Highlights
-
•
Despite the burden of gene therapy trials for DMD patients there is great enthusiasm.
-
•
Collaborating with relevant bodies (pharmacy) at an early stage can accelerate progress.
-
•
A hub and spoke model may be an option for delivering clinical trials and follow up care.
Keywords: Duchenne muscular dystrophy, Gene therapy, Collaboration, Barriers
1. Introduction
There is considerable interest, excitement and anticipation for the development of adeno associated virus (AAV) gene therapy as a treatment for Duchenne muscular dystrophy (DMD). Much of this expectation is based on promising preclinical data as well as success in other neuromuscular conditions, such as spinal muscular atrophy (SMA). However, it is also important to take an objective view of the realistic possibilities and limitations of AAV gene therapy for DMD as well as considering the likely barriers to clinical trials and the development of gene therapy as an approved and accessible treatment.
If we better understand what is possible, what is not and what hurdles need to be overcome, we can effectively prepare. We need to be educated as a community of clinicians and patients, health care providers (including pharmacy), policy makers and regulators, payers and pharmaceutical companies about the challenges of gene therapy for DMD. We should talk, understand and reach a collaborative consensus in order to move forwards with the maximum chance of successfully bringing a safe and effective therapy to market and so to patients living with DMD.
With this in mind, over 100 key stakeholders attended a 1 day meeting on the 14th November 2019 in Newcastle, UK to discuss preparing the DMD field for the imminent arrival of AAV vector gene therapy.
Stakeholders included representatives from the leading UK neuromuscular centres, patient advocacy groups, national and local pharmacy, the National Institute for Health Research (NIHR), industry, the National Institute for Health and Care Excellence (NICE), National Health Service (NHS) England and national, government funded organisations that are working to facilitate gene therapy.
The main objective of the meeting was to discuss the challenges and barriers to running gene therapy trials for DMD and to getting successful gene therapy products approved and reimbursed in the UK.
2. Shared vision and hopes
2.1. Clinical perspective
2.1.1. What is gene therapy?
Francesco Muntoni (University College London and Great Ormond Street Hospital Trust) explained that AAV gene therapy is a way of treating or changing the progression of a disease by the introduction of genetic material into the cells of a patient. This may involve different approaches, but for DMD currently relies on delivery of genetic material by an AAV vector.
AAV vectors are the current focus in many monogenic diseases because they induce a low immune response and their infection does not cause symptoms in humans [1]. After intravenous administration, they distribute in a wide variety of tissues, including muscle. In addition, unlike some other viral vectors used for example for haematological disorders, AAVs do not integrate their genetic material into the host's genome; rather they remain separate from the patient's DNA as a small episomal particle in the nucleus, making them safer in terms of unwanted, longterm potential mutagenic side effects when compared to integrating vectors [2].
However, AAV vectors have a limited capacity and can only carry genetic material of up to 4–5 kb. The DMD transcript has a coding sequence of 14 kb, which considerably exceeds the AAV capacity [2].
2.1.2. The need to develop shortened dystrophins: the role of microdystrophin
It has been possible to remove different parts of the DMD coding sequence to identify the crucial domains that allow maximum function of the translated protein to be maintained, whilst reducing the size sufficiently to be accommodated by an AAV vector. The resulting micro and mini dystrophins have been compared and changes made to reach what is felt to be the optimum sequence [3,4]. This effort was helped by truncating DMD mutations causing Becker muscular dystrophy (BMD), where large deletions of the coding region still resulted in a very mild phenotype [5].
However, it is important to recognise that even the most functional of truncated dystrophins are not as effective as wildtype dystrophin and that DMD gene replacement therapies, if successful, should not be seen as a cure for DMD. We should not either refer to ‘turning Duchenne into Becker’, as BMD patients are born with a truncated form of dystrophin and any new nuclei formed will be able to produce BMD protein. The durability of dystrophin expression in AAV treated DMD patients is currently uncertain, because of the nonintegrating nature of the vector genome and its dilution during muscle growth and cycles of de and regeneration.
And so follows the question of when to treat? To wait until a boy is older to treat will mean more nuclei produce microdystrophin but higher doses of the vector are needed and the dystrophic pathology will already be more advanced. However, to treat very young will result in dilution of the microdystrophin producing nuclei as the child increases in size.
If repeat doses of gene therapy were possible, treatment at birth could be followed by subsequent doses as a boy grows. At the moment, it seems that AAV mediated gene therapy may be a once in a lifetime treatment due to an immune response against the vector that would be induced after a first treatment. This ‘one-off’ nature of gene therapy has ethical issues for the running of trials where a dose escalation arm is included and a patient may receive a lower than efficacious dose and subsequently be unable to receive a repeat, higher dose that proved to be effective. Possibilities of redosing would need to be explored [6].
