Abstract
Simple Summary
Epstein-Barr virus (EBV) infection is known to contribute in nasopharyngeal carcinoma (NPC) carcinogenesis. The oncogenic roles of the EBV proteins and non-coding RNAs in NPC are becoming evident with the aid of current advances in genome-wide and in-depth molecular analyses. This current work provides a comprehensive overview, which covers recent understandings of the pathogenic role of EBV infection in NPC. Perspectives on molecular mechanisms, which are involved in the pathogenesis of NPC, focusing on the connection between EBV and NPC cells and the corresponding signaling pathways are highlighted. Cancer hallmarks associated with EBV in NPC development are also discussed herein.
Abstract
Nasopharyngeal carcinoma (NPC) is one of the most common tumors occurring in China and Southeast Asia. Etiology of NPC seems to be complex and involves many determinants, one of which is Epstein-Barr virus (EBV) infection. Although evidence demonstrates that EBV infection plays a key role in NPC carcinogenesis, the exact relationship between EBV and dysregulation of signaling pathways in NPC needs to be clarified. This review focuses on the interplay between EBV and NPC cells and the corresponding signaling pathways, which are modulated by EBV oncoproteins and non-coding RNAs. These altered signaling pathways could be critical for the initiation and progression of NPC.
Keywords: nasopharyngeal carcinoma, EBV, oncogenesis, infection, signaling pathway
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignant cancer, which is located behind the nose at the very upper part of the throat. As its symptoms are usually silent and painless, the patients mostly are diagnosed in the advanced stages of the disease, leading to poor prognosis [1]. Not only do inherited genetic mutations pose one of the important causes of the cancer, the Epstein–Barr virus (EBV) also plays a key role in the onset of disease and progression of NPC [1,2,3]. EBV-associated carcinogenesis is a multi-step process, involving the accumulation of genetic alterations in the cells, leading to genetic instability and dysregulation of signaling pathways, which subsequently transforms the cells allowing EBV genome maintenance and leading to the acquisition of cancerous phenotypes [3]. The dysregulation of signaling pathways in NPC has been extensively studied [4,5]. Members of such pathways can be useful for the early detection, prognosis, and targeted therapy. This review article will focus on the contribution of EBV in NPC carcinogenesis and NPC progression with the emphasis on the role of EBV oncoproteins and non-coding RNAs on altering signaling pathways in NPC cells.
2. Epidemiology of NPC
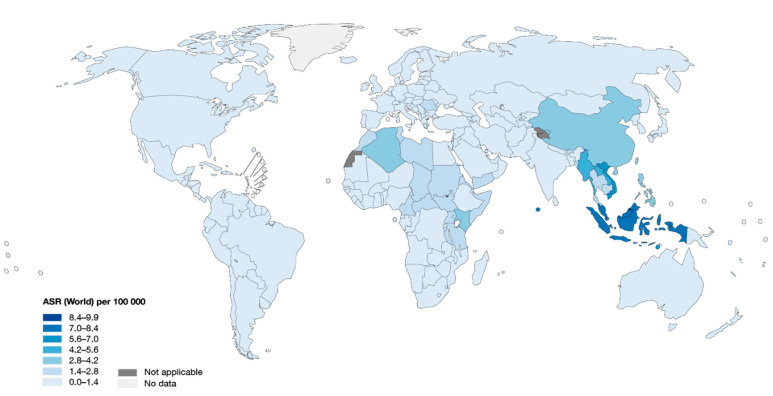
In 2018, the global estimate of cancer incidence worldwide has been reported to be approximately 18.1 million cases, with 129,079 of those cases being NPC estimating an age-standardized rate (ASR) of 1.5 per 100,000 people [6]. In the same year, a total of 72,987 mortality of NPC has been reported, accounting for ~0.8% of the total cancer deaths. Comparing incidence to other types, NPC can be considered an uncommon cancer, and its distribution worldwide is very imbalanced (Figure 1). NPC is a rare cancer in the United States (ASR 0.45) and most European countries (ASR 0.44); however, it is much more common in Asia, especially in Southeastern (ASR 5.0) and Eastern Asian countries (ASR 2.7) where 27% and 50% of the total NPC cases have been reported in the two areas, respectively [7].
Figure 1.
Global distribution of nasopharyngeal carcinoma. Nasopharyngeal carcinoma (NPC) incidence rate is represented as 100,000 person/years for all sex at all ages (0–85 years old). The map is obtained from International Agency for Research on Cancer (IARC) web-based cancer database, Global Cancer Observatory (GCO), and modified based on Globocan, 2018 [6].
Considering the uneven distribution of NPC globally, risks related to ethnicities have been suggested to contribute as a prominent risk factor. Among others, Chinese descendants are usually at higher risk, with a considerably higher incidence compared to other ethnicities in the same area [2,8,9,10]. Large-scale case-control studies have revealed an increased risk in NPC development among the Chinese population, with an increased familial risk observed among relatives and a younger age of onset in families with higher affected cases [11,12,13,14]. Furthermore, consumption of traditional diets high in nitrosamines such as salted fish and preserved vegetables is a causative factor in NPC as well [15,16,17]. Inflammatory inducing diets are also noteworthy in increasing the risk of NPC [18]. In contrast, consumption of non-preserved vegetables and fruit is inversely associated to NPC and can be considered protective in effect [15,19,20,21]. On the other hand, lifestyle habits such as smoking has also been associated with NPC [17,22,23] in addition to a higher mortality in patients [24]. Nonetheless, familial risks of NPC seems to be retained regardless of the ancestry, with similar observation found in non-Chinese populations in the United States, Europe, and Australia [25], highlighting further the role of genetic determinants underlying the development of NPC.
Looking into the host genetics, human leukocytes antigen (HLA) containing the major histocompatibility complex (MHC) region on chromosome 6p21 is recognized as the major risk loci for NPC [26]. However, single nucleotide polymorphisms (SNPs) identified through whole-genome sequencing (WGS) in DNA extracted from EBV-positive NPC tissues might reflect mutations that occur during tumorigenesis and may not be the congenital mutations of the patients. Li et al. have shown that mutations in the MHC expression inducer NLRC5, MHC class I rearrangements, and point mutations accumulated during the growth of tumor cells [27].
Not only are mutations in genes involved in immune escape of tumor cells, mutations that strongly activate the nuclear factor kappa B (NF-κB) signal in tumor cells were also found [27]. TNFRSF19, CDKN2A/2B, MDS1-EVI1, and CLPTM1L/TERT were other susceptible genes [28,29]. The whole-exome sequencing (WES) data demonstrated that several rare genes might contribute to the development of NPC such as genes involved in Notch signaling (NOTCH1, DLL3), EBV entry into epithelial cells (ITGB6), EBV modulation (BCL2L12, NEED4L), magnesium transport (NIPAL1), DNA repair (PRKDC, MLH1), and cAMP signaling (RAPGEF3) [30].
Despite the high prevalence of NPC in Asian countries, the latest cohort study showed that NPC incidence has gradually declined [10,31]. A continuous declining pattern of NPC incidence over the last few decades may be related to the changes in lifestyle and advancement in healthcare facilities [32,33].
3. EBV Strains
EBV can be classified into type 1 and type 2 based on the differences in the EBV nuclear antigen 2 (EBNA2) gene sequence. The type 1 EBV (e.g., B95-8, GP202, and Akata) exhibits worldwide distribution, while type 2 EBV (e.g., AG876) is prevalent in Africa [34]. Recently, the genomes of EBV isolates collected from various locations are fully sequenced to investigate geographic variation and its association with diseases [34,35], as shown in Table 1. The EBV strain M81 is shown to infect epithelial cells more efficiently than EBV strains isolated from B lymphoma [36], suggesting that the cell tropism of a viral strain may correlate with the type of tumors.
Table 1.
EBV strains from NPC tissue.
| EBV Genomes | Sources | GenBank Accession Number | LMP1 Strains | References |
|---|---|---|---|---|
| GD1 | Saliva of NPC patients | AY961628 | China 1 | [44] |
| GD2 | Biopsy of NPC patients | HQ020558 | China 1 | [45] |
| M81 | LCL isolated from NPC patients | KF373730 | China 1 | [46] |
| HKNPC1 | NPC patient | JQ009376 | China 1 | [47] |
| HNKPC2 | NPC patient | MH590513 | China 1 | [47] |
| HNKPC3 | NPC patient | MH590514 | China 1 | [47] |
| HNKPC4 | NPC patient | MH590515 | China 1 | [47] |
| HNKPC5 | NPC patient | MH590516 | China 1 | [47] |
| HNKPC6 | NPC patient | MH590517 | China 1 | [47] |
| HNKPC7 | NPC patient | MH590518 | China 1 | [47] |
| HNKPC8 | NPC patient | MH590519 | China 1 | [47] |
| HNKPC9 | NPC patient | MH590520 | China 1 | [47] |
| C666-1 | NPC cell lines | KC617875 | China 1 | [48] |
| M-ABA | LCL isolated from NPC patients | LN827527 | B95.8 | [49] |
The SNPs can be found in various EBV genes and transcription regulatory regions. Accordingly, latent membrane protein 1 (LMP1) genes are classified into six groups: China 1, China 2, Alaskan, Mediterranean, North Carolina, and B95-8 [37,38,39]. China 1 LMP1 strongly activates NF-κB signals compared to B95-8 LMP1 [39]. It was also reported that mutations in the BALF2 gene found in 83% of EBVs in South China were strongly correlated with NPC cases [40]. Further experiments using recombinant viruses are expected to confirm whether these SNPs are truly associated with NPC formation.
An SNP in the BamH I A rightward transcript (BART) promoter region has been shown to induce BART promoter activity and high expression of BART miRNA is expected [41]. The SNP is strongly associated with NPC cases [41]. An SNP is also found in an EBV-encoded small non-coding RNA (EBER) gene from North Chinese NPC patients and the variation is different from EBV strains isolated from gastric carcinoma [42]. The V3 polymorphism in the Zp promoter activates EBV lytic infection in NPC [43].
4. EBV and NPC Carcinogenesis
Although EBV infection is pervasive, the interplay of EBV and other NPC risk factors is also evident as an increased risk is observed for EBV-seropositive with consumption of salted fish and other NPC environmental causes among high-risk groups [2,16,50].
4.1. EBV Life Cycle in Epithelial Cells
EBV is a ubiquitous gamma herpes virus, which infects greater than 90% of the world’s population [31,51]. EBV has been known as a causative agent for diseases such as Burkitt’s lymphoma, gastric carcinoma (GC), and NPC [52,53,54]. Primary EBV infection in epithelial cells begins when the virus crosses epithelial cells of the nasopharynx, infecting the naïve B cells in Waldeyer’s tonsillar ring [55]. It is believed that pharyngeal epithelial cells act as a reservoir, in which when the lytic reactivation is induced, the EBV genomes are amplified and viral particles are released into saliva for transmission.
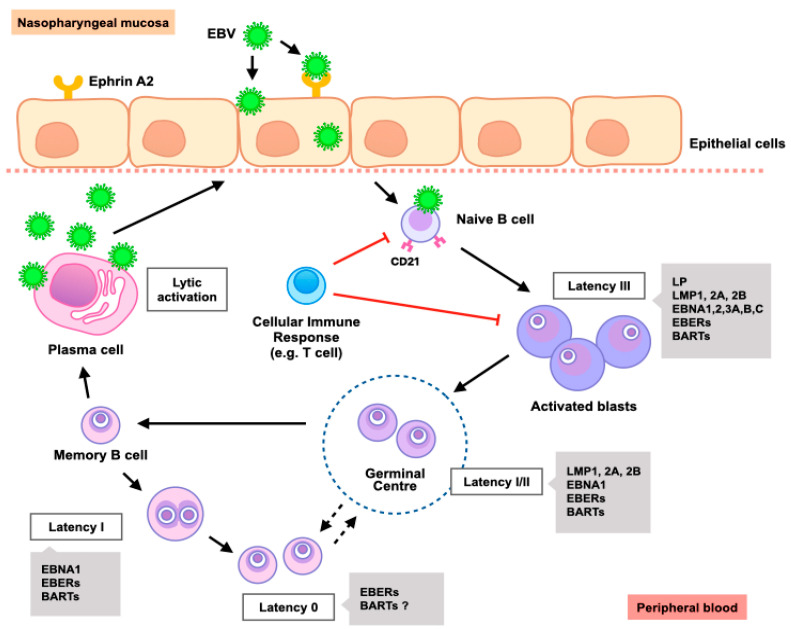
The route of entry for EBV in epithelial cells is complicated. There are several hypotheses on how EBV enters epithelial cells, which have been reviewed elsewhere [56]. In normal pharyngeal epithelium, CD21 is weakly expressed or nearly absent [57]. It has been demonstrated that dysplastic pharyngeal epithelial cells expressed CR2 gene (CD21) transcripts [58]. It is yet to be determined whether CD21 plays a role in EBV entry to epithelial cells. However, recent findings revealed that novel receptors mediated EBV entry into nasopharyngeal epithelial (NPE) cells such as neuropilin1 (NRP1) and ephrin A2. NRP1 interacts with EBV glycoprotein gB to facilitate viral internalization and membrane fusion [59,60], while ephrin A2 has been shown to induce the EBV infection with the cell-free virus in immortalized NPE cells [61,62]. The life cycle of EBV in nasopharyngeal cells is shown in Figure 2.
