rCCS (1) | 34/719 | 46/719 | 0.67 (0.44–1.03) | NA | |||
| rCCS (1) | 48/839 | 28/441 | 0.90 (0.55–1.45); NA | NA | |||
| MACCE | ACS (2) | pCCS (1) | 81/2376 | 81/971 | 0.55 (0.39–0.77) | NA | |
| rCCS (1) | 212/2756 | 281/1791 | 0.70 (0.35–1.40); NA | NA | |||
| Mixed (1) | rCCS (1) | 158/785 | 206/1224 | 0.85 (0.74–0.98) | NA | ||
| Non-fatal | ACS (3) | RCT (1) | 7/162 | 8/115 | 0.60 (0.21–1.72); NA | NA | |
| myocardial infarction | pCCS (1) | 43/2362 | 27/946 | 0.75 (0.45–1.26) | NA | ||
| pCCS (1) | 0/37 | 0/37 | 1.00 (0.02–51.73); NA | NA | |||
| CABG (1) | pCCS (1) | 3/343 | 13/334 | 0.22 (0.06–0.77); NA | NA | ||
| Mixed (3) | pCCS (1) | 15/507 | 23/507 | 0.65 (0.34–1.26) | NA | ||
| rCCS (1) | NO/785 | NO/1224 | 1.01 (0.74–1.37) | NA | |||
| rCCS (1) | 14/795 | 26/679 | 0.45 (0.23–0.87); NA | NA | |||
| Non-fatal stroke | ACS (2) | RCT (1) | 0/162 | 1/115 | 0.23 (0.01–5.81); NA | NA | |
| pCCS (1) | 10/2364 | 13/954 | 0.35 (0.14–0.85) | NA | |||
| Mixed (1) | pCCS (1) | 8/507 | 13/507 | 0.92 (0.24–3.52) | NA | ||
| Hospital readmission | ACS (3) | pCCS (2) | 794/2447 | 351/1035 | 0.96 (0.81–1.13); IV | 0%; 0; P = 0.32 | |
| for any reason | rCCS (1) | NO/878 | NO/824 | 1.00 (0.82–1.22) | NA | ||
| CABG (1) | RCT (1) | 3/18 | 1/18 | 3.40 (0.32–36.27); NA | NA | ||
| Mixed (2) | pCCS (1) | NO/2900 | NO/2432 | 0.77 (0.71–0.84) | NA | ||
| rCCS (1) | 253/795 | 258/679 | 0.76 (0.61–0.94); NA | NA | |||
| Unplanned readmission | ACS (2) | RCT (1) | 23/162 | 16/115 | 1.02 (0.51–2.04); NA | NA | |
| for any cardiovascular | pCCS (1) | 17/74 | 20/54 | 0.51 (0.23–1.10); NA | NA | ||
| event | Mixed (2) | pCCS (1) | 32/2900 | 109/2432 | 0.68 (0.55–0.84) | NA | |
| rCCS (1) | 122/839 | 119/441 | 0.46 (0.35–0.61); NA | NA | |||
| Unplanned coronary | ACS (1) | pCCS (1) | 4/69 | 7/72 | 0.57 (0.16–2.05); NA | NA | |
| revascularisation | CABG (1) | pCCS (1) | 44/343 | 49/334 | 0.86 (0.55–1.33); NA | NA | |
| Mixed (1) | pCCS (1) | 44/507 | 33/507 | 1.38 (0.88–2.16) | NA | ||
| rCCS (1) | 33/795 | 37/679 | 0.75 (0.46–1.22); NA | NA | |||
| Cardiovascular mortality | ACS (1) | pCCS (1) | 0/74 | 4/54 | 0.08 (0.00–1.43); NA | NA | |
| and readmission | Mixed (1) | rCCS (1) | 155/839 | 133/441 | 0.58 (0.43–0.77) | NA | |
| Combined endpoints | ACS (8) | RCT (1) | 5/109 | 16/95 | 0.26 (0.09–0.73) | NA | |
| RCT (1) | 24/162 | 25/115 | 0.63 (0.34–1.15); NA | NA | |||
| pCCS (1) | NO/521 | NO/522 | 0.65 (0.30–1.41) | NA | |||
| pCCS (4) | 47/620 | 69/567 | 0.58 (0.33–1.00); MH | 21%; 0.080; P = 0.28 | |||
| rCCS (1) | 183/2756 | 263/1791 | 0.41 (0.34–0.50); NA | NA | |||
| CABG (2) | RCT (1) | 2/18 | 7/18 | 0.20 (0.03–1.13); NA | NA | ||
| pCCS (1) | 44/343 | 68/334 | 0.58 (0.38–0.87); NA | NA | |||
| Mixed (2) | rCCS (1) | NO/785 | NO/1224 | 0.77 (0.65–0.91) | NA | ||
| rCCS (1) | 259/795 | 263/679 | 0.73 (0.59–0.91); NA | NA |
ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; NO: sum of events has not been calculated, if one study of a specific subgroup did not report the number of events; MH: Mantel–Haenszel pooling; NA: not applicable; IV: inverse variance pooling; RCT: randomised controlled trial; rCCS: retrospective controlled cohort study; pCCS: prospective controlled cohort study; HR: hazard ratio; CI: confidence interval; OR: odds ratio.
Table 3.
