Abstract
For much of the last century, our knowledge regarding the pancreas in type 1 and type 2 diabetes was largely derived from autopsy studies of individuals with these disorders or investigations utilising rodent models of either disease. While many important insights emanated from these efforts, the mode for investigation has increasingly seen change due to the availability of transplant-quality organ-donor tissues, improvements in pancreatic imaging, advances in metabolic assessments of living patients, genetic analyses, technological advances for laboratory investigation and more. As a result, many long-standing notions regarding the role for and the changes that occur in the pancreas in individuals with these disorders have come under question, while, at the same time, new issues (e.g., beta cell persistence, disease heterogeneity, exocrine contributions) have arisen. In this article, we will consider the vital role of the pancreas in human health and physiology, including discussion of its anatomical features and dual (exocrine and endocrine) functions. Specifically, we convey changes that occur in the pancreas of those with either type 1 or type 2 diabetes, with careful attention to the facets that may contribute to the pathogenesis of either disorder. Finally, we discuss the emerging unknowns with the belief that understanding the role of the pancreas in type 1 and type 2 diabetes will lead to improvements in disease diagnosis, understanding of disease heterogeneity and optimisation of treatments at a personalised level.
Keywords: Anatomy, Endocrine, Exocrine, Function, Pancreas, Review, Type 1 diabetes, Type 2 diabetes
Graphical Abstract
Pancreas anatomy and its exocrine and endocrine functions
In the normal, healthy human adult, the pancreas weighs approximately 100 g, has a length of 14 to 25 cm [1], a volume of approximately 72.4 ± 25.8 cm3[2] and is both lobular and elongated in shape (reviewed previously [1]). Lying obliquely behind the posterior and upper abdominal wall, this highly parenchymatous organ is divided into five anatomical parts: the head, uncinate process (located in the ventral lobe of the head), neck, body and tail (Fig. 1). The normal human pancreas grows until approximately age 30, with significant variability in adult pancreas weight or volume [2].
Fig. 1.
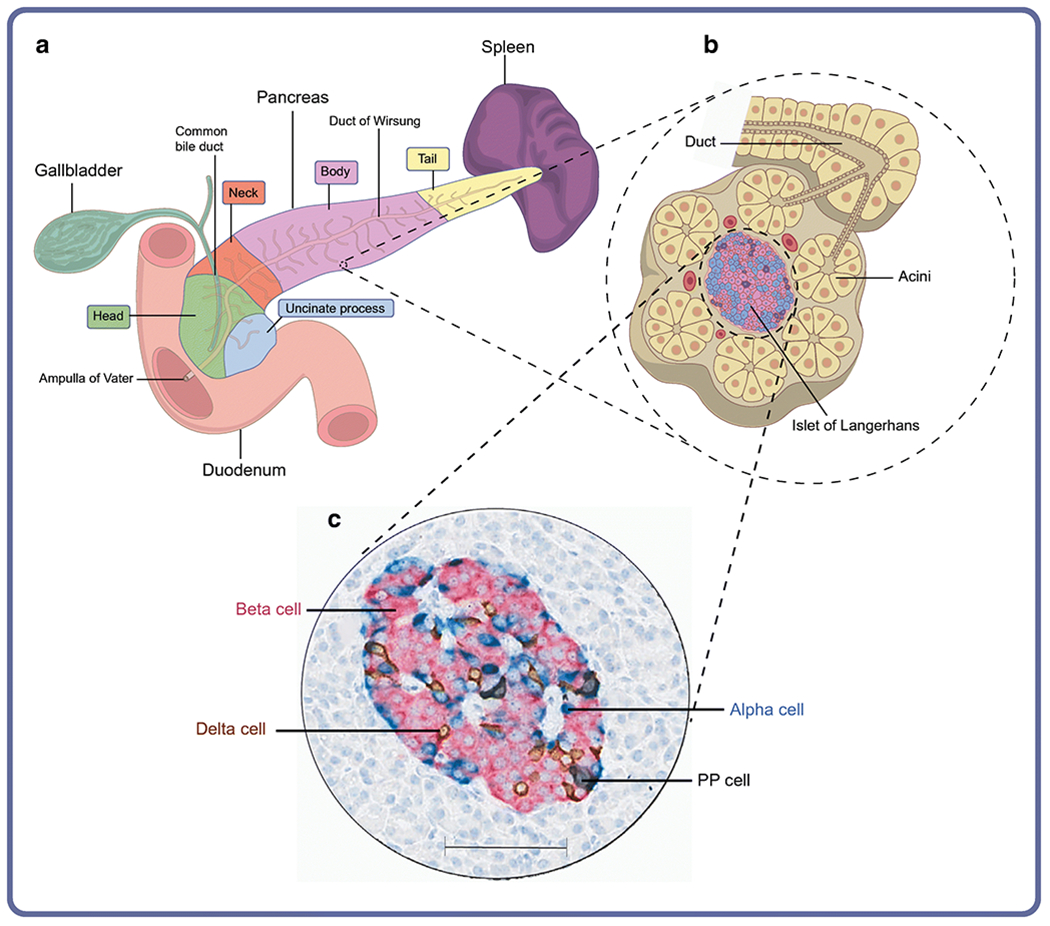
Key anatomical features of the human pancreas. (a) Diagram of the pancreas, and surrounding organs. (b) Schematic representation of organisation of the endocrine and exocrine pancreas at the cellular level. (c) Human pancreatic islet showing the four endocrine cell types. Scale bar, 100 μm. This figure is available as part of a downloadable slideset
Located in the upper abdomen, with its head residing immediately adjacent to the duodenum, the body and tail regions of the pancreas extend across the body’s midline to a point near the spleen. More specifically, the head lies directly against the descending and horizontal parts of the C-shaped duodenum. The uncinate process projects inferiorly from the head and extends posteriorly towards the superior mesenteric artery. The neck portion extends laterally from the head where it connects to the pancreatic body. Posterior to the neck is the superior mesenteric artery and vein, as well as the origin of the hepatic portal vein. The aorta, superior mesenteric artery, left renal vessels and left kidney are each situated posterior to the pancreatic body. Finally, the tail is in close proximity to the hilum of the spleen. These anatomical parameters are key to the function of the organ.
