Abstract
Adolescents with attention-deficit/hyperactivity disorder (ADHD) are at high risk for tobacco use, but tobacco use prevention strategies are not regularly incorporated into evidence-based ADHD interventions. We conducted a pilot randomized-controlled trial to determine the feasibility of integrating tobacco use prevention skills into a behavioral treatment for ADHD and to provide preliminary efficacy data comparing a combined (ADHD + tobacco) intervention (N = 40) to an ADHD only intervention (N = 23) on tobacco risk outcomes. Sixty-three adolescents (72% male; 13–17 years) with ADHD and their caregivers were randomly assigned to condition and families were masked to condition. Parent and adolescent ratings were collected at baseline, immediate post-intervention, and at 3- and 9-month follow-up assessments. The combined intervention was (1) implemented with high fidelity (94%), (2) well received by parents and adolescents as evidenced by high levels of treatment attendance (82%) and satisfaction with the intervention, and (3) associated with parent- and adolescent-reported reductions in tobacco use risk. Relative to the ADHD intervention, the combined intervention buffered against increases in tobacco risk, including reduced intentions to smoke and maladaptive social normative beliefs, and increased parental control, family cohesion, and family communication about substance use. Effect sizes at post-treatment were in the small to moderate range. Overall, this study provides preliminary support for a parent-adolescent behavioral treatment supplemented with family-based tobacco prevention strategies. This approach targets families already in treatment for ADHD, reducing barriers that occur when families attend multi-session prevention programs in addition to ADHD treatment.
Keywords: Attention-deficit/hyperactivity disorder, Prevention, Intervention, Adolescence, Tobacco, Family strategies
Tobacco use is associated with a host of significant negative health outcomes such as cancer, stroke, and lung diseases (U.S. Department of Health and Human Services 2014). Many of these health problems are the result of unhealthy behavioral choices (e.g., smoking, drinking alcohol) that are typically formed during the adolescent and young adult years. It is well established that adolescents with attention-deficit/hyperactivity disorder (ADHD) are at higher risk of tobacco use (especially cigarettes) and initiate tobacco use at younger ages compared to those without ADHD (Dunne et al. 2014; Molina et al. 2013). They also progress to heavier use more rapidly (Sibley et al. 2014b). This contributes to high rates of comorbidity between ADHD and tobacco use during emerging adulthood (Dvorsky and Langberg 2019; Mitchell et al. 2018) and adulthood (Lee et al. 2011; Wilens et al. 2011). This is concerning given that 11% of children ages 4–17 years (6.4 million children) have been diagnosed with ADHD at some point in their lives, with 8.8% currently diagnosed (Visser et al. 2014). Yet, adolescents with ADHD are seldom targeted for tobacco use prevention efforts. Moreover, ADHD medications may not reduce adolescents’ tobacco use (Molina et al. 2013) and evidence-based behavioral treatments for ADHD do not target tobacco use prevention, and have not demonstrated sustained effects on tobacco use in this population. Thus, there is an urgent need for innovative approaches that prevent the initiation of tobacco use or progression to heavier use for adolescents with ADHD.
Although family-based prevention programs have shown promise in reducing adolescent tobacco use in the general population (Spoth et al. 2015; Spoth et al. 2006), families of adolescents with ADHD are most likely to initiate treatments focused on addressing educational impairments (DuPaul and Langberg 2014). Moreover, the feasibility of families attending multiple programs is questionable. An understudied approach is to implement tobacco use prevention skills in settings where families are already engaged and receiving treatments. To our knowledge, no study to date has integrated evidence-based tobacco use prevention skills into an existing behavioral treatment for ADHD as a method of preventing adolescents’ risk of initiating tobacco use or escalating to heavier use.
Tobacco Use and Prevention among Youth with ADHD
In the Multimodal Treatment Study (MTA), which followed 579 children diagnosed with ADHD into young adulthood, adolescents with ADHD were more likely than adolescents without ADHD (36% vs. 17%) to be daily smokers at the 16-year follow-up (mean age of 24.9 years) and they progressed more rapidly from smoking initiation to daily smoking (Mitchell et al. 2018). Importantly, ADHD symptoms are uniquely responsible for the early emergence of tobacco, alcohol, and illicit drug use and predict substance use to a greater degree than childhood antisocial behaviors (Molina and Pelham 2003). In one of the first studies to examine ADHD risk for electronic cigarette (e-cigarette) use, Dvorsky and Langberg (2019) found that ADHD symptoms in high school predicted increases in e-cigarette use throughout the first year of college. The presence of ADHD symptoms also significantly increases adolescents’ risk of and progression to developing a substance use disorder (Lee et al. 2011; Molina et al. 2013). Of particular concern is that adolescents with ADHD are four to five times more likely to escalate to heavier cigarette and marijuana use after trying the substance only once compared to adolescents without ADHD (Sibley et al. 2014b). This substance use poses enormous health risks for adolescents with ADHD and can negatively impact brain development.
Much of the research examining why ADHD confers risk for tobacco and substance use has implicated that difficulties with attention, impulsivity, and poor decision-making skills via mediating cognitive (e.g., attitudes, coping skills) and social (e.g., parent/peer modeling) influences (Glass and Flory 2010 for a review). Social influences including peer and family factors have a significant role in the development of tobacco and substance use problems for adolescents with ADHD (Molina and Pelham 2014; Wilens et al. 2008). For example, Dvorsky and Langberg (2019) demonstrated that adolescents with ADHD symptoms tend to perceive greater degrees of peer use and social desirability of use, increasing their risk for reduced inhibition behaviors and subsequent tobacco use.
Although multiple evidence-based treatments for children with ADHD exist (Evans et al. 2018), none specifically target or are sufficiently effective at preventing adolescent tobacco use (Molina and Pelham 2014). Pharmacological treatment, the most common treatment for ADHD (Visser et al. 2014), can produce marked improvement in ADHD symptoms (Pliszka 2007), but these improvements often do not translate into reductions in tobacco use. Moreover, the effect of medication on tobacco use is mixed with some studies showing medication provides protective effect for tobacco use (Groenman et al. 2013; Schoenfelder et al. 2014) and other studies reporting no impact (Humphreys et al. 2013; Molina et al. 2013).
The other most commonly delivered treatments for ADHD are behavioral treatments, such as behavioral parent training (BPT; Fabiano et al. 2009), but few studies have explored whether behavioral treatments prevent or reduce adolescent tobacco use (Schoenfelder and Kollins 2014). BPT during early childhood for children with ADHD has led to reductions in early adolescent tobacco use (Molina et al. 2007); however, these differences were not sustained into later adolescence (Molina et al. 2013). This is likely because ADHD behavioral treatments studied to date have not made any specific attempt to focus on tobacco use prevention.
Integrating Tobacco Prevention Skills into an Evidence-Based ADHD Intervention
Since behavioral treatments for ADHD are widely available in the community (Loren et al. 2015) and many parents of adolescents with ADHD seek treatment for their adolescents’ academic problems (DuPaul and Langberg 2014), a parent-adolescent behavioral treatment that targets academic functioning may be an ideal context for delivering tobacco use prevention strategies. Supporting Teens’ Academic Needs Daily (STAND; Sibley et al. 2013; Sibley et al. 2014a) is a collaborative behavioral treatment that targets empirically identified adolescent (e.g., organizational problems) and parent (e.g., effective contingency management) mechanisms shown to predict positive long-term outcomes. STAND has been implemented in weekly individual therapy with adolescents with ADHD and their parents and in groups (STAND-G) with multiple families. STAND has been evaluated in multiple randomized trials with middle and high school students and found to improve ADHD symptoms, organization, parent stress, and GPA out to 6-months follow-up (Sibley et al. 2013; Sibley et al. 2014a; Sibley et al. 2016).
To our knowledge, no existing tobacco use prevention programs have been implemented and evaluated with families of adolescents with ADHD. Yet, prevention programs have been developed that target individual- and family-level risk factors associated with adolescent tobacco use and have demonstrated success in reducing tobacco use in adolescents without ADHD (Kumpfer et al. 2010; Spoth et al. 2015). One prevention program that targets parenting and family relationship factors that are associated with tobacco use is the Strengthening Families Program: For Parents and Youth 10–14 (SFP 10–14; Spoth et al. 2015; Spoth et al. 2006). Like STAND, the SFP 10–14 emphasizes parent and adolescent skill development, and provides families with opportunities to practice the skills they are taught. The SFP 10–14 Logic Model (see Supplementary File 1), which was used in the present study, specifies that the SFP 10–14 produces changes in proximal variables that are associated with decreased adolescent tobacco use such as adolescent skills (e.g., tobacco use refusal efficacy), parenting skills (e.g., risk behavior monitoring), and the family relationship (e.g., communication about substance use), which then lead to changes in distal outcomes (e.g., less tobacco use).
