Abstract
Cutaneous melanoma is a deadly skin cancer whose aggressiveness is directly linked to its metastatic potency. Despite remarkable breakthroughs in term of treatments with the emergence of targeted therapy and immunotherapy, the prognosis for metastatic patients remains uncertain mainly because of resistances. Better understanding the mechanisms responsible for melanoma progression is therefore essential to uncover new therapeutic targets. Interestingly, the sphingolipid metabolism is dysregulated in melanoma and is associated with melanoma progression and resistance to treatment. This review summarises the impact of the sphingolipid metabolism on melanoma from the initiation to metastatic dissemination with emphasis on melanoma plasticity, immune responses and resistance to treatments.
Keywords: cancer, ceramide, gangliosides, immunotherapy, metastasis, phenotype switching, sphingosine 1-phosphate
1. Introduction
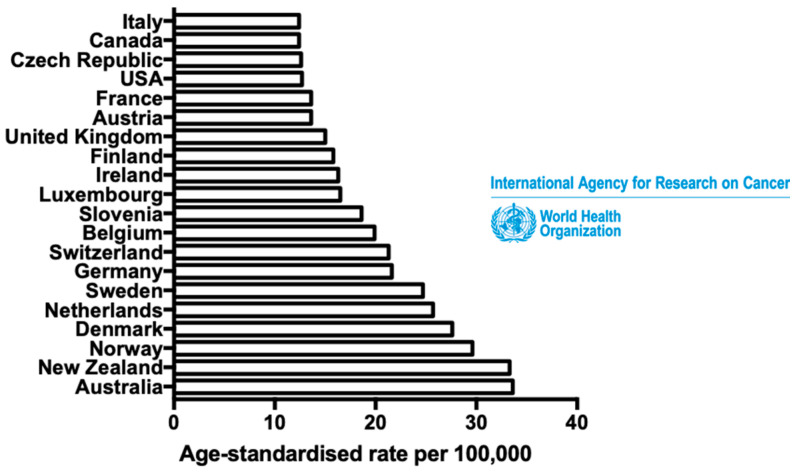
Cutaneous melanoma is a skin cancer whose incidence is increasing significantly worldwide (Figure 1). Even though melanoma is not frequent, accounting for less than 5% of skin cancers, it can be very aggressive and causes more than 75% of all skin cancer deaths [1] (Figure 1). Despite significant improvement of treatment strategies in the last decade, owing both to the emergence of BRAF- or MEK-targeted therapies and checkpoint blockade immunotherapies (i.e., anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA4) and anti-programmed cell death-1 (PD-1)), the prognosis for patients with metastatic melanoma remains uncertain, predominantly due to treatment failures and recurrences [2]. Fortunately, melanoma is usually curable by excisional surgery if detected at an early stage, with a high five-year survival rate [3,4]. Thus, a better understanding of melanoma progression processes, before dissemination, is a major public health issue in order to discern new therapeutic targets.
Figure 1.
Cutaneous melanoma: the 19th most common cancer worldwide. Estimated age-standardised incidence rates of cutaneous melanoma in the most affected countries in 2018, for both sexes and all ages. Data from the International Agency for Research on Cancer (World Health Organisation).