Figure 2.
EBV life cycle. Primary EBV infection initiates in nasopharyngeal mucosa. EBV utilizes distinct glycoproteins to infect epithelial cells through Ephrin A2 and naïve B cells via the CD21 receptor. Viral entry causes the transport of the EBV genome into the B-cell nucleus, where viral replication process begins. EBV gene products activate the B-cell growth program (Latency III), resulting in the proliferation of blasting B cells. Normally, these blasting B cells are destroyed by cellular immune responses such as cytotoxic T lymphocytes. Once in the circulation, previously activated memory B cells may continue to undergo lytic replication or, if EBV shuts down most of its protein-encoding genes, it enters latency state. At a later time, as cells recirculate between the nasopharynx mucosa and peripheral compartments, memory B cells may be activated, resulting in viral reactivation and shedding. Genes expressed during each EBV latency period are indicated in the grey boxes.
In order to understand how EBV induces tumors in epithelial cells, it is desirable to use non-transformed cells as an oncogenic model. Lymphoblastoid cell lines (LCL) represent a good tool to study the formation of EBV-associated lymphomas. However, EBV infection to primary epithelial cells induces lytic infection [63,64], therefore primary epithelial cells are not suitable for oncogenic study. Instead, cell lines originated from cancers are used, because these cell lines often show latent infection of EBV. It is well known that EBV-positive NPC tumor cells lose EBV episomes and become EBV-negative during in vitro culture [65]. EBV-positive NPC tumor cells can be isolated using the xenograft method of inoculating mice with NPC patient tissue. It is known that in vitro culture of NPC tumor cells shows lytic EBV infection, thus EBV-infected cells are killed and the remaining cells are all negative for EBV [66,67]. However, when the same cells were cultured with the addition of ROCK inhibitors, lytic infection was suppressed and EBV-positive cell lines could be established [66,67].
4.2. EBV Latency in NPC
EBV episomes replicate through the rolling circle method to generate multiple copies of the linearized EBV genome packaged into an infectious virus [68]. Upon infection, this linearized genome is circularized into episomes and heavily methylated to drive EBV into latency by limiting viral gene expression to a few coding and non-coding genes only. Homogenous length of terminal repeat is identified in NPC and pre-invasive lesions, indicating that EBV infection begins with clonal expansion of a single EBV-infected cell during the early stage of NPC development [69,70]. EBV persistently infects NPC cells as the latency II pattern that expresses episomal anchoring protein EBNA1, EBERs, BARTs, BamH I-A rightward frame-1 (BARF1), LMP1, and LMP2A. In addition, BARTs are expressed abundantly in NPC, suggesting their essential role in malignancies [71].
EBNA1 regulates cellular gene expression through binding of the viral DNA to cellular promoters [72,73]. It activates the viral promoter Cp and Wp and inhibits Qp promoters [74], which are important for the viral fate determination. EBNA1 is also essential for viral genome maintenance, chromosomal destabilization, and immune evasion.
LMP2A together with LMP2B promote motility, epithelial cell migration, and suppress differentiation [75]. In NPC, LMP2A enhances the epithelial-mesenchymal transition (EMT) via the metastatic tumor antigen 1 (MTA1) and mammalian target of rapamycin signaling [76]. BARF1 may induce carcinogenesis by immortalizing and transforming epithelial cells, thereby enabling immune evasion and cell survival [77]. Interestingly, BARF1 is exclusively expressed in EBV-positive epithelial cells, but not in the lymphoma. It is also not restricted during the lytic cycle, but also during the latency period [78].
LMP1 induces expression of intracellular adhesion molecules 1 (ICAM-1), CD40, and pro-inflammatory cytokines, such as interleukin-6 (IL-6) and IL-8 [51,79]. Overexpression of LMP1 has been shown to worsen survival ratio and increase invasiveness in an immortalized NPE cell line [80]. LMP1 reactivates EBV and regulates the EBV gene expression in differentiating epithelial cells [81,82]. These suggest that tight regulation of LMP1 may reflect the co-evolution of the oncogenesis, immune evasion, and viral pathogenesis in the host cells. LMP1 is also known to induce cell cycle disruption and genomic instability in NPC cells [83,84].
In addition, non-coding RNAs (ncRNAs) are parts of the EBV transcriptome, which are transcribed during various life cycle stages. EBV ncRNAs consist of EBERs, small nucleolar RNAs (snoRNA1), and microRNAs (BamH I fragment H rightward open reading frame miR-(BHRF)s and miR-BARTs) [85]. These ncRNAs differentially regulate host targets. While EBER and snoRNA form a secondary structure which facilitates the interaction to signaling proteins directly [86], BHRFs and BARTs interact with RNA-induced silencing complex (RISC) as a guide for complementary mRNA target that leads to its degradation [87]. During the last decade, a large number of EBV ncRNAs have been reported and investigated for their roles in NPC [88].
The host factors and genetic modification are important for maintaining EBV activity. It has been shown that EBV is unable to transform the primary and immortalized NPE cells. Rather, immortalized NPE cells infected with EBV become cell senescence and stop cell division [89]. However, cells lacking p16, a negative regulator of cyclin D1, or overexpressing cyclin D1, can grow while maintaining EBV infection [89]. It has been reported that premalignant nasopharyngeal epithelium overexpressed cyclin D1, which enables persistent infection of EBV [89,90]. The overexpression of polycomb complex protein, B lymphoma Mo-MLV insertion region 1 homolog (Bmi-1), is able to immortalize primary epithelial cells, which allows the EBV to establish latency [91,92]. These indicate the interplay of host factors in regulating the EBV proteins in the infected epithelial cells.
4.3. EBV Lytic Infection in NPC
The EBV life cycle includes both the lytic and latent infection. Lytic infection is frequently associated with apoptosis [93]. First, the expression of immediate-early (IE) genes such as BZLF1 and BRLF1 are induced by cellular stress. In turn, the expression of early genes including viral protein kinase (BGLF4), viral DNase (BGLF5), viral DNA polymerase (BALF5), EA, BALF3, BARF1, and BHRF1 are induced by BZLF1 and BRLF1. Finally, late genes such as BCRF1 and VCA are highly expressed. These lytic proteins are involved in the amplification and replication of the viral genome [93]. Interestingly, small populations of cells showing lytic infection are commonly found in EBV-infected patient-derived xenograft cell lines [67,94]. Consistently, high-risk NPC patients exhibited elevated antibody titers against EBV lytic proteins, such as BGLF5, BALF5, EA, and VCA [95,96].
BZLF1 induces the expression of inflammatory cytokines such as IL-8 [97]. Though increased inflammation can be observed in NPC, cells showing lytic infection of EBV can evade CD8+ T cell recognition [98]. Moreover, EBV lytic proteins including BGLF4, BGLF5, and BALF3, could induce chromosomal aberration, DNA damage, and genomic instability [99,100,101]. Other lytic proteins, such as BARF1, BHRF1, and BCRF1, can also induce an anti-apoptotic phenotype [102]. These suggest that EBV lytic proteins enhance the tumorigenicity of NPC cells.
5. EBV-Mediated Signaling
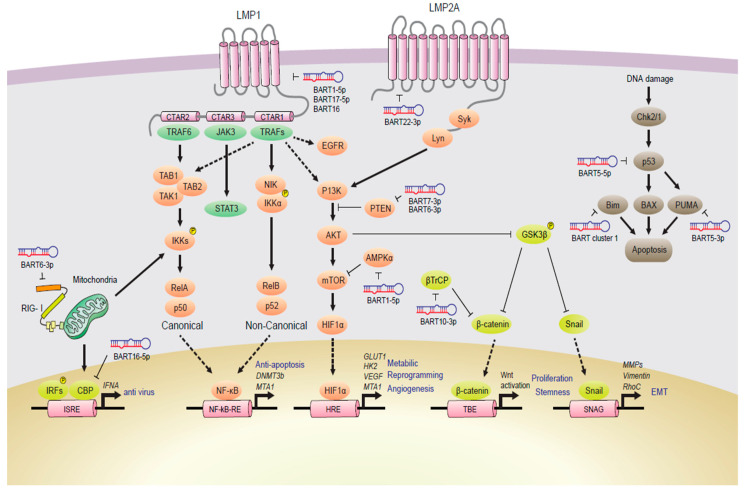
Dysregulation of intracellular signaling mediated by EBV during NPC carcinogenesis has been evident and described. The EBV-mediated signaling pathways in NPC are depicted in Figure 3.
Figure 3.
EBV-mediated signaling in NPC. An overview of signaling pathways in the NPC mediated by EBV infection. The EBV-encoded proteins including LMP1, LMP2A, and EBNA1 cause dysregulation in intracellular signaling pathways in NPC cells. This figure represents the signaling pathways, such as WNT/β-catenin, JAK/STAT, PI3K/Akt/mTOR, EGFR, and MAPK, and NF-κB, of which details are described in text. EBV ncRNA can also regulate these signals and enhances tumorigenesis and progression of NPC. The headed arrows show up-regulation and blunt-ended arrows show downregulation.
5.1. Wnt/β-Catenin
Wnt signaling pathway is a result of Wnt ligand interacting with a corresponding membrane receptor, thereby causing a nuclear translocation of β-catenin and triggering gene expression. The canonical Wnt pathway plays a role in the embryonic process and contributes to carcinogenesis and cancer progression [103,104,105]. β-catenin acts as a pivotal protein and its overexpression has been observed in cancer stem cells (CSCs) and many tumors including NPC [106]. Wnt signaling pathway is dysregulated by EBV during NPC progression. The expression of LMP1 is not correlated with the activation of β-catenin in the canonical Wnt pathway [107]. On the other hand, LMP2A induces MTA1, which stimulates the downstream Wnt cascade, such as phosphorylation of glycogen synthase kinase 3-beta (GSK3β) and nuclear translocation of β-catenin, thereby LMP2A enhances EMT process and tumor invasion [76]. Moreover, LMP2A activates the non-canonical Wnt pathway, inducing growth, and progression of NPC [108].
5.2. JAK/STAT
Janus kinases (JAK)-signal transducer and activator of transcription (STAT) signaling is composed of JAK, STAT, and cellular receptors. When cytokines bind to cellular receptors, JAK becomes phosphorylated and then phosphorylates STAT. Receptor-associated STAT is phosphorylated by JAK. Phospho-STAT is then translocated into the nucleus and regulates immune response. STAT protein members such as STAT1, STAT3, and STAT5 are upregulated in NPC tissues [109]. In addition, JAK1, a member of JAK family, promotes Qp activity of EBNA-1 and promoter of LMP1 by binding of activated STAT proteins in vitro [109,110]. JAK-STAT signaling pathway is triggered by the LMP1 through its carboxyl-terminal activation domain 3 (LMP1-CTAR3), which then activates JAK3 and promotes its phosphorylation [111], resulting in nuclear translocation [111,112,113]. STAT3 also binds to the cyclin D1 promoter, which enhances its transcription [114]. In addition, STAT3 promotes angiogenesis as it is a transcriptional-activator of the vascular endothelial growth factor (VEGF) gene [115]. Overexpression of STAT3 has been shown to link to VEGF and exhibits a negative correlation with the survival rate in NPC patients [116].