Importantly, there is considerable overlap between some of the content in SFP 10–14 and STAND (see Table 1), which facilitates the integration of tobacco prevention skills into STAND. For example, STAND includes sessions on general communication, problem solving, and using effective parenting skills, but none of this content is discussed in the context of preventing tobacco use. Further, SFP 10–14 teaches unique adolescent tobacco prevention skills (e.g., refusal self-efficacy) and parenting skills (e.g., communication about substance use) that could be incorporated into STAND. Outcome studies indicate that the SFP 10–14 has a positive impact in delaying or reducing adolescent tobacco use, and on mediators such as positive parent-child interactions and monitoring (Coatsworth et al. 2010; Spoth et al. 2015).
Table 1.
STAND-G Topics and SFP 10–14 Skills
| Parent Topic | Youth Topic | Family Topic | SFP 10–14 Skills | ||||
|---|---|---|---|---|---|---|---|
| Session 1 | Introduction to ADHD and it’s relation to youth tobacco and other substance use. | What is ADHD and it’s relation to youth tobacco and other substance use? | Discuss how ADHD symptoms impact academics and adolescent risk behaviors; help families build positive relationships | Psychoeducation about tobacco use | |||
| Session 2 | Basics of behavior management, including applications to tobacco use prevention | Problem solving, and teaching youth tobacco use refusal skills | Adolescent teaches parent problem solving steps and tobacco prevention skills |
|
|||
| Session 3 | Identifying target problems and parent expectations about adolescent tobacco use | Setting academic goals and other life goals | Create list of agreed upon problem areas and discuss parent and adolescent goals for the year, including parental expectations about tobacco use |
|
|||
| Session 4 | Monitoring of adolescent’s use of organizational skills and parental monitoring of tobacco related risk behaviors | Organizational skills | Devise a monitoring plan for planner use and school binder and bookbag organization and also for adolescent’s evening and weekend social activities. |
|
|||
| Session 5 | Homework Contract | Homework completion and study strategies | Teen teaches parent how to use homework to-do list and active studying techniques. | ||||
| Session 6 | Setting a daily routine, including parental strategies for monitoring their children’s tobacco related risk behaviors | Setting a daily routine | Parent and teen compare daily routine tasks and create a list of tasks to track for one week. Families practice tobacco monitoring and communication strategies. |
|
|||
| Session 7 | Developing a Home Privilege Program, including privileges based upon adherence to evening and weekend expectations (e.g. curfew) | Communication and Negotiation Training to develop a mutually agreed upon system of rewards and consequences (i.e. a privilege program). Following rules | Active Listening and Honest Expression role play exercise, including conversation about tobacco use |
|
|||
| Session 8 | Interfacing with Schools | Note-taking Training | Discuss level of communication that is needed between parent, teen, and teacher, including importance of ongoing family communication about tobacco use |
|
|||
Examples of components from the Strengthening Families Program 10–14 content integrated in bold
Given this strong evidence base, we integrated components of the SFP 10–14 into STAND-G to develop a combined ADHD and tobacco use prevention skills (TPS) treatment for adolescents with ADHD (STAND-G + TPS). This approach of integrating tobacco use prevention skills within the context of ADHD treatment has multiple advantages. For example, this approach targets adolescents and families already in treatment, potentially reducing barriers related to feasibility, acceptability, and cost that may occur when families are asked to attend multi-session prevention programs in addition to ADHD treatment.
The Current Study
We conducted a pilot randomized-controlled trial to determine the feasibility of implementing the STAND-G + TPS intervention, and to provide preliminary efficacy data comparing STAND-G + TPS to STAND-G alone on tobacco/substance risk outcomes. We chose to compare two active intervention conditions rather than making comparisons to a no-intervention condition. We examined the impact of STAND-G + TPS on factors that are associated with reduced adolescent tobacco use including adolescent skills, parenting skills, and the family relationship. Compared to STAND-G alone, we hypothesized that (a) STAND-G + TPS adolescents will report decreased intentions to smoke and maladaptive social normative perspectives of smoking, and increased tobacco/substance use refusal intentions, (b) STAND-G + TPS parents and adolescents will report increased parental monitoring or control of adolescents’ tobacco risk related behaviors, and (c) STAND-G + TPS parents and adolescents will report increased family cohesion and communication about substance use. Finally, we hypothesized that both conditions would be feasible to implement and acceptable to families as indicated by attendance and parent satisfaction.
Method
Participants
Participants were 63 adolescents between the ages of 13 to 17 (Mage = 15.01, SD = 1.16) with ADHD and their caregivers. Adolescents attended 27 different middle and high schools in central Virginia: 92.1% attended public school, 4.7% charter school, and 3.2% private school. Adolescents were recruited in three successive cohorts over two school years (i.e., one cohort each semester). There were no significant differences between the two intervention conditions on any of the adolescent/family demographic characteristics, adolescent’s medication status, baseline symptom severity, or co-occurring externalizing or internalizing disorders (see Table 2).
Table 2.
Adolescent demographic characteristics of the sample at baseline
| Variable | Total (N = 63) | STAND-G(N = 23) | STAND-G + TPS(N = 40) | Comparison | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | Statistica | p | |
| Gender (% male) | 45 | 71.4% | 18 | 78.3% | 27 | 67.5% | .83 | .36 |
| Grade at baseline | 2.69 | .61 | ||||||
| 8 | 11 | 17.5% | 3 | 13.0% | 8 | 20.0% | ||
| 9 | 21 | 33.3% | 6 | 26.1% | 15 | 37.5% | ||
| 10 | 17 | 27.0% | 7 | 30.4% | 10 | 25.0% | ||
| 11 | 11 | 17.5% | 6 | 26.1% | 5 | 12.5% | ||
| 12 | 3 | 4.8% | 1 | 4.3% | 2 | 5.0% | ||
| Age | M = 15.0 (SD = 1.2) | M = 15.3(SD = 1.3) | M = 14.8 (SD = 1.1) | −1.61 | .12 | |||
| Ethnicity (%Latinx) | 2 | 3.2% | 1 | 4.3% | 1 | 2.5% | .04 | .84 |
| Race | .57 | .75 | ||||||
| Black | 12 | 19.0% | 5 | 21.7% | 7 | 17.5% | ||
| White | 45 | 71.4% | 15 | 65.2% | 30 | 75.0% | ||
| Pacific Islander | 1 | 1.6% | 1 | 4.3% | 0 | 0.0% | ||
| Multiracial | 5 | 7.9% | 2 | 8.7% | 3 | 7.5% | ||
| STAND Diagnosis | .06 | .80 | ||||||
| ADHD-IN | 53 | 84.1% | 19 | 82.6% | 34 | 85.0% | ||
| ADHD-C | 10 | 15.9% | 4 | 17.4% | 6 | 15.0% | ||
| Inattention | M = 2.29 (SD = .49) | M = 2.21 (SD = .50) | M = 2.34 (SD = .48) | −1.02 | .31 | |||
| Hyperactivity/Impulsivity | M= .79 (SD = .73) | M= .72 (SD = .70) | M= .82(SD = .75) | −.63 | .53 | |||
| Co-occurring diagnoses | ||||||||
| ODD | 11 | 17.5% | 3 | 13.0% | 8 | 20.0% | .49 | .48 |
| CD | 1 | 1.6% | 0 | 0.0% | 1 | 2.5% | .58 | .45 |
| Anxiety disorder | 15 | 23.8% | 7 | 30.4% | 8 | 20.0% | .88 | .35 |
| Depressive disorder | 10 | 15.9% | 3 | 13.0% | 7 | 17.5% | .22 | .64 |
| Tobacco use | 7 | 11.1% | 1 | 4.3% | 6 | 15.0% | 1.68 | .19 |
| Substance use | 9 | 14.3% | 3 | 13.0% | 6 | 15.0% | .05 | .83 |
| WASI Estimated IQ | M= 106.5(SD = 13.2) | M= 104.2 (SD = 12.5) | M= 107.7(SD = 13.5) | .98 | .33 | |||
| WIAT Reading | M= 103.6 (SD = 10.8) | M= 103.4 (SD = 9.8) | M= 103.8 (SD = 11.5) | .11 | .91 | |||
| WIAT Math | M= 95.5 (SD = 16.1) | M = 99.1 (SD = 17.5) | M= 93.3 (SD = 15.0) | −.1.36 | .18 | |||
| Learning disability | 13 | 20.6% | 4 | 17.4% | 9 | 22.5% | 1.96 | .38 |
| Medicated | 33 | 52.4% | 11 | 47.8% | 22 | 55.0% | 1.28 | .26 |
| Parent Education | .43 | .51 | ||||||
| At least one parent with some college | 50 | 79.4% | 20 | 86.9% | 30 | 75.0% | ||
| Family income | 2.79 | .59 | ||||||
| Less than $50,000 | 7 | 11.1% | 1 | 4.3% | 6 | 15.0% | ||
| $50,000 to $74,999 | 14 | 22.2% | 7 | 30.4% | 7 | 17.5% | ||
| $75,000 to $99.999 | 9 | 14.3% | 3 | 13.0% | 6 | 15.0% | ||
| $100,000 to $149.999 | 15 | 23.8% | 6 | 26.1% | 9 | 22.5% | ||
| $150,000 or more | 18 | 28.6% | 6 | 26.1% | 12 | 30.0% | ||
Chi-square test for categorical variables and t-test for means; nonsignificant differences for all variables. ADHD-IN = Inattentive Presentation diagnosis. ADHD-C = Combined Presentation diagnosis. Medicated = medicated for ADHD; Learning disability = based on parent-report of prior diagnosis of a specific learning disability. WIAT = Wechsler Individual Achievement Test; WASI = Wechsler Abbreviated Scale of Intelligence for Children. STAND-G = Supporting Teens’ Academic Needs Daily condition. TPS = Tobacco Prevention Skills. Inattention and Hyperactivity/Impulsivity represent item means from the parent-rated Vanderbilt with scores ranging from 0 to 3
Procedures
Participants were recruited from local middle and high schools through flyers sent home and referrals from school counselors. Recruitment flyers contained descriptions of ADHD symptoms and academic impairments so that recruitment efforts were based on observed characteristics of students, rather than previous diagnoses alone. The recruitment flyers stated that parents and their adolescents have the opportunity to receive a free diagnostic assessment and if eligible, an intervention that addresses the academic impairments frequently exhibited by adolescents with ADHD. In this manner, we ensured that the sample is typical of families who frequently seek out treatment for ADHD rather than a sample specifically interested in an intervention specific to tobacco use prevention. Study flyers provided families with a number to call to receive more information about the study. When families called, research staff read a detailed description of the study to families and a phone screen was administered. In order to be scheduled for an inclusion/exclusion eligibility evaluation, parents had to endorse their child as currently exhibiting at least four of nine DSM–5 ADHD symptoms of inattention.
