Table 3.
Small molecule modulators for TRPC channels (Antagonists).
| Antagonists | Chemical Structure | TRPC Channel (EC50/IC50) | Characteristics | Reference |
|---|---|---|---|---|
| Pyrazolo [1,5-a] pyrimidine (14a) |
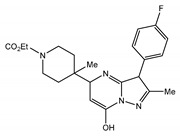
|
TRPC6 (1 μm) | Inhibits TRPC3/6/7 (TRPC6 > C7 > C3) with a very weak effect on TRPC4 and no effect on other TRP channels. | [197] |
| Pyrazole 3 (Pyr3) |
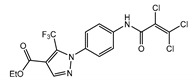
|
TRPC3 (0.5 μm) TRPC6 (> 10 μm) |
Also inhibits STIM/Orai | [274] |
| Pyrazole 10 (Pyr10) |
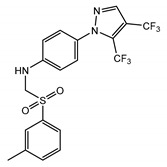
|
TRPC (0.72 μm) TRPC6 (> 10 μm) |
More selective than Pyr3; does not inhibit STIM/Orai | [160] |
| BTDM |
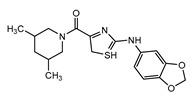
|
TRPC3 (0.01 μm) TRPC6 (0.01 μm) | The exact BTDM binding site in TRPC6 was defined by cryo-EM; wedges between the S5-S6 pore domain and voltage sensor-like domain to inhibit channel opening | [99] |
| GSK503A |
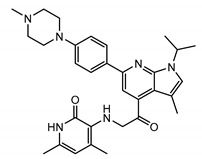
|
TRPC3 (0.003 μm) TRPC6 (0.021 μm) | Anilino thiazoles; good selectivity over other TRPA1,TRPV1, TRPV4, CaV1.2, hERG, and NaV1.5; in rodent models not orally bioavailable; high clearance, more suitable as in vitro tool | [141] |
| DS88790512 |
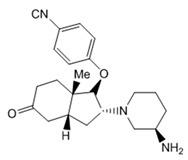
|
TRPC6 (0.011 μm) | Novel blocker of TRPC6; cyclohexanone derivative; excellent selectivity against hERG and hNaV1.5 channels | [310] |
| larixyl acetate |
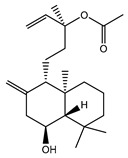
|
TRPC6 (0.1–0.6 μm) | Larch-derived labdane-type diterpenes; 12- and 5-fold selectivity compared with TRPC3 and TRPC7 | [177] |
| BI749327 |
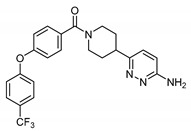
|
TRPC6 (13 nm) | BI 749327 is 85-fold more selective for mouse TRPC6 than TRPC3 (IC50 = 1100 nm) and 42-fold versus TRPC7 | [311] |
| Pico145 (HC-608) |
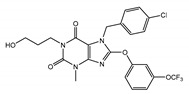
|
TRPC4 (63 pM) TRPC5 (1.3 nm) | Pico145 potency ranges from 9 to 1300 pM depending on the TRPC1/4/5 subtype while a range of other TRPC channels were unaffected | [312] |
| HC-070 |
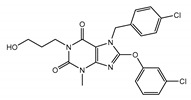
|
TRPC4 (46.0 ± 3.9 nm) TRPC5 (9.3 ± 0.9 nm) | HC-070 inhibits recombinant TRPC4 and TRPC5 homomultimers in heterologous expression systems with nanomolar potency | [220] |
| AC-1903 |
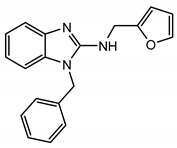
|
TRPC5 (14.7 μm) | AC1903 selectively blocks TRPC5 ion channels | [265] |
| ML204 |
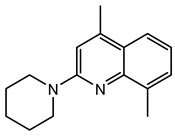
|
TRPC4 (0.99 μm) TRPC5 (9.2 μm) | ML204 exhibited modest inhibitory effects on TRPC6 | [174] |
| Galangin |
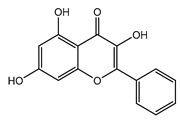
|
TRPC5 (0.45 μm) | Galangin is a natural product from the ginger family and a TRPC5 inhibitor depending on the substitution patterns of both the chromone core and the phenyl ring | [313] |
| SAR7334 |
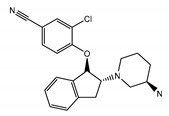
|
TRPC6 (7.9 nm) | SAR7334 inhibited TRPC3 and TRPC7-mediated Ca2+ influx into cells with IC50 s of 282 nm and 226 nm | [314] |
| SH045 |
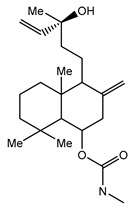
|
TRPC6(~5.8 nm) | IC50 for TRPC3 and TRPC7 are 0.84 μm and 0.22 μm, respectively | [315] |
| Bromoenol lactone (BEL) |
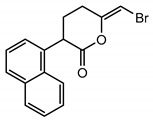
|
TRPC5 TRPC6 TRPC1–TRPC5 Cav1.2, SOCE |
TRPC5: 10.6 μm TRPC6: 7.2 μm Cav1.2: 7.6 μm |
[55] |
