External stressful stimuli are known to increase the incidence of reactivation of Alphaherpesvirinae subfamily members. Activation of the glucocorticoid receptor (GR) by the synthetic corticosteroid dexamethasone (DEX) stimulates bovine herpesvirus 1 (BoHV-1) and herpes simplex virus 1 (HSV-1) reactivation. Furthermore, GR and dexamethasone stimulate productive infection and promoters that drive expression of viral transcriptional regulators. These observations lead us to predict that stress-induced transcription is impaired by factors abundantly expressed during latency. Interestingly, activation of the Akt family of serine/threonine protein kinases is linked to maintenance of latency. New studies reveal that Akt1 and Ak2, but not Akt3, impaired GR- and dexamethasone-mediated transactivation of the BoHV-1 immediate early transcription unit 1 and HSV-1 ICP0 promoters. Strikingly, Akt3, but not Akt1 or Akt2, stimulated neurite formation in mouse neuroblastoma cells, a requirement for neurogenesis. These studies provide insight into how Akt family members may promote the maintenance of lifelong latency.
KEYWORDS: AKT signaling, HSV-1, bovine herpesvirus 1, latency, neurogenesis, stress-induced transcription
ABSTRACT
Neurotropic Alphaherpesvirinae subfamily members such as bovine herpesvirus 1 (BoHV-1) and herpes simplex virus 1 (HSV-1) establish and maintain lifelong latent infections in neurons. Following infection of ocular, oral, or nasal cavities, sensory neurons within trigeminal ganglia (TG) are an important site for latency. Certain external stressors can trigger reactivation from latency, in part because activation of the glucocorticoid receptor (GR) stimulates productive infection and promoters that drive expression of key viral transcriptional regulators. The Akt serine/threonine protein kinase family is linked to maintaining latency. For example, Akt3 is detected in more TG neurons during BoHV-1 latency than in reactivation and uninfected calves. Furthermore, Akt signaling correlates with maintaining HSV-1 latency in certain neuronal models of latency. Finally, an active Akt protein kinase is crucial for the ability of the HSV-1 latency-associated transcript (LAT) to inhibit apoptosis in neuronal cell lines. Consequently, we hypothesized that viral and/or cellular factors impair stress-induced transcription and reduce the incidence of reactivation triggered by low levels of stress. New studies demonstrate that Akt1 and Akt2, but not Akt3, significantly reduced GR-mediated transactivation of the BoHV-1 immediate early transcription unit 1 (IEtu1) promoter, the HSV-1 infected cell protein 0 (ICP0) promoter, and the mouse mammary tumor virus long terminal repeat (MMTV-LTR). Akt3, but not Akt1 or Akt2, significantly enhanced neurite formation in mouse neuroblastoma cells, which correlates with repairing damaged neurons. These studies suggest that unique biological properties of the three Akt family members promote the maintenance of latency in differentiated neurons.
IMPORTANCE External stressful stimuli are known to increase the incidence of reactivation of Alphaherpesvirinae subfamily members. Activation of the glucocorticoid receptor (GR) by the synthetic corticosteroid dexamethasone (DEX) stimulates bovine herpesvirus 1 (BoHV-1) and herpes simplex virus 1 (HSV-1) reactivation. Furthermore, GR and dexamethasone stimulate productive infection and promoters that drive expression of viral transcriptional regulators. These observations lead us to predict that stress-induced transcription is impaired by factors abundantly expressed during latency. Interestingly, activation of the Akt family of serine/threonine protein kinases is linked to maintenance of latency. New studies reveal that Akt1 and Ak2, but not Akt3, impaired GR- and dexamethasone-mediated transactivation of the BoHV-1 immediate early transcription unit 1 and HSV-1 ICP0 promoters. Strikingly, Akt3, but not Akt1 or Akt2, stimulated neurite formation in mouse neuroblastoma cells, a requirement for neurogenesis. These studies provide insight into how Akt family members may promote the maintenance of lifelong latency.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) and bovine herpesvirus 1 (BoHV-1) infections cause important diseases in their respective hosts (1, 2). Their ability to latently infect neurons and periodically reactivate from latency is crucial for virus transmission and, in the case of HSV-1, for recurrent disease. Sensory neurons in trigeminal ganglia (TG) are a primary site for latency when acute infection is initiated in the ocular, oral, or nasal cavity (1–3). Establishment and maintenance of latency requires that infected neurons survive, neuronal damage is repaired, lytic cycle viral gene expression is silenced, and little or no virus is produced (4–6). The BoHV-1-encoded latency-related (LR) RNA and the HSV-1 latency-associated transcript (LAT) are readily detected in latently infected neurons. LAT (7) and the LR gene (8) encode multiple products that can promote survival of infected neurons by inhibiting apoptosis (9–11) and expression of crucial viral regulatory proteins (12, 13). A cellular microRNA reduces expression of an HSV-1 regulatory protein (ICP0), demonstrating that cellular factors play a role in promoting latency (14). Thus, viral and cellular functions actively promote establishment and maintenance of lifelong latency.
“Stress” increases the incidence of BoHV-1 (1, 2, 15, 16), HSV-1 (17–19), and canine herpesvirus 1 reactivation from latency (20, 21). Strikingly, the genomes of BoHV-1 (22) and HSV-1 (C Jones and F. Meyer, unpublished results) contain many putative glucocorticoid receptor (GR) response elements (GREs). The immediate early transcription unit 1 (IEtu1) promoter of BoHV-1 contains two functional GREs that are essential for transactivation by GR and the synthetic corticosteroid dexamethasone (DEX) (22, 23). This viral promoter drives expression of two important viral transcriptional regulators (bICP0 and bICP4). Recent studies demonstrated that GR and Krüppel-like transcription factor 15 (KLF15) cooperatively transactivate the HSV-1 ICP0 promoter (24). While the ICP0 promoter does not contain a whole GRE, it contains five half-GREs that are important but not required for stress-induced promoter activation.
Recent studies suggested that the three Akt family members play a significant role in the latency-reactivation cycle of BoHV-1 and HSV-1. For instance, Akt3 RNA is significantly higher in TG neurons of calves latently infected with BoHV-1; conversely, 30 min after DEX is administered to initiate reactivation, Akt3 expression is reduced more than 50-fold and the number of Akt3-positive TG neurons is significantly reduced (25). Furthermore, the number of TG neurons that express Akt3 is significantly higher during latency relative to the number of TG neurons from uninfected calves or from those undergoing DEX-induced reactivation from latency. With respect to the HSV-1 latency-reactivation cycle, several studies indicate that Akt signaling is important. For example, inhibiting the phosphatidylinositol 3-kinase (PI3K)/Akt signaling axis induces reactivation from latency in two neuronal models of HSV-1 latency, primary rat sympathetic neurons (26, 27) and Lund human mesencephalic (LUHMES) neuronal cells (28). Furthermore, interfering with Akt kinase functions impairs the ability of the HSV-1 latency-associated transcript (LAT) to interfere with apoptosis and neurite formation (29, 30). The Wnt/β-catenin pathway is induced during BoHV-1 latency but repressed during DEX-induced reactivation (25, 31, 32). Mice latently infected with wild-type HSV-1 contain significantly more TG neurons that express β-catenin than those in an LAT-null mutant (33). Akt family members and the Wnt signaling pathway form a positive regulatory loop (34–39), suggesting that these signaling pathways promote the maintenance of latency.