Criteria for inclusion in the study required that adolescents (a) were between 13 and 17 years of age; (b) met full DSM–5 diagnostic criteria for ADHD (i.e., six or more symptoms present within a domain, age of onset prior to 12, symptom related impairment in multiple settings, and symptoms not accounted for by another condition) based on the Parent Children’s Interview for Psychiatric Syndromes (P-ChIPS; Weller et al. 2000); (b) demonstrated IQ of 80 or above as estimated using the Wechsler Abbreviated Scale of Intelligence Scale for Children (WASI; Wechsler 1999); and (c) did not meet diagnostic criteria for a pervasive developmental disorder, bipolar disorder, or psychosis. Adolescents were also administered four subtests from the Wechsler Individual Achievement Test, Third Edition (WIAT-III; Wechsler 2009).
Inclusion evaluations were administered by clinical psychology graduate students who were observed multiple times before implementing the evaluations independently. Participant’s assessment data were then reviewed with a licensed clinical psychologist to determine eligibility and diagnoses. Adolescents who reported current and/or prior substance use, but who did not currently meet criteria for a substance use disorder (n = 16) were allowed to participate.
The intervention is focused on preventing the initiation of tobacco use and/or progression to heavier use, so adolescents who had a substance use disorder (including tobacco use disorder) were excluded (n = 1). Adolescents, both on and off ADHD medications, were eligible to participate. After providing written verbal consent and assent, baseline measures were completed at the time of the inclusion evaluations, before the intervention started. Participants who met inclusion criteria were invited to participate in the interventions. Participants completed measures at baseline, post-intervention and 3- and 9-month post-intervention. Families received $50 for completing the baseline and post measures, and $75 for the 3-and 9-month assessments. This study was approved by the Institutional Review Board at Virginia Commonwealth University.
Randomization
After completing the baseline measures, families were randomized to either STAND-G or STAND-G + TPS. Participants were stratified to treatment condition based upon ADHD medication status. In cohorts 1 (n = 14) and 2 (n = 29), students were randomly assigned to condition within cohort in a 1:1 ratio, however in the final cohort 3 (n = 20), all participants were assigned to the STAND-G + TPS condition, for a total of N = 23 who received STAND-G and N = 40 who received STAND-G + TPS (see Fig. 1). In order to conduct the most methodologically rigorous examination of the active manipulation (i.e., addition of SFP 10–14 components to STAND-G), parents and adolescents were not told that one of the two interventions included materials focused on tobacco use prevention. Upon completion of the 9-month assessment period, families were sent a letter that explained the full purpose of the study.
Fig. 1.
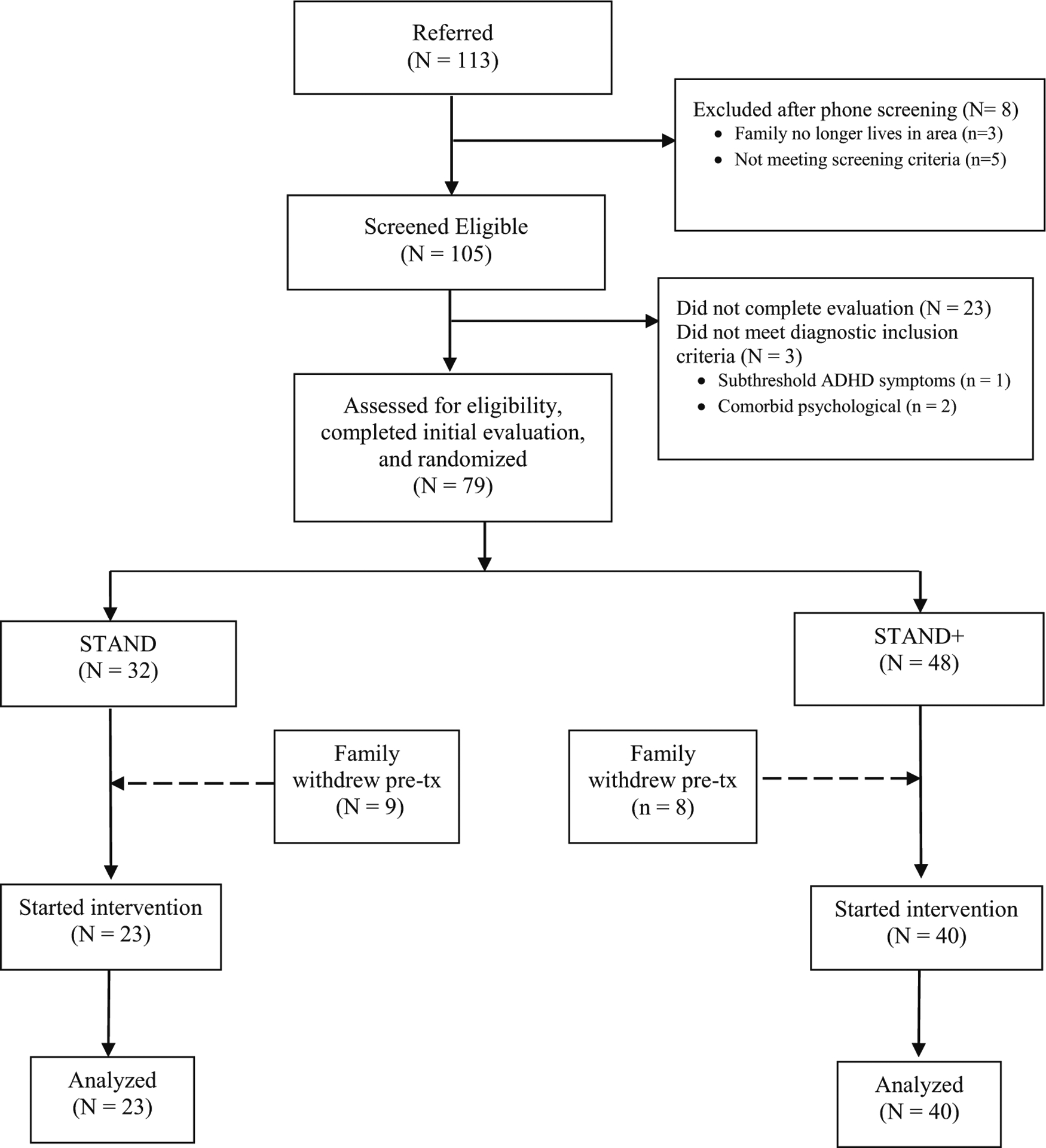
Study CONSORT diagram
Intervention Groups
Both groups were held after school or in the evenings at local high schools or in research lab space. Seven clinical psychology doctoral students facilitated the groups; 3 clinicians implemented both interventions. In supervision meetings, clinicians were reminded not to mention substance use in STAND-G only sessions. Supporting Teen’s Academic Needs Daily-Group (STAND-G) (Sibley et al. 2014a) consists of 8 weekly, 90-min sessions delivered in a group format, with 6 to 11 families per group. Sessions 1–2 build parent motivation for change through motivational interviewing, create a family-driven case conceptualization, and select modular session content through a treatment menu based on group consensus presenting problems. Sessions 3–6 are family-selected to allow for content individualization and aim to remediate organization, time management, and planning skills deficits and increase parent involvement in academics and behavioral monitoring. Sessions 7–8 enact a home academic contract to enhance adolescent motivation to use new skills, coordinating with the school as needed, and families review progress and discuss plans for continuing skill use without therapist support. Each session began with parents and adolescents together to introduce the program and to review homework. Parents and adolescents were then moved to separate groups. Adolescents learned skills to help with organization and academic skills, and parents were taught parenting skills. Parents and adolescents reunited at the end of the sessions for a collaborative activity. Table 1 shows where SFP 10–14 content was integrated into each STAND-G session. To ensure that both interventions were the same duration, we adjusted the amount of time allotted for activities/explanations in STAND-G so that we could add related SFP 10–14 skills and/or explain how STAND-G skills also apply to substance use prevention.
