This living systematic review examines evidence from randomized trials about the effectiveness and harms of remdesivir treatments for adults with suspected or confirmed COVID-19.
Abstract
Background:
Few treatments exist for coronavirus disease 2019 (COVID-19).
Purpose:
To evaluate the effectiveness and harms of remdesivir for COVID-19.
Data Sources:
Several databases, tables of contents of journals, and U.S. Food and Drug Administration and company websites were searched from 1 January through 31 August 2020.
Study Selection:
English-language, randomized trials of remdesivir treatments for adults with suspected or confirmed COVID-19. New evidence will be incorporated using living review methods.
Data Extraction:
Single-reviewer abstraction and risk-of-bias assessment verified by a second reviewer; GRADE (Grading of Recommendations Assessment, Development and Evaluation) methods used for certainty-of-evidence assessments.
Data Synthesis:
Four randomized trials were included. In adults with severe COVID-19, remdesivir compared with placebo probably improves recovery by a large amount (absolute risk difference [ARD] range, 7% to 10%) and may result in a small reduction in mortality (ARD range, -4% to 1%) and a shorter time to recovery or clinical improvement. Remdesivir may have little to no effect on hospital length of stay. Remdesivir probably reduces serious adverse events by a moderate amount (ARD range, −6% to −8%). Compared with a 10-day remdesivir course, a 5-day course may reduce mortality, increase recovery or clinical improvement by small to moderate amounts, reduce time to recovery, and reduce serious adverse events among hospitalized patients not requiring mechanical ventilation. Recovery due to remdesivir may not vary by age, sex, symptom duration, or disease severity.
Limitations:
Low-certainty evidence with few published trials, including 1 preliminary report and 2 open-label trials. Trials excluded pregnant women and adults with severe kidney or liver disease.
Conclusion:
In hospitalized adults with COVID-19, remdesivir probably improves recovery and reduces serious adverse events and may reduce mortality and time to clinical improvement. For adults not receiving mechanical ventilation or extracorporeal membrane oxygenation, a 5-day course of remdesivir may provide similar benefits to and fewer harms than a 10-day course.
Primary Funding Source:
U.S. Department of Veterans Affairs, Veterans Health Administration Office of Research and Development, Health Services Research and Development Service, and Evidence Synthesis Program.
Persons hospitalized with coronavirus disease 2019 (COVID-19) are at substantial risk for prolonged hospitalization, hypoxic respiratory failure, need for advanced airway support, end-organ damage, and death (1, 2). Because of demands for evaluating and approving safe and effective COVID-19 therapies, the U.S. Food and Drug Administration (FDA) established a Coronavirus Treatment Acceleration Program to assist manufacturers in navigating administrative requirements and to expedite the review process (3).
Remdesivir is a nucleotide analogue prodrug that inhibits viral RNA polymerases and is administered intravenously. Although developed as a potential treatment of other viral infections, including Ebola, it has shown in vitro activity against severe acute respiratory syndrome coronavirus 2 (4, 5). On 1 May 2020, remdesivir was granted an Emergency Use Authorization by the FDA for the treatment of hospitalized patients with suspected or confirmed severe COVID-19, defined as an oxygen saturation of 94% or less on room air or the need for supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) (6). This approval was based on preliminary results from the ACTT-1 (Adaptive COVID-19 Treatment Trial) funded by the National Institute of Allergy and Infectious Diseases (NCT04280705) (7) and on results of an open-label trial that evaluated different durations of remdesivir therapy (NCT04292899) (8). The Emergency Use Authorization concluded that remdesivir may be effective when used under specified conditions. However, remdesivir is not yet licensed or approved for use in the United States, and it is critical to rigorously assess premarketing evidence of drug effectiveness and safety to avoid widespread use of ineffective, harmful, and costly medications. To evaluate and disseminate information and inform the American College of Physicians Scientific Medical Policy Committee in the development of their Practice Points about the use of remdesivir, we conducted a living rapid review to assess the clinical effectiveness and harms of remdesivir in adults with COVID-19. We also sought to assess whether effectiveness and harms varied by symptom duration, disease severity, and treatment duration.
Methods
Overview
This article is an expansion of a rapid review prepared by the Minneapolis VA Evidence Synthesis Program to provide guidance for the Veterans Health Administration in the treatment of hospitalized adults with COVID-19. The literature search has been updated and expanded to include additional sources, and analyses have been revised to include recently published data.