5.3. PI3K/Akt/mTOR
Phosphoinositide 3-kinase/protein kinase B (PI3K)/Akt cascade plays a crucial role in the NPC carcinogenesis via activation of EBV oncoproteins such as LMP1 and LMP2A [117]. LMP1 stimulates the PI3K/Akt pathway, which contributes to the induction and maintenance of CD44-positive CSC properties. PI3K/Akt and LMP1 create a positive feedback loop to regulate LMP1-induced CSC in NPC cell population [118,119]. A PI3K inhibitor, LY294002, has been shown to inhibit NPC cell proliferation and induces apoptosis, revealing PI3K as a potential target for therapeutics [120]. The mammalian target of rapamycin (mTOR) is a downstream player of the PI3K/Akt pathway. It phosphorylates and activates its effectors such as ribosomal protein S6 kinases (P70S6K) and eukaryotic initiation factor 4E binding protein (4EBP1). It has been shown that LMP1, p-P70S6K, and p-4EBP1 are up-regulated in NPC clinical samples [121], but are negatively correlated with NPC patient outcome [122]. EMT can be initiated through the epithelial cell adhesion molecule (EpCAM), which is highly upregulated in both RNA and protein levels in NPC. EpCAM participates in CSC phenotypes in vitro and cellular metastasis by enhancing mTOR signalings, such as Akt, mTOR, P70S6K, and 4EBP1 [123], and is also related to survival rate in NPC patients. Furthermore, a tumor suppressor protein, WW domain-containing oxidoreductase (WWOX), which is regulated by LMP1, has been shown to be correlated with the TNM stages of NPC [124]. LMP2A also enhances the migrative and invasive properties and the expression of stem-like cell markers in NPC through AKT pathway activation [125].
5.4. EGFR and MAPK
The epidermal growth factor receptors (EGFRs) are tyrosine kinase receptors, whose dysregulation has been reported in various epithelial tumors. Co-expression of LMP1 and EGFR is found in more than 60% of NPC clinical samples and is correlated with poor prognosis [126]. The LMP1-CTAR1 activates NF-κB family members, p50/p50 and Bcl-3 complexes, and upregulates EGFR [127]. LMP1 also enhances phosphorylation and nuclear translocation of EGFR [128], which is crucial in cell proliferation and differentiation associated with mitogen-activated protein kinases (MAPK). MAPK pathway, which is known as the Ras-Raf-MEK-extracellular signal-regulated kinase (ERK) pathway, transmits the signal from a receptor on cell surface into the nucleus via phosphorylation activity of numerous kinase proteins. MAPK pathway is dysregulated in LMP1-overexpressing NPC cells [121]. LMP1-CTAR1 also activates EGFR and ERK through protein kinase C delta (PKC delta) [113], which promotes NPC cell motility and invasiveness through activation of the ERK-MAPK pathway [129]. In addition, LMP1-CTAR1 induces the transcription of hypoxia-inducible factor 1-α (HIF-1α) via recruitment of ERK1/2 and NF-κB [130]. LMP1-positive extracellular vesicle from EBV-positive NPC enhances recipient NPC cell proliferation, invasion, and radioresistance through P38 MAPK pathway [125]. Furthermore, LMP2A upregulates EGFR, and intracellular Ca2+, which promotes Ca2+-dependent protease and calpain that cleave integrin β4 (ITGβ4) from the basal layer to peripheral membrane structures, leading to the motility of NPC cells [131].
5.5. NF-κB
NF-κB functions as a transcription factor forming several dimeric combinations, including NF-κB1 (p50/p105), NF-κB2 (p52/p100), c-Rel, RelA (p65), and RelB. The NF-κB is regulated by IκBs, which are phosphorylated by IκB kinases (IKKs). The phosphorylation and subsequent degradation of IκBs enable NF-κB to translocate to the nucleus and regulates transcription of various genes involved in the inflammation, immunity, stress responses [132]. Somatic mutation in genes for NF-κB regulators has been observed in NPC cells, with dominant alteration in NF-κB inhibitor alpha (NFKBIA), CYLD lysine 63 deubiquitinase (CYLD), and tumor necrosis factor receptor-associated factor 3 (TRAF3) [27,133,134]. NFKBIA encodes IκBα. CYLD negatively regulates NF-κB by deubiquitinating target proteins, such as IKKγ (NEMO), TRAF2, and TRAF6 [134]. CYLD overexpression strongly suppressed the growth, proliferation, metastasis, and migration of NPC cells [135]. It has been reported that dysregulation of NF-κB by somatic mutations and LMP1 overexpression are mutually exclusive in NPC development [27].
LMP1 activates NF-κB pathway through recruitment and activation of TRAFs, TRADD, EGFR, and others [136,137]. LMP1 increases phosphorylation of IκB then increases nuclear translocation of NF-κB to activate target genes [138]. The effect of EBV infection in NPC cells appears to be leaning towards regulation over NF-κB activity rather than the expression of NF-κB signaling proteins. Interestingly, the IκBα phosphorylation by IKKβ is induced by LMP1 activation via mTORC1 pathway, with further modulation on aerobic glycolysis through Glut-1 transcription [139]. Constitutive activation of NF-κB is a key step in NPC development.
LMP1 promoter is also activated by NF-κB through the p50-p50 homodimer and the p65-p50 heterodimer [140]. The CTAR1 of LMP1 can activate p50-p50, p50-p52, and p52-p65 dimers through TRAF-1, 2, and 3, whereas the CTAR2 activates p52-p65 through tumor necrosis factor receptor-associated death domain protein (TRADD) and TRAF2 [141,142]. Moreover, the p52-RelB complex requires the NF-κB-inducing kinase (NIK) and IKKα interaction and translocate to the nucleus [143].
The EBV-encoded BARTs miRNAs are regulated by the interaction of p50 subunit to NF-κB site at the BART promoter. BARTs miRNA expression upregulates LMP1 level in EBV-infected NPC cells [144]. Considering the dynamic of BART and LMP1 via NF-κB, the establishment of latent EBV infection forms an oncogenic stimulatory loop in NPC cells. In addition, LMP1 inhibits phosphatase and TEN-sin homolog (PTEN) expression, because LMP1 upregulates DNA methyltransferase 3b (DNMT3b) via activation of p65 subunit, which hypermethylated the PTEN promoter [145]. The p65 subunit can also bind to the Qp EBNA1 promoter and upregulate EBNA1 expression [146].
6. Cancer Hallmarks of EBV-Associated Malignancies
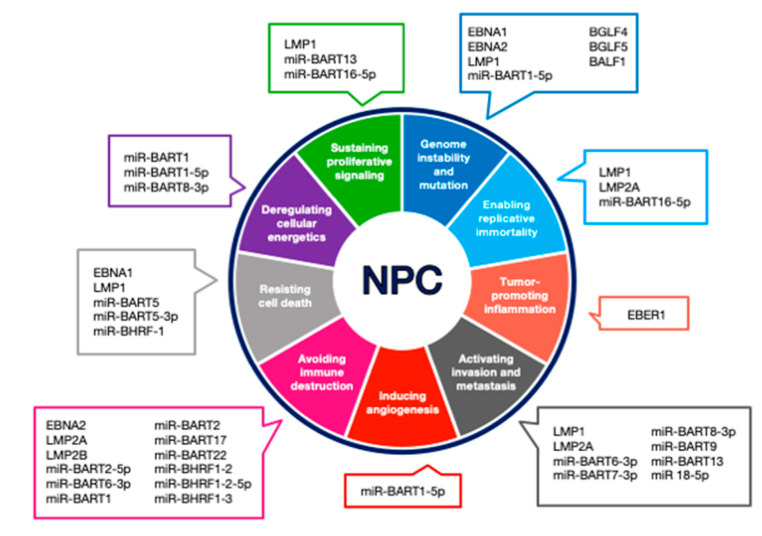
The core features of cancer have been well explored in tumor biology, and it has been further developed and revisited throughout the years. The features correspond to the hallmarks elucidated by Hanahan and Weinberg [147], described below and in Figure 4 are the hallmarks associated with EBV in NPC development.
Figure 4.
Malignancies associated with EBV. Summary of EBV major proteins, antigens, and microRNAs involved in establishment of NPC adapted from hallmarks of cancer established by Hanahan and Weinberg [147].
6.1. Immune Evasion
Immune evasion is a survival key for cancer cells. EBV sets a limited viral protein to be expressed during latency and to shed, establishing balance hiding from the host immune signaling pathway. LMP1 represses the expression of TLR9 whose ligand is viral unmethylated CpG DNA through activation of NF-κB [148]. LMP1 also promotes SUMOylation of interferon regulatory factor 7 (IRF7), which is important for the induction of type I interferon (IFN) [149]. On the other hand, LMP2A and 2B suppress IFN signaling by promoting the degradation of IFNα and IFNγ receptors [150]. Furthermore, many EBV lytic proteins are detected in NPC cells or patients, which are involved in immune evasion.
Likewise, EBV ncRNAs have been shown to allow NPC cells to avoid immune cell surveillance and escape the immune system [151,152]. Pattern recognition receptors play an important role in the innate immune response by detecting viral pathogens. During EBV infection, EBV miR-BART6-3p targets the binding sites in the 3′ untranslated region (UTR) of retinoic acid-inducible gene I (RIG-I) mRNA, resulting in a reduction of type I IFNs response [153]. Type I IFN signaling can also be inhibited at the transcription level via EBV miR-BART16-5p, which directly targets the 3′UTR of CREB-binding protein mRNA, a co-activator of IFNs [154]. Both EBV miR-BART6-3p and EBV miR-BART16-5p halt anti-proliferative properties of type I IFN, thereby enhance the tumorigenicity of NPC cells.
The cytokine network is a regulator of innate and adaptive immune systems [155]. Thus, disruption in cytokine signaling provokes NPC progression. EBV miR-BHRF1-2-5p targets the 3′UTR of IL-1 receptor mRNA, thereby reducing an early activation of the immune system from IL-1 signaling. Because EBV miR-BART1, BART2, and BART22 reduce IL-12 production, NK cells are inactivated and the transformation of immature CD4+ T cells to mature helper T cells is suspended, and activation of CD4+ effector T cells is reduced [156]. In addition, other proteins in cytokine production such as Cathepsin B, a protease in the antigen-presenting process, are targeted by EBV miR-BHRF1-2. An antigen peptide transporter 2 (TAP2) can also be disrupted by EBV miR-BHRF1-3 and BART17 [157]. EBV-infected cells evade the immune cells because EBV miR-BART2-5p directly targets the 3′UTR of MHC class I polypeptide-related sequence B (MICB) mRNA, a key ligand for NKG2D receptor in stress-induced response. Consequently, cancer cells can escape from NK cells by the loss of MICB [158]. Interestingly, RIG-I receptor can recognize the secondary structure of EBER1, which relays the signal via RIG-I/IRF-3 axis, resulting in the upregulation of IL-10 that promotes cancer cell growth [159]. To summarize the above, EBV infection can modify antigen recognition pattern and cytokine production that promotes NPC carcinogenesis through compromising the immune system.
Nevertheless, the strategies on how the virus initially escapes the immune response remain poorly understood.
6.2. Metabolic Reprogramming
The links between EBV and metabolic reprogramming have been elucidated. EBV ncRNAs manipulate metabolic pathways via suppression of metabolic genes or inhibition of LMP1 [160]. Overexpression of EBV miR-BART1 reduces the expression of glucose-6-phosphate dehydrogenase (G6PD), spermidine/spermine N1-acetyltransferase 1 (SAT1), arginosuccinate synthetase gene (ASS1), and other metabolic genes [161]. EBV miR-BART1-5p also directly targets the 3′UTR of AMP-activated protein kinase alpha 1 gene (AMPKa1) mRNA, which consequently activates AMPK/mTOR/HIF1 pathway and its downstream metabolic genes including glucose transporter 1 (GLUT1) and hexokinase 2 (HK2) gene. Because VEGF is also expressed via AMPK/mTOR/HIF1 axis, EBV miR-BART1-5p may promote both metabolic reprogramming and angiogenesis in NPC [162]. Therefore, suppression of EBV miR-BART1 will reduce the metabolic and vascular formation phenotypes of the NPC cells. Recently, EBV-miR-BART8-3p also promotes radioresistance by modulating the activity of the ATM/ATR signaling pathway [163].
EBV miR-BART1-5p, BART16, BART17-5p, and BART cluster I can indirectly modulate glycolysis by targeting LMP1 expression. GLUT1 is an example [160]. CTAR1 of LMP1 activates mTORC1 through AKT/ERK/IKKα. On the other hand, CTAR2 regulates mTROC1 via IKKβ. The mTORC1 then triggers GLUT1 expression through NF-κB signaling [139]. LMP1 also upregulates HK2 via stabilization of the HK2 transcription factor, c-MYC. c-MYC can be phosphorylated by GSK3β and is usually subject to degradation by proteasome. However, LMP1 inactivates GSK3β through PI3K/Akt signaling and stabilizes c-MYC. Consequently, overexpression of HK2 promotes glycolysis and mitochondria-dependent apoptosis [164]. In addition to glycolysis, aberrant regulation of lipid metabolism is also mediated by EBER. Overexpression of EBER leads to the upregulation of low-density lipoprotein receptor (LDLR) and fatty acid synthase (FASN). Moreover, EBER overexpression induces a lipoprotein-dependent proliferation phenotype in NPC cells [165].