Measures
Treatment Fidelity and Attendance
Clinicians completed fidelity checklists and audiotaped each weekly group session. Research staff rated clinician adherence to 25% of the sessions using a dichotomously coded treatment fidelity checklist (each item rated as 1 = implemented or 0 = not implemented). Audio-recordings were grouped by intervention session and clinician and recordings were randomly selected to ensure that sessions from the beginning, middle, and end of the intervention program were equally represented. Research staff initially listened to audio-recorded sessions together to calibrate scoring and ensure interrater reliability. Research staff double-coded the selected sessions and at no point was agreement below 80%. This review also demonstrated that substance use was not mentioned in the STAND-G only sessions. Attendance for each group session was measured from sign-in sheets completed by clinicians or research assistants at the sessions.
Treatment Satisfaction
A 12-item satisfaction questionnaire developed for this study was completed by parents and adolescents. The majority of items assessed satisfaction related to specific components of the interventions. For example, STAND-G + TPS participants were asked to rate how well the intervention improved their awareness of strategies for helping their child resist pressure to smoke or use other substances. In addition, participants in both groups responded to more general questions about overall acceptability and satisfaction with the intervention. Parents and adolescents indicated their agreement with each statement on a 5-point scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree). The present study examined the mean across items, with higher scores represented greater satisfaction.
Tobacco and Substance Use
Adolescents completed items based on the Monitoring the Future Survey (Johnston et al. 2015) assessing the frequency of tobacco use, marijuana use, and prescription drug misuse in the past month (the last 30 days). For each item, the response categories were 1 = did not use, 2 = less than 1 time per day, 3 = once/day, 4 = 2 to 5 times/day, 5 = 6 to 10 times/day, 6 = 11 to 20 times/day, and 7 = more than 20 times/day.
Smoking Intentions and Susceptibility
Adolescents answered 6 items that assessed their intention to use tobacco products within the next six months (Mahabee-Gittens et al. 2011). Items were added to assess adolescents’ intentions to use newer types of tobacco products (e.g., e-cigarettes). Adolescents also rated their susceptibility to use e-cigarettes (Pepper et al. 2013). In the present study, smoking intentions (mean of 6 items; αs = .75–.93) were rated on a 4-point scale from 1 = Definitely not, 2 = Probably not, 3 = Probably will, 4 = Definitely will; and e-cigarette susceptibility (1 item) was rated on a reverse scale: 1 = Definitely yes to 4 = Definitely not. The smoking intention measure has been correlated with lower parent-adolescent conflict, and increased parental monitoring in a sample of adolescents (Mahabee-Gittens et al. 2011).
Tobacco and Substance Use Refusal Intentions
Adolescents’ refusal intentions were assessed with 7 items including items used in previous trials of SFP 10–14 (Redmond et al. 2009). We added items to measure refusal intentions of different types of tobacco products (e.g., e-cigarettes). Items were originally rated on a 5-point scale with 1 being Definitely would say “no” and 5 being Definitely would not say “no.” Items were reverse coded so that higher scores indicated greater levels of refusal intentions. The mean across items was used. These items have shown adequate reliability in prior SFP-10–14 evaluations, as well as being sensitive to change over the course of intervention (Redmond et al. 2009). In the present study, internal reliability for tobacco (αs = .87–.94) and other substance (αs = .74–.93) refusal intentions was adequate.
Maladaptive Social Normative Beliefs about Smoking
Using items from recent studies measuring adolescents’ perceptions of social acceptability, tolerance, and desirability of smoking (Gibson et al. 2018), adolescents answered 4 items pertaining to their social normative beliefs about using cigarettes and e-cigarettes. Items were rated on a 5-point scale ranging from 0 (definitely not) to 4 (definitely yes). The mean score was used as rating of maladaptive social normative beliefs about smoking in the present study (αs = .85–.90).
Parental Monitoring of Risk Behaviors
The Parental Practices Scale (24 items; Kerr and Stattin 2000) assesses the behaviors of parents and children that relate to parents’ awareness of their children’s activities. The present study examined the Parental Control (mean of 5 items) subscale, separately rated by parents and adolescents. The Parental Control subscale has been found to be negatively associated adolescent delinquency, school problems, and poor teacher-student and father-adolescent relationships (Kerr and Stattin 2000). Parents also rated 3 items assessing their involvement with their child. These items have demonstrated sensitivity to change over the course of intervention (Kumpfer et al. 2010). Items were assessed on a 5-point scale ranging from 1 = lower to 5 = higher control/involvement and the mean for each scale was used in analyses. Internal reliability was αs = .79–.87 (parent) and αs = .78–.86 (adolescent).
Parental Communication about Substance Use
Parents and adolescents rated 8 items assessing parental messages about substance use (Ennett et al. 2001). Participants rated how many times in the last six months they talked about substance use with one another ranging from 0 (0 times) to 3 (3 or more times). This scale has been associated with adolescent report of lifetime smoking and drinking escalation over a one-year period (Ennett et al. 2001). In the present study, a mean score across items was used with higher scores representing higher frequency of communication about substance use (parent αs = .94–.98, adolescent αs = .94–.97).
Family Relationship Factors Associated with Adolescent Tobacco Use
The cohesion subscale (20 items) of the Family Adaptability and Cohesion Evaluation Scale (FACES-III; Olson et al. 1985) assessed parent and adolescent perceptions of how likely family members are to do various behaviors representing family cohesion on a 1 (almost never) to 5 (almost always) scale. This subscale has demonstrated good internal reliability, face and content validity, and has been associated with more positive communication skills (Olson 1986). In the present study, internal consistency was αs = .90–.92 (parents) and αs = .94–.96 (adolescents).
Data Analysis
All statistical analyses were performed in SAS version 9.4. Analyses are based on intent-to-treat, including all participants who participated in the interventions (attended at least one session in either condition) using all available data for participants that were assigned to either the STAND-G or STAND-G + TPS (N = 63). Analyses compared outcomes between the two conditions across three separate domains of functioning that are associated with reduced adolescent tobacco use: (1) adolescent skills; (2) parenting skills; and (3) family relationships. To test for a Group by Time interaction from post-intervention to 9-months follow-up, baseline, post, 3-months follow-up and 9-months follow-up assessment waves were added to the model. Data were analyzed using hierarchical linear modeling via linear mixed effect model (PROC MIXED). The basic model includes group assignment, time, and the interaction between group and time. In addition, this modeling approach allowed us to account for the repeated measures obtained from each adolescent across time. We used a repeated statement to account for the correlation induced by data collected from the same subject in multiple occasions.