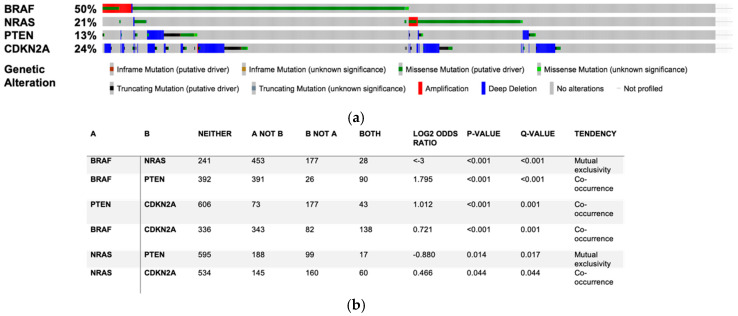
Melanoma arises from melanocytes, i.e., melanin-producing neural crest-derived cells, which are located at the junction between the epidermis and the dermis [5]. The initial stage of melanomagenesis corresponds to a radial-growth phase (RGP), in which melanoma cells invade laterally but stay confined into the epidermis. This stage is followed by a vertical-growth phase (VGP), in which melanoma cells invade the dermis and are able to reach blood vessels. Then, an extravasation stage, corresponding to the release of melanoma cells from the blood circulation into new tissues, leads to the formation of metastatic niches [6,7,8]. Melanomagenesis requires at least two key events. The first is activating mutations in oncogenes such as BRAF or NRAS. BRAF mutations are found in 50% of melanoma patients (Figure 2) and the V600E mutation accounts for approximately 75% of all BRAF mutations detected in cutaneous melanoma [9]. Conversely, the most common NRAS mutations, i.e., Q61R and Q61K, affect about 20% of melanoma patients [10]. Since BRAF and NRAS mutations are mutually exclusives, these driver mutations lead to constitutive activation of the mitogen-activated protein kinase (MAPK) pathway and aberrant cancer cell proliferation in approximately 70% of patients (Figure 2) [11]. The second event is illustrated by the loss of expression of key tumour suppressor genes such as PTEN or cell cycle checkpoint regulators such as CDKN2A, which occur in 13% and 24% of patients, respectively (Figure 2). These genetic changes can bypass oncogene-induced senescence (OIS) processes and cause the immortalisation of tumour cells [4,7,12] (Figure 2).
Figure 2.
BRAF, NRAS, PTEN and CDKN2A are the most frequently mutated genes in cutaneous melanoma. Mutation rate, genetic alteration (a) and mutual exclusivity (b) for BRAF, NRAS, PTEN and CDKN2A mutations observed in 1635 samples from 1584 patients included in 12 studies analysed on cBioportal for cancer genomics (https://www.cbioportal.org).
Metabolic reprogramming is also crucial for melanomagenesis. Indeed, a shift from mitochondrial oxidative phosphorylation to cytoplasmic anaerobic glycolysis, known as Warburg effect, is required for metastatic dissemination [13]. The present review focuses on alterations in the metabolism of sphingolipids (SL). Interestingly, several key enzymes of the glycolytic pathway can be severely affected by changes in SL metabolism in melanoma. For instance, C16-ceramide, which is the major long-chain ceramide in melanocytes and melanoma cells, impairs pyruvate kinase, hexokinase and LDH activities, consequently altering cellular glycolysis and inhibiting melanoma progression [14].
How modulations of the SL metabolism affect dermatologic diseases have long been studied [15] and accumulating evidence demonstrates the presence of alterations in the ceramide metabolism in melanoma cells. This article aims at providing a comprehensive overview of the effects dysregulations of the SL metabolism have on melanomagenesis, melanoma progression, immunity and resistance to treatment, especially linked to the phenotype switching.