Literature Search and Data Sources
We searched MEDLINE; Cochrane Central Register of Controlled Trials (CENTRAL); World Health Organization (WHO) COVID-19 Database; National Institutes of Health (NIH) COVID-19 iSearch portfolio; ClinicalTrials.gov; tables of contents of the JAMA Network, The Lancet, and the New England Journal of Medicine; and FDA and company websites from 1 January through 31 August 2020. Search terms included remdesivir and terms synonymous with COVID-19 (Supplement Table 1).
Study Selection
English-language, randomized controlled trials reporting on remdesivir for treatment of adults with confirmed or suspected COVID-19 were included. Studies were eligible if they compared remdesivir versus placebo, standard care, or another agent; different durations of remdesivir therapy versus each other; or remdesivir in combination with other agents versus remdesivir alone. The investigators screened and reviewed abstracts or company websites to identify eligible trials. Final inclusion was determined by the principal investigator (T.J.W.).
Data Abstraction and Risk-of-Bias Assessment
Study information, population, disease severity, intervention, and outcomes data were abstracted by 1 investigator (R.M.) and verified by a second (N.G.). Risk of bias was assessed using a modified approach developed by Cochrane and based on the following elements: allocation concealment, blinding, incomplete outcome data (attrition), and selective reporting (9). A study with low risk of bias would have at least 3 elements that were rated as low, with no additional elements that were rated as high. Risk of bias was assessed by 1 investigator (R.M.) and reviewed by a second (N.G.). Discrepancies were resolved through discussion.
Data Synthesis and Analysis
Data were summarized narratively because of the small number of included trials, heterogeneity in study populations, and differences in the controls used between studies. We will consider pooling in future updates if enough similar trials are identified. We used Comprehensive Meta-Analysis, version 3 (Biostat), to calculate absolute risk differences (ARDs) with corresponding 95% CIs. We present absolute rather than relative effects because risk differences are more readily interpreted and provide more directly relevant information (9). We defined critical outcomes as death, recovery, hospital length of stay, and serious adverse events. Important outcomes included clinical improvement, time to recovery or clinical improvement, receipt of invasive mechanical ventilation or ECMO, and any adverse events. We assessed outcomes for subgroups of interests when available, as provided by the study authors, including race, sex, age, baseline disease severity, symptom duration category, oxygenation, and invasive mechanical ventilation status. We assessed evidence certainty for critical and important outcomes on the basis of methods developed by the GRADE (Grading of Recommendations Assessment, Development and Evaluation) working group (Supplement Table 2) (10). We defined thresholds for determining magnitude of effect for critical and important outcomes a priori and discussed these with the American College of Physicians Scientific Medical Policy Committee.
Living Review
We plan to update our literature search, using the search strategy outlined earlier, every 2 months through December 2021 for new evidence related to the effectiveness and harms of remdesivir. Study eligibility criteria, procedures for data abstraction, risk-of-bias assessment, and data synthesis and analysis will remain the same. New evidence that does not substantially change review conclusions will be summarized every 2 months. New evidence that changes review conclusions or certainty of evidence will be incorporated into a major update. We will use previously reported statistical methods for updating meta-analyses in living reviews if meta-analyses are done (11).
Role of the Funding Source
Funding for the Evidence Synthesis Program review was provided by the Veterans Health Administration Office of Research and Development, Health Services Research and Development Service. The funding source assigned the topic but was not involved in data collection, analysis, manuscript preparation, or submission.
