Abstract
This review provides an overview of studies and case reports of neurological and neuromuscular complications associated with severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and coronavirus disease 2019 (COVID-19) and describes the possible mechanisms of viral transmission to the central nervous system (CNS). Coronavirus family has shown central and peripheral nervous system tropism in multiple retrospective studies and case reports from different parts of the world. To date, the reported cases of neurological and neuromuscular complications associated with coronaviruses, especially COVID-19, are increasing. Neurological and neuromuscular symptoms and complications ranging from headache and anosmia to more severe encephalitis and stroke have been reported in many studies. However, the neurotropism mechanism of coronaviruses is still not clear and the evidence of central nervous system (CNS) involvement is limited despite the number of studies that attempted to illustrate the possible CNS invasion mechanisms. The reported neurological complications of coronaviruses are summarized in this article.
Electronic supplementary material
The online version of this article (10.1007/s42399-020-00589-2) contains supplementary material, which is available to authorized users.
Keywords: Neurological complications, COVID-19, SARS, MERS, Encephalopathy, Stroke
Introduction
Coronaviruses are enveloped, positive-sense RNA viruses with large genomes [1]. These viruses are members of the Coronaviridae family within the order Nidovirales. Six strains of coronavirus have been identified as human pathogens [2]. These include HCoV-229E, HCoV-OC43, HCoV-HKU1, HCoV-NL63, severe acute respiratory syndrome CoV (SARS-CoV), Middle East respiratory syndrome, and more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronaviruses are mainly respiratory pathogens that cause a wide range of upper and lower respiratory tract infections with various neurological complications that will be highlighted in this review paper.
Understanding the virological background of these different viral infections might facilitate learners’ perception of the disease’s prognosis and management. SARS-CoV is an acute respiratory infection that was first reported in November 2002 in Guangdong Province [3]. It spread to other regions in America, Asia, and Europe in late 2003, infecting about 8000 people worldwide, with a mortality rate of 10%. By June 2012, a new strain of coronavirus called the Middle East respiratory syndrome coronavirus (MERS-CoV) emerged from Jeddah, Saudi Arabia [4]. MERS-CoV seems to have originated from bats and infected the intermediary reservoir (the dromedary camel) before being transmitted to humans [2]. Similarly to SARS-CoV, MERS-CoV causes severe respiratory infections that are complicated by multiorgan failure and death in some patients [2]. At the end of January 2020, a total of 2519 cases had been identified worldwide, with 866 associated deaths (34.3%) [4]. Most of the cases were reported in the Arabian Peninsula.
In December 2019, the novel coronavirus—severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—was isolated from a man who presented with severe symptoms of pneumonia in Wuhan, China. Later the WHO called the disease COVID-19 and declared that the pandemic was a public health emergency of international concern [5]. As of the end of August 2020, COVID-19 had infected about 25 million persons and killed around 800,000 patients [6]. The clinical features of COVID-19 are fever, non-productive cough, and fatigue; other symptoms might include headache, hemoptysis, and dyspnea [5]. Table 1 provides a comparison of the three viruses in terms of their epidemiology, morphogenesis, and pathogenesis.
Table 1.
Comparison between the three viruses (SARS-CoV, MERS-CoV, and COVID-19) in terms of their epidemiology, morphogenesis, and pathogenesis
| COVID-19 | MERS-CoV | SARS-CoV | |
|---|---|---|---|
| Epidemiology | |||
| Date of emergence | December 2019 [5] | June 2012 [4] | November 2002 [3] |
| Area of emergence | Wuhan, China [5] | Jeddah, Saudi Arabia [4] | Guangdong, China [3] |
| Animal host | Bats [7] | Camels and bats [7] | Bats, palm civets, and raccoon dogs [7] |
| Human–human transmission | + [8] | + [8] | + [8] |
| Nosocomial transmission | + [8] | + [8] | + [8] |
| Risk to healthcare workers | + [8] | + [8] | + [8] |
| Morphogenesis | |||
| Nature of the genome | Single-stranded positive-sense RNA genomes, linear, unimolecular, infectious, 26–32 kb in length, capped, polyadenylated, and structurally polycistronic [7, 9–14] | ||
| Presence of an envelope | + [7, 9, 11, 12, 15] | ||
| Morphology | Spherical, bacilliform, decorated with large (15–20 nm) surface projections, crown-shaped appearance [7, 9, 11] | ||
| Virion size | Spherical: 120–160 nm; bacilliform: 75–90 nm * 170–200 nm [9, 13] | ||
| Genome size | 29.9 kb [16] | 27.9 kb [13] | 30.1 kb [13] |
| Family | Coronaviridae [7, 9, 11] | ||
| Subfamily | Orthocoronaviridae [13, 15] | ||
