Abstract
Rural adults have a higher risk of developing obesity than urban adults. Several evidence-based interventions have targeted rural regions, but their impact, defined as reach (number and representativeness of participants) by effectiveness, has not been examined. The purpose of this review was to determine the impact of rural weight loss interventions and the availability of data across dimensions of the reach, effectiveness, adoption, implementation, and maintenance (RE-AIM) framework. A systematic review was conducted to identify rural weight loss interventions that targeted adults. RE-AIM related data were abstracted from each article. We performed a meta-analysis to examine effectiveness. Sixty-four articles reported on rural weight loss interventions, describing 50 unique interventions. The median number of participants was 107. Median participation rate differed between values reported by the authors (62%) and values computed using a standard method (32%). Two studies reported on sample representativeness; none reported comparisons made between target and actual delivery settings. Median weight loss per participant was 3.64kg. Meta-analyses revealed the interventions achieved a significant weight reduction, and longer-duration interventions resulted in greater weight loss. Rural weight loss interventions appear to be effective in supporting clinically meaningful weight loss but reach and cost outcomes are still difficult to determine.
Keywords: Obesity, weight loss, rural health, RE-AIM
INTRODUCTION
More than two-thirds of adults are currently overweight or obese,1 and obesity rates are even higher for those living in rural (40%), when compared to urban (33%), areas.2 More recent comparisons suggest that these obesity rates may have stayed the same or worsened in the last decade.3 The health disparities between rural and urban residents do not end with obesity–a major risk factor for chronic disease; the prevalence of diabetes and heart disease also are higher in rural, compared to urban, areas.4 For example, an analysis of national surveillance data reported that coronary heart disease prevalence was 39% higher among rural respondents when compared to urban respondents.4 The higher prevalence of obesity and other chronic diseases among rural adults may be exacerbated by the underlying social determinants of inadequate healthcare resources related to obesity prevention and treatment, as well as fundamental issues related to lower socioeconomic status.2,5,6 Indeed, about 1 in 5 adults in the U.S. resides outside of urban, metropolitan areas.11,12 To address this segment of the population, a growing body of literature has focused on weight loss interventions in rural areas.13-15
While a number of narrative and systematic reviews have demonstrated the effectiveness of weight loss interventions using lifestyle modification (e.g. diet and/or exercise counseling; behavioral therapy),7-10 it is surprising that none, to our knowledge, explicitly address rural communities. Rurality has multiple definitions,24-26 but is typically distinguished from urban by a population less than 50,000. A commentary on the definition and identification of “rural community” as it pertains to this research is included in the results and discussion.
A fundamental issue for weight loss interventions targeting those living in rural areas is reach. Reach can be defined as the number, proportion, and representativeness of individuals who participate in a given initiative.16 Due to the geographic dispersion of those living in rural areas, understanding the number, proportion, and representativeness of the population that would or could benefit from weight loss interventions is critical. When considering the public health impact of weight loss interventions, it has been recommended that researchers document intervention reach and effectiveness.29 This can be done using metrics such as the proportion of the population or sample that loses a clinically meaningful amount of weight (e.g., loss of ≥5% of initial body weight)18 and the proportion that maintains that weight loss – with a goal to compare impact across programs.19 Intervention adoption and implementation at the setting level are equally important considerations when taking an intervention to scale. Particularly, aggregated intervention cost per participant that achieves and maintains clinically meaningful weight loss, including recruitment, implementation, and sustainability costs, is a valuable metric for intervention selection.21,22 Unfortunately, factors related to external validity have traditionally been infrequently reported, such as reach, adoption, and organizational-level maintenance.17,20,29 This lack of reporting extends broadly across the field of health behavior programs,17, 29 yet has particular importance when considering the translation of evidence-based interventions into rural areas where the evidence-base may be even less externally-valid compared to urban areas where the majority of weight loss intervention research has been conducted.
Despite the growing body of literature examining the effectiveness of rural weight loss interventions, the extent to which interventions have assessed study reach and cost is unclear, and there is no synthesized evidence of the literature in this area to document intervention impact. The primary objective of this systematic review was to determine the impact of rural weight loss interventions by assessing reach and effectiveness. A secondary aim was to determine the availability of data on intervention adoption and implementation at the setting level, particularly total intervention costs per participant achieving clinically meaningful weight loss, and individual- and setting-level maintenance.
We hypothesized the effectiveness of rural weight loss interventions to be similar to those delivered in non-rural settings, but considered reasons why efficacy might be reduced. One area of concern is adapting or tailoring interventions for rural communities, which may reduce the intensity of the intervention and therefore result in smaller changes in weight. Examples of adaptations might include changing the length and/or delivery method of the intervention (i.e. in-person, telephone, or online). Related to this issue and our primary aim of assessing overall effectiveness, we included two exploratory aims. Previous systematic reviews of weight loss research demonstrated that a larger magnitude of effect was positively related to program length9; thus, we hypothesized this relationship would be present in rural settings. In addition, rural areas typically have fewer healthcare and community resources, and travel and time barriers are often cited by participants of in-person, synchronous weight loss programs. Therefore, we included an exploratory analysis to determine if there were differences in outcomes based on in-person vs technology facilitated delivery methods.
METHODS
Data Sources, Searches, and Selection
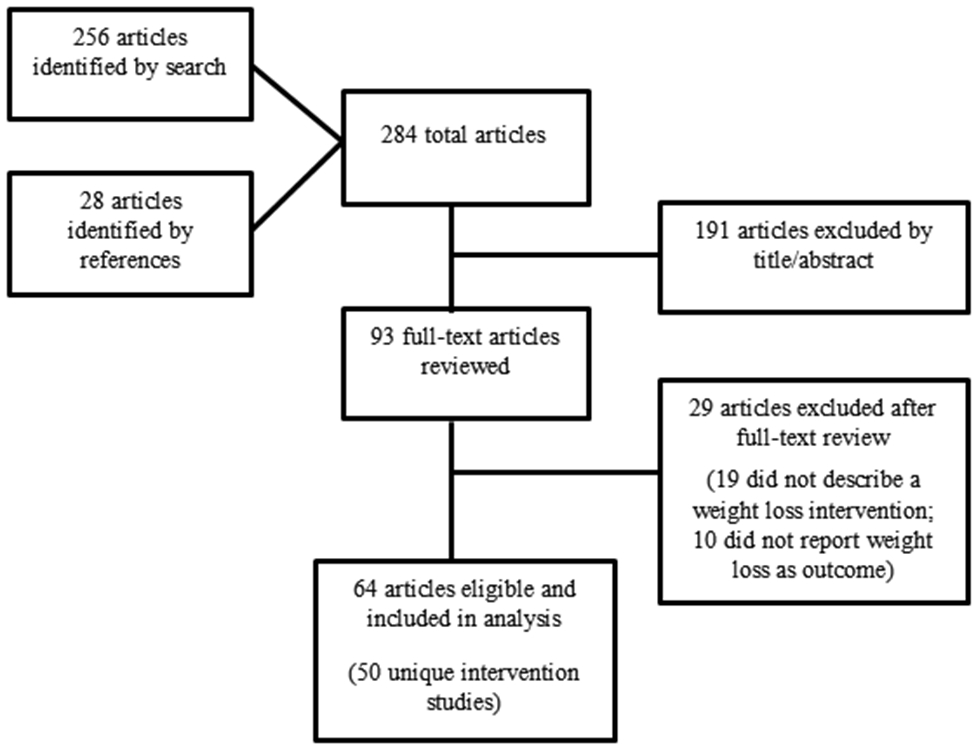
In August 2016, a systematic literature search was conducted using PubMed, Scopus, Embase, and CINAHL databases. Search terms included “intervention,” “rural,” and “weight loss.” Articles were screened using the following inclusion/exclusion criteria: 1) intervention took place in a rural setting, 2) participants were adults ≥18 years, 3) weight loss was a primary outcome reported, and 4) intervention was conducted in an English-speaking country and original article was published in English. All systematic reviews, theses, dissertations, and letters to the editor were excluded. Additionally, based on the 2013 American Heart Association, the American College of Cardiology, and The Obesity Society Guidelines for the Management of Overweight and Obesity in Adults22 – which recommends weight management interventions include a dietary component – any study that focused only on physical activity for weight loss was excluded. The study selection process is outlined in the Preferred Reporting Items for Systematic Reviews (PRISMA)23 flow diagram (Figure 1).
