Abstract
Due to the global progress of antimicrobial resistance, the World Health Organization (WHO) published the list of the antibiotic-resistant “priority pathogens” in order to promote research and development of new antibiotics to the families of bacteria that cause severe and often deadly infections. In the framework of the One Health approach, the surveillance of these pathogens in different environments should be implemented in order to analyze their spread and the potential risk of transmission of antibiotic resistances by food and water. Therefore, the objective of this work was to determine the presence of high and critical priority pathogens included in the aforementioned list in different aquatic environments in the POCTEFA area (North Spain–South France). In addition to these pathogens, detection of colistin-resistant Enterobacteriaceae was included due its relevance as being the antibiotic of choice to treat infections caused by multidrug resistant bacteria (MDR). From the total of 80 analyzed samples, 100% of the wastewater treatment plants (WWTPs) and collectors (from hospitals and slaughterhouses) and 96.4% of the rivers, carried antibiotic resistant bacteria (ARB) against the tested antibiotics. Fifty-five (17.7%) of the isolates were identified as target microorganisms (high and critical priority pathogens of WHO list) and 58.2% (n = 32) of them came from WWTPs and collectors. Phenotypic and genotypic characterization showed that 96.4% were MDR and resistance to penicillins/cephalosporins was the most widespread. The presence of bla genes, KPC-type carbapenemases, mcr-1 and vanB genes has been confirmed. In summary, the presence of clinically relevant MDR bacteria in the studied aquatic environments demonstrates the need to improve surveillance and treatments of wastewaters from slaughterhouses, hospitals and WWTPs, in order to minimize the dispersion of resistance through the effluents of these areas.
Keywords: WWTPs, collectors, rivers, antibiotic resistance bacteria, antibiotic resistance gene, ESBL
1. Introduction
One of the highest public health challenges worldwide is the increase in the number and types of antimicrobial resistances (AMR) [1]. The use and misuse of antimicrobials in human medicine is one of the main causes of this increasing problem, but inappropriate practices in intensive livestock farms have also contributed to the alarming increase in antibiotic resistant bacteria (ARB) [2,3]. Antibiotics used in animal production with different purposes (therapeutically and prophylactically) are finally disseminated through the environment. It has been estimated that about 75% of the administered antibiotics is not absorbed by animals and is excreted via the feces or urine [4]. In this sense, García-Galán et al., 2011 [5] reported the presence of emerging pollutants in the Ebro basin (area with intensive livestock farms), including at least eight types of antibiotics. As a consequence of the antibiotic pressure, ARB have been isolated from different sources such as farms (manure), water and meat products [4,6,7,8]. The dissemination of AMR throughout the environment represents a risk to human health [9,10]. In particular, one of the main routes for the dissemination of ARB and resistance genes (ARGs) is the aquatic environment [11,12]. Therefore, a One Health approach is needed to address the problem of antimicrobial resistance. Recently, the World Health Organization (WHO) has published a list of antibiotic-resistant priority pathogens with the aim to promote research and development of new antibiotics, as one of the proposed strategies to control the problem of global resistance to antimicrobial medicines [13]. The list includes 12 families of bacteria that pose the greatest threat to human health, especially if they are spread throughout the environment. Among pathogens classified as priority 1 (critical) and 2 (high), carbapenem, β-lactam, vancomycin and methicillin resistances are considered. β-lactam antibiotics have been the most extended therapeutic choice for the treatment of human and animal infections worldwide, and consequently, bacteria have developed different β-lactam resistance mechanisms, such as the production of extended spectrum β-lactamases (ESBLs) and carbapenemases [14]. The presence of bla genes encoding SHV, TEM, CTX-M groups, KPC, NDM and VIM enzymes has been frequently reported in rivers of different regions over the world [15,16]. Despite methicillin-resistant Staphylococcus aureus (MRSA) has been detected basically in clinical environments [17,18], the presence of MRSA mecA/C in river water has been described [19]. Regarding vancomycin resistant enterococci (VRE), although the presence of these bacteria seems to be related to small wild mammals, rabbits and birds [20,21], some authors described the presence of E. faecium vanA and vanB in wastewater and surface waters [22,23]. Colistin has become as the last alternative in human medicine for the treatment of infections due to multidrug-resistant Gram-negative bacteria [24]. Colistin sulfate is used for the control of Enterobacteriaceae infections in pig production in some countries [25,26], contributing to the spread of colistin resistances mediated by the transferable plasmid mcr-1 [27]. In this context, it would be very interesting to search for this type of resistance in different aquatic environments, such as rivers, WWTPs and collectors.
POCTEFA 2014-2020 is the acronym for the INTERREG V-A Spain-France-Andorra Program (https://www.poctefa.eu/). It is a European territorial cooperation program created to promote the sustainable development of the regions near to the Franco-Spanish border (Navarra, Huesca, Zaragoza, Lleida, Pyrénées-Atlantiques, Hautes-Pyrénées, Orientals-Pyrénées, Haute Garonne and Ariege). This area covers a region of 115.583 km2, populated by 15 million habitants, being the intensive livestock farms as the main rural economic engine (especially porcine, poultry and rabbit production). In this sense, the main objective of this study was to determine the presence of ARB in rivers, wastewater treatment plants (WWTPs) and collectors in the North of Spain and South of France (hereafter named POCTEFA area). Specifically, we focused the study on the isolation and characterization of critical and high priority resistant pathogens according to the WHO list: Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumanii carbapenem-resistant; Enterobacteriaceae ESBL-producing; Enterococcus faecium vancomycin-resistant and Staphylococcus aureus methicillin-resistant. In addition, due to the recent interest in colistin resistances, we also included the search for Enterobacteriaceae colistin-resistant.
2. Materials and Methods
2.1. Sample Collection
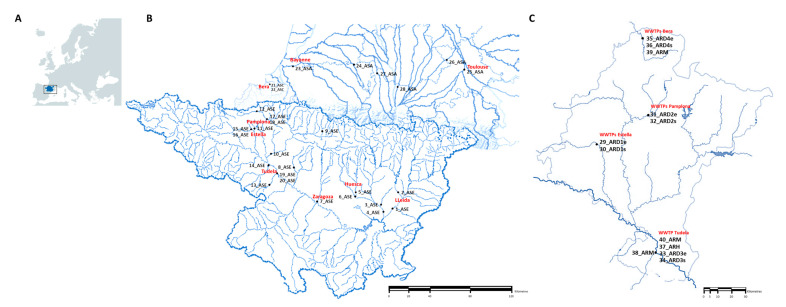
The sampling was performed by trained people from the University of Zaragoza (Laboratory of Water and Environmental Health) in 40 locations from POCTEFA area including rivers, WWTPs and collectors (hospital and slaughterhouses). A total of 80 samples were collected in two seasons of 2018 (April–May and October–November). Rivers were located in France and Spain, whereas WWTPs and collectors were present in Navarra (Northern Spain; Figure 1). Sampling of WWTPs was performed in influent and effluent water and sampling of rivers was done upstream and downstream of the WWTP (when present). Complete information of each point, provided by the Laboratory of Water and Environmental Health of University of Zaragoza, is available in the supplementary material (Tables S1 and S2).
Figure 1.
Geographical location of sampling. (A) POCTEFA area (North Spain and South France). (B) Sampling points of rivers (ASE: Ebro Basin; ASC: Cantabrian Basin; ASA: Adour-Garonne Basin); (C) Sampling points of wastewater treatment plants (WWTPs) and collectors in the Navarra region (ARDe: Discharge of wastewater entering the treatment plant. ARDs: Discharge of wastewater leaving the treatment plant. ARH: Discharge of hospital wastewater. ARM: Discharge of slaughterhouse wastewater).
Samples were taken in sterile containers in accordance with ISO 19458 [28] and ISO 5667-3 [29] standards and stored at 5 ± 3 °C in the dark until they were sent to the University of Navarra. Microbiological analysis was carried out within 24 h of arrival of samples (stored at 5 ± 3 °C).
2.2. Isolation and Identification of Resistant Bacteria
In order to detect the presence of resistant bacteria (even the lethargic ones by environmental stressors such as the temperature or the lack of nutrients), two approaches were performed. In the first method, 1 mL of each sample was spread on the surface of specific selective culture media for each resistance type (described below). In the second method, two previous enrichment processes were carried out for the recovery of stressed cells. This way, 10 mL water samples were transferred to 10 mL of double concentration EE Mossel broth (Difco, Le Pont de Claix, France) and were incubated at 37 ± 1 °C during 24 h, in order to isolate Gram negative bacteria. Similarly, enrichment in Giolitti Cantoni broth (Oxoid, Basingstoke, United Kingdom) was performed for the recovery of Gram positive bacteria (24–48 h at 37 ± 1 °C). Following the incubation periods, isolations were performed on the selected culture media. In the case of carbapenem and colistin resistances, selective culture media was changed in the second sampling in order to improve the recovery of these strains (taking into account the obtained results in the first sampling).
Chromogenic selective plates from bioMerieux (Marcy l’Etoile, France) were used for the isolation of the target resistant bacteria. Thus, ChromID ESBL plates containing a mixture of antibiotics including cefpodoxime (CPD) were used for the isolation of suspicious ESBL-producing strains. ChromID MRSA contains cefoxitin (FOX) as a selective agent and was used for the isolation of MRSA. ChromID VRE agar plates selects vancomycin (VA) resistant Enterococcus, allowing the differentiation between E. faecium and E. faecalis. Finally, ChromID CARBA SMART agar plates and ChromID CARBA agar plates were used for the isolation of carbapenemase-producing Enterobacteriaceae (CPE; first and second sampling events, respectively). In addition, Columbia CNA and MacConkey agar supplemented with 2 µg/mL of colistin (COL; Oxoid) were used for the isolation of colistin resistant bacteria (first and second sampling events, respectively). After the incubation at 37 ± 1 °C during 24–48 h, suspicious colonies were isolated on ChromID CPSE, nutrient agar or blood agar (bioMerieux). The identification was carried out using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS; bioMerieux) or biochemical tests (oxidase, API 20NE, API20E, APIstaph or API 20STREP; bioMerieux). Pure cultures were stored at −80 °C for further characterization.
