Abstract
We studied the effects of a specific cardio training program lasting 5 years on pain and quality of life in fibromyalgia patients.
Method:
An observational longitudinal pilot study was conducted in 138 fibromyalgia women. Fibromyalgia women recruited were asked to carry out three sessions per week, each lasting 45 min, of moderate-intensity continuous training (64%–75% Maximal Heart rate [HRmax]). During the first year, the patients progressively increased their training intensity. During the last 2 years, the patients were asked to associate moderate-intensity continuous training and high-intensity interval training (85%–90% HRmax). Pain on a visual analog scale, anxiety and depression state on the Hospital Anxiety and Depression Scale, impact of fibromyalgia on daily life using the Fibromyalgia Impact Questionnaire, heart rate and sleep quality (visual analog scale) were assessed at baseline and each year for 5 years.
Results:
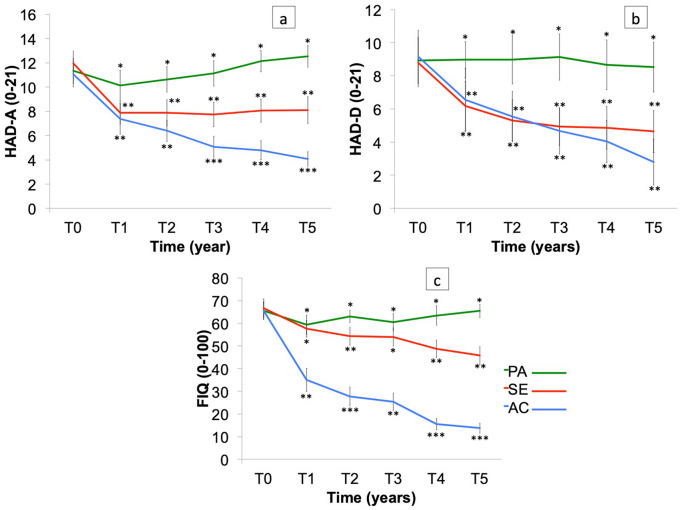
Forty-nine patients dropped out in the first year. Depending on their training status, the remaining 89 patients were retrospectively assigned to one of the three groups: Active (moderate-intensity continuous training), Semi-Active (one or two sessions, low-intensity continuous training <60% HRmax) and Passive (non-completion of training), based on their ability to comply with the program. Alleviation of all symptoms (p < 0.0001) was observed in the Active group. Increasing exercise intensity enhanced the effects obtained with moderate-intensity continuous training. Significant change in the Fibromyalgia Impact Questionnaire (p < 0.0001) and depression (Hospital Anxiety and Depression Scale; p < 0.0001), and no significant decrease in pain were noted in the Semi-Active group. No effect of the training was observed in the Passive group.
Conclusion:
The study intervention associated with multidisciplinary care alleviated pain, anxiety and depression, and improved both quality of life and quality of sleep, in fibromyalgia patients.
Keywords: Fibromyalgia, training sessions, pain, autonomic nervous system, physical activity
Introduction
Fibromyalgia (FM) syndrome is a widespread chronic pain condition characterized by heterogeneous symptoms and functional disability including pervasive pain, sleep disturbances, cognitive dysfunction, emotional disorders, and chronic fatigue. Its diagnosis is based on the symptoms and their severity as described by patients.1,2 FM’s mechanisms are currently better known. FM is considered to be a stress-related syndrome affecting the autonomic nervous system (ANS),3 the hypothalamic–pituitary–adrenal axis (HPA)4,5 and the immunity system.6–9 This stress response dysfunction was the primum movens of the condition, not only as the trigger of the condition but also as a maintaining/reinforcing factor. This leads to a state of deficient adaptation to common life events; in other words, an impairment of the physiological adaptation to trivial daily stress events.8,10,11 This stress axis deficit (HPA and ANS) may secondarily induce dysregulation of pain modulation.4,8,12,13 This neurovegetative dystonia may explain the clinical manifestations of FM (sleep disorders, anxiety, neurovegetative dystonia and associated syndromes such as irritable bowel syndrome and deconditioning syndrome).3
Pharmacological treatments fail to alleviate FM symptoms. On the contrary, all pain associations and best practice guidelines strongly recommend the practice of aerobic physical activity to alleviate symptoms in FM patients.1,14–16 Several studies have shown the effectiveness of cardio exercise training on pain, sleep, anxiety, depression and quality of life in FM patients.14,17 More recently, attention was directed toward the effects of intensity, frequency and type of exercises (endurance versus resistance, continuous training versus interval training) on pain and quality of life.18 However, the duration and especially the intensity of the exercises in these training programs are not unanimous. Bidonde et al.14 reviewed in an “umbrella” nine articles including a total of 60 randomized controlled studies, confirming the overall efficacy of physical activity on FM symptoms. However, the authors caution that, given the nature of the available studies, they are unable to make specific recommendations for an optimal physical activity program in FM patients. Furthermore, the duration of the training programs evaluated in these 60 studies ranged from 4 to 34 weeks with a mean of only 13.5 ± 6.71 weeks, the equivalent of 3 months.14
Thus, truly long-term data associating physical training and FM are almost non-existent. To date, no study has combined moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT) in a training program designed to improve pain and quality of life for FM patients.
The objective of this study was to examine whether this long-term specific exercise therapy (with MICT and HIIT) is associated with changes in pain, and quality of life, in FM women. The results of this observational pilot study might help us better understand the importance of exercise therapy dosage components and could provide a basis for future controlled randomized blinded research in this field.
Methods
The ethics committee of the University Hospital of Brest approved the study design. The patients gave their written informed consent before participating in the study. Procedures were performed in accordance with the standards of the Declaration of Helsinki, except for registration in a database.