Figure 1.
PRISMA flow diagram.
Due to the various ways that “rural” has been defined,24-26 and the varied detail of reporting intervention setting characteristics across studies, we did not apply a single taxonomy to our inclusion process when screening for interventions conducted in a rural setting. We relied on the authors’ identification of “rural” and we provide a commentary on this reporting among included interventions in the results.
The RE-AIM (reach, effectiveness, adoption, implementation, and maintenance) framework—developed to enhance the quality, speed, and influence of efforts to translate evidence-based research into practice—was used as a guide for evaluating eligible articles. The framework provides operational definitions for each of the five dimensions, and can provide summary scores for the combination of these metrics (e.g. reach and effectiveness combined to assess impact).27 Additionally, RE-AIM evaluation allows for a pragmatic assessment of cost,28 a critical step in the process of moving evidence-based strategies into typical clinical and community practice.21 Intervention costs, return on investment, and cost effectiveness per clinically meaningful unit of weight lost are important indicators of an intervention’s potential for future adoption, implementation, and sustainability.29
Data Abstraction
All members of the research team received training from a senior scientist with experience conducting systematic reviews using the RE-AIM framework. After training, each coder abstracted information from one article; a senior scientist reviewed the coding and provided feedback. This process was repeated until each coder was deemed qualified to abstract information independently.
Articles gathered from the search were first screened for inferences in the title and abstract; those that were clearly unrelated to weight loss in rural areas were excluded. Following title and abstract screening, the remaining articles underwent a full-text review and were coded across defined indicators of RE-AIM dimensions by two independent coders. Each coding pair met after the articles were reviewed to reconcile any differences in coding; disagreements were resolved by consensus and, if still unresolved, a senior scientist was consulted. If an additional article was referenced in a source article identified during the literature review, and it provided complementary information on the same study, it was also included in data abstraction and reporting. A table of all papers included in this review is included in the supplementary materials (Appendix A).
A data abstraction tool developed previously to gather RE-AIM information was adapted for use in the present review.29 This tool was used in place of risk of bias assessments to ensure all relevant data was recorded for each article, particularly regarding internal and external validity. A list of reach, effectiveness, adoption, implementation, and maintenance primary and secondary indicators (with relevant descriptions) that were used during article coding are listed in Table 1. Of note, participation rate was defined and recorded, when available, in two ways under the reach dimension. First, participation rate was recorded as it was presented in the article. Second, because participation rate was not consistently defined or presented among the articles, we used a method to standardize participation rate across studies.29 When relevant data were available, standardized participation rate was calculated using sample size as the numerator; the denominator was calculated as the product of the eligibility rate and the number of potential participants exposed to recruitment methods. Eligibility rate was calculated by subtracting the number ineligible from 1 and dividing the result by the total number exposed to recruitment activities. Additionally, standard deviations were also abstracted when available to allow for the completion of a meta-analysis.
Table 1:
RE-AIM indicators with the number and percent of unique studies (N=50) reporting each indicator. (#=number)
| Indicator | #(%) studies reporting |
|---|---|
| Reach | |
| Number of eligible participants exposed to recruitment | 22 (44%) |
| Sample size | 48 (96%) |
| Participation rate (as reported by the article authors) | 16 (32%) |
| Participation rate (number of studies that reported sufficient information to calculate standardized participation rate, using equation: sample size/ [1-(# ineligible/ # exposed to recruitment)*(# exposed to recruitment)] | 8 (16%) |
| Individual-level representativeness (Analyses conducted to examine comparisons between target population and study sample) | 2 (4%) |
| Description of broader target audience | 50 (100%) |
| Characteristics of target audience (e.g., gender, age, educational attainment, occupation, SES, behavioral outcomes) | 23 (46%) |
| Method to identify target population | 36 (72%) |
| Inclusion/exclusion criteria | 47 (94%) |
| Description of recruitment methods used | 44 (88%) |
| Efficacy/Effectiveness | |
| Weight change (weight loss in kg and/or percent of original body weight) | 45 (90%) |
| Proportion of sample that achieved ≥5% weight loss | 14 (28%) |
| Quality of life measure | 12 (24%) |
| Unintended negative consequences and results | 1 (2%) |
| Imputation of missing data | 20 (40%) |
| Mediators of weight change | 10 (20%) |
| Moderators of weight change | 17 (34%) |
| Adoption | |
| Number of eligible and invited sites | 2 (4%) |
| Number of participating sites | 32 (64%) |
| Site participation rate | 1 (2%) |
| Description of intervention setting | 40 (80%) |
| Setting representativeness (comparisons of targeted sites and study sites) | 0 (0.0%) |
| Method to identify and engage intervention setting | 17 (34%) |
| Number of staff eligible and invited to participate in intervention delivery | 2 (4%) |
| Number of staff participating in intervention delivery | 23 (46%) |
| Level of expertise of the delivery agent(s) | 42 (84%) |
| Implementation | |
| Statement of theories or principles used to develop intervention | 27 (54%) |
| Intervention duration | 47 (94%) |
| Description of encounters with participants during intervention (intervention number, timing, and/or duration of contacts) | 44 (88%) |
| Extent intervention protocol was delivered as intended | 0 (0.0%) |
| Consistency of implementation across study sites | 2 (4%) |
| Cost | |
| Cost of recruitment | 1 (2%) |
| Start-up costs | 4 (8%) |
| Ongoing cost of intervention delivery | 18 (36%) |
| Cost benefit or cost-effectiveness | 4 (8%) |
| Maintenance | |
| Weight outcome assessed at one or more points post-intervention | 17 (34%) |
| Participant attrition during follow-up period | 16 (32%) |
| Description of program continuation/institutionalization (continuation of program activities after completion of the research study or program institutionalization) | 10 (20%) |
Data Analysis
Findings for reach, adoption, implementation, and maintenance indicators are primarily reported as median values and/or in descriptive text. For effectiveness, we performed meta-analyses in weight changes (kg) between pre- and post-intervention (within group), and further separated included studies by intervention duration (≥ 6 months vs. < 6 months) and by intervention type (in-person delivered, non-in-person, and combined [both in-person and non-in-person]) in the subgroups analyses. Non-in-person delivered interventions included telephone, online, or mobile delivery, or any blend of those delivery types. We calculated the mean value of weight change within intervention groups manually if two or more intervention arms were included in one study. We also included a sensitivity analysis to determine if the inclusion of studies with both rural and urban participants influenced the meta-analytic results. The pooled effects were calculated by random-effects models using DerSimonian-Laird estimates, the most commonly used random-effect model without the assumption of the distributions of either the within- or between-study effects.30 We explored heterogeneity between the trials using the I2 statistic, where a value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity.31 The study was considered heterogeneous when the value of I2 was > 50%.32 All statistical tests and creation of forest plots were conducted with Stata 14.2 (StataCorp LP, College Station, TX) with the metaan package.33
RESULTS
Figure 1 describes the literature search and selection process. A total of 64 papers, representing 50 unique rural weight loss interventions (studies), were included. Publication dates ranged from 1989 to 2016, with 81% of the papers published between 2006 and 2016. When there were multiple papers published for one unique intervention, each paper that included new information relevant to the RE-AIM framework was included in this analysis. The number of published articles analyzed per unique intervention ranged from one to four. Nine of the papers included were design/protocol papers, four of which were not associated with a published outcome paper.