| Genus | Betacoronavirus [7, 9, 10, 13] | ||
| Subgenus | Sarbecovirus [12, 15] | Merbecovirus [13] | Sarbecovirus [15] |
| Essential structural proteins | Spike (S) glycoprotein, small envelope (E) protein, matrix (M) protein, and nucleocapsid (N) protein [10, 11] | ||
| Coding strategy | Two-thirds of the viral RNA is translated into two large polyproteins, and the remainder of the viral genome is transcribed into a nested set of subgenomic mRNAs [10] | ||
| Pathogenesis | |||
| Cellular infection | Binding of spike protein to angiotensin-converting enzyme 2 (ACE2) [7, 12] | Binding of spike protein to dipeptidyl peptidase-4 [7] | Binding of spike protein to angiotensin-converting enzyme 2 (ACE2) [7, 12] |
| Virulence factor | Spike (S) glycoprotein, others: nonstructural protein 1 (nsp1), nsp3, nsp7, nsp17, ORF3a, E protein, M protein, and the nucleocapsid protein [9, 12, 13, 17, 18] | ||
ACE2 angiotensin-converting enzyme 2, COVID-19 coronavirus disease 2019, MERS-CoV Middle East respiratory syndrome coronavirus, mRNA messenger RNA, nsp nonstructural protein, ORF3a open reading frame 3a, RNA ribonucleic acid, SARS-CoV severe acute respiratory syndrome coronavirus
In addition, infections caused by coronaviruses have also been associated with extrapulmonary complications involving renal, hematological, and hepatological manifestations [2]. Moreover, many case reports have shown that these infections were also complicated with central nervous system (CNS) disorders and were associated with a wide range of neurological symptoms in some patients. Growing evidence has suggested that coronaviruses might have the ability to invade the central nervous system and cause various neurological complications. Padda et al. reviewed the neurological complications of COVID-19 with extensive discussion on the proposed pathophysiology and prognosis [19]. Previous review paper focused on neurological complications and the utility of using certain inflammatory markers such as interleukin-6 and C-reactive protein or coagulation tests such as D-dimer, prothrombin time, and antithrombin III as a prognostication tool for disease severity. In this review, we described viral transmission to the CNS and provided a comprehensive review of the neurological complications and their reported management that were published in regard to different viral infections such as SARS-CoV, MERS-CoV, and COVID-19.
Viral Transmission to the CNS
Viruses have been known to have neuroinvasive and neurotropic properties that allow them to invade the CNS through various mechanisms and infect neurons including glial cells [2, 20, 21]. This eventually can trigger or be involved in the development of neurological sequelae showing neurovirulent properties. Previous data reported the ability of some respiratory viruses, such as respiratory syncytial virus (RSV), measles virus (MV), and influenza virus, to induce neurological complications.
Human coronaviruses have attracted interest over the years since various pathological strains have appeared, including SARS-CoV, MERS-CoV, and recently COVID-19. Human coronaviruses tend to be neuroinvasive; previous data has reported the detection of coronaviral RNA in human brain samples. Possible mechanisms of coronavirus neuroinvasiveness include two main mechanisms: hematogenous dissemination and neuronal retrograde dissemination. Hematogenous dissemination occurs secondary to viremia, which could trigger a systemic inflammation response syndrome (SIRS) with or without a cytokine storm [18, 22]. This inflammatory response may create transcellular, paracellular, or intracellular penetration mechanisms through the blood–brain barrier (BBB). The transcellular route of hematogenous dissemination is induced by infecting the leukocytes and/or phagocytes in the bloodstream that subsequently become a viral reservoir for viral dissemination reaching the CNS. The paracellular route will be through the facilitation of high degree viremia and SIRS to pass through the BBB using its tight junctions. The intracellular route may be induced by high degree viremia and cytokine storm in addition to cellular proteases.
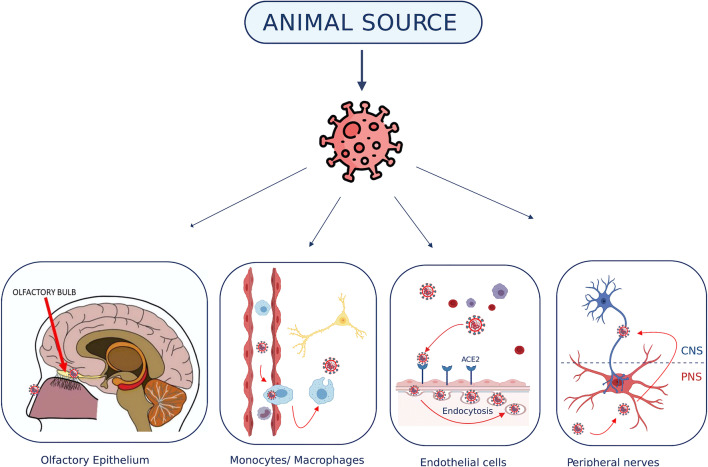
Neuronal retrograde dissemination is the second main route of viral invasion to the CNS [2, 19–21]. This mechanism happens after viruses invade the periphery neurons and enter the CNS through the active transports of these periphery neurons. For instance, the HCoV-OC43 has been observed to first invade and infect the olfactory bulb and olfactory nerve following intranasal infection and then disseminate eventually in the CNS of mice. In addition to neuroinvasive and neurotropic properties, human coronaviruses were known to potentially have neurovirulent effect. Several reports have been published and shown a clinical correlation with the human coronaviruses and cases of neurological pathologies like encephalitis and acute disseminated encephalomyelitis (ADEM) [2, 19, 23–26]. Various neurological transmission mechanisms are illustrated in Fig. 1.
Fig. 1.