Analyses were conducted to determine if there were significant pre-to-post treatment changes for adolescents and parents within each group and whether these changes differed across groups (i.e., Condition × Time interaction effects). The analytic models treated time as a dummy-coded within-person variable such that the main effect for intervention condition represented differences at pretest, time represented preto-post changes, and Intervention × Time interactions represented intervention effects on pre-to-post changes in outcomes at each follow-up wave. The models controlled for conduct problems, sex, and age, including both their main effects and conduct, sex, and age differences in change over time. The Group by Time interaction was the main outcome of interest because a significant interaction would indicate that groups changed differently over time. Group by Time interaction outcomes are presented in Table 3. In addition, the potential impact of school and cohort effects was investigated, and there were no significant effects for any of the outcomes of interest. When the Group by Time interactions were significant, they were followed with post hoc contrasts comparing the least-squared mean estimates at post-intervention. The p values from post hoc analyses were corrected for multiple comparisons across all comparisons using false discovery rate procedures (FDR; Benjamini and Hochberg 1995). The p values reported are corrected values. In order to assess maintenance of treatment effects, these models were repeated using follow-up outcomes, and post-intervention and follow-up scores were compared within and between groups. Cohen’s d effect sizes were calculated using standardized mean difference scores to examine the magnitude of between group differences (Kline 2004). The magnitude of effects was interpreted based on Cohen’s (1988) general guidelines for small (d = .20), medium (d = .50), and large (d = .80) effects. Power is an issue in that effects as large as .40 were not statistically significant. As cautioned by Cohen (1988), statistical significance is not the only factor to consider when interpreting results.
Table 3.
Adolescent- and parent-reported tobacco and substance use preventive skills and family factors at baseline, post, and 3- and 9-month follow-up assessments
| Baseline | Post | 3-Month FU | 9-Month FU | F | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group × time effects: | Est.M | SE | Est.M | SE | Est.M | SE | Est. M | SE | ||
| Adolescent Skills | ||||||||||
| E-cigarette Susceptibility (A) | 2.31 | .07 | ||||||||
| STAND-G + TPS | 3.74 | .12 | 3.42 | .12 | 3.46 | .13 | 3.34 | .15 | ||
| STAND-G | 3.43 | .15 | 3.43 | .16 | 3.57 | .15 | 3.55 | .16 | ||
| Intentions to Smoke (A) | 3.25 | .02 | ||||||||
| STAND-G + TPS | 1.47 | .10 | 1.21 | .10 | 1.12 | .08 | 1.11 | .14 | ||
| STAND-G | 1.14 | .08 | 1.54 | .10 | 1.63 | .12 | 1.52 | .16 | ||
| Tobacco use refusal intentions (A) | 2.17 | .09 | ||||||||
| STAND-G + TPS | 4.86 | .09 | 4.76 | .09 | 4.86 | .11 | 4.86 | .13 | ||
| STAND-G | 4.66 | .12 | 4.57 | .12 | 4.55 | .12 | 4.52 | .12 | ||
| Substance use refusal intentions (A) | 2.53 | .06 | ||||||||
| STAND-G + TPS | 4.41 | .14 | 4.44 | .18 | 4.58 | .29 | 4.49 | .18 | ||
| STAND-G | 4.60 | .19 | 4.25 | .16 | 4.22 | .15 | 4.33 | .18 | ||
| Social normative beliefs about smoking (A) | 2.78 | .04 | ||||||||
| STAND-G + TPS | 3.71 | .08 | 3.41 | .10 | 3.43 | .11 | 3.34 | .11 | ||
| STAND-G | 3.70 | .11 | 3.74 | .08 | 3.80 | .10 | 3.82 | .11 | ||
| Parenting Skills | ||||||||||
| Parental Control (A) | 3.07 | .03 | ||||||||
| STAND-G + TPS | 4.09 | .12 | 4.22 | .14 | 4.23 | .17 | 4.39 | .16 | ||
| STAND-G | 4.12 | .16 | 4.10 | .13 | 4.12 | .15 | 3.78 | .17 | ||
| Parental Control (P) | .07 | .97 | ||||||||
| STAND-G + TPS | 4.80 | .06 | 4.85 | .07 | 4.81 | .08 | 4.69 | .09 | ||
| STAND-G | 4.83 | .09 | 4.84 | .09 | 4.78 | .09 | 4.67 | .09 | ||
| Involvement with teen (P) | 1.49 | .22 | ||||||||
| STAND-G + TPS | 4.57 | .07 | 4.72 | .10 | 4.54 | .08 | 4.65 | .09 | ||
| STAND-G | 4.58 | .09 | 4.49 | .08 | 4.52 | .10 | 4.51 | .10 | ||
| Communication about substance use (P) | 2.97 | .03 | ||||||||
| STAND-G + TPS | 2.00 | .10 | 2.19 | .10 | 2.16 | .10 | 2.29 | .12 | ||
| STAND-G | 2.06 | .13 | 1.75 | .14 | 2.00 | .13 | 1.97 | .13 | ||
| Communication about substance use (A) | 1.26 | .28 | ||||||||
| STAND-G + TPS | 2.77 | .12 | 2.79 | .12 | 2.76 | .14 | 2.72 | .16 | ||
| STAND-G | 2.93 | .16 | 2.75 | .17 | 2.45 | .16 | 2.66 | .16 | ||
| Family Factors | ||||||||||
| Family cohesion (A) | 2.01 | .11 | ||||||||
| STAND-G + TPS | 73.46 | 3.12 | 74.56 | 3.17 | 79.61 | 3.20 | 77.88 | 3.21 | ||
| STAND-G | 71.64 | 2.37 | 67.18 | 2.40 | 68.10 | 2.69 | 70.46 | 2.98 | ||
| Family cohesion (P) | 2.86 | .04 | ||||||||
| STAND-G + TPS | 81.90 | 1.47 | 85.04 | 1.97 | 86.56 | 1.98 | 85.55 | 1.99 | ||
| STAND-G | 82.07 | 1.94 | 79.34 | 1.48 | 79.13 | 1.64 | 79.01 | 1.81 | ||
All models controlled for the conduct problems, sex, and age as covariates including both their main effects and differences in change over time. A = Adolescent-rated. P = Parent-rated. STAND-G = Supporting Teens’ Academic Needs Daily condition. STAND-G + TPS = STAND-G and Tobacco Prevention Skills condition. The goal of this study was to incorporate tobacco and substance information without reducing the efficacy of STAND on the core constructs the intervention was designed to target, such as homework problems and parent-adolescent conflict. As such, we tested for group differences on several of the main measures from the original STAND intervention trials and no significant differences were found for any outcome: parent-rated academic executive functioning skills (Adolescents Academic Problems Checklist; Sibley et al. 2014), F = 0.46, p = .71, parent-rated homework problems (Homework Problems Checklist; Power et al., 2006), F = 0.44, p = .73, adolescent-rated parent-child conflict (CBQ; Robin & Foster, 1989), F = 0.89, p = .45, and parent-rated CBQ, F = 1.66, p = .18
Adolescent-reported data on at least one measure were available for all 63 adolescents at baseline, 60 at post-test, 54 at the 3-month follow-up, and 42 at the 9-month follow-up. Parent-reported data on at least one measure were available for 63 adolescents at baseline, 60 at post-test, 53 at the 3-month follow-up, and 42 at the 9-month follow-up. Treatment conditions did not differ in the number of caregiver report waves of data available (STAND-G: M = 3.65, STAND-G + TPS: M = 3.22), t(61) = 1.92, p = .06, d = .50) or the number of adolescent report waves of data available (STAND-G: M = 3.65, STAND-G + TPS: M = 3.23), t(61) = 1.87, p = .07, d = .49), although moderate effect sizes suggest STAND-G had moderately more waves of data collected relative to STAND-G + TPS. There were no significant differences between participants who completed post- or follow-up ratings relative to those available vs. missing data either post-treatment, 6-, or 9-month follow-up waves on: adolescent baseline ADHD symptom severity, externalizing or internalizing comorbidity, medication status, tobacco use, substance use, age, sex, race/ethnicity, family income, parent marital status, or parent/adolescent-reported treatment satisfaction. As such, missing data were addressed using multilevel modeling with full-information maximum likelihood estimates. This made it possible to examine changes across all four waves of data (i.e., baseline and three posttest waves) even if individuals were missing data at one or two of the post waves.
Results
Feasibility and Acceptability
Treatment Fidelity and Attendance
Adherence across sessions and clinicians was 91.8% for STAND-G and 94.2% for STAND-G + TPS, indicating clinicians implemented both interventions with high fidelity. When averaged separately for each session, fidelity by session ranged from 84% to 100% for STAND-G and from 86% to 100% for STAND-G + TPS. The average number of sessions attended was 6.87 (86%) in STAND-G and 6.59 (82%) STAND-G + TPS, which did not differ across conditions, t(61) = .62, p = .54.