6.3. Apoptosis
Apoptosis is a programmed cell death, which is a major event in the cancer hallmarks [166]. The apoptotic pathway can be triggered via many signaling axes, such as an extrinsic pathway with death receptors through the tumor necrosis factor (TNF) or mitochondria-mediated cytochrome C pathway. Many players in these signaling pathways can be targeted by EBV miRNAs, leading to modulation of apoptosis during EBV infection [71].
The p53 is a transcription factor with pro-apoptotic properties. When DNA damages occur, p53 is phosphorylated via ATM/chk1/2 axis. Phosphorylated p53 then promotes cell cycle arrest to repair the damaged lesion. Therefore, the malfunction of p53 disrupts cell cycle arrest and allows cells to grow uncontrollably [167]. Low expression of PUMA is found in 60% of human NPC tissues. It has been shown that EBV miR-BART5 targets the 3′UTR of PUMA mRNA, resulting in the loss of mitochondrial apoptotic signaling in NPC cells [168]. EBV miR-BART5-3p and miR-BHRF-1 have been shown to inhibit p53 directly by targeting the 3′UTR of TP53 mRNA. As a result, EBV miR-BART5 enhances cell cycle progression and inhibits apoptosis [169,170]. Furthermore, Bcl-2 interacting mediator of cell death (BIM), a pro-apoptotic protein in the BAX induced mitochondrial apoptosis, is also downregulated by EBV miR-BARTs in both cluster I (miR-BART1 and BART3) and cluster II (miR-BART9, BART11, and BART12) [171]. In addition, EBNA1 interacts with USP7, the regulator of p53, and depletes p53 through proteasome degradation. EBNA1 also blocks caspase activation through Survivin [172,173]. LMP1 triggers resistance in NPC cells through PI3K/Akt/FOXO3a pathway by altering human miR-21 expression [174].
6.4. Metastasis
Cancer metastasis is a prominent feature in many NPC cases and is a major cause of poor survival in patients [175,176,177]. EBV DNA load has been used to estimate the prognosis of patients undergoing therapy and to diagnose the risk of metastasis [178]. EMT is a phenotypic change from epithelial cells to mesenchymal cells by reorganizing cell polarity, cell-cell interaction, and cell-extracellular matrix adhesion [161]. LMP2A promotes malignancy of NPC cells through the induction of matrix metalloproteinase 9 (MMP-9) [179]. Both LMP1 and LMP2A reduce the type IV collagen at the basement membrane and induce stemness-like cell growth by expressing BMI1, SOX2, and stem cell-related surface markers (CD44v6 and CD133) through the hedgehog signaling pathway. Thus, EBV oncoproteins are crucial for the maintenance and development of stemness in NPC [180]. Several EBV miRNAs have been reported to modulate the EMT process and enhance the metastatic process in NPC cells [85]. High expression of EBV miR-BART7-3p is correlated with lymph node metastasis and the clinical stage of NPC. EBV miR-BART7-3p, miR-BART9, and miR-18-5p downregulate PTEN by directly targeting 3′UTR of PTEN mRNA, which leads to translocation of Snail and β-catenin to the nucleus. PTEN knockout mimics the phenotypes of EBV miR-BART7-3p in NPC cells, while PTEN complementation reverses such phenotypes [181,182]. In addition, EBV miR-BART6-3p contributes to EMT progression by blocking cell migration via the downregulation of lncRNA LOC553103 [183]. EBV-miR-BART8-3p induces EMT by activating the NF-κB and ERK1/2 pathway [184]. On the other hand, EBV miR-BART10-3p promotes cell invasion through modulation of the stabilizer complex beta-transducin repeat containing E3 ubiquitin protein ligase (βTrCP), ZEB1, N-cadherin, Vimentin, and Slug transcription factors [185]. Promoting metastasis is not the sole function of EBV miRNAs, EBV-miR-BART13 promotes cell growth by targeting the NK1RAS2/NF-κB pathway [186].
Alteration of cell adhesion molecules is also involved in NPC cell invasion [187]. E-cadherin is a transmembrane protein that connects epithelial cells via adherent junction and loss of E-cadherin transforms pre-cancerous cells to invasive and metastatic cells [188]. EBV miR-BART9 binds to the 3′UTR of E-cadherin mRNA, thereby reducing E-cadherin production. Taken together, E-cadherin expression and EBV miR-BART9 could be effective biomarkers that predict the aggressiveness of NPC [189].
6.5. Sustaining Proliferative Signal
The human telomerase reverse transcriptase (hTERT) is a catalytic subunit of telomerase that maintains and extends telomeres in the eukaryotic chromosome, thus induces replicative senescence of cells and immortalizes cancer cells [190]. LMP1 mainly sustains proliferative signals via NF-κB pathway. LMP1 promotes nucleus translocation of NF-κB p65 then nuclear accumulation of hTERT, which upregulates telomerase activity and cell proliferation [191,192,193]. An increase in expression and phosphorylation of hTERT has been reported in LMP1-positive NPC cells [132,194], with the involvement of c-Myc [195]. Similarly, CTAR1 and CTAR2 domains of LMP1 also activate telomerase. NF-κB activation plays an important role for immortalization of NPE cells [196] with involvement of upstream EGFR/MEK/ERK/IKK/mTORC1 activation [197]. Both LMP1 and hTERT expression are important in immortalization of NPE cells [198]. It has been revealed that LMP1-NF-κB dynamic inhibits the expression of PINX1, an inhibitor to telomerase [199]. In addition to NF-κB pathway, telomerase activity is also induced by LMP1 through p16INK4A/Rb/E2F1 and JNK signaling pathways [200].
7. Conclusions
This review covers a recent understanding of the pathogenic role of EBV infection in NPC carcinogenesis. It is evident that EBV oncoproteins initiate the tumorigenesis via modulation of multiple signaling pathways including Wnt/β-catenin, JAK/STAT, PI3k/Akt/mTOR, EGFR/MAPK, and NF-κB pathways. In addition, the regulation of certain signaling pathways has been demonstrated by EBV ncRNA as summarized in Figure 4. Further investigations are required to identify the exact signaling pathways of EBV oncoproteins/ncRNA interactions and their roles in NPC initiation and progression. These molecules could provide new targets for the development of NPC therapeutics.
Acknowledgments
We acknowledge the Science Achievement Scholarship of Thailand (P.P.), the Development and Promotion of Science and Technology Talents Project scholarship (C.N.), a scholarship from Otsuka Toshimi Foundation, Hashiya Foundation and Honjo International Scholarship Foundation, Japan (T.R.), and Japanese Ministry of Education, Culture, Sports, Science, and Technology’s Scientific Research Fund [18K07147 (H.I.) and 18K07148 (H.Y.)]. We also thank Sayuri Hamada for her support in preparation of the figure.
Author Contributions
Conceived and designed the study: T.R., P.P., C.N., and T.J.; Drafted the manuscript: T.R., P.P., C.N., N.W., H.I., and H.Y.; Revised the manuscript: All authors; Supervised the project: T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by a Basic Research Grant from Thailand Research Fund (BRG5980003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Petersson F. Nasopharyngeal carcinoma: A review. Semin. Diagn. Pathol. 2015;32:54–73. doi: 10.1053/j.semdp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Chang E.T., Adami H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 3.Tsao S.W., Tsang C.M., Lo K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017;372:20160270. doi: 10.1098/rstb.2016.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tulalamba W., Janvilisri T. Nasopharyngeal carcinoma signaling pathway: An update on molecular biomarkers. Int. J. Cell Biol. 2012;2012:594681. doi: 10.1155/2012/594681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janvilisri T. Omics-based identification of biomarkers for nasopharyngeal carcinoma. Dis. Markers. 2015;2015:762128. doi: 10.1155/2015/762128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong R.W., Kutty M.K., Dharmalingam S.K., Ponnudurai J.R. Incidence of nasopharyngeal carcinoma in Malaysia, 1968–1977. Br. J. Cancer. 1979;40:557–567. doi: 10.1038/bjc.1979.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mak H.W., Lee S.H., Chee J., Tham I., Goh B.C., Chao S.S., Ong Y.K., Loh K.S., Lim C.M. Clinical outcome among nasopharyngeal cancer patients in a multi-ethnic society in Singapore. PLoS ONE. 2015;10:e0126108. doi: 10.1371/journal.pone.0126108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu W.M., Hussain S.S.M. Incidence of nasopharyngeal carcinoma in Chinese immigrants, compared with Chinese in China and South East Asia: Review. J. Laryngol. Otol. 2009;123:1067–1074. doi: 10.1017/S0022215109005623. [DOI] [PubMed] [Google Scholar]
- 11.Jia W.H., Feng B.J., Xu Z.L., Zhang X.S., Huang P., Huang L.X., Yu X.J., Feng Q.S., Yao M.H., Shugart Y.Y., et al. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer. 2004;101:363–369. doi: 10.1002/cncr.20372. [DOI] [PubMed] [Google Scholar]
- 12.Xie S.H., sun Yu I.T., Tse L.A., Au J.S.K., Lau J.S.M. Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: A case–referent study in Hong Kong Chinese. Cancer Causes Control. 2015;26:913–921. doi: 10.1007/s10552-015-0572-x. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Chang E.T., Liu Q., Cai Y., Zhang Z., Chen G., Huang Q.H., Xie S.H., Cao S.M., Shao J.Y., et al. Quantification of familial risk of nasopharyngeal carcinoma in a high-incidence area. Cancer. 2017;123:2716–2725. doi: 10.1002/cncr.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji X., Zhang W., Xie C., Wang B., Zhang G., Zhou F. Nasopharyngeal carcinoma risk by histologic type in central China: Impact of smoking, alcohol and family history. Int. J. Cancer. 2011;129:724–732. doi: 10.1002/ijc.25696. [DOI] [PubMed] [Google Scholar]
- 15.Jia W.H., Luo X.Y., Feng B.J., Ruan H.L., Bei J.X., Liu W.S., Qin H.D., Feng Q.S., Chen L.Z., Yao S.Y., et al. Traditional Cantonese diet and nasopharyngeal carcinoma risk: A large-scale case-control study in Guangdong, China. BMC Cancer. 2010;10:446. doi: 10.1186/1471-2407-10-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X., Johnson R.C., Deng H., Liao J., Guan L., Nelson G.W., Tang M., Zheng Y., De The G., O’Brien S.J., et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of southern China. Int. J. Cancer. 2009;124:2942–2947. doi: 10.1002/ijc.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okekpa S.I., Mydin R.B.S.M.N., Mangantig E., Azmi N.S.A., Zahari S.N.S., Kaur G., Musa Y. Nasopharyngeal carcinoma (NPC) risk factors: A systematic review and meta-analysis of the association with lifestyle, diets, socioeconomic and sociodemographic in asian region. Asian Pac. J. Cancer Prev. 2019;20:3505–3514. doi: 10.31557/APJCP.2019.20.11.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivappa N., Hébert J.R., Zucchetto A., Montella M., Libra M., Garavello W., Rossi M., La Vecchia C., Serraino D. Increased risk of nasopharyngeal carcinoma with increasing levels of diet-associated inflammation in an Italian case–control study. Nutr. Cancer. 2016;68:1123–1130. doi: 10.1080/01635581.2016.1216137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polesel J., Serraino D., Negri E., Barzan L., Vaccher E., Montella M., Zucchetto A., Garavello W., Franceschi S., La Vecchia C., et al. Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer Causes Control. 2013;24:1157–1165. doi: 10.1007/s10552-013-0195-z. [DOI] [PubMed] [Google Scholar]
- 20.Turkoz F.P., Celenkoglu G., Dogu G.G., Kalender M.E., Coskun U., Alkis N., Ozkan M., Mehmet Turk H., Arslan U.Y. Risk factors of nasopharyngeal carcinoma in Turkey—An epidemiological survey of the anatolian society of medical oncology. Asian Pac. J. Cancer Prev. 2011;12:3017–3021. [PubMed] [Google Scholar]
- 21.Liu Y.T., Dai J.J., Xu C.H., Lu Y.K., Fan Y.Y., Zhang X.L., Zhang C.X., Chen Y.M. Greater intake of fruit and vegetables is associated with lower risk of nasopharyngeal carcinoma in Chinese adults: A case-control study. Cancer Causes Control. 2012;23:589–599. doi: 10.1007/s10552-012-9923-z. [DOI] [PubMed] [Google Scholar]
