Myeloid cells, including monocytes, play a crucial role in immune responses to pathogens. Monocytes have also been implicated as “Trojan horses” during viral infections, carrying infectious virus particles to immune privileged sites and/or to sites protected by physical blood-tissue barriers, such as the blood-testis barrier and the blood-brain barrier. In this study, we found that myeloid cells are crucial to Zika virus (ZIKV) pathogenesis. By engineering ZIKV clones to encode myeloid-specific microRNA target sequences, viral replication was inhibited in myeloid cells by harnessing the RNA interference pathway. Severely immunodeficient mice inoculated with myeloid-restricted ZIKV did not demonstrate clinical signs of disease and survived infection. Furthermore, viral dissemination to peripheral organs was not observed in these mice. Lastly, we identified Ly6Cmid/hi murine monocytes as the major myeloid cell population that disseminates ZIKV.
KEYWORDS: myeloid, Zika virus, miRNA, monocyte, sexual transmission
ABSTRACT
Zika virus (ZIKV) can establish infection in immune privileged sites such as the testes, eye, and placenta. Whether ZIKV infection of white blood cells is required for dissemination of the virus to immune privileged sites has not been definitively shown. To assess whether initial ZIKV replication in myeloid cell populations is critical for dissemination during acute infection, recombinant ZIKVs were generated that could not replicate in these specific cells. ZIKV was cell restricted by insertion of a complementary sequence to a myeloid-specific microRNA in the 3′ untranslated region. Following inoculation of a highly sensitive immunodeficient mouse model, crucial immune parameters, such as quantification of leukocyte cell subsets, cytokine and chemokine secretion, and viremia, were assessed. Decreased neutrophil numbers in the spleen were observed during acute infection with myeloid-restricted ZIKV that precluded the generation of viremia and viral dissemination to peripheral organs. Mice inoculated with a nontarget microRNA control ZIKV demonstrated increased expression of key cytokines and chemokines critical for neutrophil and monocyte recruitment and increased neutrophil influx in the spleen. In addition, ZIKV-infected Ly6Chi monocytes were identified in vivo in the spleen. Mice inoculated with myeloid-restricted ZIKV had a decrease in Ly6Chi ZIKV RNA-positive monocytes and a lack of inflammatory cytokine production compared to mice inoculated with control ZIKV.
IMPORTANCE Myeloid cells, including monocytes, play a crucial role in immune responses to pathogens. Monocytes have also been implicated as “Trojan horses” during viral infections, carrying infectious virus particles to immune privileged sites and/or to sites protected by physical blood-tissue barriers, such as the blood-testis barrier and the blood-brain barrier. In this study, we found that myeloid cells are crucial to Zika virus (ZIKV) pathogenesis. By engineering ZIKV clones to encode myeloid-specific microRNA target sequences, viral replication was inhibited in myeloid cells by harnessing the RNA interference pathway. Severely immunodeficient mice inoculated with myeloid-restricted ZIKV did not demonstrate clinical signs of disease and survived infection. Furthermore, viral dissemination to peripheral organs was not observed in these mice. Lastly, we identified Ly6Cmid/hi murine monocytes as the major myeloid cell population that disseminates ZIKV.
INTRODUCTION
Myeloid cells are the first responders to microbial infection. Neutrophil recruitment to the site of infection induces an inflammatory response and precedes monocyte extravasation and transmigration (1). At the initial site of infection/inoculation, many flaviviruses, including dengue virus, West Nile virus (WNV), and Zika virus (ZIKV), replicate in dendritic cells and macrophages (2, 3). This causes an inflammatory response that first recruits neutrophils, followed by an influx of monocytes and monocyte-derived dendritic cells into the site of inflammation. Viruses exploit this host defense strategy by targeting these cells for infection and replication. Numerous studies support the premise that monocytes are crucial for ZIKV replication and dissemination. In humans and in nonhuman primates, intermediate (CD14+ CD16+) and nonclassical (CD14− CD16+) monocytes are productively infected by ZIKV (4–6). In mice, ZIKV inoculation results in inflammatory monocyte infiltration into the brain, testes, and placenta, but the specific subset of murine monocytes that become infected and contribute to pathogenesis have not been identified (7–10).
Myeloid cells, including monocytes and neutrophils, are intimately involved in antiviral defenses. However, these cells are also targets for viral replication. To identify whether myeloid cells are crucial for ZIKV replication, dissemination, and pathogenesis, including encephalitis and sexual transmissibility, an endogenous microRNA (miRNA) silencing approach to restrict ZIKV replication in myeloid cells in vivo was used. miRNAs are short, noncoding RNAs that posttranscriptionally regulate gene expression in all eukaryotes. miRNA precursors are processed in the nucleus and exported to the cytoplasm, where a mature miRNA is incorporated into the miRNA-induced silencing complex (miRISC). The miRISC binds an mRNA 3′ untranslated region (UTR) at target sites complementary to the miRNA sequence, resulting in translational repression, mRNA degradation, or deadenylation of target mRNAs. This regulation occurs in a cell-specific manner due to cell-specific expression of miRNAs (11). The ZIKV genome resembles mammalian mRNAs: it is a single-stranded, positive-sense RNA and has a 5′ cap. Engineering ZIKV to express complementary sequences for cell-specific miRNAs in their 3′ UTR ensures recognition of the virus by the miRISC, resulting in degradation of the viral genome in a specific cell population with no off-target effects. This strategy has been successfully used to restrict viral replication of the neurotropic virus poliovirus (12), influenza virus (13), tick-borne encephalitis/dengue chimeric vaccine candidates (14) in the central nervous system, as well as West Nile virus (15). Here, a recombinant ZIKV expressing three copies of the complementary sequence for the myeloid-specific miRNA miR-223 (ZIKV-c223) was constructed. miR-223 is conserved across all vertebrate and mammalian species. Thus, ZIKV-c223 does not replicate in myeloid cells and was used to study pathogenesis in a lethal infection model using alpha/beta interferon (IFN-α/β) and IFN-γ receptor-deficient (AG129) mice.
RESULTS
Incorporation of miRNA target sequences into the 3′ UTR of ZIKV restricts ZIKV replication in a cell-specific manner.
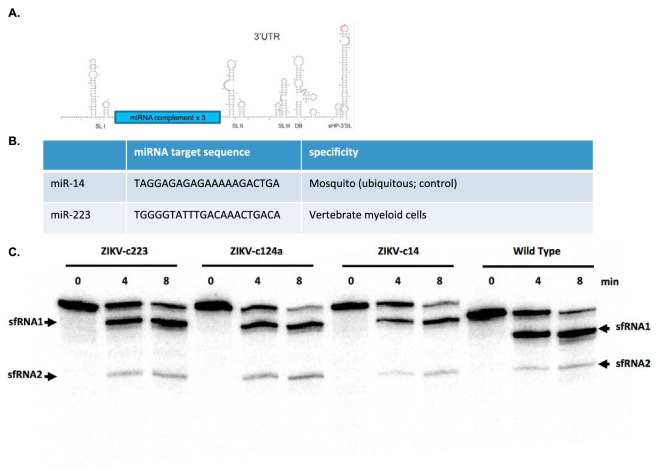
To restrict ZIKV replication in a cell-specific manner, microRNA-targeted ZIKVs were generated which could not replicate in cells expressing the cognate miRNA. The miRNA target sequences (complementary to the miRNA) were cloned into a specific site in the 3′ UTR of the PRVABC59 infectious clone system (16). The insertion site was chosen based on its proximity to, but lack of, RNA secondary elements critical for viral replication. This location was chosen to minimize the likelihood of deletion of the complementary miRNA sequence (Fig. 1A). To restrict ZIKV replication in mammalian myeloid cells, a ZIKV mutant was engineered to express the complementary miRNA sequence miR-223 (ZIKV-c223). The miRNA miR-223 is highly expressed in the bone marrow due to its involvement in differentiation of hematopoietic progenitor cells and its expression is conserved across vertebrate and mammalian species (17, 18). To control for any off-target effects caused by the insertion of the complement miRNA sequence, the complementary miRNA sequence for miR-14, a ubiquitously expressed miRNA in mosquitoes, was also cloned into the 3′ UTR of the PRVABC59 infectious clone system (ZIKV-c14) (Fig. 1B).
FIG 1.
Insertion of miRNA target sequences do not disrupt sfRNA generation. (A) Schematic of ZIKV 3′ UTR and location of miRNA target sequences. The location of target sequence insertion was chosen so that disruptions in the 3′ UTR secondary structure were avoided. Three copies of each miRNA target sequence were cloned into the 3′ UTR by Gibson assembly. (B) Sequences and description of miRNA targets used. (C) Control RNA is the entire ZIKV 3′ UTR plus the 67 nucleotides (nt) upstream of the stop codon (this was done so that there was enough resolution between input RNA and the first decay intermediate). The RNAs with the miRNA binding sites also contain the entire 3′ UTR and the upstream 67 nt. Input RNAs with miRNA sites are 561 nt, and the control input RNA is 498 nt (see reference 0 min lane). The sfRNA products (sfRNA1 and sfRNA2) are indicated by arrows.