Papers included in this review reported on interventions that took place in the United States (n=53), Australia (n=10), or Canada (n=1). Interventions targeted diet and physical activity; disease risk factor reduction; disease management (e.g. heart disease, hypertension, hyperlipidemia); and/or obesity treatment. Study settings included medical clinics, churches, and worksites, were home-based, phone-based, web-based, and/or took place in the community. Median study duration was 12 weeks (±19.84 weeks) and was reported for all but two of the studies (n=48). Table 1 provides a list of reach, effectiveness, adoption, implementation, and maintenance indicators and the number and percent of studies that reported each of those indicators.
Per the inclusion criteria for this RE-AIM review, all interventions took place in rural areas. Seven of the 50 unique interventions (14%) included both rural and urban participants.34-44 Publications for all interventions referenced a “rural” setting; however, there was not a consistent definition or approach used across interventions. Approximately 75% of the interventions (n=36) defined or described the “rural” setting of the intervention in some way (providing the size of the population, referencing a standard measure, and/or providing a narrative description of the rural nature of the setting). Approximately one-third of the interventions (n=17) provided the population size for the study area (e.g., “This program…took place in a county with a population of 12,300 and one small hospital, whose two largest towns had populations of only 6,200 and 1,300.”45). Approximately one-fourth of the interventions (n=12) described the rural nature of the study population by referencing standard measures, such as Rural-Urban Commuting Area (RUCA) codes, Rural-Urban Continuum Code, Metropolitan Statistical Areas (e.g., stating the distance from such an area), U.S. Department of Agriculture frontier and remote (FAR) area codes, or U.S. Census Bureau references, (e.g., “Participants must reside in a rural area according to the Rural-Urban Commuting Area (RUCA) Codes, Urban Influence Codes, amount of agricultural income, and/or individual commuting patterns.”46).
Sixty percent of the interventions (n=30) provided a narrative description of rural aspects of the study area, such as referencing its remote location, few resources, underserved populations, health professional shortage area designation, or shortage of healthcare facilities, (e.g., “For rural areas with few resources, such as the Dan River Region in south-central Virginia and north-central North Carolina, providing collaborative and multilevel interventions to effectively address obesity-related behaviors is challenging. The Dan River Region includes Pittsylvania and Henry counties in Virginia and Caswell County in North Carolina. A rural area with health disparities, the Dan River Region is classified as a medically underserved area.”47).
Most of the interventions (n=44) named the geographic location where the study took place, which may provide clues as to its ‘rural’ nature for those familiar with the area. However, for the purposes of this analysis, provision of the location name alone was not considered a definition of rural.
Articles generally did not provide a detailed discussion of the ways in which the intervention had been tailored for a rural target population. For those that did, information typically included a discussion of how a given intervention held potential for addressing the needs of under-resourced areas and barriers specific to rural communities (e.g., “Our findings also indicate that group facilitation via phone is a potential approach for reaching geographically dispersed, rural [breast cancer] survivors, and that phone-based group facilitation appears to cultivate the same important group mechanisms as in-person groups, such as peer accountability, information exchange, and support.”48) and to provide examples of adaptations, (e.g., “There are no large public exercise or pool facilities in Miles City. The lifestyle coaches through creative community partnerships identified resources to provide the required physical activity sessions. Aerobics, kickboxing, and circuit training were conducted in conference rooms and the physical therapy center at the health care facility.”37). By far the most detailed discussion of tailoring for rural populations can be found in the protocol paper for the RE-POWER trial,15 which includes descriptions of rural-specific issues and considerations throughout the text, as well a discussion of specific intervention components that targeted the values and culture of the target population.
Reach
All studies provided a description of the intended audience. Methods to identify the target population were described in 36 of the 50 studies and demographic and/or behavioral characteristics of the intended audience were described in 23 studies. Inclusion/exclusion criteria were detailed in 47 studies, and 44 studies described recruitment strategies.
The median sample size was 107 participants (±1217.01) and was reported in 48 of 50 studies. One study35 had a sample size of 8,412, an outlier that was considerably larger than the other studies included; with this study removed, the median sample size was 102 participants (±213.92). Sixteen studies reported participation rate, yet many did not provide a clear description of how participation rate was calculated. Only eight out of 53 studies provided adequate information to calculate a standardized participation rate. The median standardized participation rate (32%) was lower than the median reported participation rate (62%).
Only two studies provided information on the representativeness of the study sample relative to the broader target population. One study found no differences in age, education, or time since cancer treatment between women who were screened for the intervention and those who declined screening.49 Another study conducted with rural and urban cardiac rehabilitation patients41 reported significant differences between participants and nonparticipants, whereby participants were significantly more likely to be younger, from a rural location, and more socioeconomically disadvantaged than non-participants.