Study design
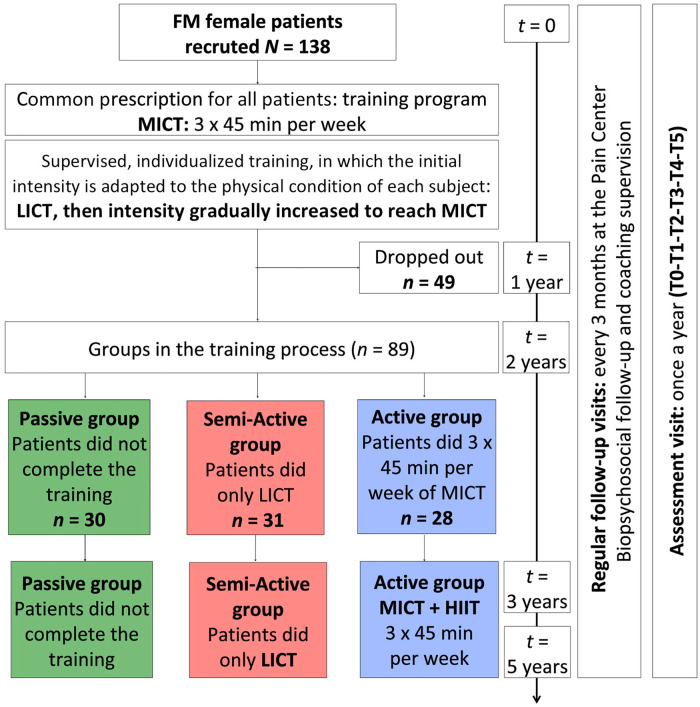
This pilot study was conducted in the Pain Center at the University Hospital of Brest. This is a prospective observational longitudinal study to assess, in a long-term study, the effectiveness of a training program combining MICT and HIIT on symptoms of FM, to provide a basis for future randomized, controlled, blind trials. As this is a pilot study, the number of subjects to be included is guided by the potential for inclusion. Participation in this study was proposed to all women (n = 173) attended the Pain Center between March 2004 and December 2006 and who met the study’s inclusion and non-inclusion criteria. One hundred thirty-eight FM women were recruited. Patients were asked to carry out three sessions per week of a specific training program, two of which were supervised by a physiotherapist. Patients were examined every 3 months at the Pain Center for biopsychosocial follow-up, which examined symptoms and training. An assessment visit was made at the end of every year for 5 years (T1–T5) from 2004 to 2011. All data were collected prospectively. For the analysis of these data, the patients were retrospectively divided into three groups according to the training performed (section “Group assignment”).
Patients
Participants met the following entry criteria: they were women aged 18–74 years, they fulfilled the American College of Rheumatology classification criteria for FM,19 they reported spontaneous pain intensity ⩾3/10 on a visual analog scale (VAS; pain had to be felt at least 3 days a week), their body mass index (BMI) was between 18.5 and 29 kg m−2, they had been on stable doses of medications for FM for ⩾4 weeks, they were covered by a social security scheme and they were aware of the limitations of the program to which gave their free informed consent.
Persons who presented any of the following were excluded: chronic pain unrelated to FM (isolated inflammatory joint, cancer, infectious, traumatic, localized neuropathic or degenerative joint pain); cardiovascular, lung, metabolic, or neurological diseases; conditions that would prohibit physical exercise; severe psychiatric disorders; use of medications that might affect chronotropic response to exercise; pregnancy or breastfeeding; or inability to speak or read French fluently (inability to understand the pain scale and cooperate in testing).
Recording of participant characteristics
During the first visit and at the end of each year for 5 years, pain was assessed on a VAS.20 Participants were asked to mark the point that best corresponded to the intensity of their pain sensation on a non-graduated straight line (scale length 100 mm). “No pain” and “the most pain imaginable” were written at the two ends of the scale. The mean and maximal spontaneous pain felt in the last 7 days was scored. To assess psychological factors such as anxiety and depression, participants filled out the Hospital Anxiety and Depression Scale (HADS).21,22 To assess FM impact in everyday life, participants filled out the French version of the Fibromyalgia Impact Questionnaire (FIQ).23 A VAS was used to assess sleep quality. The level of pharmacological pain therapies was scored on a four-step scale corresponding to the three-step “ladder” of the World Health Organization (Ladders I (paracetamol), II (codeine or tramadol), III (morphine and opioid) and 0 when no pharmacological treatment had been used). No gabapentin or pregabalin were prescribed to FM patient in the Pain Center.16 Heart rate (HR) and blood pressure were measured using an automated oscillometric blood pressure device (Dinamap Procare 400 v2). A single operator performed examinations using a standardized form.
