Abstract
Many established technologies are limited in analyzing the executive functions in motion, especially while walking. Functional near-infrared spectroscopy (fNIRS) fills this gap. The aim of the study is to investigate the inter-session reliability (ISR) of fNIRS-derived parameters at the prefrontal cortex while walking in people with multiple sclerosis (MS) and healthy control (HC) individuals. Twenty people with MS/HC individuals walked a 12 m track back and forth over 6 min. The primary outcomes were the absolute and relative reliability of the mean, slope coefficient (SC), and area under the curve (A) of the oxy-/deoxyhemoglobin concentrations (HbO/HbR) in the Brodmann areas (BA) 9/46/10. The SC and the A of HbO exhibited a fair ISR in BA10 in people with MS. For the mean and A of the HbR, almost all areas observed revealed a fair ISR. Overall, the ISR was better for HbR than HbO. A fair to excellent ISR was found for most BA of the prefrontal cortex in HC individuals. In total, the ISR of the analyzed fNIRS-derived parameters was limited. To improve the ISR, confounders such as fatigue and mind wandering should be minimized. When reporting the ISR, the focus should be on the mean/A rather than SC.
Keywords: MS, hemodynamic response, fNIRS, test–retest reliability, cortical activity, PFC
1. Introduction
Human bipedal locomotion is a central determinant of participation in daily life. Especially people suffering from inflammatory autoimmune diseases, such as multiple sclerosis (MS), often exhibit impaired locomotion [1]. These impairments can be diverse (e.g., ataxia, spasticity, or muscle weakness) and depend on the affected area in the brain or spinal cord [2]. To treat these deficits more efficiently, it is necessary to understand the underlying motor and cognitive mechanisms.
One concept that comprises both mechanisms is gait automaticity. According to Clark [3], gait automaticity is “[…] the ability of the nervous system to successfully coordinate movement with minimal use of attention-demanding executive control resources”. The interaction of automaticity and executive control are essential for executing movements. The respective contributions and the relation to each other can be shifted by different factors such as (motor-) learning progresses [4] or damage of the central nervous system [3], as it can be found in people with MS. To estimate the degree of gait automaticity, it is important to quantify the activation of the prefrontal cortex (PFC) in which the executive functions and the attention are located [5].
The established technologies (magnetic resonance imaging, positron emission tomography, and magnetoencephalography) are too limited to assess the PFC activation in motion due to the required fixed head position and non-portability [6]. Although electroencephalography (EEG) is portable, the preparation is time-consuming, and it has a high susceptibility to motion artifacts [6].
Functional near-infrared spectroscopy (fNIRS) is a promising tool that provides the following properties [7]. It is a non-invasive, easy to apply, and portable optical brain imaging method that is applicable in motion [8,9,10]. It is less affected by motion artefacts than comparable systems and has a relatively high temporal resolution up to 1 ms [8,11]. Due to these features, the interest in fNIRS is rapidly increasing in the rehabilitative context. First studies have already investigated the PFC activation while walking in people with MS [12,13]. They were able to distinguish between healthy people and people with MS and between different walking conditions based on the PFC activation. Even though these first results are promising, there is a lack of basic methodological studies on fNIRS.
To the best of our knowledge, there are no studies analyzing absolute and relative inter-session reliability (ISR) data in people with MS while walking yet, which is urgently necessary to assess changes in fNIRS-derived parameters. Especially in people with MS, it is important to verify the ISR, as the disease is accompanied by a high fluctuation in daily performance caused by, e.g., state fatigue or fatigability [14]. Moreover, there is only one study that has demonstrated moderate ISR of fNIRS-derived parameters while walking in healthy adults [15] yet.
Therefore, the present study aims to analyze the relative and absolute ISR of fNIRS-derived parameters at the PFC during single-task walking on two consecutively days in moderately affected people with MS and healthy control individuals (HC individuals).
2. Materials and Methods
2.1. Study Design and Participants
For this cross-sectional controlled ISR study, 20 people with MS (15 female/5 male) with a confirmed MS diagnosis according to the revised McDonald criteria [16] were recruited. They were 41.0 ± 12.0 years old and had an Expanded Disability Status Scale (EDSS) [17] of 2.0 ± 0.9. The patients had to be able to walk at least 300 m without walking aids. Therefore, only patients with an EDSS less than or equal to 4.5 were included. The last acute episode of MS and the last cortisone intake should date back more than 30 days. The HC individuals were age- (42.2 ± 9.8 years) and sex- (16 female/4 male) matched. They should not have orthopedic or neurologic limitations nor hypertension or obesity. The study was approved by the ethics committee of the Medical Faculty of the Otto von Guericke University (OvGU) Magdeburg (Germany) (No.: 116/18) and is registered in the German Clinical Trial Register (ID: DRKS00015190).
2.2. Study Procedure
The study was conducted by the Department of Health and Physical Activity of the OvGU Magdeburg together with the Center for Neurorehabilitation Median Klinik Flechtingen (Germany). The people with MS were recruited by health professionals at the clinic at the beginning of their six weeks rehabilitation. First, the patients were informed about the study and written informed consent was obtained. In the pre-assessment, the 6-min walk test (6MWT) was executed [18] by physiotherapists and the 12-Item Multiple Sclerosis Walking Scale (MSWS-12, German version) [19] was obtained. Subsequently, the test and retest measurements (24 h in between) were conducted in the morning on non-treatment days. The participants walked a distance of 12 m on a level floor back and forth, in their self-selected walking pace and were advised to concentrate on walking only. Due to the fNIRS system requirements, the measurement started in a standing position (baseline) for 30 s and then altered between standing and walking every 30 s (Figure 1). The test conditions standing and walking were announced by the test instructor. The number of walking intervals was chosen according the time of the 6MWT. In total, the duration of the test protocol was about 12 min and 30 s. At the beginning and the end of each test day, the subjects were asked about their perceived exhaustion using the Borg Scale [20].
Figure 1.
fNIRS test protocol.
The HC individuals were recruited from local citizens. The measurements were conducted at the facilities of the OvGU Magdeburg. The test procedure was the same as for the people with MS.
2.3. Equipment and Outcome Measures
For this study, two portable fNIRS systems (NIRSport, NIRx Medical Technologies, NY, USA) were used each attached to a standardized cap (EasyCap GmBH, Herrsching, Germany) with circumferences of 56 cm and 58 cm. Each cap was equipped with eight sources and eight detectors together with eight short separation channels according to the international 10–20 system for EEG to cover the PFC (Prefrontal cortex) (Figure 2: created with NirSite 2.0, NIRx Medical Technologies, NY, USA). The average source-detector separation distance was 30–40 mm. The arrangement of the optodes was done with the fNIRS Optodes’ Location Decider (fOLD) toolbox [21]. Additional information about the sensitivity of the channels according to the fOLD toolbox is provided in the Supplementary Material.
Figure 2.
Arrangement of sources (red dots) and detectors (blue dots) at the prefrontal cortex using fNIRS.
The cap was placed in the middle between nasion to inion and left preauricular to right preauricular point (reference point Cz). To deal with external light interferences an additional standardized cap was placed on top of the fNIRS system. The applied fNIRS system operates at two different wavelengths (760/850 nm) and at a fixed sampling frequency of 7.81 Hz. The exact subareas captured are the right, left, and medial dorsolateral PFC Brodmann area (BA) 9 and 46 (r/lDLPFC9, r/lDLPFC46, mDLPFC9) and the right, left, and medial frontopolar cortex BA10 (r/l/mFPC10). The subareas are composed of the following channels: rDLPFC9 (channels, 1, 18 and 21), rDLPFC46 (channel 6), lDLPFC9 (channels 17, 20 and 22), lDLPFC46 (channel 13), rFPC10 (channels 4, 5, 7 and 8), lFPC10 (10, 11, 12 and 14), and mFPC (channel 9).
The primary outcomes were the concentration of oxy-/deoxyhemoglobin (cHbO/cHbR) in those subareas. The secondary outcomes were the heart rate (HR) and heart rate variability (HRV) measured with a heart rate monitor (RS800CX Polar Electro Oy ®, Kempele, Finland). The HRV parameters considered were the time intervals between two R-spikes (RR interval) and the low frequency/high frequency (LF/HF) ratio. HR and HRV were used to control systemic confounders in the hemodynamic response [22]. Additionally, the perceived exhaustion was assessed on both days pre and post measurement using the Borg Scale (rating 6–20).
