Abstract
Background
The 22q11.2 microdeletion is the pathogenic copy number variation (CNV) associated with 22q11.2 deletion syndrome (22q11.2DS, formerly known as DiGeorge syndrome). Familiar endocrinological manifestations include hypoparathyroidism and hypothyroidism, with recent elucidation of elevated risk for obesity in adults. In this study, we aimed to determine whether adults with 22q11.2DS have an increased risk of developing type 2 diabetes (T2D).
Methods
We studied the effect of the 22q11.2 microdeletion on risk for T2D, defined by history and glycosylated hemoglobin (HbA1c), using weighted survey data from the adult Canadian population (based on n = 11,874) and from a clinical cohort of adults with 22q11.2DS (n = 314), aged 17–69 years. Binomial logistic regression models accounted for age, sex, non-European ethnicity, family history of T2D, obesity, and antipsychotic medication use.
Findings
The 22q11.2 microdeletion was a significant independent risk factor for T2D (OR 2·44, 95% CI 1·39–4·31), accounting for other factors (p < 0·0001). All factors except sex were also significant within 22q11.2DS. The median age at diagnosis of T2D was significantly younger in 22q11.2DS than in the Canadian population sample (32 vs 50 years, p < 0·0001). In adults without T2D, HbA1c was significantly higher in 22q11.2DS than the population (p = 0·042), after accounting for younger age of the 22q11.2DS group.
Interpretation
The results support the 22q11.2 microdeletion as a novel independent risk factor and potential model for early onset T2D. The findings complement emerging evidence that rare CNVs may contribute to risk for T2D. The results have implications for precision medicine and research into the underlying pathogenesis of T2D.
Keywords: Diabetes, Genetics, Risk, Structural variant, Molecular diagnostics, Early diagnosis, Genotype
Research in context.
Evidence before this study
Type 2 diabetes (T2D) is a common condition of complex and heterogenous etiology including genetic factors. There are few studies investigating the role of rare copy number variations (CNVs) in the development of T2D. A recent study reported six CNVs associated with T2D in older Caucasian adults, however this study was limited by healthy-volunteer selection bias. There were thus few participants with the recurrent 22q11.2 microdeletion (OMIM 188400/192430). The associated 22q11.2 deletion syndrome (22q11.2DS), formerly known as DiGeorge or velocardiofacial syndrome, affects an estimated 1 in 3–4000 live births, the most common of human microdeletion syndromes. Features include classic congenital anomalies, learning disabilities but only a minority with intellectual disability, and later onset conditions including psychiatric illness and endocrine manifestations such as hypothyroidism and hypoparathyroidism. There is recent evidence of associated obesity and medical multimorbidity in adults with 22q11.2DS. Data on type 2 diabetes are limited. We conducted a search using PubMed and Google Scholar up until March 28, 2020 using the following Medical Subject Heading terms: “copy number variation,” OR “22q11.2 deletion,” OR “DiGeorge syndrome” AND “type 2 diabetes.” We found no articles that investigated the association between 22q11.2DS and T2D.
Added value of this study
We used a cohort design to investigate a well-characterized outpatient sample of adults with 22q11.2 deletion syndrome (n = 314) and a representative Canadian population sample (based on n = 11,874). We defined T2D as clinical diagnosis of T2D or HbA1c ≥ 0·065 and investigated whether the 22q11.2 microdeletion increased the risk of developing T2D while accounting for relevant clinical and demographic variables (age, male sex, non-European ethnicity, body mass index, antipsychotic use, and family history of T2D).
Implications of all the available evidence
The results indicate that the 22q11.2 microdeletion confers an elevated risk for developing T2D, even while accounting for other risk factors, and that onset of T2D occurs at an earlier age compared with general population expectations. These findings provide further evidence that rare CNVs contribute to risk of T2D and add to previously known endocrinological features of 22q11.2DS. A relatively genetically homogenous and identifiable population such as 22q11.2DS offers a model to investigate the pathogenesis of T2D. The findings have implications for precision medicine and open new opportunities for research, especially given the array of animal and cellular models available for the 22q11.2 microdeletion. The results have implications for clinical practice in terms of anticipatory care, screening and prevention of T2D for those with 22q11.2DS, who could now be deemed a high-risk population. Increased index of suspicion for 22q11.2DS, perhaps especially for younger individuals with T2D and in other groups at elevated risk of T2D, e.g. individuals with schizophrenia, could improve molecular diagnostics.
Alt-text: Unlabelled box
1. Introduction
There is an increasing interest in developing personalized and precision medicine for the management of type 2 diabetes (T2D), a heterogeneous disease for which prevalence is rising dramatically [1]. Of clinically relevant genetic findings, rare variants in several genes including the multi-gene 17q12 deletion have been linked to maturity-onset diabetes of the young (MODY), and there are longstanding data on T2D related to familiar conditions such as Prader-Willi syndrome [1], [2], [3]. Emerging data indicate that several rare recurrent copy number variations (CNVs) may be associated with diabetes in adults [4]. However, this population-based study of over 400,000 older Caucasian adults reported ascertainment of few individuals (n = 10) with the most common pathogenic CNV, the 22q11.2 microdeletion, precluding meaningful results [4].