Results
Overview of Randomized Trials
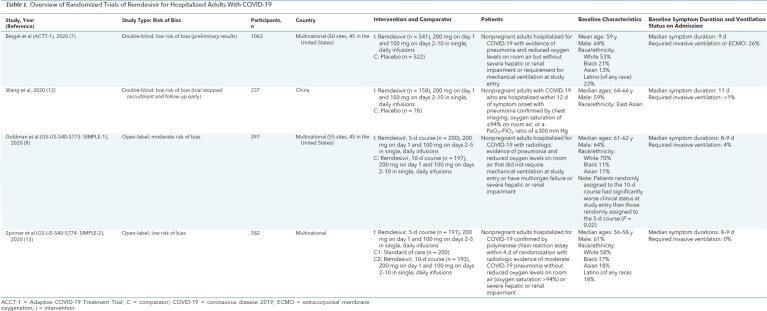
From the 645 citations in our literature search, we reviewed the full text of 89 articles and identified 4 randomized trials (Figure; Table 1; Supplement Tables 2 to 8). All included hospitalized adults. Two trials compared remdesivir with placebo (7, 12) for 10 days (12) or for up to 10 days or until hospital discharge (7). The other 2 trials assessed 5- versus 10-day remdesivir courses (8, 13), and 1 of these studies included a third, standard-of-care group (13). Risk of bias was rated as low in 3 trials (7, 12, 13) and moderate in 1 (8) (Supplement Table 9). All studies evaluated remdesivir administered intravenously, with 200 mg on day 1 and 100 mg on subsequent days. Patients were approximately 60 years old, and most were men and White. Studies excluded patients who were pregnant or had severe renal or hepatic dysfunction. Three trials (Wang and colleagues [12], ACTT-1 [7], and SIMPLE-1 [8]) included patients with severe COVID-19, defined as hospitalized patients meeting 1 or more of the following criteria: radiographic infiltrates by imaging, an oxygen saturation of 94% or less on room air, tachypnea (respiratory rate >24 breaths/min without supplemental oxygen), or a requirement for supplemental oxygen or mechanical ventilation. Goldman and colleagues (SIMPLE-1) (8) excluded patients who required invasive mechanical ventilation or ECMO at baseline. Beigel and colleagues (ACTT-1) (7) also included patients with mild to moderate disease (n = 119; 11.2% of all enrollees), defined as having an oxygen saturation greater than 94% and respiratory rate less than 24 breaths/min without supplemental oxygen. The fourth trial, SIMPLE-2 (13), included only patients with moderate COVID-19, defined as having radiographic infiltrates and oxygen saturation on room air greater than 94%. These disease severity definitions differed from those provided by the NIH, WHO, and FDA (Supplement Table 10). Median symptom duration ranged from 8 to 10 days. Primary outcomes were recovery or clinical status improvement defined according to an ordinal scale that included death and use of supplemental oxygen or mechanical ventilation. Definitions of recovery and clinical improvement varied across studies. Outcomes data are summarized in absolute terms, except for a few instances where relative effects provided by the trialists are noted.
Figure. Evidence search and selection.
Table 1. Overview of Randomized Trials of Remdesivir for Hospitalized Adults With COVID-19.
Summary of Findings
Remdesivir Compared With Placebo
Two studies (7, 12) compared remdesivir with placebo (Table 2; Supplement Tables 4 to 8). They provided information on remdesivir effectiveness and harms in adults primarily with severe COVID-19. Compared with placebo, remdesivir may result in a small reduction in mortality (ARD, −4% to 1%) (low certainty), probably increases recovery by a large amount (ARD, 7% to 10%) (moderate certainty), may result in a small reduction in time to clinical improvement and a large reduction in time to recovery (low certainty), and may result in a small reduction in the need for mechanical ventilation or ECMO (low certainty). However, remdesivir may have little to no effect on hospital length of stay (low certainty). Remdesivir probably reduces serious adverse events by a moderate amount (ARD, −8% to −6%) (moderate certainty) and provides a small reduction in nonserious adverse events (low certainty).
Table 2. Effect of Remdesivir in Randomized Controlled Studies.
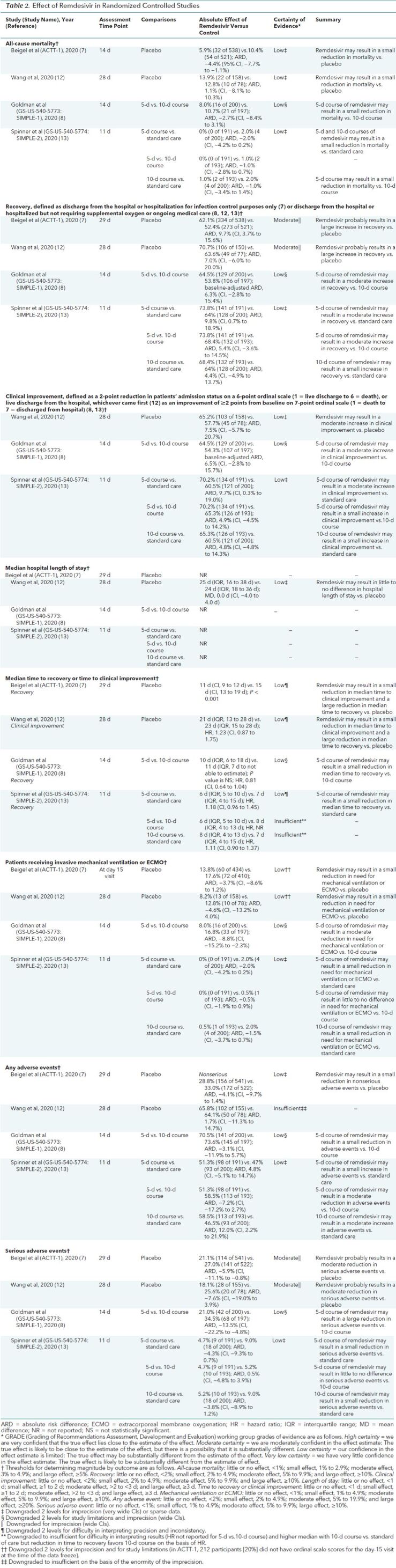