While the three Akt family members are similar and are generally associated with cell survival, regulating metabolism, and promotion of tumor development, they also have nonredundant functions, reviewed in Manning and Toker (40). For example, Akt1−/− mice exhibit growth defects and increased perinatal lethality (41). In contrast, Akt2−/− mice develop diabetes-like symptoms (42). Furthermore, Akt1 interferes with metastatic spread of breast cancer, whereas Akt2 promotes metastatic spread (43–45). Akt3−/− mice have decreased brain volume and exhibit neurodevelopmental problems (46, 47). Akt3 prevents stroke-induced neuronal injury (48), inhibits apoptosis in neurons, and stimulates axonal development more efficiently than Akt1 and Akt2 (49). Currently, proteins phosphorylated by specific Akt isoforms that mediate the unique biological properties of these kinases have not been identified.
In this study, we tested whether Akt family members regulate stress-induced transactivation of two key alphaherpesvirus promoters, BoHV-1 IEtu1 and HSV-1 ICP0. We also compared these results to the mouse mammary tumor virus long terminal repeat (MMTV-LTR) because it is widely known to be activated by GR and DEX (50). Akt1 and Akt2, but not Akt3, interfered with GR-mediated transactivation of these three promoters. Interestingly, a protein kinase dead Akt1 mutant did not inhibit stress-induced transactivation of the IEtu1 and MMTV-LTR. Conversely, this Akt1 protein kinase mutant inhibited stress-induced transactivation of the ICP0 promoter. Additional studies revealed that Akt3, but not Akt1 or Akt2, enhanced neurite formation during differentiation of Neuro-2A cells. In summary, these studies identified novel Akt functions that are predicted to be important for maintaining a lifelong latent infection.
RESULTS
Akt1 reduced GR- and DEX-mediated activation of BoHV-1 IEtu1 collapsed promoter in a dose-dependent manner.
While the antiapoptotic functions of Akt family members (40, 51) are likely to play an important role in maintaining latency, we predicted that additional Akt functions are important for this crucial phase of the latency-reactivation cycle. For example, mammals face stressful stimuli every day, but reactivation from latency is not a daily occurrence. Support for this statement comes from the finding that calves latently infected with BoHV-1 do not frequently shed virus prior to DEX treatment (C. Jones, personal communication). Interestingly, Akt1 is frequently activated in acute lymphoblastic leukemia, which correlates with development of glucocorticoid resistance (52, 53). Based on these observations, we predicted that cellular or viral factors actively restrain stress-induced stimulation of viral gene expression during latency. Consequently, we tested whether Akt1, Akt2, or Akt3 influenced GR-mediated transactivation of viral promoters in Neuro-2A cells. Neuro-2A cells were used for these studies because they are a mouse neuroblastoma cell line that can be readily transfected and differentiated into dopamine-like neurons (54)—thus, they have certain neuron-like properties.
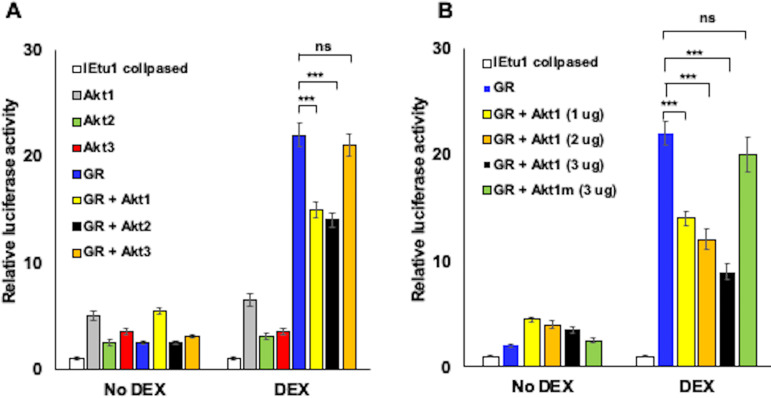
Initially we examined the IEtu1 collapsed promoter because it is a minimal IEtu1 promoter construct efficiently transactivated by GR plus DEX or by GR plus KLF15 plus DEX (22, 23, 55). The IEtu1 promoter contains two consensus GREs required for activation by GR and DEX. Akt1 or Akt2, but not Akt3, significantly reduced GR-mediated transactivation of the BoHV-1 IEtu1 collapsed promoter when cultures were treated with the synthetic corticosteroid DEX (Fig. 1A). Increasing Akt1 concentrations reduced GR-mediated activation in a dose-dependent manner (Fig. 1B). Dose-dependent effects on GR-mediated transactivation of IEtu1 collapsed promoter activity were also observed with Ak2 but not with Akt3 (data not shown). Conversely, none of the Akt family members significantly reduced basal promoter activity. Akt1 is a serine/threonine protein kinase, and the Akt1 kinase dead mutant (1014 pcDNA3 T7, which contains 3 point mutations, namely K179M, T308A, and S473A; Addgene) (56) was used to test whether kinase activity was important for inhibiting GR-mediated transcription. The Akt1 kinase mutant (Akt1m) did not significantly reduce GR-mediated transactivation of the IEtu1 collapsed promoter (Fig. 1B). These studies demonstrated that Akt1 and Akt2 impaired GR-mediated activation of the IEtu1 collapsed promoter and that Akt1 kinase activity was required for the inhibitory effect.
FIG 1.
Akt family members influence GR- and DEX-mediated transactivation of the IEtu1 collapsed promoter. (A) Neuro-2A cells were transfected with the IEtu1 collapsed promoter construct containing the firefly luciferase reporter gene (0.5 μg) and, where indicated, plasmids that expressed GR (1.0 μg), Akt1, Akt2, or Akt3 (1.0 μg). (B) Neuro-2A cells were transfected with the IEtu1 collapsed promoter (0.5 μg) and, where indicated, plasmids that expressed GR (1.0 μg), Akt1 (1.0 μg, 2.0 μg, or 3.0 μg). or Akt1 kinase mutant construct (3.0 μg). All transfections contained a plasmid that expresses Renilla luciferase (0.05 μg) to normalize firefly luciferase values. To maintain the same amount of DNA in each sample, empty vector was included in certain samples. Cells were incubated with 2% stripped fetal bovine serum (FBS) at approximately 24 h after transfection, and then certain cultures were treated with DEX (10 μM). At 48 h after transfection, cells were harvested and protein lysate subjected to a dual-luciferase assay. The results are the average of 3 independent experiments, and error bars denote the standard error. Student’s t test was used for statistical analysis. ns, not significant; **, P < 0.01; ***, P < 0.001.
Akt1 and Akt 2 reduced GR-mediated activation of the MMTV-LTR promoter.
