Abstract
Influenza remains a major cause of illness and death in geriatric populations. While the influenza vaccine has successfully reduced morbidity and mortality, its effectiveness is suspected to decrease with age. The aim of this study was to assess the impact of influenza vaccination on all-cause mortality in very old ambulatory subjects. We conducted a prospective cohort study from 1 July 2016 to 31 June 2017 in a large unselected ambulatory population aged over 80 years. We compared all-cause mortality in vaccinated versus unvaccinated subjects after propensity-score matching, to control for age, sex and comorbidities. Among the 9149 patients included, with mean age 86 years, 4380 (47.9%) were vaccinated against influenza. In total, 5253 (57.4%) had at least one chronic disease. The most commonly vaccinated patients were those with chronic respiratory failure (76.3%) and the least commonly vaccinated were those suffering from Parkinson’s disease (28.5%). Overall, 2084 patients (22.8%) died during the study. After propensity score matching, the mortality was evaluated at 20.9% in the vaccinated group and 23.9% in the unvaccinated group (OR = 0.84 [0.75–0.93], p = 0.001). This decrease in mortality in the vaccinated group persisted whatever the age and Charlson Comorbidity index. In conclusion, nearly a half of this ambulatory elderly population received Influenza vaccine. After adjustment on comorbidities, influenza vaccination was associated with a significant decrease in all-cause mortality, even in the eldest multimorbid population. Improving immunization coverage in this frail older population is urgently needed.
Keywords: flu, influenza, mortality, influenza vaccination, elderly, multimorbidity, comorbidities
1. Introduction
Influenza is a leading cause of hospitalization and death in older patients [1,2]. Every year, 290,000–650,000 people die from influenza worldwide [3,4] and 90% of these deaths occur among older adults [1,5]. In addition to the prevention of infection-related morbidity and mortality, it is now well established that influenza vaccine has a protective effect on decompensation due to underlying diseases, especially cardiovascular diseases [6,7]. Several studies have assessed the effectiveness of the influenza vaccine in preventing influenza illness and hospital admissions [8,9,10,11]. Additional recent studies have confirmed a reduction in all-cause mortality as well as influenza-related hospitalizations in elderly vaccinated individuals [12,13].
However, influenza vaccine effectiveness may decrease with advanced age [8] and the effect of vaccination on deaths from any cause is not confirmed [14]. Despite an increase in immunization coverage over time, the percentage of influenza-related deaths in winter is stable at around 5–10% [5,15]. Furthermore, vaccine efficacy is thought to be reduced in older age due to immunosenescence [16], inadequate immunization coverage and inappropriate vaccine formulation for the specific immune profile of older subjects [17]. In observational studies, comorbidities appear as confounding factors between influenza vaccination and mortality and should be more investigated to reduce biases and identify the most at-risk populations [18,19,20]. To assess the impact of vaccination on all-cause mortality, we conducted a prospective cohort study in a community-living population aged over 80 years.
2. Materials and Methods
2.1. Study Design
This prospective cohort study was conducted from 1 July 2016 to 31 June 2017, from the Mutualité Sociale Agricole de Franche-Comté (MSA) database. MSA is a French regional health insurance plan for active or retired agricultural workers that covers health costs, daily allowances and carries out preventive actions. This database prospectively records all prescriptions and chronic diseases reported by the referring physician, as these conditions (Affection Longue Durée) are fully covered by the French health insurance, leading to a systematic reimbursement of related health expenses.
The present study complied with the Declaration of Helsinki. Data were anonymously managed. No consent was required.
2.2. Patients
All patients registered in MSA and aged over 80 years were included and then divided into two groups: a group of patients for whom a prescription of influenza vaccine was delivered during the period of the study (vaccinated group) and a group with no influenza vaccine prescription (unvaccinated group).
2.3. Data Collection
Age, gender and chronic diseases (according to the International Statistical Classification of Diseases and Related Health Problems—10th edition (ICD-10)), Charlson Comorbidity Index (CCI) [21] and vital status at the end of the study period were recorded for all patients. Multimorbidity was defined as a CCI ≥ 5.
Pneumococcal vaccination and antibiotic prescription during the period of study were also collected.
2.4. Outcome
The primary outcome was adjusted all-cause mortality during the study period.