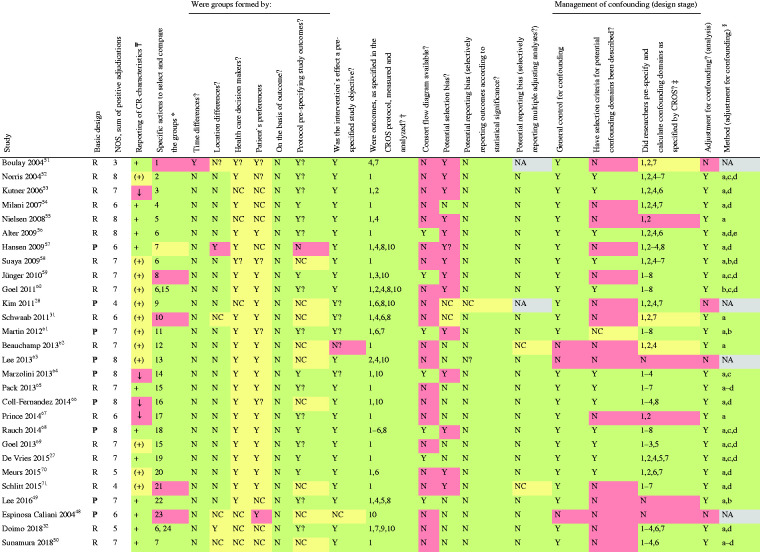
|
 Reporting of CR characteristics: +: sufficient; (+): information obtained by author or other sources; ↓: information limited.
Reporting of CR characteristics: +: sufficient; (+): information obtained by author or other sources; ↓: information limited.
Specific actions to compare groups: (1) prospectively evaluated intervention group versus retrospectively evaluated control group; (2) linkage of Canadian APPROACH and NACPR registry; (3) data extracted from the United States renal data System, USRDS; (4) retrospective identification of groups by questionnaires within a predefined study cohort; (5) retrospective identification of groups in a population surviving AMI for at least 30 days; (6) retrospective evaluation and formation of matched pairs; (7) groups were formed by two hospitals following different CR referral policies; (8) retrospective identification of groups by questionnaires and personal contact to relatives of deceased patients; (9) groups were formed prospectively according to predefined inclusion and exclusion criteria; (10) retrospective definition of the study groups out of an independent pre-existing study cohort on the basis of medical records;72 (11) propensity score matching; (12) retrospective evaluation of a pre-existing cohort of another study evaluating CR attendance after automatic referral; (13) predefinition of inclusion and exclusion criteria, but final group formation by patient`s preferences and health care decision makers; (14) selection of CAD patients with musculoskeletal disease in addition; (15) retrospective definition of the groups; CR+ group was defined as attending at least one session within 6 months after the index event; (16) prospective definition of the groups out of the FRENA registry;73 (17) patients referred to CR but not attending served as control; (18) groups were prespecified from the OMEGA trial cohort;74 (19) 180 days survival after index event required; (20) study population has been extracted from two pre-existent studies (DepeMI, MIND-IT);75,76 (21) retrospective recruitment of study population from two previous RCTs not investigating CR or prognostic CAD outcomes;71,77 (22) data extracted from ASAN Medical Center-Left MAIN Revascularisation registry and ASAN Medical Center cardiac rehabilitation database; (23) control group was formed of patients who did not accept CR programme; (24) matching pairs from the Capri Cardiac Rehabilitation database and Erasmus Medical Centre database (control).
Outcomes under investigation: the numbers refer to the predefined outcomes as outlined in Table 1.
Confounding domains as specified by CROS: 1, age; 2, sex; 3, smoker; 4, diabetes; 5, history of stroke; 6, history of acute myocardial infarction; 7, reduced left ventricular ejection fraction; 8, acute/early ercutaneous coronary intervention during acute myocardial infarction.
§Biometrical methods to manage confounding: (a) multivariable regression analysis; (b) propensity score matching; (c) propensity score-adjusted multivariable regression analysis; (d) confounders described; (e) retrospective matched pairs. Adjusting only for age and gender has been regarded as insufficient for the limitation of confounding.
APPROACH: Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease; NACRP: Northern Alberta Cardiac Rehabilitation Program; FRENA: Risk Factors and Arterial Disease registry (Factores de Riesgo y ENfermedad Arterial); OMEGA: Randomised, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy after Myocardial Infarction; DepreMI: Depression after Myocardial Infarction study; MIND-IT: Myocardial Infarction and Depression Intervention Trial.
R: retrospective cohort control study; P: prospective cohort control study; Y: yes; Y?: probably yes; N: no; N?: probably no; NC: not clear, not reported; NA: not applicable;
green → adjudication is in favor to reliability of results and reporting;
yellow → item potentially increases risk of limited reliability of results and reporting;
red → item increases risk of reliability of results and reporting.
Table 4.
Quality evaluation of randomised controlled trials included into meta-analysis (according to the Cochrane risk of bias table).
| Risk | West 201214 | Aronov 201730 | Hautala 201733 |
|---|---|---|---|
| Under-powering | High risk | High risk | Unclear risk |
| Selection bias | Unclear risk | Unclear risk | Low risk |
| Random sequence selection bias | Unclear risk | High risk | Low risk |
| Allocation concealment | Low risk | High risk | Unclear risk |
| Confounding variables | Unclear risk | High risk | Low risk |
| Performance bias | Low risk | Unclear risk | Low risk |
| Detection bias | Low risk | Unclear risk | Low risk |
| Attrition bias (incomplete outcome data) | Low risk | Low risk | Low risk |
| Groups balanced at baseline | Low risk | Unclear risk | Low risk |
| Groups not receiving the same baseline treatment | Unclear risk | Low risk | Low risk |
| Intention to treat analysis | Low risk | Low risk | Low risk |
| Reporting bias | Low risk | Low risk | Low risk |
| Comments | Low recruitment (22.5% CR arm; 22.7% control arm), study participation influenced by patient`s preferences, random sequence generation is not reported, per protocol centrally organised randomisation and blinded with respect to baseline characteristics, confirmation of exposure sufficient, CR status has been blinded before outcome assessment, follow-up reporting was completed in 95% of surviving patients, baseline treatment with respect to medication and medical supervision has to be assumed; control group may also have received life style support to a variable extend | No primary endpoint defined; no pre-estimation of sample sizes and effect sizes were described with respect to any endpoint measured), exclusively low risk patients, no randomisation method described, potential confounding variables were not assessed, no allocation concealment, interactions between the study groups confounding performance cannot be excluded, baseline values were presented in a descriptive way without statistical evaluation. At least in n = 3 relevant clinical characteristics a balance between groups was not achieved | Primary endpoint: Cost/quality-adjusted life year of a cardiac patient (QALY) Secondary endpoint: major adverse cardiac event (MACE) Statistical power of the study has not been reported with respect to either of the presented endpoints |
green → adjudication is in favour to reliability of results and reporting; yellow → item potentially increases risk of limited reliability of results and reporting; red → item increases risk of reliability of results and reporting.