The recurrent 22q11.2 microdeletion (OMIM 188400/192430) affects an estimated 1 in 3–4000 live births [5,6]. The associated 22q11.2 deletion syndrome (22q11.2DS, formerly known as DiGeorge syndrome) has high overall penetrance but a wide phenotypic variability in features and severity that, together with premature mortality, contribute to under-recognition and recruitment challenges [5,[7], [8], [9], [10]]. In addition to congenital anomalies [5,7,8,11], later onset endocrinological manifestations including hypothyroidism and hypoparathyroidism are common, as are psychiatric illnesses, including a 25-fold increased risk of schizophrenia [5,7]. In adults with 22q11.2DS, there is recent evidence of obesity, global medical multimorbidity, and other newly discovered features, including early-onset Parkinson's disease [6,9,11,12]. Data on diabetes however are limited [4,6].
In the current study, we employed a cohort design to investigate whether adults with 22q11.2DS have an increased risk of developing T2D when compared to the Canadian population. Given the rarity and other issues outlined above, we hypothesized that the 22q11.2 microdeletion would be associated with increased risk for T2D, while accounting for other T2D-relevant risk factors. We also predicted that those with 22q11.2DS would develop T2D at a younger age than in the general population.
2. Methods
2.1. Study design and setting
This was a cohort study examining risk of T2D in an outpatient, clinical sample of adults with 22q11.2DS, compared to a large community-based general population sample of the same age range, where similar data were available on T2D outcome and relevant risk variables were available and determined based on clinical guidelines [9,13,14]. A clinical cohort of individuals with a 22q11.2 microdeletion was essential for the study design, given well-recognized challenges to recruiting adequate sample sizes when the CNV is rare and has high overall penetrance, with features such as premature mortality and neuropsychiatric phenotypes that also adversely affect ascertainment as part of population-based samples [4,5,7,8].
The study was approved by the local research ethics boards of hospitals (Centr for Addiction and Mental Health and University Health Network) affiliated with the University of Toronto, with informed consent obtained in writing for participants.
2.2. 22q11.2DS cohort
From a total starting cohort of 352 adults (aged ≥17 years) ascertained through a Canadian specialty clinic for adults with 22q11.2DS, we excluded three individuals with type 1 diabetes, 33 with neither diagnosis of T2D nor usable HbA1c value to enable assessment of diabetes status (one with sickle cell anaemia and unreliable HbA1c; two with no body mass index [BMI] data). The sample thus comprised 314 individuals (153 male, 161 female) with a typical 22q11.2 deletion, i.e., involving the proximal deletion region, and most commonly (~90%) a 2.5 Mb LCR22A-LCR22D deletion [7]. 22q11.2 deletions were detected using standard clinical methods (Supplementary Table 1) [8]. Study participants were originally ascertained through referrals from various clinical sources and/or active screening at an adult congenital cardiac clinic [6]. As is typical of 22q11.2DS, phenotypes ranged in severity [[5], [6], [7], [8], [9],11]; major features are summarized in Supplementary Table 2.
We recorded a physician's diagnosis of T2D with age at onset, and/or the latest HbA1c value available, from comprehensive review of lifetime medical records [6]. Age was at latest HbA1c value (range 17·0–66·4 years; unavailable for one individual for whom we used age at diagnosis of T2D). Ethnicity was characterized as European (n = 247, 78·7%) or non-European descent. The latter comprised Asian (n = 30, 9·6%), black (n = 10, 3·2%), mixed (n = 11, 3·5%), or other ethnicities (n = 16, 5·1%) including Indigenous, Middle Eastern or Hispanic. Positive family history was defined as having an affected first degree relative (i.e. parents or siblings) diagnosed with T2D, determined from patient interviews and collateral information from family members [15]. Body mass index (BMI), as a proxy for obesity, was recorded as that closest to time of the HbA1c value; the majority were within 2 years (n = 269, 85·7%). We determined antipsychotic use from clinical assessment and/or review of medical and pharmacy records. Of 117 (37·3%) individuals on any dose of antipsychotic, most (n = 102, 87·2%) had schizophrenia or related psychotic illness.
2.3. Canadian population data
Canadian population data were accessed through the Research Data Centres (RDC) at Statistics Canada as previously described [9]. Data were ascertained from the Canadian Health Measures Survey (CHMS) Cycles 1–5 (2007–2017; n = 29,364), a national survey sampling the Canadian population aged 3–79 years [16]. This survey comprised a household visit enrolling participants into the study, gathering information on health and medical history, and demographic and other variables. Participants subsequently attended a clinic whereby additional health-related information was ascertained, physical measures (e.g. height, weight) were determined and specimens (e.g. HbA1c levels) were obtained [16]. Molecular genetic data, including assessment for CNVs such as the 22q11.2 microdeletion, are not available for the CHMS sample.
From the CHMS data, we determined a sample with comparable T2D risk-related data to that available for our 22q11.2DS sample (Supplementary Figure 1). Thus, we used Cycles 1–4 (2007–2015; n = 23,578) since Cycle 5 had no family history information, delimited as before to the age range 17–69 years [9], and excluded individuals with incomplete data (Supplementary Figure 1). Applying weights (see below) to data for the remaining sample (n = 11,874) allowed derivation of estimates representative of the Canadian population [9].
Diagnosis and age at onset of T2D was self-reported. HbA1c values were available for a single point in time (99·8% of the CHMS sample). For demographic risk variables, we used age at time of HbA1c, self-reported sex (male 49·5%, female 50·5%) and ethnicity (79·1% white, 13·2% Asian, 2·5% black, 1·5% mixed, 3·7% other, including Indigenous, Middle Eastern and Hispanic). BMI data were calculated from measured height and weight [6]. Family history was determined through a screening question; we used only data on family history of T2D. Medication history was determined through medications shown to surveyors during visits, coded based on the Anatomical Therapeutic Chemical (ATC) classification system [9]. For antipsychotic medications, we used ATC code N05A after excluding those coded N05AN (lithium).