2.3. Phenotypic and Genotypic Characterization of Resistant Strains
The antibiotic disks used for the phenotypic characterization were provided by Becton Dickinson (Le Pont de Claix, France), ROSCO Diagnostica (Taastrup, Denmark) and by Biomerieux in the case of the E-tests. The results were interpreted according to “Clinical & Laboratory Standars Institute”, CLSI [30] or “The European Committee on Antimicrobial Susceptibility Testing”, EUCAST guidelines [31]. The antimicrobials tested and resistance breakpoints can be found in the supplementary material (Table S3). The specific methodology applied for the phenotypic and/or genotypic characterization of each types of resistance is explained in the subsequent sections.
2.3.1. ESBL-Producer Enterobacteriaceae and Other β-Lactamases
ESBL production was confirmed by the double-disk synergy test (DDST) according to Jarlier et al., 1988 [32]. Basically, the amoxicillin/clavulanic acid (AMC, 30 μg) was placed in the center of the inoculated Mueller Hinton cation-adjusted agar plate (MH; Becton Dickinson) and the following β-lactam antibiotics were placed at a distance of 20 mm: ceftazidime (CAZ, 30 μg), ceftriaxone (CRO, 30 μg), aztreonam (AZT, 30 μg) and cefpodoxime (CPD, 10 μg). After incubation at 37 ± 1 °C for 18–24 h, the strain was considered as the ESBL-producer when the enhanced inhibition zone was observed between the cephalosporin disk and AMC, indicating synergy. AmpC β-lactamase production was determined following the methodology of Thean et al., 2009 [33] by comparing the diameters of each β-lactam or β-lactam with an inhibitor (ceftazidime/clavulanic acid and cefotaxime/clavulanic acid) in MH and MH supplemented with cloxacillin (250 mg/L, Sigma Aldrich, Singapore). When an increased inhibition zone of >5 mm in cloxacillin plates was observed, the microorganisms was considered to be an AmpC-producer [34]. Finally, we studied the presence of metallo-β-lactamases (MBL) according to Arakawa et al., 2000 [35], using CAZ (30 μg), imipenem (IMP, 10 μg) and EDTA (10 μL) disks in MH plates. In addition, an IMP disk was used to which 10 µL of EDTA was added. It was considered an MBL-producing strain when a synergistic effect was observed between the IMP, CAZ and EDTA discs and if the difference between the IMP + EDTA disc and the IMP disc was >5 mm.
The DNA extraction procedure was performed with the DNeasy® Blood and Tissue kit (Qiagen, Barcelona, Spain), using a pretreatment protocol for Gram-negative bacteria and following the manufacturer’s instruction. The quantity and quality of the DNA was analyzed using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
The detection of AmpC β-lactamases genes was performed using the multiplex-PCR assay described by Pérez-Pérez and Hanson, 2002 [36]. The primers, size of the amplicons and conditions followed are summarized in Table 1.
Table 1.
Primers and conditions used for the amplification of the different β-lactamases genes.
| Target | Primer | Sequence (5′–3′) | Amplicon Size (bp) | Conditions |
|---|---|---|---|---|
| bla MOXM | blaMOXM-Fw | GCTGCTCAAGGAGCACAGGAT | 520 | Initial denaturation at 94 °C for 3 min; 25 cycles of amplification: denaturation at 94 °C for 30 s, hybridization at 64 °C for 30 s and extension at 72 °C for 1 min; final elongation at 72 °C for 7 min. |
| blaMOXM-Rv | CACATTGACATAGGTGTGGTGC | |||
| bla CITM | blaCITM-Fw | TGGCCAGAACTGACAGGCAAA | 462 | |
| blaCITM-Rv | TTTCTCCTGAACGTGGCTGGC | |||
| bla DHAM | blaDHAM-Fw | AACTTTCACAGGTGTGCTGGGT | 405 | |
| blaDHAM-Rv | CCGTACGCATACTGGCTTTGC | |||
| bla ACCM | blaACCM-Fw | AACAGCCTCAGCAGCCGGTTA | 346 | |
| blaACCM-Rv | TTCGCCGCAATCATCCCTAGC | |||
| bla EBC | blaEBC-Fw | TCGGTAAAGCCGATGTTGCGG | 302 | |
| blaEBC-Rv | CTTCCACTGCGGCTGCCAGTT | |||
| bla FOX | blaFOX-Fw | AACATGGGGTATCAGGGAGATG | 190 | |
| blaFOX-Rv | CAAAGCGCGTAACCGGATTGG | |||
| bla SHV | blaSHV-Fw | AGGATTGACTGCCTTTTTG | 392 | Initial denaturation at 94 °C for 5 min; 32 cycles of amplification: denaturation at 94 °C for 30 s, hybridization at 54 °C for 30 s and extension at 72 °C for 1 min; final elongation at 72 °C for 10 min. |
| blaSHV-Rv | ATTTGCTGATTTCGCTCG | |||
| bla TEM | blaTEM-Fw | ATCAGCAATAAACCAGC | 516 | |
| blaTEM-Rv | CCCCGAAGAACGTTTTC | |||
| bla OXA | blaOXA-Fw | ATATCTCTACTGTTGCATCTCC | 619 | |
| blaOXA-Rv | AAACCCTTCAAACCATCC | |||
| bla CTX-M1 | blaCTX-M1-Fw | AAAAATCACTGGCCAGTTC | 415 | Initial denaturation at 94 °C for 5 min; 30 cycles of amplification: denaturation at 94 °C for 45 s, hybridization at 55 °C for 30 s and extension at 72 °C for 1 min; final elongation at 72 °C for 6 min. |
| blaCTX-M1-Rv | AGCTTATTCATCGCCACGTT | |||
| bla CTX-M2 | blaCTX-M2-Fw | CGACGCTACCCCTGCTATT | 552 | |
| blaCTX-M2-Rv | CCAGCGTCAGATTTTTCAGG | |||
| bla CTX-M9 | blaCTX-M9-Fw | CAAAGAGAGTGCAACGGATG | 205 | |
| blaCTX-M9-Rv | ATTGGAAAGCGTTCATCACC | |||
| bla CTX-M8 | blaCTX-M8-Fw | TCGCGTTAAGCGGATGATGC | 666 | |
| bla CTX-M25 | blaCTX-M25-Fw | GCACGATGACATTCGGG | 327 | |
| bla CTX-M8/25 | blaCTX-M1-Rv | AACCCACGATGTGGGTAGC | 666/327 |
Fw: Forward, Rv: Reverse.
The identification of blaTEM, blaSHV and blaOXA genes was performed using the multiplex-PCR assay described by Colom et al., 2003 [37] while a modification of the multiplex-PCR described by Woodford et al., 2006 [38] was used for the study of blaCTX-M genes. The reaction mixture composition and amplification conditions for the blaCTX-M genes were described in the manuscript of Ojer-Usoz et al., 2014 [8]. All the details for the several multiplex PCR assays are shown in Table 1.
A bidirectional DNA sequence analysis of the amplicons were performed by the Macrogen EZ-Seq purification service to determine the molecular types of bla genes (Macrogen Europe, Amsterdam, The Netherlands). Searches for DNA and protein homologies were carried out through the National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) using the BLAST program. The alignment of DNA and amino acids sequences was performed using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/).
2.3.2. Carbapenemase-Producing Strains
Disks of ertapenem (ETP, 10 μg, Oxoid), IMP (10 μg, Oxoid) and meropenem (MER, 10 μg, Becton Dickinson) were used to determine carbapenemase production. In addition to the screening of the presence of β-lactamase and metalo-β-lactamase (described above), OXA-48-like, KPC, NDM and VIM type carbapenemases were determined using the immunochromatography test Resist-4 O.K.N (Coris Bioconcept, Gembloux, Belgium), according to the manufacturer instructions.
2.3.3. Colistin Resistant Enterobacteriaceae
COL E-test (Biomerieux) was performed for the determination of this resistance, using EUCAST guidelines [31] for the interpretation of the inhibition zone (Table S3). The presence of mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 genes was detected by conventional PCRs using the specific primers and conditions shown in Table 2 and following the conditions described in the corresponding works [39,40,41,42,43].
Table 2.
Primers and conditions used for the amplification of the mcr genes.
| Target | Primer | Sequence (5′–3′) | Amplicon Size (bp) | Conditions |
| mcr-1 | mcr-1-Fw | CGGTCAGTCCGTTTGTTC | 309 | 20 cycles of amplification at 94 °C for 30 s, 58 °C for 90 s, 72 °C for 1 min and final 72 °C for 10 min |
| mcr-1-Rv | CTTGGTCGGTCTGTAGGG | |||
| mcr-2 | mcr-2-Fw | TGTTGCTTGTGCCGATTGGA | 567 | 33 cycles of amplification at 95 °C for 3 min, 65 °C for 30 s, 72 °C for 1 min and final 72 °C for 10 min. |
| mcr-2-Rv | AGATGGTATTGTTGGTTGCTG | |||
| mcr-3 | mcr-3-Fw | TTGGCACTGTATTTTGCATTT | 542 | 30 cycles of amplification at 95 °C for 30 s, 50 °C for 30 s, 72 °C for 45 s and final 72 °C for 7 min. |
| mcr-3-Rv | TTAACGAAATTGGCTGGAACA | |||
| mcr-4 | mcr-4-Fw | ATTGGGATAGTCGCCTTTTT | 487 | 20 cycles of amplification at 94 °C for 30 s, 58 °C for 90 s, 72 °C for 1 min and final 72 °C for 10 min |
| mcr-4-Rv | TTACAGCCAGAATCATTATCA | |||
| mcr-5 | mcr-5-Fw | ATGCGGTTGTCTGCATTTATC | 1644 | 30 cycles of amplification at 95 °C for 30 s, 50 °C for 30 s, 72 °C for 95 s and final 72 °C for 5 min. |
| mcr-5-Rv | TCATTGTGGTTGTCCTTTTCTG |
Fw: Forward, Rv: Reverse.
