Abstract
A 48-year-old male kidney-transplant recipient was bitten by a rabid dog. His immunosuppressive treatment consisted of cyclosporine 60mg b.i.d., mycophenolate mofetil (MMF) 250mg t.i.d., and prednisone 5 mg. After wound care, he received 5 doses of purified vero cell rabies vaccine on days 0, 3,7,14, and 28, and human rabies immunoglobulin, according to international guidelines. Adequate levels of rabies virus neutralizing antibodies were observed after the administration of the third vaccine dose. However, a decrease of antibody titer was detected by day 28. Immunosuppressive medication was minimized, withdrawing MMF and reducing the dose of cyclosporine Booster doses of the same vaccine were administered on days 38,41,45,52, and 66. Adequate neutralizing antibody response was recovered during the ensuing 12 months, under reduced immunosuppression. Nineteen months after the incident, the patient remains with good graft function and is asymptomatic for rabies. It remains to be determined whether the attained immune response was either the result of the booster vaccinations or the reduction of immunosuppression alone. Nevertheless, such an outcome would have been possible only with the combined management strategy implemented.
Keywords: rabies virus, kidney transplant recipient, adequate immune response
Rabies is caused by rhabdoviruses in the genus Lyssavirus, family Rhabdoviridae, typically transmitted through the saliva of infected animals (1). This disease is almost invariably fatal once clinical symptoms appear (2). According to World Health Organization (WHO) more than 55,000 human rabies cases occur worldwide each year (3), with the majority attributed to enzootic dog rabies in developing countries.
After the initiation of the ‘Urban Canine Rabies Elimination in the Americas’ program, a mass canine vaccination campaign was initiated in Mexico in 1990. During 2009, more than 17 million dogs were vaccinated in this country, compared with only 7 million in 1990 (5). Although rabies incidence in animals decreased in direct correlation to the number of vaccinated dogs, 7 human rabies cases associated with dogs were documented during 1996–2009. In addition, 26 other human rabies cases related to wildlife exposure were reported in the same period (4, 5). All over the country, the cumulative number of inhabitants attacked by dogs for the period 2000–2009 was 936,000 (an estimated average of 94,000 persons per year), all of them received medical attention, and treatment was started in 313,000 (33.4%) of them in accordance to risk evaluation (6).
During 2004,4 transplant recipients succumbed to rabies in the USA, after receiving grafts from a donor with unrecognized rabies (7). Three other organ transplant recipients died of rabies in Germany, after receiving grafts from a donor exposed to a rabid dog in India (8). In addition 8 cases have been documented historically as the result of corneal transplantation from donors with rabies (9).
No specific information is available regarding the management of transplant recipients bitten by rabid animals. Recently, Cramer et al. (10) in 2008 reported 8 pediatric solid organ transplant recipients potentially exposed to rabies. All patients received rabies vaccine and human rabies immunoglobulin (HRIG), according to the standard Advisory Committee on Immunization Practices (ACIP) recommendations (11), which resulted in the development of antibody titers above the arbitrary cutoff value considered as an indicator of adequate humoral immune response (0.5 IU/mL). These titers were attained within 1 month in 7 subjects and within 6 months for 1 individual.
The aim of this investigation was to report the management strategy and the immune response of a kidney transplant recipient under immunosuppressive medication, who was bitten by a rabid dog.
Case report
A 42-year-old male patient received a deceased donor kidney transplant on October 10,1996. The donor was a 40-year-old female with 4 human leukocyte antigen (HLA) mismatches. After 3 episodes of acute cellular rejection, treated with methylprednisolone pulses and OKT3 monoclonal antibodies, he developed lymphoproliferative disease limited to the graft. On January 1998, a graft nephrectomy was performed followed by local radiotherapy (12).