The vast majority of pancreatic tissue is devoted to its exocrine function, in which digestive enzymes are produced and secreted via a complex ductal tree into the duodenum. The cells in the pancreas that produce these digestive enzymes are acinar cells, derived from the Latin word ‘acinus’, meaning grape, as they are cellular aggregates that form bundles akin to clusters of grapes (Fig. 1) [3]. Acinar cells make up nearly 85% of the pancreas, are arranged in acini, and synthesise and secrete enzymes active in protein, fat and carbohydrate digestion, including trypsin, lipase and amylase [4]. Each acinar bundle connects to the pancreatic duct system. Centroacinar cells represent the most peripheral duct system and partially cover the apical surface of the acinar cells. Centroacinar cells connect to the intercalated ducts that converge and form the intralobular and interlobular ducts, which, in turn, eventually drain into the main pancreatic duct. The main duct, the duct of Wirsung, empties into the duodenum. The residual portion of the main pancreatic duct located in the dorsal lobe, the so-called duct of Santorin, empties into the duodenum as the accessory pancreatic duct. The main duct also connects with the bile duct in the head of the pancreas to form the hepatopancreatic duct (i.e. the ampulla of Vater). Flow through the ampulla of Vater is controlled by the muscular sphincter of Oddi, to open during digestion and to close for prevention of reflux of duodenal content into the pancreatic ductal tree postprandially.
Acinar enzymes are secreted into a bicarbonate-rich fluid produced by the ductal epithelium. Pancreatic secretions occur at a low rate between meals (0.2–0.3 ml/min) and markedly increase during meals (4.0 ml/min) for a total daily volume of ~2.5 l [5]. Pancreatic fluid output is regulated by several hormones, as well as by the autonomic nervous system. As food enters the duodenum, enteroendocrine cells found in the mucosal lining release hormones (e.g., secretin, cholecystokinin) into the bloodstream that, in turn, stimulate the pancreas to produce and release large amounts of water, bicarbonate and digestive enzymes (e.g., amylase and lipase) and zymogens (e.g., trypsinogen, chymotrypsinogen, proelastase and procarboxypeptidase), which are inactive enzyme precursors that are activated by proteolytic enzymes once they are secreted. These enzymes are critical in the digestion of food that enters the small intestine from the stomach.
Located between the clusters of acinar cells are scattered patches of endocrine secretory tissue, known as the islets of Langerhans. Approximately one million of these micro-organs [6] exist in the pancreas, altogether weighing about 1 g and forming 1–2% of the total pancreas mass [6]. In human pancreas development, islets arise from the endodermal tissue compartment and are observed in the ventral and dorsal lobes. In humans, approximately 40–60% of the endocrine cells are insulin-producing beta cells, with the remainder being alpha, delta, pancreatic polypeptide (PP; also known as F) and epsilon cells (Fig. 1), which secrete glucagon, somatostatin, pancreatic polypeptide and ghrelin, respectively. However, the proportion of these various endocrine cell types within an islet varies as a function of islet size, age and location within the organ (reviewed previously [7, 8]). Smaller islets are comprised primarily of beta cells, while larger islets may have nearly equal numbers of beta and alpha cells [7, 8]. Islets derived from the ventral lobe contain PP cell-rich areas, with few beta and alpha cells, that are found exclusively in the posterior head and uncinate regions of the pancreas [9].