2. Alterations of Sphingolipid Metabolism in Melanoma
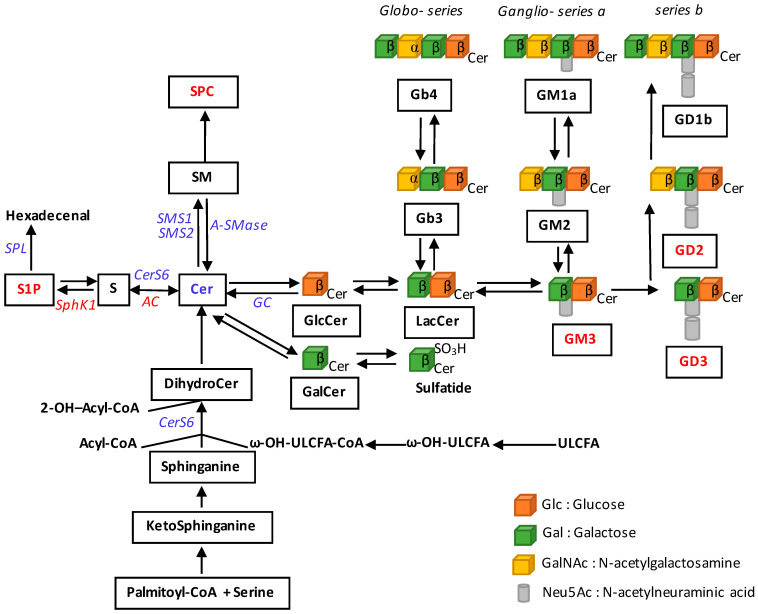
The main dysregulation affecting the SL metabolism in melanoma cells is a trend toward a reduction of ceramide, which promotes cell death (for review, see [16]). This is associated with changes in the expression and/or activity of a number of enzymes as well as the accumulation of tumour-promoting metabolites, which include sphingosine 1-phosphate (S1P) and gangliosides (Figure 3).
Figure 3.
Multiple dysregulations of sphingolipid metabolism in melanoma. SL metabolites or SL-metabolising enzymes whose levels or expression are altered in melanoma, are mentioned. Decreases are indicated in blue and increases in red. AC, acid ceramidase; Cer, ceramide; CERS, ceramide synthase; CoA, coenzyme A; DihydroCer, dihydroceramide; GalCer, galactosylceramide; GC, glucosylceramidase; GlcCer, glucosylceramide; LacCer, lactosylceramide; S, sphingosine; S1P, sphingosine 1-phosphate; SM, sphingomyelin; SMases, sphingomyelinases; SMS, sphingomyelin synthase; SPC, sphingosylphosphorylcholine; SphK, sphingosine kinase; SPL, S1P lyase; ULCFA, ultralong chain fatty acids.
The impact of SL metabolism dysregulations in melanoma cell lines and/or patients is summarised in Table 1. For instance, a low expression of the ceramide synthase 6 (CerS6) in melanoma cells is related to malignant behaviours as demonstrated in WM35, WM451 and SKMEL28 human melanoma cell lines [17]. In addition, acid ceramidase (AC), encoded by the ASAH1 gene, which hydrolyses ceramide into sphingosine, is expressed at high levels in melanocytes and proliferative melanoma cells, as observed in vitro as well as in biopsies from patients with stage II melanoma [18]. ASAH1 expression was: (i) higher in human melanoma cell lines exhibiting a proliferative phenotype as compared to invasive ones; and (ii) reduced at the invasive front on tumour specimens from melanoma patients [19]. Sphingosine kinase 1 (SphK1), which phosphorylates sphingosine to produce sphingosine-1-phosphate (S1P), also shows increased expression and/or activity in melanoma cells compared to melanocytes, not only in human and murine cell lines [20,21] but also in human biopsies [22]. Collectively, these findings suggest a shift of the S1P-ceramide balance towards S1P production in melanoma cells. In accordance, the expression of SGPL1 gene, encoding for S1P lyase (SPL), is downregulated in melanoma cell lines when compared to adult or juvenile melanocytes, suggesting that SGPL1 might be downregulated during melanomagenesis [23].
Table 1.
Impact of SL and SL-metabolising enzymes dysregulations on melanoma cell lines and patients.