2.5. Statistical Analyses
Vaccinated and unvaccinated groups were compared using univariate and multivariable analysis. Continuous variables were expressed as medians and interquartile ranges. A Kolmogorov–Smirnov test was performed to analyze the normality of continuous variables. The Student t-test or the Mann–Whitney test was used to compare continuous variables, and the Chi2 or Fisher test was used to compare dichotomous data, as appropriate.
Given the non-randomized design of the study, we used a propensity score (PS) to identify and control for confounding factors that could influence the likelihood of vaccination. A multivariable logistic regression model was built to estimate vaccination risk and calculate the PS for vaccination. The variables included in the multivariable model with a threshold at 5% were: age, gender, stroke, peripheral arterial disease, heart failure, diabetes, hypertension, coronary artery disease, respiratory failure, Parkinson’s disease, chronic kidney disease, neoplasia, other chronic diseases, number of chronic diseases and CCI.
The patients with and without vaccination were then paired 1:1 on this PS using a caliper width of 5% of the standard deviation of the PS logit.
We used the SPSS 13.0 software package (IBM Corp., Armonk, N.Y., USA) for all analyses.
3. Results
3.1. Patients Characteristics
Among the 9149 patients included, the median age was 86.5 years [83.5–90.2]; women represented 60.9% of the population.
For vaccination status, 47.9% were vaccinated against influenza and 2.5% against pneumococcal infections. The characteristics of the subjects vaccinated against influenza and unvaccinated, before and after matching, are presented in Table 1. Concerning comorbidities, 57.4% patients had at least one or more chronic diseases. The most common chronic diseases were: chronic heart failure (19.8%), diabetes (11.0%), cancer (10.7%), coronary artery disease (8.1%), cognitive disorders (6.5%), peripheral arterial disease (5.7%) and stroke (4.3%). Multimorbidity (CCI ≥ 5) was identified in 58.7% of patients.
Table 1.
Comparison of vaccinated and unvaccinated subjects against influenza before and after matching.
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Vaccinated | Unvaccinated | p | Vaccinated | Unvaccinated | p |
| n = 4380 | n = 4769 | n = 3935 | n = 3935 | |||
| Age | 86.5 [83.5–90.3] |
86.5 [83.5–90.1] |
0.8 | 86.6 [83.5–90.1] |
86.5 [83.5–90.2] |
0.7 |
| 80–90 years | 3248 (74.2) | 3488 (73.1) | 0.3 | 2919 (74.2) | 2891 (73.5) | 0.5 |
| >90 years | 1132 (25.8) | 1281 (26.9) | 0.3 | 1016 (25.8) | 1044 (26.5) | 0.5 |
| Sex | ||||||
| Female | 2538 (57.9) | 3038 (63.7) | <0.001 | 2356 (59.9) | 2348 (59.7) | 0.9 |
| Male | 1842 (42.1) | 1731 (36.3) | <0.001 | 1579 (40.1) | 1587 (40.3) | 0.9 |
