Abstract
Artemisinins are a unique class of antimalarial drugs with significant potential for drug repurposing for a wide range of diseases including cancer. Cancer is a leading cause of death globally and the majority of cancer related deaths occur in Low and Middle Income Countries (LMICs) where conventional treatment options are often limited by financial cost. Drug repurposing can significantly shorten new therapeutic discovery pathways, ensuring greater accessibility and affordability globally. Artemisinins have an excellent safety and tolerability profile as well as being affordable for deployment in Low and Middle Class Income Countries at around USD1 per daily dose. Robust, well designed clinical trials of artemisinin drug repurposing are indicated for a variety of different cancers and treatment settings.
Keywords: Artemisinins, Artesunate, Drug repurposing, Cancer, Oncology, Anti-cancer therapy
1. Introduction
Four decades since the discovery of artemisinins for the treatment of malaria by a collaborative research group led by Nobel Prize winner and eminent Chinese scientist Professor Youyou Tu, this class of drugs continue to fascinate and attract significant scientific interest as novel therapeutic agents that could be repurposed to treat a plethora of medical conditions. Artemisinins are a remarkable class of compounds that have shown promising cytotoxic effects against viruses, fungi and a variety of cancers as well as powerful anti-inflammatory effects in animal models of asthma, sepsis, arthritis, pancreatitis, systemic lupus erythematosus and haemorrhagic shock (Augustin, Krishna, Kumar, & Pantziarka, 2015; Efferth, 2017a; Efferth, 2018; Ho, Peh, Chan, & Wong, 2014; Krishna, Bustamante, Haynes, et al., 2008; Krishna, Ganapathi, Ster, et al., 2014; Mu & Wang, 2018; Shi, Li, Yang, & Hou, 2015; Sordi, Chiazza, Johnson, et al., 2015; Sordi, Nandra, Chiazza, et al., 2017; Wang, Wang, Jiang, et al., 2010). This diverse therapeutic potential has led to artemisinins being identified as potentially the new ‘aspirin’ (Krishna et al., 2008).
2. Artemisinins: From traditional Chinese medicine to anti-malarial blockbuster
Artemisinins are a family of sesquiterpene trioxane anti-malarial agents derived from Sweet wormwood (Artemisia annua L). Artesunate, artemether and arteether are derivatives of artemisinin that are wholly or partially converted into the active metabolite dihydroartemisinin (DHA) (Krishna et al., 2008). Sweet wormwood (qinghao) has been used in traditional Chinese medicine for two millenia to treat fevers and a variety of inflammatory conditions. Humanity has probably wrestled with the life-threatening global epidemic of malaria since as far back as 770 BCE with a detailed description of symptoms of malaria described in the Inner Canon of the Yellow Emperor, written around the time of the Chun Qiu and Qin Dynasties (770–207 B·C.) (Hong & fang, 2020). Renowned Chinese physician Ge Hong (284–363) listed qinghaosu as an essential remedy for fevers in Emergency Prescriptions Kept up one's Sleeve. In his treatise “On Airs, Waters, and Places” 400 B.C, Hippocrates described “agues” and “tertian fevers” frequently affecting populations living close to swamps and marshlands (Hippocrates, 1849). Although to date there are still over 200 million cases of malaria a year worldwide, the majority of these are now curable and will be treated with artemisinin combination therapy. This discovery represented a significant milestone in the history of medicine and infectious disease, a triumphant example of how scientific collaboration, commitment and traditional wisdom can converge to provide a breakthrough cure for a once lethal disease (Tu, 2015).
3. The global cancer pandemic
Whilst one epidemic may now have an elixir, another provides a global challenge for which we need an equally urgent response – the global pandemic of cancer. Cancer is now the second leading cause of death globally and accounts for an estimated 9.6 million deaths annually. Globally, cancer accounts for 1 in 6 deaths, with 70% of cancer related deaths occurring in low- and middle-income countries (LMICs) (Bray et al., 2018). In many LMICs cancer is a neglected disease with the majority of patients unable to afford novel diagnostics and effective therapeutics available in more affluent countries. Drug discovery and novel drug development takes on average 13–15 years to go from bench to bedside at an average cost of USD1-2 billion (Nosengo, 2016). Repurposing of ‘old’ drugs for new indications can shorten this pathway substantially with significant cost savings. Interestingly, drug repurposing/repositioning is not a new concept. The antimalarial quinine was used in the early 20th century as an anti-arrythmic agent to treat atrial fibrillation. Difluoromethylornithine (DFMO) which was originally developed as an anti-cancer therapy has been repurposed as a treatment for sleeping sickness. To date there are over 100 candidate drugs undergoing drug repurposing clinical trials for cancer.
The cancer burden crisis in LMICs is a humanitarian crisis that requires an urgent paradigm shift for which we (as a global community) need to utilise similar principles of multidisciplinary scientific collaboration, political commitment and research innovation that resulted in a malaria cure four decades ago. A global commitment to a dedicated programme of drug repurposing for cancer underpinned by the highest quality of robust scientific evidence and well designed clinical translational trials must form part of our roadmap to tackle this pandemic. In recent times the COVID-19 global pandemic has demonstrated how inter-reliant and connected we are as a global community. Highly coordinated, open and collaborative efforts to advance clinical translation research rapidly for affordable therapeutics through efforts such as the SOLIDARITY trial for COVID-19 have demonstrated the ability to ensure potentially life saving research in a humanitarian crisis is accessible globally (http://www.isrctn.com/ISRCTN83971151).
4. Artemisinins: Multiple modes of action against cancer
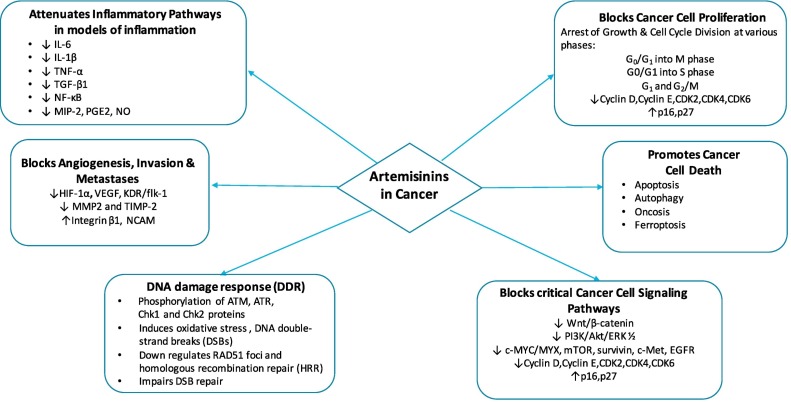
Carcinogenesis in humans is a complex process and 8 key hallmarks have been well described (Hanahan & Weinberg, 2011). These include sustained proliferative signaling, evasion of cell death and growth suppression, induction of angiogenesis, invasion and metastasis, reprogramming energy metabolism and evasion of immune destruction (Hanahan & Weinberg, 2011). In the last three decades, artemisinins have shown potent and broad anticancer properties in a range of cell lines and animal models, supporting the hypothesis that artemisinins have the potential to be developed as an effective anti-cancer therapy (Fig. 1 ). Several comprehensive reviews have examined the evidence for the anti-cancer effects of artemisinins (Efferth, 2017a; Ho et al., 2014; Krishna et al., 2008). Multiple potential mechanisms of action include anti-proliferative effects through cell-cycle disruption, reactive oxygen species (ROS) -induced DNA damage, induction of apoptosis, anti-angiogenesis, immunomodulation and induced radiosensitivity.