- 22.Long M., Fu Z., Li P., Nie Z. Cigarette smoking and the risk of nasopharyngeal carcinoma: A meta-analysis of epidemiological studies. BMJ Open. 2017;7:14–17. doi: 10.1136/bmjopen-2017-016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue W.Q., Qin H.D., Ruan H.L., Shugart Y.Y., Jia W.H. Quantitative association of tobacco smoking with the risk of nasopharyngeal carcinoma: A comprehensive meta-analysis of studies conducted between 1979 and 2011. Am. J. Epidemiol. 2013;178:325–338. doi: 10.1093/aje/kws479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J.H., Jiang C.Q., Ho S.Y., Zhang W.S., Mai Z.M., Xu L., Lo C.M., Lam T.H. Smoking and nasopharyngeal carcinoma mortality: A cohort study of 101,823 adults in Guangzhou, China. BMC Cancer. 2015;15:1–7. doi: 10.1186/s12885-015-1902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y.X., Jia W.H. Familial nasopharyngeal carcinoma. Semin. Cancer Biol. 2002;12:443–450. doi: 10.1016/S1044579X02000871. [DOI] [PubMed] [Google Scholar]
- 26.Tse K.P., Su W.H., Chang K.P., Tsang N.M., Yu C.J., Tang P., See L.C., Hsueh C., Yang M.L., Hao S.P., et al. Genome-wide sssociation study reveals multiple nasopharyngeal carcinoma-associated loci within the HLA region at chromosome 6p21.3. Am. J. Hum. Genet. 2009;85:194–203. doi: 10.1016/j.ajhg.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y.Y., Chung G.T.Y., Lui V.W.Y., To K.-F., Ma B.B.Y., Chow C., Woo J.K.S., Yip K.Y., Seo J., Hui E.P., et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat. Commun. 2017;8:14121. doi: 10.1038/ncomms14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bei J.-X., Li Y., Jia W.-H., Feng B.-J., Zhou G., Chen L.-Z., Feng Q.-S., Low H.-Q., Zhang H., He F., et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat. Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 29.Cui Q., Feng Q.-S., Mo H.-Y., Sun J., Xia Y.-F., Zhang H., Foo J.N., Guo Y.-M., Chen L.-Z., Li M., et al. An extended genome-wide association study identifies novel susceptibility loci for nasopharyngeal carcinoma. Hum. Mol. Genet. 2016;25:3626–3634. doi: 10.1093/hmg/ddw200. [DOI] [PubMed] [Google Scholar]
- 30.Yu G., Hsu W.-L., Coghill A.E., Yu K.J., Wang C.-P., Lou P.-J., Liu Z., Jones K., Vogt A., Wang M., et al. Whole-Exome Sequencing of nasopharyngeal carcinoma families reveals novel variants potentially involved in nasopharyngeal carcinoma. Sci. Rep. 2019;9:9916. doi: 10.1038/s41598-019-46137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshizaki T., Ito M., Murono S., Wakisaka N., Kondo S., Endo K. Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx. 2012;39:137–144. doi: 10.1016/j.anl.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Carioli G., Negri E., Kawakita D., Garavello W., La Vecchia C., Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: Focus on low-risk areas. Int. J. Cancer. 2017;140:2256–2264. doi: 10.1002/ijc.30660. [DOI] [PubMed] [Google Scholar]
- 33.Tang L.L., Chen W.Q., Xue W.Q., He Y.Q., Zheng R.S., Zeng Y.X., Jia W.H. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374:22–30. doi: 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Palser A.L., Grayson N.E., White R.E., Corton C., Correia S., Ba abdullah M.M., Watson S.J., Cotten M., Arrand J.R., Murray P.G., et al. Genome diversity of Epstein-Barr Virus from multiple tumor types and normal infection. J. Virol. 2015;89:5222–5237. doi: 10.1128/JVI.03614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feederle R., Klinke O., Kutikhin A., Poirey R., Tsai M.-H., Delecluse H.-J. Epstein-Barr Virus: From the detection of sequence polymorphisms to the recognition of viral types. Curr. Top. Microbiol. Immunol. 2015;390:119–148. doi: 10.1007/978-3-319-22822-8_7. [DOI] [PubMed] [Google Scholar]
- 36.Tsai M.-H., Lin X., Shumilov A., Bernhardt K., Feederle R., Poirey R., Kopp-Schneider A., Pereira B., Almeida R., Delecluse H.-J. The biological properties of different Epstein-Barr virus strains explain their association with various types of cancers. Oncotarget. 2017;8:10238–10254. doi: 10.18632/oncotarget.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanda T., Yajima M., Ikuta K. Epstein-Barr virus strain variation and cancer. Cancer Sci. 2019;110:1132–1139. doi: 10.1111/cas.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shair K.H.Y., Reddy A., Cooper V.S. New insights from elucidating the role of LMP1 in nasopharyngeal carcinoma. Cancers. 2018;10:86. doi: 10.3390/cancers10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards R.H., Seillier-Moiseiwitsch F., Raab-Traub N. Signature amino acid changes in latent membrane protein 1 distinguish Epstein-Barr virus strains. Virology. 1999;261:79–95. doi: 10.1006/viro.1999.9855. [DOI] [PubMed] [Google Scholar]
- 40.Xu M., Yao Y., Chen H., Zhang S., Cao S.M., Zhang Z., Luo B., Liu Z., Li Z., Xiang T., et al. Genome sequencing analysis identifies Epstein–Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 2019;51:1131–1136. doi: 10.1038/s41588-019-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H., Burassakarn A., Kang Y., Iizasa H., Yoshiyama H. A single nucleotide polymorphism in the BART promoter region of Epstein-Barr virus isolated from nasopharyngeal cancer cells. Biochem. Biophys. Res. Commun. 2019;520:373–378. doi: 10.1016/j.bbrc.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Zhang X., Chao Y., Jia Y., Xing X., Luo B. New variations of Epstein-Barr virus-encoded small RNA genes in nasopharyngeal carcinomas, gastric carcinomas, and healthy donors in northern China. J. Med. Virol. 2010;82:829–836. doi: 10.1002/jmv.21714. [DOI] [PubMed] [Google Scholar]
- 43.Correia S., Bridges R., Wegner F., Venturini C., Palser A., Middeldorp J.M., Cohen J.I., Lorenzetti M.A., Bassano I., White R.E., et al. Sequence variation of Epstein-Barr Virus: Viral types, geography, codon usage, and diseases. J. Virol. 2018;92:e01132-18. doi: 10.1128/JVI.01132-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng M.-S., Li D.-J., Liu Q.-L., Song L.-B., Li M.-Z., Zhang R.-H., Yu X.-J., Wang H.-M., Ernberg I., Zeng Y.-X. Genomic sequence analysis of Epstein-Barr virus strain GD1 from a nasopharyngeal carcinoma patient. J. Virol. 2005;79:15323–15330. doi: 10.1128/JVI.79.24.15323-15330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu P., Fang X., Feng Z., Guo Y.-M., Peng R.-J., Liu T., Huang Z., Feng Y., Sun X., Xiong Z., et al. Direct sequencing and characterization of a clinical isolate of Epstein-Barr Virus from nasopharyngeal carcinoma tissue by using next-generation sequencing technology. J. Virol. 2011;85:11291–11299. doi: 10.1128/JVI.00823-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai M.-H., Raykova A., Klinke O., Bernhardt K., Gärtner K., Leung C.S., Geletneky K., Sertel S., Münz C., Feederle R., et al. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep. 2013;5:458–470. doi: 10.1016/j.celrep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Kwok H., Tong A.H.Y., Lin C.H., Lok S., Farrell P.J., Kwong D.L.W., Chiang A.K.S. Genomic sequencing and comparative analysis of Epstein-Barr virus genome isolated from primary nasopharyngeal carcinoma biopsy. PLoS ONE. 2012;7:e36939. doi: 10.1371/journal.pone.0036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tso K.K.-Y., Yip K.Y.-L., Mak C.K.-Y., Chung G.T.-Y., Lee S.-D., Cheung S.-T., To K.-F., Lo K.-W. Complete genomic sequence of Epstein-Barr virus in nasopharyngeal carcinoma cell line C666-1. Infect. Agent. Cancer. 2013;8:29. doi: 10.1186/1750-9378-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arrand J.R., Young L.S., Tugwood J.D. Two families of sequences in the small RNA-encoding region of Epstein-Barr virus (EBV) correlate with EBV types A and B. J. Virol. 1989;63:983–986. doi: 10.1128/JVI.63.2.983-986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng C.X., Yan L., Nilsson B., Eklund G., Drettner B. Epstein-Barr virus infection, salted fish and nasopharyngeal carcinoma: A case-control study in southern. Acta Oncol. 1994;33:867–872. doi: 10.3109/02841869409098448. [DOI] [PubMed] [Google Scholar]
- 51.Zeng M.-S., Zeng Y.-X. Nasopharyngeal Cancer. Springer Nature; London, UK: 2010. Pathogenesis and etiology of nasopharyngeal carcinoma; pp. 9–25. [Google Scholar]
- 52.Nishikawa J., Yoshiyama H., Iizasa H., Kanehiro Y., Nakamura M., Nishimura J., Saito M., Okamoto T., Sakai K., Suehiro Y., et al. Epstein-barr virus in gastric carcinoma. Cancers. 2014;6:2259–2274. doi: 10.3390/cancers6042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang C.M., Yu K.J., Mbulaiteye S.M., Hildesheim A., Bhatia K. The extent of genetic diversity of Epstein-Barr virus and its geographic and disease patterns: A need for reappraisal. Virus Res. 2009;143:209–221. doi: 10.1016/j.virusres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]
- 55.Thorley-Lawson D.A., Hawkins J.B., Tracy S.I., Shapiro M. The pathogenesis of Epstein–Barr virus persistent infection. Curr. Opin. Virol. 2013;3:227–232. doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutt-Fletcher L.M. Epstein-Barr Virus entry. J. Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang R., Gu X., Nathan C.A., Hutt-Fletcher L. Laser-capture microdissection of oropharyngeal epithelium indicates restriction of Epstein-Barr virus receptor/CD21 mRNA to tonsil epithelial cells. J. Oral Pathol. Med. 2008;37:626–633. doi: 10.1111/j.1600-0714.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang R., Gu X., Moore-Medlin T.N., Nathan C.-A., Hutt-Fletcher L.M. Oral dysplasia and squamous cell carcinoma: Correlation between increased expression of CD21, Epstein-Barr virus and CK19. Oral Oncol. 2012;48:836–841. doi: 10.1016/j.oraloncology.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H.-B., Zhang H., Zhang J.-P., Li Y., Zhao B., Feng G.-K., Du Y., Xiong D., Zhong Q., Liu W.-L., et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 2015;6:6240. doi: 10.1038/ncomms7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong D., Du Y., Wang H.-B., Zhao B., Zhang H., Li Y., Hu L.-J., Cao J.-Y., Zhong Q., Liu W.-L., et al. Nonmuscle myosin heavy chain IIA mediates Epstein–Barr virus infection of nasopharyngeal epithelial cells. Proc. Natl. Acad. Sci. USA. 2015;112:11036–11041. doi: 10.1073/pnas.1513359112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J., Sathiyamoorthy K., Zhang X., Schaller S., Perez White B.E., Jardetzky T.S., Longnecker R. Ephrin receptor A2 is a functional entry receptor for Epstein–Barr virus. Nat. Microbiol. 2018;3:172–180. doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H., Li Y., Wang H.-B., Zhang A., Chen M.-L., Fang Z.-X., Dong X.-D., Li S.-B., Du Y., Xiong D., et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein–Barr virus entry. Nat. Microbiol. 2018;3:164–171. doi: 10.1038/s41564-017-0080-8. [DOI] [PubMed] [Google Scholar]
- 63.Temple R.M., Zhu J., Budgeon L., Christensen N.D., Meyers C., Sample C.E. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc. Natl. Acad. Sci. USA. 2014;111:16544–16549. doi: 10.1073/pnas.1400818111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eichelberg M.R., Welch R., Guidry J.T., Ali A., Ohashi M., Makielski K.R., McChesney K., Van Sciver N., Lambert P.F., Keleș S., et al. Epstein-Barr Virus infection promotes epithelial cell growth by attenuating differentiation-dependent exit from the cell cycle. mBio. 2019;10:e01332-19. doi: 10.1128/mBio.01332-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dittmer D.P., Hilscher C.J., Gulley M.L., Yang E.V., Chen M., Glaser R. Multiple pathways for Epstein-Barr virus episome loss from nasopharyngeal carcinoma. Int. J. Cancer. 2008;123:2105–2112. doi: 10.1002/ijc.23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin W., Yip Y.L., Jia L., Deng W., Zheng H., Dai W., Ko J.M.Y., Lo K.W., Chung G.T.Y., Yip K.Y., et al. Establishment and characterization of new tumor xenografts and cancer cell lines from EBV-positive nasopharyngeal carcinoma. Nat. Commun. 2018;9:4663. doi: 10.1038/s41467-018-06889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yip Y.L., Li W., Deng W., Jia L., Lo K.W., Busson P., Vérillaud B., Liu X., Tsang C.M., Lung M.L., et al. Establishment of a nasopharyngeal carcinoma cell line capable of undergoing lytic Epstein–Barr virus reactivation. Lab. Investig. 2018;98:1093–1104. doi: 10.1038/s41374-018-0034-7. [DOI] [PubMed] [Google Scholar]