The possible mechanisms of coronavirus transmission to the central nervous system. (1) Primary olfactory epithelium neurons and the olfactory bulb. (2) Infected monocytes/macrophages can cross the BBB and infect the neural cells. (3) Coronavirus interaction with ACE2 receptors on the endothelial cells of the BBB. Lastly, trans-synaptic transmission between PNS and CNS neurons. ACE2 angiotensin-converting enzyme 2, BBB blood‑brain barrier, CNS central nervous system, PNS peripheral nervous system. This figure was created using the website https://app.biorender.com
Neurological Complications of Coronaviruses (SARS-CoV, MERS-CoV, and COVID-19)
Neurological complications secondary to viral infections could be induced by infectious or non-infectious pathophysiology. Each neurological disease state will be discussed in detail based on a summary of the reported data for SARS-CoV, MERS-CoV, and COVID-19 infections.
Encephalopathy, Encephalitis, and Meningitis
Encephalopathy has been reported as a presenting symptom or manifestation of COVID-19 in 67 cases [27–40]. The first case of encephalopathy was reported by Poyiadji et al. of a 58-year-old female patient presenting with fever, cough, and altered mental status who found to be positive for COVID-19 [27]. Her imaging was consistent with an acute necrotizing encephalopathy, but cerebrospinal fluid (CSF) testing for COVID-19 was not possible, and patient was treated with intravenous immunoglobulin (IVIG) with no reported outcomes. Varatharaj et al. conducted wide surveillance that included 114 patients with neurological complications of COVID-19, of whom 16 (41%) reported to have encephalopathy [28]. Furthermore, Chen et al. conducted a retrospective case series on 113 COVID-19 patients and reported that 24 of them developed hypoxic encephalopathy, 23 died, while only 1 of them recovered [33]. Moreover, a case report by Dixon et al. describes a 59-year-old female patient with a history of transfusion-dependent aplastic anemia who presented with neurological symptoms and seizure [34]. Her overall imaging was consistent with acute necrotizing encephalopathy. The patient was given ceftriaxone, acyclovir, amoxicillin, clarithromycin, and high-dose dexamethasone; however, the team decided to withdraw care after patient showed an extremely poor prognosis with no neurological improvement, and she died on the 10th day after admission.
Encephalitis and meningitis were reported in SARS-CoV, MERS-CoV, and COVID-19 patients in nearly 39 cases [25, 26, 28, 31, 41–47]. Arabi et al. reported a case of a 45-year-old male with multiple comorbidities who presented with fever, respiratory symptoms, and diarrhea associated with MERS-CoV [25]. His hospital stay was complicated by severe shock, acute kidney injury (AKI), and severe acute respiratory distress syndrome (ARDS). The patient subsequently developed encephalitis, but the CSF was negative for MERS-CoV. The patient was treated with peginterferon alpha-2b and ribavirin and was discharged 107 days after admission. In 2017, Li et al. published a case series of 183 hospitalized children with suspected encephalitis; SARS-CoV was detected in 22 (12.02%) patients [26]. However, the study did not report the presence of the virus in the CSF. Recently, Ye et al. reported a case of encephalitis associated with COVID-19 infection [42]. The patient presented with alteration of consciousness, confusion, and meningeal irritation signs; however, the CSF RT-PCR was negative for COVID-19. The patient was treated with umifenovir and mannitol and was discharged with full recovery 31 days after admission. Moreover, Pilotto et al. reported a case of a previously healthy 60-year-old male who presented with mild respiratory symptoms and developed severe encephalitis [43]. The patient was started on antiviral therapy and antibiotics; however, due to the limited response after 3 days of therapy, high-dose methylprednisolone 1 g/day for 5 days was initiated, then oral prednisone with continuous tapering. The patient improved clinically after the first dose of steroids, and he showed a normal neurological exam and was discharged on day 11. McAbee et al. described another case of COVID-19 encephalitis in an 11-year-old child who presented with status epilepticus, requiring four antiepileptics [44]. The child recovered in 6 days without any treatment. Nevertheless, Moriguchi et al. reported a case of meningoencephalitis associated with COVID-19 in a 24-year-old male who first experienced fever, sore throat, and neurological symptoms, including headache, generalized fatigue, transient generalized seizures, and neck stiffness [47]. His neurological function deteriorated and presented to the hospital after 9 days from symptom onset with Glasgow coma score (GCS) of 6. The nasopharyngeal swab was negative for COVID-19 RNA; however, the CSF analysis was positive for COVID-19. This case is showing the neuroinvasive potential of COVID-19 and the fact that we cannot rule out the infection based on a negative nasopharyngeal swab.
Acute Disseminated Encephalomyelitis
Although ADEM is a rare immune-mediated demyelinating condition, it was reported in MERS-CoV and COVID-19 [25, 48]. Arabi et al. reported a case of a 74-year-old male with multiple comorbidities presented with 3 days history of ataxia and confusion [25]. The physical examination revealed dysmetria and decreased motor power on the left side. Virology testing was positive for MERS-CoV, and his imaging was consistent with ADEM. He was treated with broad-spectrum antibiotics, oseltamivir, bronchodilators, methylprednisolone, sedation, neuromuscular blockers, inhaled nitric oxide, vasopressors, peginterferon alpha-2b, and ribavirin. However, his condition aggravated, and he died on day 34 of admission. Moreover, Zhang et al. reported a case of ADEM associated with COVID-19 in a female in her early 40s with multiple comorbidities [48]. The patient presented with dysarthria, aphasia and headache, bulbar impairment, and inability to control secretions. The patient was treated with hydroxychloroquine, ceftriaxone, and IVIG. Later, the patient improved clinically.