The clinical perspective must include a discussion of the handling of potential adverse and serious adverse events (AEs and SAEs). Some AEs involving the liver function have been reported [7], but more serious adverse events can be associated with gene therapy approaches [8,9].
The field should learn from other disease areas about the best way to manage AEs and SAEs. For example, CAR T cell treatment has wellknown significant side effects which have been observed in immunodeficiency and cancer. However, because there is now a good understanding of when each AE may occur and how to treat or mitigate it, they can be anticipated and effectively managed [10].
Nevertheless, the burden of gene therapy trials in DMD, from ensuring patient and family understanding, to administration and monitoring, wrangling with ethical issues and management of side effects, plus the need for long term follow up, is significant. This presents capacity and resource issues for those centres running gene therapy trials, dependent on the number of patients enrolled, their age and range of disease severity and on the expertise of the centre and the teams. It is important to understand that gene therapy will not happen at a national level unless these bottlenecks are addressed.
2.2. Patient perspective
Alex Johnson (Duchenne UK and Joining Jack) informed workshop participants that the patient community is anxiously anticipating the development of gene therapies for DMD and are keen for opportunities to participate in clinical trials. A preference study conducted by Parent Project Muscular Dystrophy in 2017 [11,12] found that in the US, the potential benefits of gene therapy drive parents’ decision making more than the possible risks, uncertainties and burdens. The importance of access to gene therapy trials, as well as concerns about receiving too low a dose, were also highly prioritized. Procedures that are usually a concern for families in a clinical trial setting, such as muscle biopsies and being in a placebo group were ranked lower amongst the decision-making factors for gene therapy trials than for other types of therapy.
Additionally, the study found a relatively high tolerance for mortality risk and that this tolerance increased with disease progression. Patients who had become non-ambulatory were willing to accept more risks and risk tolerance was in fact highest where patients were in the last year of being able to bring arms to mouth.
Complementing these findings, a short UK survey looking at parent views on gene therapy revealed similar tolerance for risks. Young families want to see gene therapy in the UK and older patients are keen to look at how they can innovatively be included in these trials. The DMD Hub is planning to collaborate with PPMD in the near future to carry out a follow up study to better understand patient and caregiver preferences in the UK. As new knowledge presents itself in early gene therapy studies, it is important that patient and family preferences continue to be measured over time to understand how they think and feel about benefits, risks, and uncertainty.
It is also important to ensure that the patient community has clear and accurate information available in order to allow decision making to be fully informed.
Emily Crossley (Duchenne UK) followed this and explained that gene therapy holds great hope for patients and families of children with DMD, and it is through their collaboration with clinicians that the barriers can be identified, addressed and overcome.
Established in 2015, as a partnership between Newcastle, Great Ormond Street and Duchenne UK, the DMD Hub (www.dmdhub.org) demonstrates the powerful force of patients and clinicians working together. Since its inception, the DMD Hub has addressed a lack of clinical trial capacity for DMD. This same model is now being expanded and refocussed to help ensure that the UK is also ready for gene therapy trials.
3. Overview of the issues: what are the barriers in delivering the next generation of medicine in the UK?
3.1. Barriers and lessons learned from other NMDs
Imran Kausar (AveXis) shared experiences from gene therapy trials and treatments already underway in SMA. The development of AVXS 101 (ZolgensmaⓇ) for treatment of SMA in children under 2 years old provides a significant learning opportunity for gene therapy in DMD. For AVXS 101, time from Phase I study to approval was around five years rather than around 12 as may be normally expected. Whilst not all gene therapy approval processes will be necessarily as rapid as that for AVXS-101, it illustrates a need to move quickly, for which the community must be ready and plan for together. In particular, regulators, clinicians and payers must collaborate at an early stage to begin planning for the introduction and safe use of these therapies in clinical setting.
Imran Kausar highlighted that there is a hope for the future that viral vectors would not need to be used and that new technologies may allow different and better delivery mechanisms, maybe removing the immune response issue highlighted above [13].
He emphasised that the huge number of vector genomes needed, 100 trillion per kg bodyweight, presents manufacturing issues that need to be considered and planned for. It would not be ethical to develop an efficacious therapy that was not then possible to manufacture in large enough quantities to treat the patients that needed it.
Another challenge is screening for antibodies to the chosen viral vector because an immune response must be avoided. However, as AAV is not pathogenic in humans, it has been difficult to find a laboratory that can test for AAV antibodies. Mothers might also need to be tested when treating infants, as with AVXS 101 trial, because of the possibility of antibody transference.
Because of the novel and fast-moving nature of advanced therapies, many health authorities have little experience with gene therapies and genetically modified organisms (GMOs) and so it can be hard to get countries to participate in clinical trials. For the AveXis Phase 3 trial in SMA initially only 4 countries in Europe were able to participate. One of the problems encountered was the risk perception about viral shedding. Some countries in Europe did not approve the study as the perceived risk of viral shedding in stool and urine was assessed as too high.