Fig. 1.
Artemisinins: multiple modes of action against cancer CDK2/4/6; Cyclin dependent kinase 2/4/6:mTOR; mammalian target of rapamycin: EGFR;epidermal growth factor: HIF---1α; Hypoxia Inducible Factor 1 alpha:VEGF; Vascular endothelial growth factor: KDR/flk---1;kinase insert domain receptor MMP2; Matrix Metallopeptidase 2: TIMP---2; Tissue inhibitor of metalloproteinases 2: NCAM; Neural Cell Adhesion Molecule: IL---6;interleukin---6: IL---1β; Interleukin---1β: TNF---α; Tumour necrosis factor---α: TGF---β1; Transforming growth factor beta 1: NF---κB; nuclear factor kappa---light---chain--- enhancer of activated B cells: MIP---2; Macrophage Inflammatory Protein 2: PGE2; Prostaglandin E2: NO;Nitric Oxide.
Studies of artemisinins in in-vitro experiments and animal models have demonstrated broad anti-cancer activity including pro-apoptotic, anti-proliferative, anti-angiogenesis and anti-metastatic effects (Efferth, 2017a; Krishna et al., 2008; Ho et al., 2014). Artesunate displays cytotoxic effects against numerous cancer cell lines including colon, breast, leukaemia, melanoma, central nervous system, ovarian, renal and prostate cancers (Efferth et al., 2003; Efferth et al., 2004; Efferth, Dunstan, Sauerbrey, Miyachi, & Chitambar, 2001; Efferth, Giaisi, Merling, Krammer, & Li-Weber, 2007; Nunes, Pandey, Yadav, Goel, & Ateeq, 2017). The active metabolite of artemisinins, dihydroartemisinin (DHA), has demonstrated antineoplastic effects in breast, glioma, colon, lung, ovarian, pancreatic, renal cell and leukaemia cancer cell lines (Chauhan, Min, & Kwon, 2017; Chen et al., 2017; Chen, Li, Zhang, & Wang, 2009; Hooft van Huijsduijnen et al., 2013; Kim et al., 2006; Kumar et al., 2017; Lu, Chen, Zhang, Ding, & Meng, 2011; Mu et al., 2007; Raza, Ghoshal, Chockalingam, & Ghosh, 2017; Singh & Lai, 2001; Wang et al., 2017).
Artesunate has been shown to promiscuously target multiple critical biological pathways and over 300 specific artesunate targets using a chemical proteomics approach with artemisinin-based activity probes (Wang et al., 2017). Cellular functions associated with these target proteins include growth and proliferation, cell death and survival, protein synthesis, fatty acid metabolism, cellular movement, free radical scavenging and energy metabolism. Pleiotropic alkylation targets in different cell lines raise questions about which ones are key to anticancer effects of artemisinins, and which ones may be bystanders to the main anticancer pathways. In depth mechanism of action studies in various cancer models are needed to better understand the nature of specific molecular targets in different cellular environments.
5. Artemisinins: Effects on the cell cycle
Cell growth and repair require progression through the cell cycle which is controlled by a series of cyclins and cyclin-dependent kinases. Inhibitors of cell division include the cip/kip family of inhibitory proteins p16, p21, p27 and p57. Studies have shown that artemisinins induce cell cycle arrest via a number of pathways. Artesunate was shown to markedly impede the growth of human breast cancer cell and nasopharyngeal cancer cell lines by inducing G1 cell cycle arrest. Artesunate was show to impede proliferation in colon, small cell lung cancer, leukaemia and glioma cell lines by inducing cell cycle arrest at G2/M phase (Steinbrück, Pereira, & Efferth, 2010). Artesunate induced cell cycle arrest at the G2/M phase and induced oncosis-like cell death in renal cell cancer (Jong Da et al., 2015). Decreased levels of cyclin B, cyclin D1 and transcription factor E2F1 were also observed.
In human nasopharyngeal cancer cells, artemisinin upregulated p16 and p27 and suppressed the level of cyclin D1, cyclin E, CDK2, CDK4 and CDK6 (Wu et al., 2011). Artemisinins also induce G1 cell cycle arrest by deactivating the retinoblastoma protein (pRb), a mediator of cell cycle progression and disrupt transcription of the cyclin CDK4 promoter in prostate cancer lines (Willoughby et al., 2009) DHA was found to inhibit cell cycle progression from G0/G1 into S phase in pancreatic cell lines via reduction of CDK2, CDK4 and CDK6, cyclin E and NF-κB. Levels of the p27 inhibitory protein were amplified (Chen et al., 2010). Artesunate induced radiosensitivity in cervical cancer cell lines by inducing apoptosis and G2/M cell cycle arrest (Luo et al., 2014; Roh, Kim, Jang, & Shin, 2017).
6. Artemisinins: Effects on cancer cell death pathways
Endoperoxide bridge cleavage and the formation of alkylating radicals are thought to be key mechanisms underlying the anti-cancer effects of artesunate (Efferth et al., 2003; Efferth et al., 2004; Wang et al., 2017). Cancer cells are highly proliferative, requiring a heavy iron load which acts as a cofactor in the synthesis of deoxyriboses prior to cell division (Daniels et al., 2012). Artemisinins induce cellular damage via the formation of reactive oxygen species (ROS) such as hydroxyl and superoxide anion radicals. When free iron is available, artemisinins are converted into a highly potent alkylating radical, capable of inducing direct oxidative damage in cancer cells. Other studies in model cell lines also implicate overexpression of the transferrin receptor and enhanced anti-cancer effects of artemisinins (Efferth et al., 2003; Efferth et al., 2004; Yang et al., 2014) Artemisinin–transferrin conjugates have higher anti-cancer efficacy than artemisinins alone, (Lai et al., 2009; Wang, Hou, Liu, et al., 2015; Nakase et al., 2009).
Apoptosis (programmed cell death) is maintained by a complex balance between a family of pro-apoptotic proteins (BAX, BAK, BAD, Bid) and anti-apoptotic proteins (Bcl-2 and Bcl-xl) (Hanahan & Weinberg, 2011). When cells detect DNA damage, tumour suppressor protein TP53 is upregulated, leading to increased levels of pro-apoptotic proteins and cytochrome c (caspase activator) leading to programmed cell death. Artemisinins can induce apoptosis in lung adenocarcinoma and prostate cancer cells lines via activation of BAX (Lu et al., 2009; Nakase et al., 2009). Artesunate can also induce apoptosis in breast cancer cell lines and leukemic T cells via iron dependent ROS formation resulting in cytochrome c release and cleavage of procaspases-2, 3, 8 and (Efferth et al., 2007; Hamacher-Brady et al., 2011). Artesunate can also inhibit colorectal cancer cell proliferation via activation of mitochondrial apoptosis by suppressing the fatty acid biosynthetic pathway and nuclear factor kappa-light-chain-enhancer of activated beta (NF-κB) pathway (Chen et al., 2017). NF-κB is a family of inducible transcription factors that play a pivotal role in DNA transcription, cell survival, cytokine production and inflammation.