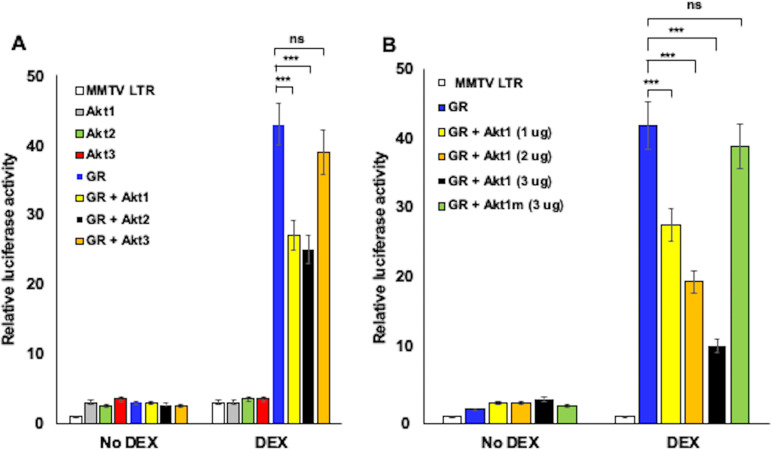
The effect of Akt family members on stress-induced activation of the mouse mammary tumor virus (MMTV) long terminal repeat (LTR) was also examined because this retroviral promoter is strongly stimulated by GR plus DEX, as well as by estrogen, due to multiple GREs in the LTR (50, 57). Consequently, the MMTV-LTR is an excellent model promoter to explore the mechanism by which stress-induced transcription occurs. Hence, we examined the effect Akt family members had on MMTV LTR promoter activity as a comparison to the BoHV-1 IEtu1 promoter. Akt1 and Akt2, but not Akt3, significantly reduced GR-mediated transactivation of the MMTV-LTR promoter in a DEX-dependent manner (Fig. 2A). However, Akt1, Akt2, and Akt3 each had little effect on basal promoter activity (no DEX addition). These studies also revealed that Akt1-dependent inhibition was dose dependent (Fig. 2B). The Akt1 kinase mutant (Akt1m) did not significantly reduce GR-mediated transactivation (Fig. 2B), indicating that Akt1 kinase activity was essential for reducing GR-mediated transactivation of the MMTV-LTR, which was similar to that of the IEtu1 promoter.
FIG 2.
Akt1 and Akt2 impair GR-mediated activation of the MMTV-LTR promoter. (A) Neuro-2A cells were transfected with the MMTV-LTR promoter construct (0.5 μg) and, where indicated, plasmids that express GR (1.0 μg), Akt1, Akt2, or Akt3 (1.0 μg). (B) Neuro-2A cells were transfected with the MMTV-LTR promoter construct (0.5 μg) and, where indicated, plasmids that expressed GR (1.0 μg), Akt1 (1.0 μg, 2.0 μg, or 3.0 μg), or Akt1 kinase mutant construct (3.0 μg). All transfections contained a plasmid that expresses Renilla luciferase (0.05 μg) to normalize firefly luciferase values. To maintain the same amount of DNA in each sample, empty vector was included in certain samples. Cells were incubated with 2% stripped FBS at approximately 24 h after transfection and then certain cultures were treated with DEX (10 μM). At 48 h after transfection, cells were harvested and protein lysate subjected to a dual-luciferase assay. The results are the average of 3 independent experiments and error bars denote the standard error. Student’s t test was used for statistical analysis. ns, not significant; **, P < 0.01; ***, P < 0.001.
Akt1 reduced GR+KLF15 mediated transactivation of the HSV-1 ICP0 promoter.
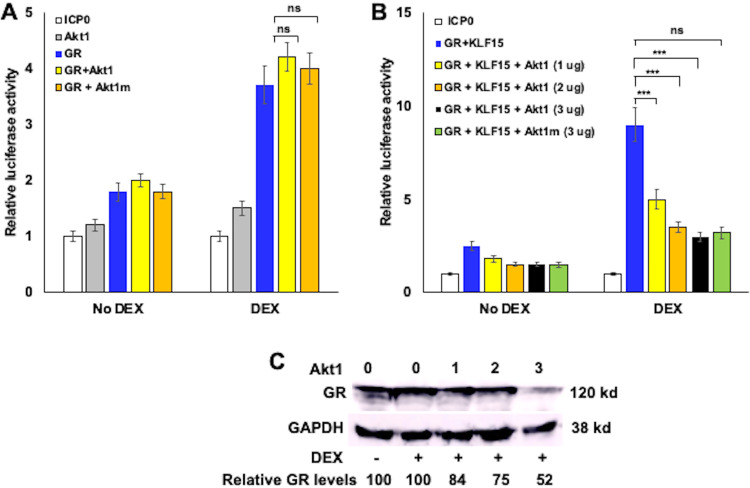
The HSV-1 ICP0 promoter has numerous transcription factor binding sites and is stimulated by heat stress-induced factors, (58). Expression of the multifunctional ICP0 protein is believed to be important for successful reactivation from latency (reviewed in reference 15). Unlike the IEtu1 collapsed promoter and MMTV-LTR, there are no consensus GREs in the ICP0 promoter (24). Akt1 had little effect on ICP0 promoter activity when cultures were not treated with DEX, in part because the basal activity of the promoter was low in Neuro-2A cells (Fig. 3A). Since DEX only stimulated promoter activity by approximately 3-fold, the effects of Akt family members were nominal. KLF15, GR, and DEX treatment cooperatively transactivate ICP0 promoter activity (24). When KLF15 was cotransfected with GR (no DEX treatment), ICP0 promoter activity was not influenced dramatically by Akt1 (Fig. 3B). However, Akt1 significantly reduced the activity of GR plus KLF15 plus DEX mediated transactivation of the ICP0 promoter in a dose-dependent manner (Fig. 3B). In contrast to the IEtu1 promoter and MMTV-LTR, the Akt1 kinase mutant (Akt1m) also significantly reduced ICP0 promoter activity (Fig. 3B). As with the MMTV-LTR and IEtu1 promoter, Ak2, but not Akt3, reduced ICP0 promoter activity stimulated by GR, KLF15, and DEX treatment (data not shown).
FIG 3.
Akt1 significantly reduces GR- and KLF15-mediated transactivation of the HSV-1 ICP0 promoter. (A) Neuro-2A cells were transfected with the HSV-1 ICP0 promoter construct (0.5 μg), plasmids that express Akt1 or Akt1m (1.0 μg), and a plasmid that expresses GR (1.0 μg). (B) Neuro-2A cells were transfected with the ICP0 promoter construct (0.5 μg) and, where indicated, plasmids that expressed GR (1.0 μg), KLF15 (1.0 μg), Akt1 (1.0 μg, 2.0 μg, or 3.0 μg), or Akt1 kinase mutant construct (3.0 μg). All transfections contained a plasmid that expresses Renilla luciferase (0.05 μg) to normalize firefly luciferase values. To maintain the same amount of DNA in each sample, empty vector was included in certain samples. Cells were incubated with 2% stripped FBS 24 h after transfection and then certain cultures were treated with DEX (10 μM). At 48 h after transfection, cells were harvested and protein lysate subjected to a dual-luciferase assay. The results are the average of 3 independent experiments, and error bars denote the standard error. Student’s t test was used for statistical analysis. ns, not significant; **, P < 0.01; ***, P < 0.001. (C) Neuro-2A cells were transfected with a plasmid that expresses Akt1 (1.0, 2.0 or 3.0 μg of the expression vector as denoted), and a plasmid that expresses GR (1.0 μg). Cells were incubated with 2% stripped FBS 24 h after transfection and then certain cultures were treated with DEX (10 μM). At 48 h after transfection, cells were harvested, cell lysate prepared, and Western blot analysis performed to detect GR and the loading control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Fifty μg of protein was loaded in each lane. The results are the average of 3 independent experiments. kd, kilodalton.
To address whether Akt1 influenced GR steady-state proteins, increasing amounts of Akt1 were cotransfected with the GR expression construct, and Western blot analysis were performed. The GR construct we used for these studies expresses a 120-kilodalton protein, as previously demonstrated (23). In the presence of DEX, we consistently observed that 3 μg Akt1 reduced GR protein levels by approximately 50% relative to the loading control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Fig. 3C).
Akt3 enhances Neuro-2A cell differentiation.
