Abstract
Solid-state NMR (ssNMR) is an indispensable tool for elucidating the structure and dynamics of insoluble and non-crystalline biomolecules. The recent advances in the sensitivity-enhancing technique Magic-Angle Spinning Dynamic Nuclear Polarization (MAS-DNP) have substantially expanded the territory of ssNMR investigations and enabled the detection of polymer interfaces in a cellular environment. This article highlights the emerging MAS-DNP approaches and their applications to the analysis of biomolecular composites and intact cells to determine the folding pathway and ligand binding of proteins, the structural polymorphism of low-populated biopolymers, as well as the physical interactions between carbohydrates, proteins, and lignin. These structural features provide an atomic-level understanding of many cellular processes, promoting the development of better biomaterials and inhibitors. It is anticipated that the capabilities of MAS-DNP in biomolecular and biomaterial research will be further enlarged by the rapid development of instrumentation and methodology.
Keywords: solid-state NMR, DNP, cell walls, plants, pathogenic fungi, human cell, polysaccharides, membrane proteins
Introduction
Solid-state Nuclear Magnetic Resonance (ssNMR) spectroscopy has been successfully employed to acquire molecular insights on the structure and dynamics of many biomolecules. Most of these studies are focused on the structural determination of purified or reconstituted biomolecules such as amyloid fibrils, membrane proteins, large protein complex, ion channels and transporters, and nucleic acids [1–9]. Nevertheless, it is technically difficult to conduct high-resolution studies of biomacromolecules in their cellular environments. This limitation is the consequence of two technical issues: the inadequate resolution due to the coexistence of many heterogeneous macromolecules and the unsatisfactory sensitivity due to the low concentration of the molecules of interest. In the past decade, the sensitivity-enhancing Dynamic Nuclear Polarization (DNP) technique has been integrated with specific isotope-labeling techniques and spectral editing approaches that efficiently attenuate spectral congestion. This has made it practicable to study protein folding, biopolymer interactions, and ligand binding using intact viruses and intact cells of bacteria, fungi, plants, and humans [10–15]. This review will summarize the technical feasibility of several emerging approaches and their applications to cellular samples as well as bio-composites. We will also elaborate on how structural restraints can be combined to conceptually comprehend the supramolecular architecture of biological constructs, which could facilitate the development of biopolymer-based materials, bio-sourced energy, and novel therapeutic agents.
MAS-DNP sensitivity enhancement enables new research avenues
NMR is a low-sensitivity technique whose signal-to-noise ratios greatly depend on the gyromagnetic ratio of spins. The cutting-edge technique MAS-DNP takes advantage of the several orders of magnitude higher gyromagnetic ratios of electrons over NMR-active nuclei, such as 13C or 1H, to boost NMR sensitivity [16–18]. An electron source, usually a stable nitroxide mono- or bi-radical, is physically or covalently incorporated into the sample, which allows the polarization of unpaired electrons to be transferred to protons under microwave irradiation (Figure 1A). The sensitivity enhancement factor (εon/off) is measured by taking the ratio of intensity turning the microwave radiation on to the intensity keeping it off while accounting for the depolarization effects [19]. Nitroxide-based radicals can be chemically reduced in biological samples. As demonstrated by McDermott and colleagues, for cells and lysates at room temperature, less than a quarter of radicals can be retained after a short time of 10 minutes, which corresponds to a reduction rate of 0.18 mmol/(L*min) [20]. The reaction rate is substantially slowed down to 0.12 mmol/(L*min) at a moderately low temperature of 4°C. Because the short lifetime presents a barrier, MAS-DNP experiments are usually conducted at a very low temperature of 90–110 K. The use of cryogenic temperature also increases the signal-to-noise ratios of all NMR spectra following the Boltzmann distribution and improves DNP efficiency by elongating both electron and proton relaxation times [17, 21]. However, this also risks the loss of spectral resolution as a broad distribution of conformations will be trapped when the dynamic components are immobilized.
Figure 1. MAS-DNP technique boosts NMR sensitivity.
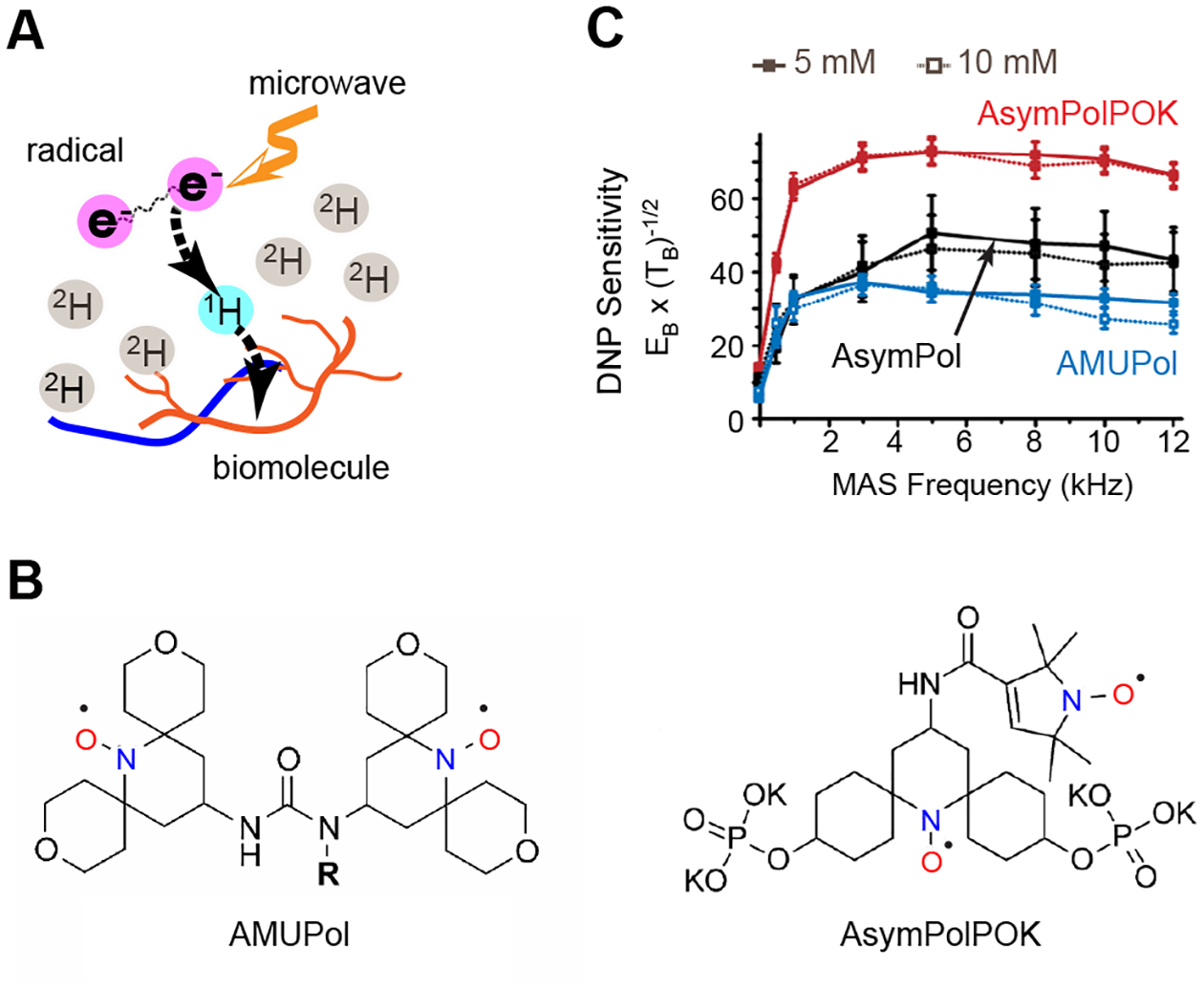
(A) Illustration of the MAS-DNP mechanism. (B) Representative structure of two bi-radicals, AMUPol and AsymPolPOK. (C) Enhancement of the sensitivity of spectra collected on 13C-urea at a variety of magic-angle spinning frequencies at 9.4 T and 105 K using three different bi-radicals, including AMUPol, AsymPolPOK, and AsymPol at two different concentrations (5 and 10 mM). The MAS-DNP sensitivity is quantified as the signal intensity per unit square root of time. Figures 1B and 1C are adapted from reference [26] with copyright permission and reference [27] (an open-access article).