Artesunate has also been shown to induce oncosis in renal cancer cell lines (Jong Da et al., 2015). Increased levels of calpain-1, calpain-2 expression and decreased levels of α-tubulin expression were exhibited in cell lines treated with artesunate. Calpains are a family of calcium-dependent cysteine proteases that exert proteolytic cleavage on a number of cellular substrates, including cytoskeletal proteins. In oncosis, ion pumps are affected by ATP depletion, leading to the collapse of mitochondrial potential resulting in cytomembrane destruction and the accumulation of reactive oxygen species(ROS) (Jong Da et al., 2015).
Ferroptosis is a novel mode of iron-dependent oxidative cell death characterized by the lethal accumulation of lipid-based reactive oxygen species (ROS). In a head and neck squamous cell cancer model, artesunate induced ferroptosis by decreasing cellular glutathione (GSH) levels and increasing lipid ROS levels (Chen et al., 2020; Roh et al., 2017).
Artemisinins have also been shown to regulate the process of autophagy (Sun et al., 2019). Autophagy is a degradative process of recycling organelles and cytoplasmic content in order to maintain cellular haemostasis and involves multiple signaling pathways. Cancer cells dysregulate autophagy as a tumour adaptation mechanism in nutrient-deplete tumour microenvironments (Sun et al., 2019) DHA has been shown to switch off activity of NF-κB, leading to upregulation of autophagy in several cancer cell lines. Accumulation of reactive oxygen species (ROS) through inhibition of NF-κB further stimulates autophagy. These various modes of cancer cell death are interlinked, for example, DHA can promote autophagic independent degradation of ferritin, resulting in free iron accumulation and dysgregulation of iron homeostasis that in turn can sensitized cancer cells to ferroptosis (Chen et al., 2020).
7. Artemisinins: Effects on critical cell signaling pathways
Artemisinins target several critical cell signal transduction pathways in cancer including inhibition of the Wnt/β-catenin pathway (Li et al., 2008; Li et al., 2007). The Wnt/beta-catenin cell signaling pathway has been identified as a critical pathway in colorectal carcinogenesis (Clevers, 2006). β-Catenin is a multifunctional protein that plays a vital role in physiological homeostasis and unregulated expression has been implicated in cancer development. β-Catenin has a role as a transcriptional co-regulator as well as an adaptor protein for intracellular adhesion. The chief regulator of β-catenin is Wnt, a family of 19 glycoproteins that regulate both β-catenin-dependent (canonical Wnt) and -independent (non-canonical Wnt) cell signaling pathways (Li et al., 2007). Artesunate was shown to inhibit the hyperactive Wnt/β-catenin pathway in colorectal cancer cell lines, thereby attenuating cancer cell growth (Li et al., 2007). Artesunate also suppressed proliferation and promoted apoptosis of colorectal cancer cells in a dose-dependent manner (Li et al., 2008). Immunohistochemical staining showed membranous translocation of regulator protein beta-catenin and inhibition of unrestricted activation of the Wnt/β-catenin pathway. In vivo studies showed that artesunate significantly slowed the growth of colorectal human tumour xenografts and delayed the development of liver metastases (Li et al., 2008). Artesunate has also been shown to induce apoptosis via inhibition of the Wnt/β-catenin pathway in myelodysplastic syndrome (MDS) cells (Xu et al., 2015).
Artemisinins also inhibit epidermal growth factor receptor (EGFR) as well as BCR/ABL signaling. Downregulation of cyclin D1, c-MYC/MYX, mTOR, survivin, c-Met, EGFR, Src, FAK, and α-tubulin expression have also been observed (Efferth, 2017a; Ho et al., 2014; Lee et al., 2012; Lu et al., 2011). Artesunate was found to induce oxidative deoxyribonucleic acid (DNA) damage, sustained DNA double-strand breaks and Ataxia telangiectasia mutated/Ataxia telangiectasis (ATM/ATR) and Rad-3 related protein damage response in glioblastoma cell lines (Berdelle et al., 2011). Artesunate was shown to be a powerful inducer of oxidative DNA damage, which was dose dependent and paralleled by cell death via apoptosis and necrosis. Artesunate was also shown to provoke a DNA damage response (DDR) with phosphorylation of ATM, ATR, Chk1 and Chk2 proteins. In an ovarian cancer cell line model, artesunate induced oxidative stress, DNA double-strand breaks (DSBs) and downregulation of RAD51 foci and homologous recombination repair (HRR), thereby impairing DSB repair (Wang et al., 2015).
8. Artemisinins: Anti-angiogenic effects
Angiogenesis plays a vital role in cancer cell growth and development. Isolated tumour cells without an adequate nutrition and oxygen supply display a growth restriction of about 1–2 mm3. Cancer cells require pro-angiogenic stimuli such as metalloproteinase (MMP) and vascular endothelial growth factor (VEGF) and a reduction in anti-angiogenic factors such thrombospondin and tissue inhibitor of metalloproteinase (TIMP) to maintain a viable blood supply required for growth (Hanahan & Weinberg, 2011). Under hypoxic conditions, hypoxia induced factor (HIF-1α) and NF-κB are activated resulting in transcription of VEGF and angiogenesis. Artemisinins were shown to reduce the levels of HIF-1α and VEGF in mouse embryonic stem cells (Wartenberg et al., 2003). In a mouse lung carcinoma model, administration of artemisinin resulted in a reduction in VEFG-C and lymphangiogenesis (Wang et al., 2008). Artesunate also suppressed angiogenesis and tumour growth and in a mouse model of Kaposi's sarcoma (KS-IMM) (Dell’Eva et al., 2004) and in a rat model of glioma model (Wu et al., 2009). Artesunate was also shown to exert anti-angiogenic effects on renal cancer cells in vitro in a dose-dependent manner (Jong Da et al., 2015). Following treatment with artesunate, renal cell tumours in a subcutaneous xenograft model showed decreased levels of proliferation marker Ki-67 and reductions in mean microvessel density compared to controls.
9. Artemisinins: Effects on tumour cell migration, invasion and metastasis
A key hallmark of cancer cells is their ability to detach from the primary tumour, degrade the extracellular matrix and metastasise through the bloodstream (Hanahan & Weinberg, 2011). Metalloproteinases (MMPs) play a critical role in tumour migration, invasion and metastases via degradation of the extracellular matrix. E-cadherin is an important cell adhesion molecule. One study in human melanoma cells, showed that artemisinin was able to reduce MMP2 levels thereby blocking cell migration (Buommino, Baroni, & Canozo, 2009). In human pancreatic and ovarian cancer cells lines, DHA suppressed the levels of MMP2, inhibiting NF-κB and metastases (Wang et al., 2011; Wu et al., 2012). Similarly, artesunate was shown to abrogate MMPs and NF-κB activity, thereby blocking metastases in non-small cell lung cancer lines. Artemisinins downregulated the levels of MMP2 and TIMP-2 whilst up-regulating E-cadherin in hepatocarcinoma cells lines (Weifeng et al., 2011). Artesunate has been shown to upregulate the expression of adhesion molecules integrin β1 and neural cell adhesion molecule (NCAM) in embryonal rhabdomyosarcoma cells, thereby reducing migration and invasion (Beccafico et al., 2015).