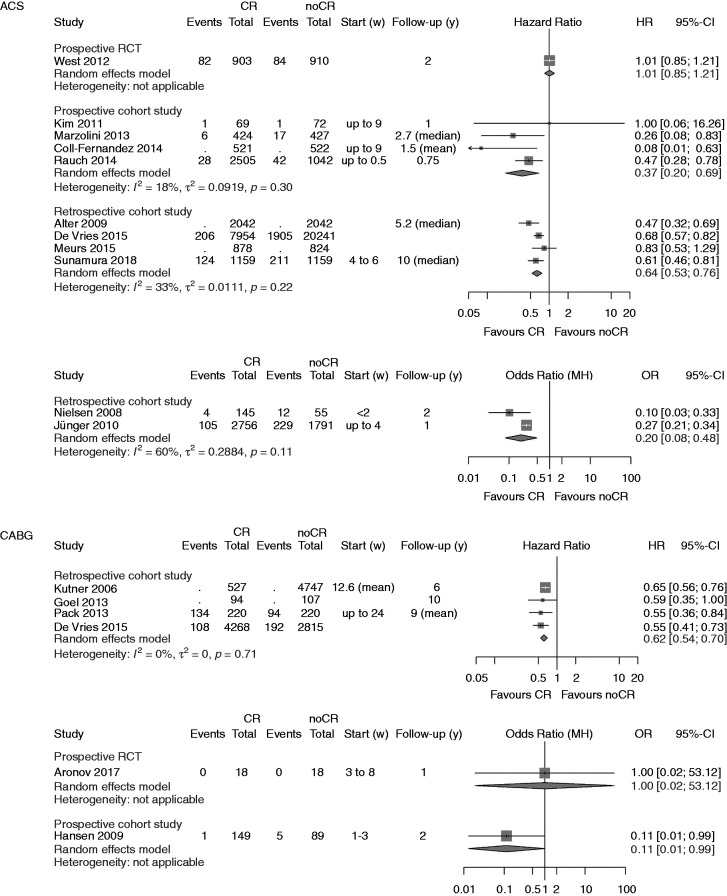
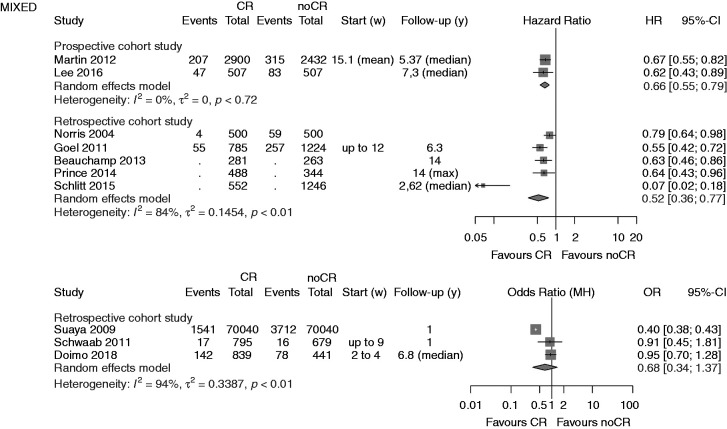
Figure 2.
Analysis of total mortality. Forest plots presenting the evaluation of the endpoint ‘total mortality’. HR: hazard ratio; OR: odds ratio; MH: Mantel–Haenszel pooling method; CR: cardiac rehabilitation; no CR: no cardiac rehabilitation (control); CI: confidence interval; Events: number of events in the evaluated group; Total: number of patients in the evaluated group; Start (w): start of cardiac rehabilitation after hospital discharge in weeks; Follow-up: follow-up in years.
Primary endpoint ‘total mortality’
A summary of the clinical outcomes is shown in Table 2. The primary endpoint ‘total mortality’ was evaluated in 27 studies, one of them evaluating both mortality after ACS and after CABG (Figure 2).27 Participation in CR was associated with a significant reduction of total mortality in all but six studies.14,28–32
After ACS a significant reduction in total mortality was confirmed by the newly added pCCSs (four studies; HR 0.37, 95% CI 0.20–0.69; I2 = 28%) and even strengthened by the newly added rCCSs (four studies; HR 0.64, 95% CI 0.53–0.76; I2 = 33%).
After CABG, the newly identified single RCT was small, only enrolling 36 low-risk patients. During a follow-up period of one year, no deaths occurred, and the risk of ‘underpowering’ has to be regarded as high in this study (see Table 4, Figure 2). No additional rCCSs or pCCSs were identified; consequently, the previous positive results on mortality reduction remained unchanged in this population.
In ‘mixed populations’ the addition of one more pCCS confirmed the significant mortality reduction in CR participants (two studies; HR 0.66, 95% CI 0.55–0.79) with zero heterogeneity. No additional rCCSs calculating HR within the mixed populations could be included by the current search (HR 0.52, 95% CI 0.36–0.77, I2 = 84%). The single rCCS newly added within the group calculating ORs did not change the neutral result reported before in this group (three studies; OR 0.68, 95% CI 0.34–1.37) but heterogeneity was high (I2 = 94%). Sensitivity analyses did not change the overall results.