A careful choice regarding the composition of the glassy matrix (typically a mixture of water with glycerol or DMSO) and the concentration of radicals is essential because MAS-DNP efficiency is influenced by the way that radicals are dispersed in the biological medium [22–24]. A homogenous mixture of d8-glycerol/D2O/H2O (60:30:10 vol%) has been widely used as the DNP juice for biomolecular samples. The formation of glassy matrix efficiently avoids the formation of the crystalline phase, thus evading radical aggregation. Multiple water-soluble bi-radicals (with two unpaired electrons), such as TOTAPOL and AMUPol [25, 26], have been widely used (Figure 1B). A recently developed, asymmetric biradical AsymPolPOK has shown meritorious performance: due to a substantial decline in the MAS-DNP buildup time, the absolute sensitivity is doubled when compared with the commercial radicals (Figure 1 B, C) [27]. The DNP buildup time limits the recycle delays between two scans, which further determines the experimental time. The DNP buildup time depends on the concentration and property of radicals; it typically ranges in the scale of 2–6 seconds when the biomolecules are well mixed with the radicals but can be as long as tens of seconds in some challenging samples [28]. The buildup time is partially controlled by the strength of electron-electron interaction; the stronger as the shorter [29]. AsympolPOK has very strong electron dipolar and exchange interaction, which accounts for the very fast buildup even with a relatively low concentration of biradicals, without affecting other characteristic times [27]. In addition, there are significant efforts in covalently incorporating radicals to biomolecules at the location of interest, which provides efficient polarization of the embedded molecules and specific interaction sites [30, 31]. These efforts have made MAS-DNP a versatile technique for addressing key biochemical questions with structural relevance as discussed below.
Membrane protein and protein-ligand binding
DNP-assisted ssNMR is uniquely capable of analyzing heterogeneous mixtures as it circumvents crystallization or solubilization; however, this method has its own challenges in sample preparation. It often requires chemically modified radicals [32–34] or special procedures to ensure the homogeneous mixing of biomolecules and polarizing agents. For example, because of the limited water accessibility and the impermeability of membranes towards water-soluble biradicals, the enhancement factors achieved on membrane protein samples (those with a reasonably low peptide-to-lipid ratio and a sufficient membrane environment) are typically below 20-fold.
In 2016, an optimized protocol that mixes radicals and membranes by direct titration has resulted in a 40–100-fold of sensitivity enhancement (εon/off) on a 400 MHz/263 GHz MAS-DNP instrument [22]. This approach has been successfully demonstrated on two ion channels: the influenza A M2 proton channel and an artificial designed protein channel that co-transports Zn2+ and H+ ions [35]. The efficient gain of sensitivity is quantified to approach 100–160-fold by comparing MAS-DNP spectra with those collected at a higher temperature (243 K). This success has been attributed to the bimodal partitioning of radicals in the phospholipid membranes, with a surface-resided portion and a membrane-inserted fraction, which can be distinguished through the paramagnetic relaxation enhancement (PRE) effects of the unpaired electrons of biradicals on the signals of phospholipids [22].
At ambient temperature, quantification of the chemical shift perturbation allows us to locate the ligand-binding sites in proteins [36, 37], but this approach is no longer efficient under MAS-DNP conditions due to the broader linewidth. As a result, innovative strategies have been developed to probe the protein surface and topology for binding cholesterol and carbohydrate-based ligands. In 2013, we have developed a method that relies on differential isotope-labeling (13C on carbohydrate components and 15N, 13C on recombinantly expressed proteins) to determine the binding of a nonenzymatic loosening protein expansin to Arabidopsis plant cell walls [12]. DNP helps to overcome the sensitivity barrier imposed by the low functional concentration of this protein, with spectral editing techniques used to detect the protein-bound carbohydrates. Expansins are recruited to the carbohydrate junctions in which the hemicellulose xyloglucan is entrapped between multiple cellulose microfibrils or several glucan chains of a single microfibril, which turns out to be the polymer nexus being released during cell elongation [12].
Recently, Hong and colleagues have developed a strategy that integrates MAS-DNP with biosynthetic 13C-labeling of cholesterols from the budding yeast Saccharomyces cerevisiae to investigate protein-cholesterol binding in lipid bilayers [38]. The yeast strain (RH6829) is genetically modified to produce cholesterols instead of ergosterols, and the cholesterols can be 13C-labeled at alternate carbon sites using either 1- or 2-13C glucose (Figure 2A). With the sensitivity enhanced by MAS-DNP, two-dimensional (2D) 13C−13C double-quantum filtered (DQF) spectra have explicitly resolved several cross peaks between the influenza A virus M2 protein and 1-13C cholesterol in lipid bilayers. These structural constraints were combined with previous findings on the helix orientation and binding stoichiometry [39] to reveal how the M2 protein utilizes its Ile, Leu and Phe sidechains on an annular binding site of the transmembrane helix to bind cholesterol asymmetrically through methyl-methyl and CH-π interactions (Figure 2B). These findings provide insights into the underlying mechanisms through which M2 proteins interact with membrane components, promote membrane curvatures [40–42], and facilitate the membrane scission process during viral budding and release [43].
Figure 2. MAS-DNP methods for probing protein-ligand binding.
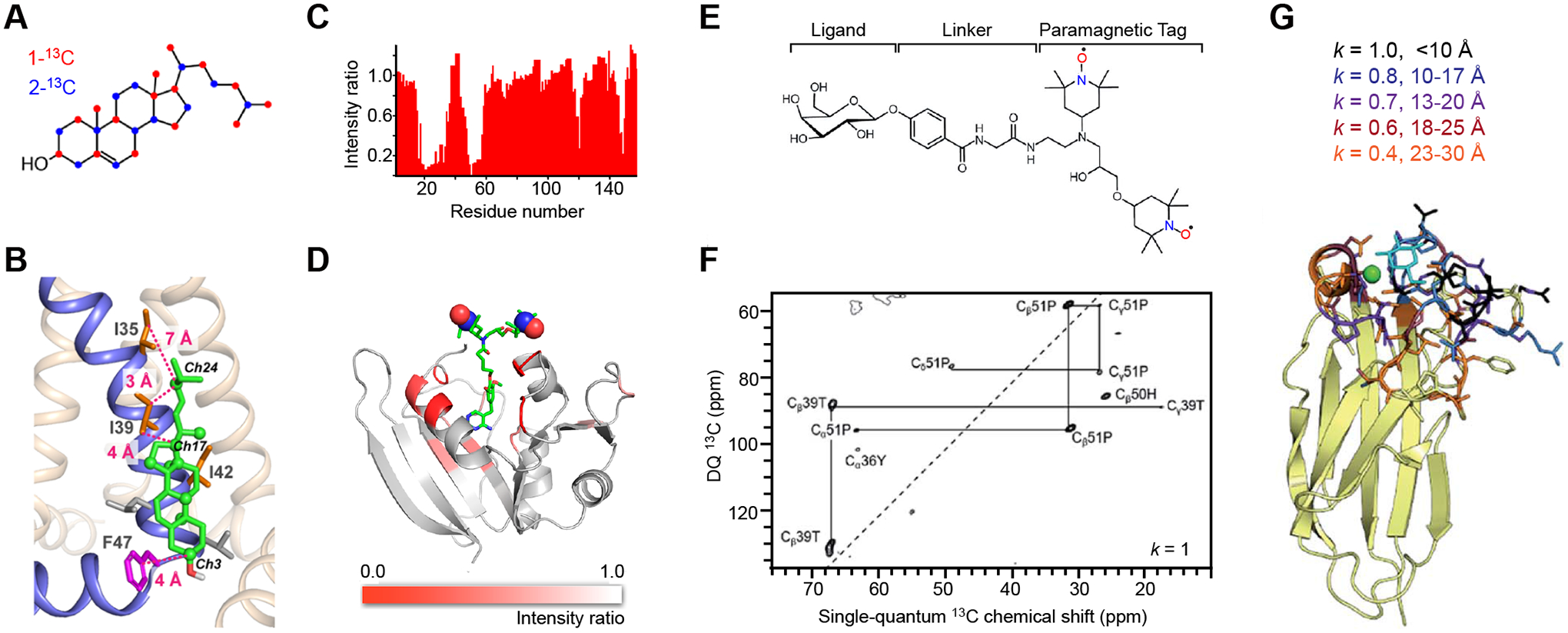
(A) Yeast-based 13C labeling of cholesterol using site-specifically 13C-labeled glucose. The labeled carbon sites on cholesterol are in red and blue for cholesterols produced from 1-13C and 2-13C glucose molecules, respectively. (B) A structural model of a cholesterol molecule bound to the influenza M2 proteins. The key Ile and Phe residues, as well as their distances to cholesterol carbons, are shown. (C) Signal bleaching quantified in solution 1H-15N HSQC spectra due to the binding of radicals to dihydrofolate reductase. (D) A model of E. coli dihydrofolate reductase with DNP bleaching information represented by the intensity ratios of 13C-13C DARR spectra collected on two samples containing either bound radicals or exogenous radicals. (E) Scheme for incorporating a carbohydrate ligand to a paramagnetic tag for selective DNP. (F) Selective DNP 13C–13C INADEQUATE difference spectrum of LecA obtained using k=1: only the tightly bound residues are observed. (G) Sideview of LecA. Residues observed using selective DNP are highlighted, with the corresponding k values given. Figures 2A-2D are adapted from references [38, 51] with copyright permission. Figures 2E-2G are adapted from reference [31], an open-access article.