10. Artemisinins: Modulation of key inflammatory pathways
Inflammatory cytokines such as Interleukin-6 (IL-6), Interleukin-1β (IL-1β),Nuclear factor kappa B (NF-κB). Nitric Oxide (NO) and Tumour Necrosis Factor alpha (TNF—α) are key regulators of cancer development (Fishbein et al., 2020; West et al., 2015). Chronic inflammation in a key characteristic of carcinogenesis and inflammation in the tumour microenvironment leads to the release of pro-inflammatory cytokines and trigger oxidative stress, DNA damage and uncontrolled cell proliferation (Fishbein et al., 2020; Kay et al., 2019; Kiraly et al., 2015; Krewski et al., 2019).
Around a quarter of cancers are attributed to infectious agents that cause chronic inflammation including hepatocellular, bladder, gastric and cervical cancer (Fishbein et al., 2020). Tumour-promoting inflammation is also induced by chemical and environmental human carcinogens, such as tobacco, alcohol, asbestos, aflatoxins and nitrosamines. It is increasingly recognized that these carcinogens can also block the resolution of inflammation resulting in cancer development and progression (Karin and Greten, 2005; Krewski et al., 2019; Fishbein et al., 2020).
Inflammation-induced cell proliferation has been linked to stimulation of carcinogen-induced mutations, demonstrating that inflammation and DNA damage can act synergistically to induce mutations that further drive carcinoma progression and recurrence (Kiraly et al., 2015). Oxidative stress and chronic inflammation can lead to pro-tumorigenic feedback loops between inflammation, DNA damage and apoptosis which then contribute to cancer progression (Nowsheen et al., 2012). Inflammation enhances the production of cytokines that drive carcinogenesis such as IL-6, IL-1β and NF-κB.
NF-κB plays a vital role in driving inflammation, oxidative stress, regulation of apoptosis and cancer cell invasion, migration and metastasis (Fishbein et al., 2020; Karin & Greten, 2005; Shi et al., 2017; Chen et al., 2017). Toll-like receptors (TLR) signaling microbes, tissue damage, or primary cytokines activate NF-κB that then triggers the production of multiple pro-inflammatory cytokines including nitric oxide (NO) synthase, prostaglandin synthesis enzymes as well as pro-angiogenic and pro-tumorigenic mediators. TNF-α stimulates anti-apoptotic signals resulting in avoidance of cell death (Amicone and Marchetti, 2018).
IL-6-induced chronic inflammation also triggers STAT3/NF-κB signaling which plays an important role in various cancer related inflammatory processes, oxidative stress and evasion of apoptosis (Colotta et al., 2009). In addition to inflammation and oxidative stress, NF-κB plays a larger role in avoidance of apoptosis, which permits cancer cells to evade death while also allowing non-cancer cells to accumulate DNA damage, mutations, and increased compensatory proliferation (Yang et al., 2019).
A recent study in a mouse model showed that artesunate attenuated the haemorrhagic shock-induced activation of NF-κB and reduced the expression of pro-inflammatory proteins including NO, TNF-α and IL-6, protecting against multi-organ failure (Sordi et al., 2017). This may also have implications for the management of oncological patients in the pre and post surgical setting.
The anti-inflammatory effects of artesunate have also been investigated in animal models of myocardial ischaemia, acute lung injury and nephritis (Khan et al., 2018; Liu et al., 2018; Wan & Li, 2017; Zhao et al., 2017). In a rat model of transient myocardial ischemia, artesunate conferred myocardial protection via inhibition of glycogen synthase kinase- 3β, inhibition of NF-κB, activation of endothelial NOS, activation of the STAT3 (SAFE) pathway and activation of the PI3K/Akt/ERK 1/2 (RISK) pathway (Khan et al., 2018). In a lung sepsis model, artesunate inhibited release of lipopolysaccharide/endotoxin (LPS)-induced IL-6 and TNF-α from bone marrow-derived monocytes, peritoneal macrophages and a RAW264.7 mouse cell line (Zhao et al., 2017). In a rat model of nephritis, artesunate conferred protection against renal failure by attenuating levels of IL-6, TNF-α, TGF-β1, TLR4, and NF-κB expression (Wan & Li, 2017). In another rat model, artesunate inhibited renal reperfusion-stimulated lung inflammation by attenuating serum and pulmonary IL-6, MIP-2, PGE2, NO and MDA levels and activating the HO-1 pathway (Liu et al., 2018).
Findings from these pump priming studies exploring the anti-inflammatory effects of artesunate in a variety of clinical conditions should be used to inform the careful design of robust mechanism of action studies for cancer.
11. Future perspectives: Strategies to enhance artesunate cytotoxicity
A number of research groups have explored the potential for synergy between artesunate and other molecular compounds and drugs as part of novel combination therapy. As previously discussed, artesunate has been shown to promiscuously target multiple critical biological pathways and over 300 specific artesunate targets using a chemical proteomics approach with artemisinin-based activity probes (Wang et al., 2017). Cellular functions associated with these target proteins include growth and proliferation, cell death and survival, protein synthesis, fatty acid metabolism, cellular movement, free radical scavenging and energy metabolism. Differential cytotoxicity of artesunate against colorectal cancer (CRC) cells compared to normal colon epithelial cells has been linked to to the increased capacity of cancer cells to synthesise heme. The addition of heme synthesis precursor aminolevulinic acid (ALA) to artesunate treatment was found to dramatically enhance cytotoxicity in a mouse xenograft CRC model (Wang et al., 2017). The ability of artemisinins to target multiple cancer pathways may allow it to target various cancer types as well as evade chemoresistance.
Artesunate combination therapy with other existing established anticancer therapies has also shown synergistic effects in a number of cancer cell line models (Sun et al., 2019). Artesunate synergistically enhanced the inhibitory effect of tyrosine kinase inhibitor sorafenib on cell growth in HepG2 and Huh7 hepatocellular cancer cells lines (Li et al., 2019). Artesunate in combination with selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor erlotinib lead to additive or synergistic inhibition of cell growth across a range of glioblastoma multiforme cancer cell lines (Efferth, 2017b). Combination therapy with paclitaxel and artesunate has shown therapeutic synergy in breast cancer cell lines (Tran et al., 2017). Artesunate has also shown ability to restore sensitivity of castrate resistant prostate cancer cell lines and mouse models to anti-androgen therapy (Nunes et al., 2017). Tamoxifen–artemisinin and estradiol-artemisinin based hybrids have also shown anticancer activity against breast and prostate cancer cell lines (Fröhlich et al., 2020). Artemisinin activity does not appear to be influenced by drug resistance related genes such as P-gp, multidrug resistance-associated protein 1 (MRP1) and breast cancer resistance protein (BCRP) (Efferth et al., 2003). In cisplatin-resistant ovarian cancer cells, combination therapy with cisplatin and DHA induced cancer cell autophagy and resulted in an enhanced antiproliferation (Feng et al., 2014).
Target versatility combined with a high specificity for cancer cells, a low toxicity profile and the absence of cross-resistance to conventional chemotherapeutic agents suggests artesunate as a potent chemotherapeutic agent.
12. Clinical translational studies of artemisinins as anti-cancer therapies
One artemisinin in particular, artesunate, has been deployed in a number of clinical translational studies (Table 1 ). Artesunate has a hemisuccinate group which confers substantial water-solubility and high oral bioavailability and therefore a convenient oral route of administration. Artesunate is extensively hydrolysed by plasma esterases and possibly CYP2A6. Its main metabolite, DHA is metabolised through glucuronidation. The plasma elimination half-life of artesunate is 3–29 min whilst its active metabolite DHA has a plasma elimination half-life of 40 to 95 minutes (World Health Organisation Public Assessment Reports, 2011).
