Abstract
In view of recent therapeutic advances in mantle cell lymphoma (MCL), the aim of this retrospective cohort analysis was to assess treatment patterns, adverse events (AEs), resource utilization, and health care costs in patients with MCL in a US-based commercial claims database. A total of 783 patients with MCL (median age=65 years) were selected. Among patients receiving systemic therapy (n=457), the most common treatment regimens were bendamustine/rituximab (BR) (41.1%), rituximab/cyclophosphamide/doxorubicin/vincristine (RCHOP) (26.7%), rituximab monotherapy (20.4%), and ibrutinib monotherapy (14.2%). Mean monthly costs during treatments with BR, RCHOP, rituximab, and ibrutinib were $12,958, $24,719, $13,153, and $21,690, respectively. Mean monthly cost during follow-up was $13,650 among patients with ≥6 AEs versus $5,131 among those without AEs. The costs of MCL varied considerably by treatment regimen and care setting. The overall economic burden of managing patients with MCL can be substantially affected by costs associated with managing AEs occurring during treatment.
Keywords: mantle cell lymphoma, MCL, economic burden, costs, treatment patterns, adverse events
Introduction
Mantle cell lymphoma (MCL) represents approximately 3% of all newly diagnosed non-Hodgkin lymphoma cases in the United States (US) [1]. MCL is incurable, often presents aggressively, and frequently infiltrates extranodal tissues [2]. Median overall survival has improved over the past several decades [3] but remains limited to approximately 5 to 7 years [4].
Patients with early-stage MCL are generally treated with chemotherapy with or without radiation therapy. Those with more advanced stages, who represent the majority of patients, or rapid disease progression are candidates for a more intensive approach. Dose-intensive chemotherapy combinations with rituximab (chemoimmunotherapy) or stem cell transplantation (SCT) are often considered for first-line treatment, especially among younger patients and medically fit older individuals [5,6]. Patients with relapsed MCL are treated with systemic therapies that may include chemotherapy, rituximab, or newer targeted agents such as bortezomib, lenalidomide, or ibrutinib [7,8].
MCL therapies are often associated with hematologic and nonhematologic adverse events (AEs) [9,10]. Newer targeted therapies such as the tyrosine kinase inhibitors have been reported to increase the risk of cardiac events, particularly atrial fibrillation (A-fib) [11]. Moderate to severe AEs, which often require medical intervention, may adversely affect adherence to planned treatment and lead to increased health care resource use (HCRU) and costs. Real-world data on HCRU and economic burden associated with MCL treatments and related AEs are sparse in the current literature [12,13].
In view of recent advances in MCL management, it is important to explore current, real-world data on treatment and costs. Therefore, the aims of this study were: (1) to assess treatment patterns, AEs, HCRU, and direct health care costs in privately insured patients in the US with diagnosis of MCL; and (2) to explore in these patients specific factors associated with AEs, inpatient admission, and health care costs.
Methods
Design and data source
Data for this retrospective cohort study were taken from the Truven MarketScan Research Databases, which contain administrative claims information on more than 60 million unique individuals enrolled in employer-sponsored private health insurance plans across the US. These databases provide longitudinal data on medical and pharmacy service utilization, and associated payments, collected from nearly 350 employers and payers. They contain health care information for employed individuals and their dependents covered under fee-for-service and various capitated health plans. Patient data for each healthcare encounter and associated diagnoses and treatments, as recorded in claims forms using applicable coding systems (e.g., International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], Current Procedural Terminology [CPT]), are recorded. Payments and charges including amounts paid by the health plan and the amount of patient responsibility (i.e., patient co-payment, coinsurance, and deductible) are also captured. The Medicare-covered portion of payment (represented as the coordination of benefits amount) and the employer-paid portion are both included.
Patient selection
Patients with a first diagnosis of MCL during the patient selection time period—July 1, 2012, through June 30, 2015—were identified using the ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes (200.4X [ICD-9-CM] and C83.1X [ICD-10-CM]). Patients were required to have at least two medical claims on separate dates with diagnosis code(s) for MCL. The date of the first observed diagnosis of MCL during the selection period defined the study index date. Eligible patients also were ≥ 18 years of age at the index date; had ≥ 12 months of continuous enrollment (with gaps ≤ 30 days permitted) in medical and drug plans, with no capitation, before the study index date; and had no evidence of MCL diagnosis or MCL-directed treatment (systemic therapy and/or SCT) during the 12-month baseline period before their index date. All patients were followed up through disenrollment from the medical and/or drug plan or end of the study period (June 30, 2016), whichever was first. A summary of the study design is presented in Figure 1.
Figure 1. Graphical Summary of the Study Design.