Each islet is supplied by one or more small arteries (arterioles) that branch into numerous capillaries. These capillaries emerge and coalesce into small veins outside the islet. Regarding pancreatic innervation, motor nerve fibers carry impulses to both acinar cells and pancreatic islets [10, 11]. Parasympathetic fibers induce secretion from acinar cells, ultimately resulting in the release of pancreatic juice, as well as stimulating islets to secrete insulin, glucagon and other polypeptide hormones required for normal blood glucose regulation. In contrast, sympathetic fibers cause inhibition of exocrine and endocrine secretions (previously reviewed [12]). Thus, islet functions are regulated by signals initiated by autonomic nerves, circulating metabolites (e.g., glucose, amino acids and ketone bodies), circulating hormones and local (paracrine) hormones.
Pancreas function and its contribution to diabetes
The specific contributions of both the endocrine and exocrine pancreas to diabetes in its many forms are described throughout this special edition in Diabetologia. However, as a collective, diabetes is a disorder of carbohydrate metabolism, characterised by the inability of the body to produce sufficient amounts of, or respond appropriately to, insulin. In addition, dysregulated glucagon secretion by alpha cells is a key feature of both type 1 and type 2 diabetes. Therefore, the importance of the endocrine pancreas lies in the fact that it secretes the two major hormones, glucagon and insulin, that play a central role in the regulation of energy metabolism.
The pancreas in type 1 diabetes
Beta cell loss
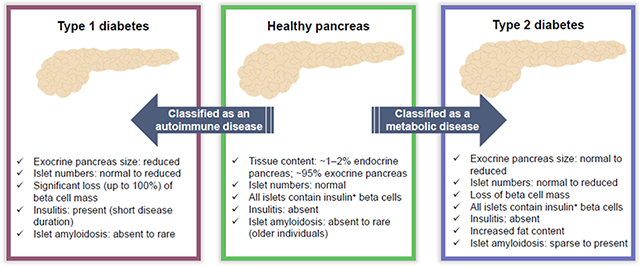
Type 1 diabetes results from autoimmune-mediated destruction of islet beta cells due to complex interactions between genetic and environmental factors [13]. The pathology of what we now consider type 1 diabetes was reported over 100 years ago, based on autopsy findings from individuals at the onset of the disease (reviewed previously [14]). These studies established the defining feature of the disease as a significant loss of islet beta cells, with 50–90% of islets having no beta cells (depending on disease duration) despite having other islet endocrine cells present at expected numbers. Loss of islet beta cells has also been observed in islet-autoantibody-positive non-diabetic organ donors [14]. Significant heterogeneity exists in the numbers of islets with residual beta cells (insulin+) vs those with partial or complete loss of beta cells (insulin−). The insulin+ islets also show a high degree of heterogeneity, ranging from normal to greatly reduced beta cell numbers. A summary of histopathological features between non-diabetic control and type 1 and type 2 diabetic organ donors is presented in Table 1.
Table 1.
Histopathological differences between organ donors without diabetes, with type 1 diabetes and with type 2 diabetes
| Characteristic | Control | Type 1 diabetes | Type 2 diabetes | |||
|---|---|---|---|---|---|---|
| Diabetes duration | – | Short duration | Long duration | Short duration | Long duration | |
| INS− islets (pseudoatrophic) | Extremely rare (<3/pancreas) | Present | Frequent | None | None | |
| INS+ islets | Normal INS:GCG:SST cell number proportions, 4:2:1 |
Patchy to absent | Reduced (up to 100%) | Normal | Reduced yet all islets have beta cells, even those with amyloid | |
| INS+:GCG+ cell ratio | >1 | <1 | <0.1 | >1 | ~Reduced | |
| Islet numbers | Normal | Normal to reduced | Reduced | Normal | Normal to reduced | |
| Islet size | Wide range in islet size, including single cells in exocrine regions, such as ducts | Normal to smaller (secondary to loss of beta cells) | Smaller (secondary to loss of beta cells) | Wide range including hypertrophic islets | Wide range including hypertrophic islets | |
| Insulitis | Absent | Present | Usually absent | Absent | Absent | |
| Amyloid | Present in older individuals | Absent/ rare | Absent/ rare | Absent/sparse | Present | |
GCG, glucagon; INS, insulin; SST, somatostatin
Insulitis
In 2013, a consensus statement was published for islet infiltration (insulitis), to provide standardisation of histopathological investigations [15]. The consensus statement defines insulitis as lesions present in a minimum of three islets, with a threshold level of ≥15 CD45+ cells immediately adjacent to islet endocrine cells. Insulitis primarily affects insulin+ islets, yet <10% of insulin+ islets are typically infiltrated [14]. The reasons for this remain unclear. Insulitic islets were rarely observed after 10 years of type 1 diabetes duration, coinciding with loss of islet beta cells [14]. Insulitis in multiple-autoantibody-positive donors was similar to that observed in donors with type 1 diabetes, with a highly variable lobular pattern and similar immune-cell phenotype and numbers [14]. The insulitic mononuclear cell infiltrate is dominated by CD3+ T lymphocytes, comprised of CD8+ T cells with lesser numbers of CD4+ T cells, and variable numbers of CD20+ B lymphocytes. Among the CD3+ T cells, CD8+ T cells are clearly