Abstract
Background
Infantile hemangioma (IH) is common in children, which may bring about cosmetically disfiguring, functional impairment, and exhibiting complications. There had been various therapies and we aimed to assess the efficacy and adverse effects of different therapies through network meta-analysis.
Methods
We searched PubMed, Embase, Cochrane Library and Web of Science (from database inception to April 11, 2020) for studies assessing the efficacy, success rate and adverse effects. Direct pairwise comparison and a network meta-analysis under random effects were performed. We also assessed the ranking probability.
Findings
A total of 30 randomized clinical trials with more than 20 different therapeutic regimens were identified. Treatment combined propranolol orally with laser could improve the curative effect than monotherapy. Laser with topical β blockers showed more efficiency than others whether in children under 6 months or not. The long-pulsed dye laser might be the best laser therapy. A higher dose and a longer treatment duration of propranolol orally achieved a higher success rate and increased side effects. Plus pulse dye laser with propranolol had the lowest incidence of adverse reactions, such as ulcer, color sink and color reduction.
Interpretation
A combination of β blockers and laser might be the first-line treatment of IHs and a longer pulsed dye laser is preferred.
Funding
No funding was received.
Keywords: Hemangioma, Therapeutics, Network meta-analysis
Abbreviation: IH, infantile hemangioma; PRO, propranolol; PDL, pulsed dye laser; LPDL, PDL at a long pulse duration; Nd, Nd:YAG laser; Laser, PDL and Nd:YAG laser; β, topical β-blockers treatment; ATL, atenolol; PED, prednisone; IMQ, imiquimod; TA, triamcinolone; CAP, captopril; OR, odds ratios; CI, confidence intervals; RCT, randomized clinical trials; NMA, network meta-analysis; SUCRA, surface under the cumulative ranking curve; H, a higher dose; L, a longer treatment duration; S, a shorter treatment duration
Panel: Research in context.
Evidence before this study
Infantile hemangiomas (IHs) are very common in children and can cause many adverse effects. With the development, there are many different treatment regimens applied to the treatment of this disease.
Added value of this study
We performed a systematic review and network meta-analysis evaluating the efficiency, success rate, and incidence of adverse events of different treatment regimens for IHs. Finally, there were 30 articles and more than 20 therapeutic regimens included in our study.
Implications of all the available evidence
We found that combined β blockers with laser might was superior to other treatments, regardless of age and type of IHs.
Alt-text: Unlabelled box
1. Introduction
Infantile hemangioma (IH) is known as one of the most common tumors of childhood, with an incidence of 5–10% in infants [1,2]. Risk factors of IHs include prematurity, low birth weight infants, white race and females [3]. IHs often involve the head and neck, and can result in local tissue damage, ulceration, infection, bleeding, functional impact, and disfigurement [2–4]. Early treatment of IHs may potentially mitigate complications. However, there are so many therapeutic protocols (systemic or topical β-blockers, corticosteroids, imiquimod, laser therapy and so on) for clinical doctors [2,4,5], it is virtually impossible to cover all the combinations in direct head-to-head RCTs in order to determine the optimal option. The application of network meta-analysis (NMA), which is able to synthesize the results of direct and indirect comparisons simultaneously to obtain a more accurate and precise statistical result, makes it possible to overcome the limitation [6]. Therefore, we aimed to perform an evidence-based systematic review and NMA so as to evaluate the efficacy and adverse effects of these therapeutic choices.
2. Methods
This review was conducted in accordance with The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions [7]. (Supplement 15)
2.1. Search strategy
We performed a systematic review and network meta-analysis. An electronic search of PubMed, Embase, Cochrane Library and Web of Science (WOS) was conducted for research published between database inception to April 11, 2020 using Medical Subject Headings (MeSH) for ‘Hemangioma’, ‘Therapeutics’, ‘Lasers’ and other drugs which might be used in the treatment of IHs, with no language restrictions. (Details in Supplement 14)
2.2. Inclusion and exclusion criteria
Inclusion criteria were:
-
(1)
studies were RCTs involving children patients with treatments for IHs
-
(2)
studies with two arms of treatments at least;
-
(3)
studies’ endpoint(s) concluded the efficacy, success rate or incidence of adverse effects. (Definition described in Supplement 3)
Exclusion criteria were:
-
(1)
studies not adhere to the inclusion criteria;
-
(2)
studies with only an abstract or could not extract available data;
-
(3)
studies with patients previously been treated
-
(4)
studies ongoing and without result.