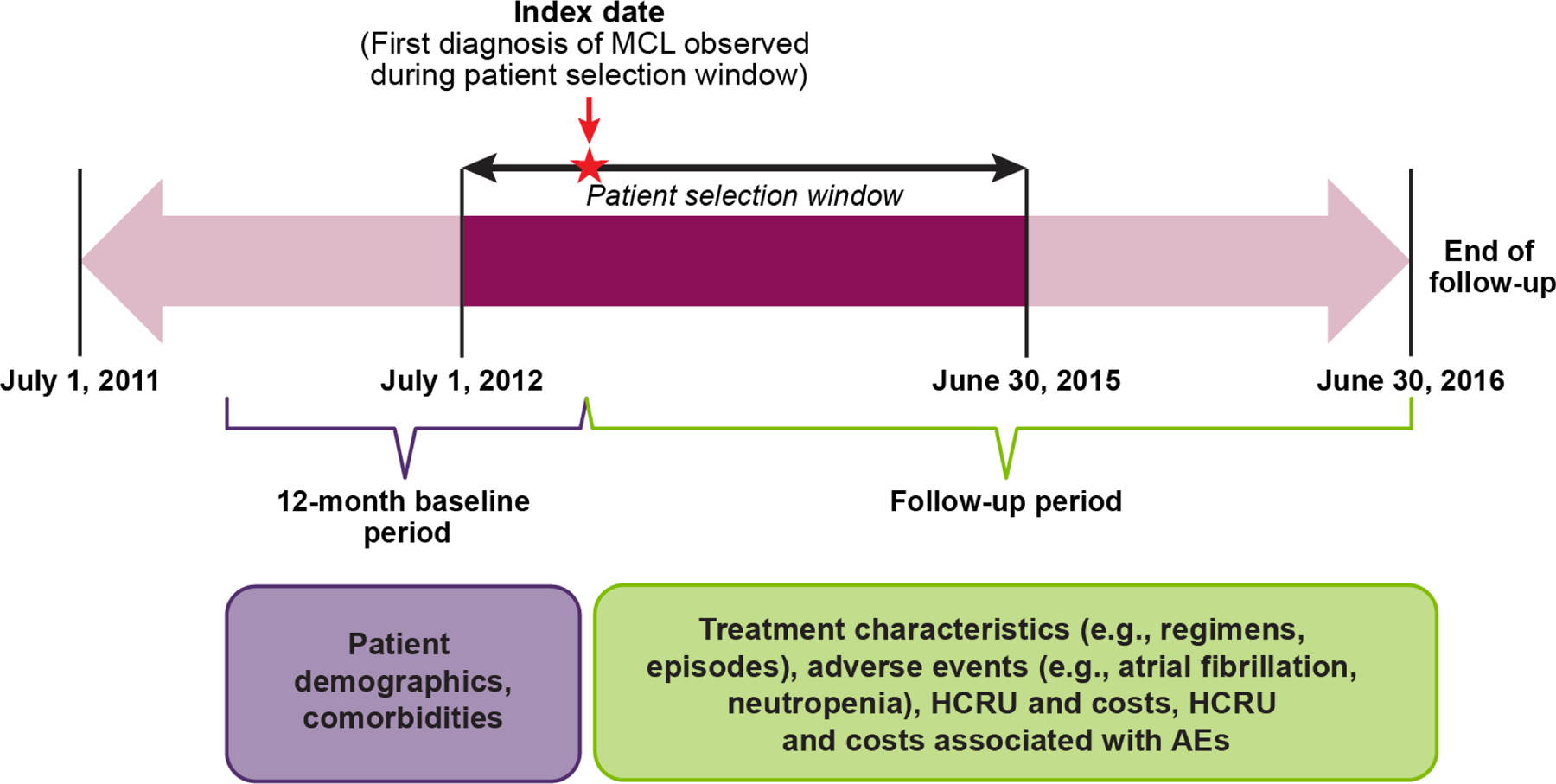
AE = adverse event; HCRU = health care resource use; MCL = mantle cell lymphoma.
Study measures
Demographic and other baseline characteristics, including Charlson Comorbidity Index (CCI) score, were assessed on the index date or during the 12-month baseline period [14]. Patients’ baseline risk of A-fib was determined based on a previously published algorithm [15] involving seven risk factors: heart failure, hypertension, diabetes, age 65 to 74 years (at index), age ≥ 75 years (at index), coronary artery disease, and chronic kidney disease. Patients were defined as ‘high risk’ for A-fib if they had evidence of at least one of the following: (1) any two of the first five risk factors listed above, (2) any three of all seven risk factors listed above, or (3) previous A-fib during the baseline period.
Mantel cell lymphoma–directed treatments included in the NCCN Clinical Practice Guidelines (version 1, 2017) [16] were identified using Healthcare Common Procedure Coding System codes, Current Procedural Terminology codes, and ICD-9-CM and ICD-10-CM procedure codes as applicable. A treatment regimen was defined as the combination of all agents observed on or within 35 days after the first claim for a systemic therapy drug (excluding claims with generic chemotherapy encounter/administration codes) [17,18]. For parenteral drugs, the first-line therapy ended 30 days after the last administration. For oral medications (e.g., ibrutinib), the prescription days’ supply was used to determine the duration of treatment. The date of the end of days’ supply based on the last observed refill of the oral regimen, with a subsequent treatment gap ≥ 90 days, defined the end of the first-line therapy. The end of the therapy line for oral regimens was calculated by adding the total number of days’ supply to the prescription fill date plus the allowed gap of 90 days. Patients who switched regimens, with or without a 90-day gap in treatment, were considered as having initiated a new line of therapy. Maintenance therapy with rituximab was defined as rituximab monotherapy initiated within 7 months after completion of a rituximab-containing combination therapy (e.g., rituximab/cyclophosphamide/doxorubicin/vincristine [RCHOP] with or without prednisone). A gap of more than 7 months after the last administration of rituximab defined the end of rituximab maintenance therapy. Detailed treatment characteristics, including composition, time to initiation, and duration, were assessed for the most common treatment regimens used in the study population, for up to four lines of therapy. We did not evaluate use of either autologous or allogeneic SCT by line of therapy as this analysis focused on receipt of routinely administered systemic therapies.