| SL or SL-Metabolising Enzymes | Dysregulation | Cell Lines or Patients | Effects | Refs |
|---|---|---|---|---|
| CerS6 | Decreased | WM35, WM451 and SKMEL28 human melanoma cells | Malignant behaviour | [17] |
| AC | Decreased | Proliferative and invasive human melanoma cells | Pro-invasive | [18,19] |
| SphK1 | Increased | Murine and human melanoma cells and biopsies | Pro-tumoral Immunosuppressive signature |
[20,21,22] |
| SPL | Decreased | Human melanoma cells | Resistance to chemotherapy Increased proliferation |
[23] |
| GD3 | Increased | GD3+ human melanoma cells with c-Yes inhibition | Reduced malignancy | [24,25,26] |
| SMS1 | Decreased | Human biopsies | Worse prognosis | [29] |
| SPC | Increased | Mel-Ab and human melanocytes | Stimulate melanomagenesis Hypopigmentation in melanocytes |
[30,31,32,33] |
| A-SMase | Decreased | Primary melanomas and lymph node metastases Pigmented murine and human melanomas |
Inverse correlation with melanin content | [34,35] |
Ceramide can also be converted into more complex SL, such as gangliosides. For instance, mono- and disialoganglioside levels are very high in human melanoma cells and tissues, especially GD3 [24]. Interestingly, levels of this latter ganglioside were correlated to the expression of the c-Yes tyrosine kinase whose activity is known to increase in melanoma cells as compared to melanocytes [25] and whose inhibition reduced the malignant potential of GD3+ melanoma cells only [26].
Alterations of the ceramide metabolism also include changes in the expression of sphingomyelin synthase 1 (SMS1), which is encoded by the SGMS1 gene and catalyses the transformation of ceramide into sphingomyelin (SM) [27,28]. SGMS1 is expressed at low levels in most of the human melanoma biopsies and low SGMS1 expression is associated with a worse prognosis in metastatic melanoma patients. Of interest, a weak expression of SMS1 was shown not to be associated with an intracellular accumulation of ceramide, most likely due to its conversion into glucosylceramide (GlcCer) through GlcCer synthase (GCS). Consequently, 6 out of 10 human melanoma cell lines exhibited higher levels of GlcCer than SM [29]. Moreover, SM can also be transformed into sphingosylphosphorylcholine (SPC) by a yet uncharacterised SM deacylase and SPC has been shown to stimulate regulators of melanomagenesis such as extracellular signal-regulated kinases (ERK), microphthalmia-associated transcription factor (MITF) and Akt/mTOR [30,31,32,33].
Finally, the expression of acid sphingomyelinase (A-SMase), which hydrolyses SM into ceramide, has been shown to be higher in benign nevi than in primary melanomas, and further reduced in the lymph-node metastases [34]. Moreover, a lower expression/activity of A-SMase was observed in hyper-pigmented murine and human melanomas as compared to the hypo-pigmented ones, suggesting an inverse correlation between A-SMase expression/activity and melanin content. In accordance, exogenous C2-ceramide decreased melanin content in melanocytes [35].
3. Role of the Sphingolipid Metabolism in Melanomagenesis
3.1. Do SL Metabolism Alterations Increase the Risk to Develop Melanoma?
A genome-wide association study has identified the 1q21.3 chromosomal region, containing LASS2 gene that encodes ceramide synthase 2 (CerS2), as a locus predisposing to cutaneous melanoma [36]. These observations suggest the involvement of some genetic determinants of the ceramide metabolism in melanoma predisposition. Interestingly, a defect in glucosylceramidase 1 (GBA1) resulting in Gaucher inborn disorder (GD), owing to a defect in GlcCer hydrolysis into ceramide, is associated with an increased risk of malignancies, including melanoma [37,38]. Indeed, accumulation of GlcCer as well as glucosylsphingosine, which arises from the cleavage of excess GlcCer by AC [39,40], occurs in macrophages and could severely alter the immune and inflammatory responses. This could create a favourable microenvironment to promote melanomagenesis (for review see [38]).