2.4. Outcome measures
We defined T2D by its recorded diagnosis, or in the absence of this, by HbA1c ≥0·065, based on standard guidelines [14,15]. Those with HbA1c value <0·065, and no recorded diagnosis of T2D, were classified as not having T2D. In a post hoc analysis we included pre-diabetes, defined in Canada as HbA1c values between 0·060 and 0·064 [15], together with T2D, as an outcome.
2.5. Weighting
Weighting of data from the CHMS sample includes a primary weight that expands the sample to provide a representation of the general Canadian population, and not only participants of the survey [9]. The CHMS sample also has bootstrap weights that allow variance calculation to account for stratification, clustering and non-response in the data. Since the 22q11.2DS sample has no weights, it was not possible to use bootstrap weights for statistical tests involving the comparison between the 22q11.2DS and CHMS samples. We therefore followed Statistics Canada regulations, as before [9], and calculated standardized primary weights that sum to the sample size of the CHMS sample used in this study. For the 22q11.2DS sample, we assigned single unit weights to allow us to merge data from both samples and perform analyses [9].
2.6. Statistical analyses
For descriptive statistics, we used univariate logistic regression models to determine whether clinical and demographic variables differed between the 22q11.2DS and the Canadian population samples after weighting. We used multivariate logistic regression models to investigate the impact of the 22q11.2 microdeletion while accounting for other T2D risk variables, based on the criteria stated above. We determined odds ratios (OR) and 95% confidence intervals (CI) for six variables: age (for every 10-year increase), sex, ethnicity, BMI (for every increase of 5), family history of T2D, and antipsychotic use. We conducted multicollinearity analyses to identify correlated variables in the models tested; variance inflation values did not exceed 2, suggesting no issues with multicollinearity. While we considered using a mediation analysis to estimate the possible effects of BMI and antipsychotic use on the risk between the 22q11.2 deletion and T2D, we decided on a more conservative approach, including both variables in our main analyses.
To determine differences in the age at onset of T2D between 22q11.2DS and the Canadian population sample we used a Wilcoxon signed-rank test limited to individuals with a T2D diagnosis. For between-group prevalence comparisons, we used the prevalence estimates to calculate prevalence ratios (PRs) [9]. We also determined approximations of 95% CIs for the PRs, and when these did not include 1, we reported significance at p < 0·05. We used PR and CI to examine group differences for age at diagnosis of T2D using age groups defined in previous reports: <25 years, 25–44 years, 45–64 years, 65–69 years [9].
As HbA1c increases with increasing age [17], we investigated those classified as not having T2D using analysis of covariance, ANCOVA, to examine whether age effects were moderated by the 22q11.2 microdeletion. Visual inspection for lack of normality, non-linearity of age and influential observations revealed no meaningful violations of assumptions of this test.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina, USA) and/or Stata version 15.1 (StataCorp LLC, College Station, Texas, USA). Values of p < 0·05 (two-tailed) were considered statistically significant.
2.7. Role of funding source
The sources of funding for this project had no role in the study design, collection, analysis and interpretation of data, nor any role in writing or submitting this paper for publication.
3. Results
3.1. 22q11.2 deletion is associated with increased risk of T2D independent of other risk factors
Univariate analyses of clinical and demographic variables found no significant between-group differences in mean HbA1c values, or in ethnicity, sex, or family history of T2D (Table 1). As expected [6,9], however, the 22q11.2DS group had significantly higher BMI and a greater proportion on antipsychotic medications than the population sample (p < 0·0001, Table 1).
Table 1.
Univariate analyses of demographic and clinical factors for adults with 22q11.2 deletion syndrome (22q11.2DS, n = 314) and weighted data for the Canadian population aged 17–69 years (based on n = 11,874)a.
| Variablesb | 22q11.2DS |
Canadian populationa |
Logistic regression analysesc | ||
|---|---|---|---|---|---|
| n | % | na | % | p-value | |
| Non-European ethnicity | 67 | 21·3 | 2482 | 20·9 | 0·85 |
| Male sex | 153 | 48·7 | 5878 | 49·5 | 0·78 |
| Family history of T2D | 53 | 16·9 | 2399 | 20·2 | 0·15 |
| Antipsychotic use | 117 | 37·3 | 142 | 1·2 | <0·0001 |
| Mean | SD | Mean | SD | ||
| Hemoglobin A1c | 0·0550 | 0·0075 | 0·0550 | 0·0060 | 0·66 |
| Body mass index | 29·6 | 8·1 | 27·1 | 5·7 | <0·0001 |
| Age (years) | 30·8 | 11·6 | 42·3 | 14·5 | <0·0001 |
SD = Standard deviation.
Based on a Canadian sample of n = 11,874 respondents who met inclusion criteria in the study and later weighted to be representative of the Canadian population (see Methods and Supplementary Figure 1), rounded to the nearest whole number.
Definitions for variables are outlined in the Methods.
Results presented are for univariate logistic regression analyses conducted individually for each variable to determine differences between the 22q11.2DS and the Canadian population data after weighting; significant results are in bold font.