Incident AEs were defined as the first occurrence of the AE over the course of a given treatment. Additionally, the total number of unique AEs recorded per patient during the follow-up period was tabulated (categorized as 0, 1–2, 3–5, or ≥ 6 AEs). Costs associated with specific MCL treatments were defined using all costs incurred over the course of the first episode of therapy (regardless of the line of therapy in which it was initiated). AE-related HCRU and costs were assessed using a subset of medical claims containing an applicable diagnosis and/or treatment code (at primary position or elsewhere) for the AE in question. AE-related monthly costs were calculated over the follow-up period and stratified by the number of unique AEs and during specific treatments.
Data analysis
All study measures were analyzed descriptively. Cost data, adjusted to 2016 US dollars using the medical care component of the US Consumer Price Index, were assessed from the payer’s perspective and included health plan paid amounts and the coordination of benefit amounts. All-cause and MCL-related HCRU and costs were estimated overall and by care setting (e.g., inpatient, emergency, hospice). To account for variability in length of follow-up, mean per-patient monthly costs were assessed. The incremental HCRU and costs associated with AEs were analyzed by stratifying these measures by the number of AEs experienced during follow-up (i.e., 0, 1–2, 3–5, and ≥ 6 AEs).
Multivariable Cox regression models were used to assess the risk of A-fib and bleeding in first-line therapy among patients who received a first-line therapy. The outcome variables (e.g., time to first occurrence of A-fib during first-line therapy) were measured from the start of the first-line treatment episode. The models controlled for baseline patient characteristics and the type of first-line treatment regimen. Hazard ratios (HR) and 95% confidence intervals (CIs) were estimated. To assess factors associated with an inpatient admission and total costs in first-line therapy, multivariable logistic regression and generalized linear models were fit, respectively. A binary variable representing the number of unique AEs observed during the first-line treatment episode (1–2 and 3–4 AEs) was included in the model as the primary independent variable to assess incremental inpatient admission and cost burdens associated with more AEs. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc.; Cary, NC; 2011).
Results
Baseline patient characteristics
A total of 783 patients with MCL met the selection criteria (median age, 65 years [range, 19–99 years]; 70% male). The mean CCI score in the 12-month baseline period was 2.4 (range: 0 to 13), with a mean daily pill burden of 2.8 (range: 0 to 17) in the month before the study index date. Approximately 46% of patients were identified as being at high risk of A-fib. The mean of monthly all-cause costs over the baseline period was $1,303 (standard deviation [SD] = $3,680). Table 1 provides a detailed description of baseline patient characteristics and costs.
Table 1.
Baseline Characteristics of Patients With MCL (Online Only)
| All Patients, n (%) | 783 (100.0%) | |
|---|---|---|
| Age at index, years | ||
| Mean (SD) | 66.8 (12.0) | |
| Median (Q1, Q3) | 65 (59, 76) | |
| Age Group, n (%) | ||
| 18–44 years | 20 | |
| 45–54 years | 79 | |
| 55–64 years | 270 | |
| 65–79 years | 283 | |
| 80+ years | 131 | |
| Health plan type, n (%) | ||
| HMO | 102 (13.0%) | |
| PPO | 403 (51.5%) | |
| POS | 49 (6.3%) | |
| Other | 211 (27.0%) | |
| Unknown | 18 (2.3%) | |
| Year of study index date (first diagnosis), n (%) | ||
| 2012e | 163 (20.8%) | |
| 2013 | 281 (35.9%) | |
| 2014 | 247 (31.6%) | |
| 2015e | 92 (11.8%) | |
| Length of follow-up (months)a | ||
| Mean (SD) | 19.4 (12.8) | |
| Median | 17.9 | |
| Min, Max | 0.1, 47.9 | |
| Atrial fibrillation risk statusb, n (%) | ||
| High risk | 363 (46.4%) | |
| Low risk | 420 (53.6%) | |
| CCI score | ||
| Mean (SD) | 2.4 (2.4) | |
| Median (Q1, Q3) | 2 (1, 4) | |
| Min, Max | 0, 13 | |
| Daily pill burdenc | ||
| Mean (SD) | 2.8 (2.8) | |
| Median (Q1, Q3) | 2 (1, 4) | |
| Min, Max | 0, 17 | |
| Average monthly costsd | ||
| Mean (SD) | $1,303 ($3,680) | |
| Median (Q1, Q3) | $429 ($203, $934) | |
| Min, Max | $0, $61,882 | |
All costs are in 2016 US dollars.
CCI = Charlson Comorbidity Index; HMO = health maintenance organization; MCL = mantle cell lymphoma; POS = point of service; PPO = preferred provider organization; SD = standard deviation.
Follow-up time calculated as the number of days between the study index date and the end of the follow-up divided by 30.5.
Atrial fibrillation risk status was defined based on the method used by Chyou et al., 2015.15
Mean number of oral medications available in-hand, on a daily basis, during the 30-day period before the study index date.
Mean monthly all-cause costs over the 12-month baseline period (includes costs for inpatient stays, emergency department visits, office visits, other outpatient and ancillary care, and pharmacy visits) as incurred by health plans.
Indicates data for partial year: For 2012, data included diagnoses from July through December, and for 2015, data include diagnoses from January through June.