2.4. Data Processing
For data processing, we used the software “HOMER2” Version 2.8 [23]. First, the data were processed with the enPruneChannels function to sort out the channels with a too weak or too strong signal or where the standard deviation was too high (data range: 1 × 10−2 to 1 × 107; signal to noise threshold: 2; source detector separation range: 0.0–45.0 mm, and reset: 0). Subsequently, the raw data were transformed to optical density data [23]. The second filter method was utilized to reduce motion artefacts based on a spline interpolation and the digital Savitzky-Golay filter (hmrMotionCorrectSplineSG) [24]. Therefore, the p value was set to 0.99 [24]. The frame size was adjusted to 15 s. The data were then processed with a 3rd order Butterworth low pass filter with a cut off frequency of 0.5 Hz [24,25]. Consecutively, the filtered optical density data were converted into the changes in cHbO/cHbR by executing the modified Beer–Lambert Law [10]. To incorporate the age-related differences, the differential path length factor was adjusted, as described in [26], for each participant. The hemodynamic response function (HRF) was appraised by a general linear model approach. Therefore, the ordinary least squares method was used [27]. The time range was set from −10 to 45 s. The basis function for the HRF is a consecutive sequence of Gaussian functions with the width of 0.5 and the temporal spacing of 0.5. For the baseline drift, a 3rd order polynomial drift correction was utilized. The regression was conducted with the nearest short separation channels. After these preprocessing steps, the block average was calculated.
The cHbO/cHbR obtained during the walking protocol (twelve times 30 s) was further processed in MATLAB (Version R2017b, The MathWorks, Natick, MA, USA). To illustrate the course of cHbO and cHbR from baseline through walking to the next baseline, the channels of each individual subject were first averaged to the corresponding subareas of the PFC (l/r/mDLPFC9/46 and l/r/mFPC10). Then, the mean and standard deviation were calculated over all subjects for the respective subareas. Here, the last 10 s of the previous baseline, the 30 s walking interval and 15 s of the succeeding baseline were included to get an impression of the signal’s increase and decrease.
To prepare the data for the absolute and relative ISR calculation, the cHbO and cHbR were averaged from all twelve walking intervals of 30 s each. The first and last 5 s were cut out due to the delay of the hemodynamic response at the beginning and to reduce possible influences of the expected end of the walking interval. Subsequently, the mean, the slope coefficient (SC) [28], and the area under the curve (A) [29] of the cHbO and cHbR of this interval (5–25 s) were calculated. The mean and the A have been applied frequently in literature [28]. The SC provides information about the magnitude and direction of the change in cHbO and cHbR and is determined by a linear regression method [28].
2.5. Statistical Analysis
The statistical analysis was performed with the IBM SPSS software (Statistical Package for social science, Version 25, Chicago, IL, USA). The normal distribution was verified using the Kolmogorov–Smirnov test. The relative ISR was determined by the intraclass correlation coefficient (ICC) estimates and their 95% confidence intervals (CI) of the mean, SC, and A of the cHbO/cHbR build on a single-rating, absolute-agreement, 2-way, mixed-effects model [30]. The ICC was classified as poor with values ≤ 0.40, fair between 0.40 and 0.59, good between 0.60 and 0.74, and excellent between 0.75 and 1.00 [31]. In addition, the absolute reliability was checked by applying Bland and Altman limits of agreement (LoA), the bias, and the CI of the lower and upper LoA [32]. The differences of the secondary outcomes between testing days were tested by paired t-tests or, in case of none, normal distribution by Wilcoxon tests.
3. Results
The data of 16 people with MS (14 female/2 male) and 19 HC individuals (15 female/4 male) with an average age of 41.0 ± 12.0 and 42.1 ± 9.8 years, respectively, were analyzed (Table 1). Four people with MS had to be excluded due to an acute episode during the study period, breathing problems (allergic coryza) during the measurement, and two for not finishing the measurement. One subject of the HC individuals had to be excluded due to obesity (body mass index: 36.5). Overall, the people with MS suffered from moderate walking limitations (MSWS-12: 45% ± 20.7%) and were able to cover 473.1 ± 109.7 m in the 6MWT (HC individuals: 533.5 ± 64.5 m).
Table 1.
Descriptive subject data.
| Age [Years] | f/m | Weight [kg] | Height [cm] | EDSS | FD [Years] | FM [years] | MSWS-12 [%] | 6MWT [m] | |
|---|---|---|---|---|---|---|---|---|---|
| MS (n = 16) | 41.0 ± 12.0 | 14/2 | 74.6 ± 18.1 | 170.1 ± 9.0 | 2.0 ± 0.9 | 5.9 ± 6.8 | 8.6 ± 8.7 | 45.0 ± 20.7 | 473.1 ± 109.7 |
| HC (n = 19) | 42.2 ± 9.8 | 15/4 | 73.0 ± 16.0 | 171.4 ± 8.8 | n.a. | n.a. | n.a. | n.a. | 533.5 ± 64.5 |
MS: multiple sclerosis; HC: healthy control; f: female; m: male; EDSS: Expanded Disability Status Scale; FD: first diagnosis; FM: first manifestation; MSWS-12: 12-Item Multiple Sclerosis Walking Scale; 6MWT: 6-min walk test; n.a.: not applicable.
3.1. Descriptive Data cHbO/cHbR
We found the highest cHbO in the l/rDLPFC46 on both days in people with MS (Table 2). The cHbR in the lDLPFC46 was lowest ranging from −0.047 to −0.036 µmol/L. In the rDLPFC46 the cHbR varied greatly between testing days in people with MS (test: −0.014 ± 0.057 µmol/L; retest: −0.081 ± 0.122 µmol/L). The only negative cHbO was found in the mFPC10 for all people with MS on both testing days ranging from −0.103 to −0.024 µmol/L.