In an unadjusted model, the crude proportion of adults with T2D was greater in the 22q11.2DS group (n = 25, 8·0%) than in the population sample (5·5%), although the result did not reach significance (p = 0·07), perhaps related to the younger age of the 22q11.2DS group (mean 30·8 [SD 11·6] years vs 42·3 [SD 14·5] years, p < 0·0001). The results of the main analysis using an adjusted model, controlling for multiple covariates, were however highly significant (χ2 = 1317·65, df = 7, p < 0·0001; Table 2); consistent with our hypothesis, the 22q11.2 deletion was a significant independent predictor of risk of T2D (OR=2·44, 95% CI = 1·39–4·31). All six other risk factors for T2D assessed (age, sex, non-European ethnicity, family history, BMI and antipsychotic use) also showed significant effects, with the greatest risk imparted by non-European ethnicity (OR = 3·48, 95% CI = 2·83–4·28; Table 2).
Table 2.
Multivariate analysis of the 22q11.2 microdeletion and other variables on type 2 diabetes risk in Canadian adults aged 17–69 yearsa.
| Variablesb | Type 2 diabetes (T2D) |
Multivariate logistic regression analysisc |
||||
|---|---|---|---|---|---|---|
| No (n = 11,506) |
Yes (n = 682) |
|||||
| na | na | OR | 95% CI | |||
| Non-European ethnicity | 2368 | 182 | 3·48 | 2·83–4·28 | ||
| Family history of T2D | 2150 | 298 | 2·57 | 2·16–3·06 | ||
| 22q11.2 microdeletiond | 289 | 25 | 2·44 | 1·39–4·31 | ||
| Antipsychotic use | 231 | 32 | 1·78 | 1·09–2·92 | ||
| Male sex | 5671 | 362 | 1·51 | 1·28–1·80 | ||
| Mean | SD | Mean | SD | |||
| Age (years) | 41·2 | 14·4 | 55·8 | 9·4 | 2·59 | 2·39–2·82 |
| Body mass index | 26·8 | 5·5 | 32·0 | 7·2 | 1·90 | 1·77–2·03 |
OR = Odds ratio; CI = Confidence interval; SD = Standard deviation.
Survey weights used to obtain population estimates for analyses were based on originating samples involving n = 12,188 participants (n = 11,874 from a Canadian population sample; n = 314 with 22q11.2 deletion syndrome); see Methods for details.
Definitions for variables are outlined in Methods. Continuous variables, age and body mass index (BMI; used as a proxy for obesity), were analysed using increments of 10 and 5, respectively.
Likelihood ratio for model: χ2 = 1317·65, df = 7, p <0·0001; significant results are in bold font.
Ascertained through specialty clinic for adults with 22q11.2 deletion syndrome (n = 314); data on 22q11.2 deletion status for the population-based sample (estimated n = 0–3) unavailable; see Methods for details.
In a post hoc analysis using the same predictors but for risk of developing either pre-diabetes or T2D (i.e., HbA1c ≥ 0·06 or previous diagnosis of T2D), the model and all variables, apart from antipsychotic use, remained significant (Supplementary Table 3).
3.2. Predictors of T2D in 22q11.2 deletion syndrome
Within the 22q11.2DS sample, results were significant using a similar regression model (χ2 = 56·80, df = 6, p < 0·0001; Table 3). Five of the six predictors examined were significant independent predictors of developing T2D; the exception was male sex, though results were in the expected direction (Table 3).
Table 3.
Multivariate analysis of clinical and demographic variables on type 2 diabetes risk within a sample of 314 adults with 22q11.2 microdeletion.
| Variablesa | Type 2 diabetes (T2D) |
Multivariate logistic regression analysisb |
||||
|---|---|---|---|---|---|---|
| No (n = 289) |
Yes (n = 25) |
|||||
| na | na | OR | 95% CI | |||
| Non-European ethnicity | 60 | 7 | 4·32 | 1·30–14·39 | ||
| Family history of T2D | 42 | 11 | 3·19 | 1·10–9·24 | ||
| Antipsychotic use | 101 | 16 | 2·98 | 1·04–8·50 | ||
| Male sex | 140 | 13 | 1·59 | 0·60–4·23 | ||
| Mean | SD | Mean | SD | |||
| Age (years) | 29·9 | 10·9 | 42·3 | 13·9 | 2·26 | 1·50–3·41 |
| Body mass index | 28·9 | 7·6 | 37·8 | 10·1 | 1·87 | 1·38–2·51 |
OR = Odds ratio; CI = Confidence interval; SD = Standard deviation.
Definitions for variables are outlined in Methods. Continuous variables, age and body mass index (BMI; used as a proxy for obesity), were analysed using increments of 10 and 5, respectively. Note: All individuals in this analysis have a 22q11.2 microdeletion.
Likelihood ratio for model: χ2 = 56·80, df = 6, p = <0·0001; significant results are in bold font.
3.3. Age at onset of T2D
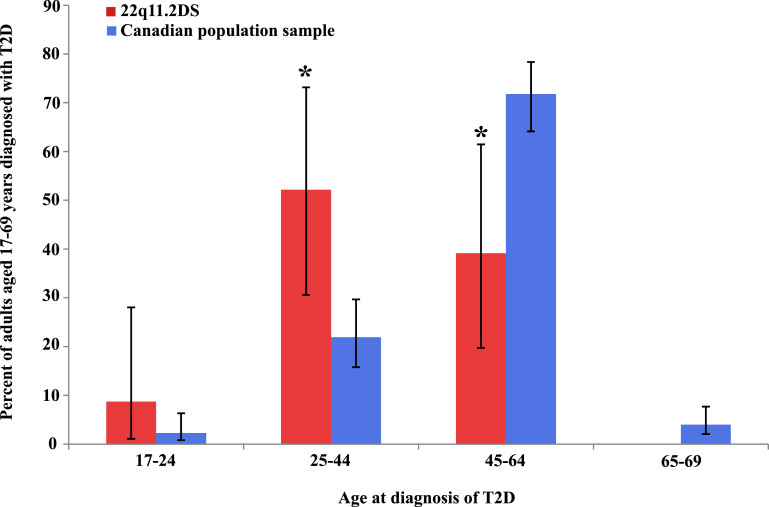
The median age at diagnosis of T2D was 32 years (Q1 30, Q3 50, years) in the 22q11.2DS group, significantly younger than in the Canadian population (median 50 years, Q1 45, Q3 56, years; Z = 4·46, p < 0·0001). Fig. 1 illustrates the age at onset distribution of T2D in the two groups. For 22q11.2DS, the distribution is overall shifted to the left. Compared with the general population, proportionately more of the initial diagnoses of T2D for the 22q11.2DS group occurred between ages 25–44 years (PR = 2·38, 95% CI = 1·39–4·08), and proportionately fewer at age 45–64 years (PR = 0·54, 95% CI = 0·31–0·97) [Supplementary Table 4].