Treatments and adverse events
Patients had a mean length of follow-up of 19.4 months (Q1 = 8.2, Q3 = 28.5) after the first MCL diagnosis. Of the total sample, 71.9% received at least one treatment for MCL. Chemotherapy was the most common category of treatment (62%), followed by biologic therapy/immunomodulators (52.4%), radiation therapy (26.2%), targeted therapy (13.9%), and autologous SCT (8.2%). No MCL-directed treatment was recorded for 28.2% of patients during follow-up. A majority of patients received an agent-specific first-line systemic therapy (n = 457; 58.4%). Among patients treated with first-line therapy (n = 457), 33.3% received second-line therapy during the follow-up period, 52 (11.4%) received third-line therapy, and 24 (5.3%) received fourth-line therapy. The most common treatment regimens, regardless of therapy line, were bendamustine/rituximab (BR) (41.1%), RCHOP (26.7%), rituximab monotherapy (20.4% [including maintenance]), and ibrutinib monotherapy (14.2%). Bortezomib, lenalidomide, and cytarabine treatments were received by 5.1%, 4.1%, and 4.0% of patients initiating a systemic therapy, respectively.
The most common therapy regimen in the first line was BR (35.4%), followed by RCHOP (24.9%) and rituximab monotherapy (12.9%). In all subsequent therapy lines, rituximab monotherapy (including maintenance) was the most common regimen (22% in the second line; 29% in both third and fourth lines), followed by ibrutinib monotherapy. Use of maintenance rituximab was observed in 6.1% (n = 28) of all patients treated with an MCL-directed systemic therapy. Among patients receiving second, third, and fourth lines of therapy, 14.5%, 21.2%, and 16.7%, respectively, received maintenance rituximab. The sequence of MCL therapies, as patients moved from first- to third-line therapy, is presented in Figure 2. In patients aged ≥ 65 years, 42% received BR as first-line therapy (vs. 28% in patients aged 18–64 years). In contrast, RCHOP was used in 19% of patients aged ≥ 65 years (vs. 32% in those aged 18–64 years).
Figure 2.
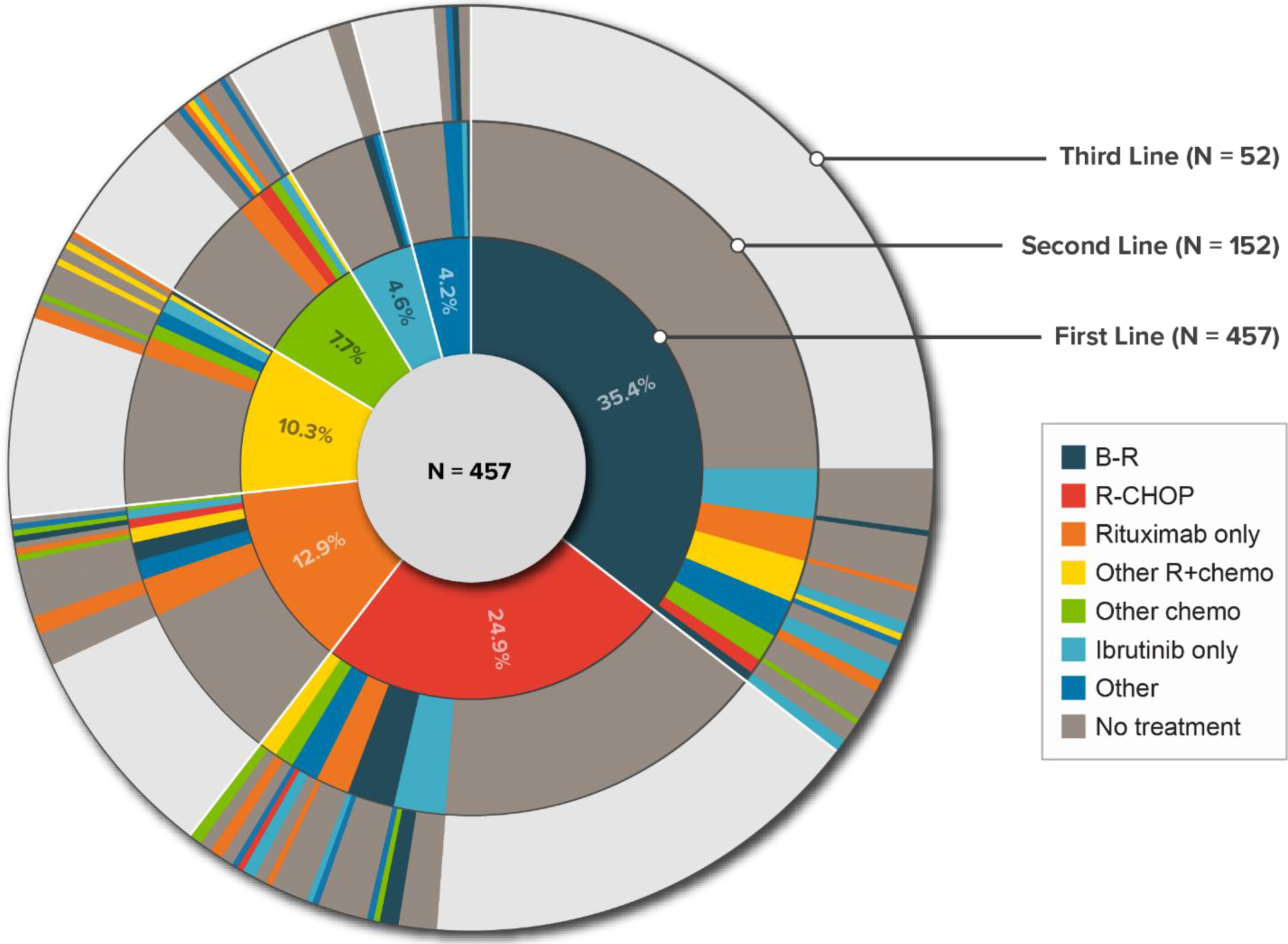
Distribution and Sequence of Treatments Received in the First Three Lines of Therapy in Patients With MCL
Note: The areas highlighted in gray color in the second and third lines of therapy represent proportions of patients who did not receive any treatment subsequent to the current line of therapy.