There are tremendous efforts to covalently link mono- or bi-radicals to proteins or membranes, which, by expectation, should provide better DNP efficiency and site-specificity. Spin-labeled phosphocholine (PC) lipids have been used as the DNP polarizing agents and the constituent molecules of lipid bilayers [30, 44]. The mono-radicals are tethered to the lipids at multiple sites, including the phosphate headgroup (TEMPO-PC), the middle segments (5-Doxyl PC and 7-Doxyl PC), and the terminal part of acyl chains (16-Doxyl PC). Consequently, efficient and homogenous polarization has been observed across the lipid bilayers and to membrane-embedded peptides such as a lung surfactant mimetic peptide KL4 inserted in these membranes. In addition, site-directed incorporation of polarizing agents has also been demonstrated on the potassium channel KcsA, the antibiotics gramicidin, and the sensory rhodopsin [45–47]. Targeted DNP has also been used to investigate the interaction between a radical‐labeled ligand with a 20 kDa protein (Bcl-xL) at a low concentration in crude cell lysates [48].
Because radicals are paramagnetic species, spins in their spatial vicinity experience faster NMR relaxation, which leads to line broadening and intensity suppression [49, 50]. The signal quenching, also called paramagnetic bleaching, can be quantified when the radical is directly bound to a protein (Figure 2C). This technique has been applied to determine the distance of a combined ligand-radical to a reductase in E. coli bacteria [51]. A comparison of the DNP spectra collected on two samples, one with a radical bound to the protein with high affinity and the other with the solvent-bearing AMUPol as a reference, has pinpointed the protein surface that is responsible for binding the target (Figure 2D).
Signal bleaching has also been employed to improve resolution when probing protein-ligand binding. This process has been successfully achieved by functionalizing the ligand (for example, carbohydrates) with a polarizing agent (TOTAPOL) covalently linked through a phenylglycine linker (Figure 2E) [31]. The conceptual setup is consistent with the studies of the E. coli reductase as discussed above, with additional assistance from difference spectroscopy [52, 53] in which the spectrum measured using the paramagnetically tagged sample is subtracted from a reference spectrum measured on a sample only containing radicals homogenously distributed in the solvent. A selective factor k is applied to the spectrum of the paramagnetically tagged sample before spectral subtraction, which renders selective DNP mimicking an atomic-resolution microscope with adjustable magnification: only the tightly bound residues (<10 Å) can be observed in the difference spectrum when k=1 is applied (Figure 2F), and the observable region gradually expands to 30 Å with a decreasing k value. This method has been applied to the study of a galactose-specific lectin LecA [31]. The spectral subtraction provides unprecedented resolution for unambiguously locating the carbohydrate-binding spots on LecA (Figure 2G). This method requires no prior knowledge of the binding site and has no limitation on the protein size.
Protein structure in cellular fractions and intact cells
The magnificent sensitivity has made DNP a suitable tool for studying highly diluted biomolecules in cellular fractions or whole cells, which are otherwise “invisible”. In 2015, the type IV secretion system core complex (T4SScc), a megadalton protein complex, has been investigated in the cell envelope fractions of E. coli [54]. In the cellular system, this protein maintains its correct folding and assembles into a structure that is consistent with the X-ray crystallography results. Similarly, a protocol has been established to study protein folding in cellular lysates, which combines different labeling schemes to produce a sample containing NMR-active prion proteins and an NMR-silent cellular environment (Figure 3A) [13, 55]. The technique was employed to determine the folding of an intrinsically disordered region of the yeast prion protein Sup35. The Sup35 fibrils were found to be restructured and different from in-vitro templated assemblies [13].
Figure 3. Cellular MAS-DNP of protein structure.
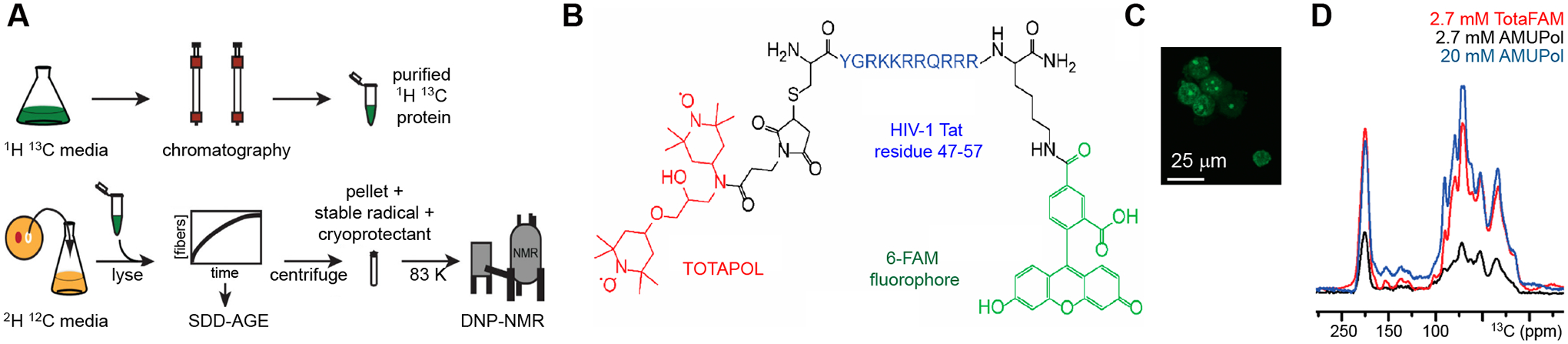
(A) Preparation of proteins at endogenous levels for MAS-DNP in biological environments. (B) Chemical structure of the trimodal polarizing agent TotaFAM. (C) A fluorescent image confirms the cellular uptake of TotaFAM. (D) 1D 13C spectra of HEK293F cells at <6 K using different radicals. Figures are adapted from references [13, 14] with copyright permission.