activated, as indicated by the dramatically elevated proportion of proliferating CD8+ T cells, even in long-standing type 1 diabetes [16]. Interestingly, numbers of CD3+ T and CD20+ B lymphocytes parallel each other, as well as total immune-cell numbers (CD45+) [14]. The same patterns were observed in two young adult organ donors with islet-associated autoantibodies but no clinical history of diabetes, suggesting infiltration may precede clinical onset of the disease. Likewise, the presence of both CD8+ T cells and CD20+ B cells was higher in islets with residual beta cells vs those without in a very recent-onset type 1 diabetes case [16]. Other studies in younger individuals at type 1 diabetes onset were conducted through retrieval of autopsy samples and reported that the highest numbers of CD20+ B cells were found in the youngest patients, suggestive of potential disease heterogeneity. However, high numbers of B cells were also observed with elevated numbers of CD8+ T cells, such that younger patients appeared to have greater numbers of islet-infiltrating cells than older patients at disease onset [17]. These findings may have implications for treatment choices. The number of islets with insulitis has been demonstrated to inversely correlate with type 1 diabetes duration (e.g., higher insulitis frequency at diabetes onset) and to positively associate with beta cell mass; age of onset was not associated with insulitis frequency [14]. In donors with insulin+ islets, insulitis was detected many years post onset of disease. Indeed, studies in the Joslin Medalist Program found residual beta cells in all donor pancreases, despite ≥50 years of type 1 diabetes [18]. Other immune cells identified within insulitic islets include CD68+ macrophages, CD11c+ dendritic cells, forkhead box P3 (FOXP3)+ regulatory T cells and mast cells [14, 19, 20]. The monocytic infiltrate in insulitic islets can be comprised of diffusely scattered cells, and as aggregates within the islet interior or in the immediately adjacent exterior.
Islet amyloidosis
Until recently, islet amyloidosis, which develops from extracellular deposition of islet amyloid polypeptide (IAPP), was widely considered pathognomonic for type 2 diabetes [21]. Islet IAPP is a 37 amino acid polypeptide that is co-produced/co-secreted with insulin from beta cells; hypersecretion of insulin with insulin resistance may result in IAPP deposition [22]. Two recent studies report the presence of islet amyloid in people with type 1 diabetes, including a 12-year-old individual [23, 24]. This observation provides an intriguing parallel between the pathological processes in type 1 and type 2 diabetes affecting at least a subset of patients, adding support to the concept that common metabolic derangements affecting beta cells are likely to occur in both diseases.
Abnormalities in islet vasculature
In comparison with control islets, islets from individuals with type 1 diabetes have smaller islet vessels that are similar in size to vessels of the exocrine region [25]. Of note, islets with residual beta cells had a similar vascular phenotype to control islets, suggesting that beta cells within islets maintain the normal microvasculature. Recent studies using optical clearing show these vessels are more tortuous in islets without beta cells [26]. In addition, the sympathetic innervation of islets from individuals with type 1 diabetes is preserved and could promote vasoconstriction, as seen with smaller vessels in these individuals by two-dimensional (2D) studies. Parasympathetic innervation of human islets is important for both insulin and glucagon responses to a meal or during fasting. Optical-clearing three-dimensional (3D) studies show abundant cholinergic innervation of human islets; however, no studies are yet available regarding parasympathetic innervation in type 1 diabetes [26].
Reduced pancreas size
The exocrine pancreas size is significantly reduced in both individuals with type 1 and type 2 diabetes, with the former exhibiting the largest difference [27–29]. Indeed, smaller pancreases in individuals with type 1 diabetes have been reported by several groups by either autopsy or radiographic studies (reviewed previously [30]). These studies show that the pancreas is 20–50% smaller in children and adults with type 1 diabetes compared with unrelated non-diabetic control participants. Potential mechanisms underlying reduced pancreas size in type 1 diabetes could include atrophy, impaired organ growth rate during fetal or postnatal life, or a combination of both. Loss of functional beta cell mass and, thereby, loss of insulinotropic effects on acinar cells, has also been proposed as a primary mechanism [31]. Since the studies reported to date are cross-sectional in nature, it is not known if people with type 1 diabetes are born with a smaller pancreas or if the organ shrinks during the disease process. In addition, several groups reported no effect of diabetes duration on pancreas weight or size, suggesting insulin therapy may not reverse exocrine changes (previously reviewed [32]).