Secondary endpoints
The results of CROS II with respect to the secondary endpoints are shown in Table 2, differentiating between the various study designs, populations and biometrical approaches. These results are summarised as follows:
Regarding the secondary endpoints ‘CV mortality’ (three additional studies, seven studies in total) and ‘MACCE’(major adverse cardiovascular and cerebrovascular events) (three studies, unchanged) all selected studies considerably differed with respect to populations and designs, and a ‘matching’ of these studies for meta-analysis was not possible (Table 2). Focusing on the endpoint ‘CV mortality’ and based on the two large controlled observational studies (pCCS, rCCS) there might be a trend in favour of CR participation after ACS and after CABG. With regard to the endpoint MACCE, however, the selected studies do not allow a final conclusion on the effect of CR participation (Table 2).
The outcomes ‘non-fatal MI’ (total seven studies) and ‘non-fatal stroke’ (total three studies) also did not show a clear trend, but all studies varied in design and population thus hindering a further evaluation by meta-analysis.
The same is true for studies investigating the variably predefined endpoints for ‘hospital readmission’ (endpoints 6–9, see Methods). Most of these studies had heterogeneous designs, and matching of the studies for meta-analysis was not possible (Table 2).
In a descriptive way the results on ‘hospital readmission’ may be summarised as follows: all studies included in CROS either showed a reduction of hospital readmissions in favour of CR participation, or there was a neutral result. In 12 studies, combined endpoints with various components were evaluated. One more RCT has been identified showing a statistically reduced combined endpoint (death, recurrent acute coronary events, or hospitalisation for HF) after CR participation compared to usual care (HR 0.26, 95% CI 0.09–0.73).33
Quality evaluation of the studies
The sum of positive adjudications estimated by NOS is presented in Table 3 (for details see online version, Supplementary Material, Supplementary Table 5). Four additional studies were graded within a range of 5–7. In total, five out of 28 studies (18%) were graded with 5 points or less. Limitations were found with respect to representativeness (six studies), comparability of the cohorts (three studies), adequacy of follow-up (five studies) and the assessment of outcomes (two studies).
On the basis of the checklist of methodological issues on non-randomised studies the following limitations were identified (Tables 3 and 4):
Three studies were based on a secondary analysis of original studies with different original objectives.
In three studies, either time or location differences between the study groups were apparent.
In most studies, the group formation was potentially influenced by healthcare decision-makers and patient preferences.
The majority of the studies had unclear study protocols and a consort flow diagram was presented in only seven out of 28 studies.
Management of confounding was not reported in three studies, whereas the description of potential confounding domains remained unclear or has not been reported in 16 studies.
Predefinition and calculation of all confounding domains as prespecified by CROS (see Materials and methods) were performed to various degrees. In only four studies all eight predefined confounders were considered for adjustment. Moreover, six studies only considered three or even fewer confounders as predefined by CROS. In general, adjustment for confounding was performed in 24 CCSs with four studies not applying adequate biometrical methods.
Both RCTs evaluating the primary endpoint ‘total mortality’ do have a considerable risk of being underpowered (Table 4).14,30,33
Discussion
This update of CROS II confirms the beneficial prognostic effect of CR in CAD patients by significantly reducing the primary endpoint ‘total mortality’ especially after ACS or CABG. However, the effects of CR participation on secondary endpoints such as ‘CV mortality’, ‘non-fatal myocardial infarction’, ‘non-fatal stroke’, ‘combined endpoints’ and various forms of ‘hospital readmission’ remain less clear. This at least in part is due to a considerable heterogeneity of the selected studies with respect to design, populations, predefined endpoints and biometry. Inconsistent results may be due to the kind of selected endpoints including ‘weak’ endpoints with increased risks of confounding. This is particularly true for the variable forms of ‘hospital readmission’, which may be influenced by local routines in medical services, individual comorbidities not necessarily associated with CVDs, and the individual’s disease perception. Moreover, a longer survival of patients after AMI/CABG may reveal other diseases that primarily determine the number of hospital admissions during prolonged follow-up.
With regard to the secondary endpoint ‘non-fatal AMI’ an overall ‘neutral’ effect has also been reported by Cochrane (Anderson et al.).9 As AMI and death are closely interrelated clinical events one might speculate that CR participation effectively prevents death initiated by AMI, but also reduces the incidence of AMI (fatal plus non-fatal) per se, resulting in an apparent ‘neutral effect’ with respect to non-fatal AMI occurrence. Unfortunately, the data sources presently available for CROS do not allow for further evaluation of this hypothesis.
One of the major strengths of this study is its robust approach to CR intervention aligned with published national CR standards and core components.5–7 Our strict definition of a comprehensive multicomponent CR underscores the importance of the amount of physical exercise provided, the adherence to exercise intervention and the adherence to non-exercise components on the patients’ prognosis. The results of recently published meta-analyses (some of them including studies of the modern era of novel medication and interventions) seem to support this approach and somehow elucidate our results. Thus, van Halewijn et al. have shown that a significant reduction in all-cause mortality was feasible in CAD patients only under the condition of a comprehensive CR programme managing six or more cardiovascular risk factors,10 while the recently published EU-CaRE study showed positive effects of comprehensive CR in 58% of older patients with three or more uncontrolled risk factors before CR.34 These findings, coupled with CROS II results, strengthen clinical recommendations that comprehensive CR is preferable to standalone exercise-based CR in reducing total and cardiac mortality, in post-myocardial infarction patients.13 The effectiveness of a comprehensive CR programme is increased by the patients’ adherence and by the shared effort consequently to assess and treat the majority of all individual cardiovascular risk factors.