Intervention
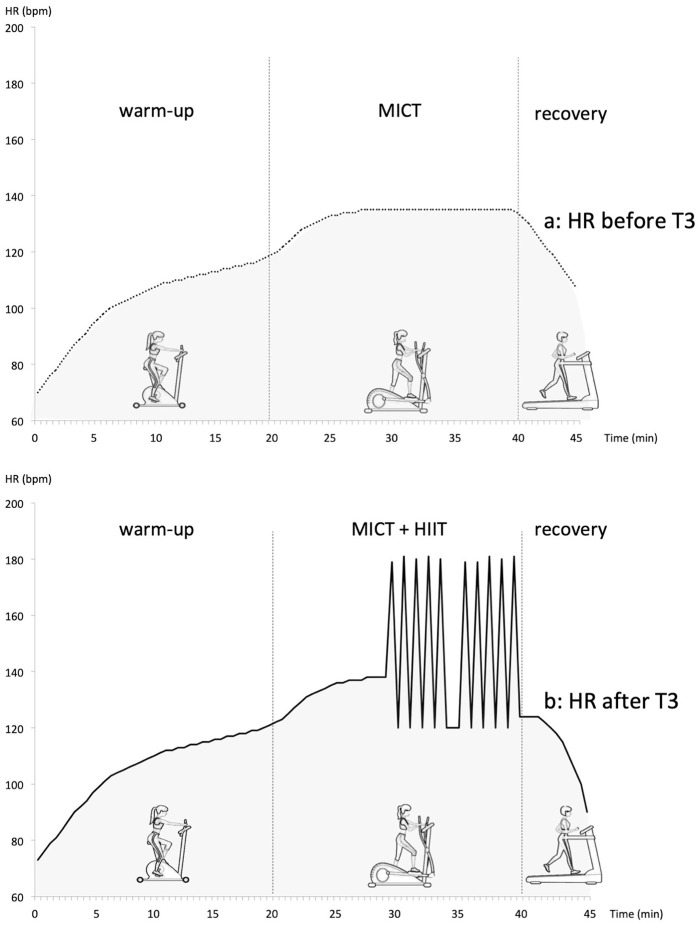
The basis of the cardio training program was common for all the patients, but was individualized for each patient (for frequency, intensity, duration and supervision) based on pain states, capacity for physical effort and other characteristics. Two sessions per week were supervised by a physiotherapist with special expertise in high-level athletic training, rehabilitation of patients and care for patients with chronic pain. One session per week was unsupervised (autonomous management). The pain care practitioner following up the patient in the Pain Center also had expertise in the physiology of physical training. Patients were asked to perform three sessions per week, each lasting 45 min, of MICT (64%–75% Maximal Heart rate [HRmax]).14 Tanaka’s age-based prediction equation (208 − 0.7 × age) is used to calculate HRmax. To favor long-term completion of the training program, patients were free to choose the physical activity type they performed unsupervised. At the early stage, the intensity and duration of the training sessions were adapted to the physical condition of each patient. This intervention had to be easy, non-traumatic and gradual.24 Accordingly, to promote patient adherence and limit pain exacerbation, exercise intensity started very low, and then gradually increased to reach the neurovegetative goal. The objective was to practice physical activity corresponding to 45 min of running at 7 km h−1, 3 times a week for women with BMI in the normal range.25 After 3 years of training, patients performing the exercises (Active group) had increased the intensity of their sessions. Gradually (from T3 to T5), MICT was associated with HIIT which consisted of five stages of 15–60 s at 85%–110% HRmax, interspersed by 15–60 s of active recovery at 64%–75% HRmax.26 The physiotherapist has assessed the patient’s HR during the sessions using an HR monitor. Session training (Figure 1) is completed as below:
Figure 1.
Program of cardio training session defined by HR (a) before T3 of the study (warm-up: biking; MICT: elliptical trainer; recovery: treadmill) and (b) after T3 of the study (warm-up: biking; MICT and HIIT: elliptical trainer; recovery: treadmill).
HR: heart rate; MICT: moderate-intensity continuous training (64%–75% HRmax); HIIT: high-intensity interval training (15–60 s at 85%–110% HRmax, interspersed by 15–60 s of active recovery).
Biking: 20 min (warm-up): 55%–65% HRmax
- Elliptical trainer:
- Before T3, progressively 20 min of MICT: 64%–75% HRmax
- After T3, 10 min of MICT followed by 10 min of HIIT
Treadmill: 5 min of recovery: 55%–65% HRmax
Whatever the exercises type, the goal to reach is neurovegetative (MICT: 64%–75% HRmax and HIIT: 85%–110% HRmax).
Group assignment
Patients were retrospectively assigned to one of the three groups (Active, Semi-Active and Passive) depending on their adhesion to the training process (Figure 2). At the end of Year 2, patients were assigned to one of the three groups: Active, Semi-Active and Passive. Patients in the Active group had reached the physiological training goal (three sessions of MICT per week each lasting 45 min: 64%–75% HRmax). Patients in the Semi-Active group had done three aerobic exercises per week, but did not reach the neurovegetative objective (intensity <60% HRmax and short duration of training session). Patients in the Passive group did not complete the training.
Figure 2.
Study design LICT: low-intensity continuous training; MICT: moderate-intensity continuous training associated with high-intensity interval training (HIIT) during the last 3 years.
Reason for drop out (49): moving out (3), pregnancy (3), discovery of an exclusion factor (4), improvement of symptoms no longer requiring treatment at the pain center (18), lack of availability/time to carry out assessments visits (9), lack of engagement in the proposed study (3) and reason indeterminate (9).
Statistics
Data analyses were performed in blind conditions. We displayed continuous variables as mean and standard deviation (SD) in centimeters for the VAS and the sleeping quality scores, in beats per minute for HR, in months for diffuse pain emergence and as dimensionless scores for HAD and FIQ. Analgesic consumption was considered an ordinal variable with four grades on the three-step “ladder.” After testing normality, we used a simple univariate general linear model (analysis of variance (ANOVA)) to compare quantitative baseline characteristics of the three groups. When ANOVA indicated a significant difference, we performed a post hoc multiple comparison procedure following Tukey’s honestly significant difference (HSD). The analyses of our longitudinal study design were performed using a mixed-model ANOVA accounting for repeated measures (T0, T1, T2, T3, T4 and T5), within each patient over time, including group effect (Active, Semi-Active and Passive). Pain (VAS), anxiety and depression (HADS), quality of life (FIQ), sleep quality (VAS) and HR were used as quantitative responses in this model testing the main effect of group and time and of analgesic consumption and time. Tukey’s HSD test was used as a post hoc test when the effects of these interactions were statically significant. To limit missing data, some data were obtained by phone call when patients missed an assessment session. The final results analysis was based on intention-to-treat analyses. Statistical analysis was performed with the Statistica 10.0 software package. The significance level was set at p < 0.05.
Results
Patient characteristics
The study involved 138 FM women. Forty-nine patients dropped out in Year 1 of the study. Data for these 49 patients were discarded. Data from 89 patients were analyzed.
At baseline, no significant difference was found between the three groups (Table 1): age (p = 0.88), BMI (p = 0.55), pain duration (p = 0.98), pain intensity (p = 0.91), painkiller step (p = 0.70), FIQ (p = 0.77), Hospital Anxiety Depression Scale (Anxiety; HADA; p = 0.23), Hospital Anxiety Depression Scale (Depression; HADD; p = 0.89), sleep quality (p = 0.35) and HR (p = 0.94).
Table 1.