Delays are also caused because different country regulators evaluate data at different speeds. Collaborative consideration of data and agreement on decision making between regulators would be a significant advantage and remove bottlenecks. Imran Kausar pointed out that it may be advantageous to select ethics committees that have approved gene therapy trials before.
This also applies to bio safety committees in hospitals. Another challenge is the frequency that committees relevant for the approval of gene therapy trials meet, as in some cases this may be only once every 6 months. If that cycle is missed, it introduces a delay that could be avoided by better planning and scheduling.
Specifically in the UK, approval is needed from the Health and Safety Executive (HSE) and possibly the Department for Environment, Food and Rural Affairs (DEFRA) for the disposal of GMOs. This should always be anticipated and added into the timeline.
Consideration also needs to be made for the involvement of other hospital departments (e.g. dialysis) for the management of possible adverse events (AEs) and serious adverse events (SAEs) and to ensure this is allowed for in costing and human resource.
3.2. What issues are industry facing? perspectives, plans and experiences in DMD gene therapy trials
Ahead of the workshop the DMD Hub engaged with industry partners preparing to run DMD gene therapy trials in the UK to understand their perspectives, plans and experience regarding setting up trials to date. Laurent Servais (University of Oxford) presented the results on behalf of the companies, highlighting the main issues and discussing how they may be addressed.
One of the main challenges industry faces relates to accurately calculating the dose of the gene therapy required for children, so that it is safe and effective whilst being affordable and reproducible in the required quantities. There is a clear need to balance the risk against the durability we want to achieve.
In addition, companies also highlighted several issues they are aware of regarding bringing gene therapy to the UK specifically, including:
-
•
Issue of GMO regulation and lack of awareness (e.g. the need for DEFRA +/− Biological Safety Committee review).
-
•
Limited pharmacy resources.
-
•
Delays in contract management.
-
•
BREXIT uncertainty is a challenge (but there may be also be opportunities within future standalone regulatory environment to create a competitive advantage for gene therapy clinical trials in the UK).
-
•
Site Capacity Constraints.
However during the discussion a number of positive opinions were also highlighted, including:
-
•
There is significant gene therapy work ongoing at UK academic and clinical institutions, second only to the USA in terms of numbers of global trials running.
-
•
The NHS provides a unique national platform with the potential for coordination of clinical expertise, data collection and long term follow up, which may be difficult to replicate in the USA or other countries.
-
•
The UK has some of the best DMD physicians in the world and is a leader in world class DMD research and the DMD investigational sites are well organised.
-
•
There already exists a national clinical network and database to facilitate the collection of postmarketing data.
Suggestions, from industry, for improvements to ensure gene therapy trials are carried out as quickly and effectively as possible in the UK include:
-
•
Adequate funding of sites (pharmacy) before the trial starts, and to ensure that adequate capacity is available.
-
•
Improving the contracting process, to reduce the set up times at sites.
-
•
Clarification of the GMO regulation to increase the understanding in hospitals and ethics committees.
-
•
Coordinate efforts to facilitate recruitment in a timely and fair manner would be welcomed.
3.3. Practical considerations for gene therapy trial implementation
As Regional Quality Assurance Specialist Pharmacist and Chair, Anne Black (Newcastle upon Tyne Hospitals NHS Foundation Trust) introduced the Pan UK Working Group for Advanced Therapy Medicinal Products (ATMPs). The group aims to establish the governance, operational and clinical roles of pharmacy in the implementation of ATMPs in both clinical trials and marketed medicines. Using experience from marketed products, a number of checklists and procedures have been developed which are now widely available as part of a suite of guidance (https://www.sps.nhs.uk/networks/pan-uk-pharmacy-working-group-for-atmps/)
For clinical trials, routine Good Clinical Practice (GCP) requirements apply to ATMPs and additionally, new guidelines were published in October 2019 covering GCP specific to ATMPs. The role of pharmacy in ATMP delivery is multifaceted, incorporating the clinical role and the operational role as well as covering organisational governance. A document was produced in 2017 addressing the role of pharmacy in successful delivery of ATMPs many organisations have embraced the advice and have an ATMP policy, which details the approval process. However, gene therapy medicinal product (GTMP), a subset of ATMPs, requirements are less well understood and there are many queries about how to undertake a first gene therapy clinical trial.
In response to this, the Pan UK Pharmacy Working Group for ATMPs developed a document on GTMPs: Governance and Preparation Requirements, which covers key practical guidelines for delivering gene therapy such as undertaking a technical feasibility assessment and local risk assessment. The group recommends that for the introduction of any GTMP (whether as part of a clinical trial or in clinical practice), a risk assessment and evaluation by a Genetic Modification Safety Committee (GMSC) is the preferred organisational governance route. The risk assessment should cover numerous aspects of implementation of the GTMP such as the product, patient and waste pathways.