| Pneumococcal vaccination | 154 (3.5) | 74 (1.6) | <0.001 | 83 (2.1) | 73 (1.9) | 0.4 |
| Chronic disease | ||||||
| Stroke | 218 (5.0) | 178 (3.7) | 0.003 | 165 (4.2) | 159 (4.0) | 0.7 |
| Peripheral arterial disease | 287 (6.6) | 234 (4.9) | 0.001 | 227 (5.8) | 227 (5.8) | 1.0 |
| Heart failure | 962 (22.0) | 849 (17.8) | <0.001 | 817 (20.8) | 769 (19.5) | 0.2 |
| Chronic liver disease | 4 (0.1) | 6 (0.1) | 0.6 | 4 (0.1) | 5 (0.1) | 0.7 |
| Diabetes | 528 (12.1) | 481 (10.1) | 0.003 | 453 (11.5) | 437 (11.1) | 0.6 |
| Hypertension | 223 (5.1) | 161 (3.4) | <0.001 | 171 (4.3) | 161 (4.1) | 0.6 |
| Coronary artery disease | 387 (8.8) | 354 (7.4) | 0.01 | 343 (8.7) | 337 (8.6) | 0.8 |
| Respiratory failure | 151 (3.4) | 47 (1.0) | <0.001 | 57 (1.4) | 46 (1.2) | 0.3 |
| Cognitive disorders | 282 (6.4) | 309 (6.5) | 0.9 | 261 (6.6) | 280 (7.1) | 0.4 |
| Parkinson’s disease | 45 (1.0) | 113 (2.4) | <0.001 | 44 (1.1) | 28 (0.7) | 0.06 |
| Chronic kidney disease | 71 (1.6) | 46 (1.0) | 0.005 | 52 (1.3) | 46 (1.2) | 0.5 |
| Psychiatric disorders | 62 (1.4) | 70 (1.5) | 0.8 | 56 (1.4) | 56 (1.4) | 1.0 |
| Neoplasia | 516 (11.8) | 464 (9.7) | 0.002 | 443 (11.3) | 431 (11.0) | 0.7 |
| Other chronic diseases | 197 (4.5) | 151 (3.2) | 0.001 | 150 (3.8) | 149 (3.8) | 1.0 |
| Other neurological diseases | 31 (0.7) | 32 (0.7) | 0.8 | 28 (0.7) | 29 (0.7) | 0.9 |
| Immunosuppression | 87 (2.0) | 88 (1.8) | 0.6 | 74 (1.9) | 76 (1.9) | 0.9 |
| Number of chronic diseases | ||||||
| 0 | 1677 (38.3) | 2219 (46.5) | <0.001 | 1633 (41.5) | 1690 (42.9) | 0.5 |
| 1 | 1651 (37.7) | 1709 (35.8) | <0.001 | 1459 (37.1) | 1451 (36.9) | 0.5 |
| 2 | 782 (17.9) | 629 (13.2) | <0.001 | 643 (16.3) | 602 (15.3) | 0.5 |
| 3 | 290 (6.2) | 212 (4.4) | <0.001 | 200 (5.1) | 192 (4.9) | 0.5 |
| Charlson Comorbidity Index | 5 [4,5] | 5 [4,6] | <0.001 | 5 [4,5] | 5 [4,6] | 0.8 |
| <5 | 1696 (38.7) | 2087 (43.8) | <0.001 | 1625 (41.3) | 1612 (41.0) | 0.8 |
| ≥5 | 2684 (61.3) | 2682 (56.2) | <0.001 | 2310 (58.7) | 2323 (59.0) | 0.8 |
| Deaths | 935 (21.3) | 1149 (24.1) | 0.002 | 827 (21.0) | 943 (24.0) | 0.002 |
3.2. Vaccination Coverage
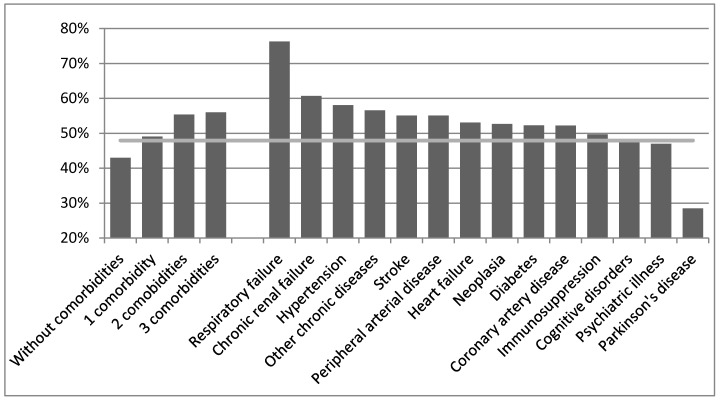
The total influenza vaccination coverage of the study population was 47.9%. The most commonly vaccinated subjects were those with chronic respiratory failure (76.3%) and those with chronic renal failure (60.7%). The least commonly vaccinated were those with Parkinson’s disease (28.5%). The results of influenza vaccine coverage by group of chronic conditions and by number of comorbidities are presented in Figure 1.
Figure 1.
Influenza vaccine coverage according to comorbidities during the 2016–2017 season. The grey line indicates the mean rate of vaccination in the whole population.