2.3.4. Methicillin Resistant Strains
Methicillin resistances were confirmed by using FOX disks (30 μg, Becton Dickinson). For the determination of gene mecA, the AlereTM PBP2a test was performed according to the manufacturer instructions (Abbot, Scarborough, Maine). This is a rapid qualitative immunochromatographic analysis for the detection of penicillin 2a binding protein (encoded by mecA).
2.3.5. Vancomycin Resistant Strains
The VA resistance of E. faecium was confirmed by the E-test (bioMerieux), in Mueller Hinton Agar with 5% sheep blood (bioMerieux). Additional E-test of teicoplanin (TEC; bioMerieux) was performed in positive strains in order to determine the presence of gene vanA or vanB. Strains with the vanA phenotype are characterized by a high level of resistance to both VA (MIC ≥ 64 µg/mL) and TEC (MIC ≥ 16 µg/mL). However, strains carrying the vanB gene are characterized by variable levels of resistance to VA (MIC between 4 and ≥ 1000 µg/mL) and sensitivity to TEC [20].
2.4. Antimicrobial Resistance Patterns
The antimicrobial susceptibility of resistant strains to additional antibiotics was obtained in the MicroScan® system (Siemens AG, Munich, Germany). NM37, PN28 and Neg Multidrug Resistant MIC 1 panels (Siemens AG, Germany) were used in combination with Lab Pro® 3.5 software for determining the minimum inhibitory concentrations (MICs). The panels included the following antimicrobials: AMC, ampicillin (AMP), ampicillin-sulbactam (AMS), azithromycin (AZI), AZT, cefazolin (CZ), cefepime (FEP), CAZ, cefuroxime (CXM), CPD, cefotaxime (CTX), FOX, chloramphenicol (CHL), ciprofloxacin (CIP), COL, clindamycin (Cd), daptomycin (DAP), ETP, erythromycin (ERY), fosfomycin (FOT), fusidic acid (FA), gentamicin (GM), IMP, levofloxacin (LV), linezolid (Lz), MER, mupirocin (MUP), moxifloxacin (MXF), mezlocillin (MZ), norfloxacin (NOR), nitrofurantoin (FD), oxacillin (OX), penicillin (P), piperacillin (PIP), piperacillin-tazobactam (TZP), rifampicin (RA), synercid (SYN), tobramycin (TO), tetracycline (TET), tigecycline (TIG), TEC, trimethoprim-sulfamethoxazole (SXT) and VA.
This automated method provided very interesting results for the study of ESBL-producing bacteria. ESBL production was confirmed when a > 3 two-fold concentration decrease occurred in an MIC for any of β-lactams tested in combination with clavulanic acid versus its MIC when tested alone [8,44]. The MIC50 and MIC90 (minimum concentration required to inhibit the growth of 50% and 90% of organisms, respectively) were used to evaluate antibiotic sensitivities. Multi-drug resistances (MDR) and extensive MDR were considered when resistances to three or at least five antimicrobial agents were detected, respectively [45].
2.5. Statistical Analysis
The results for the rates of resistances to antibiotics were subjected to statistical processing with the SPSS 15 software (SPSS Inc., Chicago, IL, USA), applying the Chi-square (X2) test with a level of significance of p < 0.05.
3. Results
3.1. Prevalence of Resistant Bacteria in Rivers and Sewage Waters
Table 3 shows the positive samples (in red) regarding antibiotic resistances in both sampling events. In relation to rivers (samples 1–28), 96.4% were carriers of antibiotic resistant bacteria for at least one of the selected family types, being only one river in the Ebro basin (9_ASE) negative in both samplings. However, a lower prevalence of positive samples was detected in the Adour-Garonne Basin (samples 23 to 28), and COL resistances were not detected in the French area.
Table 3.
Isolation of resistant bacteria in selective culture media (red: presence; green: absence). 1st SE, 2nd SE: first and second sampling events; ASE: Ebro Basin; ASC: Cantabrian Basin; ASA: Adour-Garonne Basin; ARDe: Discharge of wastewater entering the treatment plant; ARDs: Discharge of wastewater leaving the treatment plant; ARH: Discharge of hospital wastewater; ARM: Discharge of slaughterhouse wastewater; CPD (Cefpodoxime); FOX (Cefoxitin); VA (Vancomycin); CARB (Carbapenem); COL (Colistin).
| Sample point | CPD 1st SE | CPD 2nd SE | FOX 1st SE | FOX 2nd SE | VA 1st SE | VA 2nd SE | CARB 1st SE | CARB 2nd SE | COL 1st SE | COL 2nd SE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1_ASE | ||||||||||||||
| 2_ASE | ||||||||||||||
| 3_ASE | ||||||||||||||
| 4_ASE | ||||||||||||||
| 5_ASE | ||||||||||||||
| 6_ASE | ||||||||||||||
| 7_ASE | ||||||||||||||
| 8_ASE | ||||||||||||||
| 9_ASE | ||||||||||||||
| 10_ASE | ||||||||||||||
| 11_ASE | ||||||||||||||
| 12_ASE | ||||||||||||||
| 13_ASE | ||||||||||||||
| 14_ASE | ||||||||||||||
| 15_ASE | ||||||||||||||
| 16_ASE | ||||||||||||||
| 17_ASE | ||||||||||||||
| 18_ASE | ||||||||||||||
| 19_ASE | ||||||||||||||
| 20_ASE | ||||||||||||||
| 21_ASC | ||||||||||||||
| 22_ASC | ||||||||||||||
| 23_ASA | ||||||||||||||
| 24_ASA | ||||||||||||||
| 25_ASA | ||||||||||||||
| 26_ASA | ||||||||||||||
| 27_ASA | ||||||||||||||
| 28_ASA | ||||||||||||||
| 29_ARD1e | ||||||||||||||
| 30_ARD1s | ||||||||||||||
| 31_ARD2e | ||||||||||||||
| 32_ARD2s | ||||||||||||||
| 33_ARD3e | ||||||||||||||
| 34_ARD3s | ||||||||||||||
| 35_ARD4e | ||||||||||||||
| 36_ARD4s | ||||||||||||||
| 37_ARH | ||||||||||||||
| 38_ARM | ||||||||||||||
| 39_ARM | ||||||||||||||
| 40_ARM |
As shown in Table 4, the prevalence was similar in both sampling events (with the exception of carbapenem and COL, p < 0.05), the resistances to CPD and FOX being the prevalent ones. Changes in the methodology for a better isolation of COL and carbapenem resistant bacteria may be the cause of the increase of positive samples for these antibiotics in the second sampling.
Table 4.
Percentage of positive rivers and WWTPs and collectors for each antibiotic in both sampling events and the number of resistant strains isolated.
| Antimicrobial Resistance | % Positive Rivers | % Positive WWTP/C | N Total Isolates | N of Confirmed Target ARB (%) | Target ARB | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1st SE | 2nd SE | 1st SE | 2nd SE | Rivers | WWTP/C | Rivers | WWTP/C | ||
| Cefpodoxime | 75 | 50 | 83.3 | 100 | 45 | 25 | 18 (40) | 19 (75) | ESBL Enterobacteriaceae |
| Cefoxitin | 71.4 | 75 | 83.3 | 100 | 89 | 39 | 2 (2.3) | 0 | S. aureus MRSA |
| Carbapenems | 32.1 | 92.8 a | 41.6 | 91.6 a | 50 | 27 | 0 | 0 | A. baumanni |
| 0 | 2 (7.4) | P. aeruginosa | |||||||
| 0 | 5 (18.5) | Enterobacteriaceae | |||||||
| Vancomycin | 42.9 | 32.1 | 75 | 91.6 | 8 | 15 | 1 (12.5) | 4 (26.6) | E. faecium |
| Colistin | 3.6 | 53.6 b | 8.3 | 66.6 b | 8 | 5 | 2 (25) | 2 (40) | Enterobacteriaceae |
| Total | 200 | 111 | 23 (11.5) | 32 (28.9) | |||||
a and b are the statistically significant differences (p < 0.05) between the 1st and 2nd sampling events (SE); WWTP/C: wastewater treatment plant and collectors; Target ARB: antibiotic resistant bacteria included in the list of high and critical priority pathogens of WHO.
Regarding WWTP and collectors, 100% of the samples were positive for at least one type of resistance in both samplings and no differences were observed (p > 0.05) between the influent and effluent water in the treatment plants (Table 3). It must be noticed that the greatest variety and number of target resistant bacteria were isolated from wastewaters (n = 32, 28.9%). In this way, all target carbapenem resistant pathogens (n = 7) and the majority (80%) of E. faecium resistant to VA were isolated from samples of influent water of sewage treatment plants and collectors, while no MRSA was detected (Table 4).
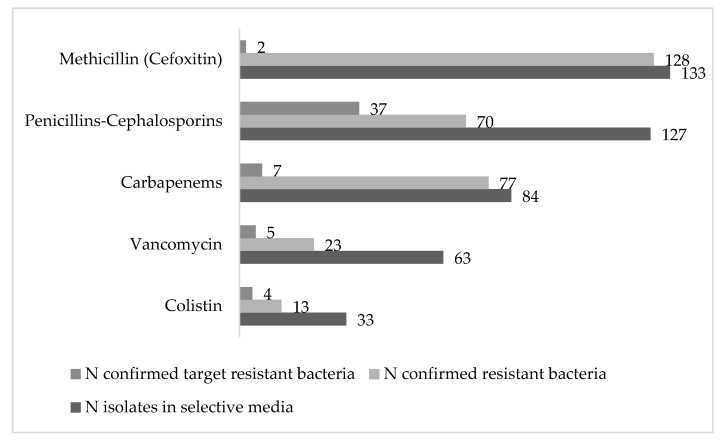
From the total of 440 strains isolated in selective media, 311 (70.7%) were confirmed as resistant by the aforementioned phenotypic methods (Figure 2).