In terms of operational guidance, the document outlines guidelines for receipt and storage, preparation and handling of the GTMP, including useful flow diagrams to support local decision making about GTMP delivery. Pathways provide clear pragmatic advice on where the GTMP delivery can happen, where the product can be made etc. This is useful not only for sites, but for trial sponsors too.
Common issues that are identified include sponsor understanding of pharmacy oversight (which incurs additional cost), packaging/storage, cryopreservation, out of specification product handling and inexperienced sponsors, contract research organisations (CROs) and sites. This is an evolving field in which everyone is learning, the key message therefore is to collaborate, using the Pan UK Pharmacy Working Group resources as a useful guide.
3.4. Overcoming local set up challenges
Maria Allen, (Newcastle upon Tyne Hospital NHS Foundation Trust) shared experiences and challenges from her perspective as a Clinical Trials Operation Manager in Pharmacy. The Pharmacy Department and Newcastle Cellular Therapies Facility have 10 years of experience of over 20 commercial and noncommercial Advanced Therapy Investigational Medicinal Product (ATIMP) trials. This experience has led to the identification of common issues that arise repeatedly when setting these trials up, such as: uncertainty of the classification of the Gene Therapy Investigational Medicinal Product (GTIMP e.g. Class I, II), lack of sponsor awareness regarding appropriate waste requirements, logistics of GTIMP delivery to site, requirement for specific storage conditions, optimal preparation location (and competency), logistics of GTIMP delivery to patient for administration (e.g. spill kit provision), optimal location for GTIMP administration, costing issues, sponsor/CRO inexperience of study delivery in the UK (e.g. regulatory requirements), and lack of information from the sponsor about the stability/expiry of product. Managing expectations of the key stakeholders such as the study sponsor, Principal Investigator (PI) and delivery team is key when dealing with these issues.
Drawing on the evolving experience of the team, some solutions to the commonly experienced problems have been identified. Early communication with all teams involved is essential and pharmacy colleagues should be involved early in the set up process. It is also vital to ensure that there is knowledge within the local hospital Trust of the key members of staff that should be included in trial set up and this should be highlighted in key organisational documents and during induction. A local process flow should be developed as a quick and easy reference guide and to ensure clarity on key steps in the set up process. Communication with the sponsor is also very important e.g. to highlight key aspects of the site set up, facilities and capabilities in the context of ATIMPs and to ensure that key documents are provided by both site and sponsor to enable full review of logistical arrangements. The NIHR costing template is difficult to use when costing ATIMPs, therefore a version specific for ATIMPs is being developed in collaboration with the NA ATTC (and DMD Hub, for DMD GTIMP trials) in order to make costing easier and more tailored to the requirements of ATIMP trials. Communication within the research team itself is also essential; to define the product pathway and outline appropriate pharmacy oversight arrangements.
Gene therapies are extremely exciting for patients and the NHS. Getting it right first time requires organisational governance and system leadership and early collaboration is key to successful trial delivery.
4. Institutional readiness
In order to understand the current readiness for gene therapy trials in the UK, it is important to get the input of potential trial sites. Anne-Marie Childs (Leeds Teaching Hospitals NHS Trust) presented the outcome of a survey designed by the DMD Hub team and analysed by Sejal Thakrar (SWS Data Analysis Ltd) that was distributed to neuromuscular clinicians at paediatric tertiary centres across the UK (North Star Clinical Network). The survey had three main aims:
-
•
To assess the experience UK sites have in setting up and delivering gene therapy clinical trials.
-
•
To assess the facilities, resources and capacity available at UK sites and to identify gaps that would need addressing prior to setting up and delivering DMD gene therapy clinical trials.
-
•
To assess the level of interest UK sites have in delivering DMD gene therapy clinical trials and any perceived barriers.
The survey was distributed to 23 sites in the UK and a total of 11 responses were received, these included 8 DMD Hub sites, the two Centres of Excellence (Newcastle and GOSH), who are also North Star centres, and one additional North Star centre.
Experience and items that were considered to be necessary for trial readiness are listed in Table 1 , along with the percentage of sites that confirmed they could deliver the items.
Table 1.
Checklist for trial readiness at potential UK trial sites.
| Checklist items | Number of sites who confirmed `Yes' | Percentage of 11 sites who responded and confirmed `Yes' |
|---|---|---|
| Gene therapy experience | 6 | 55% |
| Paediatric gene therapy experience | 4 | 36% |
| Experience in paediatric AAV gene therapy studies | 3 | 27% |
| Facilities, resources and capacity to set up and deliver gene therapy clinical trial | 8 | 73% |
| Facilities to accommodate inpatient stays as part of a clinical trial | 11 | 100% |
| Paediatric research facility (immediate access to emergency care and availability of an isolated area) | 9 | 82% |
| Facilities / resources to follow patients long- term | 11 | 100% |
| Local approval possible/achieved previously | 8 | 73% |
| Established Genetic Modification Safety / Biosafety Committee | 9 | 82% |
| Approved AAV delivery procedure | 8 | 73% |
| Remove GMO waste via established procedures and waste routes | 7 | 64% |
| Ability to handle dry ice (have training / handling equipment) | 9 | 82% |
| Ability to store product at a range of temperatures | 7 | 64% |
| Space for quarantine of product | 8 | 73% |
| Containment lab able to draw up gene therapy product | 7 | 64% |
Responses from sites surveyed revealed an interest to bring DMD gene therapy trials to the UK and those sites that have participated in previous gene therapy trials (6/11) have found that difficulties were mainly in trial setup rather than in conducting the trial.