Table 2.
Test and retest overall mean cHbO/cHbR for both groups (MS/HC).
| MS (n = 16) | HC (n = 19) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Mean | MD | SD | 25% Quartile | 75% Quartile | Mean | MD | SD | 25% Quartile | 75% Quartile | |
| Test HbO [µmol/L] |
lDLPFC9 | 0.114 # | 0.090 | 0.214 | −0.001 | 0.135 | −0.083 | −0.081 | 0.217 | −0.204 | 0.056 |
| rDLPFC9 | 0.055 # | −0.003 | 0.177 | −0.057 | 0.154 | −0.106 # | −0.075 | 0.222 | −0.283 | 0.025 | |
| lDLPFC46 | 0.315 # | 0.304 | 0.207 | 0.212 | 0.351 | 0.104 | 0.074 | 0.257 | −0.086 | 0.332 | |
| rDLPFC46 | 0.210 | 0.201 | 0.132 | 0.111 | 0.290 | 0.067 | 0.046 | 0.192 | −0.080 | 0.175 | |
| mDLPFC9 | 0.067 | 0.034 | 0.155 | −0.050 | 0.180 | −0.113 # | −0.076 | 0.207 | −0.180 | −0.029 | |
| lFPC10 | 0.122 | 0.072 | 0.237 | −0.036 | 0.218 | 0.008 | −0.034 | 0.225 | −0.141 | 0.185 | |
| rFPC10 | 0.126 | 0.055 | 0.199 | 0.000 | 0.228 | 0.008 | −0.020 | 0.179 | −0.103 | 0.084 | |
| mFPC10 | −0.058 | −0.074 | 0.250 | −0.265 | 0.081 | −0.131 | −0.188 | 0.189 | −0.213 | 0.051 | |
| Test HbR [µmol/L] |
lDLPFC9 | −0.020 | −0.033 | 0.058 | −0.066 | 0.007 | 0.015 | 0.009 | 0.051 | −0.010 | 0.047 |
| rDLPFC9 | −0.026 | −0.029 | 0.043 | −0.061 | −0.002 | 0.009 | 0.009 | 0.052 | −0.017 | 0.038 | |
| lDLPFC46 | −0.047 | −0.028 | 0.061 | −0.086 | −0.008 | 0.011 | 0.004 | 0.074 | −0.040 | 0.061 | |
| rDLPFC46 | −0.014 | 0.002 | 0.057 | −0.066 | 0.035 | 0.006 | 0.010 | 0.061 | −0.030 | 0.049 | |
| mDLPFC9 | −0.055 | −0.055 | 0.065 | −0.114 | −0.001 | 0.000 | −0.006 | 0.056 | −0.031 | 0.030 | |
| lFPC10 | −0.048 | −0.046 | 0.055 | −0.084 | 0.003 | −0.003 | 0.001 | 0.049 | −0.044 | 0.043 | |
| rFPC10 | −0.023 | −0.038 | 0.058 | −0.067 | 0.020 | 0.011 | 0.002 | 0.064 | −0.037 | 0.038 | |
| mFPC10 | −0.014 | −0.015 | 0.075 | −0.045 | 0.011 | −0.004 | 0.003 | 0.062 | −0.050 | 0.052 | |
| Retest HbO [µmol/L] |
lDLPFC9 | 0.047 | 0.016 | 0.112 | −0.026 | 0.128 | −0.121 | −0.107 | 0.237 | −0.346 | 0.058 |
| rDLPFC9 | 0.099 | 0.035 | 0.221 | −0.056 | 0.296 | −0.114 | −0.142 | 0.271 | −0.216 | 0.060 | |
| lDLPFC46 | 0.215 | 0.227 | 0.169 | 0.122 | 0.331 | 0.055 | 0.045 | 0.202 | −0.066 | 0.244 | |
| rDLPFC46 | 0.208 | 0.170 | 0.281 | 0.040 | 0.452 | 0.089 | 0.081 | 0.305 | −0.036 | 0.236 | |
| mDLPFC9 | 0.086 | 0.100 | 0.157 | −0.046 | 0.215 | −0.114 | −0.119 | 0.258 | −0.224 | 0.073 | |
| lFPC10 | 0.166 | 0.118 | 0.195 | 0.004 | 0.324 | −0.005 | 0.000 | 0.238 | −0.198 | 0.073 | |
| rFPC10 | 0.130 | 0.138 | 0.149 | 0.029 | 0.229 | −0.031 | −0.009 | 0.187 | −0.107 | 0.071 | |
| mFPC10 | −0.050 | −0.053 | 0.206 | −0.173 | 0.090 | −0.121 | −0.098 | 0.179 | −0.270 | −0.016 | |
| Retest HbR [µmol/L] |
lDLPFC9 | 0.005 # | −0.028 | 0.070 | −0.034 | 0.052 | 0.029 | 0.020 | 0.057 | −0.014 | 0.058 |
| rDLPFC9 | −0.028 | −0.031 | 0.040 | −0.067 | 0.008 | 0.010 | 0.008 | 0.076 | −0.043 | 0.078 | |
| lDLPFC46 | −0.036 | −0.050 | 0.070 | −0.086 | 0.018 | 0.019 | 0.015 | 0.056 | −0.005 | 0.044 | |
| rDLPFC46 | −0.081 # | −0.073 | 0.122 | −0.109 | 0.001 | 0.010 | 0.010 | 0.067 | −0.038 | 0.063 | |
| mDLPFC9 | −0.054 | −0.054 | 0.048 | −0.108 | −0.016 | 0.016 | 0.012 | 0.050 | −0.013 | 0.051 | |
| lFPC10 | −0.056 | −0.060 | 0.062 | −0.091 | −0.011 | 0.002 | 0.010 | 0.056 | −0.043 | 0.052 | |
| rFPC10 | −0.049 | −0.042 | 0.054 | −0.088 | −0.008 | 0.000 | −0.008 | 0.069 | −0.035 | 0.034 | |
| mFPC10 | −0.027 | −0.021 | 0.072 | −0.070 | 0.041 | 0.008 | 0.010 | 0.053 | −0.020 | 0.044 | |
MS: multiple sclerosis; HC: healthy control; MD: median; SD: standard deviation; HbO: oxyhemoglobin; HbR: deoxyhemoglobin; l/r/mDLPFC9: left/right/medial dorsolateral prefrontal cortex Brodmann area 9; l/rDLPFC46: left/right dorsolateral prefrontal cortex Brodmann area 46; l/r/mFPC10: left/right/medial frontopolar cortex Brodmann area 10; #: not normally distributed.
We further observed that the cHbO were in general close to zero and mostly negative in HC individuals. The only positive results and with it the highest activation while walking were recorded for the l/rDLPFC46.
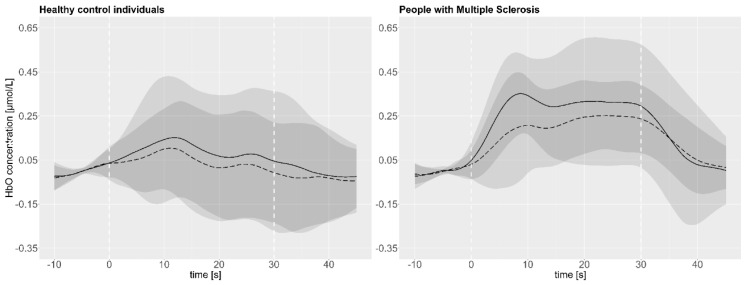
As illustrated exemplarily in Figure 3, the mean and standard deviation of the cHbO in the lDLPFC46 is higher on test than on retest day across all subjects (HC individuals: n = 19/MS: n = 16).
Figure 3.
Mean cHbO test (continuous line) and retest (dashed line) data and their respective standard deviation (test: light grey/retest: dark grey) of the lDLPFC46 over all subjects over the entire course of the measurement; 30 s walking interval is indicated by the white dashed lines.
In addition, the overall mean cHbO (Table 2) indicated also a trend that in some subareas the activation was lower on the second compared to the first day for both groups. Especially the mean cHbO in the lDLPFC9/46 and rDLPFC46 in people with MS and in the l/rDLPFC9, lDLPFC46, and l/rFPC10 in HC individuals revealed this trend. For further details regarding the cHbO and cHbR, please see Table 2.
3.2. Inter-Session Reliability cHbO/cHbR
All results regarding the ISR are listed in Table 3. For the people with MS, no ISR for the mean of the cHbO could be proven. The SC of the cHbO for the l/mFPC10 (ICC = 0.54/0.58) and the A of the cHbO for the rFPC10 (ICC = 0.42) exhibited a fair ISR. Regarding the cHbR, a fair ISR for all subareas (ICC range = 0.46-0.56) except the r/lDLPFC9 (ICC = 0.39/0.36) could be determined. The ISR of the A of the cHbR was comparable. For the SC of the cHbR, a fair ISR for the l/mFPC10 (ICC = 0.47/0.40) and the lDLPFC46 (ICC = 0.40) and a good ISR for the rFPC10 (ICC = 0.63) could be demonstrated.
Table 3.
Intraclass correlation coefficient of test and retest data of both groups (MS/HC).