With regard to the importance of the CR dose, Santiago de Araujo Pio et al. established that total mortality reduction was only possible in CVD patients experiencing medium and high doses of CR.12 Similar CR dose and volume-related effects on mortality have been published.9,35 Finally, in a systematic review of multicomponent CR, applying almost all CROS inclusion criteria, the study by Sumner et al. carried out a meta-analysis of observational studies published after the year 2000, concluding that all-cause and cardiac mortality were reduced in AMI patients following a CR programme.36
Still, one has to keep in mind that this beneficial effect of CR participation as shown in CROS may not apply to special subgroups such as elderly and frail patients who need a particularly personalised approach.37 According to Deaton,38 however, the average age of the CROS study population reflects actual clinical reality. Likewise, CR participation of patients with severe systolic heart failure may not result in mortality reduction as shown in previous meta-analyses.39–41
Apart from these limitations, CROS II presents a timely account of the effectiveness of CR when delivered to agreed published standards including scientifically confirmed CR core components.5–7 Utilising a strict approach to CR intervention study inclusion we can report a significant benefit (Table 2 and Figure 2) in favour of CR with respect to all-cause mortality. However, at the same time this approach might be viewed as a significant weakness as it makes our findings almost incompatible with previous reviews, which have been much more inclusive of CR interventions often defined by innovations in CR being evaluated as part of clinical trials rather than informed by interventions based on published CR programme standards and core components. Only three RCTs were selected for CROS II compared to 63 in the most recent Cochrane review which reported a significant reduction in cardiovascular mortality but not in all-cause mortality.9 We are not suggesting that previous trial-based reviews are erroneous. On the contrary, we agree that robust trials-based reviews remain top of the evidence base hierarchy. What we are proposing is that the CROS II approach differs to the extent that it should be viewed as an additional form of evidence that utilises registry-based research reflecting a broader population in the modern cardiology era from 1995 onwards.
For a critical estimation of the CROS II results, the following aspects have to be emphasised:
CR participation after ACS or CABG is associated with reduced total mortality if delivered on top of the current evidence-based treatment modalities (medication and acute coronary interventions). CR participation therefore may contribute to treatment adherence and further add effective individual lifestyle changes necessary to reduce patients’ cardiovascular risk significantly.42–46
This positive effect of CR participation obviously works in current clinical practice of different countries provided a minimum of CR volume and intensity is delivered. This especially refers to the individually adapted and supervised exercise training and a rigorous treatment of all individual cardiovascular risk factors. 9,12,13,47
Unfortunately, these prerequisites of successfully delivered CR – although outlined in detail in many position papers – are not necessarily followed in clinical practice. As noted in CROS II, these prerequisites are not sufficiently described in many clinical studies evaluating CR effectiveness. Therefore, there is an urgent need to translate these well-known and evidence-based minimal standards effectively into all-day clinical practice wherever CR is offered. Moreover, these clinical standards need to be the adamant basis of future CR outcome studies. To this end, minimal standards for CR interventions in clinical practice and clinical trials should be based on robust published guidelines and research. We offer the CROS II definition and criteria as a useful guide for optimal CR intervention content and delivery; including multidisciplinary and multicomponent programmes with structured, supervised exercise training delivered at least twice per week in combination with motivational techniques, risk factor modification education, dietary advice, psychosocial and vocational support delivered at least once per week. The CR setting could be inpatient, outpatient or mixed but the time between hospital discharge and CR initiation should be as low as possible, preferably within 3 months.
From this background it is one of CROS’s aims not only to evaluate the results and clinical outcomes of the studies included, but also to evaluate critically the strengths and deficiencies in detail of each single study included in the meta-analysis (see Table 3). As in the first evaluation in CROS, this update uncovers considerable deficits in current CR studies that need to be addressed and prevented in future. These deficits include predominantly insufficient description of CR content (e.g. applied components), frequency and volume of exercise sessions, CR initiation (i.e. after hospital stay for an acute cardiac event) and duration, absence of CR adherence at follow-up as well as methodological issues such as the inadequate consideration of confounding parameters at the stage of study and statistical analysis design.
Clinical implications
Together with the results of other recent reviews, minimal requirements for a successful CR after ACS or CABG are apparent and need to be ensured in clinical practice:4,9,10,12,13,45
CR is multicomponent including consequent treatment of the individual’s cardiovascular risk factors, individually adapted physical exercise, information, motivation as well as individualised psychosocial support.4
The individualised approach also reflects gender, age, frailty, heart failure, concomitant diseases, psychosocial background and effectors of the individual’s health and capabilities.
CR is supervised and carried out by adequately trained health professionals including cardiologists.4
During CR the ‘dose’ of exercise training (number of weeks of exercise training × average number of sessions/week × average duration of session in minutes) exceeds 1.000.9
The number of CR sessions (including physical exercise, information, education and psychosocial support) needs to exceed 36.12
During CR all individually recognised cardiovascular risk factors need to be addressed and treated.10
Consequently, future studies on the effect of CR need to report in detail whether these minimal requirements were rigorously followed by the participating CR centres.
Conclusions
CROS II confirms the effectiveness of CR participation after ACS and after CABG in actual clinical practice by reducing total mortality under the conditions of current evidence-based CAD treatment. The CROS approach to more strictly predefined CR intervention and to include controlled registry-based studies represents a valid hybrid approach that has clear utility in clinical decision-making.