Intact human cells have remained as a challenging system for DNP ssNMR, especially for the optimization of radicals and experimental conditions. In 2019, an original protocol has been designed to examine protein structures in mammalian cells. This approach comprises of three steps: isotope labeling the protein of interest, delivery of the protein into cells by electroporation techniques followed by a cell stimulus, and the introduction of biradicals for DNP measurement [56]. The cell integrity and biradical distribution have been simultaneously monitored with microscopy techniques. It is found that the model protein ubiquitin has remained correctly folded after delivery into the HeLa cell [56]. In the same year, another study has quantified the chemical reduction effect of nitroxide biradicals in E. coli pellets, suspensions, and lysates. Treatment of the cell using N-ethylmaleimide could neutralize pools of redox-active cysteines, suppress nitroxide reduction, and prolong the lifetime of radicals while adding potassium ferricyanide effectively re-oxidizes the reacted radicals back into their active state [20]. In 2018, a trimodal polarizing agent TotaFAM has been introduced, which contains a biradical, a targeting cell-penetrating peptide, and a fluorophore for tracking the localization of radicals in the cell (Figure 3B) [14]. The radical uptake is efficient in HEK293F cells (Figure 3C) and a high enhancement of 63-fold of the cellular signals was achieved using a low radical concentration (2.7 mM). In comparison, commercial radicals require a much higher concentration (20 mM) to reach a comparable performance (Figure 3D). These ground-breaking advances have paved the way for understanding the molecular structure, functional mechanisms, and drug inhibition [57] of protein machinery and other biomolecules within their cellular environments.
Biopolymer packing in fungal and plant cell walls
There is a growing interest in characterizing cell walls of plants and microbes because these protective armors are the resources of new energy and the targets of antimicrobial therapeutic compounds [58–60]. During the past years, many organisms have been investigated using ssNMR, including the cell walls of many plants, pathogenic fungi, microalgae, and bacteria [61–75]. The highly rigid and semi-crystalline components, for example, cellulose microfibrils in plants and chitin in fungi, are capable of retaining decent resolution under cryogenic conditions [11, 12, 76], which has made the carbohydrate-rich cell walls a preferred system for DNP investigations. In addition, radicals mainly accumulate in the cell walls due to a high binding affinity to the carbohydrate components such as the peptidoglycan in bacteria and cellulose in plants [77–79]. We can either selectively detect the cell wall molecules using a low concentration of radicals (for example, 5 mM) or observe other cellular components by bleaching the cell wall signals using a saturated concentration of radicals (for example, 60 mM) [79]. Moreover, NMR fingerprints of the highly polymerized polysaccharides in the cell wall are uniquely different from those of intracellular and metabolic carbohydrates or other molecules (e.g. proteins and nucleic acids) [23]; therefore, cell walls are spectroscopically distinguishable from other cellular components.
Recently, we have been elucidating the structural organization of cell walls in several fungal pathogens, starting from a model fungus Aspergillus fumigatus [11] and progressively outspreading to other yeasts as well as molds. A 30-fold of sensitivity enhancement (εon/off) allows us to highlight the highly polymorphic nature of biomolecules in intact fungal cell walls and efficiently probe their sub-nanometer packing. Despite its low abundance (~10% of the dry mass of A. fumigatus cell walls), chitin exists in three major forms as shown by the peak multiplicity of 2D 15N-13C correlation spectra (Figure 4A). These signals deviate from the chemical shifts of model chitin crystallites from fungi or other sources [80, 81]. This unexpected level of structural polymorphism has been attributed to the complicated patterns of hydrogen-bonding (through the amide and carbonyl groups) that form parallel, anti-parallel, and mixed ways of packing in chitin microfibrils [82, 83]. The different forms are extensively mixed in individual microfibrils as evidenced by inter-form correlations using the 2D 15N-15N Proton Assisted Recoupling (PAR) experiment [84–86]. When associated with difference spectroscopy, MAS-DNP has increased both spectral resolution and resolution so that many chitin-glucan interactions can be identified, unveiling a mechanical framework of tightly associated chitin and α−1,3-glucans. This is a novel feature that had never been discovered before [11]. Integrated with the conventional NMR data collected at room temperature, the chitin-α−1,3-glucan scaffold is further found to reside in a soft matrix of β-glucans and capped by a glycoprotein-rich shell [11].
Figure 4. Polysaccharide structure and polymer binding in plant and fungal cell walls.
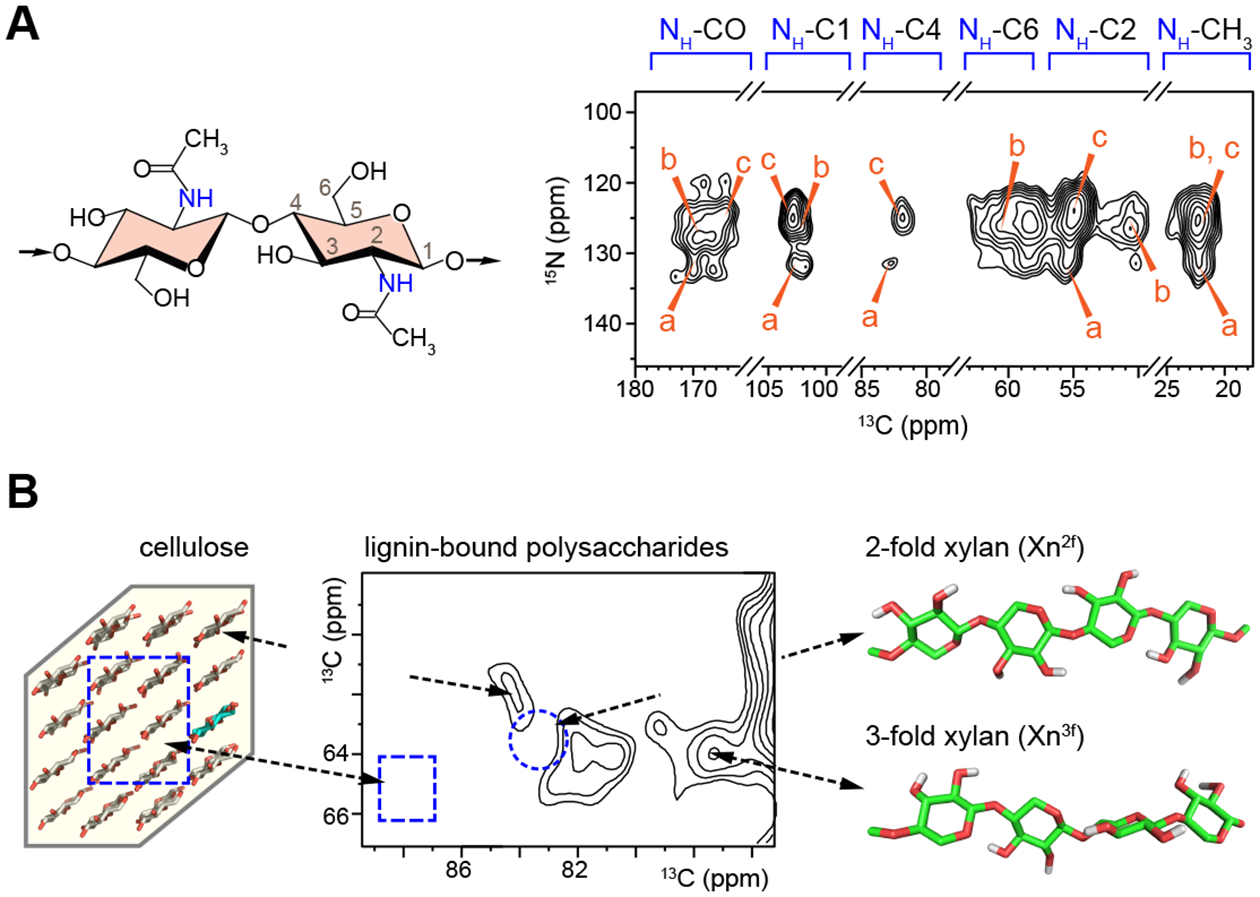
(A) Representative structure and MAS-DNP spectra of chitin in cell walls of intact A. fumigatus. Three major types of chitin signals have been resolved (Types a-c). (B) The aromatic-edited spectrum of maize stems shows the signals of lignin-bound carbohydrates. Arrows and black dotted lines connect the spectral regions with polysaccharide structures. The dashline circle and rectangle on the spectrum highlight the missing signals of the carbohydrate components that are far from lignin. Figures are adapted from references [10, 11], which are open-access publications.