| Inter-Session Reliability | Mean | Slope Coefficient | Area Under the Curve | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | F Test With True Value 0 | 95% CI | F Test With True Value 0 | 95% CI | F Test With True Value 0 | |||||||||||||||||
| ICC | Lower | Upper | Value | df1 | df2 | p | ICC | Lower | Upper | Value | df1 | df2 | p | ICC | Lower | Upper | Value | df1 | df2 | p | ||
| MS HbO (n = 16) |
lDLPFC9 | 0.172# | −0.313 | 0.598 | 1.429 | 15 | 15 | 0.249 | 0.174° | −0.096 | 0.534 | 2.630 | 15 | 15 | 0.035 | 0.172# | −0.313 | 0.598 | 1.429 | 15 | 15 | 0.249 |
| rDLPFC9 | 0.026# | −0.490 | 0.510 | 1.050 | 15 | 15 | 0.463 | 0.136 | −0.269 | 0.548 | 1.376 | 15 | 15 | 0.272 | 0.025# | −0.491 | 0.510 | 1.049 | 15 | 15 | 0.463 | |
| lDLPFC46 | −0.110# | −0.527 | 0.377 | 0.789 | 15 | 15 | 0.674 | 0.035 | −0.370 | 0.477 | 1.082 | 15 | 15 | 0.440 | −0.110# | −0.528 | 0.377 | 0.789 | 15 | 15 | 0.674 | |
| rDLPFC46 | −0.210 | −0.688 | 0.331 | 0.674 | 15 | 15 | 0.773 | 0.101 | −0.443 | 0.568 | 1.211 | 15 | 15 | 0.358 | −0.208# | −0.687 | 0.333 | 0.677 | 15 | 15 | 0.771 | |
| mDLPFC9 | 0.389 | −0.134 | 0.736 | 2.208 | 15 | 15 | 0.068 | 0.234 | −0.312 | 0.651 | 1.576 | 15 | 15 | 0.194 | 0.388 | −0.134 | 0.736 | 2.206 | 15 | 15 | 0.068 | |
| lFPC10 | 0.257 | −0.272 | 0.661 | 1.667 | 15 | 15 | 0.167 | 0.541 | 0.066 | 0.813 | 3.227 | 15 | 15 | 0.015 | 0.256 | −0.273 | 0.660 | 1.665 | 15 | 15 | 0.167 | |
| rFPC10 | 0.313 | −0.231 | 0.697 | 1.856 | 15 | 15 | 0.121 | 0.207 | −0.326 | 0.631 | 1.500 | 15 | 15 | 0.221 | 0.418 | −0.097 | 0.751 | 2.366 | 15 | 15 | 0.053 | |
| mFPC10 | 0.157 | −0.392 | 0.604 | 1.351 | 15 | 15 | 0.284 | 0.579 | 0.125 | 0.830 | 3.610 | 15 | 15 | 0.009 | 0.159 | −0.390 | 0.605 | 1.354 | 15 | 15 | 0.282 | |
| MS HbR (n = 16) |
lDLPFC9 | 0.360# | −0.113 | 0.711 | 2.190 | 15 | 15 | 0.070 | 0.097 | −0.102 | 0.406 | 1.647 | 15 | 15 | 0.172 | 0.361 | −0.112 | 0.712 | 2.193 | 15 | 15 | 0.070 |
| rDLPFC9 | 0.391 | −0.138 | 0.739 | 2.206 | 15 | 15 | 0.068 | 0.093 | −0.065 | 0.379 | 2.182 | 15 | 15 | 0.071 | 0.391 | −0.138 | 0.739 | 2.207 | 15 | 15 | 0.068 | |
| lDLPFC46 | 0.561 | 0.106 | 0.822 | 3.470 | 15 | 15 | 0.011 | 0.398 | −0.116 | 0.740 | 2.267 | 15 | 15 | 0.062 | 0.562 | 0.107 | 0.822 | 3.477 | 15 | 15 | 0.011 | |
| rDLPFC46 | 0.201# | −0.198 | 0.591 | 1.638 | 15 | 15 | 0.175 | 0.298 | −0.155 | 0.669 | 1.951 | 15 | 15 | 0.104 | 0.202 | −0.198 | 0.592 | 1.640 | 15 | 15 | 0.174 | |
| mDLPFC9 | 0.523 | 0.036 | 0.805 | 3.060 | 15 | 15 | 0.019 | 0.158 | −0.387 | 0.604 | 1.355 | 15 | 15 | 0.282 | 0.523 | 0.035 | 0.805 | 3.058 | 15 | 15 | 0.019 | |
| lFPC10 | 0.536 | 0.065 | 0.810 | 3.212 | 15 | 15 | 0.015 | 0.469 | −0.006 | 0.774 | 2.765 | 15 | 15 | 0.029 | 0.538 | 0.067 | 0.811 | 3.223 | 15 | 15 | 0.015 | |
| rFPC10 | 0.483 | 0.042 | 0.777 | 3.191 | 15 | 15 | 0.016 | 0.629 | 0.232 | 0.850 | 4.722 | 15 | 15 | 0.002 | 0.514 | 0.073 | 0.794 | 3.581 | 15 | 15 | 0.009 | |
| mFPC10 | 0.464 | −0.029 | 0.774 | 2.670 | 15 | 15 | 0.033 | 0.403 | −0.113 | 0.743 | 2.289 | 15 | 15 | 0.060 | 0.463 | −0.030 | 0.773 | 2.665 | 15 | 15 | 0.033 | |
| HC HbO (n = 19) |
lDLPFC9 | 0.744 | 0.457 | 0.892 | 6.841 | 18 | 18 | 0.000 | 0.560 | 0.162 | 0.803 | 3.535 | 18 | 18 | 0.005 | 0.745 | 0.457 | 0.892 | 6.845 | 18 | 18 | 0.000 |
| rDLPFC9 | 0.529 # | 0.099 | 0.790 | 3.132 | 18 | 18 | 0.010 | 0.481 | 0.070 | 0.758 | 3.301 | 18 | 18 | 0.008 | 0.529 # | 0.099 | 0.790 | 3.133 | 18 | 18 | 0.010 | |
| lDLPFC46 | 0.478 | 0.048 | 0.760 | 2.813 | 18 | 18 | 0.017 | 0.208 | −0.273 | 0.600 | 1.508 | 18 | 18 | 0.196 | 0.478 | 0.049 | 0.760 | 2.816 | 18 | 18 | 0.017 | |
| rDLPFC46 | 0.563 | 0.151 | 0.807 | 3.461 | 18 | 18 | 0.006 | 0.306° | −0.124 | 0.652 | 1.944 | 18 | 18 | 0.084 | 0.563 | 0.151 | 0.807 | 3.466 | 18 | 18 | 0.006 | |
| mDLPFC9 | 0.392# | −0.083 | 0.715 | 2.221 | 18 | 18 | 0.050 | 0.062 | −0.385 | 0.489 | 1.133 | 18 | 18 | 0.397 | 0.392# | −0.083 | 0.715 | 2.220 | 18 | 18 | 0.050 | |
| lFPC10 | 0.612 | 0.223 | 0.831 | 4.001 | 18 | 18 | 0.003 | 0.343° | −0.140 | 0.687 | 1.990 | 18 | 18 | 0.077 | 0.612 | 0.223 | 0.831 | 4.003 | 18 | 18 | 0.003 | |
| rFPC10 | 0.507 | 0.087 | 0.775 | 3.040 | 18 | 18 | 0.012 | 0.492 | 0.054 | 0.769 | 2.861 | 18 | 18 | 0.016 | 0.368 | −0.107 | 0.701 | 2.110 | 18 | 18 | 0.061 | |
| mFPC10 | 0.615 | 0.228 | 0.833 | 4.044 | 18 | 18 | 0.002 | 0.512 | 0.074 | 0.781 | 2.989 | 18 | 18 | 0.013 | 0.616 | 0.228 | 0.833 | 4.046 | 18 | 18 | 0.002 | |
| HbR (n = 19) |
lDLPFC9 | 0.548 | 0.153 | 0.796 | 3.488 | 18 | 18 | 0.006 | 0.395 | −0.044 | 0.710 | 2.330 | 18 | 18 | 0.040 | 0.548 | 0.153 | 0.796 | 3.490 | 18 | 18 | 0.006 |
| rDLPFC9 | 0.533 | 0.122 | 0.789 | 3.258 | 18 | 18 | 0.008 | 0.543 | 0.134 | 0.795 | 3.334 | 18 | 18 | 0.007 | 0.533 | 0.122 | 0.789 | 3.261 | 18 | 18 | 0.008 | |
| lDLPFC46 | 0.552 | 0.131 | 0.802 | 3.334 | 18 | 18 | 0.007 | 0.479 | 0.068 | 0.757 | 2.939 | 18 | 18 | 0.014 | 0.552 | 0.131 | 0.802 | 3.336 | 18 | 18 | 0.007 | |
| rDLPFC46 | 0.522 | 0.090 | 0.786 | 3.076 | 18 | 18 | 0.011 | 0.331 | −0.131 | 0.676 | 1.978 | 18 | 18 | 0.079 | 0.522 | 0.090 | 0.786 | 3.075 | 18 | 18 | 0.011 | |
| mDLPFC9 | 0.418 | −0.015 | 0.724 | 2.469 | 18 | 18 | 0.031 | 0.306 | −0.179 | 0.664 | 1.841 | 18 | 18 | 0.102 | 0.417 | −0.015 | 0.723 | 2.468 | 18 | 18 | 0.031 | |
| lFPC10 | 0.633 | 0.259 | 0.841 | 4.309 | 18 | 18 | 0.002 | 0.123 | −0.360 | 0.544 | 1.270 | 18 | 18 | 0.309 | 0.633 | 0.259 | 0.841 | 4.307 | 18 | 18 | 0.002 | |
| rFPC10 | 0.580 | 0.186 | 0.814 | 3.704 | 18 | 18 | 0.004 | 0.684 | 0.341 | 0.865 | 5.137 | 18 | 18 | 0.001 | 0.622 # | 0.245 | 0.836 | 4.182 | 18 | 18 | 0.002 | |
| mFPC10 | 0.392 | −0.061 | 0.712 | 2.272 | 18 | 18 | 0.045 | 0.206 | −0.210 | 0.581 | 1.572 | 18 | 18 | 0.173 | 0.392 | −0.062 | 0.712 | 2.270 | 18 | 18 | 0.045 | |
MS: multiple sclerosis; HC: healthy controls; ICC: intraclass correlation coefficient; CI: confidence interval; df: degree of freedom; p: p-value; HbO: oxyhemoglobin; HbR: deoxyhemoglobin; l/r/mDLPFC9: left/right/medial dorsolateral prefrontal cortex Brodmann area 9; l/rDLPFC46: left/right dorsolateral prefrontal cortex Brodmann area 46; l/r/mFPC10: left/right/medial frontopolar cortex Brodmann area 10; #: not normally distributed; bold: fair–excellent intraclass correlation coefficient.