Supplemental Material
Supplemental material, CPR905719 Supplemental Material for Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II) by Annett Salzwedel, Katrin Jensen, Bernhard Rauch, Patrick Doherty, Maria-Inti Metzendorf, Matthes Hackbusch, Heinz Völler, Jean-Paul Schmid and Constantinos H Davos in European Journal of Preventive Cardiology
Acknowledgements
EAPC Cardiac Rehabilitation Section, Nucleus members:
• Ana Abreu, Cardiology Department, Hospital Santa Marta, Lisbon, Portugal
• Marco Ambrosetti, Cardiovascular Rehabilitation Unit, ‘Le Terrazze’ Clinic, Cunardo, Italy
• Thomas Berger, Cardiomed Linz, Austria
• Véronique Cornelissen, Research Group for Rehabilitation in Internal Disorders, KU Leuven, Belgium
• Wolfram Doehner, Charité-Universitätsmedizin Berlin, Germany
• Ines Frederix, Faculty of Medicine and Life Sciences, Hasselt University, Belgium
• Andreas Gevaert, Laboratory of Cellular and Molecular Cardiology, Universität Antwerpen, Belgium
• Dominique Hansen, Biomedisch Onderzoeksinstituut (BIOMED), Universiteit Hasselt, Belgium
• Marie-Christine Iliou, Cardiac Rehabilitation and Secondary Prevention, Corentin Celton Hospital, APHP, Paris, France
• Hareld Kemps, Máxima Medisch Centrum, The Netherlands
• Nicolle Kraenkel, Medizinische Klinik für Kardiologie, Charité-Universitätsmedizin Berlin, Germany
• Jari Laukkanen, University of Jyväskylä and Central Finland Health Care District, Finland
• Roberto Pedretti, Clinic for Pressure Monitoring and Hypertension, Pavia, Italy
• Maria Simonenko, Federal Almazov North-West Medical Research Centre, St Petersburg, Russian Federation
• Heinz Völler, University of Potsdam, Human Sciences Faculty, Potsdam, Germany
• Matthias Wilhelm, University Hospital of Cardiology, Bern, Switzerland
Author contribution
All authors participated in designing the study, generating hypotheses, interpreting data, and critically reviewing the report. The special responsibilities were as follows: Initiation, organisation and leading of the project: BR, CHD, PD, JPS, HV; literature search and search strategies: MIM, BR; study selection: AS, CHD, PD, BR; study evaluation: AS, CHD, BR, KJ; statistical and biometrical analyses: KJ, MH; writing: AS, HV, CHD, PD, KJ, MIM, BR; internal reviewing: JPS, BR, HV, AS, PD, CHD, and the nucleus members of the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology (EAPC).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Pfizer AG Switzerland (unrestricted grant), Deutsche Herzstiftung eV (German Heart Foundation), Deutsche Gesellschaft für Prävention und Rehabilitation von Herz-Kreislauferkrankungen eV (DGPR; German Society of Cardiovascular Prevention and Cardiac Rehabilitation), Schweizer Arbeitsgruppe für kardiovaskuläre Prävention, Rehabilitation und Sportkardiologie (SCPRS; Swiss Working Group for Cardiovascular Prevention, Rehabilitation and Sports Cardiology). The sponsors did not have any influence on study initiation, conducting and reporting.
Previous review version
Rauch B, Davos CH, Doherty P, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies – the Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol 2016; 23: 1914–1939. doi: 10.1177/2047487316671181.
Systematic review registration
PROSPERO International prospective register of systematic reviews (registration number: CRD42014007084): http://www.crd.york.ac.uk/prospero/review_print.asp?RecordID=7084&UserID=5736.
References
- 1.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007; 356: 2388–2398. [DOI] [PubMed] [Google Scholar]
- 2.Puymirat E, Simon T, Cayla G, et al. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017; 136: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 3.Szummer K, Wallentin L, Lindhagen L, et al. Relations between implementation of new treatments and improved outcomes in patients with non-ST-elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J 2018; 39: 3766–3776. [DOI] [PubMed] [Google Scholar]
- 4.Rauch B, Davos CH, Doherty P, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies – The Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol 2016; 23: 1914–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piepoli MF, Corra U, Adamopoulos S, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol 2014; 21: 664–681. [DOI] [PubMed] [Google Scholar]
- 6.Cowie A, Buckley J, Doherty P, et al. Standards and core components for cardiovascular disease prevention and rehabilitation. Heart (British Cardiac Society) 2019; 105: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007; 115: 2675–2682. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RS, Anderson L, Oldridge N, et al. The efficacy of exercise-based cardiac rehabilitation: the changing face of usual care. J Am Coll Cardiol 2017; 69: 1207–1208. [DOI] [PubMed] [Google Scholar]
- 9.Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016; 1: Cd001800–Cd001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Halewijn G, Deckers J, Tay HY, et al. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: a systematic review and meta-analysis. Int J Cardiol 2017; 232: 294–303. [DOI] [PubMed] [Google Scholar]
- 11.Almodhy M, Ingle L, Sandercock GR. Effects of exercise-based cardiac rehabilitation on cardiorespiratory fitness: a meta-analysis of UK studies. Int J Cardiol 2016; 221: 644–651. [DOI] [PubMed] [Google Scholar]
- 12.Santiago de Araujo Pio C, Marzolini S, Pakosh M, et al. Effect of cardiac rehabilitation dose on mortality and morbidity: a systematic review and meta-regression analysis. Mayo Clin Proc 2017; 92: 1644–1659. [DOI] [PubMed] [Google Scholar]
- 13.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J 2011; 162: 571–584.e2. [DOI] [PubMed] [Google Scholar]
- 14.West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart (British Cardiac Society) 2012; 98: 637–644. [DOI] [PubMed] [Google Scholar]
- 15.Powell R, McGregor G, Ennis S, et al. Is exercise-based cardiac rehabilitation effective?: a systematic review and meta-analysis to re-examine the evidence. BMJ Open 2018; 8: e019656–e019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blokzijl F, Dieperink W, Keus F, et al. Cardiac rehabilitation for patients having cardiac surgery: a systematic review. J Cardiovasc Surg (Torino) 2018; 59: 817–829. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 19.Rauch B, Doherty P, Schmid J-P, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularization and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies. The Cardiac Rehabilitation Outcome Study (CROS). PROSPERO 2014 CRD42014007084. www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=7084 (2014, accessed 15 October 2019).
- 20.Wells GA, Shea B, Higgins JPT, et al. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Synth Methods 2013; 4: 63–77. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Ramsay C, Reeves BC, et al. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res Synth Methods 2013; 4: 12–25. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2008, accessed 20 May 2019).
- 23.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002; 55: 893–899. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT and Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://handbook.cochrane.org (2011).