- 68.Dyson P.J., Farrell P.J. Chromatin structure of Epstein-Barr Virus. J. Gen. Virol. 1985;66:1931–1940. doi: 10.1099/0022-1317-66-9-1931. [DOI] [PubMed] [Google Scholar]
- 69.Teo M.C.C., Soo K.C. Cancer trends and incidences in Singapore. Jpn. J. Clin. Oncol. 2013;43:219–224. doi: 10.1093/jjco/hys230. [DOI] [PubMed] [Google Scholar]
- 70.Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 71.Lung R.W.M., Tong J.H.M., To K.F. Emerging roles of small Epstein-Barr virus derived non-coding RNAs in epithelial malignancy. Int. J. Mol. Sci. 2013;14:17378–17409. doi: 10.3390/ijms140917378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Canaan A., Haviv I., Urban A.E., Schulz V.P., Hartman S., Zhang Z., Palejev D., Deisseroth A.B., Lacy J., Snyder M., et al. EBNA1 regulates cellular gene expression by binding cellular promoters. Proc. Natl. Acad. Sci. USA. 2009;106:22421–22426. doi: 10.1073/pnas.0911676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dresang L.R., Vereide D.T., Sugden B. Identifying sites bound by Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) in the human genome: Defining a position-weighted matrix to predict sites bound by EBNA1 in viral genomes. J. Virol. 2009;83:2930–2940. doi: 10.1128/JVI.01974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sugden B., Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J. Virol. 1989;63:2644–2649. doi: 10.1128/JVI.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen M.D., Young L.S., Dawson C.W. The Epstein-Barr Virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J. Virol. 2005;79:1789–1802. doi: 10.1128/JVI.79.3.1789-1802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin Z., Wan X., Jiang R., Deng L., Gao Y., Tang J., Yang Y., Zhao W., Yan X., Yao K., et al. Epstein-Barr Virus-encoded latent membrane protein 2A promotes the epithelial-mesenchymal transition in nasopharyngeal carcinoma via metastatic tumor antigen 1 and mechanistic target of rapamycin signaling induction. J. Virol. 2014;88:11872–11885. doi: 10.1128/JVI.01867-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoebe E.K., Le Large T.Y.S., Greijer A.E., Middeldorp J.M. BamHI-A rightward frame 1, an Epstein-Barr virus-encoded oncogene and immune modulator. Rev. Med. Virol. 2013;23:367–383. doi: 10.1002/rmv.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoebe E., Wille C., Hagemeier S., Kenney S., Greijer A., Middeldorp J. Epstein-Barr Virus gene BARF1 expression is regulated by the epithelial differentiation factor ΔNp63α in undifferentiated nasopharyngeal carcinoma. Cancers. 2018;10:76. doi: 10.3390/cancers10030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eliopoulos A.G., Stack M., Dawson C.W., Kaye K.M., Hodgkin L., Sihota S., Rowe M., Young L.S. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 80.Lo A.K.F., Huang D.P., Lo K.W., Chui Y.L., Li H.M., Pang J.C.S., Tsao S.W. Phenotypic alterations induced by the Hong Kong-prevalent Epstein-Barr virus-encoded LMP1 variant (2117-LMP1) in nasopharyngeal epithelial cells. Int. J. Cancer. 2004;109:919–925. doi: 10.1002/ijc.20051. [DOI] [PubMed] [Google Scholar]
- 81.Nawandar D.M., Ohashi M., Djavadian R., Barlow E., Makielski K., Ali A., Lee D., Lambert P.F., Johannsen E., Kenney S.C. Differentiation-dependent LMP1 expression is required for efficient lytic Epstein-Barr Virus reactivation in epithelial cells. J. Virol. 2017;91:1–18. doi: 10.1128/JVI.02438-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caves E.A., Butch R.M., Cook S.A., Wasil L.R., Chen C., Di Y.P., Lee N., Shair K.H.Y. Latent membrane protein 1 is a novel determinant of Epstein-Barr Virus genome persistence and reactivation. mSphere. 2017;2:e00453-17. doi: 10.1128/mSphereDirect.00453-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu M.-T., Chen Y.-R., Chen S.-C., Hu C.-Y., Lin C.-S., Chang Y.-T., Wang W.-B., Chen J.-Y. Epstein-Barr virus latent membrane protein 1 induces micronucleus formation, represses DNA repair and enhances sensitivity to DNA-damaging agents in human epithelial cells. Oncogene. 2004;23:2531–2539. doi: 10.1038/sj.onc.1207375. [DOI] [PubMed] [Google Scholar]
- 84.Mei Y.-P., Zhou J.-M., Wang Y., Huang H., Deng R., Feng G.-K., Zeng Y.-X., Zhu X.-F. Silencing of LMP1 induces cell cycle arrest and enhances chemosensitivity through inhibition of AKT signaling pathway in EBV-positive nasopharyngeal carcinoma cells. Cell Cycle. 2007;6:1379–1385. doi: 10.4161/cc.6.11.4274. [DOI] [PubMed] [Google Scholar]
- 85.Skalsky R.L., Cullen B.R. EBV Noncoding RNAs. Curr. Top. Microbiol. Immunol. 2015;391:181–217. doi: 10.1007/978-3-319-22834-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan C., Tang Y., Wang J., Xiong F., Guo C., Wang Y., Xiang B., Zhou M., Li X., Wu X., et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer. 2018;9:2852–2864. doi: 10.7150/jca.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spence T., Bruce J., Yip K.W., Liu F.-F. MicroRNAs in nasopharyngeal carcinoma. Chin. Clin. Oncol. 2016;5:17. doi: 10.21037/cco.2016.03.09. [DOI] [PubMed] [Google Scholar]
- 88.Young L.S., Dawson C.W. Epstein-Barr virus and nasopharyngeal carcinoma. Chin. J. Cancer. 2014;33:581–590. doi: 10.5732/cjc.014.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsang C.M., Yip Y.L., Lo K.W., Deng W., To K.F., Hau P.M., Lau V.M.Y., Takada K., Lui V.W.Y., Lung M.L., et al. Cyclin D1 overexpression supports stable EBV infection in nasopharyngeal epithelial cells. Proc. Natl. Acad. Sci. USA. 2012;109:E3473–E3482. doi: 10.1073/pnas.1202637109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hui A.B.Y., Or Y.Y.Y., Takano H., Tsang R.K.Y., To K.F., Guan X.Y., Sham J.S.T., Hung K.W.K., Lam C.N.Y., Van Hasselt C.A., et al. Array-based comparative genomic hybridization analysis identified cyclin D1 as a target oncogene at 11q13.3 in nasopharyngeal carcinoma. Cancer Res. 2005;65:8125–8133. doi: 10.1158/0008-5472.CAN-05-0648. [DOI] [PubMed] [Google Scholar]
- 91.Song L.B., Zeng M.S., Liao W.T., Zhang L., Mo H.Y., Liu W.L., Shao J.Y., Wu Q.L., Li M.Z., Xia Y.F., et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66:6225–6232. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- 92.Yip Y.L., Pang P.S., Deng W., Tsang C.M., Zeng M., Hau P.M., Man C., Jin Y., Yuen A.P.W., Tsao S.W. Efficient immortalization of primary nasopharyngeal epithelial cells for EBV infection study. PLoS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0078395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murata T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol. Immunol. 2014;58:307–317. doi: 10.1111/1348-0421.12155. [DOI] [PubMed] [Google Scholar]
- 94.Hitt M.M., Allday M.J., Hara T., Karran L., Jones M.D., Busson P., Tursz T., Ernberg I., Griffin B.E. EBV gene expression in an NPC-related tumour. EMBO J. 1989;8:2639–2651. doi: 10.1002/j.1460-2075.1989.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coghill A.E., Hsu W.-L., Pfeiffer R.M., Juwana H., Yu K.J., Lou P.-J., Wang C.-P., Chen J.-Y., Chen C.-J., Middeldorp J.M., et al. Epstein-Barr virus serology as a potential screening marker for nasopharyngeal carcinoma among high-risk individuals from multiplex families in Taiwan. Cancer Epidemiol. Biomark. Prev. 2014;23:1213–1219. doi: 10.1158/1055-9965.EPI-13-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y.Q., Khin N.S., Chua M.L.K. The evolution of Epstein-Barr virus detection in nasopharyngeal carcinoma. Cancer Biol. Med. 2018;15:1–5. doi: 10.20892/j.issn.2095-3941.2017.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsu M., Wu S.-Y., Chang S.-S., Su I.-J., Tsai C.-H., Lai S.-J., Shiau A.-L., Takada K., Chang Y. Epstein-Barr virus lytic transactivator Zta enhances chemotactic activity through induction of interleukin-8 in nasopharyngeal carcinoma cells. J. Virol. 2008;82:3679–3688. doi: 10.1128/JVI.02301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pudney V.A., Leese A.M., Rickinson A.B., Hislop A.D. CD8+ immunodominance among Epstein-Barr virus lytic cycle antigens directly reflects the efficiency of antigen presentation in lytically infected cells. J. Exp. Med. 2005;201:349–360. doi: 10.1084/jem.20041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang Y.-H., Lee C.-P., Su M.-T., Wang J.-T., Chen J.-Y., Lin S.-F., Tsai C.-H., Hsieh M.-J., Takada K., Chen M.-R. Epstein-Barr virus BGLF4 kinase retards cellular S-phase progression and induces chromosomal abnormality. PLoS ONE. 2012;7:e39217. doi: 10.1371/journal.pone.0039217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu C.-C., Liu M.-T., Chang Y.-T., Fang C.-Y., Chou S.-P., Liao H.-W., Kuo K.-L., Hsu S.-L., Chen Y.-R., Wang P.-W., et al. Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010;38:1932–1949. doi: 10.1093/nar/gkp1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiu S.-H., Wu C.-C., Fang C.-Y., Yu S.-L., Hsu H.-Y., Chow Y.-H., Chen J.-Y. Epstein-Barr virus BALF3 mediates genomic instability and progressive malignancy in nasopharyngeal carcinoma. Oncotarget. 2014;5:8583–8601. doi: 10.18632/oncotarget.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takada K. Role of EBER and BARF1 in nasopharyngeal carcinoma (NPC) tumorigenesis. Semin. Cancer Biol. 2012;22:162–165. doi: 10.1016/j.semcancer.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Li G., Su Q., Liu H., Wang D., Zhang W., Lu Z., Chen Y., Huang X., Li W., Zhang C., et al. Frizzled7 Promotes Epithelial-to-mesenchymal transition and stemness via activating canonical Wnt/β-catenin pathway in gastric cancer. Int. J. Biol. Sci. 2018;14:280–293. doi: 10.7150/ijbs.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang X., Zhu H., Gao Z., Li J., Zhuang J., Dong Y., Shen B., Li M., Zhou H., Guo H., et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018;293:6693–6706. doi: 10.1074/jbc.RA118.001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stewart D.J. Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 2014;106:djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 106.Jiang R., Niu X., Huang Y., Wang X. β-Catenin is important for cancer stem cell generation and tumorigenic activity in nasopharyngeal carcinoma. Acta Biochim. Biophys. Sin. 2016;48:229–237. doi: 10.1093/abbs/gmv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webb N., Connolly G., Tellam J., Yap A.S., Khanna R. Epstein-Barr virus associated modulation of Wnt pathway is not dependent on latent membrane protein-1. PLoS ONE. 2008;3:e3254. doi: 10.1371/journal.pone.0003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yap L.F., Ahmad M., Zabidi M.M.A., Chu T.L., Chai S.J., Lee H.M., Lim P.V.H., Wei W., Dawson C., Teo S.-H., et al. Oncogenic effects of WNT5A in Epstein-Barr virus-associated nasopharyngeal carcinoma. Int. J. Oncol. 2014;44:1774–1780. doi: 10.3892/ijo.2014.2342. [DOI] [PubMed] [Google Scholar]