Patients’ characteristics in Active, Semi-Active and Passive groups at baseline.
| Total FM (n = 89) Mean (SD) |
Active group
(n = 28) Mean (SD) |
Semi-Active group
(n = 31) Mean (SD) |
Passive group
(n = 30) Mean (SD) |
Group difference | |
|---|---|---|---|---|---|
| Age (years) | 44.02 (8.99) | 43.40 (11.19) | 44.57 (8.13) | 44.13 (7.47) | NS (p = 0.88) |
| BMI (kg m−2) | 22.53 (2.98) | 22.97 (2.79) | 22.11 (3.98) | 22.55 (1.71) | NS (p = 0.55) |
| Pain duration (months) | 62.70 (57.4) | 62.00 (50.9) | 61.75 (57.3) | 64.23 (64.9) | NS (p = 0.98) |
| Pain intensity (VAS 0–100) | 61.29 (11.12) | 60.57 (10.72) | 61.74 (11.07) | 61.50 (9.64) | NS (p = 0.92) |
| Painkiller step (0–3) | 2.35 (0.71) | 2.36 (0.73) | 2.42 (0.72) | 2.27 (0.69) | NS (p = 0.70) |
| FIQ (0–100) | 65.94 (7.61) | 65.50 (7.47) | 66.74 (7.90) | 65.53 (7.63) | NS (p = 0.77) |
| HADA (0–21) | 11.47 (2.08) | 11.07 (2.09) | 11.97 (2.01) | 11.33 (2.11) | NS (p = 0.23) |
| HADD (0–21) | 8.97 (2.94) | 9.18 (3.14) | 8.81 (2.95) | 8.93 (2.83) | NS (p = 0.89) |
| Sleep quality (VAS 0–100) | 86.75 (9.76) | 88.89 (10.49) | 86.23 (7.94) | 85.30 (10.70) | NS (p = 0.35) |
| HR (b min−1) | 75.19 (6.56) | 75.54 (6.64) | 75.10 (6.65) | 74.97 (6.61) | NS (p = 0.94) |
FM: fibromyalgia; SD: standard deviation; BMI: body mass index; VAS: visual analogue scale; FIQ: Fibromyalgia Impact Questionnaire; HAD: Hospital Anxiety Depression Scale (A: Anxiety; D: Depression); HR: heart rate.
Crossover between groups
There were few crossovers between the three groups in the course of the study (Additional Figure 1). Crossovers occurred only between Year 1 and 2 of training. Group assignments were made definitively at the end of Year 2.
Pain evaluation
The three groups showed statistically significant differences in pain intensity (F(2, 86) = 191.56, p < 0.001; Figure 3(a)). In the Passive group, pain increased significantly after Year 1 (p < 0.05) and after 5 years of study (p < 0.001). In the Semi-Active group, pain decreased significantly after Year 1 of training (p < 0.001), but there was no significant difference between T0 and T5 (p = 1.00). In the Active group, pain decreased progressively from Year 1 (p < 0.001) to the end of the Year 5 (p < 0.001). There was a significant pain difference between the Active group and both the Semi-Active group (p < 0.05) and the Passive group (p < 0.001) in Year 1 of training and until the end of the study. Pain VAS was significantly different between the Passive and Semi-Active groups (p < 0.05) throughout the study, except at T3 (p = 0.23).
Figure 3.
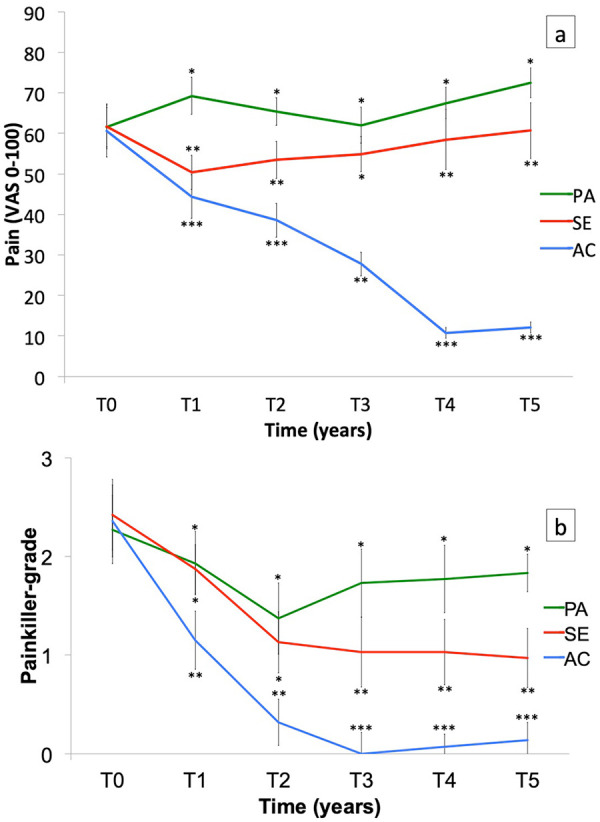
(a) Pain assessed by a visual analog scale (VAS) and (b) painkiller ladder in Active (AC), Semi-Active (SE) and Passive (PA) groups over 5 years (T0, T1, T2, T3, T4 and T5). Bars are standard error of the mean.
*Significant difference (p < 0.05) for intergroup comparison at each time.
Painkiller step
The painkiller step decreased in all groups during the first 2 years of training (Figure 3(b)). There was no difference in the painkiller step between the Semi-Active group and the Passive group (p = 1.00) in the first 2 years. The painkiller step increased significantly in the Passive group from Year 3 of training (p < 0.05). Patients in the Semi-Active group used paracetamol only during Year 2 (p < 0.001). Patients in the Active group used paracetamol only in Year 1 and took no painkiller after 2 years of training (p < 0.001).