Effectiveness
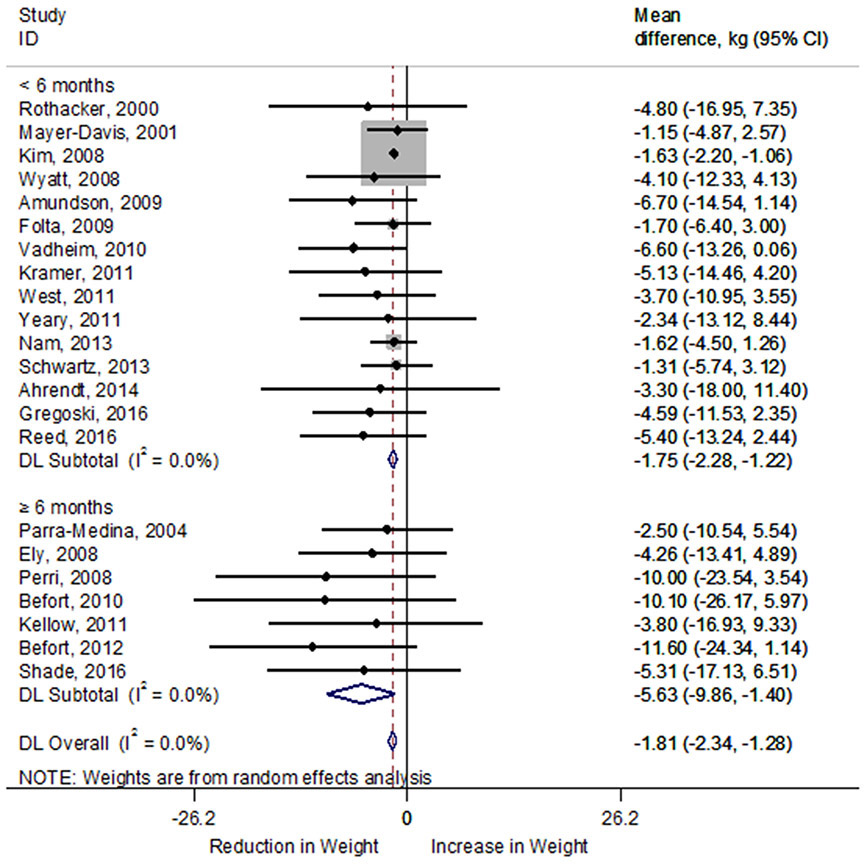
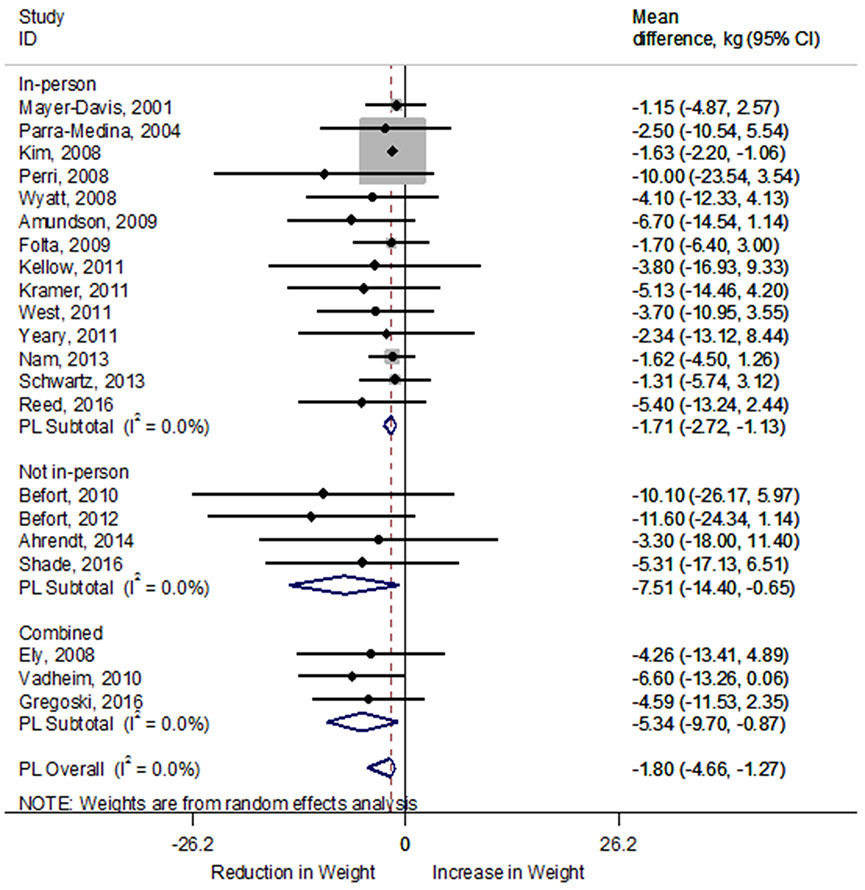
Of the 45 studies that reported intervention outcomes, 40 studies reported mean participant weight loss (median=3.64kg ± 2.72kg). Only 14 of the 45 studies reported the proportion of participants that achieved ≥5% body weight loss (median=43.91% ± 24.42%). Weight loss outcomes from 22 studies (n=3,065 participants) that included data to allow for the determination of effect size were included in the meta-analysis for the difference in weight (kg) between pre-and post-intervention. Weight loss interventions, on average, achieved a significant reduction in weight of −1.81kg (95% confidence interval [CI]: −2.34, −1.28kg). Heterogeneity among included studies did not appear to be a concern (I2 0.0%) (Figure 2). Compared to shorter duration (<6 months) interventions, longer duration (≥6 months) interventions resulted in greater weight loss (−5.63kg, 95% CI: −9.86, −1.40kg) regardless of significance. In pooled results, mean weight loss was greater in interventions that did not include in-person sessions (−7.51kg; 95% CI: −14.40, −0.65kg) than studies with in-person sessions (−1.71kg; 95% CI: −2.72, −1.13kg) or combined interventions (−5.34 kg; 95% CI: −9.70, −0.87kg; Figure 3). Of note, Rothacker 200079 was not included in the meta-analysis comparison of intervention types because it did not include a behavioral modification intervention.
Figure 2.
Forest plot of random-effects meta-analysis of mean difference (kg) in weight loss between pre- and post-intervention by intervention duration. A rhombus represents the combined effects estimate. Mean difference (circle) and 95% confidence intervals (whiskers) are presented for individual interventions. The size of the grey box is proportional to the weight assigned to each intervention. DL, DerSimonian-Laird; Kg, kilogram.
Figure 3.
Forest plot of random-effects meta-analysis of mean difference (kg) in weight loss between pre- and post-intervention by intervention type. A rhombus represents the combined effects estimate. Mean difference (circle) and 95% confidence intervals (whiskers) are presented for individual interventions. The size of the grey box is proportional to the weight assigned to each intervention. DL, DerSimonian-Laird; Kg, kilogram.
When interventions that included both rural and urban participants were removed from the meta-analysis, there remained a significant weight change of −1.76kg (95% CI: −2.29, −1.23kg; I2=0.0%). Longer duration interventions resulted in greater weight loss (−5.63kg; 95% CI: −9.86, −1.40kg) compared to shorter duration interventions (−1.70kg; 95% CI: −2.23, −1.16kg). Non-in-person delivered interventions resulted in greater weight loss (−8.64kg; 95% CI: −16.88, −0.69kg) compared to in-person delivered (−1.68kg; 95% CI: −2.53, −1.05kg) and combined interventions (−5.79kg; 95% CI: −12.02, 0.89kg).
Adoption
Overall, there was little reporting among adoption indicators across studies. Although 32 studies reported the number of sites that participated in intervention delivery, only two studies50,51 described the number of eligible sites that were asked to participate, and only one study50 reported site-level participation rate, describing in detail the number of sites contacted, assessed for eligibility, and enrolled, as well as the reasons for excluding sites. Most studies (n=40) described the intervention setting and about a third of studies (n=17) described the method to identify and engage the intervention setting; however, none of the papers in this review drew comparisons between target intervention sites and actual study sites.
Regarding intervention delivery agent(s), staff representativeness was seldom reported; nearly half (n=23) of the studies reported the number of staff participating in intervention delivery, yet only two studies reported the number of eligible staff that were contacted and asked to participate. The level of expertise of the delivery agent(s) was reported for 42 of 50 interventions.
Implementation
Program implementation was measured across six indicators, with four additional sub-indicators of cost. Authors of 27 studies explicitly identified behavioral theories or principles used to develop the intervention. Specifics about program delivery, such as program duration (n=47) and number, duration, and timing of contacts with participants during the program (n=44) were frequently reported among the 50 studies. Reporting on indicators that address program fidelity, such as the extent to which the program was delivered as intended (n=0) and measured consistency of program delivery across multiple sites (n=2), was scant.
Elements of intervention cost abstracted from the papers were: 1) start-up costs; 2) ongoing costs; 3) recruitment costs; and 4) cost effectiveness. Start-up costs were reported in four studies and typically included a report of costs for equipment, materials, food, etc. For example, one study reported a total of $1,361 for start-up costs, which included food, copies of program curriculum, balance scales, telephone calling cards, and a stove for cooking demonstrations.52 Another study reported $1,075 in start-up costs, citing paper handouts, food, scales, supplies, items distributed to participants, and postage in those costs.53 Ongoing costs were reported in 18 of 50 studies, but varied in the way the authors defined and reported ongoing costs of intervention delivery. Often this included the cost per participant in the intervention (range: $226-$571 per participant), total cost of intervention delivery (range: $1,824-$26,630), and/or a report of monetary incentives used (range: $10-$75 per session or measurement point); monetary incentives were typically distributed to participants at each completed assessment point.47,54-59
While recruitment methods were described in detail in many studies, only one59 stated the cost of recruitment. This study offered monetary incentives following participant screening and randomization visits ($10 and $25 gift certificates, respectively), and reported the “study population required substantial human and monetary resources to recruit and retain,” but otherwise did not report a total cost for recruitment.
