As of mid-September, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 30 million people, resulting in approximately 1 million deaths worldwide, including more than 200,000 deaths in the United States alone. Fever, dry cough, breathing difficulties, and gastrointestinal (GI) symptoms are typical features of coronavirus disease-2019 (COVID-19). Although 80% of infected people develop a mild disease, approximately 20% progress to severe COVID-19, which is associated with lung damage and breathing difficulties, and may lead to respiratory failure and death. Exacerbation of the COVID-19 immune response manifested by extensive cytokines release, called cytokine storm, may lead to multisystem inflammatory syndrome, which is fatal in 28% of cases.1 Children can also be infected with SARS-CoV-2 (<2%); however, most confirmed pediatric cases have a less severe outcome and milder symptoms.
In late April 2020, reports from Europe described the emergence of a new febrile pediatric entity that involved persistent fever, systemic hyperinflammation, multiorgan involvement with prominent and severe GI symptoms, and cardiogenic shock and hypotension, requiring pediatric intensive care unit care in most cases. Some who developed this syndrome, referred to as COVID-19–associated multisystem inflammatory syndrome in children (MIS-C), also demonstrated clinical findings including erythematous rashes, conjunctivitis, and inflammatory changes in the oral mucosa, features reminiscent of Kawasaki disease (KD). However, although initially designated as “Kawasaki-like,” it soon became clear that this novel syndrome was very different from KD or KD shock syndrome, because MIS-C affected different demographics, and the clinical and laboratory parameters differed greatly between the 3 conditions2 (Table I ). Although early research suggested that KD might be triggered by a superantigen (SAg), subsequent studies could not confirm Vβ2 family T-cell repertoire skewing in patients with KD. In contrast, MIS-C is more reminiscent of toxic shock syndrome (TSS).3 MIS-C cases reported from London showed similar findings, including an association with COVID-19 infection, higher incidence in children with African ancestry, and disease presentation with prominent features of shock, myocarditis, and severe GI symptoms, which are all very rare in KD.2 In addition, the overall clinical picture of MIS-C is similar in many respects to the late, severe COVID-19 phase in adults, which is characterized by a cytokine storm, hyperinflammation, and multiorgan damage, and often includes severe myocarditis and acute kidney injury, and laboratory and clinical features of TSS.4
Table I.
Demographic and clinical characteristics of patients with MIS-C, KD shock, TSS, and KD
| Characteristic | MIS-C | TSS | KD shock syndrome | KD |
|---|---|---|---|---|
| Median age (y) | 9 | 7 | 3 | 2 |
| Ethnicity | Hispanic or Latino and Black, non-hispanic | White | Asian and Asian ancestry | Asian and Asian ancestry |
| GI symptoms | Severe | Severe | Mild | Mild |
| Myocardial dysfunction/cardiovascular shock | Yes | Yes | Yes | No |
| Neuropsychological findings and CNS symptoms | Yes | Yes | No | No |
| Coronary artery dilatation/aneurysms | Transient dilation No real aneurysm |
Transient dilation No aneurysm |
Dilation and aneurysms | Dilation and aneurysms |
| D-dimers levels | High | High | Low | Low |
| Troponin levels | High | NA | Low | Low |
| Inflammation markers (ferritin, CRP, neutrophils) | Highest | Highest | Higher | High |
| Lymphopenia | Yes | Yes | No | No |
| Thrombocytopenia | Yes | Yes | No∗ | No |
| Response to IVIG and steroids | Yes | Yes | Yes | Yes |
A causal link between SARS-CoV-2 infection and MIS-C has not yet been clearly established; however, many patients with MIS-C were reportedly exposed to someone known or suspected to have COVID-19. Although only around a third of patients with MIS-C are positive for SARS-CoV-2 by PCR, a large majority are PCR-negative but positive serologically for SARS-CoV-2 antibodies and/or have a history of mild COVID-19 infection or exposure several weeks before presentation. Such timing suggests that MIS-C is a postinfectious disease or an immune or autoimmune disease. Moreover, the virus may still be present in the GI tract of these patients, because they demonstrate very severe GI symptoms.
