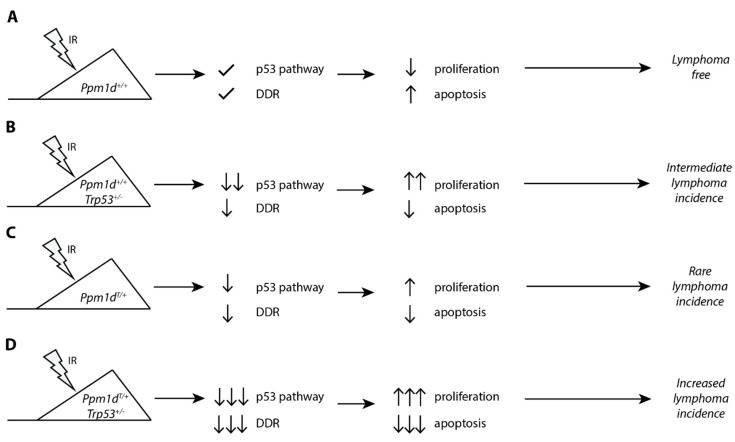
Figure 4.
A proposed model for the role of truncated PPM1D in the ionizing radiation-induced lymphoma. Mice carrying wild-type Ppm1d and Tpr53 activate the p53 pathway and DNA damage response upon exposure to ionizing radiation leading to block of the cell proliferation or to apoptosis in the thymus. This protects genome integrity and prevents tumor development (A). A partial loss of the p53 function in Trp53+/− mice suppresses activation of the checkpoint induced by ionizing radiation. This allows continuous cell proliferation in the presence of DNA damage leading to accumulation of the genetic changes and lymphoma development (B). Mice carrying C-terminally truncated Ppm1d fail to fully activate p53 and DNA damage response after exposure to ionizing radiation. In most cases, residual p53 function is sufficient to suppress proliferation and tumor formation. However, a fraction of cells proliferates despite the existing DNA damage which may occasionally result in tumor development (C). Combination of a partial loss of p53 and suppression of its function in Ppm1dT/+Trp53+/− mice allows continuous proliferation in the presence of DNA damage and prevents clearance of damaged cells by apoptosis. As a result, thymic lymphoma develops more frequently in Ppm1dT/+Trp53+/− mice compared to Ppm1dT/+ or Trp53+/− alone (D).