Another hypothetical link between glucosylceramidase (GC) and melanoma development is autophagy, which can be either cell protective, promoting their survival, or lethal, via induction of a programmed-cell death mechanism [41,42]. Defective autophagy has been reported in models of GC or saposin C deficiency [43]. Accumulation of GlcCer was associated to autophagy dysfunction in a drosophila model of GD that lacked the two fly GBA1 orthologues [44]. In accordance, GlcCer accumulation was also associated with autophagy impairment and defective autophagosome-lysosome fusion, resulting in autophagosome accumulation in induced pluripotent stem cells (iPSCs) derived from patients with GD [45]. Moreover, an hyperactivation of the autophagic inhibitor mTOR and a downregulation of the master regulator of lysosome function TFEB were reported in human neuroglioma cells treated with the GC inhibitor conduritol B epoxide [46]. Numerous studies have shown that impaired autophagy can favour melanoma development. Indeed, the activation of mTOR was associated with poor prognosis in melanoma patients [47]. In addition, ERK-induced TFEB phosphorylation impaired expression of autophagy-lysosome target genes in BRAF-mutated melanoma, which elicited the formation of TGF-β-dependent metastases [48].
All these findings indicate a possible link between GC deficiency and melanomagenesis, that may result from an altered immune response or disturbed autophagy. The underlying mechanisms remain, however, to be determined.
3.2. Sphingolipid Metabolism Modulates Melanoma Cell Proliferation and Survival
Transformation of normal melanocytes into melanoma cells is mediated by the activation of growth stimulatory pathways, typically leading to cellular proliferation as well as the inactivation of apoptotic and tumour suppressor pathways. The RAS-RAF-MEK-ERK pathway is one of the most important signalling pathways involved in melanoma cell growth and survival [49,50]. A constitutive activation of BRAF, mostly due to the substitution of valine by glutamic acid at position 600 (also known as V600E), affects about 50% of melanoma patients [9,51,52,53] (Figure 2). Therapies targeting the BRAF V600E mutation help advanced melanoma patients live longer [54,55]. Moreover, co-administration of BRAF (vemurafenib) and MEK (cobimetinib) inhibitors improves the progression-free survival [56] and extends the five-year overall survival by ~40% [57]. Unfortunately, most patients, including those who experience an initial tumour regression, exhibit disease progression within 6–8 months following the initiation of targeted therapy [58].
Multiple lines of evidence indicate that some SL-metabolising enzymes regulate melanoma cell proliferation and survival. First, SphK1 expression and activity are induced by ERK1/2 and AKT in numerous mammalian cells [59,60,61], including melanoma cells [20,21]. Moreover, SphK1 knockdown by siRNA decreased anchorage-dependent and -independent growth of human melanoma cells [20]. Similarly, targeting SphK1 using shRNA in B16F10 [62] or Yumm 1.7 [22] murine melanoma cells reduced tumour growth in syngeneic mice. Accordingly, the SphK1 inhibitor SKI-I, which increases the intracellular ceramide levels and decreases S1P levels in melanoma cells, resulted in a cell cycle arrest between G2/M and S phases as well as increased apoptotic cell death, caspase-3 activation and nuclear accumulation of cleaved PARP [20]. The intraperitoneal administration of SKI-I in mice harbouring melanoma also decreased tumour growth [20,22]. Consistently, the growth of B16F10 tumours is impaired in SphK1−/− mice as compared to wild-type animals [21].
S1P, which is mainly produced by SphK1 in melanoma cells, conveys oncogenic signals as an intracellular second messenger via a ligation of a family of G-protein coupled receptors (S1P1-5) expressed both on the malignant and their neighbouring cells [63]. We recently demonstrated that the melanoma cell-autonomous survival in response to the BRAF inhibitor vemurafenib is mediated by S1P1 and S1P3 [64].