The pancreas in type 2 diabetes
Increased amyloid deposition
Insulin-secreting beta cells are remarkable for their ability to adapt to metabolic demand. In fact, trained athletes secrete up to three times less insulin to achieve euglycaemia than untrained individuals; conversely, non-diabetic obese people can secrete five times more insulin than control participants in response to a glucose challenge [33]. However, the adaptive response of beta cells is not limitless and when it fails, type 2 diabetes ensues. In contrast to the dramatic changes in islet morphology and immune infiltration described for type 1 diabetes above, there is no stereotyped histology of the pancreas in type 2 diabetes (Table 1). One histological feature that has historically garnered interest is the deposition of amyloid, an extracellular protein aggregate derived from IAPP (Fig. 2). While islet amyloid is present in some islets of the majority of individuals with type 2 diabetes, its causal role in diabetes pathogenesis has not been established. In addition, it is not a definitive histological marker since a significant fraction of individuals with type 2 diabetes do not have amyloid in their islets, while these deposits can be present in the islets of euglycaemic individuals and, as mentioned earlier, of those with type 1 diabetes [34, 35].
Fig. 2.
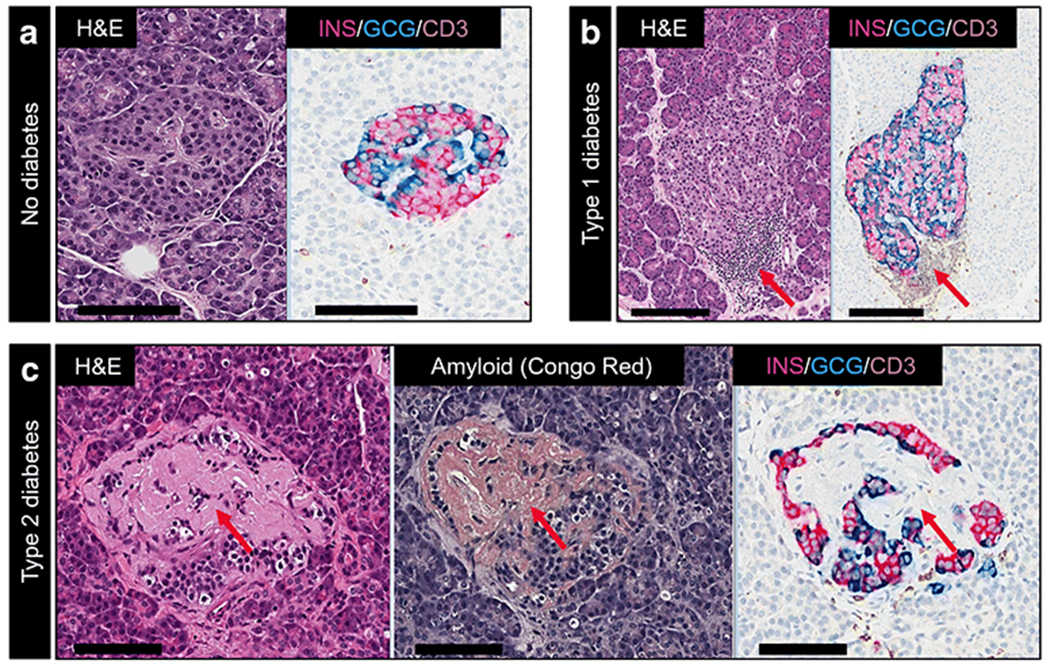
Representative images of islets of Langerhans in pancreases from individuals without diabetes and those with type 1 and type 2 diabetes. Pancreatic tissue was stained with H&E or for beta cells (insulin; INS), alpha cells (glucagon; GCG) and T cells (CD3). (a) A pancreatic islet from a healthy donor, showing normal distribution of beta and alpha cells. (b) Insulitic islet with CD3 infiltration (red arrow) in the pancreas of an individual with type 1 diabetes. (c) Islet with amyloid deposits (green arrows), demonstrated by H&E Congo Red staining in the pancreas of an individual with type 2 diabetes. INS, GCG and CD3 staining in the islet from the type 2 diabetic donor is also shown. Scale bars: 100 μm in (a) and (c); 200 μm in (b). This figure is available as part of a downloadable slideset
Reduced beta cell mass
Multiple autopsy studies have found pancreatic beta cell mass in individuals with type 2 diabetes to be about 60% of normal beta cell mass [34]. Similar findings have been obtained from organ-donor pancreatic samples [35]. Since islet and beta cell mass at birth and in childhood are highly variable, and because longitudinal determination of beta cell or, even, whole-islet mass in humans is still impossible, cause and effect cannot be determined. In other words, we do not know if beta cell-mass decline during the course of type 2 diabetes is due to genetic predisposition, or lifestyle choices and associated glucotoxicity, or if individuals born with a small islet mass are simply more likely to develop type 2 diabetes. It is clear, however, that the beta cells present in individuals with type 2 diabetes do not function normally, as was already established more than 30 years ago [36].