Table 1.
Clinical trials of artemisinins in cancer.
| Country | Study design | Dosing regimen | Study population | Outcome |
|---|---|---|---|---|
| China | Phase II open label randomised controlled trial | Vinorelbine and cisplatin chemotherapy +/− intravenous artesunate (120 mg OD IV) for 8 days (Zhang, Yu, Miao, Huang, et al., 2008) |
|
The rate of disease control rate in the trial group (88.2%) vs control group (72.7%) (p < 0.05). Progression free survival 24 weeks in artesunate treatment group compared to 20 weeks in the control arm (p < 0.05). |
| Ivory Coast | Phase I open label pilot study | In a Phase I pilot study of advanced cervical cancer, 10 patients with were treated with Dihydroartemisinin (DHA) for 28 days (Jansen et al., 2011) |
|
Patients reported symptomatic clinical benefit in terms of alleviation of vaginal discharge and pain. No G3/4 toxcities related to treatment recorded. Immunohistochemistry - tumour blocks showed a reduction in expression of p53, Epidermal growth factor receptor (EGFR), and antigen Ki-67. |
| United States | Phase I open label dose escalation study | Accelerated titration dose escalation study of IV artesunate with planned dose levels of 8, 12, 18, 25, 34 and 45 mg/kg given on days 1 and 8 of a 21-day cycle (Deeken et al., 2018) | Stage IV solid tumour malignancies | The maximum tolerated dose (MTD) in this study was determined to be 18 mg/kg. Four patients had stable disease, including three with prolonged stable disease for 8, 10, and 11 cycles, with a disease control rate of 27%. |
| United States | A Phase I dose-escalation study | Patients with CIN 2/3 received 1, 2, or 3 five-day treatment cycles (artesunate vaginal inserts) at study weeks 0, 2, and 4, respectively, prior to a planned, standard-of-care resection at study week 15 (Trimble et al., 2020) |
|
No serious adverse reactions were reported. Modified intention-to-treat analysis histologic regression 19/28 (67.9%) subjects. In patients whose lesions underwent histologic regression, clearance of HPV genotypes detected at baseline occurred in 9 of the 19 (47.4%) subjects. |
| Germany | Phase I open-label study | Oral artesunate 100, 150 or 200 mg daily as add-on therapy to 23 individual patients guideline-based oncological therapy for breast cancer for 4 ± 1 weeks. Following this, 13 patients continued the add-on therapy as compassionate use (von Hagens et al., 2019) |
|
No safety concerns. Cumulative total of 3825 treatment days were reported as a result. In individual patients up to 1115 cumulative treatment days (37 months) and cumulative artesunate doses up to 167.3 g were reached. |
| United Kingdom | Phase I randomised, double blind, placebo controlled trial | Neoadjuvant artesunate versus placebo for 14 days prior to surgery (Krishna, Ganapathi, Ster, et al., 2014) |
|
Neoadjuvant artesunate was safe, well tolerated, resulted in a reduction in Ki67 tumour proliferation index and conferred a potential survival advantage |
In a Phase II trial in advanced non-small cell lung cancer, patients were treated with standard combination vinorelbine and cisplatin +/− intravenous artesunate (120 mg OD IV) for 8 days (Zhang et al., 2008). Each treatment group consisted of 60 patients. The rate of disease control rate in the trial group (88.2%) was significantly higher than that of the control group (72.7%) (p < 0.05). Progression free survival was 24 weeks in patients treated with artesunate compared to 20 weeks in the control arm (p < 0.05). Artesunate was well tolerated and no dose limiting side effects were observed.
In a Phase I pilot study of advanced cervical cancer, 10 patients with Stage III or IV cervical cancer were treated with dihydroartemisinin for 28 days (Jansen et al., 2011). Patients reported symptomatic clinical benefit in terms of alleviation of vaginal discharge and pain and treatment was well tolerated. On immunohistochemical staining, tumour blocks showed a reduction in expression of p53, Epidermal growth factor receptor (EGFR), and antigen Ki-67.
A phase I study of intravenous artesunate in patients with advanced solid tumour malignancies enrolled 19 patients in an accelerated titration dose escalation study with planned dose levels of 8, 12, 18, 25, 34 and 45 mg/kg given on days 1 and 8 of a 21-day cycle (Deeken, Wang, Hartley, et al., 2018). The maximum tolerated dose (MTD) in this study was determined to be 18 mg/kg. Four patients had stable disease, including three with prolonged stable disease for 8, 10, and 11 cycles, for a disease control rate of 27%.
A Phase I dose-escalation study of artesunate vaginal inserts in biopsy-confirmed Cervical Intraepithelia Neoplasia (CIN)2/3 conducted in 28 patients showed that treatment was safe and well tolerated (Trimble et al., 2020). Patients with CIN 2/3 received 1, 2, or 3 five-day treatment cycles at study weeks 0, 2, and 4, respectively, prior to a planned, standard-of-care resection at study week 15. No serious adverse reactions were reported. In the modified intention-to-treat analysis histologic regression was observed in 19/28 (67.9%) subjects. In patients whose lesions underwent histologic regression, clearance of HPV genotypes detected at baseline occurred in 9 of the 19 (47.4%) subjects.
The ARTIC M33/2 reported long term results from compassionate open-label use of oral artesunate 100, 150 or 200 mg daily as add-on therapy to individual patients guideline-based oncological therapy for breast cancer (von Hagens et al., 2019). In Phase I of the trial, 23 patients received artesunate therapy for 4 ± 1 weeks. Following this, thirteen patients continued the add-on therapy as compassionate use. A cumulative total of 3825 treatment days were reported as a result. In individual patients up to 1115 cumulative treatment days (37 months) and cumulative artesunate doses up to 167.3 g were reached. Twenty five adverse events graded ≥2 and at least ‘possibly related’ to artesunate long-term add-on therapy were documented. However no major safety concerns were noted.
We conducted the first pilot randomised, double-blind, placebo-controlled trial in 20 patients with operable colorectal cancer and demonstrated that a 2 week course of neoadjuvant artesunate was safe, well tolerated, resulted in a reduction in Ki67 tumour proliferation index and conferred a potential survival advantage (Krishna, Ganapathi, Ster, et al., 2014). Two patients experienced Grade 3 neutropenia. Both cases were uncomplicated, with 1 case resolving spontaneously and 1 resolving with Granulocyte Colony Stimulating Factor (GCSF). Of note, both patients were of low body weight limit 50 kg in the pilot study (dose limiting side effects of artesunate on bone marrow suppression are seen at doses above 4 mg/kg). These results provided the basis for a Phase II randomised, double blind, placebo controlled trial of neoadjuvant artesunate versus placebo for 14 days in 200 patients (NeoART) (https://clinicaltrials.gov/ct2/show/NCT02633098) which is currently recruiting. Our primary end point is recurrence free survival 2 years after surgery. In the current Phase II NeoART study, we have set the lower limit in terms of body weight at 52 kg to reduce the risk of neutropenia. We also review patients on Day 7 and Day 14 with a toxicity check and full blood count test to monitor for neutropenia. A mirror study is also open to recruitment in Vietnam to test the potential effects of artesunate in a different ethnic and genetically diverse population (NeoART-V) (https://clinicaltrials.gov/ct2/show/NCT03093129).