BR = bendamustine/rituximab; MCL = mantle cell lymphoma; RCHOP = rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone; other R+chemo = rituximab/other chemotherapy; other chemo = other chemotherapy.
For the most common regimens, the median durations of exposure were: ibrutinib monotherapy, 6.1 months (43% still on therapy at the end of follow-up); BR, 5.6 months; RCHOP, 4.3 months; and rituximab monotherapy, 2.4 months.
The most common incident AEs (≥ 5% in patients receiving at least one of the most common treatments) are shown in Table 2. The proportions of patients experiencing hematologic AEs, fever, diarrhea, dehydration, and infection were highest among those receiving RCHOP; A-fib and renal failure/chronic kidney disease were most frequent among those receiving ibrutinib monotherapy.
Table 2.
Incident AEs During MCL Therapies
| Adverse Event | BR | RCHOP | Rituximab Monotherapy | Ibrutinib Monotherapy | ||||
|---|---|---|---|---|---|---|---|---|
| n = 188 | n = 122 | n = 93 | n = 65 | |||||
| N | % | N | % | N | % | N | % | |
| Anemia | 18 | 9.6 | 40 | 32.8 | 1 | 1.1 | 1 | 1.5 |
| Atrial fibrillation | 6 | 3.2 | 3 | 2.5 | 1 | 1.1 | 7 | 10.8 |
| Dehydration | 27 | 14.4 | 24 | 19.7 | 8 | 8.6 | 5 | 7.7 |
| Diarrhea | 12 | 6.4 | 11 | 9.0 | 3 | 3.2 | 4 | 6.2 |
| Fever/pyrexia | 19 | 10.1 | 25 | 20.5 | 7 | 7.5 | 2 | 3.1 |
| Hemorrhage/bleeding | 17 | 9.0 | 9 | 7.4 | 2 | 2.2 | 5 | 7.7 |
| Hypertension | 10 | 5.3 | 7 | 5.7 | 1 | 1.1 | 1 | 1.5 |
| Infection | 13 | 6.9 | 15 | 12.3 | 3 | 3.2 | 4 | 6.2 |
| Neutropenia | 53 | 28.2 | 40 | 32.8 | 1 | 1.1 | 3 | 4.6 |
| Renal failure/chronic kidney failure | 8 | 4.3 | 5 | 4.1 | 4 | 4.3 | 5 | 7.7 |
| Thrombocytopenia | 12 | 6.4 | 23 | 18.9 | 1 | 1.1 | 4 | 6.2 |
AE = adverse event; BR = bendamustine/rituximab; MCL = mantle cell lymphoma; RCHOP = rituximab/cyclophosphamide/doxorubicin/vincristine (with or without prednisone).
Resource use and costs
Mean (SD) total all-cause and MCL-related monthly costs during follow-up were $10,964 ($17,530) and $8,613 ($16,166), respectively. Inpatient admission costs ($5,929 [$15,623]) and outpatient office visit costs ($2,262 [$4,070]) were the largest drivers of total all-cause costs (Figure 3a). Mean (SD) monthly all-cause costs during treatments with BR, RCHOP, rituximab monotherapy, and ibrutinib monotherapy were $12,958 ($12,687), $24,719 ($44,996), $13,153 ($27,516), and $21,690 ($24,773), respectively. Mean (SD) monthly all-cause costs were $5,131 ($10,352) among those with no AEs and nearly three times higher ($13,560 [$18,466]) among those with six or more AEs during follow-up (Figure 3b).
Figure 3.