The study by Wang and colleagues (12) was done in China and was discontinued after enrolling about half of its target population because of stated control of the COVID-19 outbreak. The primary outcome was time to clinical improvement, defined as the time from randomization to the point of a decline of 2 levels on a 6-point ordinal scale of clinical status (where 1 indicates discharged and 6 indicates death) or discharge alive from the hospital, whichever came first. A 10-day course of remdesivir, compared with placebo, resulted in a nonsignificant increase in the percentage of patients with clinical improvement at day 28 (65.2% [103 of 158] vs. 57.7% [45 of 78]; ARD, 7.5% [95% CI, −5.7% to 20.7%]) and a decrease in the median time to clinical improvement (21 days [interquartile range {IQR}, 13 to 28 days] vs. 23 days [IQR, 15 to 28 days]; hazard ratio [HR], 1.23 [CI, 0.87 to 1.75]). Information was also available to develop a recovery outcome, defined as discharge from the hospital or hospitalized but not requiring supplemental oxygen (items 1 and 2 on the 6-point ordinal scale). Compared with placebo, remdesivir resulted in a nonsignificant increase in the percentage of patients that recovered at day 28 (70.7% [106 of 150] vs. 63.6% [49 of 77]; ARD, 7.0% [CI, −6.0% to 20.0%]). It did not significantly reduce median hospital length of stay (25 vs. 24 days; mean difference, 0.0 days [range, −4.0 to 4.0 days]), need for invasive mechanical ventilation (8.2% vs. 12.8%; ARD, −4.6% [CI, −13.2% to 4.0%]), or mortality at 28 days (13.9% [22 of 158] vs. 12.8% [10 of 78]; ARD, 1.1% [CI, −8.1% to 10.3%]). The effectiveness of remdesivir in reducing mortality and time to clinical improvement did not vary by symptom duration (≤10 days vs. >10 days). There was a nonsignificant moderate reduction in serious adverse events in patients receiving remdesivir compared with placebo (18.1% [28 of 155] vs. 25.6% [20 of 78]; ARD, −7.6% [CI, −19.0% to 3.9%]). Measures of viral load or undetectable viral RNA in sputum or naso-oropharyngeal swabs by day 28 did not differ between remdesivir and placebo.
The ACTT-1 study published preliminary results after 69% of enrolled participants completed follow-up (7). The primary outcome was time to recovery, defined as the first day during the 28 days of enrollment on which a patient satisfied category 1, 2, or 3 on an 8-point ordinal scale (where 1 indicates not hospitalized with no limitations of activities; 2 indicates not hospitalized but with limitation of activities, home oxygen requirement, or both; and 3 indicates hospitalized, not requiring supplemental oxygen, and no longer requiring medical care). Patients randomly assigned to remdesivir received treatment for up to 10 days or until hospital discharge. Among persons with complete adherence data available, 40.8% used a 10-day course and 38.1% received fewer than 10 doses because they recovered and were discharged from the hospital. Compared with placebo, remdesivir resulted in a shorter time to recovery (median, 11 days [CI, 9 to 12 days] vs. 15 days [CI, 13 to 19 days]; rate ratio for recovery, 1.32 [CI, 1.12 to 1.55]). Remdesivir increased the percentage of patients that recovered (62.1% [334 of 538] vs. 52.4% [273 of 521]; ARD, 9.7% [CI, 3.7% to 15.6%]) and resulted in numerically lower mortality at 14 days (5.9% [32 of 538] vs. 10.4% [54 of 521]; ARD, −4.4% [CI, −7.7% to −1.1%]; HR for death, 0.7 [CI, 0.47 to 1.04]). Compared with placebo, remdesivir did not significantly reduce the need for invasive mechanical ventilation on day 15 (13.8% vs. 17.6%; ARD, −3.7% [CI, −8.6% to 1.2%]). Remdesivir, compared with placebo, reduced serious adverse events (21.1% [114 of 541] vs. 27.0% [141 of 522]; ARD, −5.9% [CI, −11.1% to −0.8%]) and led to a small but nonsignificant reduction in nonserious adverse events (ARD, −4.1% [CI, −9.7% to 1.4%]). The effectiveness of remdesivir in shortening time to recovery did not vary by prespecified subgroups of age (categories), sex, symptom duration (≤10 days vs. >10 days), or disease severity (mild to moderate or severe). However, in patients receiving invasive mechanical ventilation or ECMO at study entry (25.7% of enrollees; critical-severity COVID-19, as defined by NIH, WHO, and FDA criteria), recovery was not improved with remdesivir (rate ratio for recovery, 0.95 [CI, 0.64 to 1.42]).