In the HC individuals, almost all subareas displayed at least a fair ISR of the mean and A of the cHbO except the mDLPFC9 (mean/A: ICC = 0.39) and the rFPC10 (A: ICC = 0.37). Furthermore, the l/mFPC10 and lDLPFC9 showed a good ISR for the mean cHbO and the lDLPFC9 an excellent ISR for the A of the cHbO. The SC of the cHbO exhibit a fair ISR for l/rDLPFC9 and r/mFPC10. The mean and the A of the cHbR were comparable concerning the ICC. In both cases, almost all subareas demonstrated a fair ISR except the mFPC10 (ICC = 0.39) with a poor and the lFPC10 with a good (ICC = 0.63) ISR. Concerning the A of the cHbR, the rFPC10 displayed also a good ISR (ICC = 0.62). The ICC for the A and the mean of the cHbO in the rDLPFC9 and for the A of the cHbR in the rFPC10 have to be interpreted with caution due to the non-normal distribution.
The highest bias/mean difference (Bland and Altman, Table 4) was shown in the lDLPFC46 (people with MS: 0.099 µmol/L; HC individuals: 0.046 µmol/L). The lowest bias was found in the rDLPFC46 (people with MS: 0.003 µmol/L) and mDLPFC9 (HC individuals: 0.000 µmol/L). For the mean cHbR the highest bias was identified in the rDLPFC46 (people with MS: 0.067 µmol/L) and mDLPFC9 (HC individuals: −0.016 µmol/L). The lowest was observed in the mDLPFC9 (people with MS: −0.001 µmol/L) and lDLPFC46 (HC individuals: 0.001 µmol/L), respectively. The LoA of the mean cHbO were smallest in mDLPFC9 (0.322/−0.361 µmol/L) in people with MS and lDLPFC9 (0.356/−0.280 µmol/L) as well as mFPC10 (0.311/−0.331 µmol/L) in HC individuals. Considering the LoA of the mean cHbR, these were narrowest in rDLPFC9 (people with MS: 0.092/−0.088 µmol/L) and lFPC10 (HC individuals: 0.084/−0.094 µmol/L).
Table 4.
Bland and Altman limits of agreement, bias and confidence intervals of both groups (MS/HC).
|
Bland and Altman
(Test–Retest) |
Mean | Area Under the Curve | ||||||||||||||||||
| Upper LoA | Lower LoA | Upper LoA | Lower LoA | |||||||||||||||||
| n | Mean | SD | SE | LoA | Upper CI | Lower CI | LoA | Upper CI | Lower CI | Mean | SD | SE | LoA | Upper CI | Lower CI | LoA | Upper CI | Lower CI | ||
| MS HbO [µmol/L] |
rDLPFC9 | 16 | −0.044 | 0.280 | 0.070 | 0.505 | 0.760 | 0.250 | −0.592 | −0.337 | −0.847 | −0.869 | 5.592 | 1.398 | 10.090 | 15.184 | 4.996 | −11.829 | −6.735 | −16.923 |
| lDLPFC9 | 16 | 0.067 | 0.219 | 0.055 | 0.497 | 0.696 | 0.297 | −0.362 | −0.163 | −0.562 | 1.341 | 4.378 | 1.094 | 9.921 | 13.909 | 5.933 | −7.239 | −3.251 | −11.227 | |
| rDLPFC46 | 16 | 0.003 | 0.339 | 0.085 | 0.667 | 0.976 | 0.358 | −0.661 | −0.353 | −0.970 | 0.057 | 6.767 | 1.692 | 13.320 | 19.485 | 7.155 | −13.207 | −7.042 | −19.372 | |
| lDLPFC46 | 16 | 0.099 | 0.282 | 0.071 | 0.652 | 0.909 | 0.395 | −0.454 | −0.197 | −0.710 | 1.986 | 5.639 | 1.410 | 13.039 | 18.176 | 7.901 | −9.067 | −3.930 | −14.204 | |
| mDLPFC9 | 16 | −0.020 | 0.174 | 0.044 | 0.322 | 0.481 | 0.163 | −0.361 | −0.203 | −0.520 | −0.395 | 3.486 | 0.871 | 6.437 | 9.613 | 3.262 | −7.227 | −4.052 | −10.403 | |
| lFPC10 | 16 | −0.044 | 0.266 | 0.066 | 0.477 | 0.719 | 0.235 | −0.565 | −0.323 | −0.807 | −0.879 | 5.309 | 1.327 | 9.526 | 14.363 | 4.690 | −11.285 | −6.449 | −16.122 | |
| rFPC10 | 16 | −0.005 | 0.208 | 0.052 | 0.403 | 0.592 | 0.213 | −0.412 | −0.223 | −0.601 | −0.419 | 3.545 | 0.886 | 6.529 | 9.759 | 3.300 | −7.368 | −4.138 | −10.597 | |
| mFPC10 | 16 | −0.008 | 0.299 | 0.075 | 0.578 | 0.851 | 0.306 | −0.593 | −0.321 | −0.866 | −0.152 | 5.967 | 1.492 | 11.543 | 16.978 | 6.107 | −11.847 | −6.411 | −17.283 | |
| MS HbR [µmol/L] |
rDLPFC9 | 16 | 0.002 | 0.046 | 0.012 | 0.092 | 0.134 | 0.050 | −0.088 | −0.046 | −0.130 | 0.043 | 0.920 | 0.230 | 1.845 | 2.683 | 1.008 | −1.759 | −0.921 | −2.597 |
| lDLPFC9 | 16 | −0.025 | 0.072 | 0.018 | 0.116 | 0.181 | 0.050 | −0.165 | −0.100 | −0.230 | −0.497 | 1.431 | 0.358 | 2.308 | 3.612 | 1.004 | −3.302 | −1.998 | −4.605 | |
| rDLPFC46 | 16 | 0.067 | 0.117 | 0.029 | 0.297 | 0.404 | 0.190 | −0.163 | −0.056 | −0.270 | 1.342 | 2.344 | 0.586 | 5.937 | 8.073 | 3.802 | −3.253 | −1.117 | −5.388 | |
| lDLPFC46 | 16 | −0.010 | 0.062 | 0.016 | 0.112 | 0.169 | 0.055 | −0.133 | −0.076 | −0.189 | −0.206 | 1.246 | 0.312 | 2.237 | 3.373 | 1.102 | −2.649 | −1.513 | −3.785 | |
| mDLPFC9 | 16 | −0.001 | 0.056 | 0.014 | 0.109 | 0.161 | 0.058 | −0.112 | −0.060 | −0.163 | −0.021 | 1.128 | 0.282 | 2.189 | 3.216 | 1.162 | −2.231 | −1.204 | −3.258 | |
| lFPC10 | 16 | 0.008 | 0.057 | 0.014 | 0.120 | 0.172 | 0.068 | −0.104 | −0.052 | −0.156 | 0.156 | 1.142 | 0.285 | 2.394 | 3.434 | 1.354 | −2.081 | −1.041 | −3.121 | |
| rFPC10 | 16 | 0.026 | 0.054 | 0.014 | 0.133 | 0.182 | 0.083 | −0.080 | −0.031 | −0.130 | 0.485 | 0.910 | 0.228 | 2.269 | 3.098 | 1.440 | −1.299 | −0.469 | −2.128 | |
| mFPC10 | 16 | 0.013 | 0.077 | 0.019 | 0.164 | 0.234 | 0.094 | −0.138 | −0.068 | −0.208 | 0.258 | 1.543 | 0.386 | 3.283 | 4.688 | 1.877 | −2.766 | −1.360 | −4.172 | |
| HC HbO [µmol/L] |
rDLPFC9 | 19 | 0.008 | 0.244 | 0.056 | 0.486 | 0.689 | 0.282 | −0.469 | −0.265 | −0.673 | 0.168 | 4.869 | 1.117 | 9.712 | 13.782 | 5.641 | −9.375 | −5.304 | −13.445 |
| lDLPFC9 | 19 | 0.038 | 0.162 | 0.037 | 0.356 | 0.492 | 0.220 | −0.280 | −0.144 | −0.416 | 0.763 | 3.243 | 0.744 | 7.119 | 9.829 | 4.408 | −5.592 | −2.882 | −8.303 | |
| rDLPFC46 | 19 | −0.022 | 0.241 | 0.055 | 0.451 | 0.653 | 0.249 | −0.495 | −0.294 | −0.697 | −0.444 | 4.822 | 1.106 | 9.007 | 13.038 | 4.976 | −9.895 | −5.864 | −13.926 | |
| lDLPFC46 | 19 | 0.049 | 0.236 | 0.054 | 0.512 | 0.710 | 0.315 | −0.414 | −0.217 | −0.612 | 0.979 | 4.723 | 1.084 | 10.237 | 14.186 | 6.288 | −8.278 | −4.330 | −12.227 | |
| mDLPFC9 | 19 | 0.000 | 0.260 | 0.060 | 0.511 | 0.728 | 0.293 | −0.510 | −0.292 | −0.728 | 0.006 | 5.206 | 1.194 | 10.209 | 14.562 | 5.857 | −10.198 | −5.846 | −14.550 | |