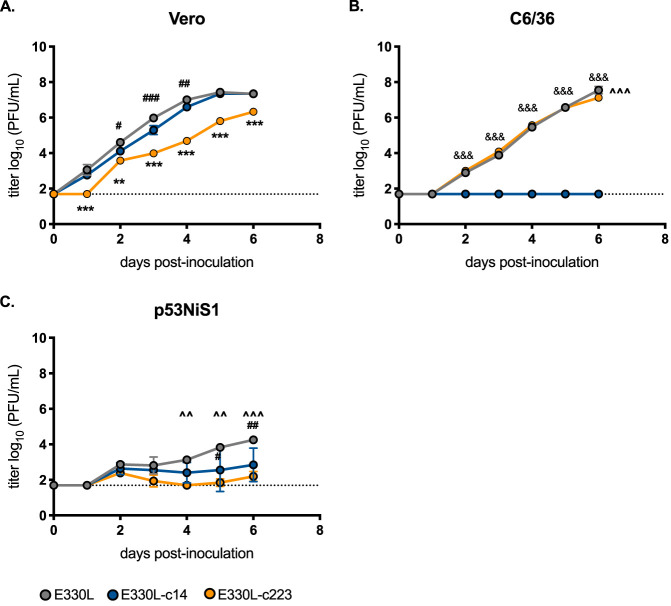
To verify that insertion of these target sequences did not disrupt viral replication, subgenomic flavivirus RNA (sfRNA) production was assessed. sfRNAs are RNA fragments arising from incomplete degradation of flaviviral genomic RNA due to host exoribonuclease (Xrn1) stallage at key secondary structures in the 3′ UTR of mosquito-borne flaviviruses (19). Thus, sfRNA production is a readout for viral replication, since sfRNAs cannot be made in the absence of viral replication. sfRNAs are vital to flavivirus replication: flaviviruses engineered to prevent the generation of sfRNAs are severely attenuated (20, 21). To verify that insertion of the miRNA target sequences did not disrupt production of sfRNAs, in vitro-transcribed RNAs from the 3′ UTR of plasmids containing the miRNA target sequences were incubated in HeLa cytoplasmic extracts. Northern blot analysis identified sfRNA-like decay intermediates similar to that observed for wild-type RNA lacking a miRNA target sequence insertion (Fig. 1C). Thus, the miRNA target sequence insertions did not result in a detectable effect on sfRNA production.miRNA target sequences were cloned into both the original ZIKV reverse genetics system based on a 2015 isolate from Puerto Rico (16). Recent work by Duggal et al. demonstrated that a nonsynonymous variant at position E-V330L resulted in less pathogenesis in immunodeficient mice (E-330L) (22). Thus, the majority of animal work was done using the E-330V infectious clone backbone (22). To demonstrate cell-specific restriction of viral replication, initial assessments were done using the E-330L infectious clone backbone (Fig. 2). In Vero cells, E330L and E330L-c14 grew to the same titer by 6 days postinoculation (dpi; 7.3 log10 PFU/ml). E330L-c223 infectious viral titers were slightly attenuated in Vero cells at all time points (Fig. 2A), but this is due to low-level expression of miR-223 in Vero cells (23). Since C6/36 cells express high levels of miR-14, E330L-c14 growth was completely abolished in C6/36 cells (Fig. 2B). E330L-c223 and E330L growth was indistinguishable until dpi 6 in C6/36 cells, when E330L reached slightly higher end titers (7.5 versus 7.1 log10 PFU/ml; P = 0.0001). Lastly, viral growth was assessed in the p53NiS1 myeloid cell line derived from a Langerhans dendritic cell skin tumor (Fig. 2C). Due to the antiviral nature of Langerhans dendritic cells (24), growth was attenuated for all three viruses, with E330L-c223 demonstrating the highest attenuation. E330L grew to higher titers than both E330L-c14 and E330L-c223 (4.3 versus 2.8 log10 PFU/ml P < 0.01), and E330L-c14 grew to higher titers than E-330L-c223, but the difference was not significant (2.8 versus 2.2 log10 PFU/ml; P = 0.3).
FIG 2.
miRNA target sequences inhibit viral replication in a cell-specific manner. The growth kinetics of E330L, E330L-c14 (insect-specific miRNA target sequence; control), and E330L-c223 (myeloid-specific miRNA target sequence) on nonhuman primate (Vero), mosquito (C6/63), and murine histiocytes (p53NiS1; mononuclear phagocytes) samples were assessed. Cells were infected with virus at an MOI of 0.1, and time point readings were taken each day. The growth kinetics were assessed by plaque assay. n = 2 biological replicates (each tested in singlicate). A two-way analysis of variance (ANOVA) was used to test for significance. Error bars represent standard deviations (SD) from the mean. E330L and E330L-c14 versus E330L-c223: **, P < 0.01; ***, P < 0.0001. E330L and E330L-c223 versus E330L-c14: &&&, P < 0.0001. E330L versus E330L-c223: ^̂, P < 0.01; ^̂̂, P < 0.0001. E330L versus E330L-c14: #, P < 0.05; ##, P < 0.01; ###, P < 0.0001.
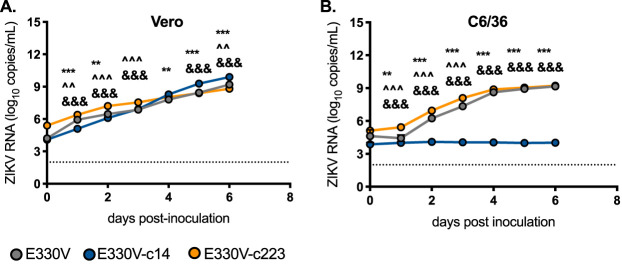
To further characterize the miRNA-restricted viruses in the E330V backbone, viral replication was assessed by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 3). Replication of E330V was assessed by qRT-PCR because the plaques formed by E330V are smaller than those formed by E330L. qRT-PCR was more robust for quantification. In Vero cells, E330V-c14 reached significantly higher viral RNA copy number by dpi 6, but the viral RNA copy number for E330V and E330V-c223 differed by only 0.7 and 1.1 log10 ZIKV RNA copies/ml, respectively (P < 0.0001). Once again, viral replication for E330V-c14 was restricted in mosquito C6/36 cells, as evidenced by the lack of an increase in viral RNA copy number across all time points. In C6/36 cells, E330V and E330V-c223 had indistinguishable endpoint viral RNA copy number titers (9.2 log10 ZIKV RNA copies/ml for both viruses).
FIG 3.
E330V-c223 and E330V-c14 replicate similarly to the parental clone in the absence of miRNA. Growth kinetics of parental clone (E-330V; gray), E330V-c14 (insect-specific miRNA target sequence; control; blue), and E330V-c223 (myeloid-specific miRNA target sequence; orange) on nonhuman primate (Vero) (A) and mosquito (C6/63) (B) cell lines. Cells were infected with virus at an MOI of 0.1, and time point readings were taken each day. Growth kinetics were assessed by qRT-PCR. n = 3 biological replicates (each tested in singlicate). A two-way ANOVA was used to test for significance. Error bars represent standard deviations from the mean. E330V versus E330V-c14: **, P < 0.01; ***, P < 0.0001. E330V versus E330V-c223: ^, P < 0.05; ^̂, P < 0.01; ^̂̂, P < 0.0001. E330V-c14 versus E330V-c223: &&&, P < 0.0001.
Myeloid restricted ZIKV does not elicit mortality or show evidence of peripheral infection of severely immunocompromised mice.
Since ZIKV infection of IFN-α/β and IFN-γ receptor-deficient (AG129) mice is lethal, this immunodeficient mouse model served as the most sensitive means to assess viral pathogenesis differences of ZIKV-miRNA inserted viruses. To initially assess pathogenesis of the control recombinant ZIKV (ZIKV-c14), 18-week-old (wko) male AG129 mice were subcutaneously (s.c.) inoculated with 103 PFU of E330V-c14 or E330L-c14. There was no significant difference in the median survival time or in viremia titers at 1, 3, 5, and 7 dpi (Fig. 4).
FIG 4.
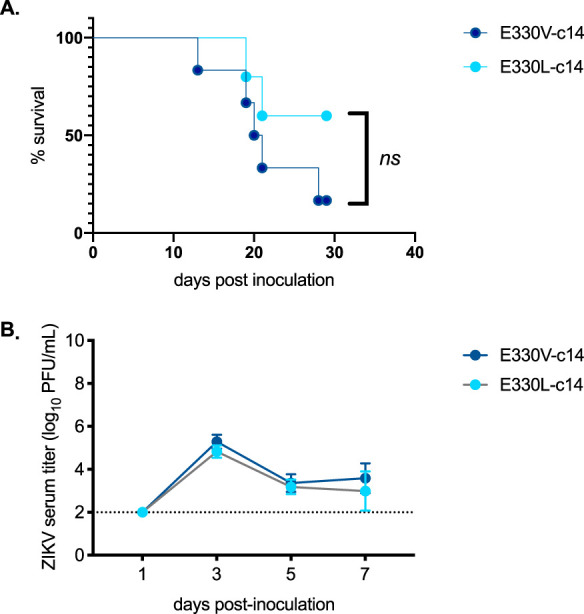
No difference in viremia or survival of mice inoculated with E330V-c14 versus E330L-c14. Groups of five to six 18-wko male AG129 mice were inoculated s.c. in their footpads with 103 PFU of either ZIKV E330V-ZIKV-14 (dark blue) or ZIKV E330L- ZIKV-14 (light blue). (A) Survival curve of mice inoculated i.c. with the ZIKV strains. No significant difference in survival of mice (a log-rank [Mantel-Cox] test was used to test for significance). (B) Mean viremia (PFU/ml) of mice inoculated with the ZIKV strains. No significant difference in mean peak viremia data (a two-way ANOVA with multiple comparisons was used to test for significance).
To determine whether ZIKV replication/growth in myeloid cells is required for pathogenesis, male AG129 mice were subcutaneously inoculated with 103 PFU of E330V, E330V-c14, or E330V-c223. The E330V backbone was used since it represents the original low-passage-number ZIKV isolate and previously demonstrated greater murine virulence; here, E330V is referred to as ZIKV (22). Of mice inoculated with ZIKV-c14, 62% succumbed to viral infection with a median survival time of 30 dpi. In contrast, 100% of the mice inoculated with ZIKV-c223 survived infection (Fig. 5A). From dpi 18 to dpi 30, significant weight loss was observed in the ZIKV-c14- inoculated mice compared to the ZIKV-c223 inoculated mice. After dpi 30, ZIKV-c14-inoculated mice did not lose further weight (Fig. 5B). Splenomegaly, quantified as spleen weight as a function of the initial weight of the mouse, was observed in mice inoculated with ZIKV-c14 on dpi 5 (P < 0.0001; Fig. 5C). In juxtaposition, there were no significant differences in spleen weight between sham-inoculated mice and mice inoculated with ZIKV-c223 (P = 0.6635).
FIG 5.