Anxiety and depression
The anxious state improved in Year 1 of training in all groups, with no significant difference between the Semi-Active group and the Active group (p = 0.99; Figure 4(a)). There was a significant difference in anxious state between the Passive group and both the Semi-Active group (p < 0.001) and the Active group (p < 0.001) in Year 1. After the first year, the anxious state significantly worsened in the Passive group (p < 0.001) and remained stable in the Semi-Active group until the end of the study (p = 1.00). The anxious state improved significantly in the Active group throughout the study (p < 0.001). Year 3 of training saw a highly significant difference in progress between the Active group and the others (p < 0.001). At the end of the program, patients in the Active group were considered normal on the HADA scale (score in the normal range).
Figure 4.
(a) Anxiety and (b) depression (assessed by the HADS) and (c) impact of fibromyalgia on daily function (assessed by the FIQ) in Active (AC), Semi-Active (SE) and Passive (PA) groups over 5 years (T0, T1, T2, T3, T4 and T5). Bars are standard error of the mean.
*Significant difference (p < 0.05) for intergroup comparison at each time.
There was no change in depression state in the Passive group at any time in the study (p = 1.00), whereas the other groups strongly improved their depression state (Active: p < 0. 001 and Semi-Active: p < 0.001; Figure 4(b)). The Semi-Active and Active groups were considered normal on the HADD scale (score in the normal range) at T1.
Impact of FM on daily function
The impact of FM on daily function as indicated by the FIQ was statistically very significantly different between the three groups (F(2, 86) = 297.95 p < 0.001; Figure 4(c)). The symptoms of FM were strongly alleviated in the Active group after Year 1 of training. Patients’ symptoms were not alleviated in the Passive group (p = 1.00). There was no difference in FM symptoms between the Passive group and the Semi-Active group (p = 0.99) after Year 1, but FIQ was significantly different between the Passive and Semi-Active groups for all the other years (p < 0.001).
Sleep quality
There was a slight improvement in sleep quality for all three groups in Year 1, significant for both the Active (p < 0.01) and Semi-active (p < 0.001) groups and non-significant for the Passive group (p = 0.33; Figure 5). Sleep quality was strongly improved in the Active group (p < 0.001) after Year 3. Adding HIIT to the training program in the Active group steepened the slope of the sleep quality improvement curve. There was no significant difference between the Passive and Semi-Active groups after Year 4 (p = 0.20) and Year 5 (p = 0.40).
Figure 5.
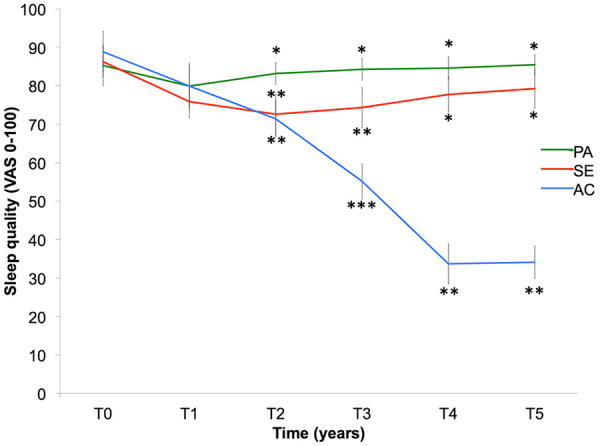
Sleep quality (assessed by a VAS) in Active (AC), Semi-Active (SE) and Passive (PA) groups over 5 years (T0, T1, T2, T3, T4 and T5). Bars are standard error of the mean.
*Significant difference (p < 0.05) for intergroup comparison at each time.
HR at rest
The Active group showed a decrease in the resting HR from the beginning of the HIIT (T3; p < 0.001; Figure 6). The low resting HR (61–65 b min−1) seen in the Active group after 5 years of training lent these patients the status of trained sportswomen.
Figure 6.
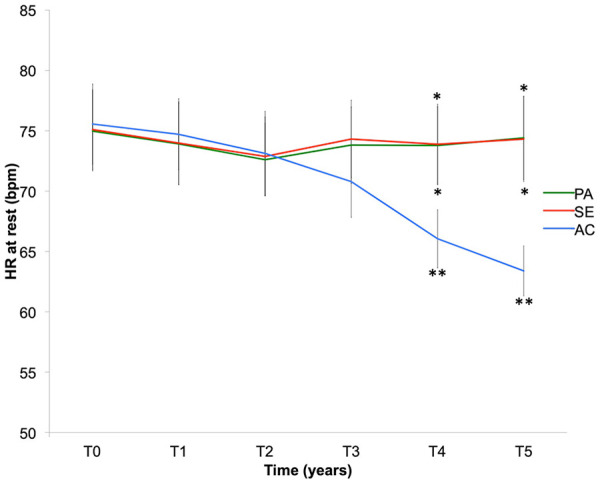
Resting heart rate (HR) in Active (AC), Semi-Active (SE) and Passive (PA) groups over 5 years (T0, T1, T2, T3, T4 and T5). Bars are standard error of the mean.
*Significant difference (p < 0.05) for intergroup comparison at each time.
Discussion
Regular physical activity is a necessary human physiological process with therapeutic power. Many short- and medium-term studies have highlighted the relative efficacy of physical training on pain and other FM symptoms.14 The present study reports a physical activity–based program leading to a dramatic alleviation of FM symptoms in a large fraction of the patients, and in almost all those who completed the program.
The content of the program must be considered, given that it is the first therapeutic proposal that includes an association of HIIT with a 5-year program. Few studies have assessed FM patients over a long period of training such as 5 years, and very few have used HIIT associated with MICT. In this study, the improvement observed in most variables was progressive and, apart from pain-related symptoms, peaked only after 4 or 5 years. The timing of the training intensity may also play an essential role in the modulation of the ANS.27,28 MICT associated with HIIT was more efficacious than MICT alone in improving autonomic functions29 and thereby in reducing FM symptoms.