3.3. Mortality
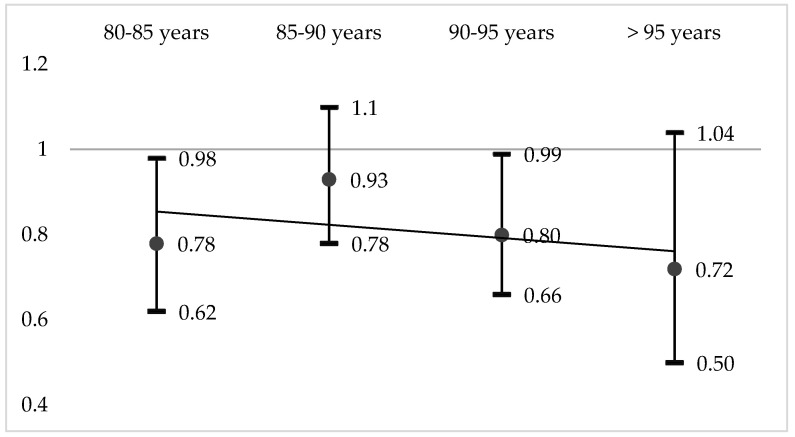
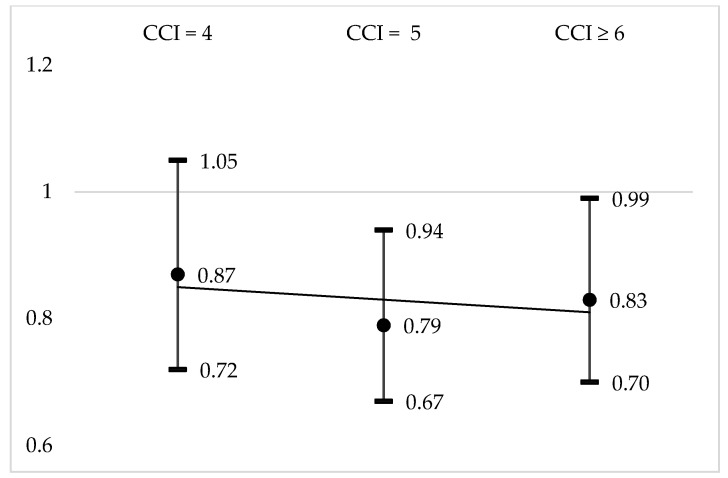
In total, 2084 subjects (22.8%), mean age 89.6 years, died during the study period. After matching on the propensity score, mortality was 20.9% in the vaccinated group and 23.9% in the unvaccinated group (OR = 0.84 [0.75–0.93], p = 0.001). Adjusted odds ratios of risk of mortality in vaccinated versus non-vaccinated groups according to age and CCI classes are presented in Figure 2 and Figure 3, respectively. As highlighted, mortality remained lower whatever the age and comorbidities in the vaccinated group compared to the non-vaccinated group.
Figure 2.
Adjusted odds ratio (95% Confidence interval) of risk of mortality in vaccinated versus non-vaccinated groups according to age (trend line in grey).
Figure 3.
Adjusted odds ratio (95% Confidence interval) of risk of mortality in vaccinated versus non-vaccinated groups according to Charlson Comorbidity Index (CCI) (trend line in grey).
4. Discussion
Older adults and those with underlying comorbidities, including chronic cardiovascular and lung diseases, immunosuppression and diabetes, have a higher risk of hospitalization and severe disease, and are consequently priority groups for influenza vaccination [22]. However, in our oldest multimorbid population at the highest risk of influenza complications, less than a half was vaccinated. The WHO goal of vaccinating 75% of high-risk patients was achieved only for the subgroup of patients with chronic respiratory failure.
However, our study suggests that influenza vaccination is associated with lower all-cause mortality in very elderly and comorbid subjects. Our results are consistent with those of other studies. In particular, Voordouw et al. showed that annual influenza vaccination and revaccination were associated with a reduction in all-cause mortality risk, similarly in healthy and comorbid subjects of 80 years and older (hazard ratio 0.81, 95% interval confidence: 0.66–1.00; and 0.69, 0.61–0.78, respectively) [23]. In accordance with our results, a recent study in an elderly Italian population highlighted that influenza vaccine was equally effective for death prevention in all age groups (≤74, 75–84 and ≥85 years old) [24]. Other authors, in a large cohort study of subjects over 65 years of age, noted a decrease in mortality of 14%, 19% and 1% associated with vaccination during three consecutive winter seasons between 1998 and 2001 [25]. Nevertheless, some other studies, after adjustment by age and type of circulating virus, failed to highlight any decrease in influenza-related excess mortality per year, despite an increase in vaccination coverage over time [5,15]. This implies an overestimation of vaccination benefit in the very elderly and the existence of bias in observational studies evaluating vaccine efficacy.