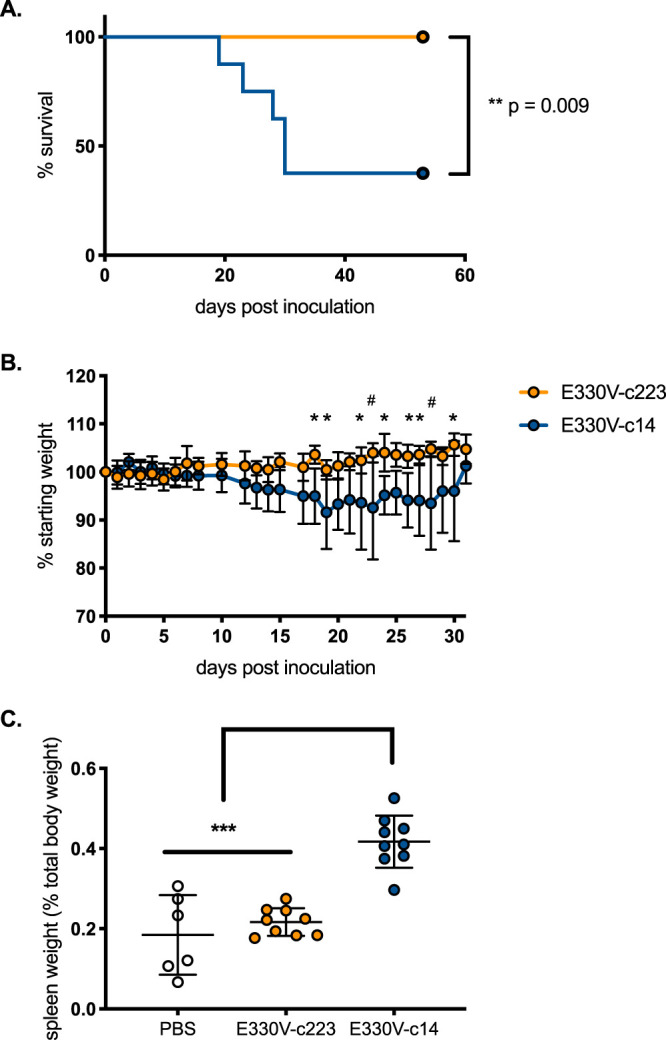
Immunodeficient mice survive inoculation with myeloid-restricted ZIKV. Groups of 32-wko male AG129 mice (n = 14/group) were s.c. inoculated with 103 PFU of E330V-c14 (blue symbols) or E330V-c223 (yellow symbols) or sham-inoculated with 10 μl of 1× PBS (white circles). (A) Percent survival of mice postinoculation. (B) Average weight of mice postinoculation, represented as a percentage of initial weight. (C) Spleen weight (as a percentage of initial body weight). Survival curves (A) were analyzed by log-rank (Mantel-Cox) test. One-way (B) and two-way (C) ANOVA was used to test for significance. Error bars represent the SD from the mean. *, P < 0.05; #, P < 0.01; ***, P < 0.0001.
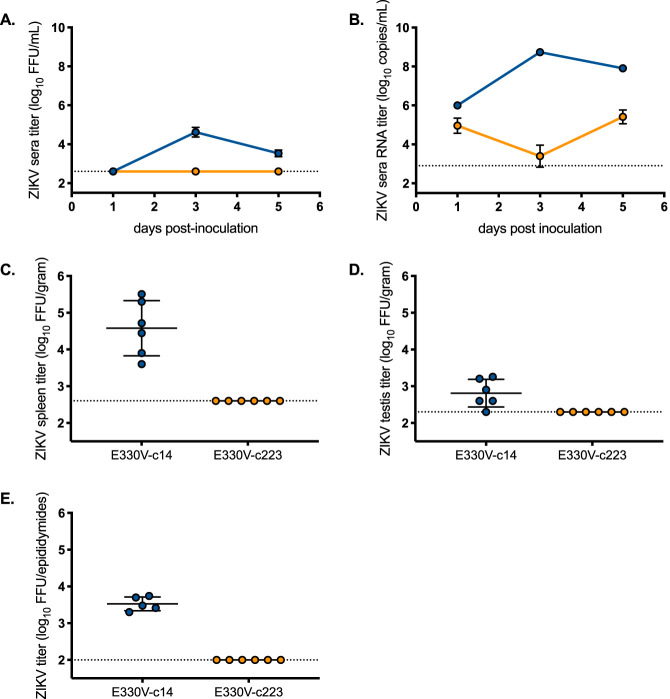
Mice inoculated with ZIKV-c14 generated viremias that reached peak titers on dpi 3. Infectious ZIKV-c14 titers were still detected in sera on dpi 5 (Fig. 6A). In marked contrast, infectious virus was never detected (limit of detection was 2.6 log10 focus-forming units [FFU]/ml), and viral RNA genome copies did not increase in the sera of ZIKV-c223-inoculated mice (Fig. 6A and B). ZIKV-c14-inoculated mice exhibited infectious virus in the spleen (4.6 log10 FFU/g tissue), testes (2.8 log10 FFU/g tissue), and epididymides (3.5 log10 FFU/epididymides) on dpi 5 (Fig. 6C to E). No infectious virus was identified in any of the organs from the ZIKV-c223-inoculated mice (limit of detection is 2.6 log10 FFU/g tissue and 2 log10 FFU/epididymides), which supports the lack of virus detected in sera. Thus, blockade of ZIKV replication in myeloid cells ablated total infection.
FIG 6.
Immunodeficient mice do not develop detectable serum viremia after inoculation with myeloid-restricted ZIKV. Infectious ZIKV titer (A) and ZIKV RNA copy number (B) in serum (n = 8 to 12 per time point). Mean titers are represented by circles. The limit of detection is represented by a dashed line (2.6 log10 FFU/ml for panel A and 2.9 log10 copies/ml for panel B). (C to E) Infectious ZIKV titers in the spleen (C), testis (D), and epididymides (E). Individual titers are represented by circles, and the mean titer is shown as a line. The limit of detection is represented by a dashed line (2.6 log10 FFU/g for spleen and testis and 2.0 log10 FFU/epididymides). Yellow circles represent mice inoculated with E330V-c223. Blue circles represent mice inoculated with E330V-c14.
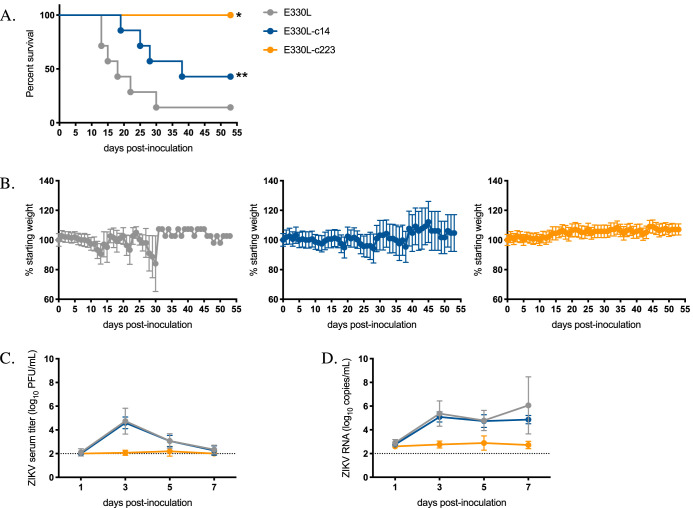
To confirm the effects of myeloid restriction on ZIKV pathogenesis were not sex dependent, these studies were repeated in AG129 female mice. In this set of studies, mice were subcutaneously inoculated with 103 PFU of E330L, E330L-c14, or E330L-c223. There was no significant difference in median survival times for E330L- and E330L-c14-inoculated mice (18 and 38 dpi, respectively; Fig. 7A). A total of 100% of female mice inoculated with E330L-c223 survived (E330L versus E330L-c223 P = 0.0013; E330L-c14 versus E330L-c223 P = 0.0039), and mice inoculated with E330L-c223 maintained their weight, in agreement with the previous study in male AG129 mice (Fig. 7A and B). Infectious virus was not detected in the sera of E330L-c223 inoculated mice (Fig. 7C). At 5 dpi, E330L-c14-inoculated female mice exhibited infectious virus in the brain, eye, spleen, ovary, uterus, and vaginal wash at titers not significantly different from the parental virus (Fig. 8). No infectious virus was detected in organs from female AG129 mice inoculated with E330L-c223, with the exception of a single mouse that exhibited viral titers near the limit of detection for the eye (2.3 log10 PFU/g, limit of detection 2 log10 PFU/g) and ovary and uterus (2.2 and 2.5 log10 PFU/g, limit of detection 1.7 log10 PFU/g). The viral titer was too low to sequence the virus to determine whether the miRNA target sequence had been deleted or mutated.
FIG 7.
Female AG129 mice are not viremic after inoculation with myeloid-restricted ZIKV. Groups of seven 16- to 18-wko female AG129 mice were inoculated s.c. in their footpads with 103 PFU of either E330L (gray symbols), E330L-c14 (dark blue symbols), or E330L-c223 (yellow symbols). (A) Percent survival of mice postinoculation. The survival curves for E330L and E330L-c14 groups were significantly different than the survival curve for E330L-c223 (significance was tested by the log-rank (Mantel-Cox) test; *, P = 0.0437 [E330L-c223 versus E330L-c14]; **, P = 0.0039 [E330L-c223 versus E330L]; survival curves were compared by the log-rank [Mantel-Cox] test). (B) Average weight of mice postinoculation, represented as a percentage of initial weight. (C and D) Infectious ZIKV titer (C) and ZIKV RNA copy number (D) in serum. Mean titers are represented by circles. The limit of detection is represented by a dashed line (2.0 log10 PFU/ml and 2.0 log10 RNA copies/ml). Two-way ANOVA with multiple comparisons was used to test for significance (B to D).
FIG 8.
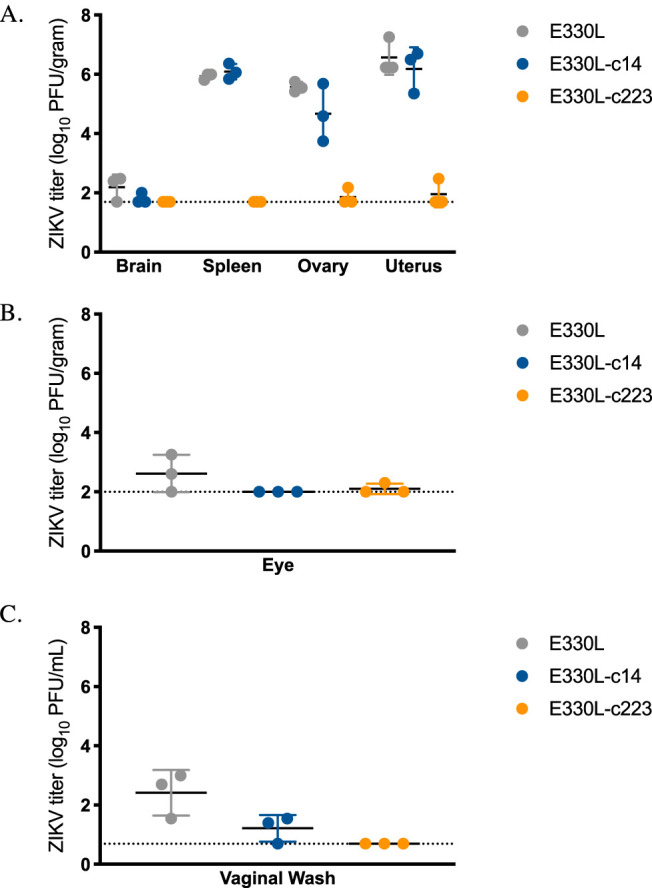
ZIKV-miR-223 does not disseminate to peripheral tissues in female AG129 mice. Groups of seven 16- to 18-wko female AG129 mice were inoculated s.c. in their footpads with 103 PFU of either E330L, E330L-c14, or E330L-c223. Infectious ZIKV titers in the brain, spleen, ovary, and uterus (A), eye (B), and vaginal wash (C) on dpi 5. The limit of detection is represented by a dashed line (2.0 log10 PFU/ml for panels A and B; 0.39 log10 PFU/ml for panel C).