2.3. Data extraction and outcomes
All basic information of studies was extracted independently by two investigators (F.Q. and L.Y.). Disagreements were settled by discussion and a senior reviewer (Dr. Yuan). The data included the first author, origin, year of publication, sample size, age, therapeutic regimens, the number of patients treated effectively and successfully, the number of adverse events. The primary outcomes were the success rate and occurrence of adverse effects of different treatments as mentioned above with dichotomous data. Secondary outcome was the efficiency of therapies.
2.4. Quality assessment and risk of bias
The risk of bias for each RCT was assessed using the Cochrane Collaboration Risk of Bias Tool [8] which examined potential selection bias, performance bias, detection bias, attrition bias, and reporting bias and other bias. Included RCTs were categorized as follows based on the risk of bias: low, high, or unclear. Funnel plots were utilized to investigate signs of publication bias. (Supplement 5 and the ‘Funnel plot’ section of Supplement 6–12) We used the Grade of Recommendations Assessment, Development, and Evaluation (GRADE) for grading the certainty of direct and network evidence (Supplement 13).
2.5. Statistical analysis
We performed a random effects network meta-analysis using STATA15 software (StataCorp, College Station, TX, USA). Evidence from both direct and indirect comparisons was combined in the analysis. The primary endpoints, the success rate and occurrence of adverse effects, were described in the inclusion criteria. A network plot was generated to represent the overall information of the RCTs included in our study. Nodes size represents the number of patients for each treatment and lines thickness represents the number of available direct comparisons (in the ‘Network plot’ section of Supplement 6–12) [9]. We evaluated summary odds ratios (ORs) for dichotomous outcomes with 95% confidence intervals (CI) (in the ‘Indirect comparisons’ section of Supplement 6–12). A surface under the cumulative ranking curve (SUCRA), considered the more precise estimation of cumulative ranking probabilities, was used to provide a hierarchy of the efficacy, success rate and adverse reactions of the treatments (in the ‘Treatment relative ranking of model’ section of Supplement 6–12), the higher the SUCRA value is, the higher efficacy and incidence of adverse effects of the treatment has [10]. (More details in Supplement 2)
2.6. Role of the funding source
This systematic review and network meta-analysis was not funded.
3. Results
Our systematic search identified 7769 citations of which we included 30 articles finally in NMA. The flow of the systematic review is presented in Fig. 1. There were 1189 duplicates. We identified a total of 89 relevant references followed by a review of titles and abstracts. We read the full-text articles and excluded 56 articles not meeting the inclusion criteria or meeting the exclusion criteria (18 were non-RCTs; 11 without any endpoint we need; 7 without full-text; 7 letters; 5 single-arm study; 3 had duplicate content; 3 studies with patients treated before; 1 review; and 1 article's study design was not eligible). Moreover, treatments of Wu's (surgical resection and pingyangmycin) [11], Jung's (soft X-ray radiation) [12] and Zhu's (90Sr-90Y therapy) [13] could not be made indirect comparison with other studies, because they were the only ones who had studied the therapies, we excluded them. Eventually, 30 RCTs met the eligibility criteria for our study with an acceptable risk of bias among them. The characteristics of the 30 included trials are summarized in ‘S Table’. The studies were conducted worldwide including Asia, Africa, Europe, Oceania and America, and were published between 2002 and 2019. One trial included 5 arms involving 446 patients, [14] 6 studies with 3 arms involving 326 patients [[15], [16], [17], [18], [19], [20]], while the others included two arms involving 1351 patients. One of the studies used self-comparison [21]. Our study was registered with PROSPERO, number CRD42020179986.
Fig. 1.
Flow diagram of search process.
Totally, there were more than 20 therapeutic regimens (Details in Supplement 4) and 7 NMAs from different perspectives conducted, the efficacy of different treatments (Supplement 6), different lasers (Supplement 8), different topical β blockers (Supplement 11), treatments used in children younger than 6 months (Supplement 10) and treatments for superficial IHs (Supplement 12), success rate (Supplement 7) and occurrence of adverse effects (Supplement 9) of different treatments. Heterogeneity and inconsistency of these NMAs were assessed (Supplement 6.1–12.1) based on a design-by-treatment interaction model, [22] no significant inconsistency between direct and indirect evidence was identified within the evidence network (P>0.05) and consistency models were supported. There was no significant publication bias in the NMAs (In the ‘Funnel plot’ section of Supplement 6–12).