Cost effectiveness was reported for four of the 53 studies. Again, each study reported different elements of cost effectiveness. One study reported the percent of participants that achieved 10% weight loss and intent-to-treat analysis to conclude average cost per successful participant was $714 for group and $1,029 for individual counseling.60 Another study61 described cost savings as $4,623 per patient educated about diabetes and able to maintain glycemic control to avoid hospitalization. A third study,62 a two-year randomized trial including 612 participants across four different intervention groups, calculated cost per participant (range: $78-$165) and cost per kg lost per participant (range: $22-$33). The fourth article that reported cost-effectiveness63 provided cost-effectiveness ratios for all three intervention groups (telephone program, face-to-face program, and education/control). The average cost per kilogram lost was higher in the face-to-face group ($47) compared to the telephone and education/control groups (both $32). This article also estimated the average cost-effectiveness ratios per kilogram weight loss and per self-rated health status point gained by the anticipated number of program participants.
Maintenance
At the individual level, weight loss maintenance was assessed at some point post-intervention in 17 of 50 studies. Fifteen of those 17 studies reported sustained positive weight results at the maintenance measurement point, and reported on attrition during the maintenance period. At the organizational level, there were 10 studies that described program continuation after the completion of the research study and/or the process by which the program was institutionalized.
DISCUSSION
The objective of this review was to determine the impact of rural weight loss interventions through an assessment of RE-AIM dimensions, particularly reach and effectiveness, and to determine the availability of information regarding intervention adoption, implementation and cost, and maintenance. Several conclusions are drawn from this work to provide insights for future research and practice. First, the meta-analysis indicates that rural weight loss interventions have been successful in helping participants lose weight. Second, it is difficult to determine the reach of these interventions, the representativeness of participants compared to the larger target population, or the adoption of these interventions by program settings due to limited or inconsistent reporting across studies. Third, cost data are provided in a larger proportion of studies when compared to other behavioral interventions29; yet nearly two-thirds of the studies included in this review did not mention any information about program costs. Of those that did mention cost, there was not a uniform approach to categorizing costs and comprehensive intervention cost information was scarce.
Rural weight loss interventions appear to be effective at reducing weight, on average; the median percent of participants that achieved clinically meaningful weight loss reached nearly half of participants. In this review, most studies that measured weight outcomes at some time post-intervention (n=17) reported encouraging results. Intervention participants sustained improvement in weight when compared to baseline measures in 15 out of 17 studies, further supporting the effectiveness of rural weight loss interventions. There were some moderating factors identified through the meta-analytic comparison, in that 1) longer-duration interventions were associated with a larger magnitude of weight loss and 2) interventions that did not include in-person sessions were associated with larger effects for participant weight loss than in-person or combined interventions. Although the second finding adds to a growing body of evidence that supports the efficacy of technology-delivered interventions over those delivered in-person,64, 65 our finding should be interpreted with caution. The number of studies used for this comparison was relatively small (n=4) and three of those studies were also categorized as having longer treatment durations.49,60,70 Further, two of those studies included meal replacement approaches to restrict caloric intake.49,60 As such, the conclusion that greater effectiveness ascribed to interventions that did not include in-person sessions is somewhat cluttered with other factors also known to result in greater weight loss. Nonetheless, this provides areas for additional investigation for those studying the potential to reduce body weight in rural populations; studies reducing the number of in-person sessions, extending the duration of the intervention, and including meal replacements to restrict calories, in combination or isolation, will help clarify our findings.
Four of the studies included in the meta-analysis included samples comprised of both rural and urban participants. Removing those studies did not change our conclusions regarding overall effectiveness of rural weight loss interventions, comparisons by intervention duration, or comparisons by intervention delivery. Still, the varying definitions of rural across these studies does not allow for a straight forward moderation analysis by type of rurality. More detailed reporting on the context or degree of rurality would benefit decision makers wishing to select and/or tailor interventions that are best suited for the resources and potential participants of a specific rural area.
Regarding reporting on intervention reach, participant number (i.e., sample size) was reported in nearly every study. However, participation rate was reported in less than one-quarter of the papers included in this review, and the definition of participation rate was inconsistent across studies that did report it. Author-reported participation rate surpassed 60% while standardized participation rate (calculated for those studies that provided a valid denominator of potentially eligible participants) was 32%. An equally important consideration is the generalizability of these interventions across different groups within a rural population. The included studies provide scarce information on representativeness, with only two studies specifically comparing characteristics of the study sample to the broader eligible population and no studies providing comparisons between target delivery sites and actual delivery sites. This lack of information regarding participant- and setting-level representativeness severely limits conclusions regarding the findings’ generalizability.
Inconsistent reporting of reach, effectiveness, adoption, and cost data is an important finding of this review. When considering the uptake and potential to scale weight loss interventions across a wide variety of rural settings, it is important to understand the proportion of the eligible population that will engage, the degree to which the participants are representative of the intended audience, the setting-level characteristics, and the cost per participant that achieves clinically meaningful weight loss.21,98 To date, the literature is clear that there is promise in rural interventions to support weight loss. However, when attempting to translate research into practice, information on the representativeness of the sample, implementation and ongoing costs, as well as any costs incurred by the participant, is critical to support community decision making.98 The lack of individual- and setting-level representativeness reporting and variability/availability in cost reporting limits researchers, practitioners, and policy makers from moving evidence-based, effective weight loss programs into clinical and community practice.
While we are limited in our ability to make cost comparisons across studies, the available cost information is encouraging. The costs reported per participant that achieved 10% weight loss60 were relatively modest; additionally, per kilogram weight loss,62 and start-up costs52,53 of interventions causing significant weight loss were arguably low as well. Furthermore, one study reported a cost savings of nearly $4,700 for each successful participant.61 Rural areas often do not have the interdisciplinary resources recommended for comprehensive weight loss interventions.1,2,5 The available data suggest it may be feasible to deliver effective weight loss interventions in rural areas that fit within the cost constraints of typical delivery organizations (e.g. health departments, health insurers, primary care clinics). For example, Perri et al.66 developed a successful weight loss intervention for obese rural women delivered via telephone through Cooperative Extension Services. Mean weight loss at the end of the 6-month intervention was 10.0kg. Following a 12-month extended care phase, which compared two intervention groups – telephone counseling and face-to-face counseling – and an educational control, intervention participants regained significantly less weight than the comparison group. Additionally, telephone counseling was found to be the least expensive intervention mode of delivery.
When considering the scale-up of evidence-based programs into clinical and community practice, there are certain implementation indicators that are valuable in program evaluation or organizational decision-making.29 Some of these indicators, such as the extent to which the protocol was delivered as intended and consistency of implementation across study sites, were rarely reported in the studies included in this review. Authors may not have valued the reporting of these indicators due to the design and aims of the study (e.g. efficacy trial), or the study having only one delivery site. Nevertheless, evaluating the fidelity of intervention delivery can influence the effectiveness and the consistency of implementation across multiple study sites, both of which are critical to the broader success of a scaled-up intervention. As dissemination and implementation scientists begin to move effective programs to scale, measuring implementation elements will be essential for the continued improvement and success of those programs.