Moreover, AC, which is expressed at high levels in proliferative melanoma cells, may also contribute to melanoma cell proliferation and survival. Indeed, AC inhibition by siRNA dramatically reduced the number of 501mel melanoma cells, as shown using short-term cell growth and colony formation assays [19]. Similarly, CRISPR/Cas9-mediated AC ablation in A375 melanoma cells blocked G1/S cell cycle progression, promoted senescence and apoptosis, resulting in reduced cell growth. These cells were unable to form spheroids and showed a lower replication rate as well as a decreased in their invasive capacity compared to controls. Mechanistically, AC ablation resulted in the accumulation of the saturated C14-, C16- and C18-ceramides and is accompanied by the down-regulation of MYC, CDK1, CHK1 and AKT [65]. In accordance, the inhibition of AC activity with a chemically stable AC inhibitor, named compound ARN14988, sensitised proliferative melanoma cells to the cytotoxic actions of various anti-tumour agents [18]. In line with these observations, we previously reported that the cytotoxic action of dacarbazine was accompanied with AC proteolysis in human melanoma cells [66]. Of interest, confocal immunofluorescence analyses revealed the nuclear localisation of AC in normal melanocytes, a phenomenon not observed in melanoma cells, suggesting that AC could activate proliferation pathways only in tumour cells [18].
By reducing ceramide levels, AC, in concert with SphK1, favours melanoma cell proliferation. This was confirmed using short-chain C2-ceramide, which was reported to inhibit AKT and ERK activation as well as proliferation in Malme-3M melanoma cells [67]. In addition, the GCS inhibitor PDMP, which increases intracellular C16-ceramide levels, inhibited cell proliferation, migration and invasion of WM35 and WM451 human melanoma cells. The effect of PDMP was associated with the inhibition of key enzymes from the glycolysis pathway including the pyruvate kinase, hexokinase and lactic acid dehydrogenase. Strikingly, the treatment of melanoma cells with exogenous C16-ceramide neither altered melanoma cell growth nor migration and invasion. In contrast, exogenous C16-ceramide was shown to promote glycolysis. This opposite effect could be explained by the reduction of endogenous C16-ceramide levels, which was induced by exogenous C16-ceramide treatment [14].
Finally, GCS, which catalyses the first committed step in the synthesis of most glycosphingolipids, i.e., the transfer of glucose to ceramide to form GlcCer, is also able to control tumorigenic capability of melanoma cells. Indeed, antisense oligonucleotide targeting the Ugcg gene, encoding GCS, reduced tumorigenicity of MEB4 murine melanoma cells [68]. Similarly, the inhibitor of GCS, OGT2378, inhibited MEB4 melanoma tumour growth in a syngeneic, orthotopic murine model [69]. In agreement with these findings, we previously showed that overexpression of GBA2 in melanoma cells, an enzyme able to degrade GlcCer into ceramide, reduced tumour cell growth both in vitro and in vivo by triggering ER stress-induced apoptosis [70]. Of note, GBA2 gene is downregulated in melanoma cells as compared to melanocytes [70]. Altogether, these observations demonstrate that the transformation of ceramide into GlcCer facilitates melanoma cell proliferation.
Interestingly, sialic acid-containing glycosphingolipids, i.e., gangliosides, can also regulate melanoma cell proliferation. First, treatment of SKMEL-28 melanoma cells with the anti-GD3 antibody R24 reduced their growth in vitro and decreased their tumorigenicity when injected in immunodeficient mice [71]. Second, Furukawa et al. demonstrated that GD3 increased the proliferation of GD3 synthase-overexpressing melanoma cells [72]. In these settings, GD3 mediated the convergence of several pro-tumoral signals, including those induced by hepatocyte growth factor (HGF) and the receptor tyrosine kinase c-MET, notably promoting cell proliferation [73].
Altogether, these data illustrate that SL metabolism alterations, which redirect ceramide metabolism towards S1P or GD3 production, can promote melanoma cell proliferation and survival in response to drugs.
4. Role of the Sphingolipid Metabolism in Melanoma Progression
4.1. SL Metabolism Regulates Melanoma Cell Adhesion
Cell junctions, which are crucial for the communication between neighbouring cells and with the extracellular environment, can be divided into three major classes: anchoring junctions (including adherens junctions, desmosomes, hemidesmosomes and focal adhesions), tight junctions and gap junctions. In the epidermis, cadherins are the major adhesion molecules, especially involved in the composition of desmosomes and adherens junctions [74], whereas integrins are the major component of hemidesmosomes [75]. Among cadherins, E-cadherin mediates the adhesion between melanocytes and keratinocytes allowing keratinocytes to control cell growth and dendricity of melanocytes [76]. E-cadherin expression is lost in melanoma cells during the first steps of tumour progression [77]. Interestingly, when E-cadherin expression is restored, keratinocytes recover control of melanoma cells thus preventing tumour progression [78].