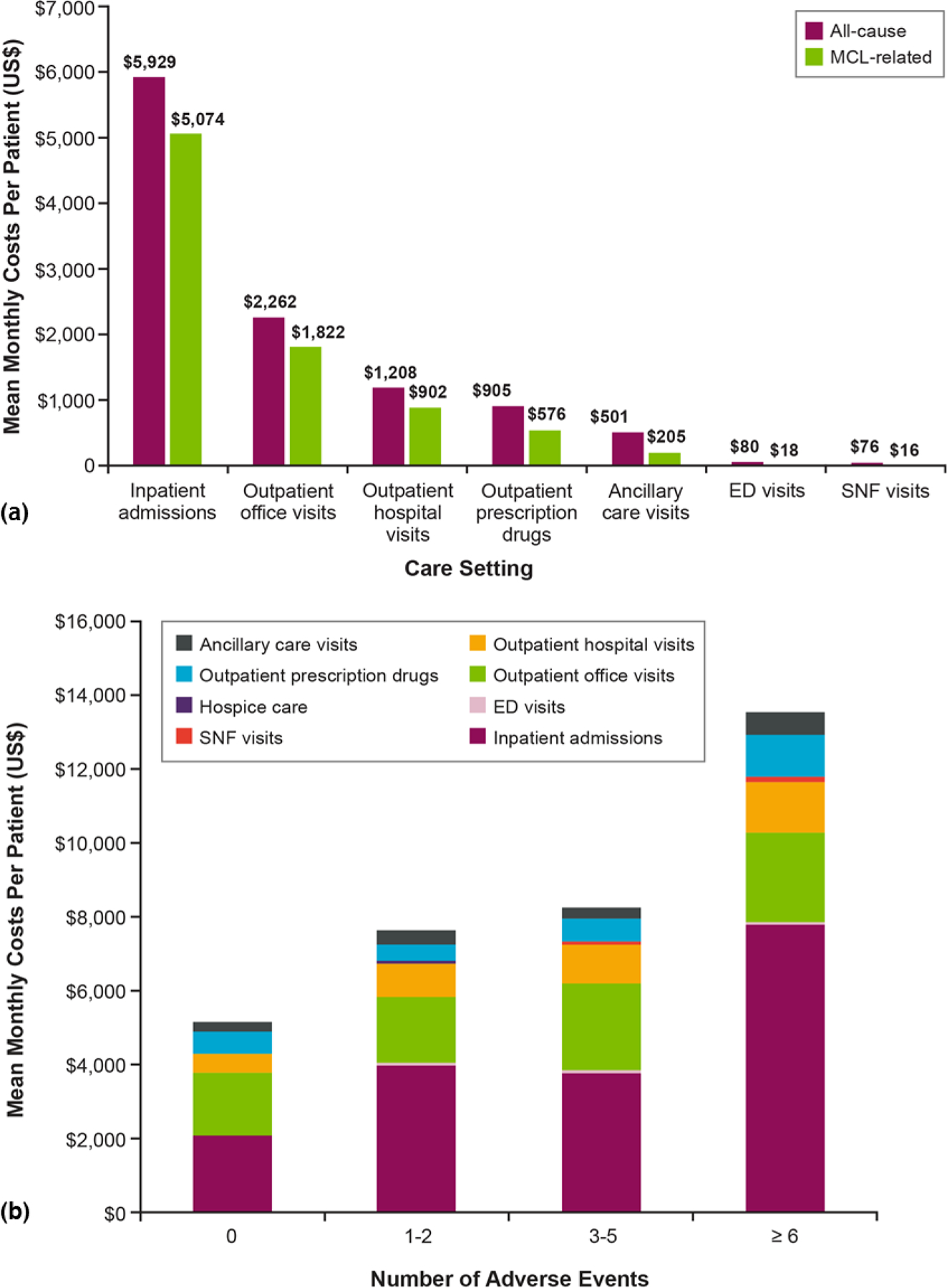
Mean Per-Patient Direct Monthly Costs in the Follow-Up Period After MCL Diagnosis. (a) All-Cause and MCL-Related Costs by Care Setting and (b) All-Cause Costs by Care Setting and Number of Adverse Events
ED = emergency department; MCL = mantle cell lymphoma; SNF = skilled nursing facility.
Factors associated with atrial fibrillation and bleeding
Patients with high A-fib risk status at baseline had a 15 times higher rate of A-fib during first-line therapy for MCL (HR, 15.21; 95% CI, 3.54–65.39). Patients with a history of pneumonia had a three times higher rate of A-fib than those without such history (HR, 3.51; 95% CI, 1.22–10.12). Patients treated with ibrutinib monotherapy (vs. BR) had an estimated hazard ratio of 2.97 (95% CI, 0.93–9.49).
The risk of bleeding during the first-line therapy was estimated to be significantly higher among patients with a history of anemia (HR, 2.71; 95% CI, 1.49–4.93) or a history of previous bleeding (HR, 2.06; 95% CI, 1.13–3.75) during the baseline period.
Factors associated with inpatient admission
Patients who experienced 3–4 AEs during first-line therapy had nearly seven times greater odds of an inpatient admission during first-line therapy than those with 1–2 AEs (odds ratio [OR], 6.90; 95% CI, 4.00–11.93). Older patients (aged ≥ 65 years) were less likely than younger patients to have an inpatient admission (OR, 0.54; 95% CI, 0.32–0.92). First-line therapy with RCHOP was associated with a nearly 2.6 times greater likelihood of an inpatient admission (OR, 2.60; 95% CI, 1.48–4.56).
Factors associated with health care costs
Patients in the older age group (≥ 65 years) had significantly lower monthly costs than younger patients during first-line therapy (cost ratio [CR], 0.70; 95% CI, 0.53–0.91). Patients diagnosed with MCL during 2014–2015 had 29% higher costs than patients diagnosed in earlier years, 2012–2013 (CR, 1.24; 95% CI, 1.00–1.53). First-line therapy with RCHOP, compared with BR, was associated with 69% higher monthly costs (CR, 1.59; 95% CI, 1.18–2.14).
Discussion
The study findings provide an overview of patient characteristics, treatment patterns, AE incidence, and health care costs among commercially enrolled patients diagnosed with MCL in the US. The median age of the study sample (65 years) is representative of the age of patients with MCL in the US population (68 years).1 Although this population is clearly heterogeneous, the findings of significant comorbidities, daily pill burden, and monthly health care costs at baseline suggest a substantial subgroup of patients who are relatively frail.