This review has limitations that should be considered when interpreting the findings. First, there is potential for selection bias as the articles reviewed for inclusion were limited to published, English language studies, and were gleaned from a search of four databases. In addition, publication bias may have inadvertently inflated our results, due to the inequality of publishing between studies with null or negative results and those with positive results. The lack of reporting on several reach and implementation indicators limits our ability to draw conclusions in these areas. Furthermore, the varied definitions of rural among included studies made it difficult to apply one overarching definition without eliminating relevant research. Despite the ubiquitous reporting of average weight loss in the included articles, the standard deviation of weight loss outcomes was not frequently reported, which limited the number of studies we were able to include in the meta-analysis.
Despite the study’s weaknesses, this systematic review includes several strengths. To our knowledge, this is the first systematic review to assess the impact of weight loss interventions that specifically address rural settings. Moreover, this review applied the RE-AIM framework to rural weight loss interventions, which allowed for a comprehensive review of each intervention across several indicators within the reach, effectiveness, adoption, implementation, and maintenance dimensions. This review was rigorously conducted using a systematic search strategy and two independent data abstractors per article. Finally, this review provides quantitative and qualitative information regarding reporting of RE-AIM indicators as well as intervention outcomes through descriptive statistics, meta-analysis, and narrative reporting.
CONCLUSION
Rural weight loss interventions appear to be effective in supporting significant and clinically meaningful weight loss, but the reach and cost outcomes of interventions delivered to rural populations are still difficult to determine. Furthermore, setting-level adoption and implementation characteristics are difficult to compare across studies due to lack of reporting or differences in how these terms are operationalized. Future research would benefit from consistent reporting of sample representativeness, participation rate, start-up and ongoing costs, cost-effectiveness, and maintenance of effects to inform decision making related to the translation of research into typical practice.
Supplementary Material
Appendix A. Summary of All Papers Included in Analysis. N=64 papers describing 50 unique studies.
ACKNOWLEDGEMENTS
We thank the reviewers of this manuscript for meaningful feedback on earlier versions of this report.
FUNDING
This work was supported by American Heart Association Grant #18PRE34060136/Gwenndolyn Porter/2018, and by the National Institute of General Medical Sciences, 1 U54 GM115458, which funds the Great Plains IDeA-CTR Network. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHA or NIH.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
None of the authors have any conflicts of interest to report.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Befort CA, Nazir N, Perri MG. Prevalence of Obesity Among Adults From Rural and Urban Areas of the United States: Findings From NHANES (2005-2008). J Rural Health 2012;28(4):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor A, Wellenius G. Rural–urban disparities in the prevalence of diabetes and coronary heart disease. Public Health 2012;126(10):813–820. [DOI] [PubMed] [Google Scholar]
- 5.Ely AC, Befort C, Banitt A, Gibson C, Sullivan D. A Qualitative Assessment of Weight Control among Rural Kansas Women. J Nutr Educ Behav 2009;41(3):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nothwehr F, Peterson NA. Healthy Eating and Exercise: Strategies for Weight Management in the Rural Midwest. Health Educ Behav 2005;32(2):253–263. [DOI] [PubMed] [Google Scholar]
- 7.Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long-term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 2014;348:g2646–g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombrowski SU, Avenell A, Sniehotta FF. Behavioural Interventions for Obese Adults with Additional Risk Factors for Morbidity: Systematic Review of Effects on Behaviour, Weight and Disease Risk Factors. Obes Facts 2010;3:377–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: A systematic review and meta-analysis. Obes Rev 2012;13(6):509–517. [DOI] [PubMed] [Google Scholar]
- 10.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation 2012;125(9):1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Census Bureau. (2016) New Census Data Show Differences Between Urban and Rural Populations. URL https://www.census.gov/newsroom/press-releases/2016/cb16-210.html [Google Scholar]
- 12.U.S. Census Bureau. (2015) 2011-2015 American Community Survey 5-Year Estimates. URL https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YR_DP05&src=pt [Google Scholar]
- 13.Wyatt HR, Jortberg BT, Babbel C, et al. Weight loss in a community initiative that promotes decreased energy intake and increased physical activity and dairy consumption: Calcium Weighs-In. J Phys Act Health 2008;5(1):28–44. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Linnan L, Kramish Campbell M, Brooks C, Koenig HG, Wiesen C. The WORD (Wholeness, Oneness, Righteousness, Deliverance): A Faith-Based Weight-Loss Program Utilizing a Community-Based Participatory Research Approach. Health Educ Behav 2008;35(5):634–650. [DOI] [PubMed] [Google Scholar]
- 15.Befort CA, VanWormer JJ, DeSouza C, et al. Protocol for the Rural Engagement in Primary Care for Optimizing Weight Reduction (RE-POWER) Trial: Comparing three obesity treatment models in rural primary care. Contemp Clin Trials 2016;47:304–314. [DOI] [PubMed] [Google Scholar]
- 16.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health 1999;89(9):1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaglio B, Shoup J, Glasgow RE. The RE-AIM Framework: A Systematic Review of Use over Time. American J Public Health 2013;103(6):e38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn G Effect of Degree of Weight Loss on Health Benefits. Obesity Research 1995;3(2S):211s–216s. [DOI] [PubMed] [Google Scholar]
- 19.Masheb RM, Chan SH, Raffa SD, et al. State of the art conference on weight management in VA: Policy and research recommendations for advancing behavioral interventions. J Gen Intern Med 2017;32(S1):74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RS, Purcell EP, Glasgow RE, Klesges LM, Benkeser RM, & Peck CJ. What does it mean to ‘employ’ the RE-AIM model? Eval Health Prof 2013;36(1):44–66. [DOI] [PubMed] [Google Scholar]
- 21.Ribisl KM, Leeman J, Glasser AM. Pricing Health Behavior Interventions to Promote Adoption: Lessons from the Marketing and Business Literature. Am J Prev Med 2014;46(6):653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 12925 suppl 2(2014):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Reprinted from Annals of Internal Medicine). Phys Ther 2009;89(9):873–880. [PubMed] [Google Scholar]
- 24.Office of Management and Budget. 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas. Fed Regist 2010;75(123):1–9. [Google Scholar]
- 25.Ratcliffe M, Burd C, Holder K, Fields A. Defining Rural at the U.S. Census Bureau. American Community Survey and Geography Brief. 2016;(December):1–8. [Google Scholar]
- 26.Centers for Disease Control and Prevention. 2013 NCHS Urban – Rural Classification Scheme for Counties. Vital Heal Stat 2 2012;2(154):1–72. [Google Scholar]
- 27.Glasgow RRE, Klesges LLM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: Using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res 2006;21(5):688–694. [DOI] [PubMed] [Google Scholar]