Fig. 1.
Age at diagnosis of type 2 diabetes in adults with 22q11.2 deletion syndrome compared to the Canadian population.
Plotted are prevalence ratios (PR, as %, bar graphs: red, 22q11.2DS; blue, Canadian population) and 95% confidence intervals (CI, vertical lines) per age group for age at diagnosis of type 2 diabetes (T2D). Asterisks (*) indicate a statistically significant difference between the two groups. The proportion of those diagnosed with type 2 diabetes for the 22q11.2DS group was significantly greater in the 25–44 year age group (PR=2·38, 95% CI=1·39–4·08), and significantly lower in the 45–64 year age group (PR=0·54, 95% CI=0·31–0·97), than in the population. Of the two individuals in the 22q11.2DS group in the 65–69 year age range, neither had T2D diagnosed. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. HbA1c in individuals without T2D
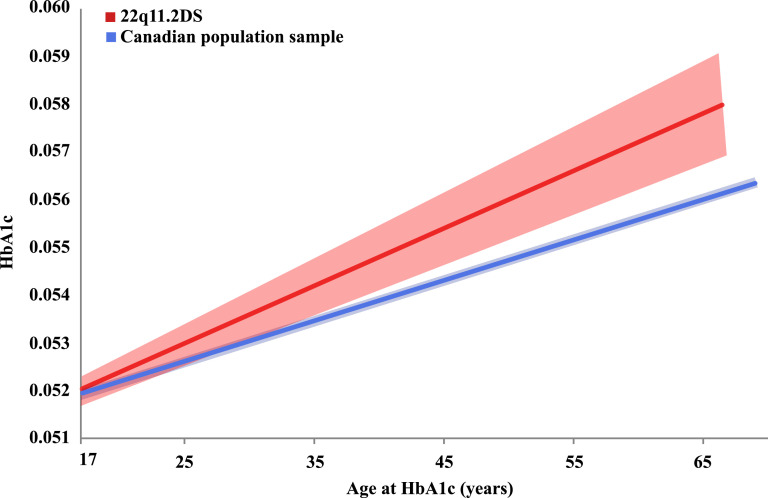
For individuals without T2D, mean HbA1c levels were not different between the 22q11.2DS group and the general population (0·054 [SD 0·0037] vs. 0·054 [SD 0·0035], respectively; OR = 0·96, 95% CI = 0·79–1·16). However, results for an ANCOVA, accounting for age, showed a significant effect of the 22q11.2 microdeletion on HbA1c values for adults without T2D (Fig. 2; F(1, 11,478) = 4·15, p = 0·042). The curves for the two groups appear to differ from an early age, with divergence of the 95% CI on either side of these curves at about age 35 years (Fig. 2).
Fig. 2.
HbA1c values by age in adults without type 2 diabetes, comparing results for 22q11.2 microdeletion and population-based samples.
The regressions of HbA1c values by age for individuals without type 2 diabetes aged 17–69 years are represented by solid lines: for those in the 22q11.2DS sample (red), and in the Canadian population-based sample (blue); the respective 95% confidence intervals (CIs) are represented by lighter coloured shaded areas around these solid lines (F(1, 11,478) = 4·15, p = 0·042). The 95% CIs for each of the two samples (shaded areas) appear to diverge at about age 35 years. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Other post hoc analyses
In post hoc analyses of data available for the 22q11.2DS group, we determined that adding intellectual disability or congenital heart disease to the model did not materially change results of the regression analysis using risk variables for T2D (Supplementary Table 5 and Supplementary Table 6, respectively). Also, neither primary hypothyroidism nor hypoparathyroidism were significantly associated with T2D in the 22q11.2DS group.
Examining data on management of T2D indicated standard treatments including metformin and other oral hypoglycemics [6,9] and dietary and lifestyle strategies; two individuals were treated with insulin, one of whom also received metformin. For those with schizophrenia and related psychotic disorders, the median difference between age at onset of psychotic illness and age at onset of T2D was 7·0 (range −0·3 to 31·4) years.