The development of standard operating procedures (SOPs) and completing a full risk assessment were reported to be particularly time consuming. Sites also reported that having AAV delivery procedures approved by their local NHS Trusts was time consuming and contributed to a delay in the activation of gene therapy trials compared to nongene therapy trials.
When asked about lessons learned from these experiences, many respondents made similar suggestions to those identified by AveXis and other industry representatives detailed above. Early discussions with all involved, consideration of the impact on other hospital departments, consultation with those who have been through the process before, use of template documents, in advance consideration of where the compound will be administered and how to handle viral shedding were all identified as important.
The need for early liaison with all those involved, including sponsors and site support departments, was identified as an important lesson learnt.
The survey results reveal that most sites have some or all of the resources, facilities and capacity needed to set up and deliver gene therapy clinical trials (Table 1). However, it is important to understand that the survey did not aim to investigate the details of those facilities/resources and whether they would meet specific clinical trial criteria.
In terms of pharmacy and waste management, some sites reported that additional facilities, personnel, training and equipment would be required to deliver gene therapy trials.
Many sites also reported a need for additional training for clinical and other staff. The DMD Hub will offer support to both central and satellite sites, by continuing to share experiences and good practice and by adapting parts of its current toolkit (https://dmdhub.org/the-dmd-hub-toolkit/) to provide specific help with gene therapy trials.
Overall the survey confirmed the interest and enthusiasm of sites for gene therapy trials. However, support is needed to develop SOPs for gene therapy to ensure a comprehensive understanding of delivery requirements, safety monitoring and pharmacy requirements. Training for staff will also be essential.
The DMD Hub will coordinate a follow up survey for sites in collaboration with industry and relevant government funded initiatives. The survey will be concerned with gene therapy trial readiness including specific standards and clinical trial criteria, utilising the new knowledge from early gene therapy studies.
4.1. NHS institutional readiness
Matthew Peak (Alder Hey Children's Hospital Trust) highlighted that it is likely that currently high performing NHS research institutions are best placed to deliver gene therapy first, as they have the culture, leadership, facilities and expertise already in place. However, the importance of developing this in other sites should also be addressed. This could be achieved through existing networks and infrastructures, as well as by the DMD Hub to coordinate and harmonise initiatives and sharing expertise and knowledge to get trials set up quickly and efficiently.
5. Sustainable access
Josie Godfrey, (JG Zebra Consulting), moderated a session on sustainable access to approved gene therapy products. In contributions from Fiona Marley (NHS England) and Ron Akehurst (University of Sheffield) it was pointed out that payers have a responsibility to consider patient access and reimbursement even if therapies have a high price attached to them. In England and Wales, NICE carries out a Health Technology Assessment (HTA) of new treatments and issues guidance based on the incremental cost of a Quality Adjusted Life Year (QALY) and budget impact. Commissioners then implement those recommendations. Some treatments that are indicated for very few patients are considered through the Highly Specialised Technologies programme at NICE, which has a higher threshold for cost per QALY. Reimbursement remains a challenge due to the expected high price of any gene therapy.
It took more than two years from marketing authorisation by the European Medicines Agency (EMA) for NICE to approve Translarna for DMD patients with nonsense mutations, in part due to the limited evidence available. Since then, Duchenne UK has established Project HERCULES to develop disease level tools and evidence in a collaborative manner that can support HTA for new treatments for DMD, including gene therapies. When individual companies have to develop this suite of evidence alone it requires more time and resources than when working together.
One of the challenges for approval and reimbursement of gene therapy will be the uncertainty as to how long a potential transformative effect will last, or what long term adverse events might arise. Different models of reimbursement, based on real world evidence, may be needed to address this uncertainty. For example, a company could be reimbursed in instalments depending on the maintenance of drug effect. Will this type of reimbursement model be acceptable to pharma and NHS England? A managed access agreement (MAA) is one way to get around an initial lack of evidence, which has been the approach for PTC with Translarna. However, for gene therapy, real life evidence could take 15 years or more to gather which is longer than the current life span of MAA. Careful consideration will need to be given as to how to address the major uncertainties of a new treatment. With any increase in MAAs, there may need to be consideration of how to reduce the cost and burden of a MAA—reducing the frequency of data collection for example. These questions need to be considered in advance in order to be ready and move quickly should gene therapy for DMD be approved by the EMA.