- 27.Vries H de, Kemps HMC, van Engen-Verheul MM, et al. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J 2015; 36: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 28.Kim C, Kim DY, Moon CJ. Prognostic influences of cardiac rehabilitation in Korean acute myocardial infarction patients. Ann Rehabil Med 2011; 35: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meurs M, Burger H, van Riezen J, et al. The association between cardiac rehabilitation and mortality risk for myocardial infarction patients with and without depressive symptoms. J Affect Disord 2015; 188: 278–283. [DOI] [PubMed] [Google Scholar]
- 30.Aronov DM, Bubnova MG, Ioseliani DG, et al. The complex program of rehabilitation of patients with ischemic heart disease after coronary artery bypass surgery in ambulatory cardiorehabilitational department: clinical effects of third stage of rehabilitation. Kardiologiia 2017; 57: 10–19. [PubMed] [Google Scholar]
- 31.Schwaab B, Waldmann A, Katalinic A, et al. In-patient cardiac rehabilitation versus medical care – a prospective multicentre controlled 12 months follow-up in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil; official journal of the European Society of Cardiology, Working Groups on Epidemiology and Prevention and Cardiac Rehabilitation and Exercise Physiology 2011; 18: 581–586. [DOI] [PubMed] [Google Scholar]
- 32.Doimo S, Fabris E, Piepoli M, et al. Impact of ambulatory cardiac rehabilitation on cardiovascular outcomes: a long-term follow-up study. Eur Heart J 2019; 40: 678–685. [DOI] [PubMed] [Google Scholar]
- 33.Hautala AJ, Kiviniemi AM, Makikallio T, et al. Economic evaluation of exercise-based cardiac rehabilitation in patients with a recent acute coronary syndrome. Scand J Med Sci Sports 2017; 27: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 34.Prescott E, Mikkelsen N, Holdgaard A, et al. Cardiac rehabilitation in the elderly patient in eight rehabilitation units in Western Europe: baseline data from the EU-CaRE multicentre observational study. Eur J Prev Cardiol 2019; 26: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 35.Abell B, Glasziou P, Hoffmann T. The contribution of individual exercise training components to clinical outcomes in randomised controlled trials of cardiac rehabilitation: a systematic review and meta-regression. Sports Med – Open 2017; 3: 19–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumner J, Harrison A, Doherty P. The effectiveness of modern cardiac rehabilitation: a systematic review of recent observational studies in non-attenders versus attenders. PloS One 2017; 12: e0177658–e0177658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigorito C, Abreu A, Ambrosetti M, et al. Frailty and cardiac rehabilitation: a call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol 2017; 24: 577–590. [DOI] [PubMed] [Google Scholar]
- 38.Deaton C. Addressing the paradox of age and participation in cardiac rehabilitation. Eur J Prev Cardiol 2019; 26: 1050–1051. [DOI] [PubMed] [Google Scholar]
- 39.Bjarnason-Wehrens B, Nebel R, Jensen K, et al. Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: the Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): a systematic review and meta-analysis. Eur J Prev Cardiol. 2020; 27: 929–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: Cochrane Systematic Review, Meta-Analysis, and Trial Sequential Analysis. JACC Heart Fail 2019; 7: 691–705. [DOI] [PubMed] [Google Scholar]
- 41.Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail 2018; 20: 1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammill BG, Curtis LH, Schulman KA, et al. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation 2010; 121: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol 2003; 42: 2139–2143. [DOI] [PubMed] [Google Scholar]
- 44.Martin BJ, Arena R, Haykowsky M, et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc 2013; 88: 455–463. [DOI] [PubMed] [Google Scholar]
- 45.Dibben GO, Dalal HM, Taylor RS, et al. Cardiac rehabilitation and physical activity: systematic review and meta-analysis. Heart (British Cardiac Society) 2018; 104: 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen V, Gucht V de, Dusseldorp E, et al. Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2013; 20: 620–640. [DOI] [PubMed] [Google Scholar]
- 47.Kavanagh T, Hamm LF, Beyene J, et al. Usefulness of improvement in walking distance versus peak oxygen uptake in predicting prognosis after myocardial infarction and/or coronary artery bypass grafting in men. Am J Cardiol 2008; 101: 1423–1427. [DOI] [PubMed] [Google Scholar]
- 48.Espinosa Caliani S, Bravo Navas JC, Gómez-Doblas JJ, et al. Rehabilitación cardíaca postinfarto de miocardio en enfermos de bajo riesgo. Resultados de un programa de coordinación entre cardiología y atención primaria. Revista Espanola de Cardiologia 2004; 57: 53–59. [PubMed] [Google Scholar]
- 49.Lee JY, Ahn JM, Park DW, et al. Impact of exercise-based cardiac rehabilitation on long-term clinical outcomes in patients with left main coronary artery stenosis. Eur J Prev Cardiol 2016; 23: 1804–1813. [DOI] [PubMed] [Google Scholar]
- 50.Sunamura M, Ter Hoeve N, van den Berg-Emons RJG, et al. Cardiac rehabilitation in patients with acute coronary syndrome with primary percutaneous coronary intervention is associated with improved 10-year survival. Eur Heart J Qual Care Clin Outcomes 2018; 4: 168–172. [DOI] [PubMed] [Google Scholar]
- 51.Boulay P, Prud’homme D. Health-care consumption and recurrent myocardial infarction after 1 year of conventional treatment versus short- and long-term cardiac rehabilitation. Prev Med 2004; 38: 586–593. [DOI] [PubMed] [Google Scholar]
- 52.Norris CM, Jensen LA, Galbraith PD, et al. Referral rate and outcomes of cardiac rehabilitation after cardiac catheterization in a large Canadian city. J Cardiopulm Rehabil 2004; 24: 392–400. [DOI] [PubMed] [Google Scholar]