Lastly, to assess sexual transmission potential, male AG129 mice were s.c. inoculated with E330L-c14 or E330L-c223 and mated with immunocompetent naive female CD-1 mice. Semen samples were collected from 7 to 21 dpi, which has previously been shown to be the infectious period for sexual transmission (7, 8, 25). As observed previously in AG129 mice inoculated with ZIKV isolates, infectious E330L-c14 was found in semen for the 3 weeks after s.c. inoculation (Fig. 9). Of the collected semen samples, 83% contained infectious virus, with titers reaching as high as 4.9 log10 PFU/ejaculate. E330L-c223-inoculated mice mated 26 times during the 3 weeks following inoculation, and only 15% (four semen samples from three different mice) contained infectious virus, with titers ranging from 3.2 to 4.1 log10 PFU/ejaculate. Viral RNA was extracted from eight plaques from three of the four semen samples and the 3′ UTR was sequenced. All three samples showed deletion of all three copies of the target sequence for miR-223. Viral RNA was also extracted from eight plaques from four unique E330L-c14-inoculated mice (from dpi 13 to 19) and sequenced. In contrast to the E330L-c223 results, all three copies of the miR-14 target sequence were present in the 3′ UTR. These data demonstrate how restrictive the myeloid-specific miRNA sequence is: only viruses that managed to delete all three copies of the miR-223 target sequence were able to disseminate to the male reproductive tract.
FIG 9.
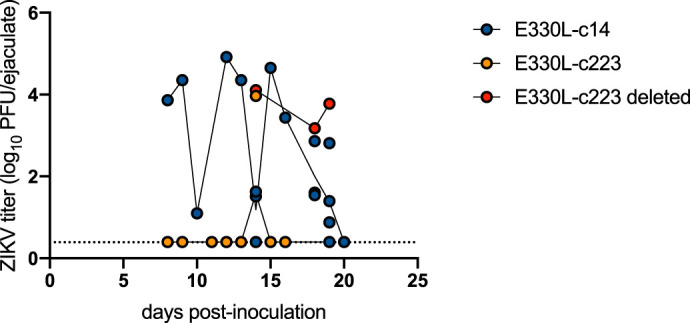
Myeloid-restricted ZIKV is not sexually transmitted. Groups of eight 17-wko male AG129 mice were inoculated s.c. in their footpads with 103 PFU of either E330L-c14 (dark blue symbols) or E330L-c223 (yellow symbols). Samples that were sequenced and shown to have a complete deletion of the miR-223 target sequence are show in red symbols. Viral titers in collected ejaculates were determined. The limit of detection is represented by a dashed line (0.4 log10 PFU/ejaculate).
Mice inoculated with myeloid-restricted ZIKV have decreased neutrophil influx into the spleen.
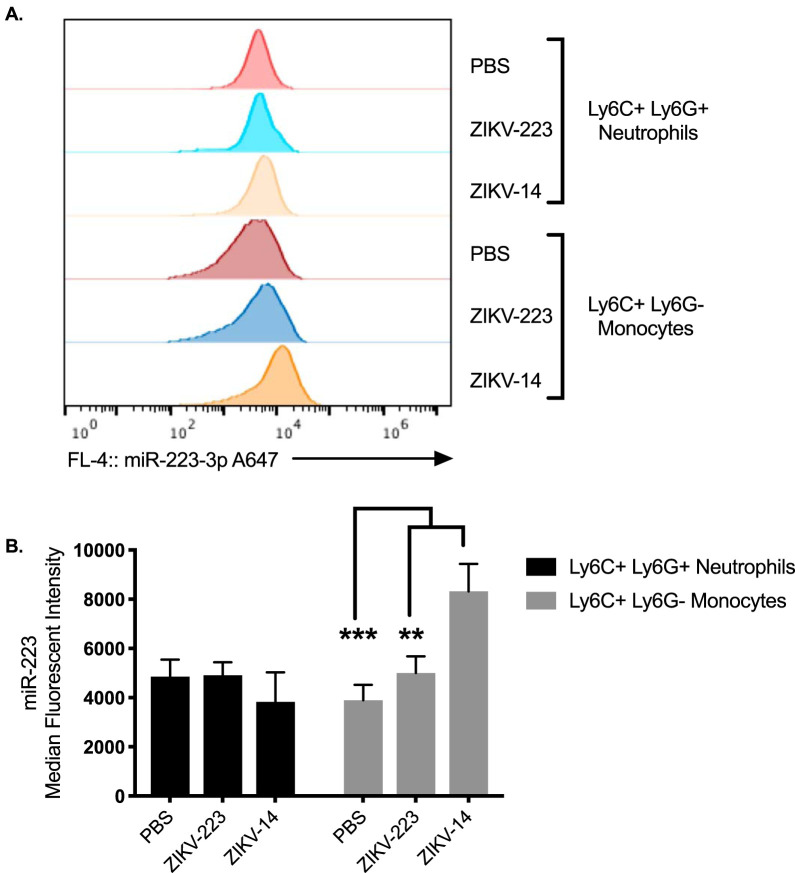
In vitro models and in vivo murine studies have demonstrated that some miRNAs have increased expression following viral infection (26, 27). To determine whether ZIKV infection modulates miR-223, miR-223 expression levels were quantified by median fluorescence intensity (MFI) by PrimeFlow. Infection with ZIKV-c14 significantly increased miR-223 MFI expression in monocytes, compared to miR-223 MFI expression in monocytes from ZIKV-c223- and PBS-sham-inoculated mice (P = 0.0012 and P < 0.0001, respectively; Fig. 10). miR-223 was highly expressed in neutrophils and did not change expression upon infection. Thus, miR-223 expression is stable and highly expressed in monocytes and neutrophils.
FIG 10.
miR-223 expression increases in monocytes following productive infection. (A) MFI histograms of miR-223 expression in Ly6C+ Ly6G+ neutrophils and Ly6C+ Ly6G– monocytes in mice sham inoculated with PBS or s.c. inoculated with ZIKV-14 or ZIKV-223. (B) Quantification of MFI of miR-223 in Ly6C+ Ly6G+ neutrophils and Ly6C+ Ly6G– monocytes in mice sham inoculated with PBS or s.c. inoculated with ZIKV-14 or ZIKV-223. Two-way ANOVA was used to test for significance. **, P < 0.01; ***, P < 0.0001.
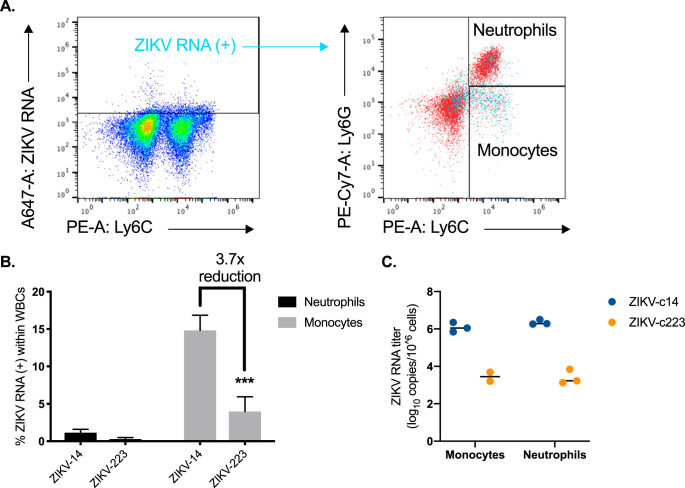
To determine whether inflammatory Ly6Cmid-hi monocytes in the spleen contained ZIKV in vivo, cells were stained for ZIKV by RNA flow cytometry (Thermo Fisher). At 5 dpi, there was a significantly higher fraction of Ly6Cmid-hi monocytes staining positive for ZIKV RNA [ZIKV RNA(+)] in ZIKV-c14- versus ZIKV-c223-inoculated mice (Fig. 11A and B). Approximately 1% of neutrophils stained positive for viral RNA [ZIKV RNA(+)] in ZIKV-c14-inoculated mice, but there was no staining above background for ZIKV RNA(+) neutrophils in ZIKV-c223-inoculated mice. Splenic Ly6Cmid-hi monocytes and neutrophils from ZIKV-c14- and ZIKV-c223-inoculated mice were isolated by fluorescent activated cell sorting (FACS), and qRT-PCR was performed on the sorted cells (Fig. 11C). Neutrophils and Ly6Cmid-hi monocytes from ZIKV-c14-inoculated mice had significantly higher levels of viral RNA versus the same cell subsets from ZIKV-c223-inoculated mice (approximately 106 ZIKV genome copies per million cells versus <103 ZIKV genome copies per million cells, respectively).
FIG 11.
Significant decrease in ZIKV RNA(+) monocytes after inoculation with myeloid-restricted ZIKV. (A) Representative dot plots showing splenic cells from ZIKV-14-inoculated mice stained with probe set to ZIKV RNA(+) and back-gated onto neutrophils and monocytes. Uninfected control mice were used to set gates for ZIKV RNA(+) cells. (B) Percentage of ZIKV RNA(+) cells within white blood cell (WBC) population. (C) Five days after inoculation with either ZIKV-c14 or ZIKV-c223, three mice from each group were euthanized, and single cell suspensions of the spleen were stained for monocytes and neutrophils and sorted by FACS. Total RNA was extracted from sorted cells, and viral RNA was quantified by qRT-PCR. A two-way ANOVA was used to test for significance (B and C). ***, P < 0.0001.