Seizures
Seizure was reported as a neurological manifestation of coronaviruses in 27 cases, and most of them were associated with encephalopathy, encephalitis, or meningitis [26, 29, 31, 32, 34, 36, 37, 40, 41, 47, 49–53]. Hung et al. reported the first case of SARS-CoV-associated seizure in a female patient with IgA nephropathy [49]. The patient had episodes of four-limb twitching, confusion, disorientation, and prolonged seizure (> 30 min) despite phenytoin administration. The virology analysis detected SARS-CoV RNA in her CSF. The patient was treated with ribavirin and pulse steroids, but her seizures continued. Nevertheless, resolution of seizures was achieved after administering extra doses of propofol and phenytoin. Likewise, Lau et al. reported a case of a pregnant lady with SARS-CoV [50]. On day 22 of her illness, the patient developed generalized tonic-clonic convulsion with loss of consciousness for 1 min [50]. Reverse transcription polymerase chain reaction (RT-PCR) of the CSF showed positive for SARS-CoV. The patient was treated with hydrocortisone, ribavirin, morphine, and midazolam, and patient was extubated on day 27 of admission. For MERS-CoV infection, Saad et al. conducted a prospective case series on 70 patients with MERS-CoV and reported that 6 (8.5%) of them had seizure [53]. Hepburn et al. reported focal seizures in two patients with COVID-19 encephalopathy, but the seizure was subsided by administering levetiracetam [29]. Another case of COVID-19-associated seizure was reported by Moriguchi et al., with a CSF RT-PCR positive for COVID-19 [47]. Additionally, in a retrospective case series of COVID-19 patients conducted by Mao et al., they reported seizure in 1 patient characterized by a sudden onset of limb twitching, and loss of consciousness for 3 min [52].
Stroke
Stroke has been identified in coronavirus-infected patients in several published articles [24, 25, 32, 35, 52, 54–78]. Most of the reported cases were associated with COVID-19, three articles for MERS-CoV, and one article for SARS-CoV. The proposed mechanism of causing stroke, as described in most of the articles, specifically in COVID-19 articles, depends on several factors [79–82]. The mechanism likely involves endothelial dysfunction, immune-mediated injury leading to cytokine storm and coagulation abnormalities, and hypoxia-induced injury. For SARS-CoV, a Singaporean study by Umapathi et al. found five out of 206 SARS-CoV-infected patients developed large-artery ischemic stroke secondary to their viral infection [74]. The study also reported that four of the five infected patients became critically ill, and three of them died. Three of the infected patients were treated with IVIG, which may play a role as cofounder since IVIG can induce and participate in thrombosis through increasing viscosity, especially in patients who have a hypercoagulable state or are at high risk for vascular disease [74, 83]. MERS-CoV can also lead and contribute to stroke, and we identified in the literature three published articles by Algahtani et al., Arabi et al., and Al-Hameed describing three cases of stroke [24, 25, 75]. The first case was a 34-year-old diabetic patient diagnosed with intracerebral hemorrhage [24]. The patient experienced the worst outcome, developing multiorgan failure followed by signs of irreversible brain stem dysfunction and ending with death. The second case was a 57-year-old patient with comorbidities, including diabetes, hypertension, and peripheral vascular disease, who was diagnosed with bilateral anterior cerebral artery stroke [25]. This patient also had the worst outcome, involving severe shock, acute kidney injury, multiple cardiac arrests, and eventually death. The last case was a 42-year-old obese patient with a new diagnosis of type 2 diabetes mellitus who developed a massive spontaneous intracranial hemorrhage (ICH) after having MERS-CoV infection [75]. The case was complicated, and the patient died after developing intra-ventricular extension and tonsillar herniation. The reporting of stroke cases in MERS-CoV and SARS-CoV was limited in the literature. We believe this may be due to the likelihood of underreporting of stroke cases or the limited ability of MERS-CoV and SARS-CoV to induce extensive inflammatory and hypercoagulable states as compared to the COVID-19 infection.
The majority of the reported stroke cases in the literature were related to COVID-19 infection [32, 35, 52, 54–73, 76–78]. Furthermore, most of the COVID-19 patients who experienced stroke had an elevated D-dimer level [32, 52, 57, 60, 62, 63, 66, 68, 76–78]. A retrospective Chinese study showed that advanced age, a high sequential organ failure assessment (SOFA) score, and an elevated D-dimer level (more than 1 μg/mL) were associated with the increased in-hospital mortality of COVID-19 patients [84]. The severity of the COVID-19 infection was also linked with the incidence of stroke [32, 52, 62, 72, 78]. According to previous reports, strokes seem to occur more in severely and critically infected patients [32, 52, 62, 72, 78]. Additionally, Mao et al.’s study found that acute cerebrovascular events, including stroke, manifested more in severely infected patients compared to non-severe patients (5.7% vs. 0.8%, p = 0.03) [52]. The study assumed that the high incidence of acute cerebrovascular events was because of the elevated D-dimer level in severely infected patients (0.9 mg/L vs. 0.4 mg/L, p = <0.001). Based on stroke types, ischemic stroke was the predominant type of stroke in COVID-19 patients [32, 52, 54, 57, 59–64, 66, 72, 73, 76–78]. Benussi et al. found that the incidence of cerebrovascular events among 56 COVID-19 patients was approximately 76.8%; most of them had an ischemic stroke, and only 7.0% had hemorrhagic stroke [54]. Another study of retrospective case series reported all six infected patients presented with ischemic stroke except for two patients who were diagnosed with hemorrhagic stroke [32]. Interestingly, there is one report published about a COVID-19 patient diagnosed with cerebral venous sinus thrombosis (CVST) [55]. The patient was obese, with a medical history of diabetes and hypertension. The patient received low molecular weight heparin (LMWH) for CVST management. The patient showed improvement after receiving LMWH and then switched to apixaban, 10 mg twice a day for 7 days, at discharge. The etiology of this patient having CVST was most likely due to the COVID-19 virus’s ability to induce hypercoagulopathy, with a hypercoagulable state being considered one of the risk factors for CVST development.