- 28.Estabrooks PA, Allen KC. Updating, Employing, and Adapting: A Commentary on What Does It Mean to “Employ” The RE-AIM Model. Eval Health Prof 2013;36(1):67–72. [DOI] [PubMed] [Google Scholar]
- 29.Harden SM, Gaglio B, Shoup JA, et al. Fidelity to and comparative results across behavioral interventions evaluated through the RE-AIM framework: A systematic review. Syst Rev 2015;4(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 33.Kontopantelis E, Reeves D. metaan: Random-effects meta-analysis. Stata J 2010;10(3):395. [Google Scholar]
- 34.Harris MF, Fanaian M, Jayasinghe UW, et al. A cluster randomised controlled trial of vascular risk factor management in general practice. Med J Aust 2012;197(7):387–393. [DOI] [PubMed] [Google Scholar]
- 35.Dunbar JA, Jayawardena A, Johnson G, et al. Scaling Up Diabetes Prevention in Victoria, Australia: Policy Development, Implementation, and Evaluation. Diabetes Care 2014;37(4):934–942. [DOI] [PubMed] [Google Scholar]
- 36.Amundson HA, Butcher MK, Gohdes D, et al. Translating the diabetes prevention program into practice in the general community: Findings from the Montana cardiovascular disease and diabetes prevention program. Diabetes Educ 2009;35(2):209–223. [DOI] [PubMed] [Google Scholar]
- 37.Vadheim LM, Brewer KA, Kassner DR, et al. Effectiveness of a Lifestyle Intervention Program Among Persons at High Risk for Cardiovascular Disease and Diabetes in a Rural Community. J Rural Health 2010;26(3):266–272. [DOI] [PubMed] [Google Scholar]
- 38.Fanaian M, Laws RA, Passey M, et al. Health Improvement and Prevention Study (HIPS): Evaluation of an intervention to prevent vascular disease in general practice. BMC Family Practice 2010;11(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahrendt AD, Kattelmann KK, Rector TS, Maddox DA. The Effectiveness of Telemedicine for Weight Management in the MOVE! Program. J Rural Health 2014;30(1):113–119. [DOI] [PubMed] [Google Scholar]
- 40.Sangster J, Furber S, Allman-Farinelli M, et al. A population-based lifestyle intervention to promote healthy weight and physical activity in people with cardiac disease: The PANACHE (Physical Activity, Nutrition And Cardiac HEalth) study protocol. BMC Cardiovasc Disord 2010;10(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangster J, Furber S, Allman-Farinelli M, et al. Effectiveness of a Pedometer-Based Telephone Coaching Program on Weight and Physical Activity for People Referred to a Cardiac Rehabilitation Program: A randomized controlled trial. J Cardiopulm Rehabil Prev 2015;35(2):124–129. [DOI] [PubMed] [Google Scholar]
- 42.Kramer MK, McWilliams JR, Chen H-Y, Siminerio LM. A Community-Based Diabetes Prevention Program. Diabetes Educ 2011;37(5):659–668. [DOI] [PubMed] [Google Scholar]
- 43.Kramer MK, Miller RG, Siminerio LM. Evaluation of a community Diabetes Prevention Program delivered by diabetes educators in the United States: One-year follow up. Diabetes Res Clin Pract 2014;106(3):e49–e52. [DOI] [PubMed] [Google Scholar]
- 44.Gregoski MJ, Newton J, Ling CG, et al. Effective weight-loss using an e-health delivered physical activity and dietary intervention: A federal credit union pilot study. Work 2016;54(1):127–134. [DOI] [PubMed] [Google Scholar]
- 45.Schafer E, Anderson P. Heart*Style: A Worksite Nutrition Education Program in a Rural Setting. J Nutr Educ Behav 1998;30(1):62–65. [Google Scholar]
- 46.Befort CA, Klemp JR, Fabian C, et al. Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemp Clin Trials 2014;37(2):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zoellner J, Hill JL, Grier K, et al. Randomized Controlled Trial Targeting Obesity-Related Behaviors: Better Together Healthy Caswell County. Prev Chronic Dis 2013;10(2):120296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fazzino TL, Sporn NJ, Befort CA. A qualitative evaluation of a group phone-based weight loss intervention for rural breast cancer survivors: Themes and mechanisms of success. Support Care Cancer 2016;24(7):3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Befort CA, Klemp JR, Austin HL, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat 2012;132(2):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West DS, Bursac Z, Cornell CE, et al. Lay health educators translate a weight-loss intervention in senior centers: A randomized controlled trial. Am J Prev Med 2011;41(4):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keyserling TC, Ammerman AS, Atwood JR, et al. A cholesterol intervention program for public health nurses in the rural southeast: Description of the intervention, study design, and baseline results. Public Health Nurs 1999;16(3):156–167. [DOI] [PubMed] [Google Scholar]
- 52.Anderson-Loftin W, Barnett S, Bunn P, Sullivan P, Hussey J, Tavakoli A. Soul Food Light: Culturally Competent Diabetes Education. Diabetes Educ 2005;31(4):555–563. [DOI] [PubMed] [Google Scholar]
- 53.Davis-Smith YM, Boltri JM, Seale JP, Shellenberger S, Blalock T, Tobin B. Implementing a diabetes prevention program in a rural African-American church. J Natl Med Assoc 2007;99(4):440–446. [PMC free article] [PubMed] [Google Scholar]
- 54.Folta SC, Lichtenstein AH, Seguin RA, Goldberg JP, Kuder JF, Nelson ME. The StrongWomen—Healthy Hearts program: Reducing cardiovascular disease risk factors in rural sedentary, overweight, and obese midlife and older women. Am J Public Health 2009;99(7):1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ely AC, Banitt A, Befort C, et al. Kansas Primary Care Weighs In: A Pilot Randomized Trial of a Chronic Care Model Program for Obesity in 3 Rural Kansas Primary Care Practices. J Rural Heal 2008;24(2):125–132. [DOI] [PubMed] [Google Scholar]
- 56.Foley P, Steinberg D, Levine E, et al. Track: A randomized controlled trial of a digital health obesity treatment intervention for medically vulnerable primary care patients. Contemp Clin Trials 2016;48:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed JR, Yates BC, Houfek J, Briner W, Schmid KK, Pullen CH. Motivational Factors Predict Weight Loss in Rural Adults. Public Health Nurs 2016;33(3):232–241. [DOI] [PubMed] [Google Scholar]
- 58.Befort CA, Klemp JR, Fabian C, et al. Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemp Clin Trials 2014;37(2):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parra-Medina D, D’Antonio A, Smith SM, Levin S, Kirkner G, Mayer-Davis E. Successful recruitment and retention strategies for a randomized weight management trial for people with diabetes living in rural, medically underserved counties of South Carolina: the POWER study. J Am Diet Assoc 2004;104(1):70–75. [DOI] [PubMed] [Google Scholar]
- 60.Befort CA, Donnelly JE, Sullivan DK, Ellerbeck EF, Perri MG. Group versus individual phone-based obesity treatment for rural women. Eat Behav 2010;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrock LE. Review of cost efficiency & efficacy of delivering diabetes education program in southwest rural healthcare facility. Diabetes Educ 1998;24(4):485–492. [DOI] [PubMed] [Google Scholar]
- 62.Perri MG, Limacher MC, von Castel-Roberts K, et al. Comparative effectiveness of three doses of weight-loss counseling: Two-year findings from the rural LITE trial. Obesity 2014;22(11):2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radclif TA, Bobroff LB, Lutes LD, et al. Comparing costs of telephone versus face-to-face extended care programs for the management of obesity in rural settings. J Acad Nutr Diet 2012;112(9):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Y, You W, Almeida F, Estabrooks P, Davy B. The Effectiveness and Cost of Lifestyle Interventions Including Nutrition Education for Diabetes Prevention: A Systematic Review and Meta-Analysis. J Acad Nutr Diet 2017;117(3):404–421.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pellegrini CA, Verba SD, Otto AD. The Comparison of a Technology-Based System and an In-Person Behavioral Weight Loss Intervention. Obesity 2012. 202:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perri MG, Limacher MC, Durning PE, et al. Extended-Care Programs for Weight Management in Rural Communities. JAMA Intern Med 2008;168(21):2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeary KHCK, Cornell CE, Moore P, Bursac Z, Prewitt TE, West DS, & Turner J. Peer Reviewed: Feasibility of an Evidence-Based Weight Loss Intervention for a Faith-Based, Rural, African American Population. Prev Chronic Dis 2011;8(6). [PMC free article] [PubMed] [Google Scholar]