When applied to the plant secondary cell walls, MAS-DNP is employed to probe the physical contacts between multiple polysaccharides and the aromatic polymer lignin, which is a polymer interface with a low occurrence. Assisted by dipolar and frequency filters as well as a mechanical shutter that regulates microwave on the millisecond timescale [87], we have cleanly selected the aromatic signals from lignin and further determined the composition of polysaccharides in the vicinity. Contradictory to the prevailing knowledge, cellulose is found to lack interactions with lignin in maize stems because the signals from the internal and surface glucan chains in the microfibrils are either missing or weak (Figure 4B) [10]. The polysaccharide interactor of lignin is found to be the hemicellulose xylan, which relies on its twisted 3-fold conformers (3 residues per helical turn) to bind lignin and uses the 2-fold flat-ribbon domains to bind cellulose microfibrils [10]. The molecular information of the lignin-carbohydrate interface provides an understanding of the polymer interactions underlying the nanoscale architecture of this bio-complex, which has revised the structural concepts of lignocellulosic biomass. In addition, MAS-DNP has been employed to screen the carbohydrate and lignin constituents of poplar and its genetic variants following chemical treatments, which will aid the improvement of biomass conversion technology [88, 89].
Beyond these studies, there are many other DNP investigations focused on complex biosystems, for example, the DNA and coat proteins of the filamentous phage Pf1 in an intact virus, the supramolecular assembly of HIV capsid, the peptidoglycans of Bacillus subtilis bacterial cell walls, the nucleic acids in bones, the post-translational collagen modification in muscle cell extracellular matrix, and the interfaces of biominerals [15, 79, 90–96]. The scope of MAS-DNP should be substantially broadened by high-field DNP that provides better resolution, the natural-abundance technique that eliminates the need for isotope-enrichment, and the analytical software for spectral and structural comparisons [97–101]. These efforts have the potential for revolutionizing biomolecular and biomaterial research.
Perspectives
Importance of the field: MAS-DNP has vastly broadened the horizon of solid-state NMR spectroscopy and enabled the atomic-level view of polymorphic biomolecules in their native cellular environments. The structural insights of protein machinery and structural carbohydrates also provide an in-depth understanding of many cellular processes to guide the development of functional biomaterials, bio-renewable energy, and novel inhibitors.
Summaries of the current thinking: The molecular structure and binding interactions of many carbohydrate- or protein-based biopolymers have been successfully investigated using biomolecular mixtures, cellular fractions, and intact cells. Due to the complex nature of cellular polymers, MAS-DNP investigations should be coupled with selective labeling or site-directed polarization technology to efficiently alleviate spectral congestion.
Future directions: The rapid development of high-field and fast-MAS DNP as well as natural-abundance approaches have established a new avenue of biochemical research, especially for the complex biosystems with a high demand for resolution or the biomedical samples that are difficult to replicate in vitro.
Acknowledgment
This work was supported by the National Institutes of Health grant AI149289. T.W. thanks the support ofthe Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by theUS Department of Energy, Office of Science, Basic Energy Sciences under award number DE-SC0001090 forsolid-state NMR studies of plant cell walls. The National High Magnetic Field Laboratory is supported byNational Science Foundation through NSF/DMR-1644779 and the State of Florida. The MAS-DNP program atNHMFL is funded by the NIH P41 GM122698.
Abbreviations used:
- MAS
magic-angle spinning
- ssNMR
solid-state Nuclear Magnetic Resonance
- MAS-DNP
magic-angle spinning dynamic nuclear polarization
- PRE
paramagnetic relaxation enhancement
References
- 1.McDermott A (2009) Structure and Dynamics of Membrane Proteins by Magic Angle Spinning Solid-State NMR. Annu. Rev. Biophys 38, 385–403 [DOI] [PubMed] [Google Scholar]
- 2.Marchanka A, Simon B, Althoff-Ospelt G and Carlomagno T (2015) RNA structure determination by solid-state NMR spectroscopy. Nat. Commun 6, 7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier BH, Riek R and Bockmann A (2017) Emerging Structural Understanding of Amyloid Fibrils by Solid-State NMR. Trends Biochem. Sci 42, 777–787 [DOI] [PubMed] [Google Scholar]
- 4.Comellas G and Rienstra CM (2013) Protein Structure Determination by Magic-Angle Spinning Solid-State NMR, and Insights into the Formation, Structure, and Stability of Amyloid Fibrils. Annu. Rev. Biophys 42, 515–536 [DOI] [PubMed] [Google Scholar]
- 5.Mandala VS, Williams JK and Hong M (2018) Structure and Dynamics of Membrane Proteins from Solid-State NMR. Annu. Rev. Biophys 47, 201–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn CM and Polenova T (2017) Structural biology of supramolecular assemblies by magic-angle spinning NMR spectroscopy. Q. Rev. Biophys 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ader C, Schneider R, Seidel K, Etzkorn M and Baldus M (2007) Magic-angle-spinning NMR spectroscopy applied to small molecules and peptides in lipid bilayers. Biochem. Soc. Trans 35, 991–995 [DOI] [PubMed] [Google Scholar]
- 8.Middleton DA (2007) Solid-state NMR spectroscopy as a tool for drug design: from membrane-embedded targets to amyloid fibrils. Biochem. Soc. Trans 35, 985–990 [DOI] [PubMed] [Google Scholar]
- 9.Uluca B, Viennet T, Petrovic D, Shaykhalishahi H, Weirich F, Gonulalan A, Strodel B, Etzkorn M, Hoyer W and Heise H (2018) DNP-Enhanced MAS NMR: A Tool to Snapshot Conformational Ensembles of alpha-Synuclein in Different States. Biophys. J 114, 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang X, Kirui A, Dickwella Widanage MC, Mentink-Vigier F, Cosgrove DJ and Wang T (2019) Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun 10, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang X, Kirui A, Muszynski A, Widanage MCD, Chen A, Azadi P, Wang P, Mentink-Vigier F and Wang T (2018) Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat. Commun 9, 2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Park YB, Caporini MA, Rosay M, Zhong LH, Cosgrove DJ and Hong M (2013) Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc. Natl. Acad. Sci. USA 110, 16444–16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frederick KK, Michaelis VK, Corzilius B, Ong TC, Jacavone AC, Griffin RG and Lindquist S (2015) Sensitivity-Enhanced NMR Reveals Alterations in Protein Structure by Cellular Milieus. Cell. 163, 620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert BJ, Gao CK, Sesti EL, Saliba EP, Alaniva N, Scott FJ, Sigurdsson ST and Barnes AB (2018) Dynamic Nuclear Polarization Nuclear Magnetic Resonance in Human Cells Using Fluorescent Polarizing Agents. Biochemistry. 57, 4741–4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sergeyev IV, Itin B, Rogawski R, Day LA and McDermott AE (2017) Efficient assignment and NMR analysis of an intact virus using sequential side-chain correlations and DNP sensitization. Proc. Natl. Acad. Sci. USA 114, 5171–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mentink-Vigier F, Akbey U, Oschkinat H, Vega S and Feintuch A (2015) Theoretical aspects of Magic Angle Spinning - Dynamic Nuclear Polarization. J. Magn. Reson 258, 102–120 [DOI] [PubMed] [Google Scholar]