Neuromuscular Disorders
Multiple reports have documented the link between coronaviruses and neuromuscular disorders. To date, there have been 23 reported cases of Guillain-Barre syndrome (GBS) associated with COVID-19 infections [85–101]. These case reports indicate the following results. CSF analysis revealed albumin-cytological dissociation in most patients; the polymerase chain reaction assay was negative for COVID-19 in all patients. Nineteen patients were treated with IV immunoglobulins—eight received a dose of 0.4 g/kg for 5 days, and two received a dose of 2 g/kg for 3‑5 days based on the dosing strategy—while information about IVIG dosing was not available in other patients. After treatment with IVIG, outcomes were reported for 14 patients. Four patients fully recovered, four patients were discharged to the neurorehabilitation unit to receive physical therapy, five patients were critically ill, and one patient died. In two patients, therapeutic plasma exchange was started after IVIG administration due to inadequate response, and two other patients received a second course of IVIG [91, 100]. In contrast, two patients were not given any specific therapy for managing the GBS symptoms, and they showed full recovery [89, 92]. Moreover, Caamaño et al. reported a case of a 61-year-old male with a diagnosis of COVID-19 who presented with symptoms of atypical GBS [93]. His GBS symptoms were managed with low-dose oral prednisone and showed mild improvement after 2 weeks of follow-up. Before the COVID-19 pandemic, coronavirus-associated GBS was reported in only two cases [85, 102]. The first was a man with MERS-CoV who had Bickerstaff’s encephalitis overlapping with Guillain-Barré syndrome and was treated with IVIG with full recovery. Another patient had respiratory infections caused by HCoV-OC43 complicated with atypical GBS.
Other types of polyneuropathy were also reported with SARS-CoV, MERS-CoV, and COVID-19 [24, 85, 103–106]. In SARS-CoV, a prospective case series from Taiwan reported four patients who developed neuromuscular symptoms mainly consistent with either critical illness polyneuropathy (CIP) or a myopathy with elevated creatinine kinase [103]. All of those patients had received a high dose of steroid therapy, and three of them were intubated and admitted to the ICU prior to the development of neurological symptoms. Other case reports from South Korea and Saudi Arabia presented four patients with MERS-CoV who had a diagnosis of ICU-acquired weakness, toxic or infectious neuropathies, and critical illness polyneuropathy [24, 85]. Patient with CIP was managed with IVIG 0.4 g/kg daily for 5 days and was slowly improving after 6 months of follow-up [24]. The remaining three patients were only treated with antivirals consisting of pegylated interferon, ribavirin, and lopinavir/ritonavir and made full recovery [85]. In the COVID-19 pandemic, a case report presented a 69-year-old male patient who developed bilateral lower limb weakness, numbness in both legs, and gait ataxia 7 days prior to the typical symptoms of COVID-19, which was consistent with motor peripheral neuropathy [104]. There was no specific treatment given, and the patient had a spontaneous recovery. Another case report from Iran reported a 68-year-old female who developed generalized hypotonia in her lower extremities and bilateral weakness 6 days after the onset of COVID-19 [105]. The patient was considered to have a virus-related immune reaction, and the neurologist decided to administer methylprednisolone. After 3 days of treatment, the patient developed acute respiratory distress syndrome followed by cardiac arrest and subsequently died. Direct causality between the neuromuscular disorders and coronavirus infection is not established yet, and both para- and post-infectious mechanisms were proposed. As mentioned before, coronavirus infection might cause immune system dysregulation, activation of macrophages (cytokine storm), and lymphocyte alterations leading to autoimmune damage to the peripheral nervous system [2, 20, 21]. Further studies and investigations should be conducted to better understand the mechanism of neuromuscular disorders in coronavirus infection to help improve the management of these conditions.
Anosmia and Dysgeusia
Patients infected with COVID-19 commonly presented with sensory symptoms, including anosmia and ageusia. According to the initial finding of Kaye et al., 73% of 237 patients had anosmia prior to the diagnosis of COVID-19, and anosmia was the first symptom in around 26% of the patients [107]. Other cross-sectional studies from different countries reported an incidence of anosmia ranging between 33.9 and 68%, with predominance among females in comparison to males [108]. Another finding from Meng et al. revealed a predominance of the chemosensory dysfunction in European and North American countries while it was rarely reported in China. These findings suggest that chemosensory dysfunction could be a prominent and critical sign for the early detection of COVID-19 infections. The study performed by Lechien et al. analyzed the frequency of olfactory and gustatory dysfunctions in 417 patients with confirmed COVID-19 infection, identifying that 85.6% had olfactory dysfunction with 79.6% being anosmic while the remaining 20.4% were hyposemic [109]. In addition, it was discovered that 63% of the patients who had the chemosensory dysfunction were females, and they were significantly more affected than males (p = 0.001). The treatment most commonly used for managing the olfactory dysfunction were nasal saline irrigations in 16.7% of the patients followed by nasal corticosteroids in 8.1%, oral corticosteroids in 2.5%, and others including vitamins, non-corticoid decongestants, and trace elements in about 2.5%. Moreover, this study reported that 88.0% of the patients had gustatory dysfunctions and were managed in only 1.4% of the patients with l-carnitine or trace elements and vitamins. However, most of the chemosensory dysfunctions caused by COVID-19 were reversible and spontaneously treated.