Duration of Remdesivir Treatment: 5 Days Versus 10 Days Versus Standard of Care
Information on the effectiveness, comparative effectiveness, and harms of shorter (5 days) versus longer (10 days) durations of remdesivir therapy was available from 2 randomized, open-label trials—1 in hospitalized adults with severe disease who did not require mechanical ventilation (SIMPLE-1) (8) and the other in those with moderate COVID-19 (SIMPLE-2) (13) (Table 2; Supplement Tables 4 to 8). The SIMPLE-2 study also included a standard-of-care comparison. The primary outcome for both trials was clinical status on day 11 (13) or 14 (8) based on a predefined 7-point scale, ranging from hospital discharge to increasing levels of oxygen support to death. Both studies also reported clinical improvement by day 11 or 14, defined as an improvement of 2 or more points from baseline on this 7-point scale.
In SIMPLE-1 (severe COVID-19), patients randomly assigned to the 10-day course had significantly worse clinical status at study entry than those randomly assigned to the 5-day course (P = 0.020) (8). After adjustment for baseline differences in clinical status, clinical status distribution at day 14 was similar between groups (P = 0.140). A 5-day course of remdesivir may result in a moderate increase in recovery at 14 days (defined as discharge from the hospital or hospitalized but not requiring supplemental oxygen or ongoing care) compared with a 10-day course (64.5% [129 of 200] vs. 53.8% [106 of 197]; baseline-adjusted ARD, 6.3% [CI, −2.8% to 15.4%]) (low certainty). A small reduction in mortality was also observed at day 14 for a 5-day versus a 10-day course (8.0% [16 of 200] vs. 10.7% [21 of 197]; ARD, −2.7% [CI, −8.4% to 3.1%]) (low certainty). The percentage of patients having clinical improvement was moderately higher with a 5-day course than a 10-day course (64.5% [129 of 200] vs. 54.3% [107 of 197]; baseline-adjusted ARD, 6.5% [CI, −2.8% to 15.7%]) (low certainty). Compared with a 10-day course, a 5-day course of remdesivir may result in a moderate reduction in the need for mechanical ventilation (8.0% vs. 16.8%; ARD, −8.8% [CI, −15.2% to −2.3%]) and a small reduction in median time to recovery (10 days [IQR, 6 to 18 days] vs. 11 days [IQR, 7 days to not able to estimate]; HR, 0.81 [CI, 0.64 to 1.04]). Numerically, more patients in the 5-day group than in the 10-day group were discharged from the hospital (60% [120 of 200] vs. 52.3% [103 of 197]; ARD, 7.7% [CI, −2.0% to 17.4%]). In post hoc analyses, treatment beyond 5 days among patients who were receiving noninvasive positive-pressure ventilation, high-flow oxygen, or low-flow oxygen or were breathing ambient air did not improve outcomes. However, among patients who progressed to require mechanical ventilation or ECMO at day 5, mortality was higher in the 5-day group than in the 10-day group (40.0% [10 of 25] vs. 17.1% [7 of 41]; ARD, 22.9% [CI, 0.5% to 45.3%]). In post hoc analyses based on pooling of data across remdesivir treatment duration groups, the percentage of patients discharged from the hospital was numerically higher among those who received remdesivir within 10 days of symptom onset than among those treated after more than 10 days of symptoms (62% vs. 49%). Compared with the 10-day course of remdesivir, the 5-day course may result in a large reduction in serious adverse events (21.0% [42 of 200] vs. 34.5% [68 of 197]; ARD, −13.5% [CI, −22.2% to −4.8%]) and a small reduction in any adverse events.