If treating early is more effective, the implementation of a neonatal screening programme might need to be considered. NICE would seek to understand the consequences of earlier treatment, but a nationwide neonatal screening programme is out of their remit. NHS England should be involved in this discussion. The UK National Screening Committee would need to assess evidence for the benefits of any neonatal or other screening programme—one issue is the potentially high cost of screening programmes for a relatively small return.
6. Existing initiatives and collaboration
Several existing initiatives were presented to explore potential avenues for collaboration and ensure no duplication of effort, these included Catapult, NA ATTC and TREAT NMD.
Anna Outhwaite, (Catapult) head of ATTC Network Coordination Cell and Gene Therapy Catapult, introduced Catapult, a network of world leading centres designed to enhance the UK's capability for innovation and to drive economic growth. One key area of focus is cell and gene therapy, and Catapult is working with researchers and companies to help overcome some of the challenges around cell and gene therapies. Complementing Catapult, the Northern Alliance Advanced Therapies Treatment Centre (NA-ATTC) is an initiative to increase patient access to advanced therapies. James Shaw, (Newcastle University and Newcastle Hospitals NHS Foundation Trust) director for NA ATTC, explained that the alliance develops the systems and infrastructure required to support cell and gene therapies. The DMD Hub is working with NA ATTC to ensure that efforts are harmonised and lessons shared.
Volker Straub, (Newcastle University and Newcastle Hospitals NHS Foundation Trust) former coordinator of TREAT NMD, a global network of neuromuscular specialists and patient organisations working in close partnership with industry to enable new therapies to reach patients as quickly as possible, informed workshop participants about an initiative to develop a post marketing surveillance registry. The primary objective of the registry would be for industry, in partnership with TREAT NMD, to monitor safety and long term efficacy after a drug has been approved. This could be particularly useful for gene therapy, given our limited knowledge of its long-term effects.
7. Prioritisation of barriers
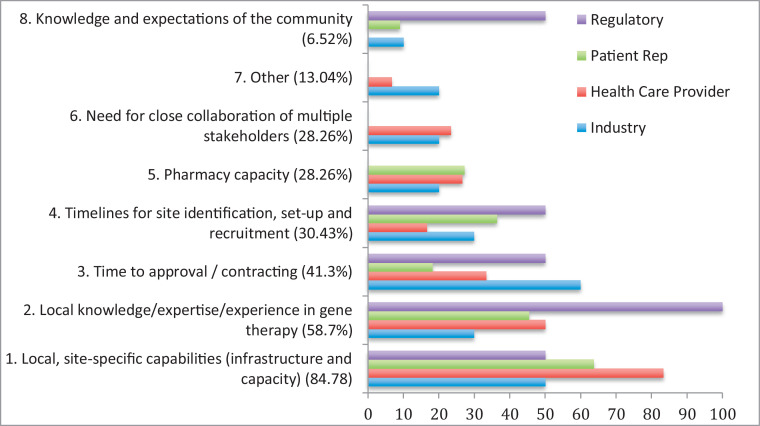
The meeting participants were asked to answer specific questions on identifying potential barriers to delivering gene therapy in the UK (Fig 1 ).
Fig. 1.
Barriers to delivering gene therapy clinical trials in the UK, listed in order of importance (1–8) as identified by all participants then broken down by stakeholders.
The graph (Fig 1) shows the results arranged in order of priority for all participants. The results, broken down by stakeholder, show that the same 4 main barriers were identified by all 4 stakeholders, only in a slightly different order of priority when asked to identify their ‘top 3′. The regulators were the only group to include ‘knowledge and expectations of the community’ with equal importance. ‘Other’ barriers suggested by 13% of participants include environmental risk assessments for a genetically modified organism (GMOs).
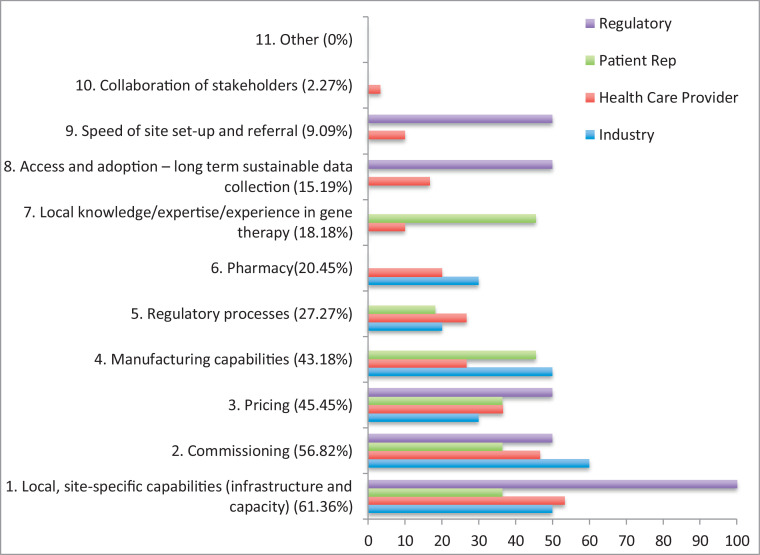
Participants were then asked to rank potential barriers to providing access to gene therapy following successful delivery of gene therapy trials in the UK (Fig 2 ).