This study identifies the most common treatment regimens used in the management for MCL in routine practice during a period when targeted therapies were approved and readily available in clinical practice. The distribution of treatment regimens and incidence of potentially treatment-related AEs observed in this study are largely consistent with those previously reported in clinical studies [19,20]; such data from real-world settings are absent. BR was the preferred first-line regimen, which aligns with the findings of a randomized, phase 3 trial reporting that BR increased progression-free survival and was associated with fewer hematological and other AEs than RCHOP, a historically common first-line regimen for older patients with MCL [19]. Concordant with the NCCN guideline recommendations, our study shows that most patients (68%) received treatment with immunochemotherapy regimens (BR and RCHOP) in the first line and then with noncytotoxic targeted/biologic therapy regimens in the second and subsequent therapy lines. Rituximab monotherapy was also received by almost 13% of the patients during the first line and 20.4% during the overall follow-up period. Results from an observational claims database study also reported a similar proportion of MCL patients receiving rituximab monotherapy (20.0%) during the follow-up period [21]. Although not a suggested treatment regimen according to the NCCN Clinical Practice Guidelines, the higher than expected usage of rituximab monotherapy in our population may be a reflection of the inclusion of elderly patients who may be less able to tolerate chemotherapy, for whom rituximab monotherapy could be considered an alternative in community practice.
The results of this study indicate that resource utilization and the economic burden associated with MCL are substantial across all lines of therapy and with all agents studied, with mean monthly costs varying considerably by treatment and care setting. Inpatient admissions and office visits were the largest drivers of total monthly all-cause and MCL-related costs, consistent with findings of a similar analysis by Wade and colleagues [22].
The high burden of AEs in patients treated with the common MCL therapies, as observed here, is largely consistent with reports from clinical trials [19,20,23]. Hematologic AEs were more commonly observed in patients treated with RCHOP than with the other regimens. Noncytotoxic regimens (rituximab and ibrutinib monotherapy) were generally less toxic, but ibrutinib was associated with the highest incidence of A-fib (10.8%). In clinical trials, ibrutinib-associated A-fib occurred in 6%−16% of patients [24–28].
Several AEs—including anemia, neutropenia, thrombocytopenia, infection, and nausea—were associated with substantial costs, which varied by type of treatment. The mean monthly costs increased with the number of unique AEs experienced during the follow-up period. In the adjusted analysis, patients at high baseline risk of A-fib (vs. low risk) had a substantially higher rate of A-fib during the first-line therapy—a finding consistent with results indicated in randomized trials [29,30], although, the magnitude of incremental rate observed in our study is higher than those seen in these trials. Patients with a greater number of AEs were observed to have higher utilization of inpatient care. The finding that older patients aged ≥ 65 years were less likely to have an inpatient admission and had lower monthly costs than their younger counterparts is potentially due to differences in treatment selection. A relatively higher proportion of older patients in this study received less aggressive outpatient therapies (e.g., BR), which are associated with lower severity and frequency of toxicities than the more intense therapies that require more health care resources spent to manage toxicities.
The findings of this analysis should be viewed in the context of certain limitations. The analyses are based on a nationally representative database of individuals enrolled in employer-sponsored health plans in the US. As such, individuals who are enrolled only in public health insurance program (e.g., Medicare, Medicaid) with no supplemental private insurance, or those who are unemployed and/or uninsured, are not represented, and therefore, findings cannot be generalized to the general US population. The selection of the study cohort was based on diagnosis codes indicative of MCL as recorded in insurance claims, and any erroneous coding could have misclassified patients. In the absence of access to patients’ medical records, this study assumed that claims associated with treatments, AEs, and costs were accurately coded. Because all payments associated with claims containing a diagnosis and/or procedure code for the AE in question were attributed to the AE, there may be overlaps and possible overestimation of individual AE-related costs. Conversely, costs not coded as relating to an AE but still spent toward managing the AE were unaccounted for, which may have offset some of the overestimation. In assessing the proportion of patients with incident AEs, those with evidence of AEs prior to treatment initiation were excluded from the numerator but were retained in the denominator to reflect the proportion of new cases in the total cohort. Although a 12-month baseline period was required to identify patients with newly diagnosed MCL, it is possible that some patients with more than 12 months of encounter-free time related to previously diagnosed MCL may still have been included. Finally, patients had different observation periods depending on the timing of MCL diagnosis in relation to acquisition of data for this study, and treatment practices may have changed during the study entry period. Despite these limitations, the findings from this study are important in that they set a comprehensive benchmark against which the future therapeutic landscape—potentially affected by increasing uptake of novel agents including idelalisib, venetoclax, and acalabrutinib—can be evaluated.
In conclusion, this study demonstrates that chemoimmunotherapy combinations, particularly BR, remain the most common choice for initial treatment for patients with MCL. After rituximab, ibrutinib was the most commonly used targeted agent, with few patients receiving lenalidomide- or bortezomib-based therapies during the study period. The burden of AEs among patients receiving systemic therapy is considerable, and the overall cost of managing patients with MCL can be substantially affected by the costs associated with managing AEs occurring during treatment.
Funding:
This work was supported by AstraZeneca.