| lFPC10 | 19 | 0.013 | 0.207 | 0.048 | 0.419 | 0.593 | 0.246 | −0.393 | −0.220 | −0.567 | 0.262 | 4.144 | 0.951 | 8.384 | 11.849 | 4.920 | −7.861 | −4.397 | −11.326 | |
| rFPC10 | 19 | 0.038 | 0.182 | 0.042 | 0.396 | 0.548 | 0.243 | −0.319 | −0.167 | −0.471 | 0.244 | 3.039 | 0.697 | 6.201 | 8.742 | 3.660 | −5.713 | −3.172 | −8.254 | |
| mFPC10 | 19 | −0.010 | 0.164 | 0.038 | 0.311 | 0.448 | 0.174 | −0.331 | −0.194 | −0.468 | −0.196 | 3.272 | 0.751 | 6.218 | 8.954 | 3.483 | −6.610 | −3.874 | −9.345 | |
| HC HbR [µmol/L] |
rDLPFC9 | 19 | −0.011 | 0.052 | 0.012 | 0.092 | 0.136 | 0.048 | −0.114 | −0.070 | −0.157 | −0.214 | 1.049 | 0.241 | 1.842 | 2.719 | 0.965 | −2.270 | −1.393 | −3.147 |
| lDLPFC9 | 19 | −0.014 | 0.051 | 0.012 | 0.085 | 0.128 | 0.043 | −0.114 | −0.071 | −0.156 | −0.285 | 1.015 | 0.233 | 1.704 | 2.552 | 0.855 | −2.274 | −1.426 | −3.122 | |
| rDLPFC46 | 19 | −0.004 | 0.063 | 0.015 | 0.120 | 0.173 | 0.067 | −0.128 | −0.075 | −0.181 | −0.083 | 1.267 | 0.291 | 2.400 | 3.459 | 1.341 | −2.565 | −1.506 | −3.624 | |
| lDLPFC46 | 19 | 0.001 | 0.072 | 0.017 | 0.142 | 0.203 | 0.082 | −0.140 | −0.080 | −0.201 | 0.019 | 1.443 | 0.331 | 2.846 | 4.052 | 1.640 | −2.809 | −1.603 | −4.015 | |
| mDLPFC9 | 19 | −0.016 | 0.057 | 0.013 | 0.096 | 0.144 | 0.048 | −0.128 | −0.080 | −0.175 | −0.316 | 1.140 | 0.262 | 1.918 | 2.871 | 0.965 | −2.551 | −1.598 | −3.504 | |
| lFPC10 | 19 | −0.005 | 0.045 | 0.010 | 0.084 | 0.122 | 0.046 | −0.094 | −0.056 | −0.132 | −0.103 | 0.907 | 0.208 | 1.674 | 2.432 | 0.916 | −1.880 | −1.122 | −2.638 | |
| rFPC10 | 19 | 0.011 | 0.061 | 0.014 | 0.131 | 0.182 | 0.080 | −0.109 | −0.058 | −0.160 | 0.133 | 0.981 | 0.225 | 2.055 | 2.875 | 1.235 | −1.790 | −0.970 | −2.610 | |
| mFPC10 | 19 | −0.012 | 0.064 | 0.015 | 0.113 | 0.166 | 0.059 | −0.137 | −0.084 | −0.191 | −0.247 | 1.275 | 0.292 | 2.251 | 3.317 | 1.186 | −2.745 | −1.680 | −3.811 | |
|
Bland and Altman
(Test–Retest) |
Slope Coefficient | |||||||||||||||||||
| Upper LoA | Lower LoA | |||||||||||||||||||
| n | Mean | SD | SE | LoA | upper CI | lower CI | LoA | upper CI | lower CI | |||||||||||
| MS HbO [µmol/L] |
rDLPFC9 | 16 | 0.167 | 0.329 | 0.082 | 0.812 | 1.111 | 0.512 | −0.478 | −0.178 | −0.777 | |||||||||
| lDLPF9 | 16 | 0.385 | 0.225 | 0.056 | 0.826 | 1.031 | 0.621 | −0.055 | 0.149 | −0.260 | ||||||||||
| rDLPFC46 | 16 | −0.014 | 0.417 | 0.104 | 0.803 | 1.183 | 0.423 | −0.831 | −0.452 | −1.211 | ||||||||||
| lDLPFC46 | 16 | 0.179 | 0.394 | 0.099 | 0.952 | 1.311 | 0.593 | −0.594 | −0.235 | −0.953 | ||||||||||
| mDLPFC9 | 16 | 0.028 | 0.404 | 0.101 | 0.818 | 1.186 | 0.451 | −0.763 | −0.396 | −1.131 | ||||||||||
| lFPC10 | 16 | 0.022 | 0.247 | 0.062 | 0.506 | 0.731 | 0.281 | −0.461 | −0.236 | −0.686 | ||||||||||
| rFPC10 | 16 | −0.041 | 0.269 | 0.067 | 0.486 | 0.731 | 0.241 | −0.568 | −0.323 | −0.813 | ||||||||||
| mFPC10 | 16 | −0.033 | 0.296 | 0.074 | 0.547 | 0.817 | 0.278 | −0.613 | −0.343 | −0.883 | ||||||||||
| MS HbR [µmol/L] |
rDLPFC9 | 16 | −0.108 | 0.049 | 0.012 | −0.012 | 0.033 | −0.057 | −0.205 | −0.160 | −0.249 | |||||||||
| lDLPFC9 | 16 | −0.105 | 0.073 | 0.018 | 0.038 | 0.104 | −0.029 | −0.248 | −0.182 | −0.315 | ||||||||||
| rDLPFC46 | 16 | 0.062 | 0.144 | 0.036 | 0.345 | 0.477 | 0.213 | −0.221 | −0.090 | −0.353 | ||||||||||
| lDLPFC46 | 16 | −0.015 | 0.103 | 0.026 | 0.186 | 0.280 | 0.093 | −0.217 | −0.123 | −0.311 | ||||||||||
| mDLPFC9 | 16 | 0.009 | 0.115 | 0.029 | 0.233 | 0.338 | 0.129 | −0.216 | −0.112 | −0.321 | ||||||||||
| lFPC10 | 16 | −0.018 | 0.072 | 0.018 | 0.123 | 0.189 | 0.058 | −0.159 | −0.093 | −0.224 | ||||||||||
| rFPC10 | 16 | 0.022 | 0.054 | 0.014 | 0.128 | 0.177 | 0.079 | −0.085 | −0.035 | −0.134 | ||||||||||
| mFPC10 | 16 | 0.013 | 0.098 | 0.025 | 0.205 | 0.294 | 0.116 | −0.179 | −0.090 | −0.269 | ||||||||||
| HC HbO [µmol/L] |
rDLPFC9 | 19 | 0.065 | 0.120 | 0.027 | 0.300 | 0.400 | 0.200 | −0.169 | −0.069 | −0.270 | |||||||||
| lDLPF9 | 19 | −0.029 | 0.129 | 0.030 | 0.224 | 0.331 | 0.116 | −0.281 | −0.173 | −0.388 | ||||||||||
| rDLPFC46 | 19 | −0.107 | 0.305 | 0.070 | 0.490 | 0.745 | 0.236 | −0.704 | −0.449 | −0.959 | ||||||||||
| lDLPFC46 | 19 | 0.046 | 0.312 | 0.071 | 0.656 | 0.917 | 0.396 | −0.565 | −0.305 | −0.826 | ||||||||||
| mDLPFC9 | 19 | −0.071 | 0.284 | 0.065 | 0.486 | 0.724 | 0.248 | −0.629 | −0.391 | −0.867 | ||||||||||
| lFPC10 | 19 | 0.010 | 0.245 | 0.056 | 0.490 | 0.694 | 0.285 | −0.469 | −0.265 | −0.674 | ||||||||||
| rFPC10 | 19 | −0.018 | 0.154 | 0.035 | 0.284 | 0.413 | 0.155 | −0.320 | −0.191 | −0.449 | ||||||||||
| mFPC10 | 19 | −0.001 | 0.198 | 0.045 | 0.387 | 0.552 | 0.221 | −0.388 | −0.223 | −0.553 | ||||||||||
| HC HbR [µmol/L] |
rDLPFC9 | 19 | 0.008 | 0.042 | 0.010 | 0.090 | 0.125 | 0.055 | −0.074 | −0.039 | −0.109 | |||||||||
| lDLPF9 | 19 | 0.012 | 0.043 | 0.010 | 0.097 | 0.133 | 0.060 | −0.073 | −0.037 | −0.110 | ||||||||||
| rDLPFC46 | 19 | −0.016 | 0.083 | 0.019 | 0.147 | 0.216 | 0.077 | −0.180 | −0.110 | −0.249 | ||||||||||
| lDLPFC46 | 19 | −0.023 | 0.069 | 0.016 | 0.114 | 0.172 | 0.055 | −0.159 | −0.101 | −0.217 | ||||||||||
| mDLPFC9 | 19 | 0.006 | 0.074 | 0.017 | 0.151 | 0.213 | 0.089 | −0.140 | −0.078 | −0.202 | ||||||||||
| lFPC10 | 19 | −0.009 | 0.070 | 0.016 | 0.128 | 0.186 | 0.070 | −0.145 | −0.087 | −0.203 | ||||||||||
| rFPC10 | 19 | −0.005 | 0.045 | 0.010 | 0.084 | 0.121 | 0.046 | −0.093 | −0.055 | −0.130 | ||||||||||
| mFPC10 | 19 | −0.021 | 0.054 | 0.012 | 0.085 | 0.131 | 0.040 | −0.128 | −0.082 | −0.173 | ||||||||||
MS: multiple sclerosis; HC: healthy control; CI: confidence interval; SD: standard deviation; SE: standard error; LoA: limits of agreement; HbO: oxyhemoglobin; HbR: deoxyhemoglobin; l/r/mDLPFC9: left/right/medial dorsolateral prefrontal cortex Brodmann area 9; l/rDLPFC46: left/right dorsolateral prefrontal cortex Brodmann area 46; l/r/mFPC10: left/right/medial frontopolar cortex Brodmann area 10.