For the success rate, the network map was shown in Fig. 2A. There were 11 various treatments included, not all of which had a placebo-controlled trial, but they were more effective than placebo or observation (SUCRA of Placebo: 0.037, ranking 11th of 11 kinds of treatments). Combination therapy of propranolol orally with Nd:YAG did excellent (SUCRA of PRO-L+Nd: 0.98, ranking 1st of 11). Different doses and different durations of propranolol were also compared (PRO-S vs PRO-L: OR: 0.07, 95%CI: 0.02–0.32; H-PRO-S vs H-PRO-L: OR: 0.09, 95%CI: 0.02–0.37).
Fig. 2.
(A). Network meta-analysis of eligible comparisons for success rate. (B). Network meta-analysis of eligible comparisons for incidence of adverse effects. Placebo: placebo or observation; β: topical β-blockers treatment; PRO-L: propranolol orally at 1–2 mg per kilogram per day for a longer treatment duration (6 months or so); PRO-S: propranolol orally at 1–2 mg per kilogram per day for a shorter treatment duration (3 months or so); H-PRO-L: propranolol orally at a higher dose (3 mg per kilogram per day) for a longer treatment duration (6 months or so); H-PRO-S: propranolol orally at a higher dose (3 mg per kilogram per day) for a shorter treatment duration (3 months or so); PDL: pulsed dye laser; Nd: Nd:YAG laser; ATL: atenolol at 1 mg per kilogram per day for 6 months; PRO-L+Nd: PRO-L with Nd:YAG laser; PRO-L+β: PRO-L with topical β-blockers.
In terms of efficacy, combined propranolol orally with laser could improve the curative effect than monotherapy (PRO-L vs PRO-L+Laser: OR: 0.16, 95%CI: 0.03–0.81; Laser vs PRO-L+Laser: OR: 0.21, 95%CI: 0.06–0.69). Laser with topical β blockers showed more efficiency than others (β+Laser vs PRO-L: OR: 50.69, 95%CI: 1.01–2566.32; β+Laser vs Laser: OR: 39.00, 95%CI: 0.99–1539.15). SUCRA analysis indicated that β+Laser was likely to be the most effective treatment (SUCRA=0.92, PrBest 0.731, ranking 1st). This result also held for children under 6 months (SUCRA of β+Laser=0.806, ranking 1st). However, the comparison between different topical β blockers was not statistically significant (β-PRO vs β-TIM, OR=3.15, CI: 0.09–110.53). In addition, effects varied across different lasers, among which the long-pulsed dye laser (LPDL) might be the best (SUCRA of LPDL: 0.730, ranking 1st of 4 kinds of lasers). For superficial IHs therapy, the combination of propranolol orally and laser ranked in 1st of 6 regimens.
For the occurrence of side effects, the network plot was shown in Fig. 2B. There were twenty-five out of thirty trials included and eighteen various therapy regimens compared, of which PRO-L had the largest sample size. The treatment of plus PDL with propranolol orally had the lowest incidence of adverse reactions (SUCRA of PDL+PRO-L=0.086, ranking 18th of 19th, the 19th was Placebo) followed by topical use of β blockers alone (SUCRA of β=0.156). PDL had fewer adverse reactions than Nd:YAG (PDL vs Nd, OR=0.04, CI: 0.00–0.74). The most adverse reactions occurred in intravenous therapy of triamcinolone (SUCRA of TA=0.867, ranking 1st).
Table 1 shows the primary results of our NMA. There were eleven treatment regimens compared both success rate and incidence of adverse reactions. SUCRA rankings for success rate and incidence of adverse reactions were evaluated in Fig. 3.
Table 1.
Comparisons for success rate and incidence of adverse effects of the 11 treatments.