4. Discussion
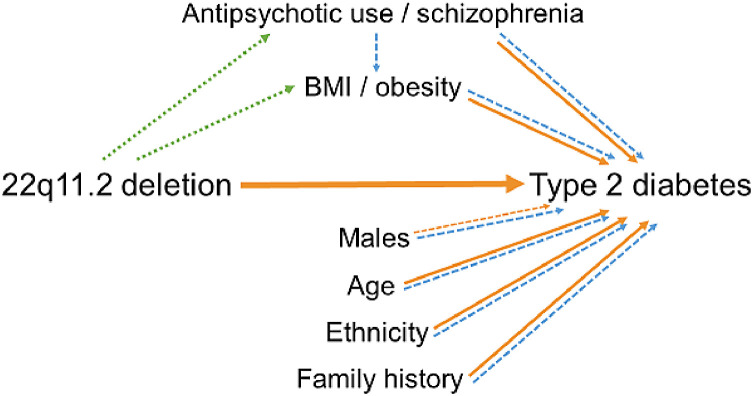
The results of this study indicate that, even while accounting for other risk factors, the 22q11.2 microdeletion imparts a significant risk for T2D, with an effect size comparable to that of positive family history of T2D. Notably, the findings show that the effects of other T2D risk factors within 22q11.2DS share the same direction and approximate magnitude as in the general population (Fig. 3). The age at onset of T2D was also significantly younger in 22q11.2DS, on average by nearly two decades, compared to population expectations [15]. Collectively, the results have implications for clinical practice and diabetes research.
Fig. 3.
Proposed model of increased risk for type 2 diabetes in individuals with a 22q11.2 microdeletion.
Diagram illustrating the proposed relationships between the 22q11.2 deletion and risk of type 2 diabetes, including clinical variables with known enrichment in individuals with 22q11.2DS. The direction of proposed relationships is indicated by arrows. Orange arrows (solid, significant findings) indicate evidence derived from the current study. Green dashed arrows indicate relationships suggested by previous studies of 22q11.2DS; blue dashed arrows indicate evidence from previous studies of T2D risk factors in the general population [5,6,11,21]. The green and blue dashed lines unaccompanied by orange lines indicate possible mediation effects that the current study did not investigate. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In contrast to MODY [1], [2], [3], relatively few associated rare variants with large effect size have previously been identified for T2D [18]. The findings of the current study complement a recent report that other rare clinically relevant CNVs may contribute to risk for T2D [4]. The paucity of individuals recruited with a 22q11.2 microdeletion however, reflects the challenges in population-based studies to ascertain sufficient numbers of rare CNVs with high penetrance
The significantly younger age at onset of T2D in 22q11.2DS, together with the elevated HbA1c values for age observed for individuals without T2D, are consistent with evidence for premature mortality, early multimorbidity, and other age-related conditions including early-onset Parkinson disease, compared to adult norms [9,10,12]. Given the ~25-fold increased risk for schizophrenia in 22q11.2DS [7], the results of the current study may also be consistent with previous research showing idiopathic schizophrenia to be associated with elevated risk for T2D, even in the absence of antipsychotic exposure [19].
There are several immediate clinical implications deserving of consideration. First, with respect to molecular diagnostics, there could be an increased index of suspicion for 22q11.2DS particularly in individuals with relatively early onset T2D and any of the many multisystem features of this condition [5,7,9]. Under-recognition of 22q11.2DS is particularly common in adults [5,7]. Notably, clinical genetic testing with genome-wide methods such as microarray would detect this and other pathogenic CNVs that may be associated with T2D [4]. Most gene panels, e.g., for MODY, cannot detect such structural variants; improved molecular testing methodology may reveal novel genetic causes of diabetes [3,20]. Second, for individuals with 22q11.2DS, early monitoring for, and assessment and treatment of T2D, would appear warranted [13,14,18]. Those of non-European ethnicity, with family history of T2D, with schizophrenia and/or other standard risk factors for T2D, would appear to deserve particular attention [15,21,22]. Amendment of clinical practice guidelines for 22q11.2DS and T2D could be considered [5,15]. Also, these results may encourage efforts to introduce dietary and lifestyle measures in early adolescence, as suggested previously due to risk of adult-onset obesity in 22q11.2DS [5,6].
The results support the potential for the recurrent 22q11.2 microdeletion, the most common human microdeletion, as a genetic model to investigate T2D. Further studies are needed to establish whether the increased T2D risk observed in 22q11.2DS is due to insulin resistance and/or β-cell dysfunction. The propensity for autoimmune disease in 22q11.2DS may also be of interest [7,23]. Mechanistic studies will be aided by the availability of multiple animal and cellular models [7,24,25]. Further understanding of how 22q11.2 microdeletions increase risk for T2D could also shed new light on general mechanisms, risk algorithms, and personalized treatment of T2D [1]. For example, one could examine whether polygenic risk scores for T2D could help with risk stratification within a high-risk group such as 22q11.2DS, over and above family history and other risk factors.
Research to establish whether 22q11.2DS is associated with increased risk of T2D-related complications such as cardiovascular and renal disease will be important, especially given the earlier age at onset of T2D and the congenital and later onset cardiac and renal conditions associated with 22q11.2DS [5,7,8,11,26]. Perhaps most important are investigations to determine the optimal treatment and efficacy of early treatment with hypoglycaemic medications. Prospective designs will be important; feasibility is enhanced given the relative youth of most patients known to have 22q11.2DS, and previous international consortium studies of other conditions [7].
With respect to genetic mechanisms, although structural variants comprise a substantial proportion of genomic variation, their detection requires specialized analytic techniques, and even CNVs remain little studied, as the main focus has been on common single nucleotide variants in diabetes research [27]. Recent findings for MODY, and now for T2D, however, indicate the potential promise of novel insights into pathogenesis from rare structural variants, including CNVs [[1], [2], [3], [4],20,27].