Unlike in COVID-19, anosmia and dysgeusia were reported rarely in MERS-CoV and SARS-CoV infections. Only one case report from Taiwan presented a 27-year-old female who had complete anosmia 3 weeks after her almost complete recovery from the SARS-CoV infection [110]. Her anosmia was managed with a nasal spray steroid and oral vitamin B12, but with no improvement and her complete anosmia persisted during 2 years of follow-up. Based on the current literature, there are no case reports of permanent anosmia in COVID-19 patients, and longitudinal studies are insufficient to prove these findings. The summary of the reported neurological complications associated with SARS-CoV, MERS-CoV, and COVID-19 is provided in the supplementary table.
Conclusion
In conclusion, SARS-CoV, MERS-CoV, and COVID-19 tend to have neuroinvasive properties. COVID-19 has more neurological complications than SARS-CoV and MERS-CoV, considering the risk of reporting bias about the COVID-19 pandemic. Awareness about these pathological coronaviruses’ neurological properties can help identify at-risk patients and take the necessary measures to prevent the development of neurological complications while guiding the appropriate management.
Electronic Supplementary Material
(DOCX 78 kb).
Authors’ Contributions
A Alshaya contributed to the design and idea of this study. R Alshouimi, M Alshebri, and H Alhumidi substantially contributed in the drafting of manuscript. All authors equally participated in the editing and reviewing of the final manuscript.
Data Availability
All data generated during this study are included in this published article and its supplementary information files.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Munirah Saad Alshebri, Email: Alshebrimu@hotmail.com.
Reema Abdulaziz Alshouimi, Email: r.alshouimi@hotmail.com.
Hadeel Aqeel Alhumidi, Email: Alhumidi.ha@outlook.com.
Abdulrahman I. Alshaya, Email: shayaab@ksau-hs.edu.sa
References
- 1.McIntosh K, Perlman S. Coronaviruses, including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Mand Douglas Bennetts Princ Pract Infect Dis. 2015:1928–1936.e2. 10.1016/B978-1-4557-4801-3.00157-0.
- 2.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:1–28. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 4.Middle East respiratory syndrome coronavirus (MERS-CoV). n.d. https://www.who.int/emergencies/mers-cov/en/ (accessed July 22, 2020).
- 5.Rothan HA, Byrareddy SN. The epidemeology and pathogensis of coronavirus (Covid-19) outbreak. J Autoimmun. 2020;109:1–4. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus disease (COVID-19) situation reports. n.d. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed July 25, 2020).
- 7.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. 2020. 10.1007/s00134-020-05955-1. [DOI] [PMC free article] [PubMed]
- 9.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds.). Family iridoviridae. In: Virus taxon. San Diego: Elsevier Academic Press; 2012. p 193–210.
- 10.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orenstein JM, Banach BS, Baker SC. Morphogenesis of coronavirus HCoV-NL63 in cell culture: a transmission electron microscopic study. Open Infect Dis J. 2008;2:52–58. doi: 10.2174/1874279300802010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabassum Hakim S, Tilghman Boyd F, Angel de Soto J. The pathophysiology of virulence of the COVID-19 virus, Prepr. 2020. 10.20944/preprints202004.0077.v1.
- 13.Li X, Luk HKH, Lau SKP, Woo PCY. Human coronaviruses: general features. Ref Modul Biomed Sci. 2019. 10.1016/b978-0-12-801238-3.95704-0.