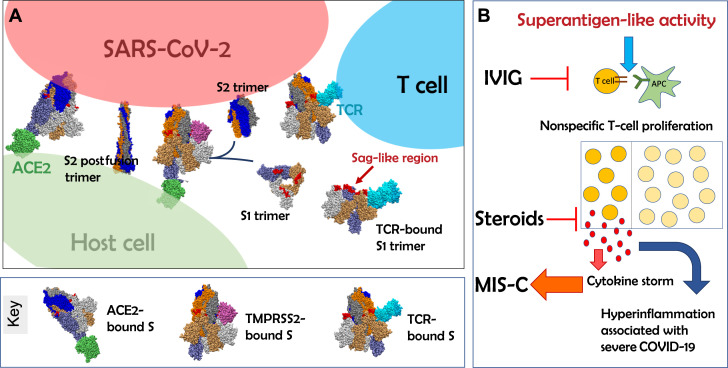
Through structure-based computational modeling, we discovered that the SARS-CoV-2 spike protein encodes a high-affinity SAg-like sequence motif near the S1/S2 cleavage site of the spike protein. The region containing this motif exhibits a high affinity to bind to T-cell receptors (TCRs) by closely associating with the variable domains’ complementarity-determining regions of both the α and β chains(Fig 1 ).5 , 6 Notably, this region (containing the SAg-like motif) is highly similar in sequence and 3-dimensional structure to a fragment of the superantigenic Staphylococcal Enterotoxin B (SEB), which is known to interact with the TCR and CD28.6 SEB triggers large-scale T-cell activation and proliferation, resulting in massive production of a proinflammatory cytokine profile typical of TSS, similar to that which entails severity and death from COVID-19. Next-generation immunosequencing analysis of T-cell repertoires from patients with COVID-19 indicated that severe COVID-19 was associated with a TCRVβ skewing, enrichment of selected Vβ genes, and increased J diversity, consistent with SAg activity.5 These data support our hypothesis that MIS-C, as well as cytokine storm observed in adult patients with severe COVID-19, is mediated by SAg activity of the SARS-CoV-2 spike protein. Additional, prospective studies in adult and pediatric cohorts are warranted to test this hypothesis.
Fig 1.
SARS-CoV-2 spike superantigen (SAg)-like motif exposure and immune activation following SARS-CoV-2 infection potentially leads to hyperinflammation in MIS-C and cytokine storm. A, Schematic representation of the interaction between SARS-CoV-2 spike and host cell ACE2 receptor (green) and transmembrane protease TMPRSS2 (purple). TMPRSS2 binds the spike trimer near the P681RRA684 insert (red) unique to the SARS-CoV-2 spike. P681RRA684 is located in an SAg-like region, which also exhibits a high affinity to bind TCRs (cyan), -and is adjacent to the cleavage site R685-S686, the breakage of which separates each subunit of the spike trimer into 2 subunits, S1 and S2, resulting in the S2 fusion trimer (bound to viral membrane) and the S1 trimer (released to extracellular space). The diagram displays S2 prefusion and postfusion conformations (see the key for diagrams in A at the bottom). B, Exposure of the SAg-like region prompts nonspecific T-cell activation and cytokine storm, leading to hyperinflammation associated with MIS-C and severe COVID-19. IVIG and steroids may block the SAg-triggered activity. Note, we show the catalytic domain of membrane-bound TMPRSS2, and 1 monomer of ACE2 receptor, for simplicity. Spike monomers are colored blue, gray, and orange, with lighter/darker shades for S1/S2 subunits. ACE2, Angiotensin-converting enzyme 2; APC, Antigen-presenting cell; TMPRSS2, Transmembrane Serine Protease 2.