13. Conclusion
In summary, artemisinins have an excellent safety and tolerability profile, having been used to treat tens of millions of adults and children globally. This class of drugs are off patent and affordable at USD1 per dose, thereby representing a feasible treatment option for patients in LMICs. An artemisinin drug repurposing programme for a number of cancer types, with a variety of clinical settings and combination therapies from pump priming window studies to Phase III clinical trials is urgently needed. This includes neoadjuvant, adjuvant and combination therapy, strategies for re-sensitisation of drug resistant cancers and radiosensitisation. Mechanism of action studies deploying proteomics, metabolomics, genomics and immunology studies will be central to understanding how artemisinins exert their anticancer effects and enable optimal deployment from bench to bedside. Once new anti-cancer indications for existing drugs are confirmed through robust clinical trials established pipelines must then be ready for licensing, technology transfer and local manufacturing in partner LMICs.
Declaration of Competing Interest
No competing interests declared.
Acknowledgment
HMS is supported by the Wellcome Trust Institutional Strategic Support Fund (204809/Z/16/Z) awarded to St. George's University of London.
References
- Amicone L., Marchetti A. Microenvironment and tumor cells: two targets for new molecular therapies of hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2018;3:24. doi: 10.21037/tgh.2018.04.05. PMID: 29971255; PMCID: PMC6002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin Y., Krishna S., Kumar D., Pantziarka P. The wisdom of crowds and the repurposing of artesunate as an anticancer drug. Ecancermedicalscience. 2015;9 doi: 10.3332/ecancer.2015.ed50. ed50. Published 2015 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccafico S., Morozzi G., Marchetti M.C., Riccardi C., Sidoni A., Donato R., Sorci G. Artesunate induces ROS- and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis. 2015;36(9):1071–1083. doi: 10.1093/carcin/bgv098. [DOI] [PubMed] [Google Scholar]
- Berdelle N., Nikolova T., Quiros S., Efferth T., Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Molecular cancer therapeutics. 2011;10(12):2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Buommino E, Baroni A, Canozo N. Artemisinin reduces human melanoma cell migration by downregulating αVβ3 iintegrin and reducing metalloproteinase- 2 production. Invest New Drugs. 2009;27:412–418. doi: 10.1007/s10637-008-9188-2. [DOI] [PubMed] [Google Scholar]
- Chauhan A.K., Min K.J., Kwon T.K. RIP1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma Caki cells. Molecular and Cellular Biochemistry. 2017 doi: 10.1007/s11010-017-3052-7. [DOI] [PubMed] [Google Scholar]
- Chen G.Q. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death and Differentiation. 2020;27:242–254. doi: 10.1038/s41418-019-0352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Sun B., Wang S., Pan S., Gao Y., Bai X., Xue D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. Journal of cancer research and clinical oncology. 2010;136(6):897–903. doi: 10.1007/s00432-009-0731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Li M., Zhang R., Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. Journal of Cellular and Molecular Medicine. 2009;13:1358–1370. doi: 10.1111/j.1582-4934.2008.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wong Y.K., Lim T.K., Lim W.H., Lin Q., Wang J., Hua Z. Artesunate activates the intrinsic apoptosis of HCT116 cells through the suppression of fatty acid synthesis and the NF-κB pathway. Molecules. 2017;22(8):1272. doi: 10.3390/molecules22081272. PMID: 28786914; PMCID: PMC6152404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. Epub 2009 May 25. PMID: 19468060. [DOI] [PubMed] [Google Scholar]
- Daniels T.R., Bernabeu E., Rodríguez J.A., Patel S., Kozman M., Chiappetta D.A.…Penichet M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochimica et biophysica acta. 2012;1820(3):291–317. doi: 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken J.F., Wang H., Hartley M. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemotherapy and Pharmacology. 2018;81(3):587–596. doi: 10.1007/s00280-018-3533-8. [DOI] [PubMed] [Google Scholar]
- Dell’Eva R., Pfeffer U., Vené R., Anfosso L., Forlani A., Albini A., Efferth T. Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochemical pharmacology. 2004;68(12):2359–2366. doi: 10.1016/j.bcp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in Cancer Biology. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Efferth T. Cancer combination therapy of the sesquiterpenoid artesunate and the selective EGFR-tyrosine kinase inhibitor erlotinib. Phytomedicine. 2017;37:58–61. doi: 10.1016/j.phymed.2017.11.003. Epub 2017 Nov 10. PMID: 29174651. [DOI] [PubMed] [Google Scholar]
- Efferth T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnology Advances. 2018;36(6):1730–1737. doi: 10.1016/j.biotechadv.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Efferth T., Benakis A., Romero M.R., Tomicic M., Rauh R., Steinbach D.…Marschall M. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radical Biology & Medicine. 2004;37(7):998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Efferth T., Dunstan H., Sauerbrey A., Miyachi H., Chitambar C. The anti-malarial artesunate is also active against cancer. International Journal of Oncology. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- Efferth T., Giaisi M., Merling A., Krammer P.H., Li-Weber M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T., Sauerbrey A., Olbrich A., Gebhart E., Rauch P., Weber H.O. Molecular modes of action of artesunate in tumor cell lines. Molecular Pharmacology. 2003;64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- Feng X., Li L., Jiang H., Jiang K., Jin Y., Zheng J. Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: Involvement of apoptosis and autophagy. Biochemical and Biophysical Research Communications. 2014;444(3):376–381. doi: 10.1016/j.bbrc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- Fishbein A., Hammock B.D., Serhan C.N., Panigrahy D. Carcinogenesis: Failure of resolution of inflammation? Pharmacology & Therapeutics. 2020:107670. doi: 10.1016/j.pharmthera.2020.107670. Epub ahead of print. PMID: 32891711; PMCID: PMC7470770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich T., Mai C., Bogautdinov R.P., Morozkina S.N., Shavva A.G., Friedrich O.…Tsogoeva S.B. Synthesis of tamoxifen-artemisinin and estrogen-artemisinin hybrids highly potent against breast and prostate cancer. ChemMedChem. 2020;15(15):1473–1479. doi: 10.1002/cmdc.202000174. Epub 2020 Jun 30. PMID: 32374071; PMCID: PMC7496903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher-Brady A., Stein H.A., Turschner S., Toegel I., Mora R., Jennewein N. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. The Journal of Biological Chemistry. 2011;286:6587–6601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hippocrates . 1849. On airs, waters, and places. 400 B.C. London: Sydenham Society; pp. 179–222. [Google Scholar]
- Ho W.E., Peh H.Y., Chan T.K., Wong W.S. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacology & Therapeutics. 2014;142(1):126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Hong G., fang Z.h.b.j. 2020. [Emergency Prescriptions kept up one’s Sleeve] Jin, 4th c. (340 AD). Si ku quan shu [Collection of the Works from the Four Storehouses] [Google Scholar]