The tailoring of the program according to the patient could also explain the efficacy of the program: individual mood characteristics, physical shape and sport habits were considered when giving the first instructions, and these were constantly adjusted in the course of the 5 years. For example, MICT/HIIT association was not used at the beginning of the program, since HIIT and even MICT can significantly exacerbate pain.4,30 Only low-intensity continuous training (LICT) was used at that stage, often for very short duration of a few minutes. Training intensity was gradually increased to a moderate level (MICT), and then to a vigorous level (HIIT) to become efficacious toward both pain and other symptoms. The time required to reach this goal might be several weeks, months or even several years, according to the program stage, and the patient’s rhythms, abilities and limits such as exacerbation of pain.
Symptom alleviation depended on the level of training attained
The Passive group patients did not complete the training program. FM symptoms were not alleviated. On the contrary, pain and anxiety state actually worsened. Discontinuing the program was probably related to personality profiles such as willingness, pain acceptance and secondary gain.31–34
The Semi-Active group patients also reported acute pain induced by the exercises.24,30 This led to a decrease in frequency, intensity and/or duration of their training despite coaching by the physiotherapist. LICT as performed by the Semi-Active group had an effect on chronic pain for the first year only. LICT had no effect on the ANS. Practice of LICT, however, meant reduced inactivity and less sedentary living. Social life was improved in relation to life quality betterment shown by FIQ and HAD changes.
In its review of reviews, Bidonde et al. (2014) advised doing three sessions per week, each lasting 45 min, of physical training at moderate intensity. However, in many cases reported in medium-term studies, many FM patients did only LICT,35,36 like our Semi-Active group. Intensity of physical activity was lower than prescribed when the patients were free to choose the intensity of exercises.37 We note that determining factors affecting the compliance to this type of training program associating MICT and HIIT were neither pain and symptom duration nor anxiety or depression, but patients’ personality, coping style and sports background.38 Finally, it is clear that implementing the training program required motivation and a significant investment of patients.
Active group patients complied with the prescribed specific training (MICT for 3 years followed by the association of MICT and HIIT for 2 years). A physiotherapist performed the follow-up twice a week with very few missing visits over the 5 years. During these frequent sessions, the physiotherapist and other pain care practitioners coordinated their efforts to induce patients to behave with an independent mind. Finally, patients reached a near-total improvement in pain, sleep quality, life quality (FIQ) and FM symptoms after 4 years of training. The 15–20 min of HIIT performed during the last year or years of the program in addition to MICT was concomitant with a final enhancement of FM symptomatology. High-intensity training led to more autonomic adaptations than moderate-intensity exercise.39 In the same line, several studies have shown that supervised aerobic exercise at moderate to vigorous intensity reduces pain perception and improves mental health and sleep quality. The efficacy of these effects was higher with HIIT.18,40,41 The training program must be continued over long term to maintain the beneficial effects of physical activity on FM symptoms.42
The pathophysiological mechanisms underlying the improvement of these symptoms will be explored in a future study. This new study should evaluate the effects of this specific cardio training on ANS and on the mechanisms of pain neuromodulation. Several studies have already found dysfunction of the physiological response to stress is observed in FM8,43–46 involving both the ANS and the HPA axis.17,47,48 Physical activity (MICT and HIIT) has been shown to be effective in regulating autonomic balance.29 This future study would validate the hypothesis that central nervous system plasticity induced by physical training may regulate cardiovascular adaptations not only through the ANS49 but also through endogenous pain control mechanisms,18,30,50 helping to alleviate FM symptoms.3,4,18,51
Limits of the study
Several limits of the study are listed below:
Thirty-five per cent of the patients dropped out during the first year (before T1). This drop-out rate is consistent with studies assessing the adherence of patients with chronic conditions to a maintained exercise program (36.7% drop-out rate during the first year in Heerema-Poelman et al.52 However, all patients present at the first assessment visit (T1) went on until the end of the program. This high adherence is probably due to the selection of a motivated sample of FM patients together with a high level of coaching.
Two thirds of the patients encompassing Passive and Semi-Active groups completed the 5 years of the study, but did not perform the training as initially prescribed probably because of a lack of motivation.53 However, patients in the Passive group continued to take responsibility for their own care and to visit the Pain Center. Patients in the Semi-Active group did three sessions a week,36 although the intensity required for neurovegetative rehabilitation was not reached.
There was no calculation of sample size in the methodology of this study. As this is a pilot study, the number of subjects to be included was guided by the potential for inclusion.
The three groups (Passive, Semi-Active and Active) were not set up randomly. Group assignment was retrospective as befitted the observational nature of the observational pilot study. Due to this feature, the benefits observed in the Active group during the 5 years in comparison with the other two could include other factors than this training program and results should be interpreted with some caution. However, this design allowed observation of the true effect of the program independently of patients’ adherence.54
Parameters of both personality and emotional profiles were not assessed, even though they may be decisive in predicting a person’s ability to complete the program.34,54 Profile subgroups based on the style of coping might allow a better choice of treatment for FM patients,54 thereby limiting the risk of therapeutic failures. Such prediction could considerably reduce the cost of the program.
More monitoring would have been useful. For example, measurement of sleep disturbance with a VAS does not assess quantitative sleep and objective sleep measures. The Pittsburgh Sleep Quality Index (PSQI)55 or polysomnography could be used in future work to assess sleep quality and quantity. Also, the neurovegetative system should be evaluated by HR variability and skin conductance56 to better assess the modulation of both parasympathetic and sympathetic systems. This study was devised to evidence clinical results. Future studies will need to focus on mechanisms.57
Conclusion
This pilot study found an alleviation of psychological and organic FM symptoms. FM patients who were active (with both MICT and HIIT) during the 5 years have a very significant improvement in overall symptoms compared to the other two groups (LICT and passive). A multicenter randomized controlled trial could further confirm the hypothesis supported by this observational pilot study. From the results of this observational pilot study, we hypothesize that FM could be cured by both MICT and HIIT associated with psychosocial care.