Figure 2.
Total number of resistant strains isolated from water samples in the POCTEFA area.
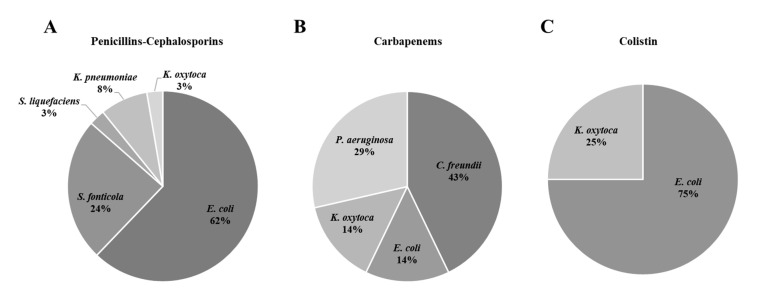
In general, penicillin and cephalosporin resistances were the most extended ones (63.7%), followed by carbapenem resistances (24.7%) and a lower prevalence of VA and COL resistances was detected (7.4% and 4.2%, respectively). Due to the large number of environmental strains isolated with innate resistance to these groups of antibiotics (mainly penicillin resistance), only 17.7% (n = 55) of the isolates were identified as target priority pathogens (with acquired resistance), with a high presence of ESBL-producers (52.8% n = 37). The majority of isolates of this target group were identified as E. coli (62%) followed by Serratia (Figure 3A). Similarly, only 2 out of 128 isolates resistant to FOX (1.6%) were confirmed as MRSA. Regarding carbapenemase-producing isolates 7 out of 77 (9%) corresponded to the list of priority pathogens (P. aeruginosa and Enterobacteriaceae) and no A. baumannii was isolated (Figure 3B). In addition, 4 out of the 13 COL resistant Enterobacteriaceae (30.8%) were identified, E. coli being the prevalent one (Figure 3C). Finally, five E. faecium (21.7%) were identified from the total of 23 VA resistant isolates.
Figure 3.
Genus distribution of isolated Gram negative target resistant bacteria. (A) Penicillins-Cephalosporins; (B) Carbapenems and (C) Colistin.
3.2. Characteristics of the Target Isolated Pathogens
A summary of the antimicrobial resistance patterns and antimicrobial resistance genes (ARG) of the 48 target Gram negative isolates is present in Table 5.
Table 5.
Characteristics and antibiotic resistance profile of isolated Gram negative bacteria.
| Nº Strain | Samples Code | Species | Resistance Genes | AmpC | Antimicrobial Resistance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillins | Cephalosporins | Monobactams | β-lactamase Inhibitors | Carbapenems | AminogLycosides | Quinolones | Tetracyclines | Others | MDR | Extensive MDR | |||||
| 1 | 3_ASE | E. coli | TEML-278, CTX-M14 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMS | - | GM, TO | LV, CIP, MXF, NOR | TET | SXT, FOT, CHL | + | + |
| 2 | 3_ASE | E. coli | TEML-278, CTX-M14 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, FOX, FEP | AZT | AMC, AMS | - | GM, TO | LV, CIP, MXF, NOR | TET | SXT, FOT, CHL | + | + |
| 3 | 4_ASE | E. coli | TEML-278, CTX-M14 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, FOX, FEP | AZT | AMC, AMS | - | GM, TO | LV, CIP, MXF, NOR | TET | SXT, FOT, CHL | + | + |
| 4 | 6_ASE | E. coli | CTX-M15 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS | MER | - | LV, CIP, MXF, NOR | TET, TIG | SXT, CHL | + | + |
| 5 | 8_ASE | E. coli | TEML-278, CTX-M14 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMS | - | - | LV, CIP, MXF, NOR | TET | - | + | + |
| 6 | 17_ASE | E. coli | CTX-M1 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | - | - | TET | CHL | + | + |
| 7 | 17_ASE | E. coli | CTX-M1 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | - | - | TET | CHL | + | + |
| 8 | 29_ARD1e | E. coli | CTX-M1 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMS | - | - | - | TET | - | + | + |
| 9 | 30_ARD1s | E. coli | TEML-278, CTX-M14 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | - | - | - | - | LV, CIP, MXF, NOR | - | - | + | - |
| 10 | 30_ARD1s | E. coli | CTX-M14 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMS | - | - | - | - | SXT, FOT | + | + |
| 11 | 31_ARD2e | E. coli | CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | - | - | - | SXT | + | - |
| 12 | 32_ARD2s | E. coli | CTX-M1, SHV-12 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | - | LV, CIP, MXF, NOR | - | CHL | + | + |
| 13 | 32_ARD2s | E. coli | TEML-278, CTX-M1, SHV-12 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMC, AMS, TZP | - | - | LV, CIP, MXF, NOR | TET | SXT, CHL | + | + |
| 14 | 33_ARD3e | E. coli | TEM-171, CTX-M1 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMS | - | - | - | - | - | + | - |
| 15 | 34_ARD3s | E. coli | TEML-278, CTX-M14 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | - | AMS | - | GM, TO | - | TET | SXT | + | + |
| 16 | 34_ARD3s | E. coli | TEML-278, CTX-M14 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | - | AMS | - | GM, TO | - | TET | SXT | + | + |
| 17 | 35_ARD4e | E. coli | TEML-278, CTX-M1 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | GM, TO | LV, CIP, MXF, NOR | TET | SXT, FOT | + | + |
| 18 | 7_ASE | E. coli | TEML-278, SHV-12 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | - | - | TET | + | - | |
| 19 | 30_ARD1s | E. coli | TEM-171, CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | - | - | - | - | TET | + | - | |
| 20 | 32_ARD2s | E. coli | TEML-278, CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMC, AMS | - | - | - | - | FOT | + | + |
| 21 | 35_ARD4e | E. coli | TEM-176, CTX-M15, CTX-M14 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | - | - | - | - | - | - | - | - | |
| 22 | 36_ARD4s | E. coli | OXA-1, CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMC, AMS | - | TO | LV, CIP, MXF, NOR | - | SXT | + | + |
| 23 | 40_ARM | E. coli | CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMC, AMS | - | - | LV, CIP, MXF, NOR | TET | SXT, CHL | + | + |
| 24 | 7_ASE | S. fonticola | CTX-M1 | + | AMP, MZ | CZ, CXM, CPD, CTX, CAZ | AZT | AMC | - | - | - | - | - | + | - |
| 25 | 11_ASE | S. fonticola | - | + | AMP | CZ, CXM, CPD, CTX | - | AMC | - | - | - | - | - | + | - |
| 26 | 11_ASE | S. fonticola | CTX-M1 | + | AMP | CZ, CXM, CPD, CTX, CAZ | AZT | AMC, AMS | - | - | - | - | - | + | - |
| 27 | 11_ASE | S. fonticola | CTX-M15 | + | AMP, PIP | CZ, CXM, CPD, CTX | - | AMC, AMS | - | - | MXF | - | FOT | + | + |
| 28 | 20_ASE | S. fonticola | - | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ | AZT | AMC, AMS | - | - | - | - | - | + | - |
| 29 | 3_ASE | S. fonticola | TEM-171 | + | AMP | CZ, CXM, CPD, CTX | - | AMC | ETP | - | - | - | - | + | - |
| 30 | 5_ASE | S. fonticola | TEM-171, ACC | + | AMP, PIP | CZ, CXM, CPD, CTX | - | AMC, AMS, TZP | - | - | - | - | - | + | - |
| 31 | 11_ASE | S. fonticola | TEM-171 | + | AMP | CZ, CXM, CPD, CTX | - | AMC | - | - | - | - | - | + | - |
| 32 | 39_ARM | S. fonticola | CTX-M15, ACC | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX | AZT | AMC, AMS | - | - | - | - | COL | + | + |
| 33 | 8_ASE | S. liquefaciens | - | + | AMP | CZ, CXM, CPD | - | AMC | - | - | - | - | - | + | - |
| 34 | 33_ARD3e | K. pneumoniae | TEM-171, SHV-12, CTX-M1, DHA | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS | MER | - | LV, CIP, MXF, NOR | TET | SXT, FOT, CHL | + | + |
| 35 | 35_ARD4e | K. pneumoniae | CTX-M14 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS, TZP | MER | - | LV, CIP, MXF, NOR | TET | SXT, FOT, CHL | + | + |
| 36 | 35_ARD4e | K. pneumoniae | TEML-278, SHV-12, OXA-1, CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMC, AMS | - | TO | LV, CIP, MXF, NOR | - | SXT, CHL | + | + |
| 37 | 11_ASE | K. oxytoca | - | + | AMP | CZ, FOX, FEP | AZT | AMC | - | - | - | - | - | + | - |
| 38 | 33_ARD3e | E. coli | TEML-278, KPC | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, FOX, FEP | AZT | AMC, AMS, TZP | ETP, MER, IMP | - | LV, CIP, MXF, NOR | - | SXT, FOT | + | + |
| 39 | 33_ARD3e | K. oxytoca | TEML-278, KPC | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FEP | AZT | AMC, AMS, TZP | ETP, MER, IMP | TO | LV, CIP, MXF, NOR | - | SXT, FOT | + | + |
| 40 | 36_ARD4s | C. freundii | CTX-M1 | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | - | AMC, AMS, TZP | IMP | - | MXF | - | SXT | + | + |
| 41 | 33_ARD3e | C. freundii | TEML-278, EBC, DHA, KPC | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS, TZP | ETP, MER, IMP | GM, TO | MXF | - | FOT | + | + |
| 42 | 39_ARM | C. freundii | TEML-278, EBC, KPC | + | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS, TZP | ETP, MER, IMP | GM, TO | NOR | - | FOT | + | + |
| 43 | 33_ARD3e | P. aeruginosa | TEML-278 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS | MER, IMP | AM, GM, TO | LV, CIP, NOR | TET | SXT, FOT, CHL | + | + |
| 44 | 29_ARD1e | P. aeruginosa | - | + | AMP, MZ | CZ, CXM, CPD, CTX, FOX | - | AMC, AMS | ETP, MER, IMP | - | - | TET | SXT, FOT, CHL | + | + |
| 45 | 22_ASC | E. coli | - | - | - | - | - | - | - | - | - | - | COL | - | - |
| 46 | 39_ARM | E. coli | CTX-M15 | - | AMP, PIP, MZ | CZ, CXM, CPD, CTX, CAZ, FOX, FEP | AZT | AMC, AMS, TZP | - | - | - | - | COL, FOT | + | + |
| 47 | 40_ARM | E. coli | mcr-1 | - | - | - | - | - | - | - | LV, CIP, MXF, NOR | TET | COL, SXT | + | - |
| 48 | 1_ASE | K. oxytoca | - | - | AMP | - | - | - | - | - | - | - | COL, FOT | + | - |
AMP, ampicillin; PIP, piperacillin; MZ, mezlocillin; CZ, cefazolin; CXM, cefuroxime; CPD, cefpodoxime; CTX, cefotaxime; CAZ, ceftazidime; FOX, cefoxitin; FEP, cefepime; AZT, aztreonam; AMC, amoxicillin-clavulanic acid; AMS, ampicillin-sulbactam; TZP, piperacillin-tazobactam; ETP, ertapenem; MER, meropenem; IMP, imipenem; GM, gentamicin; TO, tobramycin; LV, levofloxacin; CIP, ciprofloxacin; MXF, moxifloxacin; NOR, norfloxacin; TET, tetracycline; TIG, tigecycline; SXT, trimethoprim-sulfamethoxazole; COL, colistin; FOT, fosfomycin; CHL, chloramphenicol.