Fig. 2.
Barriers to delivering access to gene therapy in the UK following clinical trials, listed by order of importance (1–11) by all participants then broken down by stakeholders.
Again local, site specific capabilities were ranked as the main barrier. When broken down by stakeholder, there was a slightly greater variation in responses regarding the implementation of treatment access with a total of 6 potential barriers (out of 11) appearing in the top 3 identified by the 4 stakeholders.
8. DMD Hub call to action
8.1. Proposed models of delivery
Michela Guglieri (Newcastle University and Newcastle Hospitals NHS Foundation Trust) highlighted that several aspects need to be considered when developing models for gene therapy delivery in clinical trials and in a clinical setting, once the drug is approved. These may include capacity limitations across different sites as also shown in Figs. 1 and 2, the level of expertise in terms of setting up and delivering gene therapy clinical trials and the ability to provide patient follow up over a prolonged period of time.
As detailed in the results of the DMD Hub Institutional Readiness survey, only a limited number of sites in the UK are currently able to deliver gene therapy trials in paediatric patients. Therefore, it is important to be realistic about the number of sites that should be involved in gene therapy trials whilst exploring how follow up care can be delivered. A ‘hub and spoke’ model may be one option, where the gene therapy is delivered in a small number of centres and follow up is carried out elsewhere, more local to the patient. This model relies on ensuring that the knowledge and expertise required for safety follow up is in place in the ‘spoke’ centres.
The impact of gene therapy trials on the general trial capacity at sites in the UK should be evaluated and might require a redistribution of other nongene therapy studies to those DMD Hub sites that may not yet be able to deliver gene therapy trials. The DMD Hub could help to coordinate this trial allocation with input from sites and trial sponsors.
Patient burden and retention is another issue that must be considered, as the follow up period for gene therapy trials is considerable and the importance of ensuring that patients continue to comply with the follow up assessments cannot be overlooked. Equitable access to clinical trials might become an even bigger issue with gene therapy trials. The DMD Hub initiative to develop a procedure for Fair and Equitable Access to Clinical Trials aims to ensure that not only patients seen at delivery sites will have access to gene therapy trials.
There is interest and enthusiasm to take on gene therapy trials in the UK and there are established networks of clinical trial sites and clinicians, such as the North Star Network, to facilitate sharing knowledge and experience, which will be essential for the long term planning for delivery and follow up of approved treatments. Coordination is required to be able to optimise delivery and access, and the DMD Hub is well placed to be able to lead on this.
9. Workshop key deliverables
A key outcome of the meeting was to ensure the community works together as effectively as possible to bring gene therapy trials to the UK in a timely manner. In order to facilitate this, a number of deliverables were identified as a result of this workshop and are detailed below:
-
1.
Develop a process for central coordination between sites interested in participating in gene therapy trials. This would include developing standardised procedures for recruitment to clinical trials, implementation of a centralised costing model and central coordination of interested sites.
-
2.
Develop and agree a model for delivering DMD gene therapy trials in the UK, possibly via a hub and spoke model.
-
3.
Expand the DMD Hub Toolkit to act as a repository for shared resources and guidance documents developed by key collaborators relevant to gene therapy whilst also developing DMD specific documents.
-
4.
Ensure links with existing initiatives such as Catapult, NAATTC, TREAT NMD and UKRD are maintained and utilised.
-
5.
Outline plans for postmarketing data collection requirements in collaboration with existing initiatives such as Hercules and TREAT NMD to understand what data is required to be collected postapproval.
-
6.
Facilitate engagement with UK commissioners early in the process to discuss issues such as interrogation of data at an early stage, how to deliver the therapies (following approval) as part of the standards of care, the role of newborn screening and innovative payment mechanisms.
-
7.
Provide the appropriate education and communication required for patients to manage expectations relating to gene therapy and provide appropriate training to sites on how to disseminate information relating to gene therapy trials to patients.
-
8.
Build on the DMD gene therapy patient preferences information currently available in light of the new data in the human population, specific to the UK.
-
9.
Conduct a more detailed version of the institutional readiness survey to drill down on the true capabilities and facilities at the sites, in collaboration with DMD Hub, North Star sites, industry, NA ATTC and Catapult.
-
10.
Consider how the DMD Hub can influence the speed of R&D processes and ethics board reviews for gene therapy trials including exploring the idea of centralised gene therapy ethics committees, central coordination of costing and contracting and harmonisation of risk assessment review committees.
-
11.