Currently, for ethical reasons, only observational studies can assess the effectiveness of influenza vaccine within a population. Nevertheless, one randomized placebo-controlled trial was carried out during the 1991–1992 season in patients over the age of 60 in Holland [8]. In this study, vaccine efficacy was 50% (95% CI 39–65%) against influenza confirmed by serology, but only 23% (95% CI 51–61%) in subjects 70 years and over, suggesting a decrease in efficiency with increasing age. More recently, meta-analyses have evaluated an influenza vaccine efficacy of 50% (95% CI 45–56%) and 47% (95% CI 39–54%) to prevent all-cause deaths in subjects 65 and over [10,11]. By contrast, the Cochrane Database of Systematic Reviews published a meta-analysis in 2018 showing no reduction in mortality from influenza or all causes in elderly subjects vaccinated against influenza (RR 1.02, 95% CI 0.11–9.72) [14]. Additionally, authors have highlighted the existence of biases since they observed a greater reduction in the risk of death from all causes before the influenza season when there should be no vaccine effect [26,27], assuming preferential reception of the vaccine by healthy subjects.
The influenza vaccination coverage of our population was comparable to those observed in the elderly in France (50%) [28,29] and Europe (47.1%) [30] in the same year. This rate remains far below the target of 75% vaccination coverage recommended by the World Health Organization [22]. The flu epidemic strain in France during 2016–2017 was A(H3N2) at 98% and was covered by all the influenza vaccines prescribed.
Our results indicate that certain comorbidities associated with higher or lower rates of vaccine coverage. Subjects with respiratory failure were more vaccinated against influenza than the rest of the study population. Other reports also highlight higher vaccination coverage in pulmonary diseases compared with other groups at risk [28]. Patients with respiratory failure or heart disease appear to have the highest risk of mortality attributable to influenza [20]. Conversely, subjects with Parkinson’s disease were significantly less vaccinated than the rest of the study population. Interestingly, lower vaccination rates have already been reported in patients with neurodegenerative disorders compared with other patients [31,32]. We can assume that practitioners are more likely to abandon preventive for palliative care, limited to symptom reduction, in patients with neurocognitive disorders, as observed in cardiovascular care [33].
Even though vaccine effectiveness seems to decrease in frail subjects [34,35], similar to other authors [23], we observed a persistent lower risk of mortality in vaccinated patients with multimorbidity (CCI > 5) compared with non-vaccinated patients.
Some limitations should be mentioned. First, as for all observational studies, it cannot be excluded that the decrease in mortality observed in the vaccinated group was linked to confounding factors. To minimize these biases, we performed a propensity score analysis. Nevertheless, certain characteristics such as performance status, socioeconomic status, body mass index, smoking and alcohol consumption were not collected, even though they are known to be factors associated with higher mortality. In particular, material deprivation and low access to health care services are associated with a lower frequency of influenza vaccination [36]. Unfortunately, as the date of death was not recorded in this series, we were unable to compare mortality in vaccinated and non-vaccinated groups before the flu season, as suggested by others to assess the existence of biases [27]. Moreover, it remains undetermined for which causes of death influenza vaccination could be associated with a protective effect in this study. Second, only chronic diseases were recorded in the present study, although acute diseases also have a major impact on mortality. Third, only vaccine prescriptions and not effective administrations were recorded. Finally, our population is mostly composed of retired French farmers and it remains uncertain that these results can be extrapolated to the general population.
5. Conclusions
Notwithstanding its observational design, this study suggests that influenza vaccination does have a benefit for very elderly people. More specifically, it appears to reduce all-cause mortality, whatever the age and comorbidities. Patient comorbidities were associated with highly variable vaccination rates: subjects with chronic respiratory disease were the most vaccinated and those suffering from neurodegenerative disorders were the least vaccinated from this cohort of older patients. Vaccination coverage needs to be urgently improved in this very-high-risk population.
Acknowledgments
The authors thank Suzanne Rankin, a native English speaker, for reviewing the manuscript.