- 14.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Coronaviruses methods protoc. Springer, New York; 2015. p. 1–23. 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed]
- 15.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19:100682. doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antivir Res. 2018;149:58–74. doi: 10.1016/j.antiviral.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020. 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed]
- 19.Padda I, Khehra N, Jaferi U, Parmar MS. The neurological complexities and prognosis of COVID-19. SN Compr Clin Med. 2020:1–12. 10.1007/s42399-020-00527-2. [DOI] [PMC free article] [PubMed]
- 20.Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desforges M, Le Coupanec A, Brison É, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miner JJ, Diamond MS. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. 2015;38:18–23. 10.1016/j.coi.2015.10.008. [DOI] [PMC free article] [PubMed]
- 23.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113. 10.1542/peds.113.1.e73. [DOI] [PubMed]
- 24.Algahtani H, Subahi A, Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. 2016;2016:1–6. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, Saeed BT, Wahbi A, Saedy A, AlDabbagh T, Okaili R, Sadat M, Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, Wang C, Song Z, Li S, Li X, Lv X, Qu X, Huang R, Liu W. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2017;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296:201187–20E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020. 10.1016/s2215-0366(20)30287-x. [DOI] [PMC free article] [PubMed]
- 29.Hepburn M, Mullaguri N, George P, Hantus S, Punia V, Bhimraj A, et al. Acute symptomatic seizures in critically ill patients with COVID-19: is there an association? Neurocrit Care. 2020:1–5. 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed]
- 30.Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)–associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020. 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed]
- 31.Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26. 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed]
- 32.Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267:1–8. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 368(2020). 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed]
- 34.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, Luqmani A, Jenkins IH, Nicholas R, Jones B, Everitt A. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7:789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-COV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12. 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed]
- 37.Farhadian S, Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, Zhou J, Odio C, Vijayakumar P, Geng B, Fournier J, Bermejo S, Fauver JR, Alpert T, Wyllie AL, Turcotte C, Steinle M, Paczkowski P, Dela Cruz C, Wilen C, Ko AI, Ko AI, MacKay S, Grubaugh ND, Spudich S, Barakat LA. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi M, Sahashi Y, Baba Y, Okura H, Shimohata T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415:116941. doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franceschi AM, Ahmed O, Giliberto L, Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. Am J Neuroradiol. 2020;41. 10.3174/ajnr.a6595. [DOI] [PMC free article] [PubMed]
- 40.Haddad S, Tayyar R, Risch L, Churchill G, Fares E, Choe M, et al. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020;21. 10.1016/j.idcr.2020.e00814. [DOI] [PMC free article] [PubMed]
- 41.Bernard-Valnet R, Pizzarotti B, Anichini A, Demars Y, Russo E, Schmidhauser M, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27. 10.1111/ene.14298. [DOI] [PMC free article] [PubMed]
- 42.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020. 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed]
- 43.Pilotto A, Odolini S, Stefano Masciocchi S, Comelli A, Volonghi I, Gazzina S, Nocivelli S, Pezzini A, Focà E, Caruso A, Leonardi M, Pasolini MP, Roberto Gasparotti R, Francesco Castelli F, Ashton NJ, Blennow K, Zetterberg H, Padovani A. Steroid-responsive encephalitis in Covid-19 disease. Ann Neurol. 2020;88:423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109. 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed]
- 45.Packwood R, Galletta G, Tennyson J. An unusual case report of COVID-19 presenting with meningitis symptoms and shingles. Clin Pract Cases Emerg Med. 2020;4:316–5. doi: 10.5811/cpcem.2020.4.47557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87. 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed]
- 47.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang T, Rodricks MB, Hirsh E. COVID-19-associated acute disseminated encephalomyelitis: a case report. MedRxiv. 2020;2020.04.16.20068148. 10.1101/2020.04.16.20068148.
- 49.Hung ECW, Chim SSC, Chan PKS, Tong YK, Ng EKO, Chiu RWK, Leung CB, Sung JJY, Tam JS, Lo YMD. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sohal S, Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20. 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed]
- 52.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- 53.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MAA, Al Mutairi M, Al Nakhli D, Aidaroos AYA, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020. 10.1212/WNL.0000000000009848. [DOI] [PubMed]
- 55.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7:001691. doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tejada Meza H, Lambea Gil Á, Sancho Saldaña A, Villar Yus C, Pardiñas Barón B, Sagarra Mur D, Marta Moreno J. Ischaemic stroke in the time of coronavirus disease 2019. Eur J Neurol. 2020;27:1788–1792. doi: 10.1111/ene.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.González-Pinto T, Luna-Rodríguez A, Moreno-Estébanez A, Agirre-Beitia G, Rodríguez-Antigüedad A, Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischemic stroke and SARS-COV2 infection. Eur J Neurol. 2020;1:1–2. doi: 10.1111/ene.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T, Mouchtouris N, Philipp L, Peiper SC, Wang ZX, Rincon F, Tjoumakaris SI, Jabbour P, Rosenwasser RH, Gooch MR. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91:1–3. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 59.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020;18:2031–2. doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020;12:648–653. doi: 10.1136/neurintsurg-2020-016220. [DOI] [PubMed] [Google Scholar]
- 61.Kihira S, Schefflein J, Chung M, Mahmoudi K, Rigney B, Delman BN, Mocco J, Doshi A, Belani P. Incidental COVID-19 related lung apical findings on stroke CTA during the COVID-19 pandemic. J Neurointerv Surg. 2020;12:669–672. doi: 10.1136/neurintsurg-2020-016188. [DOI] [PubMed] [Google Scholar]
- 62.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, Sanger M, Kim S, Scher E, Dehkharghani S, Wachs M, Tanweer O, Volpicelli F, Bosworth B, Lord A, Frontera J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020;51:1–4. doi: 10.1161/strokeaha.120.030153. [DOI] [PubMed] [Google Scholar]