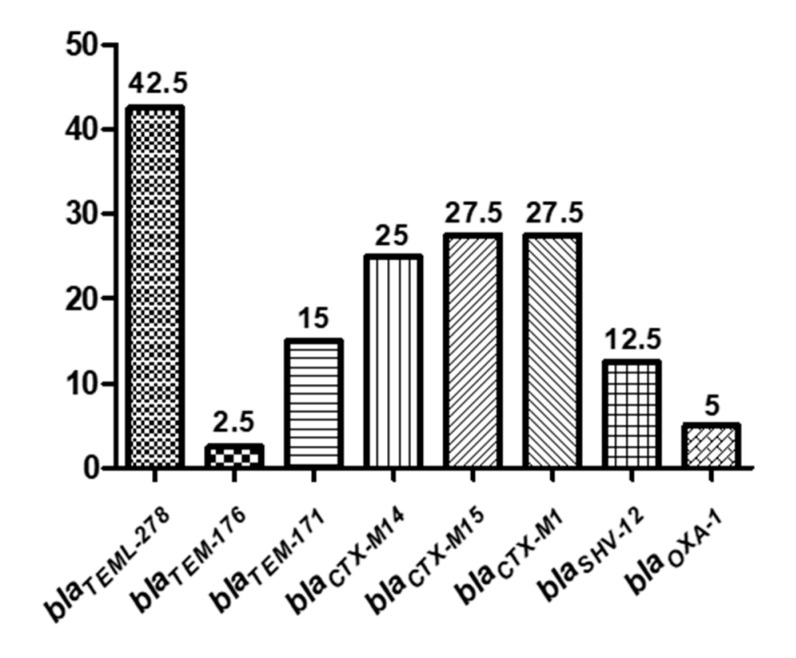
ESBL production was confirmed by the double-disk synergy test (DDST) and MicroScan® system in a 93.7% (n = 45) of the strains. However, the presence of bla genes was confirmed by PCR and sequencing in 88.9% (n = 40) of them. This could be probably related with the higher specificity of these genotypic methods in E. coli strains rather than in other species (Serratia, Klebsiella or Pseudomonas). Regarding the incidence of bla genes, the prevalent one was blaCTX-M (80%), followed by blaTEM (60%), blaSHV-12 (12.5%) and blaOXA-1 (5%; Figure 4). The sequence analysis demonstrated that genes from CTX-M1 group (blaCTX-M1 and blaCTX-M15) were present in 55% of ESBL-producing isolates (Figure 4). Regarding the CTX-M-9 group, it was present in 25% of ESBL-producing isolates and the sequences shown that all of them corresponded to the blaCTX-M-14 gene. Genes encoding for blaCTX-M9, blaCTX-M2, blaCTX-M8 and blaCTX-M25 were not detected. Sequence analysis demonstrates that all isolates carrying the β-lactamase TEM (Temoneira) belonged to the class TEM-1, being blaTEML-278 the prevalent one. In general, CTX-M and TEM bla genes were widely distributed among all the water sources, but blaSHV-12 and blaOXA-1 were mainly detected in WWTPs plants. Furthermore, 18 (45%) of the isolates had two or more bla genes (Table 5). Specifically, one of the K. pneumoniae strains carried four different bla types (blaTEML-278, blaSHV-12, blaOXA-1 and blaCTX-M15). In addition, blaOXA-1 and blaSHV-12 genes were always detected together with other β-lactamase genes.
Figure 4.
Prevalence (percentage) of β-lactamase genes in Gram negative isolates.
Otherwise, the presence of metallo-β-lactamases was not observed in any of the selected strains, while 50% of isolates (n = 24) were confirmed as AmpC-β-lactamases-producers (Table 5). Nevertheless, AmpC genes (ACC, DHA and EBC) were detected in only five strains (numbers 30, 32, 34, 41 and 42 in Table 5). With the exception of the river isolate (number 30), the remaining four strains came from a WWTP and a duck slaughterhouse collector located in the same geographical area. Furthermore, all the strains had been characterized as carriers of other types of bla genes. In particular, the K. pneumoniae isolate number 34, contained three types of bla genes (blaTEM-171, blaSHV-12 and blaCTX-M1). Regarding carbapenemase-producing isolates, all of them were negative for carbapenemases type OXA-48, NDM and VIM. However, the presence of KPC-type carbapenemases was confirmed in four strains (36.3%), corresponding with the strains isolated from the aforementioned WWTPs and the duck slaughterhouse collector (numbers 38, 39, 41 and 42), in which AmpC genes were detected (Table 5). Furthermore, all the carbapenemase-producing bacteria were ESBL carriers, blaTEML-278 being the most extended bla gen (71.4%). In another way, despite all Enterobacteriaceae being confirmed as COL resistant by the E-test and MicroScan® system, the presence of the phosphoethanolamine transferase mcr-1 gene was confirmed in only one E. coli (number 47 in Table 5) and all the strains were negative for the rest of the variants of mcr. This E. coli mcr-1 was isolated from the collector of a rabbit slaughterhouse and was resistant to quinolones, tetracyclines and SXT, while it was not resistant to β-lactam antibiotics (Table 5). In fact, from the four COL-resistant isolates, only one strain of E. coli isolated from duck slaughterhouse collector (number 32) was resistant to this group of antibiotics and carried the blaCTX-M15 gene
With respect to the target Gram positive resistant isolates (n = 7, Table 6), the five strains identified as VA resistant E. faecium (VRE) were sensitive for TEC and considered as the vanB phenotype according to the recommendations (CLSI, 2018). Finally, the two MRSA isolates were negative in the AlereTM PBP2a test for checking the presence of the mecA gene, the most widespread genetic mechanism involved in this resistance.
Table 6.
Characteristics and antibiotic resistance profile of isolated Gram positive bacteria.
| Nº Strain | Samples Code | Species | Resistance Genes | Antimicrobial Resistance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillins | Cephalosporins | Carbapenems | Glycopeptides | Aminoglycosides | Macrolides | Quinolones | Tetracyclines | Others | MDR | Extensive MDR | ||||
| 49 | 14_ASE | E. faecium | vanB | OX | CXM, FEP | ETP, MER | VA | GM | ERY | - | TET | FA, Cd, FOT, SYN | + | + |
| 50 | 40_ARM | E. faecium | vanB | - | CXM, FEP | - | VA | - | ERY | - | - | FA, MUP, DAP, Cd, FOT | + | + |
| 51 | 29_ARD1e | E. faecium | vanB | OX | CXM, FEP | ETP, MER | VA | GM, TO | ERY | LV, CIP, MXF | TET | FA, SXT, Cd, FOT, SYN | + | + |
| 52 | 33_ARD3e | E. faecium | vanB | P, OX | CXM, FEP | ETP, MER | VA | GM, TO | ERY | LV, CIP, MXF | TET | FA, SXT, DAP, Cd, FOT, RA, FD, SYN | + | + |
| 53 | 40_ARM | E. faecium | vanB | OX | CXM, FEP | ETP, MER | VA | GM | ERY | LV, CIP, MXF | TET | FA, SXT, Cd, FD, SYN | + | + |
| 54 | 4_ASE | S. aureus | - | AMP, P, OX | CXM, FOX, FEP | ETP, MER, IMP | - | - | AZI, ERY | - | - | MUP, Cd, FOT | + | + |
| 55 | 15_ASE | S. aureus | - | AMP, P, OX | FOX | - | VA, TEC | - | ERY | - | - | LZ, DAP, Cd, SYN | + | + |
AMP, ampicillin; P, Penicillin; OX, oxacilin; CXM, cefuroxime; FOX, cefoxitin; FEP, cefepime; ETP, ertapenem; MER, meropenem; IMP, imipenem; VA, vancomycin; TEC, teicoplanin GM, gentamicin; TO, tobramycin; AZI, azithromycin; ERY, erythromycin; LV, levofloxacin; CIP, ciprofloxacin; MXF, moxifloxacin; TET, tetracycline; FA, fusidic acid; SXT trimethoprim-sulfamethoxazole; Lz, Linezolid; MUP mupirocin; DAP, Daptomycin; Cd, clindamycin; FOT, fosfomycin; RA, rifampicin; FD, nitrofurantoin; SYN, synercid.