- 53.Kutner NG, Zhang R, Huang Y, et al. Cardiac rehabilitation and survival of dialysis patients after coronary bypass. J Am Soc Nephrol JASN 2006; 17: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 54.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120: 799–806. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen KM, Faergeman O, Foldspang A, et al. Cardiac rehabilitation: health characteristics and socio-economic status among those who do not attend. Eur J Public Health 2008; 18: 479–483. [DOI] [PubMed] [Google Scholar]
- 56.Alter DA, Oh PI, Chong A. Relationship between cardiac rehabilitation and survival after acute cardiac hospitalization within a universal health care system. Eur J Prev Cardiol Rehabil; official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology 2009; 16: 102–113. [DOI] [PubMed] [Google Scholar]
- 57.Hansen D, Dendale P, Leenders M, et al. Reduction of cardiovascular event rate: different effects of cardiac rehabilitation in CABG and PCI patients. Acta Cardiol 2009; 64: 639–644. [DOI] [PubMed] [Google Scholar]
- 58.Suaya JA, Stason WB, Ades PA, et al. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54: 25–33. [DOI] [PubMed] [Google Scholar]
- 59.Junger C, Rauch B, Schneider S, et al. Effect of early short-term cardiac rehabilitation after acute ST-elevation and non-ST-elevation myocardial infarction on 1-year mortality. Curr Med Res Opinion 2010; 26: 803–811. [DOI] [PubMed] [Google Scholar]
- 60.Goel K, Lennon RJ, Tilbury RT, et al. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011; 123: 2344–2352. [DOI] [PubMed] [Google Scholar]
- 61.Martin BJ, Hauer T, Arena R, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation 2012; 126: 677–687. [DOI] [PubMed] [Google Scholar]
- 62.Beauchamp A, Worcester M, Ng A, et al. Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart (British Cardiac Society) 2013; 99: 620–625. [DOI] [PubMed] [Google Scholar]
- 63.Lee HY, Kim JH, Kim BO, et al. Regular exercise training reduces coronary restenosis after percutaneous coronary intervention in patients with acute myocardial infarction. Int J Cardiol 2013; 167: 2617–2622. [DOI] [PubMed] [Google Scholar]
- 64.Marzolini S, Leung YW, Alter DA, et al. Outcomes associated with cardiac rehabilitation participation in patients with musculoskeletal comorbidities. Eur J Phys Rehabil Med 2013; 49: 775–783. [PubMed] [Google Scholar]
- 65.Pack QR, Goel K, Lahr BD, et al. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation 2013; 128: 590–597. [DOI] [PubMed] [Google Scholar]
- 66.Coll-Fernandez R, Coll R, Pascual T, et al. Cardiac rehabilitation and outcome in stable outpatients with recent myocardial infarction. Arch Phys Med Rehabil 2014; 95: 322–329. [DOI] [PubMed] [Google Scholar]
- 67.Prince DZ, Sobolev M, Gao J, et al. Racial disparities in cardiac rehabilitation initiation and the effect on survival. PM & R; the Journal of Injury, Function, and Rehabilitation 2013; 6: 486–492. [DOI] [PubMed] [Google Scholar]
- 68.Rauch B, Riemer T, Schwaab B, et al. Short-term comprehensive cardiac rehabilitation after AMI is associated with reduced 1-year mortality: results from the OMEGA study. Eur J Prev Cardiol 2014; 21: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 69.Goel K, Pack QR, Lahr B, et al. Cardiac rehabilitation is associated with reduced long-term mortality in patients undergoing combined heart valve and CABG surgery. Eur J Prev Cardiol 2015; 22: 159–168. [DOI] [PubMed] [Google Scholar]
- 70.Meurs M, Burger H, Riezen J, et al. The association between cardiac rehabilitation and mortality risk for myocardial infarction patients with and without depressive symptoms. J Affect Disord 2015; 188: 278–283. [DOI] [PubMed] [Google Scholar]
- 71.Schlitt A, Wischmann P, Wienke A, et al. Rehabilitation in patients with coronary heart disease: participation and its effect on prognosis. Dtsch Arztebl Int 2015; 112: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldmann A, Katalinic A, Schwaab B, et al. The TeleGuard trial of additional telemedicine care in CAD patients. 2 Morbidity and mortality after 12 months. J Telemed Telecare 2008; 14: 22–26. [DOI] [PubMed] [Google Scholar]
- 73.Barba R, Bisbe J, Pedrajas JNA, et al. Body mass index and outcome in patients with coronary, cerebrovascular, or peripheral artery disease: findings from the FRENA registry. Eur J Cardiovasc Prev Rehabil: Official journal of the European Society of Cardiology, Working Groups on Epidemiology and Prevention and Cardiac Rehabilitation and Exercise Physiology 2009; 16: 457–463. [DOI] [PubMed] [Google Scholar]
- 74.Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010; 122: 2152–2159. [DOI] [PubMed] [Google Scholar]
- 75.Spijkerman TA, van den Brink RHS, May JF, et al. Decreased impact of post-myocardial infarction depression on cardiac prognosis? J Psychosom Res 2006; 61: 493–499. [DOI] [PubMed] [Google Scholar]
- 76.van den Brink RHS, van Melle JP, Honig A, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: rationale and outline of the Myocardial INfarction and Depression-Intervention Trial (MIND-IT). Am Heart J 2002; 144: 219–225. [PubMed] [Google Scholar]
- 77.Schulz S, Schlitt A, Lutze A, et al. The importance of genetic variants in TNFα for periodontal disease in a cohort of coronary patients. J Clin Periodontol 2012; 39: 699–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CPR905719 Supplemental Material for Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II) by Annett Salzwedel, Katrin Jensen, Bernhard Rauch, Patrick Doherty, Maria-Inti Metzendorf, Matthes Hackbusch, Heinz Völler, Jean-Paul Schmid and Constantinos H Davos in European Journal of Preventive Cardiology