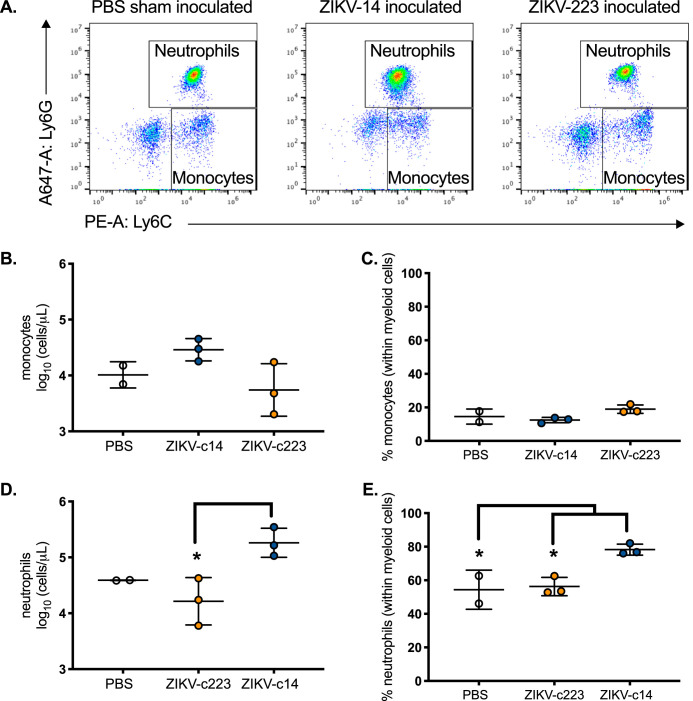
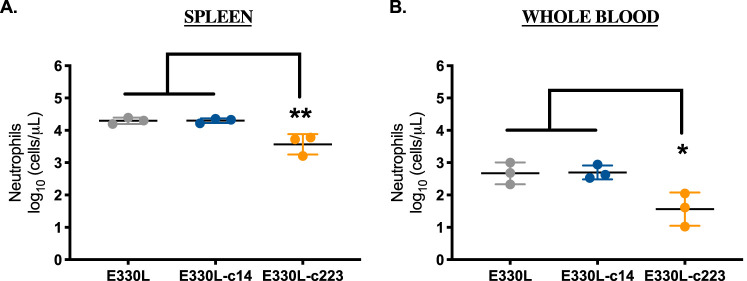
Next, myeloid cell influx into the spleen was quantified by flow cytometry (Fig. 12A). ZIKV-c14-inoculated mice showed a slight increase in total splenic monocyte numbers by dpi 5 compared to phosphate-buffered saline (PBS)-sham-inoculated mice and ZIKV-c223-inoculated mice (P = 0.1; Fig. 12B and C). Total neutrophil numbers and the fraction of neutrophils (within white blood cells) were significantly increased in the spleen of ZIKV-c14-inoculated mice (P = 0.02; Fig. 12D and E). ZIKV-c223-inoculated mice more closely resembled PBS-sham-inoculated mice and thus did not demonstrate mobilization of neutrophils into the spleen by 5 dpi. The total neutrophil counts in the spleen and whole blood at 5 dpi were also significantly reduced in female AG129 mice inoculated with E330L-c223 compared to mice inoculated with the parental clone or E330L-c14 (Fig. 13).
FIG 12.
Significant decrease in neutrophil influx in mice inoculated with myeloid-restricted ZIKV. (A) Representative FACS plots from PBS-sham-inoculated (left), ZIKV-miR-14-inoculated (center), or ZIKV-miR-223-inoculated male AG129 mice. The neutrophils are Ly6G+ Ly6C+; the monocytes are Ly6G– Ly6C+. (B to E) Flow cytometric quantification of the absolute number (B and D) and percentage (C and E) of monocytes and neutrophils in the spleen 5 days after PBS (clear), ZIKV-miR-14 (blue), or ZIKV-miR-223 (orange) inoculation. n = 2 for PBS and n = 3 for ZIKV-miR-14 and ZIKV-miR-223. *, P < 0.05 (one-way ANOVA with a Tukey’s post hoc test). The data represent means and SD.
FIG 13.
Significant decrease in neutrophil influx in mice inoculated with myeloid-restricted ZIKV. Flow cytometric quantification of the absolute number of neutrophils in the spleen (A) and blood (B) 5 days after inoculation. n = 2 for PBS and n = 3 for E330L-c14- and E330L-c223-inoculated mice. One-way ANOVA with a Tukey’s post hoc test. The data represent means and SD. *, P < 0.05; **, P < 0.01.
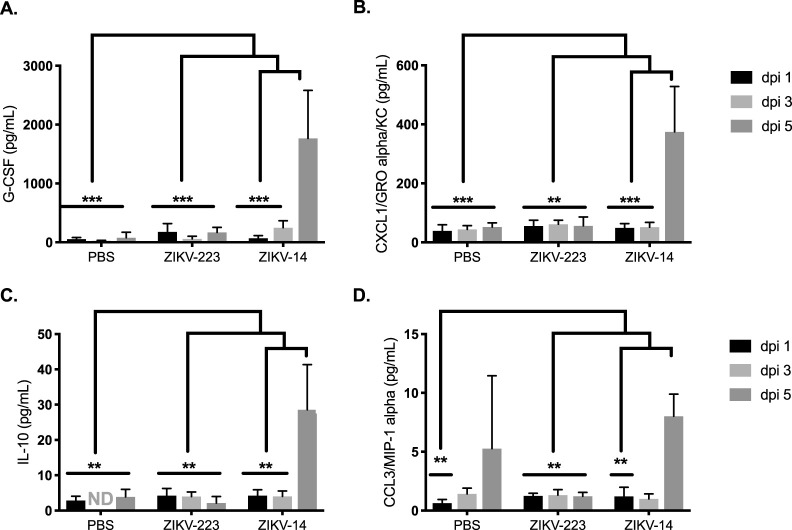
Serum levels of select cytokines and chemokines associated with mobilization and chemoattraction of monocytes and neutrophils were quantified by multiplexed Luminex assay. G-CSF, CXCL1/KC, and CCL3/MIP-1α cytokines were all significantly increased by dpi 5 in the sera of ZIKV-c14-inoculated mice compared to the other two groups of mice (Fig. 14). The levels of these cytokines were similar between PBS-sham-inoculated mice and ZIKV-c223-inoculated mice at all time points (dpi 1, 3, and 5). Interleukin-10 (IL-10) is an anti-inflammatory cytokine that acts as a countermeasure to limit inflammation during acute infection. IL-10 was significantly increased in ZIKV-c14-inoculated mice by dpi 5, compared to dpi 1 and dpi 3 sera from this group. Also, IL-10 was significantly increased in the ZIKV-c14-inoculated mice compared to PBS-sham-inoculated mice and ZIKV-c223-inoculated mice. No differences in IL-10 levels were found between PBS-sham-inoculated or ZIKV-c223-inoculated mice at any of the time points. Lastly, IFN-γ and tumor necrosis factor alpha were significantly increased in the sera of ZIKV-c14-inoculated mice (dpi 3 versus dpi 5; P < 0.01). These cytokines were below the limit of quantitation in the sera of ZIKV-c223- or PBS-sham-inoculated mice.
FIG 14.
Cytokines and chemokines involved in myeloid mobilization and chemoattraction are increased in the sera of ZIKV-14-inoculated mice. The levels of cytokines and chemokines in sera from AG129 mice inoculated with ZIKV-c14 or ZIKV-c223 or sham inoculated with PBS at 1, 3, and 5 dpi are shown. Significant differences in cytokine/chemokine production from mice inoculated with ZIKV-c14 were observed for G-CSF (A), CXCL1/GROα/KC (B), IL-10 (C), and CCL3/MIP-1α (D). Data were obtained after measurements from six mice per group, are expressed as means ± the SD, and were analyzed using two-way ANOVA. This experiment was repeated (three mice per group), and similar results were obtained. **, P < 0.01; ***, P < 0.0001.
Inoculation with myeloid restricted ZIKV protects mice from lethal challenge with MR766.
Protection from lethal challenge was compared between mice initially challenged with the miRNA restricted viruses. Surviving mice from the ZIKV-c14-inoculated group (n = 3) and from the ZIKV-c223-inoculated group (n = 8) were s.c. inoculated with a lethal dose of 103 PFU of the virulent African ZIKV genotype MR766. PBS-sham-inoculated mice (n = 3) were also challenged with the same dose of MR766. All PBS-sham-inoculated mice were euthanized at 7 days postchallenge due to signs of significant morbidity. All mice in the ZIKV-c14-inoculated group survived to the experimental endpoint, which was 21 days postchallenge. Six of the eight mice in the ZIKV-c223-inoculated group survived challenge (Fig. 15A). Two mice were euthanized 7 days postchallenge due to weight loss (Fig. 15B).
FIG 15.
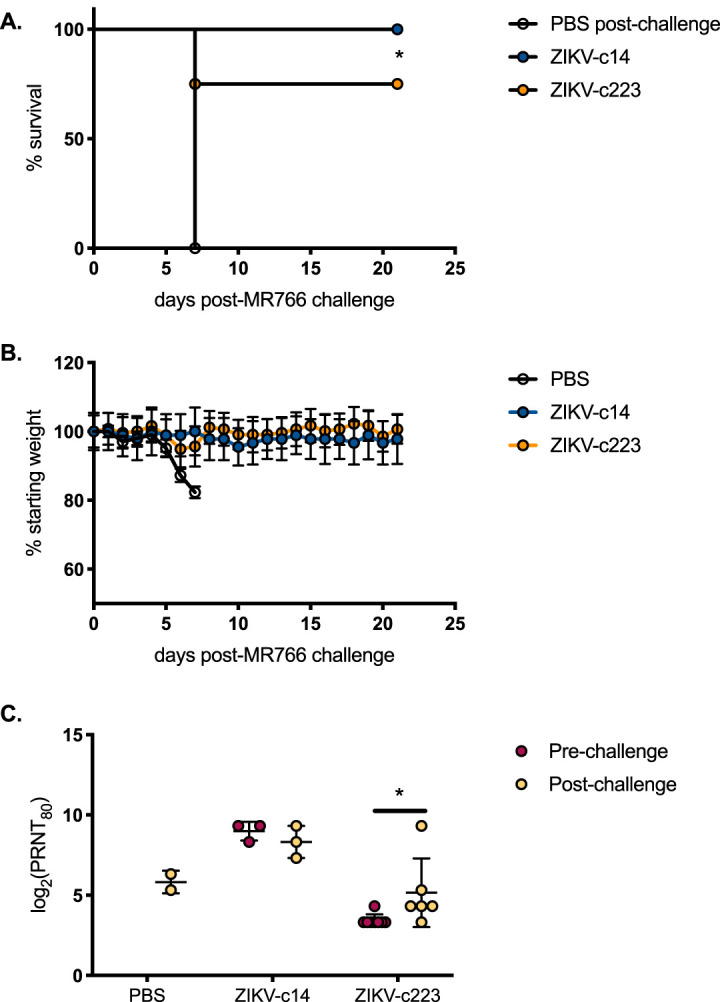
Mice inoculated with myeloid-restricted ZIKV produce ZIKV-neutralizing antibodies. Mice surviving s.c. inoculation with E330V-c14 (blue symbols; n = 3) or E330V-c223 (yellow symbols; n = 8) and all mice previously sham inoculated with 10 μl of 1× PBS (white circles; n = 3) were s.c. challenged with 103 PFU of MR766. (A) Percent survival of mice postinoculation. (B) Average weight of mice postinoculation, represented as a percentage of initial weight. (C) Neutralization potency of each serum sample against PRVABC59. Survival curves were compared by log-rank (Mantel-Cox) test (A). A Tukey’s multiple-comparison test was used to detect a significant difference between ZIKV-14 pre- and postchallenge and ZIKV-223 pre- and postchallenge (C). *, P < 0.05.