Notably, both T and B cells can be triggered by SAgs to contribute to the innate immune response. Multiple autoantigenic immunoglobulins have been identified in children with MIS-C,7 raising the possibility that the SARS-CoV-2 SAg-like structure we identified may also possess B-cell SAg-like function. Furthermore, T-cell SAgs can interact with MHCII expressed on B cells to induce B-cell signaling pathways.8 It will be important to explore this potential in future studies.
Some children with MIS-C develop neurological symptoms,3 including headache, altered mental state, and confusion, and similar neurological complications are reported in adult patients with COVID-19.9 The pathologic mechanisms leading to these symptoms remain unknown. Interestingly, SAg-induced TSS has been associated with long-term neuropsychologic deficits in adults, including cognitive decline,10 and we identified a homology between the SAg motif of SARS-CoV-2 and neurotoxin-like sequences that are able to bind the TCR.5 Notably, SARS-CoV-2 spike contains other neurotoxin-like motifs as well, including in particular the segment T299-Y351, which has been recently observed to be a highly cross-reactive epitope that triggers CD4+ T-cell response.5 It will be interesting to determine whether these neurotoxin-like sequences in the SARS-CoV-2 spike protein contribute to the neurological manifestations observed in children with MIS-C and adults with severe COVID-19.
Why only a small fraction of SARS-CoV-2–infected children develop MIS-C remains unclear. It is possible that a poor initial antibody response to the virus in a subset of children fails to produce neutralizing antibodies, leading to immune enhancement following SARS-CoV-2 reexposure. Alternatively, some HLA types may be more permissive, and respond more robustly to certain viral antigenic structures.5 Indeed, among the reported cases from London,2 50% of patients with MIS-C were of Afro-Caribbean descent, which suggests a possible genetic component for MIS-C susceptibility.
Finally, our findings suggest that immunomodulatory therapeutic approaches used for TSS, such as intravenous immunoglobulin (IVIG) and steroids, may also be effective for MIS-C. Indeed, most patients with MIS-C respond well to IVIG (2 g/kg) and aspirin, with or without steroids.3 Given the structural similarities between SEB and the SARS-CoV-2 spike SAg motif,5 it is possible that antibodies within IVIG that neutralize SEB cross-react with the SARS-CoV-2 spike, which may in part explain the beneficial response of MIS-C cases to IVIG. In addition, in the mouse model of TSS, lethal SEB SAg challenge can be prevented by short peptide mimetics of the SAg motif.6 Therefore, it would be important to investigate the therapeutic potential of peptide mimetics of SARS-CoV-2 spike SAg-like region in COVID-19–induced hyperinflammatory syndromes in future studies. Further elucidation of the parameters affecting the interaction between SARS-CoV-2 spike glycoprotein and immune cells will be necessary to design effective preventive and therapeutic interventions.
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health.
Footnotes
This study was supported by the National Institutes of Health awards (grant no. P41 GM103712 to I.B. and grant no. R01 AI072726 to M.A.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pain C.E., Felsenstein S., Cleary G., Mayell S., Conrad K., Harave S. Novel paediatric presentation of COVID-19 with ARDS and cytokine storm syndrome without respiratory symptoms. Lancet Rheumatol. 2020;2:e376–e379. doi: 10.1016/S2665-9913(20)30137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng M.H., Zhang S., Porritt R.A., Noval Rivas M., Paschold L., Willscher E. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci U S A. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arad G., Levy R., Nasie I., Hillman D., Rotfogel Z., Barash U. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020 Sep 6. 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed]
- 8.Morio T., Geha R.S., Chatila T.A. Engagement of MHC class II molecules by staphylococcal superantigens activates src-type protein tyrosine kinases. Eur J Immunol. 1994;24:651–658. doi: 10.1002/eji.1830240325. [DOI] [PubMed] [Google Scholar]
- 9.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosene K.A., Copass M.K., Kastner L.S., Nolan C.M., Eschenbach D.A. Persistent neuropsychological sequelae of toxic shock syndrome. Ann Intern Med. 1982;96:865–870. doi: 10.7326/0003-4819-96-6-865. [DOI] [PubMed] [Google Scholar]