- 109.Chen H., Lee J.M., Zong Y., Borowitz M., Ng M.H., Ambinder R.F., Hayward S.D. Linkage between STAT regulation and Epstein-Barr virus gene expression in tumors. J. Virol. 2001;75:2929–2937. doi: 10.1128/JVI.75.6.2929-2937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen H., Lee J.M., Wang Y., Huang D.P., Ambinder R.F., Hayward S.D. The Epstein-Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity. Proc. Natl. Acad. Sci. USA. 1999;96:9339–9344. doi: 10.1073/pnas.96.16.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y.-P., Tan Y.-N., Wang Z.-L., Zeng L., Lu Z.-X., Li L.-L., Luo W., Tang M., Cao Y. Phosphorylation and nuclear translocation of STAT3 regulated by the Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Int. J. Mol. Med. 2008;21:153–162. doi: 10.3892/ijmm.21.2.153. [DOI] [PubMed] [Google Scholar]
- 112.Kung C.-P., Raab-Traub N. Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J. Virol. 2008;82:5486–5493. doi: 10.1128/JVI.00125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kung C.-P., Meckes D.G., Raab-Traub N. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J. Virol. 2011;85:4399–4408. doi: 10.1128/JVI.01703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu Y., Shi Y., Yuan Q., Liu X., Yan B., Chen L., Tao Y., Cao Y. Epstein-Barr Virus encoded LMP1 regulates cyclin D1 promoter activity by nuclear EGFR and STAT3 in CNE1 cells. J. Exp. Clin. Cancer Res. 2013;32:90. doi: 10.1186/1756-9966-32-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Z., Luo F., Li L., Yang L., Hu D., Ma X., Lu Z., Sun L., Cao Y. STAT3 activation induced by Epstein-Barr virus latent membrane protein1 causes vascular endothelial growth factor expression and cellular invasiveness via JAK3 And ERK signaling. Eur. J. Cancer. 2010;46:2996–3006. doi: 10.1016/j.ejca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Cheng J.-Z., Chen J.-J., Xue K., Wang Z.-G., Yu D. Clinicopathologic and prognostic significance of VEGF, JAK2 and STAT3 in patients with nasopharyngeal carcinoma. Cancer Cell Int. 2018;18:110. doi: 10.1186/s12935-018-0605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J. Roles of the PI3K/Akt pathway in Epstein-Barr virus-induced cancers and therapeutic implications. World J. Virol. 2012;1:154–161. doi: 10.5501/wjv.v1.i6.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang C.-F., Yang G.-D., Huang T.-J., Li R., Chu Q.-Q., Xu L., Wang M.-S., Cai M.-D., Zhong L., Wei H.-J., et al. EB-virus latent membrane protein 1 potentiates the stemness of nasopharyngeal carcinoma via preferential activation of PI3K/AKT pathway by a positive feedback loop. Oncogene. 2016;35:3419–3431. doi: 10.1038/onc.2015.402. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H., Wang J., Yu D., Liu Y., Xue K., Zhao X. Role of Epstein-Barr Virus in the Development of Nasopharyngeal Carcinoma. Open Med. 2017;12:171–176. doi: 10.1515/med-2017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiang H., Fan D., Zhou G., Li X., Deng H. Phosphatidylinositol 3-kinase inhibitor(LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010;29:34. doi: 10.1186/1756-9966-29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J., Hu C.-F., Hou J.-H., Shao Q., Yan L.-X., Zhu X.-F., Zeng Y.-X., Shao J.-Y. Epstein-Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. J. Transl. Med. 2010;8:30. doi: 10.1186/1479-5876-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang W., Wen Q., Xu L., Xie G., Li J., Luo J., Chu S., Shi L., Huang D., Li J., et al. Activation of Akt/mTOR pathway is associated with poor prognosis of nasopharyngeal carcinoma. PLoS ONE. 2014;9:e106098. doi: 10.1371/journal.pone.0106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang M.-H., Sun R., Zhou X.-M., Zhang M.-Y., Lu J.-B., Yang Y., Zeng L.-S., Yang X.-Z., Shi L., Xiao R.-W., et al. Epithelial cell adhesion molecule overexpression regulates epithelial-mesenchymal transition, stemness and metastasis of nasopharyngeal carcinoma cells via the PTEN/AKT/mTOR pathway. Cell Death Dis. 2018;9:2. doi: 10.1038/s41419-017-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Z., Lan H., Chen X., Li P., Li S., Mo W., Tang A. Molecular alterations of the WWOX gene in nasopharyngeal carcinoma. Neoplasma. 2014;61:170–176. doi: 10.4149/neo_2014_023. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Z., Yu X., Zhou Z., Li B., Peng J., Wu X., Luo X., Yang L. LMP1-positive extracellular vesicles promote radioresistance in nasopharyngeal carcinoma cells through P38 MAPK signaling. Cancer Med. 2019;8:6082–6094. doi: 10.1002/cam4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma B.B.Y., Poon T.C.W., To K.F., Zee B., Mo F.K.F., Chan C.M.L., Ho S., Teo P.M.L., Johnson P.J., Chan A.T.C. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma—A prospective study. Head Neck. 2003;25:864–872. doi: 10.1002/hed.10307. [DOI] [PubMed] [Google Scholar]
- 127.Thornburg N.J., Raab-Traub N. Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-κB p50 homodimer/Bcl-3 complexes. J. Virol. 2007;81:12954–12961. doi: 10.1128/JVI.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tao Y., Song X., Tan Y., Lin X., Zhao Y., Zeng L., Tang M., Li W., Wu Q., Cao Y. Nuclear translocation of EGF receptor regulated by Epstein-Barr virus encoded latent membrane protein 1. Sci. China. Ser. C Life Sci. 2004;47:258–267. doi: 10.1007/BF03182771. [DOI] [PubMed] [Google Scholar]
- 129.Dawson C.W., Laverick L., Morris M.A., Tramoutanis G., Young L.S. Epstein-Barr virus-encoded LMP1 regulates epithelial cell motility and invasion via the ERK-MAPK pathway. J. Virol. 2008;82:3654–3664. doi: 10.1128/JVI.01888-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sung W.-W., Chu Y.-C., Chen P.-R., Liao M.-H., Lee J.-W. Positive regulation of HIF-1A expression by EBV oncoprotein LMP1 in nasopharyngeal carcinoma cells. Cancer Lett. 2016;382:21–31. doi: 10.1016/j.canlet.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 131.Liang J., Zheng S., Xiao X., Wei J., Zhang Z., Ernberg I., Matskova L., Huang G., Zhou X. Epstein-Barr virus-encoded LMP2A stimulates migration of nasopharyngeal carcinoma cells via the EGFR/Ca2+/calpain/ITGβ4 axis. Biol. Open. 2017;6:914–922. doi: 10.1242/bio.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng H., Li L.L., Hu D.S., Deng X.Y., Cao Y. Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell. Mol. Immunol. 2007;4:185–196. [PubMed] [Google Scholar]
- 133.Chung G.T.Y., Lou W.P.K., Chow C., To K.F., Choy K.W., Leung A.W.C., Tong C.Y.K., Yuen J.W.F., Ko C.W., Yip T.T.C., et al. Constitutive activation of distinct NF-κB signals in EBV-associated nasopharyngeal carcinoma. J. Pathol. 2013;231:311–322. doi: 10.1002/path.4239. [DOI] [PubMed] [Google Scholar]
- 134.Zheng H., Dai W., Cheung A.K.L., Ko J.M.Y., Kan R., Wong B.W.Y., Leong M.M.L., Deng M., Kwok T.C.T., Chan J.Y.-W., et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma. Proc. Natl. Acad. Sci. USA. 2016;113:11283–11288. doi: 10.1073/pnas.1607606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deng M., Dai W., Yu V.Z., Tao L., Lung M.L. Cylindromatosis Lysine 63 Deubiquitinase (CYLD) regulates NF-kB signaling pathway and modulates fibroblast and endothelial cells recruitment in nasopharyngeal carcinoma. Cancers. 2020;12:1924. doi: 10.3390/cancers12071924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ersing I., Bernhardt K., Gewurz B.E. NF-κB and IRF7 pathway activation by Epstein-Barr virus Latent Membrane Protein 1. Viruses. 2013;5:1587–1606. doi: 10.3390/v5061587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L.W., Jiang S., Gewurz B.E. Epstein-Barr Virus LMP1-mediated oncogenicity. J. Virol. 2017;91:e01718-16. doi: 10.1128/JVI.01718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lo A.K.F., Lo K.W., Tsao S.W., Wong H.L., Hui J.W.Y., To K.F., Hayward D.S., Chui Y.L., Lau Y.L., Takada K., et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia. 2006;8:173–180. doi: 10.1593/neo.05625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang J., Jia L., Lin W., Yip Y.L., Lo K.W., Lau V.M.Y., Zhu D., Tsang C.M., Zhou Y., Deng W., et al. Epstein-Barr Virus-encoded latent membrane protein 1 upregulates glucose transporter 1 transcription via the mTORC1/NF-κB signaling pathways. J. Virol. 2017;91:e02168-16. doi: 10.1128/JVI.02168-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johansson P., Jansson A., Rüetschi U., Rymo L. Nuclear factor-kappaB binds to the Epstein-Barr Virus LMP1 promoter and upregulates its expression. J. Virol. 2009;83:1393–1401. doi: 10.1128/JVI.01637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Devergne O., Hatzivassiliou E., Izumi K.M., Kaye K.M., Kleijnen M.F., Kieff E., Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: Role in NF-κB activation. Mol. Cell. Biol. 1996;16:7098–7108. doi: 10.1128/MCB.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thornburg N.J., Pathmanathan R., Raab-Traub N. Activation of nuclear factor-κB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003;63:8293–8301. [PubMed] [Google Scholar]
- 143.Luftig M., Yasui T., Soni V., Kang M.-S., Jacobson N., Cahir-McFarland E., Seed B., Kieff E. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc. Natl. Acad. Sci. USA. 2004;101:141–146. doi: 10.1073/pnas.2237183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verhoeven R.J.A., Tong S., Zhang G., Zong J., Chen Y., Jin D.-Y., Chen M.-R., Pan J., Chen H. NF-κB signaling regulates expression of Epstein-Barr Virus BART microRNAs and long noncoding RNAs in nasopharyngeal carcinoma. J. Virol. 2016;90:6475–6488. doi: 10.1128/JVI.00613-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peng H., Chen Y., Gong P., Cai L., Lyu X., Jiang Q., Wang J., Lu J., Yao K., Liu K., et al. Higher methylation intensity induced by EBV LMP1 via NF-κB/DNMT3b signaling contributes to silencing of PTEN gene. Oncotarget. 2016;7:40025–40037. doi: 10.18632/oncotarget.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verhoeven R.J.A., Tong S., Zong J., Chen Y., Tsao S.-W., Pan J., Chen H. NF-κB signaling regulates Epstein-Barr Virus BamHI-Q-driven EBNA1 expression. Cancers. 2018;10:119. doi: 10.3390/cancers10040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 148.Fathallah I., Parroche P., Gruffat H., Zannetti C., Johansson H., Yue J., Manet E., Tommasino M., Sylla B.S., Hasan U.A. EBV latent membrane protein 1 is a negative regulator of TLR9. J. Immunol. 2010;185:6439–6447. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 149.Bentz G.L., Shackelford J., Pagano J.S. Epstein-Barr virus latent membrane protein 1 regulates the function of interferon regulatory factor 7 by inducing its sumoylation. J. Virol. 2012;86:12251–12261. doi: 10.1128/JVI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shah K.M., Stewart S.E., Wei W., Woodman C.B.J., O’Neil J.D., Dawson C.W., Young L.S. The EBV-encoded latent membrane proteins, LMP2A and LMP2B, limit the actions of interferon by targeting interferon receptors for degradation. Oncogene. 2009;28:3903–3914. doi: 10.1038/onc.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang M., Yu F., Wu W., Wang Y., Ding H., Qian L. Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int. J. Biol. Sci. 2018;14:565–576. doi: 10.7150/ijbs.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 153.Lu Y., Qin Z., Wang J., Zheng X., Lu J., Zhang X., Wei L., Peng Q., Zheng Y., Ou C., et al. Epstein-Barr Virus miR-BART6-3p inhibits the RIG-I pathway. J. Innate Immun. 2017;9:574–586. doi: 10.1159/000479749. [DOI] [PubMed] [Google Scholar]
- 154.Hooykaas M.J.G., van Gent M., Soppe J.A., Kruse E., Boer I.G.J., van Leenen D., Groot Koerkamp M.J.A., Holstege F.C.P., Ressing M.E., Wiertz E.J.H.J., et al. EBV MicroRNA BART16 suppresses type I IFN signaling. J. Immunol. 2017;198:4062–4073. doi: 10.4049/jimmunol.1501605. [DOI] [PubMed] [Google Scholar]