In the 3-group SIMPLE-2 study (moderate COVID-19) (13), a 5-day course of remdesivir compared with standard of care may result in a greater percentage of patients with recovery (defined as discharge from the hospital or hospitalized but not requiring supplemental oxygen or ongoing care) at day 11 (73.8% [141 of 191] vs. 64% [128 of 200]; ARD, 9.8% [CI, 0.7% to 18.9%]) (low certainty) and clinical improvement at day 11 (70.2% [134 of 191] vs. 60.5% [121 of 200]; ARD, 9.7% [CI, 0.3% to 19.0%]) (low certainty). Clinical improvement was similar in the 5-day versus 10-day groups and 10-day versus standard-of-care groups (ARD, 5%) (low certainty). Although deaths were infrequent in each group, both 5-day and 10-day courses of remdesivir may result in a small reduction in mortality versus standard care, and a 5-day course may result in a small reduction in mortality versus a 10-day course. A 5-day course of remdesivir may result in a small reduction in median time to recovery versus standard of care (6 days [IQR, 5 to 10 days] vs. 7 days [IQR, 4 to 15 days]; HR, 1.18 [CI, 0.96 to 1.45]) (low certainty). There may be a small difference between the 5-day course and standard of care (4.7% [9 of 191] vs. 9.0% [18 of 200]; ARD, −4.3% [CI, −9.3% to 0.7%]) and no difference between the 5-day and 10-day courses (4.7% [9 of 191] vs. 5.2% [10 of 193]; ARD, 0.5% [CI, −4.8% to 3.9%]) (low certainty). There may be a moderate increase in any adverse event with a 10-day course compared with standard of care (58.5% [113 of 193] vs. 46.5% [93 of 200]; ARD, 12.0% [CI, 2.2% to 21.9%]) (low certainty).
Ongoing Studies
Several trials are ongoing or in development to evaluate the comparative effectiveness and harms of remdesivir alone or in combination with other potential therapies (14). The ACTT-1 study is continuing to evaluate enrolled patients and is expected to provide additional information based on longer follow-up. The SIMPLE-1 study evaluating 5 versus 10 days of remdesivir therapy in adults with severe COVID-19 is enrolling up to 5600 additional patients, including evaluating the 10-day course for patients receiving mechanical ventilation. The SIMPLE-2 study among adults with moderate disease is enrolling up to 1000 additional patients. Additional, large randomized trials are evaluating the effectiveness and comparative effectiveness of remdesivir alone or in combination with other agents or potentially active comparators, including baricitinib (ACTT-II) (15), interferon-β1a (ACTT-III) (16), tocilizumab (REMDACTA [A Study to Evaluate the Efficacy and Safety of Remdesivir Plus Tocilizumab Compared With Remdesivir Plus Placebo in Hospitalized Participants With Severe COVID-19 Pneumonia]) (17), lopinavir with ritonavir plus interferon-β1a versus standard of care (Solidarity) (18), and lopinavir and ritonavir versus interferon-β1a versus hydroxychloroquine versus standard of care (DisCoVeRy [Trial of Treatments for COVID-19 in Hospitalized Adults]) (19). An inhaled, nebulized version of remdesivir to treat patients with COVID-19 in outpatient settings is also being evaluated (20). Other trials of remdesivir in combination with anti-inflammatory drugs in vulnerable patient populations and in outpatient settings are ongoing or planned for future initiation. An open-label, single-group study (CARAVAN [Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir {GS-5734™} in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019]) is planning to enroll pediatric patients (including newborns and adolescents) hospitalized with moderate COVID-19, with the primary outcome measures being treatment-emergent adverse events and laboratory abnormalities (21). Secondary outcomes will include oxygen use, mechanical ventilation, clinical improvement, and time to hospital discharge.
Discussion
This living systematic review of 4 published randomized trials found that among adults hospitalized with severe COVID-19, remdesivir for up to 10 days, compared with placebo, may result in a small reduction in mortality and probably results in a large improvement in recovery and decrease in time to recovery but may have little to no effect on hospital length of stay (7, 12). Remdesivir probably reduces serious adverse events by a moderate amount and may reduce any adverse event by a small amount. Recovery due to remdesivir may not vary by patient age, sex, race, or time from symptom onset but may be limited to patients who are not already receiving invasive mechanical ventilation or ECMO (critical-severity COVID-19) (7).
Compared with a 10-day treatment course, a 5-day course may reduce mortality by a small amount, increase recovery by a moderate amount, and reduce serious adverse events by a large amount among hospitalized patients with severe COVID-19 who do not require mechanical ventilation at study entry (8). However, among patients whose symptoms worsen and who require mechanical ventilation or ECMO on day 5 of the remdesivir course, continuing treatment through 10 days may be beneficial versus discontinuing it on day 5. For adults hospitalized with moderate COVID-19, a 5-day course of remdesivir compared with standard of care may result in small decreases in mortality and serious adverse events and a greater percentage of persons having clinical improvement at day 11 (13). A 10-day course was not more effective than 5 days or standard of care.