- Hooft van Huijsduijnen R., Guy R.K., Chibale K., Haynes R.K., Peitz I., Kelter G. Anticancer properties of distinct antimalarial drug classes. PLoS One. 2013;8(12):e82962. doi: 10.1371/journal.pone.0082962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F.H., Adoubi I., Comoe J.C. First study of oral artenimol-R in advanced cervical cancer: Clinical benefit, tolerability and tumor marke rs. Anticancer Research. 2011;31:4417–4422. [PubMed] [Google Scholar]
- Jong Da E., Song H.J., Lim S., Lee S.J., Lim J.E., Nam D.H., Lee H.W. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget. 2015;6(32):33046–33064. doi: 10.18632/oncotarget.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Greten F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nature Reviews. Immunology. 2005;5(10):749–759. doi: 10.1038/nri1703. PMID: 16175180. [DOI] [PubMed] [Google Scholar]
- Kay J., Thadhani E., Samson L., Engelward B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst) 2019;83:102673. doi: 10.1016/j.dnarep.2019.102673. Epub 2019 Jul 25. PMID: 31387777; PMCID: PMC6801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.I., Kapoor A., Chen J. The antimalarial drug artesunate attenuates cardiac injury in a rodent model of myocardial infarction. Shock. 2018;49(6):675–681. doi: 10.1097/SHK.0000000000000963. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Kim M.S., Lee J.W., Lee C.H., Yoo H., Shin S.H. Dihydroartemisinin enhances radiosensitivity of human glioma cells in vitro. Journal of Cancer Research and Clinical Oncology. 2006;132:129–135. doi: 10.1007/s00432-005-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly O., Gong G., Olipitz W., Muthupalani S., Engelward B.P. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genetics. 2015;11(2):e1004901. doi: 10.1371/journal.pgen.1004901. PMID: 25647331; PMCID: PMC4372043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D., Rice J.M., Bird M., Milton B., Collins B., Lajoie P.…Zielinski J.M. Concordance between sites of tumor development in humans and in experimental animals for 111 agents that are carcinogenic to humans. Journal of Toxicology and Environmental Health. Part B, Critical Reviews. 2019;22(7–8):203–236. doi: 10.1080/10937404.2019.1642586. PMID: 31795923; PMCID: PMC7139235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S., Bustamante L., Haynes R.K. Artemisinins: Their growing importance in medicine. Trends in Pharmacological Sciences. 2008;29(10):520–527. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S., Ganapathi S., Ster I.C. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2014;2(1):82–90. doi: 10.1016/j.ebiom.2014.11.010. Published 2014 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Kalvala A., Chu S., Rosen S., Forman S.J., Marcucci G.…Pullarkat V. Antileukemic activity and cellular effects of the antimalarial agent artesunate in acute myeloid leukemia. Leukemia Research. 2017;59:124–135. doi: 10.1016/j.leukres.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Lai H., Nakase I., Lacoste E., Singh N.P., Sasaki Artemisinin–transferrin conjugate retards growth of breast tumors in the rat. Anticancer Research. 2009;29:3807–3810. [PubMed] [Google Scholar]
- Lee J., Zhang G., Wu X., Xu F., Zhou J., Zhang X. Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation. Journal of cancer research and clinical oncology. 2012;138(12):2095–2102. doi: 10.1007/s00432-012-1292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu K., Pian G., Sun S. Artesunate and sorafenib: Combinatorial inhibition of liver cancer cell growth. Oncology Letters. 2019;18(5):4735–4743. doi: 10.3892/ol.2019.10810. Epub 2019 Sep 5. PMID: 31611983; PMCID: PMC6781774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.N., Zhang H.D., Yuan S.J., Tian Z.Y., Wang L., Sun Z.X. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. International Journal of Cancer. 2007;121(6):1360–1365. doi: 10.1002/ijc.22804. [DOI] [PubMed] [Google Scholar]
- Li L.N., Zhang H.D., Yuan S.J., Yang D.X., Wang L., Sun Z.X. Differential sensitivity of colorectal cancer cell lines to artesunate is associated with expression of β-catenin and E-cadherin. European Journal of Pharmacology. 2008;588:1–8. doi: 10.1016/j.ejphar.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhang J., Li S., Jiang J. Artesunate inhibits renal ischemia reperfusion-stimulated lung inflammation in rats by activating HO-1 pathway. Inflammation. 2018;41(1):114–121. doi: 10.1007/s10753-017-0669-3. [DOI] [PubMed] [Google Scholar]
- Lu J.J., Chen S.M., Zhang X.W., Ding J., Meng L.H. The anti-cancer activity of dihydroartemisinin is associated with induction of iron-dependent endoplasmic reticulum stress in colorectal carcinoma HCT116 cells. Investigational New Drugs. 2011;29:1276–1283. doi: 10.1007/s10637-010-9481-8. [DOI] [PubMed] [Google Scholar]
- Lu Y.Y., Chen T.S., Qu J.L., Pan W.L., Sun L., Wei X.B. Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. Journal of biomedical science. 2009;16(1):16. doi: 10.1186/1423-0127-16-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zhu W., Tang Y., Cao H., Zhou Y., Ji R.…Cao J. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiation oncology (London, England) 2014;9:84. doi: 10.1186/1748-717X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D., Chen W., Yu B., Zhang C., Zhang Y., Qi H. Calcium and survivin are involved in the induction of apoptosis by dihydroartemisinin in human lung cancer SPC-A-1 cells. Methods and Findings in Experimental and Clinical Pharmacology. 2007;29(1):33–38. doi: 10.1358/mf.2007.29.1.1063493. [DOI] [PubMed] [Google Scholar]
- Mu X., Wang C. Artemisinins-a promising new treatment for systemic lupus erythematosus: a descriptive review. Current Rheumatology Reports. 2018;20(9):55. doi: 10.1007/s11926-018-0764-y. Published 2018 Jul 28. [DOI] [PubMed] [Google Scholar]
- Nakase I., Gallis B., Takatani-Nakase T., Oh S., Lacoste, Singh N. Transferrin receptor-dependent cytotoxicity of artemisinin–transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Letters. 2009;274:290–298. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534(7607):314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- Nowsheen S., Aziz K., Kryston T.B., Ferguson N.F., Georgakilas A. The interplay between inflammation and oxidative stress in carcinogenesis. Current Molecular Medicine. 2012;12(6):672–680. doi: 10.2174/156652412800792642. PMID: 22292435. [DOI] [PubMed] [Google Scholar]