Treatment Satisfaction
Parent and adolescent satisfaction with STAND-G was compared to STAND-G + TPS satisfaction. Parent and adolescent satisfaction were highly correlated for STAND-G (r = .78, p < .001) and STAND-G + TPS (r = .58, p < .001). T-test comparisons indicated that parents who received STAND-G + TPS expressed very high treatment satisfaction (M = 4.42, SD = .42), and this did not significantly differ from those in STAND-G (M = 4.30, SD = .61, t(60) = .89, p = .38, d = .24). Adolescents who received STAND-G + TPS were also satisfied with treatment (M = 3.89, SD = .61), but not significantly more so than STAND-G adolescents (M = 4.16, SD = .52, t(60) = −1.78 p = .08, d = .47).
Preliminary Treatment Effects
The intervention groups did not differ on baseline demographic and clinical characteristics (see Table 2). Overall, rates of adolescent tobacco use (4.3–15.0% at wave 1, 5.3–9.1% at wave 2, 6.9–9.1% at wave 3, and 5.3–13/6% at wave 4), substance use (13.0–15.0% at wave 1, 13.2–13.6% at wave 2, 9.1–13.8% at wave 3, and 9.1–15.8% at wave 4) were low. Given this low prevalence and that the primary focus of prevention, frequency of use was not explored in subsequent longitudinal mixed models. Using hierarchical linear modeling, we conducted a series of linear mixed effect models with each outcome variable as the dependent variable and group (STAND-G vs. STAND-G + TPS) as the between-subjects predictor and time (baseline, post, 3 months, 9 months) as the within-subjects predictor. To consider relative change between the groups, we also calculated a Cohen’s d effect sizes based on the mean baseline to post-treatment change in the STAND-G group minus the mean baseline to post-treatment change in the STAND-G + TPS group, divided by the pooled baseline standard deviation (Morris 2008).
Adolescent Skills Associated with Decreased Tobacco Risk
Table 3 displays the results of the group × time analyses of all adolescent outcomes including susceptibility, intentions, and social normative beliefs, controlling for sex, conduct problems, and age as covariates, including both their main effects and differences in change over time. There was a significant linear group × time intervention effect for reducing adolescents’ intentions to smoke and maladaptive social normative beliefs about smoking from baseline to follow-up (see Figure 2). These findings suggested that compared to STAND-G alone, participants who received STAND-G + TPS experienced greater reductions in tobacco risk over time. The magnitude of the difference in smoking intentions between STAND-G + TPS and STAND-G from baseline to post-intervention was large (t = −2.80, p = .005, d = .75). Although no longer significant, the magnitude of the difference in smoking intentions groups from post-intervention to 3-months follow-up (t = −1.94, p = .06, d = .53) and from 3-months to 9-months follow-up (t = −1.91, p = .06, d = .59) was moderate, and suggested that, compared to STAND-G alone, participants who received STAND-G + TPS had greater reductions in intentions to smoke over time. Group differences on social normative beliefs was moderate from baseline to post-intervention (t = −2.27, p = .03, d = .61); however, reduced from post to 3-months follow-up (t = −.45, p = .65, d = .12) and from 3- to 9-months follow-up (t = −1.20, p = .24, d = .37). Each represented a preventative effect such that adolescents in STAND-G + TPS did not show the significant increase in intentions to smoke or increase in maladaptive social norm beliefs found for adolescents in the STAND-G condition. Adolescents in the STAND-G + TPS condition demonstrated reduced e-cigarette susceptibility and stronger tobacco refusal intentions, although the group × time interactions were not significant (ps = .07 to .09). Post hoc analyses compared differences between groups at post-intervention and follow-up and revealed significant differences in the hypothesized direction between STAND-G + TPS and STAND-G, with effect sizes in the moderate range at post intervention (ds = .20 to .55) for stronger refusal intentions and reduced susceptibility, however group differences were reduced at follow-up waves.
Fig. 2.
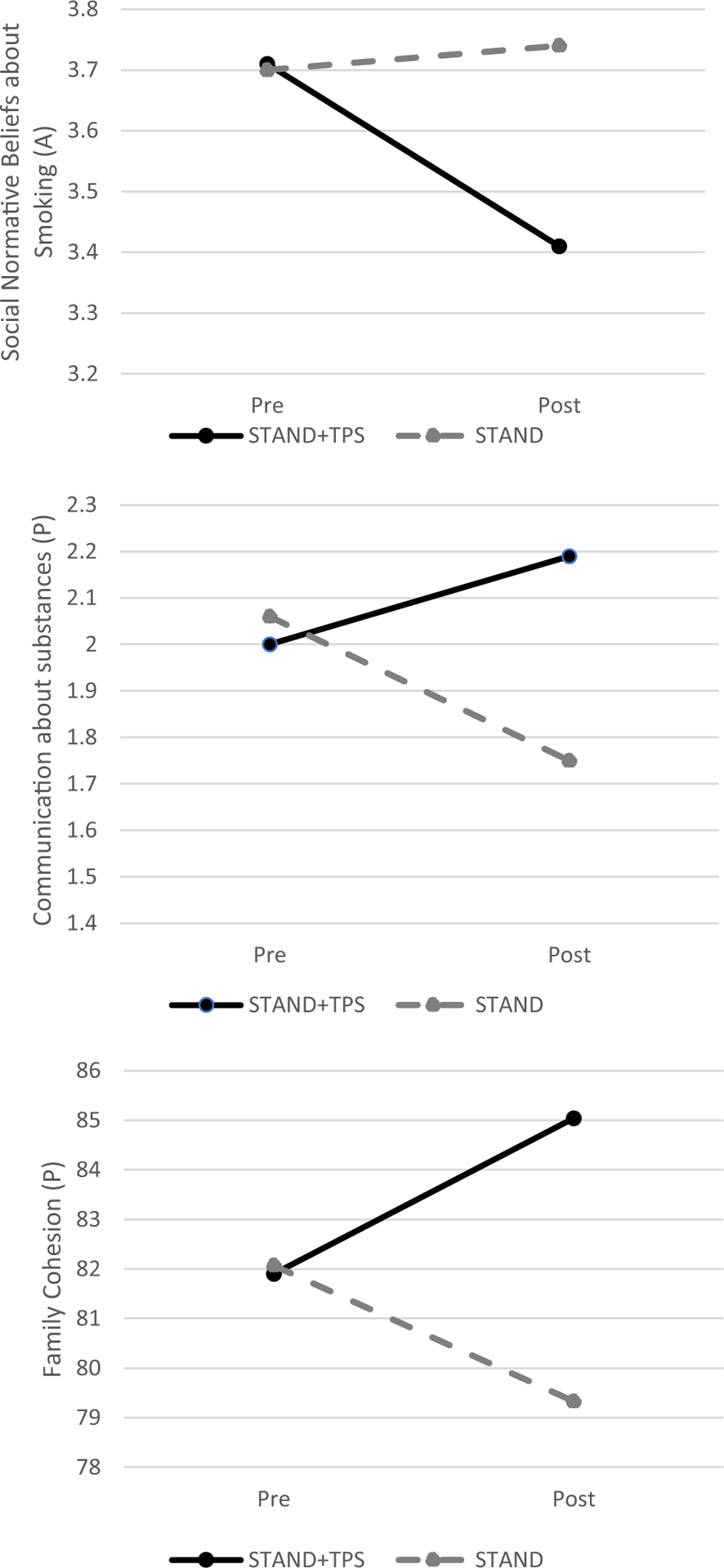
Group by time effects at baseline and post intervention
Parenting Skills Associated with Decreased Adolescent Tobacco Risk
There was also a significant group by time effects on the adolescent report of parental control in parenting practices and parent report of communication about substances (Table 3). Post hoc analyses revealed no significant differences between groups at immediate post-intervention (t = .35, p = .72, d = .10) and 3-month follow-up (t = .12, p = .90, d = .03), however significant differences were found in the hypothesized direction at the 9-month follow-up (t = 2.75, p = .006, d = .85). The magnitude of the difference between groups increased substantially with moderate preventative effects at 9-months follow-up. In contrast to adolescents in the STAND-G + TPS condition, those in the STAND-G condition reported a significant decrease in adolescent-rated parental control at the 9-month follow-up. Group differences on parent-reported communication about substance risk was moderate from baseline to post-intervention (t = 2.10, p = .03, d = .56); however reduced from post to 3-months follow-up (t = .84, p = .39, d = .23) and from 3- to 9-months follow-up (t = 1.16, p = .24, d = .36).
Family Relationship Factors Associated with Decreased Tobacco Risk
There was a significant group by time interaction for parent-rated family cohesion across waves. Post hoc analyses, found a significant effect favoring the STAND-G + TPS for enhancing family cohesion, with moderate effect sizes from baseline to post-intervention (t = 2.13, p = .03, d = .58) and from post to 3-months follow-up (t = 2.73, p = .007, d = .74). Although there was no significant between group differences on parent-rated family cohesion at 9-months follow-up, the magnitude of this difference was moderate (t = 1.84, p = .07, d = .55).