Establish subgroups to discuss specific areas of work identified during the course of the workshop to address and discuss the issues whilst relevant. The subcommittees to include: a) Engagement with commissioners, b) Post Marketing, c) Education, d) Risk Assessments, R&D and Ethics committees.
-
12.
Work with Contract Research Organisations to discuss and prepare for gene therapy trials to enhance their understanding, promoting competent CROs.
-
13.
Publish a lay workshop report to share lessons learnt with the patient community.
10. Conclusions
Participants agreed that the meeting was very timely and the feedback to the discussions was generally positive. There was a clear need for the discussion to take place and the key deliverables agreed upon following the workshop will ensure that the DMD Hub has a significant impact on facilitating and delivering of gene therapy clinical trials in the UK.
Since the workshop was held and the report written clinical trials in the UK have been delayed by the impact of COVID-19. It is a real testament to the drive to set up clinical trials in gene therapy that despite the crisis, gene therapy trials have been the least affected. NHS Trusts are keen to push forward with the required setup documentation and the DMD Hub has continued to liaise with patients, sites and industry to move forward as quickly as possible to ensure that DMD gene therapy trial in the UK become a reality in 2020.
10.1. Workshop speakers
Ron Akehurst (Sheffield, UK),
Zoya Alhaswani (Birmingham, UK),
Maria Allen (Newcastle, UK),
Michael Binks (Cambridge, US),
Anne Black (Newcastle, UK),
Anne-Marie Childs (Leeds, UK),
Emily Crossley (London, UK),
Josie Godfrey (London, UK),
Michela Guglieri (Newcastle, UK),
Geraldine Honnet (Paris, France),
Alex Johnson (Preston, UK),
Imran Kausar (London, UK),
Fiona Marley (London, UK),
Gary McCullagh (Manchester, UK),
Francesco Muntoni (London, UK),
Anna Outhwaite (London, UK),
Matthew Peak (Liverpool, UK),
Laurent Servais (Oxford, UK),
James Shaw (Newcastle, UK),
Teji Singh (Cambridge, US),
Stefan Spinty (Liverpool, UK),
Volker Straub (Newcastle, UK),
Tracey Willis (Oswestry, UK).
References
- 1.Wang D., Tai W.L., Guangping G. Adeno associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milone M.C., O'Doherty U. Acute lymphoblastic leukemia. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athanasopoulos T., Foster H., Foster K., Dickson G. Codon optimization of the microdystrophin gene for Duchene muscular dystrophy gene therapy. Methods Mol Biol. 2011;709:21–37. doi: 10.1007/978-1-61737-982-6_2. [DOI] [PubMed] [Google Scholar]
- 4.Duan D. Systemic AAV microdystrophin gene therapy for duchenne muscular dystrophy. Mol Ther. 2018 Oct 3;26(10):2337–2356. doi: 10.1016/j.ymthe.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.England S.B., Nicholson L.V., Johnson M.A., Forrest S.M., Love D.R., Zubrzycka-Gaarn E.E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 6.Verdera H.C., Kuranda K., Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W. Single dose gene replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. doi: 10.1056/nejmoa1706198. [DOI] [PubMed] [Google Scholar]
- 8.Feldman A.G., Parsons J.A., Dutmer C.M., Veerapandiyan A., Hafberg E., Maloney N. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type I. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.05.044. S0022-3476(20)30682-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gansbacher B. Report of a second serious adverse event in a clinical trial of gene therapy for X linked severe combined immune deficiency (X SCID). Position of the European Society of Gene Therapy (ESGT) J Gene Med. 2003;5(3):261–262. doi: 10.2165/00137696-200604020-00003. [DOI] [PubMed] [Google Scholar]
- 10.Oved J.H., Barrett D.M., Teachey D.T. Cellular therapy: immune related complications. Immunol Rev. 2019;290:114–126. doi: 10.1111/imr.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landrum Peay H., Fischer R., Tzeng J.P., Hesterlee S.E., Morris C., Strong Martin A. Gene therapy as a potential therapeutic option for Duchenne muscular dystrophy: a qualitative preference study of patients and parents. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0213649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paquin R.S., Fischer R., Mansfield C., Mange B., Beaverson K., Ganot A. Priorities when deciding on participation in early-phase gene therapy trials for Duchenne muscular dystrophy: a best worst scaling experiment in caregivers and adult patients. Orphanet J Rare Dis. 2019;14:102. doi: 10.1186/s13023-019-1069-6. https://dx.doi.org/10.1186%2Fs13023-019-1069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahane A., Waghmode A., Kapphahn A., Dhuri K., Gupta A., Bahal R. Role of lipid-based and polymer-based nonviral vectors in nucleic acid delivery for next generation gene therapy. Molecules. 2020 Jun 22;25(12):E286. doi: 10.3390/molecules25122866. [DOI] [PMC free article] [PubMed] [Google Scholar]