3.3. Multidrug Resistance Profiles
MDR (resistance to at least three antibiotics families) and extensive MDR (at least to five families) were observed in 96.4% and 67.2% of the strains respectively, with MDR being observed in 100% of the Gram positive strains (Table 5 and Table 6). High levels of resistance against penicillins, cephalosporins and β-lactamase inhibitors were detected in the Gram negative isolates (n = 48). In fact, the higher resistances were observed in AMP (95.8%), CZ (93.75%), CXM (91.6%), CPD (91.6%) and CTX (89.6%), followed by FEP (70.8%) and CAZ (66.6%). Furthermore, the majority of the isolates (77%) showed susceptibility to carbapenems (ETP, MER and IMP), despite the fact that some isolates showed MIC values in the MicroScan® close to the breaking point for IMP. In relation to monobactams, the 68.7% of strains were resistant against AZT, while more reduced resistance against tetracyclines (41.6%), CHL (29.2%) and aminoglycosides (25%) was observed. The percentage of resistance against quinolones and sulfonamides was approximately 50%. Finally, resistance to COL was the least prevalent (10.4%), with only five positive confirmed strains.
Regarding Gram positive strains (n = 7), 100% of isolates were resistant to ERY and the majority (85.7%) were resistant to OX and fourth generation cephalosporins (FEP). Furthermore, resistance to glycopeptides, such as VA, was also prevalent (85.7%). In addition, one of the MRSA isolates was also resistant to VA and TEC, an important fact since MRSA with intermediate resistance to VA are also considered in the WHO list. Likewise, 71.4% of isolates were resistant to carbapenems, whereas the resistance against aminoglycosides (57.1% GM and 28.6% TO), tetracyclines (57.1%) and quinolones (42.9%) was lower.
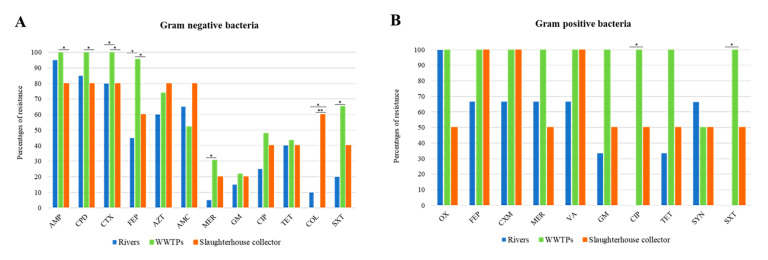
The resistance rates to each individual antibiotic according to the isolates origin is represented in Figure 5A,B, for Gram negative and Gram positive strains, respectively. Regarding Gram negative bacteria, strains isolated from wastewater had the highest resistance rates for AMP, CPD, CTX, FEP, MER and SXT (p < 0.05). Besides, significant differences were found between rivers and WWTPs for COL resistance (p < 0.05). In fact, very significant differences were found among WWTPs and collectors (p = 0.0001). Concerning Gram positive bacteria, only significant differences were found between rivers and WWTPs for CIP and SXT (p < 0.05).
Figure 5.
Resistance rate to each antibiotic according to the isolates origin. (A) Gram negative strains and (B) Gram positive strains. AMP (ampicillin); CPD (cefpodoxime); CTX (cefotaxime); FEP (cefepime); AZT (aztreonam); AMC (amoxicillin-clavulanic acid); MER (meropenem); GM (gentamicin); CIP (ciprofloxacin); TET (tetracycline); COL (colistin); SXT (trimethoprim-sulfamethoxazole); OX (oxacilin); CXM (cefuroxime); VA (vancomycin); SYN (synercid). (*, p < 0.05, **, p < 0.01).
4. Discussion
This study aimed to determine the prevalence of antibiotic resistant bacteria in aquatic environments of the POCTEFA area, a region of intensive livestock activity. The widespread presence of resistant bacteria observed in rivers, WWTPs and collectors (96.4% and 100%, respectively), highlight the impact of human activity on the spread of these resistances, especially from hospital and livestock production. In fact, 55 resistant strains identified as critical and high priority resistant pathogens (according to WHO list) were isolated in the study.
The wastewater from slaughterhouses is considered a relevant source of antimicrobial resistant bacteria and consequently may be important for its diffusion into the environment [46]. The livestock pressure in most of the Spanish rivers studied in this work was high, where pig farms stood out mainly (Table S1). This could be the reason of the higher prevalence of resistances in the Spanish rivers than the French ones (Table 3). In addition, the unique negative river regarding the presence of resistant bacteria (9_ASE) was located in the Pyrenees area, where no relevant cattle exploitations such as pigs, birds and rabbits were reported.
In agreement with our results, previous studies reported an increase of ESBL and carbapenemase-producing Enterobacteriaceae (CPE) in rivers and WWTPs, with high percentages of clinically relevant multidrug resistant bacteria and related genes (intI1, sul1, blaOXA, mcr-1, blaCTX-M15, blaKPC and blaVIM, among others) that were still present in effluent samples, indicating an insufficient reduction during conventional wastewater treatment [47]. In this line, our results are in agreement with previous work published by Ojer-Usoz in the same region of Navarra [8], with a similar prevalence of ESBL after 6 years. In addition, the association between blaOXA-1 and resistance to aminoglycosides and quinolones reported by Osińska et al., 2016 [48] was confirmed in the present study, because the two WWTP isolates carrying blaOXA-1 were resistant to TO, LV, CIP, MXF and NOR. Indeed, the increased quinolone resistance rate in the isolates (50%) may be caused by the use of enrofloxacin in slaughtered broiler herds [46]. Furthermore, the high prevalence regarding AmpC β-lactamases could be related with the large number of Serratia and Citrobacter strains (carriers of chromosomal AmpC). In addition, it is interesting to keep in mind that ESBL and AmpC coproduction was detected in 19 strains, despite only five strains being confirmed as AmpC-producers by molecular methods. Moreover, two of them (numbers 39 and 42 in Table 5), were also carriers of carbapenemase gene KPC. The isolation of different KPC producing species (E. coli, K. oxytoca and C. freundii) in the same water sample (33_ARD3e) reinforced the hypothesis that a horizontal gene transfer is taking place between different bacterial species. Finally, in this study we did not isolate A. baumanii resistant to penicillins, cephalosporins or carbapenems. This could be related to the low presence of this pathogen or with methodological problems on the isolation of this species.
Colistin (polymyxin E) is currently used as a last alternative drug against MDR Gram negative bacteria. However, its resistance has even emerged in humans since it has been widely used in pig production and in some countries in veterinary (especially in cows) for the treatment of gastrointestinal infections caused by Enterobacteriaceae [26]. This resistance is frequently related to chromosomal mutations, nonetheless, the mechanism by which the mcr-1 gene confers resistance to COL was the first one that described plasmid mediated transmission antibiotic resistance and was first discovered in China on a pig farm [39]. Despite this gene being widespread in the environment [49] and it having been documented in 30 strains isolated from three Spanish WWTPs [50], only one E. coli strain isolated from a rabbit slaughterhouse collector (number 47 in Table 5) was positive for mcr-1 in our study.
Enterococci are recognized as important nosocomial pathogens due to their natural intrinsic resistance and their ability to acquire resistance to multiple drugs [49]. The resistance to VA in enterococci (VRE) is associated with the use of this antibiotic in clinics and, as a consequence, effluents from hospitals constitute an important point for the transmission of this resistance [51]. In this sense, despite the fact that VA resistant bacteria were isolated from the hospital collector (37_ARH), none of these isolates was identified as VRE. However, VRE were present in the influent waters of the WWTPs of two points near hospitals (29_ARD1e and 33_ARD3e), in accordance with other studies [52]. In general, VA resistances are specially linked to vanA and vanB genes and represent a major public health problem, due to their resistant gene transfer capacity [53]. In this sense, vanB carriers are characterized by high levels of VA resistance and TEC sensitivity, and the resistance is transferred by conjugation associated with the mobilization of genetic material through the acquisition and/or exchange of transposons [20]. In agreement with that, all our VRE isolates showed the vanB phenotype. As VA is not used in veterinary medicine, the use of other glycopeptides as an animal growth promoter (such as avoparcin), was associated with the increase in VRE in the 70s [20]. Numerous studies have shown that VRE persisted in animals for a long time after avoparcin was banned [49]. Therefore, the presence of identical resistance genes in animal and human enterococci, suggest the spread between isolates from different environments [53]. The isolation of E. faecium VA resistance in samples from a rabbit slaughterhouse collector (40_ARM) reinforces this hypothesis. Finally, it is known that VRE can rapidly develop resistance after the introduction of new antimicrobial agents in the clinic, such as quinupristin-dalfopristin (SYN), Lz and DAP [54]. So, it should be noted that 80% of the E. faecium vanB isolates of this study were resistant to SYN and 40% were resistant to DAP, whereas no resistances to Lz were observed.