- 17.Ni QZ, Daviso E, Can TV, Markhasin E, Jawla SK, Swager TM, Temkin RJ, Herzfeld J and Griffin RG (2013) High Frequency Dynamic Nuclear Polarization. Acc. Chem. Res 46, 1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossini AJ, Zagdoun A, Lelli M, Lesage A, Coperet C and Emsley L (2013) Dynamic Nuclear Polarization Surface Enhanced NMR Spectroscopy. Acc. Chem. Res 46, 1942–1951 [DOI] [PubMed] [Google Scholar]
- 19.Hediger S, Lee D, Mentink-Vigier F and De Paepe G (2018) MAS-DNP Enhancements: Hyperpolarization, Depolarization, and Absolute Sensitivity. eMagRes. 7, 105–116 [Google Scholar]
- 20.McCoy KM, Rogawski R, Stovicek O and McDermott AE (2019) Stability of nitroxide biradical TOTAPOL in biological samples. J. Magn. Reson 303, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurber KR, Yau WM and Tycko R (2010) Low-temperature dynamic nuclear polarization at 9.4 T with a 30 mW microwave source. J. Magn. Reson 204, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao SY, Lee M, Wang T, Sergeyev IV and Hong M (2016) Efficient DNP NMR of membrane proteins: sample preparation protocols, sensitivity, and radical location. J. Biomol. NMR 64, 223–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirui A, Dickwella Widanage MC, Mentink-Vigier F, Wang P, Kang X and Wang T (2019) Preparation of Fungal and Plant Materials for Structural Elucidation Using Dynamic Nuclear Polarization Solid-State NMR. J. Vis. Exp 144, e59152. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi H, Fernandez-de-Alba C, Lee D, Maurel V, Gambarelli S, Bardet M, Hediger S, Barra AL and De Paepe G (2014) Optimization of an absolute sensitivity in a glassy matrix during DNP-enhanced multidimensional solid-state NMR experiments. J. Magn. Reson 239, 91–99 [DOI] [PubMed] [Google Scholar]
- 25.Song CS, Hu KN, Joo CG, Swager TM and Griffin RG (2006) TOTAPOL: A biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media. J. Am. Chem. Soc 128, 11385–11390 [DOI] [PubMed] [Google Scholar]
- 26.Sauvee C, Rosay M, Casano G, Aussenac F, Weber RT, Ouari O and Tordo P (2013) Highly Efficient, Water-Soluble Polarizing Agents for Dynamic Nuclear Polarization at High Frequency. Angew. Chem. Int. Edit 52, 10858–10861 [DOI] [PubMed] [Google Scholar]
- 27.Mentink-Vigier F, Marin-Montesinos I, Jagtap AP, Halbritter T, van Tol J, Hediger S, Lee D, Sigurdsson ST and De Paepe G (2018) Computationally Assisted Design of Polarizing Agents for Dynamic Nuclear Polarization Enhanced NMR: The AsymPol Family. J. Am. Chem. Soc 140, 11013–11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linden AH, Lange S, Franks WT, Akbey U, Specker E, van Rossum BJ and Oschkinat H (2011) Neurotoxin II Bound to Acetylcholine Receptors in Native Membranes Studied by Dynamic Nuclear Polarization NMR. J. Am. Chem. Soc 133, 19266–19269 [DOI] [PubMed] [Google Scholar]
- 29.Mentink-Vigier F, Vega S and De Paepe G (2017) Fast and accurate MAS-DNP simulations of large spin ensembles. Phys Chem Chem Phys. 19, 3506–3522 [DOI] [PubMed] [Google Scholar]
- 30.Smith AN, Caporini MA, Fanucci GE and Long JR (2015) A method for dynamic nuclear polarization enhancement of membrane proteins. Angew. Chem. Int. Ed. Engl 54, 1542–1546 [DOI] [PubMed] [Google Scholar]
- 31.Marin-Montesinos I, Goyard D, Gillon E, Renaudet O, Imberty A, Hediger S and De Paepe G (2019) Selective high-resolution DNP-enhanced NMR of biomolecular binding sites. Chem. Sci 10, 3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-de-Alba C, Takahashi H, Richard A, Chenavier Y, Dubois L, Maurel V, Lee D, Hediger S and De Paepe G (2015) Matrix-Free DNP-Enhanced NMR Spectroscopy of Liposomes Using a Lipid-Anchored Biradical. Chem. Eur. J 21, 4512–4517 [DOI] [PubMed] [Google Scholar]
- 33.Lim BJ, Ackermann BE and Debelouchina GT (2020) Targetable Tetrazine-Based Dynamic Nuclear Polarization Agents for Biological Systems. ChemBioChem, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salnikov ES, Abel S, Karthikeyan G, Karoui H, Aussenac F, Tordo P, Bechinger B and Ouari O (2017) Dynamic Nuclear Polarization/Solid-State NMR Spectroscopy of Membrane Polypeptides: Free-Radical Optimization for Matrix-Free Lipid Bilayer Samples. ChemPhysChem. 18, 2103–2113 [DOI] [PubMed] [Google Scholar]
- 35.Joh NH, Wang T, Bhate MP, Acharya R, Wu YB, Grabe M, Hong M, Grigoryan G and DeGrado WF (2014) De novo design of a transmembrane Zn2+-transporting four-helix bundle. Science. 346, 1520–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson MP (2013) Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Mag. Res. Sp 73, 1–16 [DOI] [PubMed] [Google Scholar]
- 37.Charlton AJ, Baxter NJ, Khan ML, Moir AJG, Haslam E, Davies AP and Williamson MP (2002) Polyphenol/peptide binding and precipitation. J. Agr. Food Chem 50, 1593–1601 [DOI] [PubMed] [Google Scholar]
- 38.Elkins MR, Sergeyev IV and Hong M (2018) Determining Cholesterol Binding to Membrane Proteins by Cholesterol 13C Labeling in Yeast and Dynamic Nuclear Polarization NMR. J. Am. Chem. Soc 140, 15437–15449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkins MR, Williams JK, Gelenter MD, Dai P, Kwon B, Sergeyev IV, Pentelute BL and Hong M (2017) Cholesterol-binding site of the influenza M2 protein in lipid bilayers from solid-state NMR. Proc. Natl. Acad. Sci. USA 114, 12946–12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt NW, Mishra A, Wang J, DeGrado WF and Wong GCL (2013) Influenza Virus A M2 Protein Generates Negative Gaussian Membrane Curvature Necessary for Budding and Scission. J. Am. Chem. Soc 135, 13710–13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, Cady SD and Hong M (2012) NMR Determination of Protein Partitioning into Membrane Domains with Different Curvatures and Application to the Influenza M2 Peptide. Biophys. J 102, 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T and Hong M (2015) Investigation of the curvature induction and membrane localization of the influenza virus M2 protein using static and off-magic-angle spinning solid-state nuclear magnetic resonance of oriented bicelles. Biochemistry. 54, 2214–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossman JS, Jing XH, Leser GP and Lamb RA (2010) Influenza Virus M2 Protein Mediates ESCRT-Independent Membrane Scission. Cell. 142, 902–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith AN, Twahir UT, Dubroca T, Fanucci GE and Long JR (2016) Molecular Rationale for Improved Dynamic Nuclear Polarization of Biomembranes. J. Phys. Chem. B 120, 7880–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Cruijsen EAW, Koers EJ, Sauvee C, Hulse RE, Weingarth M, Ouari O, Perozo E, Tordo P and Baldus M (2015) Biomolecular DNP-Supported NMR Spectroscopy using Site-Directed Spin Labeling. Chem. Eur. J 21, 12971–12977 [DOI] [PubMed] [Google Scholar]