Author Contributions
Conceptualization, A.P., J.B. and P.M.; methodology, A.P.; validation, A.P., D.M. and P.M.; formal analysis, A.P. and C.E.; investigation, P.W. and C.E.; resources, C.C. and D.M.; data curation, D.M.; writing—original draft preparation, P.W.; writing—review and editing, all authors; visualization, A.P.; supervision, A.P.; and project administration, A.P., P.M. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thompson W., Shay D., Weintraub E., Brammer L., Cox N., Anderson L.J., Fukuda K. Mortality Associated with Influenza and Respiratory Syncytial Virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Thompson W., Shay D., Weintraub E., Brammer L., Bridges C.B., Cox N., Fukuda K. Influenza-Associated Hospitalizations in the United States. JAMA. 2004;292:1333. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.OMS Grippe. [(accessed on 28 September 2019)]; Available online: http://www.who.int/topics/influenza/fr/
- 4.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D.L., Cowling B.J., et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2017;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonsen L., Reichert T.A., Viboud C., Blackwelder W.C., Taylor R.J., Miller M.A. Impact of Influenza Vaccination on Seasonal Mortality in the US Elderly Population. Arch. Intern. Med. 2005;165:265. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 6.Nichol K.L., Mullooly J., Lask R., Fillbrandt K., Iwane M., Nordin J. Influenza Vaccination and Reduction in Hospitalizations for Cardiac Disease and Stroke among the Elderly. N. Engl. J. Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 7.Clar C., Oseni Z., Flowers N., Rees K., Jahromi M.K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst. Rev. 2015;5:CD005050. doi: 10.1002/14651858.cd005050.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govaert T.M., Thijs C.T., Masurel N., Sprenger M.J., Dinant G.J., Knottnerus J.A. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–1665. doi: 10.1001/jama.1994.03520210045030. [DOI] [PubMed] [Google Scholar]
- 9.Gross P.A., Hermogenes A.W., Sacks H.S., Lau J., Levandowski R.A. The Efficacy of Influenza Vaccine in Elderly Persons. Ann. Intern. Med. 1995;123:518. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Vu T., Farish S., Jenkins M.A., Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. doi: 10.1016/S0264-410X(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson T., Rivetti D., Rivetti A., Rudin M., Di Pietrantonj C., Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y.-C., Tung H.-J., Huang Y.-T., Lu C.-T., Ernawaty E., Wu S.-Y. Effect of Influenza Vaccination on Mortality and Risk of Hospitalization in Elderly Individuals with and without Disabilities: A Nationwide, Population-Based Cohort Study. Vaccines. 2020;8:112. doi: 10.3390/vaccines8010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y.-C., Yu-Tung H., Chen L.-S., Tung H.-J., Huang K.-H., Ernawaty E., Wu S.-Y. Protective Effect of Seasonal Influenza Vaccination in Elderly Individuals with Disability in Taiwan: A Propensity Score–Matched, Nationwide, Population-Based Cohort Study. Vaccines. 2020;8:140. doi: 10.3390/vaccines8010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demicheli V., Jefferson T., Pietrantonj C.D., Ferroni E., Thorning S., Thomas R.E., Rivetti A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2018;2018:CD004876. doi: 10.1002/14651858.CD004876.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzo C., Viboud C., Montomoli E., Simonsen L., Miller M.A. Influenza-related mortality in the Italian elderly: No decline associated with increasing vaccination coverage. Vaccine. 2006;24:6468–6475. doi: 10.1016/j.vaccine.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 16.McElhaney J.E., Verschoor C.P., Andrew M.K., Haynes L., Kuchel G.A., Pawelec G. The immune response to influenza in older humans: Beyond immune senescence. Immun. Ageing. 2020;17:1–10. doi: 10.1186/s12979-020-00181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin K., Viboud C., Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 18.Jackson L.A., Nelson J.C., Benson P., Neuzil K.M., Reid R.J., Psaty B.M., Heckbert S.R., Larson E.B., Weiss N.S. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int. J. Epidemiol. 2005;35:345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez-González E., Cantero-Escribano J.M., Redondo-Bravo L., Juan-Sanz I.S., Robustillo-Rodela A., Cendejas-Bueno E., Influenza Working Group Effect of vaccination, comorbidities and age on mortality and severe disease associated with influenza during the season 2016–2017 in a Spanish tertiary hospital. J. Infect. Public Health. 2019;12:486–491. doi: 10.1016/j.jiph.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Schanzer D.L., Langley J.M., Tam T.W. Co-morbidities associated with influenza-attributed mortality, 1994–2000, Canada. Vaccine. 2008;26:4697–4703. doi: 10.1016/j.vaccine.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 21.Charlson M.E., Pompei P., Ales K.L., MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Vaccines against influenza WHO position paper—November 2012. Wkly. Epidemiol. Rec. 2012;47:461–476. [PubMed] [Google Scholar]