During February 2002, at age 48, the patient received a second kidney transplant from a 16-year-old deceased male with 6 HLA mismatches. By January 2009, his renal function was stable with a serum creatinine (SCr) of 1.35 mg/dL and an estimated glomerular filtration rate (eGFR) by modification of diet in real disease (MDRD) of 60.2 mL/min, receiving cyclosporine (CsA) 60 mg twice daily (mean CsA trough levels of 60 ng/mL), mycophenolate mofetil (MMF) 250 mg 3 times a day, and prednisone 5 mg/day to maintain moderate immunosuppression.
During January 2009, the patient was bitten on the leg by a suspected rabid dog. The dog was captured and euthanized on the same day. Rabies was confirmed at the State Laboratory of Public Health in Hidalgo, and re-confirmed by the National Reference Laboratory (InDRE) in Mexico City. Diagnosis was performed by the direct fluorescent antibody test, and rabies virus (RABV) was subsequently characterized as a dog-related RABV variant VI, by antigenic and gene sequencing typing.
On the day of exposure, the patient received immediate care in the emergency room. The wound was cleaned thoroughly, and post-exposure rabies prophylaxis (PEP) was administered according to Mexican Ministry of Health guidelines (13), which essentially corresponds to the ACIP recommendations. Briefly, prophylaxis consisted of a single dose of HRIG, 20IU/kg body weight, resulting in the infiltration of 795 IU around the wound and the remaining 780 IU applied intramuscularly. Active immunization was also administered with 5 doses of purified vero cell vaccine (PVCV, 0.5 mL each), on days 0,3,7,14, and 28, in the deltoid muscle.
Rabies-virus neutralizing antibody (VNA) titers were determined by the rapid fluorescent focus inhibition test (14). AdequateVNA titers were obtained on days 10 and 16 after the beginning of PEP. However, a decrease of VNA titers below 0.5 IU/mL was detected by the end of the vaccination scheme, on day 28. Consequently, immunosuppressive medication was minimized, withdrawing MMF and reducing the dose of CsA to levels of ~ 50 ng/mL. In addition, further PVCV doses were administered on days 38, 41,45, 52, and 66, followed by sequential antibody monitoring over a year. An adequate humoral response was observed throughout the observation period (Table 1), even with immunosuppressive medication, consisting of CsA with a dose of 60 mg in the morning and 50 mg in the evening, together with prednisone at a dose of 7.5 mg/day (Fig. 1,Table 1).
Table 1.
Patient rabies virus antibody titer, post Immunization
| Dose number/date of vaccine application | Date ofsampleforAbtesting,1 month/day/year (#of days after PEP started) | Abs titer2 IU/mL |
|---|---|---|
| First vaccine scheme | ||
| 1st 01/21/2009 | – | – |
| 2nd 01/23/2009 | – | – |
| 3rd 01/28/2009 | – | – |
| 01/31/2009 (10) | 0.69 | |
| 4th 02/03/2009 | – | – |
| 02/06/2009 (16) | 0.7 | |
| 5th 02/17/20093 | 02/17/2009 (28)4 | 0.314 |
| Second vaccine scheme | ||
| 1st 02/27/2009 | – | – |
| 2nd 03/02/2009 | 03/02/2009 (41) | 0.97 |
| 3rd 03/06/2009 | – | – |
| 03/09/2009 (48) | 4.5 | |
| 4th 03/13/2009 | – | – |
| 5th 03/29/2009 | - | - |
| 05/06/2009 (106) | 4.5 | |
| 09/14/2009 (236) | 0.8 | |
| 11/25/2009 (308) | 0.8 | |
| 01/21/2010 (365) | 0.54 | |
Samples tested using the rapid fluorescent focus inhibition titer test.
liters > 0.5 lU/mL are considered adequate.
Fifth dose administration of the first vaccine scheme.
Antibody (Abs) titer on day 28 th of first PVCV dose administration.
PEP, post-exposure rabies prophylaxis; PVCV, purified vero cell vaccine.
The bold represents the date when VNA decreased below 0.5 lU/mL.
Fig 1.