Supplemental Material
Supplemental material, additional_Table_ for A training program for fibromyalgia management: A 5-year pilot study by Céline Bodéré, Mathilde Cabon, Alain Woda, Marie-Agnès Giroux-Metges, Youenn Bodéré, Philippe Saliou, Bertrand Quinio, Laurent Misery and Anais Le Fur-Bonnabesse in SAGE Open Medicine
Supplemental material, cross_over for A training program for fibromyalgia management: A 5-year pilot study by Céline Bodéré, Mathilde Cabon, Alain Woda, Marie-Agnès Giroux-Metges, Youenn Bodéré, Philippe Saliou, Bertrand Quinio, Laurent Misery and Anais Le Fur-Bonnabesse in SAGE Open Medicine
Acknowledgments
The authors thank Prof. François Carré for his guidance and support and R. Ryan for his help in language editing. The authors thank the University Hospital of Brest.
Footnotes
Author contributions: C.B. conceived the study design and the outcome assessments. C.B. and A.W. designed the training protocol. C.B. and B.Q. conducted the recruitment. M.C., C.B., A.L.F.-B., and P.S. analyzed the data. C.B. and A.L.F.-B. wrote the article. A.L.F.-B. prepared figures and/or tables. M.-A.G.-M., Y.B., B.Q., A.W., and L.M. provided advice for the study design. Y.B. conducted the training program. C.B., M.C., M.-A.G.-M., Y.B., P.S., B.Q., A.W., L.M., and A.L.F.-B. reviewed drafts of the article. All authors approved the final version of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval and informed consent: Ethical approval for this study was obtained from the ethics committee of the University Hospital of Brest (CE150901). Written informed consent was obtained from all subjects before the study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anais Le Fur-Bonnabesse  https://orcid.org/0000-0002-5495-821X
https://orcid.org/0000-0002-5495-821X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Fitzcharles MA, Ste-Marie PA, Goldenberg DL, et al. 2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag 2013; 18(3): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016; 46(3): 319–329. [DOI] [PubMed] [Google Scholar]
- 3. Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther 2007; 9(4): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci 2018, https://www.frontiersin.org/articles/10.3389/fncel.2018.00035/full [DOI] [PMC free article] [PubMed]
- 5. Coaccioli S, Varrassi G, Sabatini C, et al. Fibromyalgia: nosography and therapeutic perspectives. Pain Pract 2008; 8(3): 190–201. [DOI] [PubMed] [Google Scholar]
- 6. Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain 2009; 10(8): 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodere C, Woda A. Effect of a jig on EMG activity in different orofacial pain conditions. Int J Prosthodont 2008; 21(3): 253–258. [PubMed] [Google Scholar]
- 8. Woda A, L’heveder G, Ouchchane L, et al. Effect of experimental stress in 2 different pain conditions affecting the facial muscles. J Pain 2013; 14(5): 455–466. [DOI] [PubMed] [Google Scholar]
- 9. Del Giorno R, Skaper S, Paladini A, et al. Palmitoylethanolamide in fibromyalgia: results from prospective and retrospective observational studies. Pain Ther 2015; 4(2): 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clauw D, Ablin J. The relationship between “stress” and pain: lessons learned from fibromyalgia and related conditions. In: Castro-Lopes J. (ed.) Current topics in pain (XIIth World Congress on Pain). Seattle, WA: IASP Press, 2009, pp. 245–270. [Google Scholar]
- 11. Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology 2016; 71: 127–135. [DOI] [PubMed] [Google Scholar]
- 12. Martinez-Lavin M. Fibromyalgia: when distress becomes (Un)sympathetic pain. Pain Res Treat 2012; 2012: 981565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thieme K, Turk DC, Gracely RH, et al. The relationship among psychological and psychophysiological characteristics of fibromyalgia patients. J Pain 2015; 16(2): 186–196. [DOI] [PubMed] [Google Scholar]
- 14. Bidonde J, Busch AJ, Bath B, et al. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev 2014; 10(1): 45–79. [DOI] [PubMed] [Google Scholar]
- 15. Macfarlane GJ, Kronisch C, Atzeni F, et al. EULAR recommendations for management of fibromyalgia. Ann Rheum Dis 2017; 76(12): e54. [DOI] [PubMed] [Google Scholar]
- 16. Thieme K, Mathys M, Turk DC. Evidenced-based guidelines on the treatment of fibromyalgia patients: are they consistent and if not, why not? Have effective psychological treatments been overlooked? J Pain off J Am Pain Soc 2017; 18(7): 747–756. [DOI] [PubMed] [Google Scholar]
- 17. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev 2017; 216: CD012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naugle KM, Naugle KE, Fillingim RB, et al. Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc 2014; 46(4): 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990; 33(2): 160–172. [DOI] [PubMed] [Google Scholar]
- 20. Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983; 16(1): 87–101. [DOI] [PubMed] [Google Scholar]
- 21. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67(6): 361–370. [DOI] [PubMed] [Google Scholar]
- 22. Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002; 52(2): 69–77. [DOI] [PubMed] [Google Scholar]
- 23. Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol 1991; 18(5): 728–733. [PubMed] [Google Scholar]
- 24. Nijs J, Kosek E, Van Oosterwijck J, et al. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012; 15(3 Suppl.): ES205–ES213. [PubMed] [Google Scholar]
- 25. Carré F. Cardiologie du sport. de Boeck: Brussels, 2013, p.280 (Sciences et pratique du sport). [Google Scholar]
- 26. Haykowsky MJ, Daniel KM, Bhella PS, et al. Heart failure: exercise-based cardiac rehabilitation: who, when, and how intense. Can J Cardiol 2016; 32(10 Suppl.2): S382–S387 [DOI] [PubMed] [Google Scholar]
- 27. Cornelissen VA, Verheyden B, Aubert AE, et al. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens 2010; 24(3): 175–182. [DOI] [PubMed] [Google Scholar]