Increased pancreatic fat content
One frequently, though not consistently, reported feature of the pancreas in type 2 diabetes is its increased fat content, as determined by computed tomography (CT) [2, 37] and MRI [38,39]. While Saisho and colleagues found pancreatic fat content increased with age, but not further with type 2 diabetes, multiple other studies documented additional lipid accumulation in individuals with type 2 diabetes and suggested that intra-organ fat might contribute to beta cell dysfunction [2, 38]. Hepatic steatosis is, of course, a common feature in obesity and insulin resistance. Therefore, it is not surprising that steatosis also occurs in the pancreas; in fact, pancreatic steatosis co-occurs in more than two thirds of individuals with type 2 diabetes [39]. But does it play a role in islet dysfunction? This question is much more difficult to answer; although, increased pancreatic fat content precedes the development of diabetes in some animal models [39]. One possibility is that, in the initial stages of the disease, pancreatic steatosis may contribute to a decline in function, but once overt diabetes ensues, multiple deleterious factors, such as increased inflammation and oxidative stress, play a much more determinative role than pancreatic lipid content [40].
Ongoing trials and human tissue studies
At present, no clear histological marker specific to type 2 diabetes has emerged. Currently, large-scale efforts directed at the analysis of the human pancreas from deceased organ donors using multiple experimental modalities include the European RHAPSODY, INNODIA and Innovative Medicines Initiative for Diabetes: Improving beta-cell function and identification of diagnostic biomarkers for treatment monitoring in Diabetes (IMIDIA) projects (summarised previously [41]), and the NIH-funded Human Pancreas Analysis Program (HPAP) [42], which was recently extended to the analysis of type 2 diabetes. It can be hoped that these projects will shed new light on the pathogenic events that occur in the pancreas in diabetes. For instance, until recently, insulin resistance, particularly of the liver, was considered by many to be the most relevant cause of type 2 diabetes [43, 44]. Now, the concept of islet failure as key to both forms of diabetes is gaining acceptance among diabetes researchers worldwide and represents a substantial step forward.
Why understanding the pancreas in type 1 and type 2 diabetes is important
With an ever-increasing incidence of type 1 and type 2 diabetes at a global level, the need to identify methods capable of averting what has become an international healthcare crisis has never been greater. Despite improvements in a variety of tools for disease management, the determination of markers capable of predicting risk for disease and identification of genetic regions having a potential contributory role in disease development, our basic understanding of the pathogenic mechanisms underlying both disorders remain in an unsatisfactory position. As evidenced throughout this article, and in articles elsewhere in this special issue, an understanding of the changes that occur at the level of the pancreas has the potential to lead to dramatic changes in this unacceptable situation. This being said, while the pancreas may play a central role in diabetes pathogenesis, all research-derived observations must be considered in the context of the body’s entirety, which includes the circulatory network and many organ systems that may not be reproducible in an experimental context. We remain optimistic that advances in pancreatic research will lead to improvements in disease diagnosis, understanding of disease heterogeneity and optimisation of treatments at a personalised level.
Acknowledgements
The authors would like to thank A. Posgai and S. Williams (University of Florida, Gainesville, FL, USA) for their technical assistance in writing this manuscript. In addition, we respectfully acknowledge the organ donors and their family members that make intellectual advances regarding diabetes, especially the role of the pancreas, possible.
Funding Relevant work in the authors’ laboratories is supported by the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R to MAA) and the Leona M. & Harry B. Helmsley Charitable Trust (2018PG-T1D053 to MAA). Additional support was provided by the NIH NIDDK Human Islet Research Network (RRID:SCR_014393; https://hirnetwork.org; UC4DK112217 to KHK and UC4DK108132 to MAA), NIH NIAID (P0142288 to MAA), and NIH SPARC OT2 OD023861 and JDRF 2-SRA-2019-697-S-B to MCT.