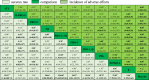 |
Placebo: placebo or observation; β: topical β-blockers treatment, like propranolol ointment, 2% carteolol hydrochloride, 0.5% timolol maleate eye drops or gel; H-PRO-L: propranolol orally at a higher dose (3 mg per kilogram per day) for a longer treatment duration (6 months or so); PDL: pulsed dye laser; PRO-L+β: combined propranolol orally with topical β-blockers; PRO-L: propranolol orally at 1–2 mg per kilogram per day for a longer treatment duration (6 months or so); PRO-L+Nd: combined propranolol orally with Nd:YAG laser; Nd: Nd:YAG laser; PRO-S: propranolol orally at 1–2 mg per kilogram per day for a shorter treatment duration (3 months or so); H-PRO-S: propranolol orally at a higher dose (3 mg per kilogram per day) for a shorter treatment duration (3 months or so); ATL: atenolol at 1 mg per kilogram per day for 6 months; *: Moderate quality of evidence; †: Low quality of evidence; ‡: Very low quality of evidence.
Fig. 3.
Two-dimensional graphs about success rate and incidence of adverse effects in all studies by SUCRA. Placebo: placebo or observation; β: topical β-blockers treatment; PRO-L: propranolol orally at 1–2 mg per kilogram per day for a longer treatment duration (6 months or so); PRO-S: propranolol orally at 1–2 mg per kilogram per day for a shorter treatment duration (3 months or so); H-PRO-L: propranolol orally at a higher dose (3 mg per kilogram per day) for a longer treatment duration (6 months or so); H-PRO-S: propranolol orally at a higher dose (3 mg per kilogram per day) for a shorter treatment duration (3 months or so); PDL: pulsed dye laser; Nd: Nd:YAG laser; ATL: atenolol at 1 mg per kilogram per day for 6 months; PRO-L+Nd: PRO-L with Nd:YAG laser; PRO-L+β: PRO-L with topical β-blockers.
4. Discussion
This systematic review and NMA included 30 RCTs which assigned 2123 children patients diagnosed IH to more than 20 kinds of therapeutic regimens randomly. There was no head-to-head comparison of most therapies, so indirect comparison played a major role in our study. We analyzed from the aspects of efficiency, success rate, adverse reactions, different lasers, age of patients, and superficial IHs. We found that all the treatments included in this analysis were more efficacious than placebo or ‘wait-and-see’ in children with IHs.
Our study found that combined β blockers with laser might be the most appropriate treatment for IHs, whether superficial or not. This conclusion also applied to children under 6 months which was known as the proliferative phase [23]. There were 4 kinds of β blockers (propranolol, timolol, atenolol and carteolol) included in our analysis and both systemic and topical usage of propranolol were compared. The applications of propranolol orally and 0.5% timolol maleate eye drops for IHs therapy were first reported by Leaute-Labreze et al. in 2009 and Guo et al. in 2010 respectively, which turned out well [24,25]. From then on, more and more studies on β blockers in IH treatment reported [14,[26], [27], [28]]. The mechanism of β-blockers on IHs may include constriction of capillaries, inhibition of angiogenesis, and trigger of capillary endothelial cell apoptosis [29,30]. A higher dose and a longer treatment duration of propranolol orally achieved a higher success rate and increased side effects. And topical β blockade therapy, either propranolol or timolol, had similar therapeutic efficacy to 1–2 mg/kg per day propranolol treatment orally with fewer adverse effects. Intralesional propranolol hydrochloride had fewer side effects than oral administration, but its efficiency is not satisfactory. The common side effects of propranolol include sleep disorders, peripheral coldness and peripheral coldness, [31] which attributed to propranolol's β blockade activity and lipophilicity. Atenolol, a hydrophilic, selective beta-blocker, might be as effective as propranolol and was well tolerated.
The laser acts on the chromophore with oxygen-containing hemoglobin being predominant in IHs. The chromophore absorbs light to heat the lesion and causes coagulation, thereby exerting therapeutic effects [5]. Different lasers have different scopes of application, we compared four laser modalities used in IHs, PDL, LPDL, Nd: YAG and PDL+ Nd: YAG. The pulse dye laser is usually the first choice of vascular laser therapy and mostly reported and applied in IHs laser therapy [32]. A longer pulse has a higher efficiency due to its advantage in transdermal depth. Nd:YAG laser is an infrared laser with a wavelength of 1064 nm that can penetrate deeply into the skin, showing good therapeutic effects on thick tumors [33]. Combined Nd: YAG with PDL was not superior than PDL alone. In addition, compared with PDL, Nd:YAG had worse efficiency and more adverse reactions occurred. What's more, the adverse effects, such as ulcer, color sink and color reduction, were occurred the least with combined using of propranolol orally and PDL. The reason should be that the usage of PDL could reduce the dosage and duration of propranolol, thus effectively shortening the treatment cycle while reducing the adverse reaction [34].