- 68.Yeary KHCK, Cornell CE, Prewitt E, et al. The WORD (Wholeness, Oneness, Righteousness, Deliverance): Design of a randomized controlled trial testing the effectiveness of an evidence-based weight loss and maintenance intervention translated for a faith-based, rural, African American population using a community-based participatory approach. Contemp Clin Trials 2015;40:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hageman PA, Pullen CH, Hertzog M, Boeckner LS, & Walker SN. Web-based interventions for weight loss and weight maintenance among rural midlife and older women: protocol for a randomized controlled trial. BMC Public Health 2011;11(1):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shade MY, Berger AM, Dizona PJ, Pozehl BJ, & Pullen CH. Sleep and health-related factors in overweight and obese rural women in a randomized controlled trial. J Behav Med 2016;39(3):386–397. [DOI] [PubMed] [Google Scholar]
- 71.Befort CA, Bennett L, Christafano D, Klemp JR, & Krebill H. Effective recruitment of rural breast cancer survivors into a lifestyle intervention. Psycho-oncology 2015;24(4):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vadheim LM, McPherson C, Kassner DR, et al. Adapted diabetes prevention program lifestyle intervention can be effectively delivered through telehealth. Diabetes Educ 2010;36(4):651–656. [DOI] [PubMed] [Google Scholar]
- 73.Mayer-Davis EJ, D’Antonio A, Martin M, Wandersman A, Parra-Medina D, & Schulz R. Pilot study of strategies for effective weight management in type 2 diabetes: Pounds Off with Empowerment (POWER). Fam Community Health 2001;24(2):27–35. [DOI] [PubMed] [Google Scholar]
- 74.Mayer-Davis EJ, D’Antonio AM, Smith SM, Kirkner G, Levin Martin S, Parra-Medina D, & Schultz R. Pounds Off With Empowerment (POWER): A clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J of Public Health 2004;94(10):1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garies S, Irving A, Williamson T, & Drummond N. Using EMR data to evaluate a physician-developed lifestyle plan for obese patients in primary care. Can Fam Physician 2015;61(5):e225–e231. [PMC free article] [PubMed] [Google Scholar]
- 76.Cummings DM, Lutes LD, Littlewood K, Dinatale E, Hambidge B, & Schulman K. EMPOWER: A randomized trial using community health workers to deliver a lifestyle intervention program in African American women with Type 2 diabetes: Design, rationale, and baseline characteristics. Contemp Clin Trials 2013;36(1):147–53. [DOI] [PubMed] [Google Scholar]
- 77.Lyle D, Hobba J, Lloyd K, et al. Mobilising a rural community to lose weight: Impact evaluation of the WellingTonne Challenge. Aust J Rural Health 2008;16(2):80–5. [DOI] [PubMed] [Google Scholar]
- 78.Parker VG, Coles C, Logan BN, & Davis L. The LIFE project: A community-based weight loss intervention program for rural African American women. Fam Community Health 2010;33(2):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothacker DQ. Five-year self-management of weight using meal replacements: Comparison with matched controls in rural Wisconsin. Nutrition 2000;16(5):344–8. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland M, Cowart M, & Heck CA. Rural senior citizens health promotion demonstration project. AM J Health Educ 1989;20(6):40–43. [PubMed] [Google Scholar]
- 81.Carter VL, Dawkins NL, & Howard B. Weight and blood pressure reduction among participants engaged in a cancer awareness and prevention program. Preventive Med Reports 2015;2:858–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daniel EL. A multi-intervention weight management program for low-income rural women. Journal of the American Dietetic Association 1989;89(9):1310–1311. [PubMed] [Google Scholar]
- 83.Aoun S, & Rosenberg M. Are Rural People Getting HeartSmart? Aust J Rural Health 2004;12:81–88. [DOI] [PubMed] [Google Scholar]
- 84.Saleh ZT, Lennie TA, Mudd-Martin G, et al. Decreasing sedentary behavior by 30 minutes per day reduces cardiovascular disease risk factors in rural Americans. Heart Lung 2015;44(5):382–386. [DOI] [PubMed] [Google Scholar]
- 85.Kellow N Evaluation of a rural community pharmacy-based Waist Management Project: Bringing the program to the people. Aust J Prim Health 2011;17(1):16–22. [DOI] [PubMed] [Google Scholar]
- 86.Kilkkinen A, Heistaro S, Laatikainen T, Janus E, Chapman A, Absetz P, & Dunbar J. Prevention of type 2 diabetes in a primary health care setting. Interim results from the Greater Green Triangle (GGT) Diabetes Prevention Project. Diabetes Res Clin Pract 2007;76(3):460–2. [DOI] [PubMed] [Google Scholar]
- 87.Laatikainen T, Dunbar JA, Chapman A, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health 2007;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwartz R, Powell L, & Keifer M. Family-based risk reduction of obesity and metabolic syndrome: An overview and outcomes of the Idaho partnership for Hispanic health. J of Health Care Poor Underserved 2013;24(2):129–144. [DOI] [PubMed] [Google Scholar]
- 89.Rigsby BD. Hypertension improvement through healthy lifestyle modifications. The ABNF Journal: Official Journal of the Association of Black Nursing Faculty in Higher Education 2011;22(2):41–3. [PubMed] [Google Scholar]
- 90.Whisenant D, Cortes C, & Hill J. Is faith-based health promotion effective? Results from two programs. J Christ Nurs 2014;31(3):188–93. [DOI] [PubMed] [Google Scholar]
- 91.Ammerman AS, Keyserling TC, Atwood JR, Hosking JD, Zayed H, & Krasny C. A randomized controlled trial of a public health nurse directed treatment program for rural patients with high blood cholesterol. Prev Med 2003;36(3):340–51. [DOI] [PubMed] [Google Scholar]
- 92.Piatt GA, Seidel MC, Powell RO, & Zgibor JC. Comparative effectiveness of lifestyle intervention efforts in the community: Results of the Rethinking Eating and ACTivity (REACT) study. Diabetes Care 2013;36(2):202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piatt GA, Seidel MC, Powell RO, & Zgibor JC. Influence of Patient-Centered Decision Making on Sustained Weight Loss and Risk Reduction Following Lifestyle Intervention Efforts in Rural Pennsylvania. Diabetes Educ 2016;42(3):281–90. [DOI] [PubMed] [Google Scholar]
- 94.Nam S Effects of social support and spirituality on weight loss for rural African-American women. The ABNF Journal: Official Journal of the Association of Black Nursing Faculty in Higher Education 2013;24(3):71–6. [PubMed] [Google Scholar]
- 95.Rickel KA, Milsom VA, Ross KM, Hoover VJ, Peterson ND, & Perri MG. Differential response of African American and Caucasian women to extended-care programs for obesity management. Ethn & Dis 2011;21(2):170–5. [PMC free article] [PubMed] [Google Scholar]
- 96.Milsom VA, Middleton KMR, & Perri MG. Successful long-term weight loss maintenance in a rural population. Clin Interv Aging 2011;6:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pullen CH, Hageman PA, Boeckner L, Walker SN, & Oberdorfer MK. Feasibility of Internet-delivered weight loss interventions among rural women ages 50-69. J Geriatr Phys Ther 2008;31(3):105–12. [DOI] [PubMed] [Google Scholar]
- 98.Estabrooks PA, Wilson KE, McGuire TJ, et al. A Quasi-Experiment to Assess the Impact of a Scalable, Community-Based Weight Loss Program: Combining Reach, Effectiveness, and Cost. J Gen Intern Med 2017;32(S1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Summary of All Papers Included in Analysis. N=64 papers describing 50 unique studies.