Abbreviations
- IAPP
Islet amyloid polypeptide
- PP
Pancreatic polypeptide (cells)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Longnecker DS, Gorelick F, Thompson ED (2018) Anatomy, histology, and fine structure of the pancreas In: Beger HG, Warshaw AL, Hruban RH et al. (eds) The pancreas: an integrated textbook of basic science, medicine, and surgery, 3rd edn John Wiley & Sons Ltd, Hoboken, NJ, pp 10–23. [Google Scholar]
- [2].Saisho Y, Butler AE, Meier JJ, et al. (2007) Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 20(8): 933–942. 10.1002/ca.20543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Williams JA (2010) Regulation of acinar cell function in the pancreas. Curr Opin Gastroenterol 26(5): 478–483. 10.1097/MOG.0b013e32833d11c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Matsuda Y (2019) Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol Int 69:450–462. 10.1111/pin.12837 [DOI] [PubMed] [Google Scholar]
- [5].Pandol SJ (2010) Water and ion secretion from the pancreatic ductal system. In: Pandol SJ (ed) The Exocrine Pancreas. Morgan & Claypool Life Sciences, San Rafael, CA: Available from: www.ncbi.nlm.nih.gov/books/NBK54130/ Accessed: 2 February 2020. [PubMed] [Google Scholar]
- [6].In’t Veld P, Marichal M (2010) Microscopic anatomy of the human islet of Langerhans. Adv Exp Med Biol 654: 1–19. 10.1007/978-90-481-3271-3_1 [DOI] [PubMed] [Google Scholar]
- [7].Kilimnik G, Jo J, Periwal V, Zielinski MC, Hara M 2012) Quantification of islet size and architecture. Islets 4(2): 167–172. 10.4161/isl.19256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bonner-Weir S, Sullivan BA, Weir GC (2015) Human islet morphology revisited: human and rodent islets are not so different after all. J Histochem Cytochem. 63(8): 604–612. 10.1369/0022155415570969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Savari O, Zielinski MC, Wang X, et al. (2013) Distinct function of the head region of human pancreas in the pathogenesis of diabetes. Islets 5(5): 226–228. 10.4161/isl.26432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ahrén B (2012) Islet nerves in focus—defining their neurobiological and clinical role. Diabetologia 55(12): 3152–3154. 10.1007/s00125-012-2727-6 [DOI] [PubMed] [Google Scholar]
- [11].Schwartz MW, Seeley RJ, Tschöp MH, et al. (2013) Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503(7474): 59–66. 10.1038/nature12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. (2011) Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14(1): 45–54. 10.1016/j.cmet.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383(9911): 69–82. 10.1016/S0140-6736(13)60591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Campbell-Thompson M, Fu A, Kaddis JS, et al. (2016) Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 65(3): 719–731. 10.2337/db15-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Campbell-Thompson ML, Atkinson MA, Butler AE, et al. (2013) The diagnosis of insulitis in human type 1 diabetes. Diabetologia 56(11): 2541–2543. 10.1007/s00125-013-3043-5 [DOI] [PubMed] [Google Scholar]
- [16].Wang YJ, Traum D, Schug J, et al. (2019) Multiplexed in situ imaging mass cytometry analysis of the human endocrine pancreas and immune system in type 1 diabetes. Cell Metab 29(3): 769–783. 10.1016/j.cmet.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Leete P, Willcox A, Krogvold L, et al. (2016) Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 65(5): 1362–1369. 10.2337/db15-1615 [DOI] [PubMed] [Google Scholar]
- [18].Yu MG, Keenan HA, Shah HS, et al. (2019) Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest 129(8):3252–3263. 10.1172/JCI127397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seay HR, Yusko E, Rothweiler SJ, et al. (2016) Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes. JCI Insight 1(20): e88242 10.1172/jci.insight.88242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martino L, Masini M, Bugliani M, et al. (2015) Mast cells infiltrate pancreatic islets in human type 1 diabetes. Diabetologia 58(11):2554–2562. 10.1007/s00125-015-3734-1 [DOI] [PubMed] [Google Scholar]