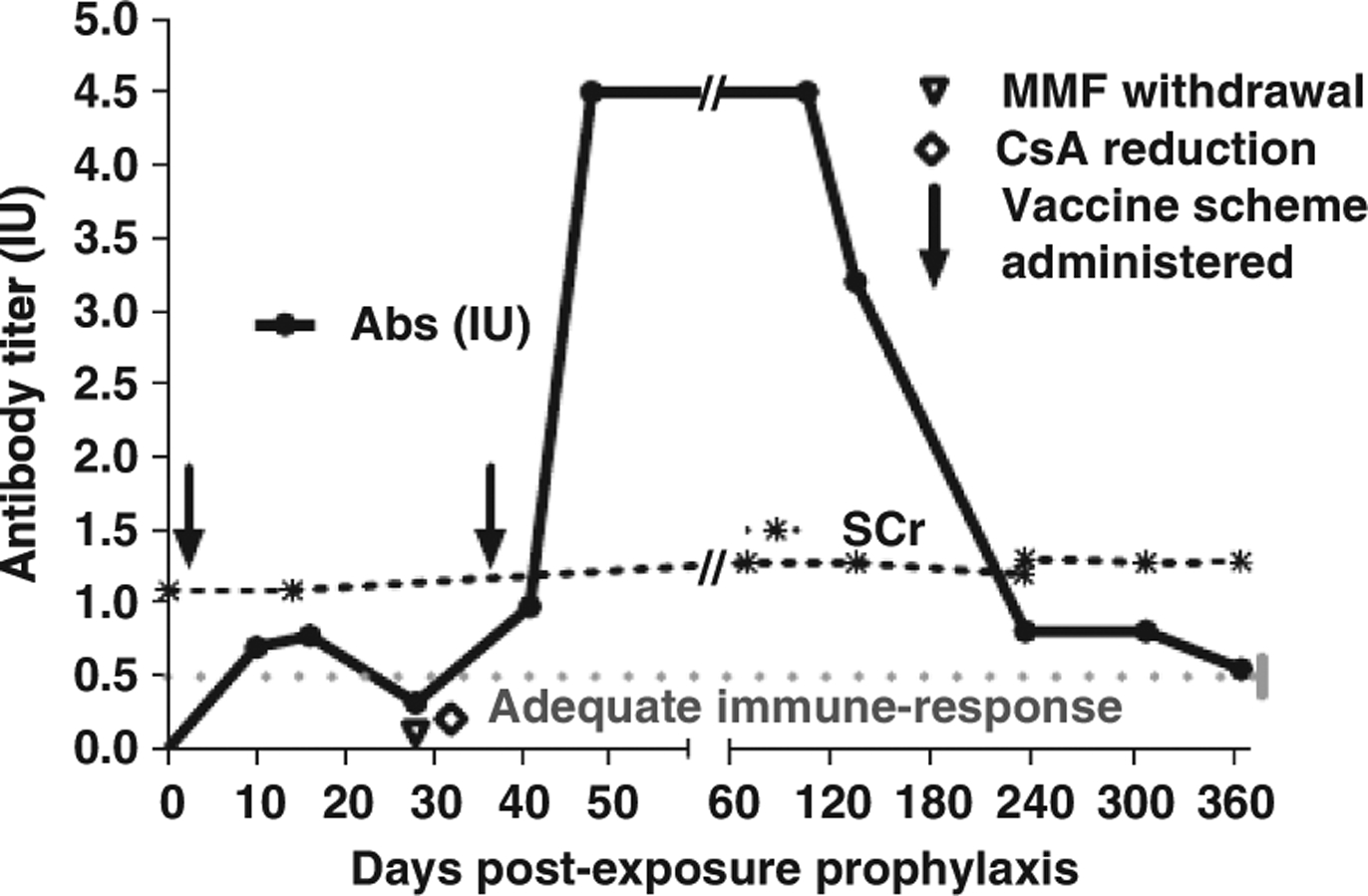
Dynamics of rabies virus neutralizing antibodies (Abs), and serum creatinine (SCr) in the patient’s serum. Timing of vaccine schemes, and immunosuppression minimization, are also depicted. MMF, mycophenolate mofetil; CsA,cyclosporine.