- Nunes J.J., Pandey S.K., Yadav A., Goel S., Ateeq B. Targeting NF-kappa B signaling by artesunate restores sensitivity of castrate-resistant prostate cancer cells to antiandrogens. Neoplasia. 2017;19(4):333–345. doi: 10.1016/j.neo.2017.02.002. (Epub 2017 Mar 19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A., Ghoshal A., Chockalingam S., Ghosh S.S. Connexin-43 enhances tumor suppressing activity of artesunate via gap junction-dependent as well as independent pathways in human breast cancer cells. Scientific Reports. 2017;7(1):7580. doi: 10.1038/s41598-017-08058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.L., Kim E.H., Jang H., Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biology. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Li H., Yang Y., Hou L. Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Mediators of Inflammation. 2015;2015:435713. doi: 10.1155/2015/435713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Chen F., Zhang X., Clinton S.K., Tang X., Sun Z., Chen T. Suppression of oxidative stress and NFκB/MAPK signaling by lyophilized black raspberries for esophageal cancer prevention in rats. Nutrients. 2017;9(4):413. doi: 10.3390/nu9040413. PMID: 28441719; PMCID: PMC5409752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.P., Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sciences. 2001;70:49–56. doi: 10.1016/s0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- Sordi R., Chiazza F., Johnson F.L. Inhibition of IκB kinase attenuates the organ injury and dysfunction associated with hemorrhagic shock. Molecular Medicine. 2015;21(1):563–575. doi: 10.2119/molmed.2015.00049. Published 2015 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordi R., Nandra K.K., Chiazza F. Artesunate protects against the organ injury and dysfunction induced by severe hemorrhage and resuscitation. Annals of Surgery. 2017;265(2):408–417. doi: 10.1097/SLA.0000000000001664. [DOI] [PubMed] [Google Scholar]
- Steinbrück L., Pereira G., Efferth T. Effects of artesunate on cytokinesis and G₂/M cell cycle progression of tumour cells and budding yeast. Cancer genomics & proteomics. 2010;7(6):337–346. [PubMed] [Google Scholar]
- Sun X., Yan P., Zou C., Wong Y.K., Shu Y., Lee Y.M.…Zhang J. Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Medicinal Research Reviews. 2019;39(6):2172–2193. doi: 10.1002/med.21580. Epub 2019 Apr 11. PMID: 30972803. [DOI] [PubMed] [Google Scholar]
- Tran B.N., Nguyen H.T., Kim J.O., Yong C.S., Nguyen C.N. Developing combination of artesunate with paclitaxel loaded into poly-d,l-lactic-co-glycolic acid nanoparticle for systemic delivery to exhibit synergic chemotherapeutic response. Drug Development and Industrial Pharmacy. 2017;43(12):1952–1962. doi: 10.1080/03639045.2017.1357729. Epub 2017 Aug 3. PMID: 28724314. [DOI] [PubMed] [Google Scholar]
- Trimble C.L., Levinson K., Maldonado L. A first-in-human proof-of-concept trial of intravaginal artesunate to treat cervical intraepithelial neoplasia 2/3 (CIN2/3) Gynecologic Oncology. 2020;157(1):188–194. doi: 10.1016/j.ygyno.2019.12.035. [DOI] [PubMed] [Google Scholar]
- Tu Y. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences; Beijing, China: 2015. Artemisinin — A gift from traditional Chinese medicine to the world. Nobel Lecture, December 7.https://www.nobelprize.org/uploads/2018/06/tu-lecture.pdf [Google Scholar]
- von Hagens C., Walter-Sack I., Goeckenjan M. Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2) Phytomedicine. 2019;54:140–148. doi: 10.1016/j.phymed.2018.09.178. [DOI] [PubMed] [Google Scholar]
- Wan R.J., Li Y.H. Effects of artesunate prevent nephritis via the toll-like receptor 4/nuclear factor-κB signaling pathway in rats. Molecular Medicine Reports. 2017;16(5):6389–6395. doi: 10.3892/mmr.2017.7362. [DOI] [PubMed] [Google Scholar]
- Wang B., Hou D., Liu Q. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biology & Therapy. 2015:1548–1556. doi: 10.1080/15384047.2015.1071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang H., Jiang B. Artesunate relieves lupus nephritis by inhibiting the expression of ICAM-1. Journal of Clinical Medicine in Practice. 2010;14(17):1–3. doi: 10.3969/j.issn.1672-2353.2010.17.001. [DOI] [Google Scholar]
- Wang J., Zhang B., Guo Y., Li G., Xie Q., Zhu B.…Chen Z. Artemisinin inhibits tumor lymphangiogenesis by suppression of vascular endothelial growth factor C. Pharmacology. 2008;82(2):148–155. doi: 10.1159/000148261. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang J., Shi Y., Xu C., Zhang C., Wong Y.K.…Lin Q. Mechanistic investigation of the specific anticancer property of artemisinin and its combination with aminolevulinic acid for enhanced anticolorectal cancer activity. ACS Central Science. 2017;3(7):743–750. doi: 10.1021/acscentsci.7b00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Sun B, Cheng ZX, Zho HX, Gao Y, Kong R. Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by targeting the NFXκB pathway. Cancer Chemother Pharmacol. 2011;68:1421–1430. doi: 10.1007/s00280-011-1643-7. [DOI] [PubMed] [Google Scholar]
- Wartenberg M., Wolf S., Budde P., Grünheck F., Acker H., Hescheler J.…Sauer H. The antimalaria agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Laboratory investigation; a journal of technical methods and pathology. 2003;83(11):1647–1655. doi: 10.1097/01.lab.0000098424.38003.ff. [DOI] [PubMed] [Google Scholar]
- Weifeng T, Feng S, Xiangji L, Changqing S, Zhiquan Q, Huazhong Z. Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine. 2011;18:158–162. doi: 10.1016/j.phymed.2010.07.003. [DOI] [PubMed] [Google Scholar]
- West N.R., McCuaig S., Franchini F., Powrie F. Emerging cytokine networks in colorectal cancer. Nature Reviews. Immunology. 2015;15(10):615–629. doi: 10.1038/nri3896. (Epub 2015 Sep 11) [DOI] [PubMed] [Google Scholar]
- Willoughby J.A., SrSundar S.N., Cheung M., Tin A.S., Modiano J., Firestone G.L. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. The Journal of biological chemistry. 2009;284(4):2203–2213. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation Public Assessment Reports Summary of product characteristics for Artesunate. 2011. http://apps.who.int/prequal/whopar/whoparproducts/MA044part4v2.pdf
- Wu B, Hu K, Li S, Zhu J, Gu L, Shen H. Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Reports. 2012;27:101–108. doi: 10.3892/or.2011.1505. [DOI] [PubMed] [Google Scholar]
- Wu J., Hu D., Yang G., Zhou J., Yang C., Gao Y., Zhu Z. Down-regulation of BMI-1 cooperates with artemisinin on growth inhibition of nasopharyngeal carcinoma cells. Journal of cellular biochemistry. 2011;112(7):1938–1948. doi: 10.1002/jcb.23114. [DOI] [PubMed] [Google Scholar]
- Wu Z.P., Gao C.W., Wu Y.G., Zhu Q.S., Chen Yan, Liu Xin, Chuen Liu Inhibitive effect of artemether on tumor growth and angiogenesis in the rat C6 orthotopic brain gliomas model. Integrative cancer therapies. 2009;8(1):88–92. doi: 10.1177/1534735408330714. [DOI] [PubMed] [Google Scholar]
- Xu N., Zhou X., Wang S., Xu L.L., Zhou H.S., Liu X.L. Artesunate Induces SKM-1 Cells Apoptosis by Inhibiting Hyperactive β-catenin Signaling Pathway. International journal of medical sciences. 2015;12(6):524–529. doi: 10.7150/ijms.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N.D., Tan S.H., Ng S., Shi Y., Zhou J., Tan K.S.…Shen H.M. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. The Journal of Biological Chemistry. 2014;289(48):33425–33441. doi: 10.1074/jbc.M114.564567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.M., Kim S.Y., Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Seminars in Liver Disease. 2019;39(1):26–42. doi: 10.1055/s-0038-1676806. Epub 2019 Jan 17. PMID: 30809789; PMCID: PMC6616367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Y., Yu S.Q., Miao L.Y., Huang X.Y., Zhang X.P., Zhu Y.P.…Li D.Q. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2008;6(2):134–138. doi: 10.3736/jcim20080206. Chinese. PMID: 18241646. [DOI] [PubMed] [Google Scholar]
- Zhao D., Zhang J., Xu G., Wang Q. Artesunate protects LPS induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation. 2017;40(3):798–805. doi: 10.1007/s10753-017-0524-6. [DOI] [PubMed] [Google Scholar]