Our study has some limitations and strengths. The quality of some indirect comparisons was low according to GRADE. No study was reported on the success rate of PRO-L+PDL (it had the lowest incidence of adverse reactions) or β+PDL/Nd (β+Laser ranked first in the efficacy), so that PRO-L+Nd (it ranked first in the success rate) could not be compared with them. We did not group the children by sex. However, this is the first comprehensive synthesis of various treatments for IHs that allows estimates of head-to-head comparisons for different therapies from different angles. Pediatricians and researchers could more visibly judge the treatment differences among them.
In conclusion, a combination of β blockers and laser might be the first-line treatment of IHs and a longer pulsed dye laser is preferred. More well-designed RCTs are needed to confirm our findings.
Data sharing statement
No additional unpublished data are available.
Declaration of Competing Interest
All the authors declare no competing interests.
Acknowledgments
Acknowledgements
We thank Dr. Yuan Tian-Ming for helping us solve the problem of data extraction.
Funding
No funding was received.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100506.
Appendix. Supplementary materials
References
- 1.Greenberger S., Bischoff J. Infantile hemangioma-mechanism(s) of drug action on a vascular tumor. Cold Spring Harb Perspect Med. 2011;1(1) doi: 10.1101/cshperspect.a006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satterfield K.R., Chambers C.B. Current treatment and management of infantile hemangiomas. Surv Ophthalmol. 2019;64(5):608–618. doi: 10.1016/j.survophthal.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Darrow D.H., Greene A.K., Mancini A.J. Diagnosis and Management of Infantile Hemangioma. Pediatrics. 2015;136(4) doi: 10.1542/peds.2015-2485. e1060-104. [DOI] [PubMed] [Google Scholar]
- 4.Krowchuk D.P., Frieden I.J., Mancini A.J. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143(1) doi: 10.1542/peds.2018-3475. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z.Y., Wang Q.N., Zhu Y.H. Progress in the treatment of infantile hemangioma. Ann Transl Med. 2019;7(22):692. doi: 10.21037/atm.2019.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell D.M., Ades A.E., Higgins J.P. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutton B., Salanti G., Caldwell D.M. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 8.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 10.Chaimani A., Higgins J.P., Mavridis D., Spyridonos P., Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D., Yang Y., Pi G., Zhang Y. Clinical efficacy of pingyangmycin-assisted hemangioma surgery at peculiar sites in children and effects on expression of ER, VEGF and bF-GF proteins. Int J Clin Exp Med. 2019;12(8) 10851-+ [Google Scholar]
- 12.Jung EG K.U. Regression of haemangiomata in infants after x-ray treatment and mock-radiation [Rückbildung frühkindlicher hämangiome nach röntgenund pseudobestrahlung] Arch. Dermatol. Res. 1977;259(1):21–28. doi: 10.1007/BF00562734. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H.-.J., Liu Q., Deng X.-.L., Guan Y.-.X. Efficacy of low-dose Sr-90-Y-90 therapy combined with topical application of 0.5% timolol maleate solution for the treatment of superficial infantile hemangiomas. Exp Ther Med. 2015;10(3):1013–1018. doi: 10.3892/etm.2015.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leaute-Labreze C., Hoeger P., Mazereeuw-Hautier J. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372(8):735‐46. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- 15.Kader M., Fawzy A. β-Blocker versus triamacinolone acetate in the treatment of infantile periocular hemangioma. Egyptian J Cataract Refract Surgery. 2016;22(2) [Google Scholar]
- 16.Zhong S., Tao Y., Zhou J. Evaluation on efficacy of low dose propranolol combined with 1 064nm Nd: YAG laser on mixed and deeper infantile hemangioma. J Jilin Univ Med Ed. 2015;41(5):1032–1035. [Google Scholar]