Nineteen months after the exposure and PEP initiation, under the same immunosuppressive scheme and doses mentioned previously, the patient remains asymptomatic with stable graft function, SCr 1.3mg/dL, and eGFR by MDRD 62.36 mL/min.
Discussion
This report describes PEP and the resulting immune response in an immunosuppressed kidney transplant recipient bitten by a rabid dog. The standard PEP resulted in VNA titers in serum, transiently above an adequate value of 0.5 IU/mL. Thereafter, this was followed by a decrease in VNA by day 28 when the 5th dose of PVCV was administered (Table 1).This observation likely reflected the effects of the patient’s immunosuppressive regimen, and additional interventions were required to achieve an efficient and sustained immune response. Thus, immunosuppressive treatment was decreased by MMF withdrawal and a tapering of CsA, even though the maintenance dosages of the immunosuppressive scheme before exposure were relatively low Furthermore, a second series of 5 doses of rabies vaccine was administered, and an adequate immune response was finally achieved. Whether this patient would have showed a suitable increase in VNA titers solely by reducing the immunosuppression by the end of the first PVCV scheme remains uncertain. However, given the risk of rabies, the patient was given an additional 5 doses of rabies vaccine. The PVCV has safety and efficacy records comparable to those of the human diploid cell vaccine, and complies with the WHO recommended potency (15).
Safety and efficacy of such vaccines, particularly in combination with proper wound care and administration of HRIG, has been shown repeatedly in animal experiments, clinical trials, and evidence-based observations (11,16).
Although immunosuppression compromises host defenses and may create a favorable environment for viral replication, the effect of therapeutic immunosuppression on RABV is not well studied. Information is scarce about PEP in immunocompromised organ-transplanted individuals and their subsequent immune response. Cramer et al. (10) in 2008 reported on 8 pediatric solid organ transplant recipients (5 liver, 3 kidney) potentially exposed to a rabid bat found in their cabin at a summer camp. All patients received standard PEP which resulted in adequate antibody production. None of these patients developed rabies, or any severe adverse reactions to PEP and none developed allograft rejection. According to the serologic response, patients on tacrolimus monotherapy achieved higher VNA titers compared with transplant recipients on triple drug therapy (10). It is important to stress even further that the patient reported herein had no allograft rejection or decline in graft function after receiving the immunization scheme, which included extra doses of vaccine.
Our case report provides new information on management of an immunosuppressed patient who received PEP, with successful development of immune response, as indicated by VNA titers, after modification of immunosuppressive treatment and a secondary PEP regimen. The monitoring schedule of VNAtiters was decided by the medical group in charge; it is important to mention this, because no national or international established scheme exists for immune response follow-up after rabies vaccine application in an immunosuppressed patient.
Several essential questions remain to be resolved. Is the recommended PEP schedule equally effective in all transplant recipients? How is the immune response post-vaccination linked to the intensity of immunosuppression and specific immunosuppressive medication? Is there a need for an extended PEP series in some patients? How long should rabies VNA titers be monitored after immunization of such patients? Is it critical to reduce immunosuppressive medication in such patients to achieve an adequate immune response? As at the moment we do not have adequate experimental and clinical data to address these questions, cases like the one described in our report must be considered individually, via collegial decision of experts in relevant medical areas based upon the evidence at hand.
Acknowledgements:
The authors recognize the coordinated and valuable work performed by the members of the Mexican Healthcare System regarding the entire follow-up of this case:
National Center of Preventive Programs and Diseases Control: Drs Miguel Angel Lezama Fernández, Carlos H. Alvarez Lucas, Fernando Vargas Pino, and Veronica Gutiérrez Cedillo. Ministry of Health in Hidalgo State: Drs David Cabrera Gaytán, Llurely Islas Téllez, Miriam Veraz Godoy, Yolanda García Anaya, and Erick J. Canales Vargas, Antonio Lechuga Traspeña, Carlos Avila Zúñiga, Luis Rene Gress Ortega, Ana B. Alvarado Melquiades, and Alejandro Fernández Gallegos. Mexican Institute of Social Security: Rosalinda G. Ponce Escamilla.