Shared mechanisms for diabetes could include those related to gene functions affected by dosage changes engendered by multi-genic CNVs. Although single gene effects remain possible, to our knowledge, no individual gene at the 22q11.2 locus has been associated with elevated risk for T2D (OMIM 125853) [24]. Nevertheless, there may be candidate mechanisms of interest, including regulatory mechanisms of broader applicability to other rare and common variants, that are consistent with the undoubted polygenicity of T2D and that present potential for novel therapeutic interventions [24,25,27]. For T2D, there may also be parallels to recent evidence indicating that genetic risk for expression of schizophrenia in 22q11.2DS was not related to rare or common variants at the 22q11.2 locus, but rather was conferred by the same common genome-wide polygenic risk factors that contribute to idiopathic schizophrenia [28]. Shared polygenic risk between schizophrenia and T2D has also been reported in general population samples [19]; studies of 22q11.2 deletions and other CNVs may help elucidate such common mechanisms.
Strengths of the study include the data available for both 22q11.2DS and population-based samples from the same country, allowing simultaneous investigation of multiple risk factors for T2D, and the sample size of adults with 22q11.2 microdeletion. The sample was larger than that for other rare CNVs recently shown to be associated with T2D [4], and to our knowledge, is the world's largest adult 22q11.2DS cohort with such multi-system phenotypic data available. Notwithstanding, larger 22q11.2DS samples would increase power to detect other T2D-related findings, e.g., in those aged <25 years.
A major advantage of the CHMS data is its representativeness of the Canadian population, however no genetic data were available. Thus, given its rarity, we assumed there were no individuals with a 22q11.2 microdeletion in the population sample, a conservative assumption that would be unlikely to have materially affected the results. A more ideal study design would entail comparable population-based sampling but include clinical data verified for accuracy, high quality genomic data that included CNV analyses, and assurance that selection bias against people with neuropsychiatric features and other multimorbidity was minimized. The rarity of the 22q11.2 microdeletion would predict however that several million individuals with such data would need to be recruited in order to obtain a comparable sample size of those with 22q11.2 deletions as the clinical sample studied here. Replication of this study may require an international consortium, ensuring comparable depth and quality of 22q11.2DS and population-based data, and the ability to account for possible differences, e.g., in healthcare availability, between countries/sites.
Screening guidelines suggest that for HbA1c ≥0·065, further tests are needed to make the diagnosis of T2D [13]. Nevertheless, in community-based non-diabetic adults, single-point HbA1c testing is associated with risk of developing diabetes [26]. It is possible that by instituting routine HbA1c testing at our 22q11.2DS clinic we may have increased the diagnosis of T2D.
Family history of T2D in the CHMS group was ascertained by asking whether participants had an immediate family member with T2D. Some may have interpreted this to include individuals other than those with first-degree genetic relationships. However, given that 43.7% of those with T2D had a family history of T2D, compared to 18.7% of those without T2D, there would have to have been a substantial differential reporting of non-genetically related individuals between these sub-groups to have materially affected the overall results. Also, given missing data for age at onset of T2D in the CHMS data, we opted not to attempt a Cox regression model. In the CHMS cycles 1–4, we dealt with missing data by excluding participants, and this may have introduced bias into the study. However as only ~6% of participants were excluded due to this decision (Supplementary Figure 1), we believe that this is unlikely to have substantively affected the overall results.
We used an inclusive definition for antipsychotic medications as a proxy for psychiatric illness and considered any antipsychotic use regardless of classification (i.e. first or second generation) or dose to be a positive exposure. However, this was not the most significant factor in analyses of T2D risk, and results were not significant in post hoc studies when pre-diabetes was included together with T2D as an outcome. Possible mediating effects of antipsychotics/schizophrenia and obesity were not examined in this study but are indicated from other evidence in a proposed model of T2D risk (Fig. 3).
The results indicate that the 22q11.2 microdeletion confers an elevated risk for developing T2D, and at an earlier age, compared with general population-based expectations. The findings add to previously known endocrinological comorbidities for 22q11.2DS and will be of importance to both clinicians and researchers. Relatively genetically homogenous populations such as 22q11.2DS offer a model to investigate the pathogenesis of T2D. The findings have implications for precision medicine and open new opportunities for research. Investigating the mechanisms involved in the risk attributable to the 22q11.2 microdeletion and impaired glucose metabolism may involve genome sequencing and other “omics” strategies, using the array of animal and cellular models available for this CNV. Determining the proportion of individuals with a 22q11.2 microdeletion in young T2D cohorts may be helpful. The results allow for screening, anticipatory care, and prevention of T2D for those with known 22q11.2DS. Future research to investigate management of T2D and risk for T2D in this newly identified high-risk population will provide immediately translatable results.
Declaration of Competing Interest
The authors have declared no conflicts of interest.
Acknowledgments
Data sharing
Some data that support the findings of this study are provided by Statistics Canada. These data are not publicly available due to privacy restrictions; interested parties may apply to Statistics Canada for access to these. Other data that support the findings are available from the corresponding author upon reasonable request.
Author contributions
Lily Van - Conceptualization, data curation, data analysis, data collection/investigation, methodology, writing, reviewing and editing
Tracy Heung - Conceptualization, data curation, data analysis, data collection/investigation, methodology, reviewing and editing
Sarah L. Malecki - Methodology, reviewing and editing
Christian Fenn - Conceptualization, data collection/investigation
Andrea Tyrer - Conceptualization, data collection/investigation
Marcos Sanches - Data analysis, methodology
Eva W. C. Chow - Data collection/investigation, methodology
Erik Boot - Data collection/investigation, reviewing and editing
Maria Corral - Data collection/investigation
Satya Dash - Conceptualization, reviewing and editing
Susan R. George - Conceptualization, reviewing and editing
Anne S. Bassett - Conceptualization, data curation, data collection/investigation, methodology, writing, reviewing and editing
Funding
This work was supported in part by the Canadian Institutes of Health Research (CIHR) [MOP-313331 and MOP-111238 to A.S.B.], and the Clinician-Scientist Program at the University of Toronto [L.V.]; A.S.B. holds the Dalglish Chair in 22q11.2 Deletion Syndrome at the University of Toronto and University Health Network.