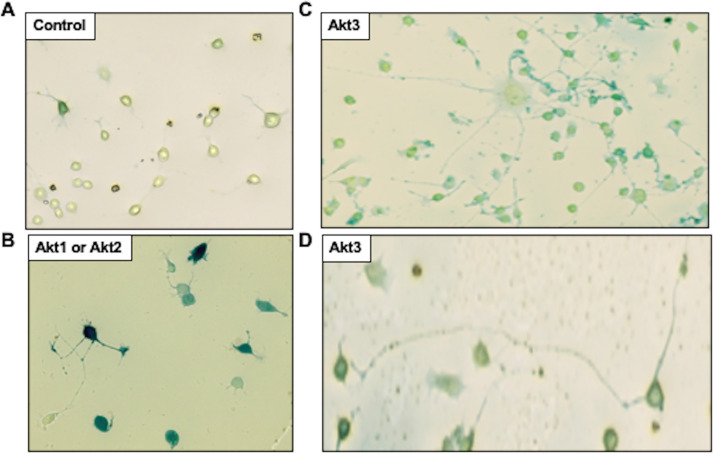
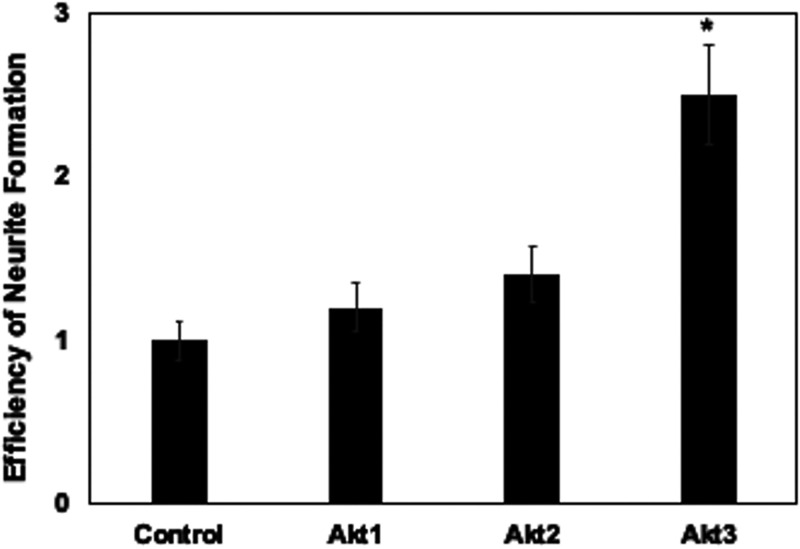
When Neuro-2A cells are transfected with a LacZ expression vector and growth factors are removed, β-galactosidase-positive (β-Gal+) neurites were detected when cells were seeded at a low density and then serum starved for 3 days (Fig. 4A, “control”), which is consistent with previous studies (54). Numerous cells detach from the plate because they do not survive growth factor withdrawal and consequently undergo apoptosis (27). When Akt1 or Akt2 was transfected with the LacZ expression vector and neurite formation assessed, Akt1 and Akt2 appeared to slightly increase the length of neurites (Fig. 4B). However, Akt1 and Akt2 did not significantly increase the frequency of neurite formation (Fig. 5). In stark contrast to Akt1 or Akt2, Akt3 dramatically increased the efficiency of neurite formation (Fig. 4C and D and Fig. 5). For example, clusters of neurite-positive cells that contained β-Gal+ cells were readily detected (Fig. 4C). Furthermore, neurites were generally longer relative to controls or Neuro-2A cells transfected with Akt1 or Akt2. In fact, we observed β-Gal+ Neuro-2A cells that had differentiated and contained neurites more than 20 times longer than the cell body (Fig. 4D). Since neurite sprouting is synonymous with regeneration of damaged axons and dendrites (59), the ability of Akt3 to enhance neurite formation and length of neurites may be important for repairing and restoring normal neuronal functions following infection.
FIG 4.
Akt3 efficiently promotes neurite formation in Neuro-2A cells. Neuro-2A cells were cotransfected with an empty vector (pcDNA3.1) (A), a plasmid expressing Akt1 or Akt2 (Panel B), or Akt3 (C and D) (1 μg plasmid DNA) and a plasmid expressing the lacZ gene (0.1 μg plasmid) to mark transfected cells. (B) A typical result from cells transfected with Akt1 or Akt2. To induce neurite sprouting, 24 h after transfection, cells were seeded into new plates at a low density (2,000 cells/cm2) and then incubated with minimal essential medium (MEM) that contained 0.5% serum for 3 days. Cells were fixed, and β-Gal+ cells were detected by staining.
FIG 5.
Akt3, but not Akt1or Akt2, significantly increased neurite formation in Neuro-2A cells. The relative efficiency of β-Gal+ cells containing neurites was calculated by dividing the number of β-Gal+ cells with a neurite length at least twice the diameter of the cell by the total number of β-Gal+ cells. The % of β-Gal+ cells with neurites in the control was set at 1. The other samples were compared to the control to obtain the relative efficiency of neurite formation. The average of three independent experiments is shown with the respective standard deviation. An asterisk denotes significant differences (P < 0.05) in β-Gal+ Neuro-2A cells containing neurites following transfection with the Akt family member relative to the number of β-Gal+ Neuro-2A cells with neurites following transfection with an empty vector, as determined by the one-way analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) multiple means comparison tests.
DISCUSSION
UV light, heat stress (fever), trauma, and increased corticosteroids as a result of stress increase the incidence of reactivation from latency in humans (1–3, 17, 19, 60, 61). While these reactivation stimuli appear to be dissimilar, heat stress and UV light activate the GR. For example, cyanoketone, a glucocorticoid synthesis inhibitor, reduces corticosterone synthesis and the efficiency of HSV-1 reactivation in latently infected mice (62). Furthermore, heat stress or DEX increased the incidence of reactivation from latency in cultured TG cells (63). UV light-induced GR phosphorylation occurs via a ligand-independent mechanism that correlates with GR-mediated transcriptional activation (64, 65). UV light also induces expression of certain enzymes regulated by GR activation (66) and activates a serine/threonine protein kinase, c-Jun N-terminal kinase (JNK) (65). JNK is crucial for remodeling HSV-1 chromatin during reactivation from latency in an in vitro neuronal model for latency (67). These studies suggest that several known reactivation stimuli activate GR; consequently, GR-mediated transactivation of viral and cellular promoters is predicted to trigger early events during reactivation from latency.
Several independent studies concluded that the phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR signaling axis promotes HSV-1 latent or quiescent infections in certain neuronal cell culture models of latency. For example, inhibiting nerve growth factor, PI3K, or Akt signaling induces reactivation from latency in two in vitro models for latency, primary rat sympathetic neurons (26, 27, 68) and human LUHMES cells (a human mesencephalic neuronal cell line) (28). Numerous growth factor signaling pathways activate PI3K/Akt signaling pathways (38–40, 69). PI3K activation increases phosphatidylinositol(3,4,5)-trisphosphate (PIP3); consequently, 3-phosphoinositide-dependent protein kinase-1 (PDK1) and Akt signaling are activated (reviewed in references 40 and 70). Akt can directly phosphorylate β-catenin, which leads to increased β-catenin-dependent transcription (71). N-cadherin is also activated by Wnt/β-catenin and N-cadherin activates Akt, which promotes neuronal differentiation during cortical development (38).