- 46.Wylie BJ, Dzikovski BG, Pawsey S, Caporini M, Rosay M, Freed JH and McDermott AE (2015) Dynamic nuclear polarization of membrane proteins: covalently bound spin-labels at protein-protein interfaces. J. Biomol. NMR 61, 361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voinov MA, Good DB, Ward ME, Milikisiyants S, Marek A, Caporini MA, Rosay M, Munro RA, Ljumovic M, Brown LS, Ladizhansky V and Smirnov AI (2015) Cysteine-Specific Labeling of Proteins with a Nitroxide Biradical for Dynamic Nuclear Polarization NMR. J. Phys. Chem. B 119, 10180–10190 [DOI] [PubMed] [Google Scholar]
- 48.Viennet T, Viegas A, Kuepper A, Arens S, Gelev V, Petrov O, Grossmann TN, Heise H and Etzkorn M (2016) Selective Protein Hyperpolarization in Cell Lysates Using Targeted Dynamic Nuclear Polarization. Angew. Chem. Int. Ed 55, 10746–10750 [DOI] [PubMed] [Google Scholar]
- 49.Sengupta I, Nadaud PS and Jaroniec CP (2013) Protein Structure Determination with Paramagnetic Solid-State NMR Spectroscopy. Acc. Chem. Res 46, 2117–2126 [DOI] [PubMed] [Google Scholar]
- 50.Corzilius B, Andreas LB, Smith AA, Ni QZ and Griffin RG (2014) Paramagnet induced signal quenching in MAS-DNP experiments in frozen homogeneous solutions. J. Magn. Reson 240, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogawski R, Sergeyev IV, Zhang Y, Tran TH, Li YJ, Tong L and McDermott AE (2017) NMR Signal Quenching from Bound Biradical Affinity Reagents in DNP Samples. J. Phys. Chem. B 121, 10770–10781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Williams JK, Schmidt-Rohr K and Hong M (2015) Relaxation-compensated difference spin diffusion NMR for detecting 13C-13C long-range correlations in proteins and polysaccharides. J. Biomol. NMR 61, 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T, Chen YN, Tabuchi A, Cosgrove DJ and Hong M (2016) The Target of β-Expansin EXPB1 in Maize Cell Walls from Binding and Solid-State NMR Studies. Plant Physiol. 172, 2107–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplan M, Cukkemane A, van Zundert GCP, Narasimhan S, Daniels M, Mance D, Waksman G, Bonvin AMJJ, Fronzes R, Folkers GE and Baldus M (2015) Probing a cell-embedded megadalton protein complex by DNP-supported solid-state NMR. Nat. Methods 12, 649–652 [DOI] [PubMed] [Google Scholar]
- 55.Frederick KK, Michaelis VK, Caporini MA, Andreas LB, Debelouchina GT, Griffin RG and Lindquist S (2017) Combining DNP NMR with segmental and specific labeling to study a yeast prion protein strain that is not parallel in-register. Proc. Natl. Acad. Sci. USA 114, 3642–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narasimhan S, Scherpe S, Paioni AL, van der Zwan J, Folkers GE, Ovaa H and Baldus M (2019) DNP-Supported Solid-State NMR Spectroscopy of Proteins Inside Mammalian Cells. Angew. Chem. Int. Edit 58, 12969–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlagnitweit J, Sandoz SF, Jaworski A, Guzzetti I, Aussenac F, Carbajo RJ, Chiarparin E, Pell AJ and Petzold K (2019) Observing an Antisense Drug Complex in Intact Human Cells by in-Cell NMR Spectroscopy. ChemBioChem. 20, 2474–2478 [DOI] [PubMed] [Google Scholar]
- 58.Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol. 125, 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG and White TC (2012) Hidden Killers: Human Fungal Infections. Sci. Transl. Med 4 [DOI] [PubMed] [Google Scholar]
- 60.Fontaine T, Mouyna I, Hartland RP, Paris S and Latge JP (1997) From the surface to the inner layer of the fungal cell wall. Biochem. Soc. Trans 25, 194–199 [DOI] [PubMed] [Google Scholar]
- 61.Nygaard R, Romaniuk JAH, Rice DM and Cegelski L (2015) Spectral snapshots of bacterial cell-wall composition and the influence of antibiotics by whole-cell NMR. Biophys. J 108, 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold AA, Bourgouin JP, Genard B, Warschawski DE, Tremblay R and Marcotte I (2018) Whole cell solid-state NMR study of Chlamydomonas reinhardtii microalgae. J. Biomol. NMR 70, 123–131 [DOI] [PubMed] [Google Scholar]
- 63.Chatterjee S, Prados-Rosales R, Itin B, Casadevall A and Stark RE (2015) Solid-state NMR Reveals the Carbon-based Molecular Architecture of Cryptococcus neoformans Fungal Eumelanins in the Cell Wall. J. Biol. Chem 290, 13779–13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chatterjee S, Prados-Rosales R, Tan S, Phan VC, Chrissian C, Itin B, Wang H, Khajo A, Magliozzo RS, Casadevall A and Stark RE (2018) The melanization road more traveled by: Precursor substrate effects on melanin synthesis in cell-free and fungal cell systems. J. Biol. Chem 293, 20157–20168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terrett OM, Lyczakowski JJ, Yu L, Iuga D, Franks WT, Brown SP, Dupree R and Dupree P (2019) Molecular architecture of softwood revealed by solid-state NMR. Nat. Commun 10, 4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simmons TJ, Mortimer JC, Bernardinelli OD, Poppler AC, Brown SP, deAzevedo ER, Dupree R and Dupree P (2016) Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun 7, 13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang T, Salazar A, Zabotina OA and Hong M (2014) Structure and dynamics of Brachypodium primary cell wall polysaccharides from two-dimensional 13C solid-state nuclear magnetic resonance spectroscopy. Biochemistry. 53, 2840–2854 [DOI] [PubMed] [Google Scholar]
- 68.Wang T, Yang H, Kubicki JD and Hong M (2016) Cellulose Structural Polymorphism in Plant Primary Cell Walls Investigated by High-Field 2D Solid-State NMR Spectroscopy and Density Functional Theory Calculations. Biomacromolecules. 17, 2210–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phyo P, Wang T, Kiemle SN, O’Neill H, Pingali SV, Hong M and Cosgrove DJ (2017) Gradients in Wall Mechanics and Polysaccharides along Growing Inflorescence Stems. Plant Physiol. 175, 1593–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phyo P, Wang T, Xiao CW, Anderson CT and Hong M (2017) Effects of Pectin Molecular Weight Changes on the Structure, Dynamics, and Polysaccharide Interactions of Primary Cell Walls of Arabidopsis thaliana: Insights from Solid-State NMR. Biomacromolecules. 18, 2937–2950 [DOI] [PubMed] [Google Scholar]
- 71.Thongsomboon W, Serra DO, Possling A, Hadjineophytou C, Hengge R and Cegelski L (2018) Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science. 359, 334–338 [DOI] [PubMed] [Google Scholar]
- 72.Romaniuk JA and Cegelski L (2015) Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Phil. Trans. R. Soc. B 370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao W, Fernando LD, Kirui A, Deligey F and Wang T (2020) Solid-state NMR of plant and fungal cell walls: A critical review. Solid State Nucl. Magn. Reson 107, 101660. [DOI] [PubMed] [Google Scholar]