- 23.Voordouw A.C.G., Sturkenboom M.C.J.M., Dieleman J., Stijnen T., Smith D.J., Van Der Lei J., Stricker B.H. Annual Revaccination Against Influenza and Mortality Risk in Community-Dwelling Elderly Persons. JAMA. 2004;292:2089–2095. doi: 10.1001/jama.292.17.2089. [DOI] [PubMed] [Google Scholar]
- 24.Bellino S., Piovesan C., Bella A., Rizzo C., Pezzotti P., Ramigni M. Determinants of vaccination uptake, and influenza vaccine effectiveness in preventing deaths and hospital admissions in the elderly population; Treviso, Italy, 2014/2015–2016/2017 seasons. Hum. Vaccines Immunother. 2019;16:301–312. doi: 10.1080/21645515.2019.1661754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortqvist A., Granath F., Askling J., Hedlund J. Influenza vaccination and mortality: Prospective cohort study of the elderly in a large geographical area. Eur. Respir. J. 2007;30:414–422. doi: 10.1183/09031936.00135306. [DOI] [PubMed] [Google Scholar]
- 26.Campitelli M.A., Rosella L.C., Stukel T.A., Kwong J.C. Influenza vaccination and all-cause mortality in community-dwelling elderly in Ontario, Canada, a cohort study. Vaccine. 2010;29:240–246. doi: 10.1016/j.vaccine.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 27.Jackson L.A., Jackson M.L., Nelson J.C., Neuzil K.M., Weiss N.S. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int. J. Epidemiol. 2005;35:337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 28.Robert J., Detournay B., Levant M., Uhart M., Gourmelen J., Cohen J. Flu vaccine coverage for recommended populations in France. Med. Mal. Infect. 2019 doi: 10.1016/j.medmal.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Santé Publique France Influenza Vaccine Coverage by Age Group. [(accessed on 30 October 2019)]; Available online: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-grippe-par-groupe-d-age.
- 30.Bossuyt N., Damme P.V., Grammens T., Nadezhda V., Filipova R., Kyncl J., Havlickova M., Kostalova J., Koliou M., Visekruna V.V., et al. Seasonal Influenza Vaccination and Antiviral Use in EU/EEA Member States—Overview of Vaccine Recommendations for 2017–2018 and Vaccination Coverage Rates for 2015–2016 and 2016–2017 Influenza Seasons. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2018. [Google Scholar]
- 31.Shah S.M., Harris T., Harris T., Dewilde S., Cook D. The impact of dementia on influenza vaccination uptake in community and care home residents. Age Ageing. 2011;41:64–69. doi: 10.1093/ageing/afr135. [DOI] [PubMed] [Google Scholar]
- 32.Landi F., Onder G., Carpenter I., Garms-Homolová V., Bernabei R. Prevalence and predictors of influenza vaccination among frail, community-living elderly patients: An International Observational Study. Vaccine. 2005;23:3896–3901. doi: 10.1016/j.vaccine.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Liu E., Dyer S.M., O’Donnell L.K., Milte R., Bradley C., Harrison S.L., Gnanamanickam E., Whitehead C., Crotty M. Association of cardiovascular system medications with cognitive function and dementia in older adults living in nursing homes in Australia. J. Geriatr. Cardiol. 2017;14:407–415. doi: 10.11909/j.issn.1671-5411.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangtani P., Cumberland P., Hodgson C.R., Roberts J.A., Cutts F., Hall A. A Cohort Study of the Effectiveness of Influenza Vaccine in Older People, Performed Using the United Kingdom General Practice Research Database. J. Infect. Dis. 2004;190:1–10. doi: 10.1086/421274. [DOI] [PubMed] [Google Scholar]
- 35.Andrew M.K., Shinde V., Ye L., Hatchette T., Haguinet F., Dos Santos G., McElhaney J.E., Ambrose A., Boivin G., Bowie W., et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J. Infect. Dis. 2017;216:405–414. doi: 10.1093/infdis/jix282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meshefedjian G.A., Ouimet M.-J., Frigault L.-R., Leaune V., Azzou S.A.K., Simoneau M.-È. Association of Material Deprivation Status, Access to Health Care Services, and Lifestyle With Screening and Prevention of Disease, Montreal, Canada, 2012. Prev. Chronic Dis. 2016;13 doi: 10.5888/pcd13.160157. [DOI] [PMC free article] [PubMed] [Google Scholar]