3.3. Secondary Outcomes
The mean HR did not differ significantly between test and retest in people with MS (test: 98.1 ± 12.2 bpm/retest: 97.3 ± 16.4 bpm) and in HC individuals (test: 91.1 ± 10.2 bpm/retest: 91.5 ± 8.2 bpm). In addition, the mean RR interval did not reveal any difference in people with MS (test: 618.09 ± 72.52 ms/retest: 626.37 ± 104.60 ms) and in HC individuals (test: 674.58 ± 80.24 ms/retest: 660:39 ± 59.63 ms) as well as the standard deviation of the RR interval (people with MS test: 19.51 ± 9.08 ms/retest: 22.75 ± 14.02 ms and HC individuals (test: 60.78 (44.20/90.78) ms/retest: 70.25 (45.77/128.56) ms)). Furthermore, no difference could be found for the mean LF/HF ratio in people with MS (test: 3.51 ± 2.06/retest: 4.67 ± 4.25) and HC individuals (test: 2.72 ± 1.06/retest: 2.77 ± 1.32).
The perceived exhaustion (Borg Scale) was rated as “very light” to “light” in people with MS (median (25%/75% quartile) test: 8 (8/10); retest: 8 (8/12)) and in HC individuals (test: 9 (8/11); retest: 10 (8/11)).
4. Discussion
Clinicians need precise diagnostic tools with reasonable reliability to be able to deduct specially tailored intervention strategies. Therefore, the aim of the study was to verify the ISR of the fNIRS-derived parameters HbO and HbR while single-task walking in people with MS and HC individuals.
Basically, a fair to excellent ISR of the fNIRS-derived parameters in the subareas of the PFC could be proven for the HC individuals in our study. Our results are partly congruent with those of Stuart et al. [15] who found a moderate ISR in the overall PFC.
In people with MS, the ISR was very limited in our study. One explanation could be that the daily performance of people with MS can fluctuate greatly mainly due to fatigue symptoms [14]. It is also known that the motor and cognitive performance of people with MS decline over the course of the day [14]. However, we have tried to keep these influences as low as possible by performing the measurement in the morning in a rested state and without prior treatment. We also checked the exhaustion state pre and post measurement on both days, and there was no difference between days nor before and after the walking trial in both groups.
An interesting outcome for both groups is a relatively high activation of the lDLPFC46 in comparison to the other subareas and the poor ISR. It is known that in the case of mind wandering during simple tasks the lDLPFC46 is involved [33]. Single-task walking might provoke mind wandering due to its low requirements. Mind wandering is not a constant factor between days and could be an explanation for the partly poor ISR in both groups.
Another interesting result is that the ICCs were higher for the cHbR than for the cHbO in people with MS. This is in line with the results of a study by Plichta et al. [34] that quantified ISR of fNIRS measures during different finger-tapping tasks. In contrast, other studies verifying the ISR of fNIRS for motor [35] or cognitive [36] tasks demonstrated that cHbO is more reliable than cHbR. However, these studies are only comparable to a limited extent, as different brain areas and cohorts were investigated. Nevertheless, an explanation for a better ISR of the HbR could be that it is less affected by physiological noise [35,37,38] and that it is spatially more concentrated [35,36] than HbO. Another explanation could be that HbO is more sensitive to changes in blood flow [37] and therefore may be more susceptible to fluctuations from day to day.
Overall, it has been shown that the ISR for the mean and A of cHBO and cHbR are comparable among each other. However, the ISR of the SC of the cHbO and cHbR was worse. The mean is known to be relatively robust against motion artefacts [39]. The SC was only reported in the context of cognitive tasks [28], and it is not yet clear how robust this parameter would be in regard to movement artefacts.
One limitation in the experimental procedure, which is perhaps responsible for the relatively high bias (Bland and Altman), is that the fNIRS cap was manually aligned using anatomical landmarks without any other technical aids. Nevertheless, the cap was always fitted by the same experienced investigator according to current 10–20 EEG system standards.
Furthermore, we have assumed that single-task walking does not need to be familiarized. However, in both groups, it was observed that the activation of some subareas of the PFC were lower on the retest than on the test day. Therefore, there might have been a certain learning effect even though the task was simple. A familiarization could have improved the reliability, as Hamacher et al. have already demonstrated for kinematic gait parameters [40].
In conclusion, it would be helpful for future studies (i) to control state fatigue in people with MS more adequately by applying, e.g., the Profile of Mood States questionnaire [41], (ii) to add an easy cognitive task guiding the attention to minimize possible mind wandering, (iii) to report the mean/A of the cHbO/cHbR rather than the SC, (iv) to improve the placement of the fNIRS cap by applying a 3D digitizer, and (v) to familiarize even very simple tasks.
Acknowledgments
There is nothing to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
| 6MWT | 6-min walk test |
| A | area under the curve |
| BA | Brodmann area |
| cHbO | concentration of oxyhemoglobin |
| cHBR | concentration of deoxyhemoglobin |
| CI | confidence interval |
| df | degree of freedom |
| DLPFC | dorsolateral prefrontal cortex |
| EDSS | Expanded Disability Status Scale |
| EEG | electroencephalography |
| FD | first diagnosis |
| FM | first manifestation |
| fNIRS | functional near-infrared spectroscopy |
| fOLD | fNIRS Optodes’ Location Decider |
| FPC | frontopolar cortex |
| HbO | oxyhemoglobin |
| HbR | deoxyhemoglobin |
| HC individuals | healthy control individuals |
| HR | heart rate |
| HRF | hemodynamic response function |
| HRV | heart rate variability |
| ICC | intraclass correlation coefficient |
| l | left |
| LF/HF | low frequency/high frequency |
| LoA | limits of agreement |
| m | medial |
| MS | multiple sclerosis |
| MSWS-12 | 12-Item Multiple Sclerosis Walking Scale |
| OvGU | Otto von Guericke University |
| PFC | prefrontal cortex |
| people with MS | people with MS |
| r | right |
| SC | slope coefficient |
| ISR | inter-session reliability |
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/10/9/643/s1, where a supplementary table is included listing the sensitivity of the channels. The table was generated using the fOLD-software [21].