Plaque reduction neutralization tests (PRNTs) were performed on sera collected from mice 21 days postchallenge with MR766. After challenge, the reciprocal mean titers were 7.8, 9.0, and 6.2 log2 for PBS-sham-, ZIKV-c14-, and ZIKV-c223-inoculated mice, respectively (Fig. 15C). The PRNT90 titers for ZIKV-c14 pre- and postchallenge were not statistically different from one another. The PRNT90 titers did significantly differ between the ZIKV-c14-inoculated group and the ZIKV-c223-inoculated mice at both pre- and postchallenge. ZIKV-c223-inoculated mice had a significant, albeit slight, increase in PRNT90 titers after challenge (4.3 versus 6.2 log2), despite the lack of viremia and lack of infectious virus dissemination to peripheral organs.
DISCUSSION
Complete knockout of myeloid cells is lethal in mice, and it is impossible to completely deplete these populations by use of specific antibodies. Thus, to study the role of these immune cells in ZIKV dissemination and pathogenesis, an alternative approach is required. By utilizing the power of endogenous miRNA silencing, recombinant ZIKV incapable of replicating in myeloid cells (ZIKV-c223) was generated. Using a highly sensitive mouse model, we demonstrated that neutrophil recruitment and Ly6Cmid-hi monocytes are essential for ZIKV dissemination and pathogenesis in IFN-α/β and IFN-γ receptor-deficient mice.
Many viruses, including arboviruses, infect monocytes; infected monocytes can circulate in the blood and thus are potential vehicles for viral dissemination. Dengue virus replicates in classical dendritic cells and macrophages at the site of the inoculation, leading to monocyte infiltration, which are major target for dengue virus replication (28). West Nile virus (WNV) infection of mice demonstrated that neutrophils are robustly recruited to the site of inoculation (29). In this study, depletion of neutrophils prior to WNV inoculation not only reduced WNV RNA levels in sera but also significantly increased survival. Myeloid cells control WNV infection via type I interferons, WNV is still able to replicate in monocytes. This has been shown in crow, mice, and equine models and through ex vivo infection of human monocytes (30–33). As demonstrated above, many flaviviruses infect monocytes, but only ZIKV gains access to the female and male reproductive tracts. Thus, ZIKV-infected monocytes can act as Trojan horses specifically for these reproductive tissues. Do monocytes have different “Trojan horse” tissue specificities depending on which pathogen infects them? In this study, we propose a murine model whereby active ZIKV replication in myeloid cells at the site of inoculation is required for eventual dissemination of virus to peripheral organs (Fig. 16). Inoculation of AG129 mice, a lethal mouse model, with ZIKV-c223 resulted in no morbidity or mortality, a complete lack of infectious virus in the sera, and no dissemination of virus to peripheral organs. In addition, neutrophil influx into the spleen was not observed, and key cytokines and chemokines involved in leukocyte recruitment during inflammation were observed at levels indistinguishable from baseline PBS-sham-inoculated controls. Despite the lack of virus replication, most mice inoculated with ZIKV-c223 were protected from challenge with a lethal dose of African genotype ZIKV MR766. All the mice inoculated with control recombinant ZIKV (ZIKV-c14) survived challenge, demonstrating that the slight attenuation of ZIKV-c223 leads to less protection overall in the vaccinated mice. This trade-off between attenuation and efficacy has been demonstrated in many studies of live-attenuated vaccines (34).
FIG 16.
Restricting ZIKV replication in myeloid cells inhibits ZIKV pathogenesis. Insertion of three copies of the miRNA miR-223 complement sequence into the 3′ UTR of ZIKV blocks replication in myeloid cells. Insertion of a nontarget control sequence (the complement of miR-14, an insect-specific miRNA) does not block ZIKV replication in myeloid cells. After inoculation of miR-223-restricted ZIKV in a sensitive mouse model, viremia is not observed, and virus does not disseminate to peripheral organs. Neutrophil numbers and key cytokines and chemokines involved in leukocyte recruitment during inflammation were observed at levels indistinguishable from baseline PBS-sham-inoculated controls.
During human infection, monocytes have been proposed to act as “Trojan horses” for ZIKV, allowing the virus direct access to immune privileged sites such as the testes and placenta. In mice, ZIKV-infected monocytes have been shown in the brain (22) and eye (35, 36), in addition to the immune privileged sites mentioned above. ZIKV can directly infect monocytes from humans, nonhuman primates, and mice (4–6, 8, 37). Furthermore, ex vivo assays using human cerebellar microvascular endothelial cell transwells and cerebral organoids have shown that monocytes transmigrate faster if infected with ZIKV (38). Faster dissemination kinetics were attributed to increased expression of adhesion molecules on monocytes and higher arresting properties of monocytes in the bloodstream. In humans, classical monocytes (CD14+ CD16−) are the most prevalent class of monocytes in the blood. Upon inflammation, classical monocytes differentiate into intermediate (CD14+ CD16+) and then into nonclassical (CD14+ CD16hi) monocytes. Subtypes of human peripheral blood mononuclear cells undergo a dramatic shift in frequency after ex vivo infection with ZIKV, with “intermediate” CD14+ CD16+ and “nonclassical” CD14+ CD16− monocytes expanding (4, 6).
In mice, the counterparts to classical and intermediate monocytes are Ly6Cmid and Ly6Chi monocytes, which are proinflammatory and whose main function is phagocytosis. Using a highly sensitive fluorescence in situ hybridization flow cytometry assay, we demonstrate in vivo that Ly6Cmid/hi monocytes harbor ZIKV RNA. Furthermore, monocytes from mice inoculated with myeloid-restricted ZIKV are not infected, do not suffer from viral pathogenesis, and do not succumb to infection.
Although AG129 mice are devoid of type I and type II interferon signaling, they are still able to mount B-cell and T-cell responses to viral infections (39–42). In addition, since ZIKV inoculation is lethal in AG129 mice, any decrease in pathogenesis or mortality is significant and warrants future study in immunocompetent models, such as in human STAT2 knock-in mice (43). Using endogenous miRNA silencing to create recombinant ZIKVs that are restricted from replicating in specific cellular populations is a promising vaccine strategy; however, additional work needs to be done to determine the likelihood of mutations that either delete or alter the miRNA complementary sequences, ablating miRNA-mediated silencing. In the present study, E330L-c223 revertants, in which the miRNA target sequence had been deleted, were possible. Furthermore, of all the tissues tested for infectious virus, E330L-c223 revertants were only found in the male reproductive tract, an immune privileged site. Thus, outside immune privileged sites, the immune system exerts a greater restriction on the myeloid-restricted virus, preventing sufficient levels of viral replication and persistence that would result in reversions.
Myeloid cells, especially monocytes, are critical for antiviral defense. However, they are also essential for dissemination of many viruses. Activating type I and type II interferon responses in these cells by viral replication and preventing viral dissemination in these same cells must be balanced. For example, Eastern equine encephalitis virus (EEEV) has multiple target sites for miR-142-3p (another myeloid-specific miRNA) in its 3′ UTR, which leads to limited replication in myeloid cells. If these sites are deleted, the virus strongly induces host innate immune signaling pathways, resulting in decreased disease severity and increased survival (44). In this example, preventing overt activation of the immune system by limiting replication in myeloid cells is a strategy that benefits EEEV.
MATERIALS AND METHODS
Plasmid construction for miRNA-restricted viruses.
To construct miRNA-restricted viruses, we used the previously described two-plasmid infectious clone system (16). Single-strand ultramers containing three repeat copies of each miRNA target sequence (complement sequence of miRNA) flanked by 31 nucleotides of overlap with the second plasmid were ordered from IDT (Duluth, GA). The miRNA target sequences used in this study were the myeloid-specific miR-223 miRNA (ZIKV-c223) and the ubiquitously expressed mosquito miRNA miR-14 (ZIKV-c14; control). The 3′ plasmid was linearized into two fragments using PCR (Q5 high-fidelity polymerase; NEB, Ipswich, MA). Gibson cloning was used to join the plasmid fragments and 0.5 μM concentrations of single-strand ultramers. Gibson products were transformed into OneShot Top10 chemically competent Escherichia coli (Thermo Fisher, USA) and colonies were screened by PCR. Sanger sequencing was used to verify the plasmid contained all three repeats of the miRNA target sequence insertion at plasmid nucleotide position 7578. This corresponds to nucleotide position 10476 in the 3′ UTR of the ZIKV genome (PRVABC59, KU501215).
RNA transfection and virus recovery.
The 5′ plasmid was linearized using the restriction enzymes ApaLI and BamHI-HF, and the 3′ plasmid was linearized using the restriction enzymes ApaLI and EcoRI-HF. After ligation of the 5′ and 3′ linearized plasmids, RNA was transcribed from the full-length infectious clone DNA. Transcribed RNA was transfected into Vero (for ZIKV-c14 and wild-type viruses) or C6/36 (for ZIKV-c223 virus) cells using MessengerMax Lipofectamine transfection reagent, according to the manufacturer’s instructions. Cell supernatant was harvested from both Vero cells and C6/36 cells when a cytopathic effect was observed in Vero cells. Supernatant was centrifuged to remove cell debris and supplemented to 20% FBS. Titers of all recombinant viruses were determined by plaque assay on Vero cells. All recovered recombinant viruses were full-length sequenced: viral RNA was extracted from titered stocks and Qiagen one-step RT-PCR was used to generate overlapping cDNA amplicons spanning the entire genome. Sanger sequencing was performed on each amplicon.
Reconstituted Xrn1 assay to generate sfRNA.