Acknowledgments
The authors thank the patients and their families for their participation, colleagues for referring patients, and research assistants, administrative staff and students who assisted in the collection of data for the study. The authors also thank the staff at the Toronto Region Statistics Canada Research Data Centre for their support of this project.
Footnotes
Funding: in part by the Canadian Institutes of Health Research (CIHR) [MOP-313331 and MOP-111238 to A.S.B.], as well as the Dalglish Chair in 22q11.2 Deletion Syndrome [A.S.B].
Supplementary material associated with this article can be found in the online version, at doi:10.1016/j.eclinm.2020.100528.
Appendix. Supplementary materials
References
- 1.Gloyn A.L., Drucker D.J. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6:891–900. doi: 10.1016/S2213-8587(18)30052-4. [DOI] [PubMed] [Google Scholar]
- 2.Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes. 2019;12:1047–1056. doi: 10.2147/DMSO.S179793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mefford H.C., Clauin S., Sharp A.J. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am J Hum Genet. 2007;81:1057–1069. doi: 10.1086/522591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford K., Bracher-Smith M., Owen D. Medical consequences of pathogenic CNVs in adults: analysis of the UK biobank. J Med Genet. 2019;56:131–138. doi: 10.1136/jmedgenet-2018-105477. [DOI] [PubMed] [Google Scholar]
- 5.Fung W.L., Butcher N.J., Costain G. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genet Med. 2015;17:599–609. doi: 10.1038/gim.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voll S.L., Boot E., Butcher N.J. Obesity in adults with 22q11.2 deletion syndrome. Genet Med. 2017;19:204–208. doi: 10.1038/gim.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald-McGinn D.M., Sullivan K.E., Marino B. 22q11.2 deletion syndrome. Nat Rev Dis Primer. 2015;1:15071. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer L.D., Butcher N.J., Boot E. Elucidating the diagnostic odyssey of 22q11.2 deletion syndrome. Am J Med Genet A. 2018;176:936–944. doi: 10.1002/ajmg.a.38645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malecki S.L., Van Mil S., Graffi J. A genetic model for multimorbidity in young adults. Genet Med. 2020;22:132–141. doi: 10.1038/s41436-019-0603-1. [DOI] [PubMed] [Google Scholar]
- 10.Van L., Heung T., Graffi J. All-cause mortality and survival in adults with 22q11.2 deletion syndrome. Genet Med. 2019;21:2328–2335. doi: 10.1038/s41436-019-0509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Rivera E., Liu Y.P., Verbitsky M. Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med. 2017;376:742–754. doi: 10.1056/NEJMoa1609009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher N.J., Kiehl T.R., Hazrati L.N. Association between early-onset Parkinson disease and 22q11.2 deletion syndrome: identification of a novel genetic form of Parkinson disease and its clinical implications. JAMA Neurol. 2013;70:1359–1366. doi: 10.1001/jamaneurol.2013.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Canada Clinical Practice Guidelines Expert Committee. Punthakee Z., Goldenberg R., Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 15.Diabetes Canada Clinical Practice Guidelines Expert Committee. Ekoe J.M., Goldenberg R., Katz P. Screening for diabetes in adults. Can J Diabetes. 2018;42(Suppl 1):S16–S19. doi: 10.1016/j.jcjd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Statistics Canada. Canadian health measures survey (CHMS). 2019. https://www.statcan.gc.ca/eng/survey/household/5071#info2019 (Accessed 12 August 2019).
- 17.Pani L.N., Korenda L., Meigs J.B. Effect of aging on A1c levels in individuals without diabetes: evidence from the Framingham offspring study and the national health and nutrition examination survey 2001-2004. Diabetes Care. 2008;31:1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 19.Hackinger S., Prins B., Mamakou V. Evidence for genetic contribution to the increased risk of type 2 diabetes in schizophrenia. Transl Psychiatry. 2018;8:252. doi: 10.1038/s41398-018-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berberich A.J., Huot C., Cao H. Copy number variation in GCK in patients with maturity-onset diabetes of the young. J Clin Endocrinol Metab. 2019;104:3428–3436. doi: 10.1210/jc.2018-02574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lascar N., Brown J., Pattison H., Barnett A.H., Bailey C.J., Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen J., Skadhede S., Correll C.U. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–2004. doi: 10.1038/npp.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemminki K., Liu X., Forsti A., Sundquist J., Sundquist K., Ji J. Subsequent type 2 diabetes in patients with autoimmune disease. Sci Rep. 2015;5:13871. doi: 10.1038/srep13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guna A., Butcher N.J., Bassett A.S. Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. J Neurodev Disord. 2015;7:18. doi: 10.1186/s11689-015-9113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stark K.L., Xu B., Bagchi A. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E., Steffes M.W., Zhu H. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jesus Ascencio-Montiel I., Pinto D., Parra E.J., Valladares-Salgado A., Cruz M., Scherer S.W. Characterization of large copy number variation in Mexican type 2 diabetes subjects. Sci Rep. 2017;7:17105. doi: 10.1038/s41598-017-17361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleynen I., Engchuan W., Hestand M.S. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion. Mol Psychiatry. [Epub ahead of print] 2020 doi: 10.1038/s41380-020-0654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.