- 155.Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tagawa T., Albanese M., Bouvet M., Moosmann A., Mautner J., Heissmeyer V., Zielinski C., Lutter D., Hoser J., Hastreiter M., et al. Epstein-Barr viral miRNAs inhibit antiviral CD4+ T cell responses targeting IL-12 and peptide processing. J. Exp. Med. 2016;213:2065–2080. doi: 10.1084/jem.20160248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Albanese M., Tagawa T., Bouvet M., Maliqi L., Lutter D., Hoser J., Hastreiter M., Hayes M., Sugden B., Martin L., et al. Epstein-Barr virus microRNAs reduce immune surveillance by virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2016;113:E6467–E6475. doi: 10.1073/pnas.1605884113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nachmani D., Stern-Ginossar N., Sarid R., Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5:376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 159.Samanta M., Takada K. Modulation of innate immunity system by Epstein-Barr virus-encoded non-coding RNA and oncogenesis. Cancer Sci. 2010;101:29–35. doi: 10.1111/j.1349-7006.2009.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y., Guo Z., Shu Y., Zhou H., Wang H., Zhang W. BART miRNAs: An unimaginable force in the development of nasopharyngeal carcinoma. Eur. J. Cancer Prev. 2017;26:144–150. doi: 10.1097/CEJ.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ye Y., Zhou Y., Zhang L., Chen Y., Lyu X., Cai L., Lu Y., Deng Y., Wang J., Yao K., et al. EBV-miR-BART1 is involved in regulating metabolism-associated genes in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2013;436:19–24. doi: 10.1016/j.bbrc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 162.Lyu X., Wang J., Guo X., Wu G., Jiao Y., Faleti O.D., Liu P., Liu T., Long Y., Chong T., et al. EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14:e1007484. doi: 10.1371/journal.ppat.1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou X., Zheng J., Tang Y., Lin Y., Wang L., Li Y., Liu C., Wu D., Cai L. EBV encoded miRNA BART8-3p promotes radioresistance in nasopharyngeal carcinoma by regulating ATM/ATR signaling pathway. Biosci. Rep. 2019;39:1–12. doi: 10.1042/BSR20190415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiao L., Hu Z.-Y., Dong X., Tan Z., Li W., Tang M., Chen L., Yang L., Tao Y., Jiang Y., et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33:4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Daker M., Bhuvanendran S., Ahmad M., Takada K., Khoo A.S.-B. Deregulation of lipid metabolism pathway genes in nasopharyngeal carcinoma cells. Mol. Med. Rep. 2013;7:731–741. doi: 10.3892/mmr.2012.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wong R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ozaki T., Nakagawara A. Role of p53 in cell death and human cancers. Cancers. 2011;3:994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Choy E.Y.-W., Siu K.-L., Kok K.-H., Lung R.W.-M., Tsang C.M., To K.-F., Kwong D.L.-W., Tsao S.W., Jin D.-Y. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J. Exp. Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zheng X., Wang J., Wei L., Peng Q., Gao Y., Fu Y., Lu Y., Qin Z., Zhang X., Lu J., et al. Epstein-Barr Virus microRNA miR-BART5-3p inhibits p53 expression. J. Virol. 2018;92:e01022-18. doi: 10.1128/JVI.01022-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Z., Chen X., Li L., Liu S., Yang L., Ma X., Tang M., Bode A.M., Dong Z., Sun L., et al. EBV encoded miR-BHRF1-1 potentiates viral lytic replication by downregulating host p53 in nasopharyngeal carcinoma. Int. J. Biochem. Cell Biol. 2012;44:275–279. doi: 10.1016/j.biocel.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 171.Marquitz A.R., Mathur A., Nam C.S., Raab-Traub N. The Epstein-Barr Virus BART microRNAs target the pro-apoptotic protein Bim. Virology. 2011;412:392–400. doi: 10.1016/j.virol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saridakis V., Sheng Y., Sarkari F., Holowaty M.N., Shire K., Nguyen T., Zhang R.G., Liao J., Lee W., Edwards A.M., et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol. Cell. 2005;18:25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 173.Lu J., Murakami M., Verma S.C., Cai Q., Haldar S., Kaul R., Wasik M.A., Middeldorp J., Robertson E.S. Epstein-Barr Virus nuclear antigen 1 (EBNA1) confers resistance to apoptosis in EBV-positive B-lymphoma cells through up-regulation of survivin. Virology. 2011;410:64–75. doi: 10.1016/j.virol.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang G.-D., Huang T.-J., Peng L.-X., Yang C.-F., Liu R.-Y., Huang H.-B., Chu Q.-Q., Yang H.-J., Huang J.-L., Zhu Z.-Y., et al. Epstein-Barr Virus encoded LMP1 upregulates microRNA-21 to promote the resistance of nasopharyngeal carcinomacells to cisplatin-induced apoptosis by suppressing PDCD4 and Fas-L. PLoS ONE. 2013;8:e78355. doi: 10.1371/journal.pone.0078355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Valastyan S., Weinberg R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liao W., Tian M., Chen N. Characteristic and novel therapeutic strategies of nasopharyngeal carcinoma with synchronous metastasis. Cancer Manag. Res. 2019;11:8431–8442. doi: 10.2147/CMAR.S219994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li A.C., Xiao W.W., Shen G.Z., Wang L., Xu A.A., Cao Y.Q., Huang S.M., Lin C.G., Han F., Deng X.W., et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: Long-term results and benefits of chemotherapy. Oncotarget. 2015;6:24511–24521. doi: 10.18632/oncotarget.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yip T.T.C., Ngan R.K.C., Fong A.H.W., Law S.C.K. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol. 2014;50:527–538. doi: 10.1016/j.oraloncology.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 179.Lan Y.-Y., Hsiao J.-R., Chang K.-C., Chang J.S.-M., Chen C.-W., Lai H.-C., Wu S.-Y., Yeh T.-H., Chang F.-H., Lin W.-H., et al. Epstein-Barr Virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J. Virol. 2012;86:6656–6667. doi: 10.1128/JVI.00174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Port R.J., Pinheiro-Maia S., Hu C., Arrand J.R., Wei W., Young L.S., Dawson C.W. Epstein-Barr virus induction of the Hedgehog signalling pathway imposes a stem cell phenotype on human epithelial cells. J. Pathol. 2013;231:367–377. doi: 10.1002/path.4245. [DOI] [PubMed] [Google Scholar]
- 181.Cai L.-M., Lyu X.-M., Luo W.-R., Cui X.-F., Ye Y.-F., Yuan C.-C., Peng Q.-X., Wu D.-H., Liu T.-F., Wang E., et al. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene. 2015;34:2156–2166. doi: 10.1038/onc.2014.341. [DOI] [PubMed] [Google Scholar]
- 182.Wong A.M.G., Kong K.L., Tsang J.W.H., Kwong D.L.W., Guan X.-Y. Profiling of Epstein-Barr virus-encoded microRNAs in nasopharyngeal carcinoma reveals potential biomarkers and oncomirs. Cancer. 2012;118:698–710. doi: 10.1002/cncr.26309. [DOI] [PubMed] [Google Scholar]
- 183.He B., Li W., Wu Y., Wei F., Gong Z., Bo H., Wang Y., Li X., Xiang B., Guo C., et al. Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell metastasis and invasion by targeting long non-coding RNA LOC553103. Cell Death Dis. 2016;7:e2353. doi: 10.1038/cddis.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lin C., Zong J., Lin W., Wang M., Xu Y., Zhou R., Lin S., Guo Q., Chen H., Ye Y., et al. EBV-miR-BART8-3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways. J. Exp. Clin. Cancer Res. 2018;37:1–14. doi: 10.1186/s13046-018-0953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yan Q., Zeng Z., Gong Z., Zhang W., Li X., He B., Song Y., Li Q., Zeng Y., Liao Q., et al. EBV-miR-BART10-3p facilitates epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma by targeting BTRC. Oncotarget. 2015;6:41766–41782. doi: 10.18632/oncotarget.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu Y.J., Zhou R., Zong J.F., Lin W.S., Tong S., Guo Q.J., Lin C., Lin S.J., Chen Y.X., Chen M.R., et al. Epstein-Barr virus-coded miR-BART13 promotes nasopharyngeal carcinoma cell growth and metastasis via targeting of the NKIRAS2/NF-κB pathway. Cancer Lett. 2019;447:33–40. doi: 10.1016/j.canlet.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 187.Pećina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17. doi: 10.1186/1475-2867-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Onder T.T., Gupta P.B., Mani S.A., Yang J., Lander E.S., Weinberg R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 189.Hsu C.-Y., Yi Y.-H., Chang K.-P., Chang Y.-S., Chen S.-J., Chen H.-C. The Epstein-Barr virus-encoded microRNA miR-BART9 promotes tumor metastasis by targeting E-cadherin in nasopharyngeal carcinoma. PLoS Pathog. 2014;10:e1003974. doi: 10.1371/journal.ppat.1003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leão R., Apolónio J.D., Lee D., Figueiredo A., Tabori U., Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: Clinical impacts in cancer. J. Biomed. Sci. 2018;25:1–12. doi: 10.1186/s12929-018-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kawagoe J., Ohmichi M., Takahashi T., Ohshima C., Mabuchi S., Takahashi K., Igarashi H., Mori-Abe A., Saitoh M., Du B., et al. Raloxifene inhibits estrogen-induced up-regulation of telomerase activity in a human breast cancer cell line. J. Biol. Chem. 2003;278:43363–43372. doi: 10.1074/jbc.M304363200. [DOI] [PubMed] [Google Scholar]
- 192.Akiyama M., Hideshima T., Hayashi T., Tai Y.T., Mitsiades C.S., Mitsiades N., Chauhan D., Richardson P., Munshi N.C., Anderson K.C. Nuclear factor-κB p65 mediates tumor necrosis factor α-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 2003;63:18–21. [PubMed] [Google Scholar]
- 193.Ding L., Li L.L., Yang J., Yong G.T., Ye M., Shi Y., Tang M., Yi W., Xiao L.L., Jian P.G., et al. Epstein-Barr virus encoded latent membrane protein 1 modulates nuclear translocation of telomerase reverse transcriptase protein by activating nuclear factor-κB p65 in human nasopharyngeal carcinoma cells. Int. J. Biochem. Cell Biol. 2005;37:1881–1889. doi: 10.1016/j.biocel.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 194.Yang L., Xu Z., Liu L., Luo X., Lu J., Sun L., Cao Y. Targeting EBV-LMP1 DNAzyme enhances radiosensitivity of nasopharyngeal carcinoma cells by inhibiting telomerase activity. Cancer Biol. Ther. 2014;15:61–68. doi: 10.4161/cbt.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang J., Deng X., Deng L., Gu H., Fan W., Cao Y. Telomerase activation by Epstein-Barr virus latent membrane protein 1 is associated with c-Myc expression in human nasopharyngeal epithelial cells. J. Exp. Clin. Cancer Res. 2004;23:495–506. [PubMed] [Google Scholar]
- 196.Li H.M., Man C., Jin Y., Deng W., Yip Y.L., Feng H.C., Cheung Y.C., Lo K.W., Meltzer P.S., Wu Z.G., et al. Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int. J. Cancer. 2006;119:1567–1576. doi: 10.1002/ijc.22032. [DOI] [PubMed] [Google Scholar]
- 197.Zhu D.D., Zhang J., Deng W., Yip Y.L., Lung H.L., Tsang C.M., Law W.T., Yang J., Lau V.M.Y., Shuen W.H., et al. Significance of NF-κB activation in immortalization of nasopharyngeal epithelial cells. Int. J. Cancer. 2016;138:1175–1185. doi: 10.1002/ijc.29850. [DOI] [PubMed] [Google Scholar]
- 198.Yip Y.-L., Tsang C.-M., Deng W., Cheung P.-Y., Jin Y., Cheung A.L., Lung M.L., Tsao S.-W. Expression of Epstein-Barr Virus-encoded LMP1 and hTERT extends the life span and immortalizes primary cultures of nasopharyngeal epithelial cells. J. Med. Virol. 2010;82:1711–1723. doi: 10.1002/jmv.21875. [DOI] [PubMed] [Google Scholar]
- 199.Liu Y., Gong P., Zhou N., Zhang J., He C., Wang S., Peng H. Insufficient PINX1 expression stimulates telomerase activation by direct inhibition of EBV LMP1-NF-κB axis during nasopharyngeal carcinoma development. Biochem. Biophys. Res. Commun. 2019;514:127–133. doi: 10.1016/j.bbrc.2019.04.104. [DOI] [PubMed] [Google Scholar]
- 200.Ding L., Li L., Yang J., Zhou S., Li W., Tang M., Shi Y., Yi W., Cao Y. Latent membrane protein 1 encoded by Epstein–Barr Virus induces telomerase activity via p16INK4A/Rb/E2F1 and JNK signaling pathways. J. Med. Virol. 2007;79:1153–1163. doi: 10.1002/jmv.20896. [DOI] [PubMed] [Google Scholar]