DNA segments containing the entire 3′ UTR, along with 67 upstream nucleotides of the translation stop codon, were amplified and gel purified from plasmids containing the wild-type, miR-223, miR-124a, or miR-14 complementary sequences. The primers used for amplification were 5′-GATCATCGAATTTAGGTGACACTATAGGTACATGGACTACCTATCCACCC and 5′-AGACCCATGGATTTCCCCA. The forward primer contained a SP6 promoter element at the 5′ portion of the oligonucleotide sequence. RNAs were generated by in vitro transcription using SP6 polymerase in the presence of a 10× molar excess of 5′ GMP to GTP in the reaction in order to obtain transcripts with a 5′ monophosphate. Approximately 30 fmol of radioactive RNA was incubated in HeLa cytoplasmic S100 extracts prepared as described previously (45) under conditions that strongly favor 5′-to-3′ exonucleolytic decay by Xrn1 (46, 47) for 4 and 8 min after enzymatic exposure. Reaction products were analyzed on 5% polyacrylamide gels containing 7 M urea and visualized by phosphorimaging.
Growth kinetics.
Nonhuman primate (Vero), mouse (p53NiS1), and mosquito (C6/36) cell lines were used to assess viral replication. Cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 5% CO2 and 37°C, with the exception that C6/36 cells were grown at 28°C. To assess viral replication, cells were seeded in 12-well tissue culture treated plates and a day later infected with recombinant virus at a multiplicity of infection (MOI) of 0.1. Supernatants were harvested daily from dpi 0 through dpi 6, and infectious titers were quantified by plaque assay on Vero cells or viral RNA quantified by qRT-PCR.
AG129 pathogenesis.
An IFN-α/β and IFN-γ receptor-deficient (AG129) mouse colony was bred in-house, and the receptor knockout genotype of the mice was confirmed as described previously (7). Female and male mice were inoculated subcutaneously (s.c.) in the rear footpad with 103 PFU of recombinant ZIKVs diluted in PBS (mouse ages ranged from 16 to 32 weeks). Mice were monitored daily. At experimental time points and endpoint, the mice were humanely euthanized by isoflurane-induced deep anesthesia, followed by cervical dislocation. Mice were not perfused prior to necropsy and tissues were collected at time of euthanasia. All animal studies were conducted under approved IACUC protocols at the Centers for Disease Control and Prevention. All protocols and practices for the handling and manipulation of mice were in accordance with the guidelines of the American Veterinary Medical Association for humane treatment of laboratory animals.
Challenge with MR766 and neutralizing antibody titrations (PRNT90).
PBS-sham-inoculated mice and all mice surviving inoculation with ZIKV-c14 and ZIKV-c223 were challenged by s.c. inoculation of 103 PFU of MR766 on dpi 54. Mice were monitored daily and euthanized when they met clinical criteria. Blood was collected in serum separator tubes by submandibular bleed on dpi 53 (the day prior to challenge with MR766) or by cardiac puncture at time of euthanasia. Collected sera was spun at 3,500 × g for 5 min and frozen at –80°C. For PRNT90 determination, a portion of the serum was heat inactivated at 56°C for 1 h, serially diluted 2-fold, and incubated with ∼100 PFU of PRVABC59, and plaque assays were then performed on the diluted serum/virus mixed samples (48). Sera from PBS-sham-inoculated mice at dpi 53 were used as negative controls, and a 90% reduction in plaques in these negative-control sera was defined as the neutralization activity threshold.
Virus titration by plaque assay and focus-forming assay.
Tissues (testes, seminal vesicles, spleens, and brains) were weighed and homogenized in an equal volume of BA-1 media. These tissues were then clarified by centrifugation at 10,000 × g for 4 min. To quantify viral titer in the epididymides, an epididymis was minced in 250 μl of BA-1 media. Semen samples from male AG129 mice were collected as previously described (7, 25). In brief, male mice were housed individually during the day and, beginning on dpi 7, three female CD-1 mice were introduced into each cage every evening. The following morning, mated females were identified by the presence of a copulatory plug and were euthanized. Semen was collected by flushing the uterine horns with 500 μl of BA-1 media. All semen samples were stored at –80°C until processed for infectious virus and viral RNA. All tissue homogenates and semen samples were serially diluted in BA-1 media and plated on Vero cells for quantification by either plaque assay (7, 8) or focus-forming assay (49, 50). For the focus-forming assay, Vero cells were seeded at 2 × 104 cells/well in a 96-well flat-bottom tissue culture treated plate in 100 μl of complete DMEM. The following day, 50 μl of serially diluted tissue homogenate was added to the wells. Plates were incubated at 37°C for 1 h, and then 150 μl of prewarmed methylcellulose diluted 2-fold in Barry’s Ye-Lah medium was added to each well. The plates were incubated at 37°C for 48 h. After 48 h, the methylcellulose was removed, and the cells were fixed in 4% paraformaldehyde. Fixed cells were then blocked in 2.5% nonfat milk in PBST (BBL FTA hemagglutination buffer plus 0.5% Triton X-100 and 0.05% Tween 20). After blocking, the cells were incubated with 4G2 antibody (diluted 1:800 in milk block buffer). Cells were washed and then incubated in horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) at a 1:250 dilution in milk block buffer. All incubations were carried out at 37°C for 1 h each. Lastly, an ImmPACT NovaRED kit (Vector Labs) was used to detect the HRP substrate. An AID enzyme-linked immunospot assay reader was used to visualize and capture images of plates. Focus-forming units were manually counted using ImageJ.
Flow cytometry and fluorescence activated cell sorting.
Spleens were removed from euthanized AG129 mice and weighed. Using the plunger end of a syringe, spleens were pressed through a 70-μm strainer attached to a 50-ml conical tube. Strainers were rinsed with 10 ml of 1× PBS to collect all cells. The resulting cell suspension was centrifuged at 500 × g for 5 min at 4°C. The cell pellet was resuspended in 1 ml of 1× RBC lysis buffer (BioLegend) for ∼2 min, before being diluted out in excess 1× PBS. The cells were washed in 1× PBS before being stained with Zombie Green live/dead stain ([BioLegend] [1:1,000] dilution in 1× PBS) for 15 min in the dark at 4°C, followed by a single wash. The cells were then incubated with FcBlock (BioLegend) at a 1:50 dilution in 1× PBS for 15 min at room temperature. Surface staining was performed in the dark at 4°C. Cells were stained with anti-Ly6CPE (clone HK1.4), anti-Ly6GAF647 (IA8), anti-CD11bPE-Cy7 (clone M1/70), anti-CD19FITC (clone 1D3/CD19), anti-NK-1.1AF488, and anti-CD3AF488 (clone 17A2). A dump channel was used to exclude dead cells, B cells, T cells, and NK cells.
To stain for ZIKV RNA and the miRNA miR-223, cells were prepared as described above and treated according to PrimeFlow RNA assay kit instructions (Thermo Fisher Scientific). To detect miRNA expression, the PrimeFlow microRNA pretreatment buffer was used in combination with the PrimeFlow RNA assay, following the instructions provided by the manufacturer. Probe sets targeting the positive-strand ZIKV RNA and miR-223 miRNA (designed and synthesized by Thermo Fisher Scientific; assay ID VF-19981 and VB6-3224052-VC) were used at the recommended dilution.
An Accuri C6 flow cytometer equipped with blue and red lasers was used to analyze fixed and stained cells. For FACS, cells were surface stained (as described above). Live cells were sorted on a FACSAria III with four lasers (violet, blue, yellow, and red lasers) and 16 detection channels. Single-color controls were used to set compensation, and fluorescence minus one controls were used to set gates. Two-way sorting was used to collect neutrophils and monocytes. The average postsort purity was 89.4% for monocytes and 98.6% for neutrophils. Sorted cells were kept on ice and then spun down and frozen at –80°C. A fraction of the sorted cells was used for plaque assay to determine viral titer in monocytes and neutrophils, and the remaining fraction was used for total RNA extraction.
Luminex.
Magnetic Luminex assays were used to simultaneously quantify protein levels of cytokines and chemokines in mouse sera. All magnetic microparticle-analyte-specific antibodies and standards were purchased from R&D Systems (LXSAMSM). The assays were performed according to manufacturer’s instructions. The data were collected on a Luminex 200 instrument that was verified and calibrated before each use. Fifty magnetic beads were collected per region, and the MFI was measured. Standard curves were calculated for each analyte by using a Logistic 5p weighted-curve fit using xPONENT software (Luminex 200 system). Sera was diluted 1:4 in diluent RD6-52.
RNA quantification.
RNA was extracted from 50 μl of diluted sera using the MagMAX viral RNA isolation kit (Ambion). RNA was extracted from sorted monocytes and neutrophils using the miRVana miRNA isolation kit (Thermo Fisher). ZIKV RNA was quantified using real-time qRT-PCR primers and probe, as described previously (51). A standard curve was generated by in vitro transcription (AmpliScribe T7 high-yield transcription kit) of a plasmid containing a fragment of ZIKV spanning genomic nucleotides at positions 859 to 1278. The detection limit for this assay was 2.0 log10 RNA copies per ml. Next, 25 μl of mouse sera was diluted 1:1 by adding 25 μl of BA-1.
ACKNOWLEDGMENTS
This study was funded by National Institutes of Health grant AI 129593 (G.D.E. and A.C.B.).
We thank the Colorado State University Flow Cytometry and Cell Sorting Facility for their help in analyzing samples. We thank Division of Vector-Borne Diseases staff members Jason Velez for cell culture support and Sean Masters for excellent contributions to animal husbandry and animal care needs throughout this study.
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the U.S. Agency for International Development.