- 28. Morseth B, Graff-Iversen S, Jacobsen BK, et al. Physical activity, resting heart rate, and atrial fibrillation: the Tromsø study. Eur Heart J 2016; 37(29): 2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alansare A, Alford K, Lee S, et al. The effects of high-intensity interval training vs. moderate-intensity continuous training on heart rate variability in physically inactive adults. Int J Environ Res Public Health 2018; 15(7): 1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J Physiol 2017; 595(13): 4141–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Egmond JJ. Beyond secondary gain. Am J Psychoanal 2005; 65(2): 167–177. [DOI] [PubMed] [Google Scholar]
- 32. Rodero B, Casanueva B, Luciano JV, et al. Relationship between behavioural coping strategies and acceptance in patients with fibromyalgia syndrome: elucidating targets of interventions. BMC Musculoskelet Disord 2011; 12: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patrick RE, Horner MD. Psychological characteristics of individuals who put forth inadequate cognitive effort in a secondary gain context. Arch Clin Neuropsychol 2014; 29(8): 754–766. [DOI] [PubMed] [Google Scholar]
- 34. Ablin JN, Zohar AH, Zaraya-Blum R, et al. Distinctive personality profiles of fibromyalgia and chronic fatigue syndrome patients. PeerJ 2016; 4: e2421, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5028783/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arcos-Carmona IM, Castro-Sánchez AM, Matarán-Peñarrocha GA, et al. [Effects of aerobic exercise program and relaxation techniques on anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia: a randomized controlled trial]. Med Clin 2011; 137(9): 398–401. [DOI] [PubMed] [Google Scholar]
- 36. Kayo AH, Peccin MS, Sanches CM, et al. Effectiveness of physical activity in reducing pain in patients with fibromyalgia: a blinded randomized clinical trial. Rheumatol Int 2012; 32(8): 2285–2292. [DOI] [PubMed] [Google Scholar]
- 37. Newcomb LW, Koltyn KF, Morgan WP, et al. Influence of preferred versus prescribed exercise on pain in fibromyalgia. Med Sci Sports Exerc 2011; 43(6): 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrari R, Louw D. Coping style as a predictor of compliance with referral to active rehabilitation in whiplash patients. Clin Rheumatol 2011; 30(9): 1221–1225. [DOI] [PubMed] [Google Scholar]
- 39. Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol 2006; 97(1): 141–147. [DOI] [PubMed] [Google Scholar]
- 40. Gerber M, Brand S, Herrmann C, et al. Increased objectively assessed vigorous-intensity exercise is associated with reduced stress, increased mental health and good objective and subjective sleep in young adults. Physiol Behav 2014; 135: 17–24. [DOI] [PubMed] [Google Scholar]
- 41. Hartescu I, Morgan K, Stevinson CD. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res 2015; 24(5): 526–534. [DOI] [PubMed] [Google Scholar]
- 42. Andrade CP, Zamunér AR, Forti M, et al. Effects of aquatic training and detraining on women with fibromyalgia: controlled randomized clinical trial. Eur J Phys Rehabil Med 2019; 55(1): 79–88. [DOI] [PubMed] [Google Scholar]
- 43. McBeth J, Chiu YH, Silman AJ, et al. Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents. Arthritis Res Ther 2005; 7(5): R992–R1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alok R, Das SK, Agarwal GG, et al. Relationship of severity of depression, anxiety and stress with severity of fibromyalgia. Clin Exp Rheumatol 2011; 29(6 Suppl. 69): S70–S72. [PubMed] [Google Scholar]
- 45. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15): 1547–1555. [DOI] [PubMed] [Google Scholar]
- 46. Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016; 338: 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kadetoff D, Kosek E. Evidence of reduced sympatho-adrenal and hypothalamic-pituitary activity during static muscular work in patients with fibromyalgia. J Rehabil Med 2010; 42(8): 765–772. [DOI] [PubMed] [Google Scholar]
- 48. Bote ME, Garcia JJ, Hinchado MD, et al. Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS ONE 2013; 8(9): e74524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martins-Pinge MC. Cardiovascular and autonomic modulation by the central nervous system after aerobic exercise training. Braz J Med Biol Res 2011; 44(9): 848–854. [DOI] [PubMed] [Google Scholar]
- 50. Law LF, Sluka KA. How does physical activity modulate pain. Pain 2017; 158(3): 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Da Silva Santos R, Galdino G. Endogenous systems involved in exercise-induced analgesia. J Physiol Pharmacol 2018; 69(1): 3–13. [DOI] [PubMed] [Google Scholar]
- 52. Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout. J Cardiopulm Rehabil Prev 2013; 33(6): 419–426. [DOI] [PubMed] [Google Scholar]
- 53. Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014; 17(10): 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Häuser W, Perrot S, Clauw DJ, et al. Unravelling Fibromyalgia-Steps Toward Individualized Management. J Pain 2018; 19(2): 125–134. [DOI] [PubMed] [Google Scholar]
- 55. Omachi TA. Measures of sleep in rheumatologic diseases: Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Insomnia Severity Index (ISI), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res 2011; 63(Suppl. 11): S287–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mackersie CL, Calderon-Moultrie N. Autonomic nervous system reactivity during speech repetition tasks: heart rate variability and skin conductance. Ear Hear 2016; 37 (Suppl 1): 118S–125S. [DOI] [PubMed] [Google Scholar]
- 57. Le Fur Bonnabesse A, Cabon M, L’Heveder G, et al. Impact of a specific training programme on the neuromodulation of pain in female patient with fibromyalgia (DouFiSport): a 24-month, controlled, randomised, double-blind protocol. BMJ Open 2019; 9(1): e023742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, additional_Table_ for A training program for fibromyalgia management: A 5-year pilot study by Céline Bodéré, Mathilde Cabon, Alain Woda, Marie-Agnès Giroux-Metges, Youenn Bodéré, Philippe Saliou, Bertrand Quinio, Laurent Misery and Anais Le Fur-Bonnabesse in SAGE Open Medicine
Supplemental material, cross_over for A training program for fibromyalgia management: A 5-year pilot study by Céline Bodéré, Mathilde Cabon, Alain Woda, Marie-Agnès Giroux-Metges, Youenn Bodéré, Philippe Saliou, Bertrand Quinio, Laurent Misery and Anais Le Fur-Bonnabesse in SAGE Open Medicine