- 64.Escalard S, Maïer B, Redjem H, Delvoye F, Hébert S, Smajda S, Ciccio G, Desilles J-P, Mazighi M, Blanc R, Piotin M. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19. Stroke. 2020;51:1–4. doi: 10.1161/strokeaha.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudilosso S, Laredo C, Vera V, Vargas M, Renú A, Llull L, Obach V, Amaro S, Urra X, Torres F, Jiménez-Fàbrega FX, Chamorro Á. Acute stroke care is at risk in the era of COVID-19. Stroke. 2020;51:1991–1995. doi: 10.1161/strokeaha.120.030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Co COC, Yu JRT, Laxamana LC, David-Ona DIA. Intravenous thrombolysis for stroke in a COVID-19 positive Filipino patient, a case report. J Clin Neurosci. 2020;77:5–7. doi: 10.1016/j.jocn.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegler JE, Heslin ME, Thau L, Smith A, Jovin TG. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center: cover title: falling stroke rates during COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104953. doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:1–3. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191. 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed]
- 71.Zayet S, Klopfenstein T, Kovẚcs R, Stancescu S, Hagenkötter B. Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg Infect Dis. 2020;26. 10.3201/eid2609.201791. [DOI] [PMC free article] [PubMed]
- 72.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H. C or r e sp ondence Coagulopathy and antiphospholipid antibodies in patients with Covid-19. Nejm. 2020;38:1–3. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deliwala S, Abdulhamid S, Abusalih MF, Al-Qasmi MM, Bachuwa G. Encephalopathy as the sentinel sign of a cortical stroke in a patient infected with coronavirus disease-19 (COVID-19). Cureus. 2020;19. 10.7759/cureus.8121. [DOI] [PMC free article] [PubMed]
- 74.Umapathi T, Kor AC, Venketasubramanian N, Lim CCT, Pang BC, Yeo TT, Lee CC, Lim PL, Ponnudurai K, Chuah KL, Tan PH, Tai DYH, Ang SPB. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Hameed FM. Spontaneous intracranial hemorrhage in a patient with Middle East respiratory syndrome corona virus. Saudi Med J. 2017;38:196–200. doi: 10.15537/smj.2017.2.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–1. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020;382. 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed]
- 78.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jäger HR, Losseff NA, Perry RJ, Shah S, Simister RJ, Turner D, Chandratheva A, Werring DJ. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:8–11. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. Coronavirus disease 2019 and the cerebrovascular-cardiovascular systems: what do we know so far? J Am Heart Assoc. 2020;9. 10.1161/jaha.120.016793. [DOI] [PMC free article] [PubMed]
- 81.Archie SR, Cucullo L. Cerebrovascular and neurological dysfunction under the threat of COVID-19: is there a comorbid role for smoking and vaping? Int J Mol Sci. 2020;21. 10.3390/ijms21113916. [DOI] [PMC free article] [PubMed]
- 82.Boukhris M, Hillani A, Moroni F, Annabi MS, Addad F, Ribeiro MH, Mansour S, Zhao X, Ybarra LF, Abbate A, Vilca LM, Azzalini L. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dalakas MC, Clark WM. Strokes, thromboembolic events, and IVIg: rare incidents blemish an excellent safety record. Neurology. 2003;60:1736–1737. doi: 10.1212/01.WNL.0000074394.15882.83. [DOI] [PubMed] [Google Scholar]
- 84.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, Ahn JY, Kim MK, Choi JP. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ottaviani D, Boso F, Tranquillini E, Gapeni I, Pedrotti G, Cozzio S, Guarrera GM, Giometto B. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41:1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25:204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;241. 10.1212/WNL.0000000000009619. [DOI] [PubMed]
- 90.Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020. 10.1212/WNL.0000000000009700. [DOI] [PubMed]
- 91.Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, Aufenanger J, Nowak-Machen M, Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. 2020;267:1883–1884. doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ebrahimzadeh SA, Ghoreishi A, Rahimian N. Guillain-Barré syndrome associated with the coronavirus disease 2019 (COVID-19). Neurol Clin Pract. 2020;2019. 10.1212/CPJ.0000000000000879. [DOI] [PMC free article] [PubMed]
- 93.Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, Viganò M, Giovannelli G, Pirro F, Montisano DA, Appollonio I, Ferrarese C. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:1–4. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce Guillain–Barré syndrome. Rev Neurol (Paris). 2020;176. 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed]
- 97.Padroni M, Mastrangelo V, Asioli GM, Pavolucci L, Abu-Rumeileh S, Piscaglia MG, Querzani P, Callegarini C, Foschi M. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020;267:1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Virani A, Rabold E, Hanson T, Haag A, Elrufay R, Cheema T, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20. 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed]
- 99.El Otmani H, El Moutawakil B, Rafai MA, El Benna N, El Kettani C, Soussi M, et al. Covid-19 and Guillain-Barré syndrome: more than a coincidence! Rev Neurol (Paris). 2020. 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed]
- 100.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2577. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coen M, Jeanson G, Culebras Almeida LA, Hübers A, Stierlin F, Najjar I, Ongaro M, Moulin K, Makrygianni M, Leemann B, Kronig I, Bertrand J, Reny JL, Schibler M, Serratrice J. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. 2020;87:111–112. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma K, Tengsupakul S, Sanchez O, Phaltas R, Maertens P. Guillain–Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: a case report. SAGE Open Med Case Rep. 2019;7:2050313X1983875. doi: 10.1177/2050313x19838750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, Chang SC, Chang YC. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 104.Abdelnour L, Eltahir Abdalla M, Babiker S. COVID 19 infection presenting as motor peripheral neuropathy. J Formos Med Assoc. 2020;119:1119–1120. doi: 10.1016/j.jfma.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghiasvand F, Ghadimi M, Ghadimi F, Safarpour S, Hosseinzadeh R, SeyedAlinaghi SA. Symmetrical polyneuropathy in coronavirus disease 2019 (COVID-19) IDCases. 2020;21:e00815. doi: 10.1016/j.idcr.2020.e00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Freitas Ferreira ACA, Romão TT, Silva Macedo Y, Pupe C, Nascimento OJM. COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27:1748–1750. doi: 10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163:132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 108.Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol Head Neck Med Surg. 2020;41:102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;2. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed]
- 110.Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15:26–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 78 kb).
Data Availability Statement
All data generated during this study are included in this published article and its supplementary information files.