- 74.Wang T, Park YB, Cosgrove DJ and Hong M (2015) Cellulose-Pectin Spatial Contacts Are Inherent to Never-Dried Arabidopsis thaliana Primary Cell Walls: Evidence from Solid-State NMR. Plant Physiol. 168, 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang T, Phyo P and Hong M (2016) Multidimensional solid-state NMR spectroscopy of plant cell walls. Solid State Nucl. Magn. Reson 78, 56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirui A, Ling Z, Kang X, Dickwella Widanage MC, Mentink-Vigier F, French AD and Wang T (2019) Atomic Resolution of Cotton Cellulose Structure Enabled by Dynamic Nuclear Polarization Solid-State NMR. Cellulose. 26, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi H, Lee D, Dubois L, Bardet M, Hediger S and De Paepe G (2012) Rapid Natural-Abundance 2D 13C-13C Correlation Spectroscopy Using Dynamic Nuclear Polarization Enhanced Solid-State NMR and Matrix-Free Sample Preparation. Angew. Chem. Int. Edit 51, 11766–11769 [DOI] [PubMed] [Google Scholar]
- 78.Takahashi H, Hediger S and De Paepe G (2013) Matrix-free dynamic nuclear polarization enables solid-state NMR 13C-13C correlation spectroscopy of proteins at natural isotopic abundance. Chem. Commun 49, 9479–9481 [DOI] [PubMed] [Google Scholar]
- 79.Takahashi H, Ayala I, Bardet M, De Paepe G, Simorre JP and Hediger S (2013) Solid-state NMR on bacterial cells: selective cell wall signal enhancement and resolution improvement using dynamic nuclear polarization. J. Am. Chem. Soc 135, 5105–5110 [DOI] [PubMed] [Google Scholar]
- 80.Kono H (2004) Two-dimensional magic angle spinning NMR investigation of naturally occurring chitins: Precise 1H and 13C resonance assignment of alpha- and beta-chitin. Biopolymers. 75, 255–263 [DOI] [PubMed] [Google Scholar]
- 81.Kameda T, Miyazawa M, Ono H and Yoshida M (2004) Hydrogen bonding structure and stability of alpha-chitin studied by 13C solid-state NMR. Macromol. Biosci 5, 103–106 [DOI] [PubMed] [Google Scholar]
- 82.Sikorski P, Hori R and Wada M (2009) Revisit of alpha-Chitin Crystal Structure Using High Resolution X-ray Diffraction Data. Biomacromolecules. 10, 1100–1105 [DOI] [PubMed] [Google Scholar]
- 83.Yui T, Taki N, Sugiyama J and Hayashi S (2007) Exhaustive crystal structure search and crystal modeling of beta-chitin. Int. J. Biol. Macromol 40, 336–344 [DOI] [PubMed] [Google Scholar]
- 84.Lewandowski JR, De Paepe G, Eddy MT and Griffin RG (2009) 15N-15N proton assisted recoupling in magic angle spinning NMR. J. Am. Chem. Soc 131, 5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Paepe G, Lewandowski JR, Loquet A, Bockmann A and Griffin RG (2008) Proton assisted recoupling and protein structure determination. J. Chem. Phys 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donovan KJ, Jain SK, Silvers R, Linse S and Griffin RG (2017) Proton-Assisted Recoupling (PAR) in Peptides and Proteins. J. Phys. Chem. B 121, 10804–10817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dubroca T, Smith AN, Pike KJ, Froud S, Wylde R, Trociewitz B, Mckay J, Mentink-Vigier F, van Tol J, Wi S, Brey W, Long JR, Frydman L and Hill S (2018) A quasi-optical and corrugated waveguide microwave transmission system for simultaneous dynamic nuclear polarization NMR on two separate 14.1 T spectrometers. J. Magn. Reson 289, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Viger-Gravel J, Lan W, Pinon AC, Berruyer P, Emsley L, Bardet M and Luterbacher J (2019) Topology of Pretreated Wood Fibers Using Dynamic Nuclear Polarization. J. Phys. Chem C 123, 30407–30415 [Google Scholar]
- 89.Perras FA, Luo H, Zhang X, Mosier NS, Pruski M and Abu-Omar MM (2017) Atomic-Level Structure Characterization of Biomass Pre- and Post-Lignin Treatment by Dynamic Nuclear Polarization-Enhanced Solid-State NMR. J. Phys. Chem. A 121, 623–630 [DOI] [PubMed] [Google Scholar]
- 90.Quinn CM, Wang MZ, Fritz MP, Runge B, Ahn J, Xu CY, Perilla JR, Gronenborn AM and Polenova T (2018) Dynamic regulation of HIV-1 capsid interaction with the restriction factor TRIM5 alpha identified by magic-angle spinning NMR and molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 115, 11519–11524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta R, Lu MM, Hou GJ, Caporini MA, Rosay M, Maas W, Struppe J, Suiter C, Ahn J, Byeon IJL, Franks WT, Orwick-Rydmark M, Bertarello A, Oschkinat H, Lesage A, Pintacuda G, Gronenborn AM and Polenova T (2016) Dynamic Nuclear Polarization Enhanced MAS NMR Spectroscopy for Structural Analysis of HIV-1 Protein Assemblies. J. Phys. Chem. B 120, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sergeyev IV, Day LA, Goldbourt A and McDermott AE (2011) Chemical Shifts for the Unusual DNA Structure in Pf1 Bacteriophage from Dynamic-Nuclear-Polarization-Enhanced Solid-State NMR Spectroscopy. J. Am. Chem. Soc 133, 20208–20217 [DOI] [PubMed] [Google Scholar]
- 93.Azais T, Von Euw S, Ajili W, Auzoux-Bordenave S, Bertani P, Gajan D, Emsley L, Nassif N and Lesage A (2019) Structural description of surfaces and interfaces in biominerals by DNP SENS. Solid State Nucl. Magn. Reson 102, 2–11 [DOI] [PubMed] [Google Scholar]
- 94.Goldberga I, Li R, Chow WY, Reid DG, Bashtanova U, Rajan R, Puszkarska A, Oschkinat H and Duer MJ (2019) Detection of nucleic acids and other low abundance components in native bone and osteosarcoma extracellular matrix by isotope enrichment and DNP-enhanced NMR. RSC Adv. 9, 26686–26690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chow WY, Li R, Goldberga I, Reid DG, Rajan R, Clark J, Oschkinat H, Duer MJ, Hayward R and Shanahan CM (2018) Essential but sparse collagen hydroxylysyl post-translational modifications detected by DNP NMR. Chem. Commun 54, 12570–12573 [DOI] [PubMed] [Google Scholar]
- 96.Lu MM, Wang MZ, Sergeyev IV, Quinn CM, Struppe J, Rosay M, Maas W, Gronenborn AM and Polenova T (2019) 19F Dynamic Nuclear Polarization at Fast Magic Angle Spinning for NMR of HIV-1 Capsid Protein Assemblies. J. Am. Chem. Soc 141, 5681–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jaudzems K, Bertarello A, Chaudhari SR, Pica A, Cala-De Paepe D, Barbet-Massin E, Pell AJ, Akopjana I, Kotelovica S, Gajan D, Ouari O, Tars K, Pintacuda G and Lesage A (2018) Dynamic Nuclear Polarization-Enhanced Biomolecular NMR Spectroscopy at High Magnetic Field with Fast Magic-Angle Spinning. Angew. Chem. Int. Edit 57, 7458–7462 [DOI] [PubMed] [Google Scholar]
- 98.Smith AN, Marker K, Hediger S and De Paepe G (2019) Natural Isotopic Abundance 13C and 15N Multidimensional Solid-State NMR Enabled by Dynamic Nuclear Polarization. J. Phys. Chem. Lett 10, 4652–4662 [DOI] [PubMed] [Google Scholar]
- 99.Smith AN, Marker K, Piretra T, Boatz JC, Matlahov I, Kodali R, Hediger S, van der Wel PCA and De Paepe G (2018) Structural Fingerprinting of Protein Aggregates by Dynamic Nuclear Polarization-Enhanced Solid-State NMR at Natural Isotopic Abundance. J. Am. Chem. Soc 140, 14576–14580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marker K, Paul S, Fernandez-de-Alba C, Lee D, Mouesca JM, Hediger S and De Paepe G (2017) Welcoming natural isotopic abundance in solid-state NMR: probing pi-stacking and supramolecular structure of organic nanoassemblies using DNP. Chem. Sci 8, 974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang X, Zhao W, Dickwella Widanage MC, Kirui A, Ozdenvar U and Wang T (2019) CCMRD: A Solid-State NMR Database for Complex Carbohydrates. J. Biomol. NMR in press. DOI: 10.1007/s10858-020-00304-2 [DOI] [PubMed] [Google Scholar]