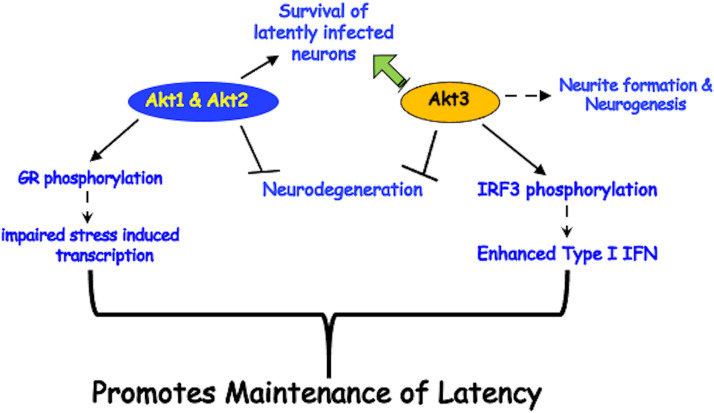
We propose that Akt signaling has multiple effects on the ability of neurotropic Alphaherpesvirinae subfamily members to maintain latency (summarized in Fig. 6). This model is based on published studies using the following different latency models: (i) Akt3 is expressed in more TG neurons during BoHV-1 latency (25) and (ii) the PI3K/Akt/mTOR pathway maintain an HSV-1 latent/quiescent infection in neuronal models of latency (26–28, 68). Strikingly, Akt3 RNA levels in TG are reduced more than 50-fold, and the number of Akt3+ TG neurons is significantly reduced during DEX-induced reactivation of BoHV-1. In contrast, Akt1 and Akt2 RNA levels are similar in TG of uninfected calves and in those with latency or DEX-induced reactivation. Studies presented in Fig. 1 and 3 revealed that Akt1 and Akt2 can potentially support maintenance of latency by impairing stress-induced activation of viral promoters that drive expression of transcriptional regulators. The finding that Akt2 inhibited GR-mediated transactivation is supported by a published study demonstrating that Akt2 kinase activity interferes with glucocorticoid resistance in certain lymphoid malignancies (72). Furthermore, Akt1 directly phosphorylates GR at position serine-134, which interferes with GR nuclear localization and transactivation (53). Multiple GR isoforms are generated by alternative splicing and alternative translation initiation at the amino terminus of the GR (73, 74), suggesting that certain GR isoforms may not be phosphorylated by Akt1 and Akt2. While our studies indicated that overexpression of Akt1 in Neuro-2A cells slightly reduced GR steady protein levels (Fig. 3C), we suggest that the ability of Akt1 to directly phosphorylate GR at serine-134 was more important.
FIG 6.
Schematic of Akt functions that can influence maintenance of latency. Summary of how Akt family members may contribute to maintenance of latency. For specific details, see the text.
Surprisingly, Akt1 kinase activity was not important for interfering with the HSV-1 ICP0 promoter. In contrast to IEtu1 and MMTV-LTR, the ICP0 promoter lacks “whole” GREs but contains half-GREs that are important but not required for stress-induced transcription (24). GR and KLF15 stably interact with each other to form a feed-forward transcription loop that cooperatively transactivates the ICP0 promoter and the BoHV-1 IEtu1 promoter (23, 24, 75). While GR monomers can bind and transactivate certain half-GREs (76), we do not fully understand how GR and KLF15 transactivate promoters that lack “whole” GREs. Of note, Akt1 was reported to stably interact with transcriptional activators or repressors (77, 78), including an H3 methyltransferase that coordinates gene silencing (79). Based on these observations, we suggest that interactions between the Akt1 kinase mutant and transcriptional repressors serve as a molecular scaffold that impair GR- and KLF15-mediated transactivation of the HSV-1 ICP0 promoter. Studies designed to determine whether specific Akt family members regulate the BoHV-1 or HSV-1 latency-reactivation cycle in vivo need to be performed. However, these studies will be complicated and are not within the scope of this study.
While Akt3 had no obvious effect on interfering with GR-mediated transactivation, novel functions of Akt3 are predicted to mediate certain aspects of maintaining latency. For example, Akt3 enhanced neurite formation in Neuro-2A cells significantly better than Akt1 or Akt2. In general, Akt signaling pathways stimulate neurite outgrowth (80), impair neurodegeneration (81), promote neuronal survival following stressful stimuli (82), and coregulate neuronal differentiation (83). However, Akt3, but not the other two Akt family members, is required for nerve growth factor (NGF)-mediated antiapoptotic signaling in PC12 neuron-like cells (84) and apoptosis in motor neurons (85). As discussed above, Akt3 is more important than Akt1 and Akt2 for preventing stroke-induced neuronal injury (48) and for promoting the growth of axons (49). Interestingly, a recent study concluded that Akt3 directly binds and phosphorylates interferon response factor 3 (IRF3), thus stimulating expression of antiviral type I interferons (86), which may interfere with reactivation via the antiviral activity of IRF3 and type 1 interferons. In summary, novel Akt3 functions are predicted to contribute to the maintenance of latency (summarized in Fig. 6).
Several independent studies concluded that the PI3k/Akt signaling pathways is coopted by several different viruses, including HSV-1 (87), to enhance virus entry, cell survival, transcription, protein synthesis, and virus transmission (88, 89). HSV-1-encoded protein kinases (US3 and UL13) regulate PI3K/Akt kinase activity to enhance virion packaging, which increases virus yield (90). During BoHV-1 productive infection, Akt phosphorylation is also increased dramatically (91) suggesting that these virally encoded kinases have similar effects on virus yield. While these findings appear to be discordant with the findings presented in this study and the model proposed in Fig. 6, tissue-specific effects of the three Akt family members in terminally differentiated sensory neurons are predicted to be responsible for their putative roles during lifelong latent infections.
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
Murine neuroblastoma cells (Neuro-2A; CCL-131) were obtained from ATCC (Manassas, VA) and grown in minimal essential medium (MEM; Life Technology) supplemented with 10% fetal bovine serum (FBS), penicillin (10 U/ml), and streptomycin (100 μg/ml).
A plasmid that expresses Akt1 was a gift from Jie Chen (pCDNA3-HA-Akt1, plasmid 73408; Addgene). A plasmid that expresses Akt3 was a gift from William Sellers (1236 pcDNA3 Myr HA Akt3, plasmid 9017; Addgene). A plasmid that expresses Akt2 was a gift from William Sellers (1227 pcDNA3 Myr HA Akt2, plasmid 9016; Addgene). The Akt1 kinase dead mutant (1014 pcDNA3 T7; Addgene) includes 3 points mutations, K179M, T308A, and S473A. The HSV-1 ICP0 luciferase construct (−800 to +150) was obtained from Priscilla Schaffer and was described in previous studies (58, 92). The BoHV-1 IEtu1 collapsed promoter construct was previously described (93). The MMTV LTR luciferase reporter construct (pGL3-MMLV-LTR-Luc) was obtained from Stephen Goff (catalog no. 67831; Addgene). All plasmids were transfected into Neuro-2A cells in 60-mm dishes or 12-well plates using Lipofectamine 3000 transfection reagent (L3000075; Invitrogen) according to the manufacturer’s instructions.
Dual-luciferase assay.