Our findings are generally in line with prior reviews, although none included the trial of remdesivir in patients with moderate COVID-19 (13), and outcomes reported in prior reviews were limited (22–26). Our living review uses methods specifically derived for continual updating and is intended, in part, to inform the work of the American College of Physicians Scientific Medical Policy Committee. We derived thresholds to define magnitude of benefit to inform policymakers and assist in the development of certainty of evidence. Stakeholders may select different thresholds to define magnitude of clinical benefit, which may alter evidence certainty and decision making. We included new information from the SIMPLE-2 study and will update our report with information from ongoing studies evaluating remdesivir alone or in combination with other therapies. We also provide specific information on findings about treatment duration and tradeoffs in benefits, harms, and costs for patients with different disease severities.
Current research has limitations. Few studies exist, some information is based on preliminary findings of published results, and 2 of the 4 studies are open-label. Most studies use time to recovery or improvement as their primary outcome. However, in comparative clinical studies of COVID-19, patients may die before recovery or improvement occurs, which can bias treatment effect estimates. This, combined with other issues relevant to short-term studies in critical care, has led to recommendations for how such studies should quantify and interpret treatment effects (27). Disease severity definitions varied slightly across studies and did not fully align with those provided by the NIH, WHO, or FDA (Supplement Table 8). Changing definitions of disease severity may alter the benefit–risk profile of remdesivir from that currently reported. The ACTT-1 study enrolled hospitalized patients with mild to moderate disease but did not provide a definition or outcomes separately for those with mild versus moderate disease, thus making interpretation of these findings difficult. Pregnant women and patients with severe renal and hepatic dysfunction were excluded from trials. Therefore, results may not apply to these persons, including the finding of lack of serious harms. The FDA advises caution in the use of remdesivir among pregnant women and recommends against use in patients with an estimated glomerular filtration rate less than 30 mL/min/1.73 m2, unless the potential benefits outweigh the potential risks. Additional harms reported to the FDA from clinical studies of remdesivir, which were not identified in the current studies, include infusion-related allergic reactions and elevated liver transferase levels (6). The FDA recommends that clinicians assess kidney and hepatic function at baseline and during treatment. Remdesivir should be withdrawn if alanine aminotransferase levels increase to 5 or more times the upper limit of normal or if any alanine aminotransferase elevation is accompanied by signs or symptoms of liver inflammation or increasing conjugated bilirubin levels, alkaline phosphatase levels, or international normalized ratio. The FDA has also recommended against the coadministration of chloroquine or hydroxychloroquine because of the potential for reduced antiviral activity of remdesivir. More important, remdesivir has not yet received FDA approval for use. It is available through an Emergency Use Authorization to treat hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19.
The manufacturer has announced that it will charge governments in the developed world, including the U.S. government's Indian Health Service and the Department of Veterans Affairs, $2340 for a 5-day course of remdesivir. Insurers in the United States, in addition to Medicare and Medicaid, will pay 33% more, or $3120 for a 5-day treatment course ($520 per vial). The price for those without insurance will be $390 per vial (28). Drug availability is limited, and measures to allocate this scarce resource equitably are needed. Treating most patients for 5 days rather than 10 days may result in similar outcomes and would lower drug cost and increase availability of limited drug supplies.
In conclusion, our review of the published studies indicates that in hospitalized adults with COVID-19, remdesivir probably improves recovery and reduces serious adverse events and may reduce mortality and time to clinical improvement, although with little to no difference in hospital length of stay. Recovery due to remdesivir may not vary by age, sex, symptom duration, or disease severity. For patients not receiving mechanical ventilation or ECMO, a 5-day course may provide similar benefits to a 10-day course, with fewer harms and lower drug costs. New evidence will be incorporated using living review methods and may alter these conclusions.
Supplementary Material
Footnotes
This article was published at Annals.org on 5 October 2020
Update Alerts: The authors have specified in the Methods section the interval and stop date for updates to this living review. As Annals receives updates, they will appear in the Comments section of the article on Annals.org. Reader inquiries about updates that are not available at approximately the specified intervals should be submitted as comments to the article.
References
- 1. Richardson S, Hirsch JS, Narasimhan M, et al; Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052-2059. [PMID: 32320003] doi:10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed]
- 2. Duan-Porter W. COVID-19: intensive care unit length of stay and ventilation days. Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP project no. 09-009. 2020. Accessed at https://mfr.osf.io/render?url=https://osf.io/peyux/?direct%26mode=render%26action=download%26mode=render on 15 June 2020.