Author Contributions
Conceptualization, K.-C.B.; formal analysis, K.-C.B. and J.L.; investigation, K.-C.B.; methodology, K.-C.B.; project administration, L.S.; resources, K.-C.B. and L.S.; supervision, L.S.; validation, D.H., M.S. and L.S.; visualization, K.-C.B.; writing—original draft, K.-C.B.; writing—review & editing, D.H., J.L., M.S. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.LaRocca N.G. Impact of walking impairment in multiple sclerosis: Perspectives of patients and care partners. Patient. 2011;4:189–201. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti M.G., Piperno R., Simoncini L., Bonato P., Tonini A., Giannini S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. J. 1999;5:363–368. doi: 10.1177/135245859900500510. [DOI] [PubMed] [Google Scholar]
- 3.Clark D.J. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Front. Hum. Neurosci. 2015;9:246. doi: 10.3389/fnhum.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochester L., Baker K., Hetherington V., Jones D., Willems A.-M., Kwakkel G., van Wegen E., Lim I., Nieuwboer A. Evidence for motor learning in Parkinson’s disease: Acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010;1319:103–111. doi: 10.1016/j.brainres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Yogev G., Hausdorff J.M., Giladi N. The Role of Executive Function and Attention in Gait. Mov. Disord. 2008;23:329–472. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gramann K., Ferris D.P., Gwin J., Makeig S. Imaging natural cognition in action. Int. J. Psychophysiol. 2014;91:22–29. doi: 10.1016/j.ijpsycho.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamacher D., Herold F., Wiegel P., Hamacher D., Schega L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015;57:310–327. doi: 10.1016/j.neubiorev.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari M., Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012;63:921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Gramigna V., Pellegrino G., Cerasa A., Cutini S., Vasta R., Olivadese G., Martino I., Quattrone A. Near-Infrared Spectroscopy in Gait Disorders: Is It Time to Begin? Neurorehabil. Neural Repair. 2017;31:402–412. doi: 10.1177/1545968317693304. [DOI] [PubMed] [Google Scholar]
- 10.Izzetoglu M., Bunce S., Izzetoglu K., Onaral B., Pourrezaei A. Functional brain imaging using near-infrared technology. IEEE Eng. Med. Biol. Mag. 2007;26:38–46. doi: 10.1109/MEMB.2007.384094. [DOI] [PubMed] [Google Scholar]
- 11.Strangman G., Culver J.P., Thompson J.H., Boas D.A. A Quantitative Comparison of Simultaneous BOLD fMRI and NIRS Recordings during Functional Brain Activation. NeuroImage. 2002;17:719–731. doi: 10.1006/nimg.2002.1227. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez M.E., Holtzer R., Chaparro G., Jean K., Balto J.M., Sandroff B.M., Izzetoglu M., Motl R.W. Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J. Neurol. Sci. 2016;370:277–283. doi: 10.1016/j.jns.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Chaparro G., Balto J.M., Sandroff B.M., Holtzer R., Izzetoglu M., Motl R.W., Hernandez M.E. Frontal brain activation changes due to dual-tasking under partial body weight support conditions in older adults with multiple sclerosis. J. NeuroEng. Rehabil. 2017;14:65. doi: 10.1186/s12984-017-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell D.J.H., Liossi C., Schlotz W., Moss-Morris R. Tracking daily fatigue fluctuations in multiple sclerosis: Ecological momentary assessment provides unique insights. J. Behav. Med. 2017;40:772–783. doi: 10.1007/s10865-017-9840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart S., Belluscio V., Quinn J.F., Mancini M. Pre-frontal Cortical Activity During Walking and Turning Is Reliable and Differentiates Across Young, Older Adults and People With Parkinson’s Disease. Front. Neurol. 2019;10:536. doi: 10.3389/fneur.2019.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Wetzel J.L., Fry D.K., Pfalzer L.A. Six-minute walk test for persons with mild or moderate disability from multiple sclerosis: Performance and explanatory factors. Physiother. Can. 2011;63:166–180. doi: 10.3138/ptc.2009-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobart J.C., Riazi A., Lamping D.L., Fitzpatrick R., Thompson A.J. Measuring the impact of MS on walking ability: The 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60:31–36. doi: 10.1212/WNL.60.1.31. [DOI] [PubMed] [Google Scholar]
- 20.Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL, USA: 1998. [Google Scholar]
- 21.Zimeo Morais G.A., Balardin J.B., Sato J.R. fNIRS Optodes’ Location Decider (fOLD): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018;8:3341. doi: 10.1038/s41598-018-21716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholkmann F., Kleiser S., Metz A.J., Zimmermann R., Mata Pavia J., Wolf U., Wolf M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage. 2014;85:6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Huppert T.J., Diamond S.G., Franceschini M.A., Boas D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009;48:D280–D298. doi: 10.1364/AO.48.00D280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahani S., Setarehdan S.K., Boas D.A., Yücel M.A. Motion artifact detection and correction in functional near-infrared spectroscopy: A new hybrid method based on spline interpolation method and Savitzky-Golay filtering. Neurophotonics. 2018;5:15003. doi: 10.1117/1.NPh.5.1.015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herold F., Wiegel P., Scholkmann F., Thiers A., Hamacher D., Schega L. Functional near-infrared spectroscopy in movement science: A systematic review on cortical activity in postural and walking tasks. Neurophotonics. 2017;4:041403. doi: 10.1117/1.NPh.4.4.041403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholkmann F., Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013;18:105004. doi: 10.1117/1.JBO.18.10.105004. [DOI] [PubMed] [Google Scholar]
- 27.Ye J.C., Tak S., Jang K.E., Jung J., Jang J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Mandrick K., Derosiere G., Dray G., Coulon D., Micallef J.P., Perrey S. Utilizing slope method as an alternative data analysis for functional near-infrared spectroscopy-derived cerebral hemodynamic responses. Int. J. Ind. Ergon. 2013;43:335–341. doi: 10.1016/j.ergon.2013.05.003. [DOI] [Google Scholar]
- 29.Gagnon C., Desjardins-Crépeau L., Tournier I., Desjardins M., Lesage F., Greenwood C.E., Bherer L. Near-infrared imaging of the effects of glucose ingestion and regulation on prefrontal activation during dual-task execution in healthy fasting older adults. Behav. Brain Res. 2012;232:137–147. doi: 10.1016/j.bbr.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Koo T.K., Li M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicchetti D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994;6:284–290. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 32.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 33.Turnbull A., Wang H.T., Murphy C., Ho N.S.P., Wang X., Sormaz M., Karapanagiotidis T., Leech R.M., Bernhardt B., Margulies D.S., et al. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat. Commun. 2019;10:3816. doi: 10.1038/s41467-019-11764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plichta M.M., Herrmann M.J., Baehne C.G., Ehlis A.C., Richter M.M., Pauli P., Fallgatter A.J. Event-related functional near-infrared spectroscopy (fNIRS) based on craniocerebral correlations: Reproducibility of activation? Hum. Brain Mapp. 2007;28:733–741. doi: 10.1002/hbm.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dravida S., Noah J.A., Zhang X., Hirsch J. Comparison of oxyhemoglobin and deoxyhemoglobin signal reliability with and without global mean removal for digit manipulation motor tasks. Neurophotonics. 2018;5:11006. doi: 10.1117/1.NPh.5.1.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plichta M.M., Herrmann M.J., Baehne C.G., Ehlis A.C., Richter M.M., Pauli P., Fallgatter A.J. Event-related functional near-infrared spectroscopy (fNIRS): Are the measurements reliable? NeuroImage. 2006;31:116–124. doi: 10.1016/j.neuroimage.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Herold F., Wiegel P., Scholkmann F., Müller N.G. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise-Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018;7:466. doi: 10.3390/jcm7120466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirilina E., Jelzow A., Heine A., Niessing M., Wabnitz H., Brühl R., Ittermann B., Jacobs A.M., Tachtsidis I. The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy. NeuroImage. 2012;61:70–81. doi: 10.1016/j.neuroimage.2012.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vitorio R., Stuart S., Rochester L., Alcock L., Pantall A. fNIRS response during walking—Artefact or cortical activity? A systematic review. Neurosci. Biobehav. Rev. 2017;83:160–172. doi: 10.1016/j.neubiorev.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Hamacher D., Hamacher D., Krowicki M., Schega L. Between-day test–retest reliability of gait variability in older individuals improves with a familiarization trial. Aging Clin. Exp. Res. 2017;29:327–329. doi: 10.1007/s40520-016-0536-3. [DOI] [PubMed] [Google Scholar]
- 41.McNair D., Lorr M., Droppleman L. Manual for Profile of Mood States. Educational and Industrial Testing Services; San Diego, CA, USA: 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.