To test the effect of Akt1, Akt2 and Akt3 on GR-mediated activation, Neuro-2A cells were seeded into 60-mm dishes containing MEM with 10% FBS at 24 h prior to transfection. At the time of transfection, approximately 6 × 105 Neuro-2A cells were present in each 60-mm dish. Two h before transfection, cells were cultured with antibiotic-free medium containing 2% stripped FBS. Cells were cotransfected with the designated plasmids and a plasmid carrying Renilla luciferase under the control of a minimal herpesvirus thymidine kinase (TK) promoter (50 ng). To maintain equal plasmid amounts in the transfection mixtures, an empty expression vector was added as needed. At approximately 24 h after transfection, water soluble DEX (Sigma; catalog no. D2915) number was added to the designated cultures. At 48 h after transfection, cells were harvested, and protein lysate subjected to a dual-luciferase assay by using a commercially available kit (catalog number E1910; Promega) according to the manufacturer’s instructions. Luminescence was measured with a GloMax 20/20 luminometer (catalog number E5331; Promega).
Neurite formation assay.
Neuro-2A cells grown in 24-well plates were cotransfected with plasmids that express Akt1, Akt2, or Akt3 (1 μg) and a pCMV–β-Gal plasmid (1 μg). At the time of transfection, approximately 6 × 105 Neuro-2A cells were present in each 60-mm dish. To induce neurite sprouting, 24 h after transfection cells were seeded onto 60-mm dishes at a low density (2,000 cells/cm2) and cells were starved in medium with 0.5% serum for 3 days. Cells were then fixed, stained, and a β-Gal assay was performed as previously described (94). The percentage of cells with β-Gal+ neurites was calculated by dividing the number of β-Gal+ cells with neurite length at least twice the diameter of the cell by the total number of β-Gal+ cells. The results are averages of three independent experiments.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS111167, by the USDA-NIFA Competitive Grants Program (grants 2016-09370 and 2018-06668), by the Oklahoma Center for Respiratory and Infectious Diseases (National Institutes of Health Centers for Biomedical Research Excellence Grant P20GM103648), and by funds from the Sitlington Endowment.
REFERENCES
- 1.Jones C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv Virus Res 51:81–133. doi: 10.1016/s0065-3527(08)60784-8. [DOI] [PubMed] [Google Scholar]
- 2.Jones C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin Microbiol Rev 16:79–95. doi: 10.1128/cmr.16.1.79-95.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perng G-C, Jones C. 2010. Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdiscip Perspect Infect Dis 2010:262415–262418. doi: 10.1155/2010/262415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera C, Wohlenberg C, Openshaw H, Rey-Mendez M, Puga A, Notkins AL. 1980. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature 288:288–290. doi: 10.1038/288288a0. [DOI] [PubMed] [Google Scholar]
- 5.Fraser N, Lawrence WC, Wroblewska Z, Gilde DH, Koprowski H. 1981. Herpes simplex virus type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A 78:6461–6465. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock D, Fraser NW. 1983. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature 302:523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- 7.Phelan D, Barrozo ER, Bloom DC. 2017. HSV1 latent transcription and non-coding RNA: a critical retrospective. J Neuroimmunol 308:65–101. doi: 10.1016/j.jneuroim.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Jones C, da Silva LF, Sinani D. 2011. Regulation of the latency-reactivation cycle by products encoded by the bovine herpesvirus 1 (BHV-1) latency-related gene. J Neurovirol 17:535–545. doi: 10.1007/s13365-011-0060-3. [DOI] [PubMed] [Google Scholar]
- 9.Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina SM, Hofman FM, Ghiasi H, Nesburn AB, Wechsler SL. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript (LAT). Science 287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 10.Ciacci-Zanella J, Stone M, Henderson G, Jones C. 1999. The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death. J Virol 73:9734–9740. doi: 10.1128/JVI.73.12.9734-9740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W, Jones C. 2008. Open reading frame 2, encoded by the latency-related gene of bovine herpesvirus 1, has antiapoptotic activity in transiently transfected neuroblastoma cells. J Virol 82:10940–10945. doi: 10.1128/JVI.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaber T, Workman A, Jones C. 2010. Small noncoding RNAs encoded within the bovine herpesvirus 1 latency-related gene can reduce steady-state levels of infected cell protein 0 (bICP0). J Virol 84:6297–6307. doi: 10.1128/JVI.02639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. 2008. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–785. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan D, Flores O, Umbach JL, Pesola JM, Bentley P, Rosato PC, Leib DA, Cullen BR, Coen DM. 2014. A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency. Cell Host Microbe 15:446–456. doi: 10.1016/j.chom.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones C. 2014. Reactivation from latency by alpha-herpesvirinae subfamily members: a stressful situation. Curr Top Virol 12:99–118. [Google Scholar]
- 16.Jones C, Geiser V, Henderson G, Jiang Y, Meyer F, Perez S, Zhang Y. 2006. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet Microbiol 113:199–210. doi: 10.1016/j.vetmic.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Padgett DA, Sherida J, Dorne FJ, Berntson GG, Candelora J, Glaser R. 1998. Social stress and the reactivation of latent herpes simplex virus type 1. Proc Natl Acad Sci U S A 95:7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. 2007. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infection. J Immunol 179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. 1985. Stress, loneliness, and changes in herpesvirus latency. J Behav Med 8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- 20.Ledbetter EC, Kim SG, Dubovi EJ, Bicalho RC. 2009. Experimental reactivation of latent canine herpesvirus-1 and induction of recurrent ocular disease in adult dogs. Vet Microbiol 138:98–105. doi: 10.1016/j.vetmic.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Ledbetter EC, Kice NC, Matusow RB, Dubovi EJ, Kim SG. 2010. The effect of topical ocular corticosteroid administration in dogs experimentally induced latent canine herpesvirus-1 infection. Exp Eye Res 90:711–717. doi: 10.1016/j.exer.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Kook I, Henley C, Meyer F, Hoffmann F, Jones C. 2015. Bovine herpesvirus 1 productive infection and the immediate early transcription unit 1 are stimulated by the synthetic corticosteroid dexamethasone. Virology 484:377–385. doi: 10.1016/j.virol.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.El-Mayet FS, Sawant L, Thunuguntla P, Jones C. 2017. Combinatorial effects of the glucocorticoid receptor and Krüppel-like transcription factor 15 on bovine herpesvirus 1 transcription and productive infection. J Virol 91:e00904-17. doi: 10.1128/JVI.00904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostler J, Harrison KS, Schroeder K, Thunuguntla P, Jones C. 2019. The glucocorticoid receptor (GR) stimulates herpes simplex virus 1 productive infection, in part because the infected cell protein 0 (ICP0) promoter is cooperatively transactivated by the GR and Krüppel-like transcription factor 15. J Virol 93:e02063-18. doi: 10.1128/JVI.02063-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Workman A, Zhu L, Keel BN, Smith TPL, Jones C. 2018. The Wnt signaling pathway is differentially expressed during the bovine herpesvirus 1 latency-reactivation cycle: evidence that two protein kinases associated with neuronal survival, Akt3 and BMPR2, are expressed at higher levels during latency. J Virol 92:e01937-17. doi: 10.1128/JVI.01937-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC. 2012. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of HSV-1 in neurons. PLoS Pathog 8:e1002540. doi: 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camarena V, Kobayashi M, Kim JK, Roehm P, Perez R, Gardner J, Wilson AC, Mohr I, Chao MV. 2010. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe 8:320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards TG, Bloom DC. 2019. Lund human mesencephalic (LUHMES) neuronal cell line supports herpes simplex virus 1 latency in vitro. J Virol 93:e02210-18. doi: 10.1128/JVI.02210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter D, Hsiang C, Jiang X, Osorio N, BenMohamed L, Jones C, Wechsler SL. 2015. The herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) protects cells against cold-shock-induced apoptosis by maintaining phosphorylation of protein kinase B (AKT). J Neurovirol 21:568–575. doi: 10.1007/s13365-015-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Carpenter D, Hsiang C, Wechsler SL, Jones C. 2010. The herpes simplex virus type 1 latency-associated transcript (LAT) locus inhibits apoptosis and promotes neurite sprouting in neuroblastoma cells following serum starvation by maintaining active AKT (protein kinase B). J Gen Virol 91:858–866. doi: 10.1099/vir.0.015719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Hancock M, Workman A, Doster A, Jones C. 2016. Beta-catenin, a transcription factor activated by canonical Wnt signaling, is expressed in sensory neurons of calves latently infected with bovine herpesvirus 1. J Virol 90:3148–3159. doi: 10.1128/JVI.02971-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Workman A, Eudy J, Smith L, Frizzo da Silva L, Sinani D, Bricker H, Cook E, Doster A, Jones C. 2012. Cellular transcription factors induced in trigeminal ganglia during dexamethasone-induced reactivation from latency stimulate bovine herpesvirus 1 productive infection and certain viral promoters. J Virol 86:2459–2473. doi: 10.1128/JVI.06143-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison K, Zhu L, Thunuguntla P, Jones C. 2020. Herpes simplex virus 1 regulates beta-catenin expression in TG neurons during the latency-reactivation cycle. PLoS One 15:e0230870. doi: 10.1371/journal.pone.0230870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukumoto S, Hsieh C-M, Maemura K, Layne MD, Yet S-F, Lee K-H, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee M-E. 2001. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem 276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 35.Kim S-E, Lee W-J, Choi K-Y. 2007. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cell Signal 19:511–518. doi: 10.1016/j.cellsig.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Langhammer T-S, Roolf C, Krohn S, Kretzschmar C, Huebner R, Rolfs A, Freund M, Junghanss C. 2013. PI3/K/Akt signaling interacts with Wnt/Beta-catenin signaling but does not induce an accumulation of beta-catenin in the nucleus of acute lymphoblastic leukemia cell lines. Blood 122:4886–4886. doi: 10.1182/blood.V122.21.4886.4886. [DOI] [Google Scholar]
- 37.Tenbaum SP, Ordóñez-Morán P, Puig I, Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert JD, Mendizabal L, Aguilar S, Ramón y Cajal S, Schwartz S, Vivancos A, Espín E, Rojas S, Baselga J, Tabernero J, Muñoz A, Palmer HG. 2012. β-Catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med 18:892–902. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Shemezis JR, McQuinn ER, Wang J, Sverdlov M, Chenn A. 2013. AKT activation by N-cadherin regulates beta-catenin signaling and neuronal differentiation during cortical development. Neural Dev 8:7. doi: 10.1186/1749-8104-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y. 2012. Activation of Akt/GSK-3beta/beta-catenin signaling pathway is involved in survival of neurons after traumatic brain injury in rats. Neurol Res 34:400–407. doi: 10.1179/1743132812Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 40.Manning B, Toker A. 2017. AKT/PKB signaling: navigating the network. Cell 169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev 15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 43.Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. 2009. Akt1 and Akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res 69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. 2005. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. 2007. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/Neu and MMTV-polyoma middle T transgenic mice. Cancer Res 67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 46.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM-Y, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. 2005. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 25:1869–1879. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tschopp O, Yang Z-Z, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. 2005. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 48.Xie R, Cheng M, Li M, Xiong X, Daadi M, Sapolsky RM, Zhao H. 2013. Akt isoforms differentially protect against stroke-induced neuronal injury by regulating mTOR activities. J Cereb Blood Flow Metab 33:1875–1885. doi: 10.1038/jcbfm.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diez H, Garrido JJ, Wandosell F. 2012. Specific roles of Akt isoforms in apoptosis and axon growth regulation in neurons. PLoS One 7:e32715. doi: 10.1371/journal.pone.0032715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richard-Foy H, Hager GL. 1987. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J 6:2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martelli AM, Tabellini G, Bressanin D, Ognibene A, Goto K, Cocco L, Evangelisti C. 2012. The emerging multiple roles of nuclear Akt. Biochim Biophys Acta 1823:2168–2178. doi: 10.1016/j.bbamcr.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Habib T, Sadoun A, Nader N, Suzuki S, Liu W, Jithesh PV, Kino T. 2017. AKT1 has dual actions on the glucocorticoid receptor by cooperating with 14-3-3. Mol Cell Endocrinol 439:431–441. doi: 10.1016/j.mce.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Piovan E, Yu J, Tosello V, Herranz D, Ambesi-Impiombato A, Da Silva AC, Sanchez-Martin M, Perez-Garcia A, Rigo I, Castillo M, Indraccolo S, Cross JR, de Stanchina E, Paietta E, Racevskis J, Rowe JM, Tallman MS, Basso G, Meijerink JP, Cordon-Cardo C, Califano A, Ferrando AA. 2013. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell 24:766–776. doi: 10.1016/j.ccr.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tremblay R, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M. 2010. Differentiation of mouse Neuro-2A cells into dopamine neurons. J Neurosci Methods 186:60–67. doi: 10.1016/j.jneumeth.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 55.El-Mayet F, El-Habbaa AS, El-Bagoury GF, Sharawi SSA, El-Nahas EM, Jones C. 2018. The glucocorticoid receptor and certain KRÜPPEL-like transcription factors have the potential to synergistically stimulate bovine herpesvirus 1 transcription and reactivation from latency In Kais G. (ed), Transcriptional regulation. Intech, Rijeka, Croatia. [Google Scholar]
- 56.Ramaswamy S, Nakamura N, Valquez F, Batt DB, Perera S, Roberts TM, Sellers WS. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A 96:2119–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricousse S, Gouilleux F, Fortin D, Joulin V, Richard-Foy H. 1996. Glucocorticoid and progestin receptors are differently involved in the cooperation with a structural element of the mouse mammary tumor virus promoter. Proc Natl Acad Sci U S A 93:5072–5077. doi: 10.1073/pnas.93.10.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kushnir AS, Davido DJ, Schaffer PA. 2010. Role of nuclear factor Y in stress-induced activation of the herpes simplex virus type 1 ICP0 promoter. J Virol 84:188–200. doi: 10.1128/JVI.01377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Bejjani R, Hammarlund M. 2012. Notch signaling inhibits axon regeneration. Neuron 73:268–278. doi: 10.1016/j.neuron.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassidy L, Meadows J, Catalan J, Barton S. 1997. Are stress and coping style associated with frequent recurrence of genital herpes? Genitourin Med 73:263–266. doi: 10.1136/sti.73.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rooney JT, Straus SE, Mannix ML, Wohlenberg CR, Banks S, Jagannath S, Brauer JE, Notkins AL. 1992. UV light-induced reactivation of herpes simplex virus type 2 and prevention by acyclovir. J Infect Dis 166:500–506. doi: 10.1093/infdis/166.3.500. [DOI] [PubMed] [Google Scholar]