- 17.Gong H., Xu D.P., Li Y.X., Cheng C., Li G., Wang X.K. Evaluation of the efficacy and safety of propranolol, timolol maleate, and the combination of the two, in the treatment of superficial infantile haemangiomas. Br J Oral Maxillofac Surg. 2015;53(9):836–840. doi: 10.1016/j.bjoms.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Zaher H., Rasheed H., Esmat S. Propranolol and infantile hemangiomas: different routes of administration, a randomized clinical trial. Eur J Dermatol. 2013;23(5):646‐52. doi: 10.1684/ejd.2013.2146. [DOI] [PubMed] [Google Scholar]
- 19.Malik M.A., Menon P., Rao K.L.N., Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: a randomized controlled study. J. Pediatr. Surg. 2013;48(12):2453–2459. doi: 10.1016/j.jpedsurg.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Tan M., Duan B., C-m Zhou, Gong H. The therapeutic effect of propranolol with 1064nm Nd: YAG laser on proliferating hemangioma in body surface. Zhonghua Zheng Xing Wai Ke Za Zhi. 2012;28(3):164–168. [PubMed] [Google Scholar]
- 21.Ying H., Zou Y., Yu W. Prospective, open-label, rater-blinded and self-controlled pilot study of the treatment of proliferating superficial infantile hemangiomas with 0.5% topical timolol cream versus 595-nm pulsed dye laser. J Dermatol. 2017 doi: 10.1111/1346-8138.13747. (no pagination) [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou H., Xu G., Huo R. Curative effect and safety of propranolol combined with prednisone in the treatment of infantile hemangiomas. Exp Ther Med. 2018;15(6):4677–4682. doi: 10.3892/etm.2018.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leaute-Labreze C., Dumas de la Roque E., Hubiche T., Boralevi F., Thambo J.B., Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 25.Guo S., Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128(2):255–256. doi: 10.1001/archophthalmol.2009.370. [DOI] [PubMed] [Google Scholar]
- 26.Zhong S.X., Tao Y.C., Zhou J.F., Yao L., Li S.S. Evaluation on efficacy of different doses of propranolol in treatment of infantile hemangioma. J Jilin Univ Med Ed. 2014;(4):880–883. [Google Scholar]
- 27.Aly M.M.D., Hamza A.F., Abdel Kader H.M., Saafan H.A., Ghazy M.S., Ragab I.A. Therapeutic superiority of combined propranolol with short steroids course over propranolol monotherapy in infantile hemangioma. Eur. J. Pediatr. 2015;174(11):1503–1509. doi: 10.1007/s00431-015-2561-1. [DOI] [PubMed] [Google Scholar]
- 28.Li G. Xu d-p, Tong S, Xue L, Sun N-n, Wang X-k. Oral Propranolol With Topical Timolol Maleate Therapy for Mixed Infantile Hemangiomas in Oral and Maxillofacial Regions. J Craniofacial Surgery. 2016;27(1):56–60. doi: 10.1097/SCS.0000000000002221. [DOI] [PubMed] [Google Scholar]
- 29.Laken P.A. Infantile hemangiomas: pathogenesis and review of propranolol use. Adv Neonatal Care. 2016;16(2):135–142. doi: 10.1097/ANC.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y., Li K., Xiao X., Zheng S., Xu T., Chen S. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg. 2012;47(12):2216–2223. doi: 10.1016/j.jpedsurg.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Leaute-Labreze C., Boccara O., Degrugillier-Chopinet C. Safety of oral propranolol for the treatment of infantile hemangioma: a systematic review. Pediatrics. 2016;138(4) doi: 10.1542/peds.2016-0353. [DOI] [PubMed] [Google Scholar]
- 32.Chinnadurai S., Sathe N.A., Surawicz T. Laser treatment of infantile hemangioma: a systematic review. Lasers Surg Med. 2016;48(3):221–233. doi: 10.1002/lsm.22455. [DOI] [PubMed] [Google Scholar]
- 33.Zhong S.X., Tao Y.C., Zhou J.F., Liu Y.Y., Yao L., Li S.S. Infantile Hemangioma: clinical Characteristics and Efficacy of Treatment with the Long-Pulsed 1,064-nm Neodymium-Doped Yttrium Aluminum Garnet Laser in 794 Chinese Patients. Pediatr Dermatol. 2015;32(4):495–500. doi: 10.1111/pde.12593. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Yang W., Tai M., Li Y., Wu X., Chen X. Combinational effect of pulsed dye laser and propranolol on treatment of superficial hemangioma. Biomed Res (India) 2017;28(20):8951‐3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