- 3. Rome BN, Avorn J. Drug evaluation during the covid-19 pandemic. N Engl J Med. 2020;382:2282-2284. [PMID: 32289216] doi:10.1056/NEJMp2009457 [DOI] [PubMed]
- 4. Mulangu S, Dodd LE, Davey RT Jr, et al; PALM Writing Group. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303. [PMID: 31774950] doi:10.1056/NEJMoa1910993 [DOI] [PMC free article] [PubMed]
- 5. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [PMID: 31924756] doi:10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed]
- 6. U.S. Food and Drug Administration. Fact sheet for health care providers: Emergency Use Authorization (EUA) of Veklury (remdesivir). Accessed at www.fda.gov/media/137566/download on 24 July 2020.
- 7. Beigel JH, Tomashek KM, Dodd LE, et al; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. 2020. [PMID: 32445440] doi:10.1056/NEJMoa2007764 [DOI] [PubMed]
- 8. Goldman JD, Lye DCB, Hui DS, et al; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe covid-19. N Engl J Med. 2020. [PMID: 32459919] doi:10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed]
- 9. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Accessed at http://handbook.cochrane.org on 24 July 2020.
- 10. Schünemann H, Broźek J, Guyatt G, et al, eds. GRADE Handbook. Accessed at https://gdt.gradepro.org/app/handbook/handbook.html on 2 June 2020.
- 11. Simmonds M, Salanti G, McKenzie J, et al; Living Systematic Review Network. Living systematic reviews: 3. Statistical methods for updating meta-analyses. J Clin Epidemiol. 2017;91:38-46. [PMID: 28912004] doi:10.1016/j.jclinepi.2017.08.008 [DOI] [PubMed]
- 12. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [PMID: 32423584] doi:10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed]
- 13. Spinner CD, Gottlieb RL, Criner GJ, et al; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048-1057. [PMID: 32821939] doi:10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed]
- 14. Gilead Sciences. Remdesivir clinical trials. Accessed at www.gilead.com/purpose/advancing-global-health/covid-19/remdesivir-clinical-trials on 14 September 2020.
- 15. Adaptive COVID-19 Treatment Trial 2 (ACTT-2) [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04401579 on 10 September 2020.
- 16. Adaptive COVID-19 Treatment Trial 3 (ACTT-3) [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04492475 on 10 September 2020.
- 17.A Study to Evaluate the Efficacy and Safety of Remdesivir Plus Tocilizumab Compared With Remdesivir Plus Placebo in Hospitalized Participants With Severe COVID-19 Pneumonia (REMDACTA) [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04409262 on 10 September 2020.
- 18. World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. Accessed at www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments on 15 June 2020.
- 19.Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04315948 on 15 June 2020.
- 20.Study in Participants With Early Stage Coronavirus Disease 2019 (COVID-19) to Evaluate the Safety, Efficacy, and Pharmacokinetics of Remdesivir Administered by Inhalation [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04539262 on 14 September 2020.
- 21.Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734™) in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019 (COVID-19) (CARAVAN) [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04431453 on 10 September 2020.
- 22. Jiang Y, Chen D, Cai D, et al. Effectiveness of remdesivir for the treatment of hospitalized Covid-19 persons: a network meta-analysis. J Med Virol. 2020. [PMID: 32813283] doi:10.1002/jmv.26443 [DOI] [PMC free article] [PubMed]
- 23. Misra S, Nath M, Hadda V, et al. Efficacy of various treatment modalities for nCOV-2019: a systematic review and meta-analysis. Eur J Clin Invest. 2020:e13383. [PMID: 32810285] doi:10.1111/eci.13383 [DOI] [PMC free article] [PubMed]
- 24. Yokoyama Y, Briasoulis A, Takagi H, et al. Effect of remdesivir on patients with COVID-19: a network meta-analysis of randomized control trials. Virus Res. 2020;288:198137. [PMID: 32827627] doi:10.1016/j.virusres.2020.198137 [DOI] [PMC free article] [PubMed]
- 25. Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. [PMID: 32732190] doi:10.1136/bmj.m2980 [DOI] [PMC free article] [PubMed]
- 26. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America. 11 April 2020. Accessed at www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management on 14 September 2020. [DOI] [PMC free article] [PubMed]
- 27. McCaw ZR, Tian L, Vassy JL, et al. How to quantify and interpret treatment effects in comparative clinical studies of COVID-19. Ann Intern Med. 2020. [PMID: 32634024] doi:10.7326/M20-4044 [DOI] [PMC free article] [PubMed]
- 28. O'Day D. An open letter from Daniel O'Day, Chairman & CEO, Gilead Sciences. Stories @ Gilead. 29 June 2020. Accessed at https://stories.gilead.com/articles/an-open-letter-from-daniel-oday-june-29 on 24 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.