Discussion
This study evaluated the impact of two brief interventions for adolescents with ADHD. To reduce potential expectancy bias (Sonuga-Barke et al. 2013), parents and adolescents were masked to intervention condition and were not aware that one condition included tobacco prevention strategies, while the other condition did not. Parents and adolescents expressed equivalent satisfaction with both interventions, providing evidence that observed differences between STAND-G and STAND-G + TPS cannot be attributed to bias. Overall, this study provides preliminary support for a parent-adolescent treatment for academic impairment, STAND-G (Sibley et al. 2013, 2014a), supplemented with tobacco prevention strategies from the Strengthening Families Program 10–14 (SFP 10–14; Spoth et al. 2006). The combined intervention was 1) implemented by novice clinicians with high fidelity, 2) well received by parents and adolescents as evidenced by high levels of treatment attendance and satisfaction with the intervention, and 3) associated with parent- and adolescent-reported reductions in tobacco use risk behaviors and attitudes. Specifically, relative to STAND-G, the STAND-G + TPS buffered against increases in tobacco risk, including reduced adolescents’ intentions to smoke and maladaptive social normative beliefs, and increased parental control, and family cohesion, with small to moderate effect sizes.
On average, families attended 82–86% of the STAND-G and STAND-G + TPS sessions, which is typical for other family-based approaches to treating adolescents with ADHD (18–38% dropout; Barkley et al. 2001), and consistent with attendance rates in prior examinations of STAND in groups (Sibley et al. 2013, 2014a). In addition, parents and adolescents in both conditions maintained that group sessions were very helpful with satisfaction ratings (on a scale of 1 to 5) ranging from 3.9–4.2 for adolescents and 4.3–4.4 for parents across STAND-G and STAND-G + TPS. Once families engaged in the treatment, they displayed openness to the family-based approach, and learned a range of tools to promote academic success (e.g., organizational skills, study skills, conflict resolution) and families in the STAND-G + TPS group learned strategies for promoting tobacco use prevention (e.g., tobacco use refusal skills, parent monitoring of adolescent risk behaviors).
Treatment effects were present for adolescent- and parent-rated tobacco risk prevention outcomes. Significant effects were generally moderate in size (.52–.85), which is impressive given the use of an active control group who received the same dosage of intervention. Most prior examinations of the SFP 10–14 report modest effects for adolescents on similar outcomes of family cohesion and family communication (Kumpfer et al. 2010). Both adolescent and parent ratings suggested that families in the combined group improved their family cohesion, family communication, increased parent control, and reduced adolescent intentions to smoke as well as social normative beliefs about smoking during the intervention and throughout follow-up, while those who received STAND-G alone had declining or stable family and adolescent tobacco prevention skills. Post-hoc follow-up analyses suggested the greatest effects were present between baseline and post treatment, although the effect of family cohesion continued to increase up through 3-months follow-up. Despite these effects, the STAND-G + TPS group displayed only minor improvements in tobacco susceptibility and tobacco refusal intentions.
Our approach has several innovative aspects that are consistent with national recommendations for tobacco use prevention among adolescents (NCI Working Group Report 2016). First, we intervened with adolescents with ADHD who have not been targeted in tobacco use prevention trials but who are at high risk of tobacco use (Lee et al. 2011) and at risk of escalating to more frequent and heavier use of substances after trying a substance only once (Sibley et al. 2014b). In addition, our intervention focused on the use of alternative tobacco products among adolescents with ADHD. Finally, and perhaps most importantly, our approach of teaching tobacco prevention skills to adolescents with ADHD and their parents by integrating them into an existing evidence-based treatment for ADHD (e.g., a “stealth” intervention; NCI Working Group Report 2016) could significantly increase the likelihood that this high-risk group will be exposed to tobacco use prevention messages and strategies. In this pilot feasibility study, we were able to create changes in adolescent and parent behaviors related to tobacco use without lengthening the ADHD intervention. As noted above, adolescents with ADHD and their families frequently seek out and initiate treatment for school-related problems such as low and failing grades (DuPaul and Langberg 2014). Therefore, if effective tobacco use prevention strategies could be incorporated in a feasible manner, this could facilitate widespread dissemination to community clinics already serving adolescents with ADHD.
Limitations and Future Directions
There are a few limitations to this study. First, as a preliminary investigation, our sample size limited our statistical power. Therefore, some medium treatment effects were nonsignificant in some models. Second, we did not measure parents’ adherence to or between session implementation of STAND-G or STAND-G + TPS strategies at home, so it is possible that there was variability in practice of these techniques throughout treatment and it is unknown whether parent adherence to these strategies was maintained at follow-up assessments. Third, although the study recruitment procedures were designed to align with typical school identification practices for services and as such, there are lower rates of conduct problems than may be present in clinic-based studies, average cognitive function was in the average range (and slightly above 100), the sample was predominately White, and about half the sample was higher income level and with a parent who attended college so our findings may not generalize to more diverse or severe populations (e.g., youth with comorbid ADHD and CD). Further, it is important to acknowledge that the group leaders were graduate level research assistants and aware that sessions were being audio-recorded and reviewed for fidelity, which likely affected how the interventions were implemented. Therefore, it is unclear how the findings will generalize to other providers including school mental health providers and community clinicians. Finally, several of the outcomes showed trends for group differences but were not significant. It might be that to generate larger effects, increased dosage (attention to those areas – e.g., adding a HW assignment; adding more group practice) is needed.
In sum, results from this study demonstrate that an evidence-based ADHD intervention supplemented with tobacco prevention strategies is a promising behavioral treatment approach for adolescents with ADHD. This brief, 8-week treatment may be an appropriate prevention approach for promoting adolescent, parent, and family skills that that are critical for substance use risk prevention. Future examinations of STAND-G + TPS with a larger sample size and longer follow-up periods are needed to fully demonstrate the efficacy of this approach. Larger samples will also facilitate exploring whether the treatment is similarly effective across varying levels of risks common to adolescents with ADHD including co-occurring symptomology (e.g., oppositional defiant behaviors, internalizing problems), executive dysfunctions, or social difficulties, which may moderate the effects of treatment on substance use risk outcomes. Moreover, given evidence of heightened risk for substance use problems during developmental transitions (Dvorsky and Langberg 2019; Mitchell et al. 2018), it is important that future research explore intervention effectiveness across key periods such as the transition to high school or into emerging adulthood. Further, despite the promise of this approach, future modifications may be needed. For example, group leaders in this study were well supervised in their implementation of the interventions. To facilitate the translation of research-based treatments into community settings, implementation researchers have recommended the use of fidelity measurements as a training and supervision tool (McLeod et al. 2013). Future work should examine whether this type of process would help better train school mental health and community clinicians in tobacco prevention skills that are integrated into an intervention focused on improving academic skills.
Funding from the Virginia Foundation for Healthy Youth supported this project. The findings and conclusions do not necessarily reflect those of the foundation. Melissa Dvorsky is supported by award number T32MH018261 from the from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Footnotes
Conflicts of Interest The authors declare that they have no conflict of interest.
Human Participants and/or Animals Ethical clearance was obtained by Virginia Commonwealth University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research ethics committee (BSSERC #2015000875) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Written informed consent was obtained from parents and informed assent from adolescents after their parent had granted permission for them to participate.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10802-020-00689-6) contains supplementary material, which is available to authorized users.