REFERENCES
- 1.Soehnlein O, Lindbom L, Weber C. 2009. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 2.Johnston LJ, Halliday GM, King NJ. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol 114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 3.Laureti M, Narayanan D, Rodriguez-Andres J, Fazakerley JK, Kedzierski L. 2018. Flavivirus receptors: diversity, identity, and cell entry. Front Immunol 9:2180. doi: 10.3389/fimmu.2018.02180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michlmayr D, Andrade P, Gonzalez K, Balmaseda A, Harris E. 2017. CD14+ CD16+ monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol 2:1462–1470. doi: 10.1038/s41564-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor MA, Tisoncik-Go J, Lewis TB, Miller CJ, Bratt D, Moats CR, Edlefsen PT, Smedley J, Klatt NR, Gale M Jr, Fuller DH. 2018. Early cellular innate immune responses drive Zika viral persistence and tissue tropism in pigtail macaques. Nat Commun 9:3371. doi: 10.1038/s41467-018-05826-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, Yoo JS, Ge J, Cheng G, Bonnin A, Nielsen-Saines K, Brasil P, Jung JU. 2017. Asian Zika virus strains target CD14+ blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol 2:1558–1570. doi: 10.1038/s41564-017-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang GJ, Bowen RA, Brault AC. 2017. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep 18:1751–1760. doi: 10.1016/j.celrep.2017.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald EM, Duggal NK, Ritter JM, Brault AC. 2018. Infection of epididymal epithelial cells and leukocytes drives seminal shedding of Zika virus in a mouse model. PLoS Negl Trop Dis 12:e0006691. doi: 10.1371/journal.pntd.0006691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hastings AK, Uraki R, Gaitsch H, Dhaliwal K, Stanley S, Sproch H, Williamson E, MacNeil T, Marin-Lopez A, Hwang J, Wang Y, Grover JR, Fikrig E. 2019. Aedes aegypti NeSt1 protein enhances Zika virus pathogenesis by activating neutrophils. J Virol 93:e00395-19. doi: 10.1128/JVI.00395-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu YH, Tseng CK, Lin CK, Wei CK, Lee JC, Young KC. 2018. ICR suckling mouse model of Zika virus infection for disease modeling and drug validation. PLoS Negl Trop Dis 12:e0006848. doi: 10.1371/journal.pntd.0006848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preall JB, Sontheimer EJ. 2005. RNAi: RISC gets loaded. Cell 123:543–545. doi: 10.1016/j.cell.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Barnes D, Kunitomi M, Vignuzzi M, Saksela K, Andino R. 2008. Harnessing endogenous miRNAs to control virus tissue tropism as a strategy for developing attenuated virus vaccines. Cell Host Microbe 4:239–248. doi: 10.1016/j.chom.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waring BM, Sjaastad LE, Fiege JK, Fay EJ, Reyes I, Moriarity B, Langlois RA. 2017. MicroRNA-based attenuation of influenza virus across susceptible hosts. J Virol 92:e01741-17. doi: 10.1128/JVI.01741-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiss BL, Maximova OA, Pletnev AG. 2011. Insertion of microRNA targets into the flavivirus genome alters its highly neurovirulent phenotype. J Virol 85:1464–1472. doi: 10.1128/JVI.02091-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brostoff T, Pesavento PA, Barker CM, Kenney JL, Dietrich EA, Duggal NK, Bosco-Lauth AM, Brault AC. 2016. MicroRNA reduction of neuronal West Nile virus replication attenuates and affords a protective immune response in mice. Vaccine 34:5366–5375. doi: 10.1016/j.vaccine.2016.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weger-Lucarelli J, Duggal NK, Bullard-Feibelman K, Veselinovic M, Romo H, Nguyen C, Ruckert C, Brault AC, Bowen RA, Stenglein M, Geiss BJ, Ebel GD. 2017. Development and characterization of recombinant virus generated from a New World Zika virus infectious clone. J Virol 91:e00172-17. doi: 10.1128/JVI.00172-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsitsiou E, Lindsay MA. 2009. microRNAs and the immune response. Curr Opin Pharmacol 9:514–520. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CZ, Li L, Lodish HF, Bartel DP. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science 303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama BM, Laurence HM, Massey AR, Costantino DA, Xie X, Yang Y, Shi PY, Nix JC, Beckham JD, Kieft JS. 2016. Zika virus produces noncoding RNAs using a multi-pseudoknot structure that confounds a cellular exonuclease. Science 354:1148–1152. doi: 10.1126/science.aah3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roby JA, Pijlman GP, Wilusz J, Khromykh AA. 2014. Noncoding subgenomic flavivirus RNA: multiple functions in West Nile virus pathogenesis and modulation of host responses. Viruses 6:404–427. doi: 10.3390/v6020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon SL, Dodd BJ, Brackney DE, Wilusz CJ, Ebel GD, Wilusz J. 2015. Flavivirus sfRNA suppresses antiviral RNA interference in cultured cells and mosquitoes and directly interacts with the RNAi machinery. Virology 485:322–329. doi: 10.1016/j.virol.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggal NK, McDonald EM, Weger-Lucarelli J, Hawks SA, Ritter JM, Romo H, Ebel GD, Brault AC. 2019. Mutations present in a low-passage Zika virus isolate result in attenuated pathogenesis in mice. Virology 530:19–26. doi: 10.1016/j.virol.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu N, Gao N, Fan D, Wei J, Zhang J, An J. 2014. miR-223 inhibits dengue virus replication by negatively regulating the microtubule-destabilizing protein STMN1 in EAhy926 cells. Microbes Infect 16:911–922. doi: 10.1016/j.micinf.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham AL, Carbone F, Geijtenbeek TB. 2008. Langerhans cells and viral immunity. Eur J Immunol 38:2377–2385. doi: 10.1002/eji.200838521. [DOI] [PubMed] [Google Scholar]
- 25.McDonald EM, Duggal NK, Brault AC. 2017. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl Trop Dis 11:e0005990. doi: 10.1371/journal.pntd.0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Song Y, He L, Wan X, Lai L, Dai F, Liu Y, Wang Q. 2016. MicroRNA-223 promotes type I interferon production in antiviral innate immunity by targeting forkhead box protein O3 (FOXO3). J Biol Chem 291:14706–14716. doi: 10.1074/jbc.M115.700252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preusse M, Schughart K, Pessler F. 2017. Host genetic background strongly affects pulmonary microRNA expression before and during influenza A virus infection. Front Immunol 8:246. doi: 10.3389/fimmu.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid MA, Harris E. 2014. Monocyte recruitment to the dermis and differentiation to dendritic cells increases the targets for dengue virus replication. PLoS Pathog 10:e1004541. doi: 10.1371/journal.ppat.1004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. 2010. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis 202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto AK, Ramos HJ, Wu X, Aggarwal S, Shrestha B, Gorman M, Kim KY, Suthar MS, Atkinson JP, Gale M Jr, Diamond MS. 2014. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. PLoS Pathog 10:e1004086. doi: 10.1371/journal.ppat.1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich EA, Bowen RA, Brault AC. 2015. An ex vivo avian leukocyte culture model for West Nile virus infection. J Virol Methods 218:19–22. doi: 10.1016/j.jviromet.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rios M, Zhang MJ, Grinev A, Srinivasan K, Daniel S, Wood O, Hewlett IK, Dayton AI. 2006. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion 46:659–667. doi: 10.1111/j.1537-2995.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Tapia D, Loiacono CM, Kleiboeker SB. 2006. Replication of West Nile virus in equine peripheral blood mononuclear cells. Vet Immunol Immunopathol 110:229–244. doi: 10.1016/j.vetimm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Lauring AS, Jones JO, Andino R. 2010. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol 28:573–579. doi: 10.1038/nbt.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manangeeswaran M, Kielczewski JL, Sen HN, Xu BC, Ireland DDC, McWilliams IL, Chan CC, Caspi RR, Verthelyi D. 2018. Zika virus infection causes persistent chorioretinal lesions. Emerg Microbes Infect 7:96. doi: 10.1038/s41426-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Ruckert C, Govero J, Noguchi KK, Ebel GD, Diamond MS, Apte RS. 2016. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep 16:3208–3218. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lum FM, Lin C, Susova OY, Teo TH, Fong SW, Mak TM, Lee LK, Chong CY, Lye DCB, Lin RTP, Merits A, Leo YS, Ng LFP. 2017. A sensitive method for detecting Zika virus antigen in patients’ whole-blood specimens as an alternative diagnostic approach. J Infect Dis 216:182–190. doi: 10.1093/infdis/jix276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayala-Nunez NV, Follain G, Delalande F, Hirschler A, Partiot E, Hale GL, Bollweg BC, Roels J, Chazal M, Bakoa F, Carocci M, Bourdoulous S, Faklaris O, Zaki SR, Eckly A, Uring-Lambert B, Doussau F, Cianferani S, Carapito C, Jacobs FMJ, Jouvenet N, Goetz JG, Gaudin R. 2019. Zika virus enhances monocyte adhesion and transmigration favoring viral dissemination to neural cells. Nat Commun 10:4430. doi: 10.1038/s41467-019-12408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumathy K, Kulkarni B, Gondu RK, Ponnuru SK, Bonguram N, Eligeti R, Gadiyaram S, Praturi U, Chougule B, Karunakaran L, Ella KM. 2017. Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep 7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zompi S, Santich BH, Beatty PR, Harris E. 2012. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J Immunol 188:404–416. doi: 10.4049/jimmunol.1102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs J, Chu H, O’Day P, Pyles R, Bourne N, Das SC, Milligan GN, Barrett AD, Partidos CD, Osorio JE. 2014. Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice. Vaccine 32:6537–6543. doi: 10.1016/j.vaccine.2014.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partidos CD, Weger J, Brewoo J, Seymour R, Borland EM, Ledermann JP, Powers AM, Weaver SC, Stinchcomb DT, Osorio JE. 2011. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine 29:3067–3073. doi: 10.1016/j.vaccine.2011.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorman MJ, Caine EA, Zaitsev K, Begley MC, Weger-Lucarelli J, Uccellini MB, Tripathi S, Morrison J, Yount BL, Dinnon KH III, Ruckert C, Young MC, Zhu Z, Robertson SJ, McNally KL, Ye J, Cao B, Mysorekar IU, Ebel GD, Baric RS, Best SM, Artyomov MN, Garcia-Sastre A, Diamond MS. 2018. An immunocompetent mouse model of Zika virus infection. Cell Host Microbe 23:672–685.e6. doi: 10.1016/j.chom.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trobaugh DW, Gardner CL, Sun C, Haddow AD, Wang E, Chapnik E, Mildner A, Weaver SC, Ryman KD, Klimstra WB. 2014. RNA viruses can hijack vertebrate microRNAs to suppress innate immunity. Nature 506:245–248. doi: 10.1038/nature12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ford LP, Watson J, Keene JD, Wilusz J. 1999. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev 13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukherjee D, Gao M, O’Connor JP, Raijmakers R, Pruijn G, Lutz CS, Wilusz J. 2002. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J 21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon SL, Anderson JR, Kumagai Y, Wilusz CJ, Akira S, Khromykh AA, Wilusz J. 2012. A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability. RNA 18:2029–2040. doi: 10.1261/rna.034330.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duggal NK, Ritter JM, McDonald EM, Romo H, Guirakhoo F, Davis BS, Chang GJ, Brault AC. 2017. Differential neurovirulence of African and Asian genotype Zika virus isolates in outbred immunocompetent mice. Am J Trop Med Hyg 97:1410–1417. doi: 10.4269/ajtmh.17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. 2016. Zika virus infects human placental macrophages. Cell Host Microbe 20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George SL, Wong MA, Dube TJ, Boroughs KL, Stovall JL, Luy BE, Haller AA, Osorio JE, Eggemeyer LM, Irby-Moore S, Frey SE, Huang CY, Stinchcomb DT. 2015. Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis 212:1032–1041. doi: 10.1093/infdis/jiv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]