Abbreviations;
- WHO
World Health Organization
- FVCX
purified vero cell rabies vaccine
- Abs
antibodies
- ACIP
Advisory Committee on Immunization Practice
- IS
immunosuppression
- SCr
serum creatinine
- eGFR
estimate glomerular filtration rate
- MDRD
modification of diet in renal disease
- InDRE
Institute of Epidemiological Diagnostic and Reference
- PEP
post-exposure rabies prophylaxis
- HRIG
human rabies immunoglobulin
- MMF
mycophenolate mofetil
- RABX
rabies virus
- VNA
rabies-virus neutralizing antibody
Footnotes
Disclosure: Findings and conclusions of this study do not reflect CDC’s policies or opinions and represents those of the authors based on the scientific evidence presented. Trademarks and products brands described here are presented for comparative purposes do not imply any endorsement by the any of the author s affiliation agencies.
References
- 1.Jackson AC. Rabies. Neurol Clin 2008: 26:717–726. [DOI] [PubMed] [Google Scholar]
- 2.Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection: post-exposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA 1976; 236: 2751–2754. [PubMed] [Google Scholar]
- 3.World Health Organization. WHO expert consultation on rabies: first report Geneva: WHO, 2005. [PubMed] [Google Scholar]
- 4.CENAVECE, Ministry of Health, Mexico. Available at http://www.cenave.gob.mx/zoonosis/rabia/principal2.asp. Accessed March 2, 2011.
- 5.Lucas CH, Pino FY Baer G, et al. Rabies control in Mexico. Dev Biol (Basel) 2008; 131:167–175 [PubMed] [Google Scholar]
- 6.CENAVECE, Ministry of Health, Mexico. Gufa para la atención médica y antirrábica de la persona expuesta al virus de la rabia. Available at http://www.cenavece.salud.gob.mx/programas/descargas/pdf/guiatxrab.pdf. Accessed March 2,2011.
- 7.Srinivasan A, Burton EC, Kuehnert MJ, et al. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med 2005; 352:1103–1111. [DOI] [PubMed] [Google Scholar]
- 8.Johnson N, Brookes SM, Fooks AR, Ross RS. Review of human rabies cases in the UK and in Germany. Vet Rec 2005; 157:715. [DOI] [PubMed] [Google Scholar]
- 9.Jackson AC. Human disease In: Jackson AC, Wunner WH, eds. Rabies (2nd edn). Amsterdam: Elsevier Academic Press, 2007: 309–340. [Google Scholar]
- 10.Cramer CH II, Shiecky Thomas SE, Kershaw DB, Magee JC, Lopez MJ. Immune response to rabies vaccination in pediatric transplant patients. Pediatr Transplant 2008; 12: 874–877. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Human rabies prevention-United States, 2008: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–28. [PubMed] [Google Scholar]
- 12.Alberu J, Villasís A. Virus, inmunosupresióny el receptor detrasplante renal. Rev Invest Clin 2005; 57: 582–595. [PubMed] [Google Scholar]
- 13.Modificación a la Norma Oficial Mexicana NOM-OH-SSA2–1993, para la prevencion y control de la rabia, publicada el 25 de Enero de 1995
- 14.Smith JS,Yager PA, Baer GM. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibodies In: Kaplan MM, Koprowski H, Meslin FX, eds. Laboratory Techniques in Rabies. 4th edn. Geneva: WHO, 1996. :181–192. [Google Scholar]
- 15.WHO. Weekly epidemiological record, August 6,2010,85th year. No. 32, 2010; 85,309–320. Available at http://www.who.int/wer. Accessed March 2, 2011.
- 16.WHO. Rabies immunization. Available at http://www.who.int/entity/immunization/rabies.grad.efficacy.pdf. Accessed March 2, 2011.