- 62.Noisakran S, Halford WP, Veress L, Carr DJJ. 1998. Role of the hypothalamic pituitary adrenal axis and IL-6 in stress-induced reactivation of latent herpes simplex virus type 1. J Immunol 160:5441–5447. [PubMed] [Google Scholar]
- 63.Halford WP, Gebhardt BM, Carr DJJ. 1996. Mechanisms of herpes simplex virus type 1 reactivation. J Virol 70:5051–5060. doi: 10.1128/JVI.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galliher-Beckley A, Williams JG, Anthony Cidlowski J. 2011. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol 31:4663–4675. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies L, Karthikeyan N, Lynch JT, Sial E-A, Gkourtsa A, Demonacos C, Krstic-Demonacos M. 2008. Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol Endocrinol 22:1331–1344. doi: 10.1210/me.2007-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. 2013. Ultraviolet radiation regulates cortisol activity in a waveband dependent manner in human skin ex vivo. Br J Dermatol 168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cliffe A, Arbuckle JH, Vogel JL, Geden MJ, Rothbart SB, Cusack CL, Strahl BD, Kristie TM, Deshmukh M. 2015. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 18:649–658. doi: 10.1016/j.chom.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu H-L, Shiflett LA, Kobayashi M, Chao MV, Wilson AC, Mohr I, Huang TT. 2019. TOP2β-dependent nuclear DNA damage shapes extracellular growth factor responses via dynamic AKT phosphorylation to control virus latency. Mol Cell 74:466–480. doi: 10.1016/j.molcel.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheid MP, Woodgett JR. 2003. Unraveling the activation of protein kinase B/Akt. FEBS Lett 546:108–112. doi: 10.1016/S0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 70.Franke TF, Kaplan DR, Cantley LC. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435–437. doi: 10.1016/S0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 71.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. 2007. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie M, Yang A, Ma J, Wu M, Xu H, Wu K, Jin Y, Xie Y. 2018. Akt2 mediates glucocorticoid resistance in lymphoid malignancies through FoxO3a/Bim axis and serves as a direct target for resistance reversal. Cell Death Dis 9:1013. doi: 10.1038/s41419-018-1043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu NZ, Cidlowski JA. 2006. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol 16:301–307. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Lu N, Cidlowski JA. 2005. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 75.Sasse SK, Mailloux CM, Barczak AJ, Wang Q, Altonsy MO, Jain MK, Haldar SM, Gerber AN. 2013. The glucocorticoid receptor and KLF15 regulate gene expression dynamics and integrate signals through feed-forward circuitry. Mol Cell Biol 33:2104–2115. doi: 10.1128/MCB.01474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiller B, Chodankar R, Watson LC, Stallcup MR, Yamamoto KR. 2014. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol 15:418. doi: 10.1186/s13059-014-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pande P, Browne G, Padmanabhan S, Zaidi SK, Lian JB, van Wijnen AJ, Stein JL, Stein GS. 2013. Oncogenic cooperation between PI3K/Akt signaling and transcription factor Runx2 promotes the invasive properties of metastatic breast cancer cells. J Cell Physiol 228:1784–1792. doi: 10.1002/jcp.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun L, Liu L, Yang X-J, Wu Z. 2004. Akt binds prohibitin 2 and relieves its repression of MyoD and muscle differentiation. J Cell Sci 117:3021–3028. doi: 10.1242/jcs.01142. [DOI] [PubMed] [Google Scholar]
- 79.Gao H, Yu Z, Bi D, Jiang L, Cui Y, Sun J, Ma R. 2007. Akt/PKB interacts with the histone H3 methyltransferase SETDB1 and coordinates to silence gene expression. Mol Cell Biochem 305:35–44. doi: 10.1007/s11010-007-9525-3. [DOI] [PubMed] [Google Scholar]
- 80.Liu X, Wang X, Lu J. 2015. Tenuifoliside A promotes neurite outgrowth in PC12 cells via the PI3K/AKT and MEK/ERK/CREB signaling pathways. Mol Med Rep 12:7637–7642. doi: 10.3892/mmr.2015.4397. [DOI] [PubMed] [Google Scholar]
- 81.Singh AK, Kashyap MP, Tripathi VK, Singh S, Garg G, Rizvi SI. 2017. Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol Neurobiol 54:5815–5828. doi: 10.1007/s12035-016-0129-3. [DOI] [PubMed] [Google Scholar]
- 82.Zeng B, Li Y, Niu B, Wang X, Cheng Y, Zhou Z, You T, Liu Y, Wang H, Xu J. 2016. Involvement of PI3K/Akt/FoxO3a and PKA/CREB signaling pathways in the protective effect of fluoxetine against corticosterone-induced cytotoxicity in PC12 cells. J Mol Neurosci 59:567–578. doi: 10.1007/s12031-016-0779-7. [DOI] [PubMed] [Google Scholar]
- 83.Peltier J, O'Neill A, Schaffer DV. 2007. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 84.Ahn J, Rong R, Liu XS, Ye KQ. 2004. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic functions of NGF in the nucleus. EMBO J 23:3995–4006. doi: 10.1038/sj.emboj.7600392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanekura K, Hashimoto Y, Kita Y, Sasabe J, Aiso S, Nishimoto I, Matsuoka M. 2005. A Rac1/phosphatidylinositol 3-kinase/Akt3 anti-apoptotic pathway, triggered by AlsinLF, the product of the ALS2 gene, antagonizes Cu/Zn-superoxide dismutase (SOD1) mutant-induced motoneuronal cell death. J Biol Chem 280:4532–4543. doi: 10.1074/jbc.M410508200. [DOI] [PubMed] [Google Scholar]
- 86.Xiao J, Li W, Zheng X, Qi L, Wang H, Zhang C, Wan X, Zheng Y, Zhong R, Zhou X, Lu Y, Li Z, Qiu Y, Liu C, Zhang F, Zhang Y, Xu X, Yang Z, Chen H, Zhai Q, Wei B, Wang H. 2020. Targeting 7-dehydrocholesterol reductase integrates cholesterol metabolism and IRF3 activation to eliminate infection. Immunity 52:109–114. doi: 10.1016/j.immuni.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 87.Agelidis A, Shukla D. 2015. Cell entry mechanisms of HSV: what we have learned in recent years. Future Virol 10:1145–1154. doi: 10.2217/fvl.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooray S. 2004. The pivotal role of phosphatidylinositol 3-kinase–Akt signal transduction in virus survival. J Gen Virol 85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 89.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol 6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eaton H, Saffran HA, Wu FW, Quach K, Smiley JR. 2014. Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J Virol 88:7379–7388. doi: 10.1128/JVI.00712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu L, Thompson J, Ma F, Eudy J, Jones C. 2017. Effects of the synthetic corticosteroid dexamethasone on early events during bovine herpesvirus 1 productive infection. Virology 505:71–79. doi: 10.1016/j.virol.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Sinani D, Cordes E, Workman A, Thunuguntia P, Jones C. 2013. Stress-induced cellular transcription factors expressed in trigeminal ganglionic neurons stimulate the herpes simplex virus 1 ICP0 promoter. J Virol 87:13042–13047. doi: 10.1128/JVI.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El-Mayet FS, El-Habbaa AS, D’Offay J, Jones C. 2018. Synergistic activation of bovine herpesvirus 1 productive infection and viral regulatory promoters by the progesterone receptor and Krüppel-like transcription factor 15. J Virol 93:e01519-18. doi: 10.1128/JVI.01519-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinani D, Jones C. 2011. Localization of sequences in a protein (ORF2) encoded by the latency-related gene of bovine herpesvirus 1 that inhibits apoptosis and interferes with Notch1-mediated trans-activation of the bICP0 promoter. J Virol 85:12124–12133. doi: 10.1128/JVI.05478-11. [DOI] [PMC free article] [PubMed] [Google Scholar]