Footnotes
Declaration of interest:
Ravi K. Goyal, Saurabh P. Nagar, and James A. Kaye are full-time employees of RTI Health Solutions, which received funding from AstraZeneca to conduct this research. RTI Health Solutions is a business unit of Research Triangle Institute, which is an independent, nonprofit, research organization that does work for government agencies and private companies. Shaum Kabadi and Brian Seal are full-time employees of AstraZeneca, the funding organization. Anthony Mato has received research funding and/or has consulting relationship with the following organizations: AbbVie, Acerta, AstraZeneca, Celgene, DTRM, Gilead Sciences, Janssen, Kite, Pharmacyclics, Portola, Regeneron, and TG Therapeutics.
Prior Presentation
Part of this research was presented at the 59th Annual Meeting of the American Society of Hematology, December 9–12, 2017, Atlanta GA.
References
- 1.Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008. August 15;113(4):791–798. [DOI] [PubMed] [Google Scholar]
- 2.Lu NN, Li YX, Wang WH, et al. Clinical behavior and treatment outcome of primary nasal diffuse large B-cell lymphoma. Cancer. 2012. March 15;118(6):1593–1598. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsson A, Dahle N, Jerkeman M. Marked improvement of overall survival in mantle cell lymphoma: a population based study from the Swedish Lymphoma Registry. Leuk Lymphoma. 2011. October;52(10):1929–1935. [DOI] [PubMed] [Google Scholar]
- 4.Leukemia and Lymphoma Society. Mantle cell lymphoma facts. 2014. [cited 2018 Jan 12] Available from: https://www.lls.org/sites/default/files/file_assets/mantlecelllymphoma.pdf. [Google Scholar]
- 5.Fakhri B, Kahl B. Current and emerging treatment options for mantle cell lymphoma. Ther Adv Hematol. 2017. August;8(8):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Zhang X, Zhong J. Current approaches and advance in mantle cell lymphoma treatment. Stem Cell Investig. 2015. [cited 2018 Jan 12];2:18 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923656/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009. March;20(3):520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015. August 6;126(6):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan J, Martin P, Shah B, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med. 2015. November 5;373(19):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013. August 8;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang CPS, McMullen J, Tam C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. 2017. September 13:1–11. [DOI] [PubMed] [Google Scholar]
- 12.Kozma CM, Slaton T, Ellis R, McKenzie RS. Healthcare resource utilization (HRU) in treated mantle cell lymphoma (MCL) patients. [abstract]. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5–9; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2014;74(19 Suppl): Abstract 4139. doi: 10.1158/1538-7445.AM2014-4139. [DOI] [Google Scholar]
- 13.Feinberg B, Schenkel B, McBride A, et al. Predictors of emergency room (ER) visits and hospitalizations in patients with mantle cell lymphoma (MCL) treated with chemotherapy. Blood. 2015;126:4526. [Google Scholar]
- 14.Charlson ME, Charlson RE, Peterson JC, et al. The Charlson Comorbidity Index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–1240. [DOI] [PubMed] [Google Scholar]
- 15.Chyou JY, Hunter TD, Mollenkopf SA, et al. Individual and Combined risk factors for incident atrial fibrillation and incident stroke: an analysis of 3 million at-risk US Patients. J Am Heart Assoc. 2015. July 23;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: B-cell lymphomas. Version 1. 2017. [cited 2018 Feb] Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 17.Danese MD, Reyes CM, Gleeson ML, et al. Estimating the population benefits and costs of rituximab therapy in the United States from 1998 to 2013 using real-world data. Med Care. 2016. April;54(4):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvitz S, Guerin A, Brammer M, et al. Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist. 2014. September;19(9):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013. April 6;381(9873):1203–1210. [DOI] [PubMed] [Google Scholar]
- 20.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or RCHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014. May 8;123(19):2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozma C, Slaton T, McKenzie RS, et al. Mantle Cell Lymphoma (MCL) Treatment Patterns, Healthcare Resource Use (HRU), and Costs in the United States. Clinical Lymphoma, Myeloma and Leukemia. 2015. June 1;15:S226. [Google Scholar]
- 22.Wade RL, Hu HX, Chen JY, et al. Increased treatment cost associated with mantle cell lymphoma disease progression. Poster presentation. EHA Learning Center. June 12, 2015. [cited 2018 Feb 28] Available from: https://learningcenter.ehaweb.org/eha/2015/20th/100659/rolin.wade.increased.treatment.cost.associated.with.mantle.cell.lymphoma.html?f=m1. [Google Scholar]
- 23.Czuczman MS, Goy A, Lamonica D, et al. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann Hematol. 2015. December;94(12):2025–2032. [DOI] [PubMed] [Google Scholar]
- 24.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. [DOI] [PubMed] [Google Scholar]
- 25.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. [DOI] [PubMed] [Google Scholar]
- 27.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farooqui M, Valdez J, Soto S, et al. Atrial fibrillation in CLL/SLL patients on ibrutinib. Presented at the American Society of Hematology’s 57th Annual Meeting and Exhibition; December 5–8, 2015; Orlando, FL. [Google Scholar]
- 29.Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017. October;102(10):1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrontikis A, Carey J, Gilreath JA, et al. Proposed Algorithm for Managing Ibrutinib-Related Atrial Fibrillation. Oncology (Williston Park). 2016. November 15;30(11):970–974, 980–981, C3. [PubMed] [Google Scholar]