One of the most important acquired resistances in S. aureus is methicillin resistance (MRSA) and is mainly due to the acquisition of the mecA gene, encoding a β-lactam low affinity penicillin binding protein (PBP) called PBP2a [49]. In general MRSA isolates from surface water are quite rare, with only a low number of isolates [51]. Despite this, MRSA mecA has been reported to survive in rivers and municipal wastewater and had been associated with colonized people [19]. In addition, the presence of gene mecC has been reported for the first time in a Spanish river, highlighting the potential role of water in the dissemination of mecC MRSA [19]. S. aureus mecC was also isolated from animals and an urban wastewater treatment plant [55] and other studies highlighted the emergence of S. aureus mecC in livestock production, particularly in pigs in European countries [56]. In this sense, our two MRSA strains (negative for the mecA gene in the PBP2a test) were isolated from rivers (numbers 54 and 55 in Table 6) with high incidence from pig exploitations, which would reinforce this hypothesis.
In general, the main objective of wastewater treatment is to eliminate organic (chemical and biological) components, phosphorous and nitrogen nutrients as well as suspended solids. Directive 91/271/EEC [57] establishes the guidelines to be followed by the Member States of the European Union to ensure that urban wastewater receives adequate treatment before discharge, but it does not include disinfection processes that reduce the microbiological charge and ARGs in the effluents [58]. Consequently, these bacteria are incorporated into the environment through the direct or indirect discharge of treated water or through sludge, which finally is used as a fertilizer in agricultural practices. In the same way, the directive does not provide specific restrictions for effluents from hospital wastewater, which also constitute an important reservoir of ARB [52]. It is known that some ARB can be removed through conventional wastewater treatment processes [6], but still large numbers that survive in the effluent. Therefore, tertiary treatment methods or advanced treatment technologies are those that manage to eliminate some bacterial load and genes [47]. In this sense, UV and ozone-treatment have been investigated for a long time with the aim of reducing these microbial loads. UV disinfection contributes to the effective reduction of some bacteria, like 99.9% of MRSA or VRE [6]. However, Munir et al., 2011 [59] founded that this disinfection did not contribute to the reduction of TET and sulfonamide resistant bacteria. Moreover, ozonation is an efficient process to eliminate organic microcontaminants and for inactivating bacteria through the production of highly reactive radical [60]. Other tertiary treatments are based on the water exposure to solar radiation in the lagoon and according to López Martínez [61] are able to reduce the microbiological concentration up to four orders of magnitude at the longest time of exposure to solar radiation. However, these advanced wastewater treatment technologies are also known to accelerate horizontal gene transfer due to the activation of different repair mechanisms involved in the dissemination of antibiotic resistance genes [6]. Consequently, it is necessary to develop other additional strategies and guidelines for the elimination of microbial contaminants in wastewater, which included surveillance of pathogenic bacteria and ARGs. For that reason, there is a need to improve effective disinfection measures and treatments in WWPTs and animal slaughterhouses to avoid environmental contamination and prevent the evolution of antibiotic resistance.
5. Conclusions
The results of this study highlight that resistant bacteria to clinically relevant antibiotics were present in the different water samples examined in the POCTEFA area, with a higher presence in wastewaters from slaughterhouses, hospitals and WWTPs. In order to minimize the dispersion of resistances through the effluents of these areas, it is necessary to implement effective methods of wastewater disinfection and surveillance programs of ARB.
Acknowledgments
We thank the staff of the Laboratory of Water and Environmental Health from the University of Zaragoza, for their useful collaboration in carrying out the sampling.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/9/1425/s1, Table S1. Characteristics of sampling points in rivers for the north of Spain and south of France. Table S2. Characteristics of sampling points for sewage water in the Navarra Region. Table S3. Zone diameter breakpoints for the different antibiotics tested.
Author Contributions
L.P.-E. performed the experiments, analyzed the data and wrote the paper. J.L. performed the mcr PCRs, D.G. and A.I.V. conceived, designed the experiments, supervised data analysis and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant of the “la Caixa” Banking Foundation and the “Asociación de Amigos de la Universidad de Navarra” and by the European Regional Development Fund (ERDF) through the Interreg V-A Spain-France-Andorra programme (POCTEFA 2014–2020, cofinancing rate 65%). Proyect EFA183/16/OUTBIOTICS. POCTEFA aims to reinforce the economic and social integration of the French–Spanish–Andorran border. Its support is focused on developing economic, social and environmental cross-border activities through joint strategies favouring sustainable territorial development.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- 1.Blaak H., Lynch G., Italiaander R., Hamidjaja R.A., Schets F.M., De Husman A.M.R. Multidrug-resistant and extended spectrum beta-lactamase-producing Escherichia coli in dutch surface water and wastewater. PLoS ONE. 2015;10:e0127752. doi: 10.1371/journal.pone.0127752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kümmerer K. Resistance in the environment. J. Antimicrob. Chemother. 2004;54:311–320. doi: 10.1093/jac/dkh325. [DOI] [PubMed] [Google Scholar]
- 3.Woolhouse M. Antimicrobial resistance in humans, livestock and the wider environment. J. High Energy Phys. 2015;6:1575–1589. doi: 10.1098/rstb.2014.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chee-Sanford J.C., Mackie R.I., Koike S., Krapac I.G., Lin Y.-F., Yannarell A.C., Maxwell S., Aminov R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes following Land Application of Manure Waste. J. Environ. Qual. 2009;38:1086–1108. doi: 10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- 5.García-Galán M.J., Díaz-Cruz M.S., Barceló D. Occurrence of sulfonamide residues along the Ebro river basin. Removal in wastewater treatment plants and environmental impact assessment. Environ. Int. 2011;37:462–473. doi: 10.1016/j.envint.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Jäger T., Hembach N., Elpers C., Wieland A., Alexander J., Hiller C., Krauter G., Schwartz T. Reduction of Antibiotic Resistant Bacteria During Conventional and Advanced Wastewater Treatment, and the Disseminated Loads Released to the Environment. Front. Microbiol. 2018;9:2599. doi: 10.3389/fmicb.2018.02599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ojer-Usoz E., González D., Vitas A.I., Leiva J., García-Jalón I., Febles-Casquero A., de la Soledad Escolano M. Prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in meat products sold in Navarra, Spain. Meat Sci. 2013;93:316–321. doi: 10.1016/j.meatsci.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Ojer-Usoz E., González D., García-Jalón I., Vitas A.I. High dissemination of extended-spectrum β-lactamase-producing Enterobacteriaceae in effluents from wastewater treatment plants. Water Res. 2014;56:37–47. doi: 10.1016/j.watres.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Leonard A.F.C., Zhang L., Balfour A.J., Garside R., Gaze W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015;82:92–100. doi: 10.1016/j.envint.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y., Guo C., Luo Y., Lv J., Zhang Y., Lin H., Wang L., Xu J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016;213:833–840. doi: 10.1016/j.envpol.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Guo M.T., Yuan Q.-B., Yang J. Microbial selectivity of UV treatment on antibiotic-resistant heterotrophic bacteria in secondary effluents of a municipal wastewater treatment plant. Water Res. 2013;47:6388–6394. doi: 10.1016/j.watres.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Hembach N., Schmid F., Alexander J., Hiller C., Rogall E.T., Schwartz T. Occurrence of the mcr-1 colistin resistance gene and other clinically relevant antibiotic resistance genes in microbial populations at different municipal wastewater treatment plants in Germany. Front. Microbiol. 2017;8:1282. doi: 10.3389/fmicb.2017.01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. WHO; Geneva, Switzerland: 2017. [Google Scholar]
- 14.Falagas M.E., Karageorgopoulos D.E. Extended-spectrum β-lactamase-producing organisms. J. Hosp. Infect. 2009;73:345–354. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Zurfluh K., Hächler H., Nüesch-Inderbinen M., Stephan R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013;79:3021–3026. doi: 10.1128/AEM.00054-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarfel G., Lipp M., Gürtl E., Folli B., Baumert R., Kittinger C. Troubled water under the bridge: Screening of River Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci. Total Environ. 2017;593–594:399–405. doi: 10.1016/j.scitotenv.2017.03.138. [DOI] [PubMed] [Google Scholar]
- 17.Stryjewski M.E., Corey G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014;58:10–19. doi: 10.1093/cid/cit613. [DOI] [PubMed] [Google Scholar]
- 18.Vaz-Moreira I., Nunes O.C., Manaia C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014;38:761–778. doi: 10.1111/1574-6976.12062. [DOI] [PubMed] [Google Scholar]
- 19.Porrero M.C., Harrison E., Fernández-Garayzábal J.F., Paterson G.K., Díez-Guerrier A., Holmes M.A., Domínguez L. Detection of mecC-Methicillin-resistant Staphylococcus aureus isolates in river water: A potential role for water in the environmental dissemination. Environ. Microbiol. Rep. 2014;6:705–708. doi: 10.1111/1758-2229.12191. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed M.O., Baptiste K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018;24:590–606. doi: 10.1089/mdr.2017.0147. [DOI] [PubMed] [Google Scholar]
- 21.Lozano C., González-Barrio D., García J.T., Ceballos S., Olea P.P., Ruiz-Fons F., Torres C. Detection of vancomycin-resistant Enterococcus faecalis ST6-vanB2 and E. faecium ST915-vanA in faecal samples of wild Rattus rattus in Spain. Vet. Microbiol. 2015;177:168–174. doi: 10.1016/j.vetmic.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Nakipoğlu M., Yilmaz F., Icgen B. vanA Gene Harboring Enterococcal and Non-enterococcal Isolates Expressing High Level Vancomycin and Teicoplanin Resistance Reservoired in Surface Waters. Bull. Environ. Contam. Toxicol. 2017;98:712–719. doi: 10.1007/s00128-016-1955-8. [DOI] [PubMed] [Google Scholar]
- 23.Lata P., Ram S., Shanker R. Multiplex PCR based genotypic characterization of pathogenic vancomycin resistant Enterococcus faecalis recovered from an Indian river along a city landscape. Springerplus. 2016;5:1199. doi: 10.1186/s40064-016-2870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller H., Sib E., Gajdiss M., Klanke U., Lenz-Plet F., Barabasch V., Albert C., Schallenberg A., Timm C., Zacharias N., et al. Dissemination of multi-resistant Gram-negative bacteria into German wastewater and surface waters. FEMS Microbiol. Ecol. 2018;94:1–11. doi: 10.1093/femsec/fiy057. [DOI] [PubMed] [Google Scholar]
- 25.Guyonnet J., Manco B., Baduel L., Kaltsatos V., Aliabadi M.H.F.S., Lees P. Determination of a dosage regimen of colistin by pharmacokinetic/pharmacodynamic integration and modeling for treatment of G.I.T. disease in pigs. Res. Vet. Sci. 2010;88:307–314. doi: 10.1016/j.rvsc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Rhouma M., Beaudry F., Letellier A. Resistance to colistin: What is the fate for this antibiotic in pig production? Int. J. Antimicrob. Agents. 2016;48:119–126. doi: 10.1016/j.ijantimicag.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 27.McGann P., Snesrud E., Maybank R., Corey B., Ong A.C., Clifford R., Hinkle M., Whitman T., Lesho E., Schaecher K.E. Erratum for McGann et al. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: First report of mcr-1 in the United States. Antimicrob. Agents Chemother. 2016;60:5107. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Organization for Standardization . Calidad del Agua. Muestreo para el Análisis Microbiológico. ISO; Geneva, Switzerland: 2007. ISO 19458:2007. [Google Scholar]
- 29.International Organization for Standardization . Calidad del Agua. Muestreo Parte 3: Conservación y Manipulación de las Muestras de Agua. ISO; Geneva, Switzerland: 2012. ISO 5667-3:2012. [Google Scholar]
- 30.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI; Wayne, PA, USA: 2018. [Google Scholar]
- 31.The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Breakpoint tables for interpretation of MICs and zone Diameters. [(accessed on 15 September 2018)];2018 Version 8.0. Available online: http://www.eucast.org.