- [21].Jurgens CA, Toukatly MN, Fligner CL, et al. (2011) β-Cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol 178(6): 2632–2640. 10.1016/j.ajpath.2011.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bhowmick DC, Singh S, Trikha S, Jeremic AM (2018) The molecular physiopathogenesis of islet amyloidosis. Handb Exp Pharmacol 245: 271–312. 10.1007/164_2017_62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beery ML, Jacobsen LM, Atkinson MA, Butler AE, Campbell-Thompson M (2019) Islet amyloidosis in a child with type 1 diabetes. Islets 11(2): 44–49. 10.1080/19382014.2019.1599707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Westermark GT, Krogvold L, Dahl-Jørgensen K, Ludvigsson J (2017) Islet amyloid in recent-onset type 1 diabetes-the DiViD study. Ups J Med Sci 122(3): 201–203. 10.1080/03009734.2017.1359219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Canzano JS, Nasif LH, Butterworth EA, Fu DA, Atkinson MA, Campbell-Thompson M (2018) Islet microvasculature alterations with loss of beta-cells in patients with type 1 diabetes. J Histochem Cytochem 67(1):41–52. 10.1369/0022155418778546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tang SC, Jessup CF, Campbell-Thompson M (2018) The role of accessory cells in islet homeostasis. Curr Diab Rep 18(11): 117 10.1007/s11892-018-1096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Silva ME, Vezozzo DP, Ursich MJ, Rocha DM, Cerri GG, Wajchenberg BL (1993) Ultrasonographic abnormalities of the pancreas in IDDM and NIDDM patients. Diabetes Care 16(9): 1296–1297. 10.2337/diacare.16.9.1296 [DOI] [PubMed] [Google Scholar]
- [28].Alzaid A, Aideyan O, Nawaz S (1993) The size of the pancreas in diabetes mellitus. Diabet Med 10(8): 759–763. 10.1111/j.1464-5491.1993.tb00160.x [DOI] [PubMed] [Google Scholar]
- [29].Garcia TS, Rech TH, Leitao CB (2017) Pancreatic size and fat content in diabetes: a systematic review and meta-analysis of imaging studies. PLoS One 12(7): e0180911 10.1371/journal.pone.0180911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Campbell-Thompson ML, Filipp SL, Grajo JR, et al. (2019) Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care 42(2): 281–287. 10.2337/dc18-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Henderson JR, Daniel PM, Fraser PA (1981) The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut 22(2): 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Di Gialleonardo V, de Vries EF, Di Girolamo M, Quintero AM, Dierckx RA, Signore A (2012) Imaging of β-cell mass and insulitis in insulin-dependent (type 1) diabetes mellitus. Endocrine Reviews 33(6):892–919. 10.1210/er.2011-1041 [DOI] [PubMed] [Google Scholar]
- [33].Moghetti P, Bacchi E, Brangani C, Donà S, Negri C (2016) Metabolic effects of exercise. Frontiers of hormone research 47:44–57. 10.1159/000445156 [DOI] [PubMed] [Google Scholar]
- [34].Westermark P, Wilander E, Westermark G, Johnson K (1987) Islet amyloid polypeptide-like immunoreactivity in the islet B cells of type 2 (non-insulin-dependent) diabetic and non-diabetic individuals. Diabetologia 30(11):887–892. 10.1007/bf00274799 [DOI] [PubMed] [Google Scholar]
- [35].Clark A, Saad MF, Nezzer T, et al. (1990) Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia 33(5): 285–289. 10.1007/bf00403322 [DOI] [PubMed] [Google Scholar]
- [36].Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D (1984) Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 74(4): 1318–1328. 10.1172/JCI111542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma J, Song Z, Yan F (2014) Detection of hepatic and pancreatic fat infiltration in type II diabetes mellitus patients with IDEAL-Quant using 3.0T MR: comparison with single-voxel proton spectroscopy. Chin Med J 127(20): 3548–3552 [PubMed] [Google Scholar]
- [38].Ou HY, Wang CY, Yang YC, Chen MF, Chang CJ (2013) The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One 8(5): e62561 10.1371/journal.pone.0062561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee JS, Kim SH, Jun DW, et al. (2009) Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol 15(15): 1869–1875. 10.3748/wjg.15.1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guglielmi V, Sbraccia P (2018) Type 2 diabetes: does pancreatic fat really matter? Diabetes Metab Res Rev 34(2):e2955 10.1002/dmrr.2955 [DOI] [PubMed] [Google Scholar]
- [41].Marchetti P, Schulte A, Marselli L, et al. (2019) Fostering improved human islet research: a European perspective. Diabetologia 62(8): 1514–1516. 10.1007/s00125-019-4911- [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kaestner KH, Powers AC, Naji A, Atkinson MA, Consortium H (2019) NIH initiative to improve understanding of the pancreas, islet, and autoimmunity in type 1 diabetes: the Human Pancreas Analysis Program (HPAP). Diabetes 68(7): 1394–1402. 10.2337/db19-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Taylor R (2012) Insulin resistance and type 2 diabetes. Diabetes 61(4):778–779. 10.2337/db12-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Petersen K, Dufour S, Befroy D, Garcia R, Shulman G (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. The New England journal of medicine 350(7): 664–671. 10.1056/NEJMoa031314 [DOI] [PMC free article] [PubMed] [Google Scholar]