References
- Barkley RA, Edwards G, Laneri M, Fletcher K, & Metevia L (2001). The efficacy of problem-solving communication training alone, behavior management training alone, and their combination for parent–adolescent conflict in teenagers with ADHD and ODD. Journal of Consulting and Clinical Psychology, 69(6), 926–941. [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B: Methodological, 57, 289–300. [Google Scholar]
- Coatsworth JD, Duncan LG, Greenberg MT, & Nix RL (2010). Changing parent’s mindfulness, child management skills and relationship quality with their youth: Results from a randomized pilot intervention trial. Journal of Child and Family Studies, 19(2), 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Hillsdale: Erlbaum. [Google Scholar]
- Dunne EM, Hearn LE, Rose JJ, & Latimer WW (2014). ADHD as a risk factor for early onset and heightened adult problem severity of illicit substance use: An accelerated gateway model. Addictive Behaviors, 39(12), 1755–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ & Langberg JM (2014). Educational impairments in children with ADHD: In Barkley R.a. (Ed.) Attention-Deficit/Hyperactivity Disorder: A Handbook for Diagnosis and Treatment, 4thEdition, New York, NY: Guilford publications. [Google Scholar]
- Dvorsky MR, & Langberg JM (2019). Cigarette and e-cigarette use and social perceptions over the transition to college: The role of ADHD symptoms. Psychology of Addictive Behaviors, 33(3), 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennett ST, Bauman KE, Foshee VA, Pemberton M, & Hicks KA (2001). Parent-child communication about adolescent tobacco and alcohol use: What do parents say and does it affect youth behavior? Journal of Marriage and Family, 63(1), 48–62. [Google Scholar]
- Evans SW, Owens JS, Wymbs BT, & Ray AR (2018). Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology, 47(2), 157–198. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Coles EK, Gnagy EM, Chronis-Tuscano A, & O’Connor BC (2009). A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clinical Psychology Review, 29(2), 129–140. [DOI] [PubMed] [Google Scholar]
- Glass K, & Flory K (2010). Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clinical Child and Family Psychology Review, 13(3), 291–313. [DOI] [PubMed] [Google Scholar]
- Gibson LA, Creamer MR, Breland AB, Giachello AL, Kaufman A, Kong G, Pechacek TF, Pepper JK, Soule EK, & Halpern-Felsher B (2018). Measuring perceptions related to e-cigarettes: Important principles and next steps to enhance study validity. Addictive Behaviors, 79, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenman A, Oosterlaan J, Rommelse N, Franke B, Greven CU, Hoekstra P, et al. (2013). Stimulant treatment for attention-deficit hyperactivity disorder and risk of developing substance use disorder. The British Journal of Psychiatry: Mental Science, 203(2), 112–119. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, & Lee SS (2013). Stimulant medication and substance use outcomes: A meta-analysis. JAMA Psychiatry, 70(7), 740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, & Schulenberg JE (2015). Monitoring the future national results on adolescent drug use: Overview of key findings, 2014. Ann Arbor: Institute for Social Research, the University of Michigan. [Google Scholar]
- Kerr M, & Stattin H (2000). What parents know, how they know it, and several forms of adolescent adjustment: Further support for a reinterpretation of monitoring. Developmental Psychology, 36(3), 366–380. [PubMed] [Google Scholar]
- Kline RB (2004). Beyond significance testing. Washington, DC: American Psychological Association. [Google Scholar]
- Kumpfer KL, Whiteside HO, Greene JA, & Allen KC (2010). Effectiveness outcomes of four age versions of the strengthening families program in statewide field sites. Group Dynamics: Theory, Research, and Practice, 14(3), 211–229. [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, & Glass K (2011). Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review, 31(3), 328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loren R, Vaughn A, Langberg J, Cyran J, Proano-Raps T, et al. (2015). Effects of an 8-session behavioral parent training group for parents of children with ADHD on child impairment and parenting confidence. Journal of Attention Disorders, 19(2), 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabee-Gittens EM, Huang B, Chen C, Dorn LD, Ammerman RT, & Gordon JS (2011). The association of parental self-efficacy and parent-youth connectedness with youth smoking intentions. Journal of Prevention & Intervention in the Community, 39, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BD, Southam-Gerow MA, Tully CB, Rodríguez A, & Smith MM (2013). Making a case for treatment integrity as a psychosocial treatment quality indicator for youth mental health care. Clinical Psychology: Science and Practice, 20(1), 14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Howard A, Belendiuk K, Kennedy T, Stehli A, et al. (2018). Cigarette smoking progression among young adults diagnosed with ADHD in childhood: A 16-year longitudinal study of children with and without ADHD. Nicotine & Tobacco Research, 1, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, et al. (2007). Delinquent behavior and emerging substance use in the MTA at 36 months: Prevalence, course, and treatment effects. Journal of the American Acad of Child & Adolescent Psychiatry, 46(8), 1028–1040. [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Arnold L, Swanson J, Pelham W, et al. (2013). Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Acad of Child & Adolescent Psychiatry, 52(3), 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, & Pelham WE (2003). Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology, 112(3), 497. [DOI] [PubMed] [Google Scholar]
- Molina BS, & Pelham WE (2014). Attention-deficit/hyperactivity disorder and risk of substance use disorder: Developmental considerations, potential pathways, and opportunities for research. Annual Review of Clinical Psychology, 10, 607–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SB (2008). Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods, 11(2), 364–386. [Google Scholar]
- NCI Working Group Report (2016). Tobacco control research priorities for the next decade: Working group recommendations for 2016–2025. Retrieved on September 26, 2019 from https://www.cancer.gov/news-events/cancer-currents-blog/2016/tobacco-control-plan
- Olson DH (1986). Circumplex model VII: Validation studies and FACES III. Family Process, 25(3), 337–351. [DOI] [PubMed] [Google Scholar]
- Olson DH, Portner J, & Lavee Y (1985). Family adaptability and cohesion evaluation scales (FACES III). St.Paul: University of Minnesota, Family Social Science. [Google Scholar]
- Pepper J, Reiter P, McRee A, Cameron L, Gilkey M, & Brewer N (2013). Adolescent males’ awareness of and willingness to try electronic cigarettes. Adolescent Health, 52(2), 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR (2007). Pharmacologic treatment of attention-deficit/hyper-activity disorder: Efficacy, safety and mechanisms of action. Neuropsychology Review, 17(1), 61–72. [DOI] [PubMed] [Google Scholar]
- Power TJ, Werba BE, Watkins MW, Angelucci JG, & Eiraldi RB (2006). Patterns of parent-reported homework problems among ADHD-referred and non-referred children. School Psychology Quarterly, 21(1), 13–33. [Google Scholar]
- Redmond C, Spoth R, Shin C, Schainker L, Greenberg M, & Feinberg M (2009). Long-term protective factor outcomes of evidence-based interventions implemented by community teams through a community–university partnership. The Journal of Primary Prevention, 30(5), 513–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AL & Foster SL (1989). Negotiating parent-adolescent conflict: A behavioral-family systems approach. New York: Guilford. [Google Scholar]
- Schoenfelder EN, & Kollins SH (2014). Prevention of health risk behaviors in ADHD youth: Is ADHD treatment enough? The ADHD Report, 22(4), 1–8. [Google Scholar]
- Schoenfelder EN, Faraone SV, & Kollins SH (2014). Stimulant treatment of ADHD and cigarette smoking: A meta-analysis. Pediatrics, 133(6), 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham W, Derefinko K, Kuriyan A, Sanchez F, & Graziano P (2013). A pilot trial of supporting teens’ academic needs daily (STAND): A parent-adolescent collaborative intervention for ADHD. Journal of Psychopathology and Behavioral Assessment, 35(4), 436–449. [Google Scholar]
- Sibley MH, Altszuler AR, Ross JM, Sanchez F, Pelham WE Jr., & Gnagy EM (2014a). A parent-teen collaborative treatment model for academically impaired high school students with ADHD. Cognitive and Behavioral Practice, 21(1), 32–42. [Google Scholar]
- Sibley MH, Pelham W, Molina BS, Coxe S, Kipp H, Gnagy EM, et al. (2014b). The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. Journal of Abnormal Psychology, 123(2), 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Graziano PA, Kuriyan AB, Coxe S, Pelham WE, Rodriguez L, Sanchez F, Derefinko K, Helseth S, & Ward A (2016). Parent–teen behavior therapy+ motivational interviewing for adolescents with ADHD. Journal of Consulting and Clinical Psychology, 84(8), 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. (2013). Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. American Journal of Psychiatry, 170(3), 275–289. [DOI] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Mason WA, Schainker L, & Borduin L (2015). Research on the strengthening families program for parents and youth 10–14: Long-term effects, mechanisms, translation to public health, PROSPER partnership scale up. In Scheier LM (Ed.), Handbook of drug prevention. DC. American Psychological Association: Washington. [Google Scholar]
- Spoth R, Shin C, Guyll M, Redmond C, & Azevedo K (2006). Universality of effects: An examination of the comparability of long-term family intervention effects on substance use across risk-related subgroups. Prevention Science, 7, 209–224. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. (2014). Trends in the parent-report of health care provider-diagnosed and medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 34–46 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller EB, Weller R, Fristad M, Rooney MT, & Schecter J (2000). Children’s interview for psychiatric syndromes (ChIPS). Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 76–84. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence-II. San Antonio: Psych Corp. [Google Scholar]
- Wechsler D (2009). Wechsler individual achievement test-III. San Antonio: Psych Corp. [Google Scholar]
- Wilens T, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Utzinger L, et al. (2008). Cigarette smoking associated with attention deficit hyperactivity disorder. The Journal of Pediatrics, 153(3), 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, & Biederman J (2011). Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 50(6), 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