- 32.Jarlier V., Nicolas M., Fournier G., Philippon A. Extended Broad-Spectrum β-Lactamases Conferring Transferable Resistance to Newer β- Lactam Agents in Enterobacteriaceae: Hospital Prevalence and Susceptibility Patterns. Rev. Infect. Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 33.Thean Y.T., Ng L.S.Y., He J., Tse H.K., Li Y.H. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob. Agents Chemother. 2009;53:146–149. doi: 10.1128/AAC.00862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derbyshire H., Kay G., Evans K., Vaughan C., Kavuri U., Winstanley T. A simple disc diffusion method for detecting AmpC and extended-spectrum β-lactamases in clinical isolates of Enterobacteriaceae. J. Antimicrob. Chemother. 2009;63:497–501. doi: 10.1093/jac/dkn535. [DOI] [PubMed] [Google Scholar]
- 35.Arakawa Y., Shibata N., Shibayama K., Kurokawa H., Yagi T., Fujiwara H., Goto M. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 2000;38:40–43. doi: 10.1128/jcm.38.1.40-43.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-pérez F.J., Hanson N.D. Detection of Plasmid-Mediated AmpC β-Lactamase Genes in Clinical Isolates by Using Multiplex PCR. J. Clin. Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colom K., Pérez J., Alonso R., Fernández-Aranguiz A., Lariño E., Cisterna R. Simple and reliable multiplex PCR assay for detection of blaTEM, bla(SHV) and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol. Lett. 2003;223:147–151. doi: 10.1016/S0378-1097(03)00306-9. [DOI] [PubMed] [Google Scholar]
- 38.Woodford N., Fagan E.J., Ellington M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases [4] J. Antimicrob. Chemother. 2006;57:154–155. doi: 10.1093/jac/dki412. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 40.Xavier B.B., Lammens C., Ruhal R., Butaye P., Goossens H. Identification of a novel plasmid-mediated colistin- resistance gene, mcr-2, in Escherichia coli, Belgium, June. Euro Surveill. 2016;21:6–11. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 41.Yin W.J., Li H., Shen Y.B., Liu Z., Wang S., Shen Z., Zhang R., Timothy R., Walsh J.S., Wanga Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli Wenjuan. MBio. 2017;8:e00543-17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carattoli A., Villa L., Feudi C., Curcio L., Orsini S., Luppi A., Pezzotti G., Magistrali C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance. 2017;22:1–5. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borowiak M., Fischer J., Hammerl J.A., Hendriksen R.S., Szabo I., Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017;72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M., Aihara M., Shimakawa K., Iwasaki M., Nagasaka Y., Fukuda S., Matsuo S., Iwatani Y. Evaluation of MicroScan ESBL confirmation panel for Enterobacteriaceae-producing, extended-spectrum β-lactamases isolated in Japan. Diagn. Microbiol. Infect. Dis. 2003;46:125–130. doi: 10.1016/S0732-8893(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 45.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 46.Savin M., Bierbaum G., Hammerl J.A., Heinemann C., Parcina M., Sib E., Voigt A., Kreyenschmidt J. ESKAPE Bacteria and Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolated from Wastewater and Process Water from German Poultry Slaughterhouses. Appl. Environ. Microbiol. 2020;86:e02748-19. doi: 10.1128/AEM.02748-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makowska N., Philips A., Dabert M., Nowis K., Trzebny A., Koczura R., Mokracka J. Metagenomic analysis of β-lactamase and carbapenemase genes in the wastewater resistome. Water Res. 2020;170:115277. doi: 10.1016/j.watres.2019.115277. [DOI] [PubMed] [Google Scholar]
- 48.Osińska A., Harnisz M., Korzeniewska E. Prevalence of plasmid-mediated multidrug resistance determinants in fluoroquinolone-resistant bacteria isolated from sewage and surface water. Environ. Sci. Pollut. Res. 2016;23:10818–10831. doi: 10.1007/s11356-016-6221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Argudín M.A., Deplano A., Meghraoui A., Dodémont M., Heinrichs A., Denis O., Nonhoff C., Roisin S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics. 2017;6:12. doi: 10.3390/antibiotics6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ovejero C.M., Delgado-Blas J.F., Calero-Caceres W., Muniesa M., Gonzalez-Zorn B. Spread of mcr-1-carrying Enterobacteriaceae in sewage water from Spain. J. Antimicrob. Chemother. 2017;72:1050–1053. doi: 10.1093/jac/dkw533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galler H., Feierl G., Petternel C., Reinthaler F.F., Haas D., Habib J., Kittinger C., Luxner J., Zarfel G. Multiresistant bacteria isolated from activated sludge in Austria. Int. J. Environ. Res. Public Health. 2018;15:479. doi: 10.3390/ijerph15030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulshus E., Kühn I., Möllby R., Colque P., O’Sullivan K., Midtvedt T., Lingaas E., Holmstad R., Sørum H. Diversity and antibiotic resistance among Escherichia coli populations in hospital and community wastewater compared to wastewater at the receiving urban treatment plant. Water Res. 2019;161:232–241. doi: 10.1016/j.watres.2019.05.102. [DOI] [PubMed] [Google Scholar]
- 53.Lozano C., Gonzalez-Barrio D., Camacho M.C., Lima-Barbero J.F., de la Puente J., Höfle U., Torres C. Characterization of fecal vancomycin-resistant Enterococci with acquired and intrinsic resistance mechanisms in wild animals, Spain. Microb. Ecol. 2016;72:813–820. doi: 10.1007/s00248-015-0648-x. [DOI] [PubMed] [Google Scholar]
- 54.Kristich C.J., Rice L.B., Arias C.A. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Massachusetts Eye and Ear Infirmary; Boston, MA, USA: 2014. Enterococcal Infection−Treatment and Antibiotic Resistance; pp. 123–185. [Google Scholar]
- 55.Porrero M.C., Valverde A., Fernández-Llario P., Díez-Guerrier A., Mateos A., Lavín S., Cantón R., Fernández-Garayzabal J.F., Domínguez L. Staphylococcus aureus carrying mecC gene in animals and Urban Wastewater, Spain. Emerg. Infect. Dis. 2014;20:899–901. doi: 10.3201/eid2005.130426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boswihi S.S., Udo E.E., Mathew B., Noronha B., Verghese T., Tappa S.B. Livestock-Associated Methicillin-Resistant Staphylococcus aureus in Patients Admitted to Kuwait Hospitals in 2016–2017. Front. Microbiol. 2020;10:2912. doi: 10.3389/fmicb.2019.02912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.EU Directive 91/271/ECC sobre el tratamiento de las aguas residuales urbanas. Diario oficial de la Unión Europea L135; European Union; Brussels, Belgium: 1991. [Google Scholar]
- 58.Pallares-Vega R., Blaak H., van der Plaats R., de Roda Husman A.M., Hernandez Leal L., van Loosdrecht M.C.M., Weissbrodt D.G., Schmitt H. Determinants of presence and removal of antibiotic resistance genes during WWTP treatment: A cross-sectional study. Water Res. 2019;161:319–328. doi: 10.1016/j.watres.2019.05.100. [DOI] [PubMed] [Google Scholar]
- 59.Munir M., Wong K., Xagoraraki I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011;45:681–693. doi: 10.1016/j.watres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 60.Jia S., Bian K., Shi P., Ye L., Liu C.H. Metagenomic profiling of antibiotic resistance genes and their associations with bacterial community during multiple disinfection regimes in a full-scale drinking water treatment plant. Water Res. 2020;176:115721. doi: 10.1016/j.watres.2020.115721. [DOI] [PubMed] [Google Scholar]
- 61.López Martínez A. Evaluación de Tencologías en el Tratamiento de Aguas y Fangos en EDARs, para la Reducción de Microorganismos con Riesgo Sanitario y Ambiental. Universidad de Zaragoza; Zaragoza, España: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.