Here, we review studies documenting the role of the SL metabolism in the control of the expression of adhesion molecules as well as melanoma cell adhesion capacity. Previous reports have shown that E-cadherin loss was observed in SphK1-overexpressing cancer cells [79,80]. S1P-induced E-cadherin downregulation could be mediated by S1P2 and S1P3, as shown in alveolar epithelial cells [81] and lung fibroblasts [82]. This phenomenon could be indirect in melanoma cells as the SphK1/S1P pathway is able to stimulate TGF-β1 production [62], which may trans-activate S1P2 and S1P3 [82]. Interestingly, overexpression of S1P2, but not S1P1, in B16F10 melanoma cells resulted in the inhibition of the small GTPase Rac activity as well as tumour progression in mice [83]. Importantly, Rac is crucial to create E-cadherin-dependent cell-cell contacts [84].
Moreover, downregulation of AC in melanoma cells induced E-cadherin loss and, inversely, increased expression of the epithelial-mesenchymal transition (EMT)-associated protein TWIST1, which is in accordance with a more aggressive phenotype [19].
Finally, GD3 was shown to favour the recruitment of integrins through glycolipid-enriched microdomains in GD3 synthase-overexpressing melanoma cells. Under these conditions, melanoma cell adhesion to the extracellular matrix (ECM) was increased [85]. Similarly, Ohmi et al. demonstrated that cell adhesion increased in GD2 synthase-overexpressing melanoma cells as compared control cells [86].
Thus, SL alterations appear to impact on melanoma cell adhesion, particularly through E-cadherin loss, which promotes melanoma progression.
4.2. SL Metabolism as a Determinant of Melanoma Plasticity
To colonise distant organs, tumour cells need, besides losing their cell junctions, to acquire invasive capacities. In skin cancers, EMT plays a key role in this process. This fundamental mechanism allows epithelial cells to gain mesenchymal features, increasing their migration and invasion abilities (for review, see [87]). However, unlike other skin cancers, melanoma does not arise from epithelial cells but from neural crest-derived melanocytes. For this reason, EMT stricto sensu cannot be considered in melanoma progression. Nevertheless, an EMT-like phenomenon has been described, in which melanoma cells can dynamically and reversibly switch between a proliferative and an invasive state; this is known as “phenotype switching”. Indeed, the microarray analysis of DNA from different human melanoma cell lines allowed Hoek et al. to determine a transcriptional signature representative of metastatic cell behaviour. The authors indeed demonstrated that MITF is one crucial actor in this switch, particularly in maintaining the proliferative state [88].
MITF represents a melanocytic lineage-specific transcription factor that regulates melanocyte differentiation, function and survival as well as melanoma progression [89]. MITF regulates pigment cell-specific transcription of genes encoding melanogenic enzymes such as TYR, DCT and TYRP1, as well as proteins involved in melanosome formation and maturation such as Melan-A, Premelanosome Protein and G Protein-Coupled Receptor 143. As a matter of fact, MITF expression and activity are modulated by a range of activators and suppressors operating at transcriptional, post-transcriptional and post-translational levels (for review, see [90]). MITF function has been tightly connected to melanoma cell plasticity. It is now well accepted that melanoma cells expressing moderate to high levels of MITF proliferate rapidly and are poorly invasive, whereas melanoma cells characterised by low MITF levels grow more slowly and are more invasive. Thereby, low levels of MITF correlate with a worse prognosis for melanoma patients [19,89,91].
Interestingly, numerous studies showed that the SL metabolism regulates MITF expression. Firstly, A-SMase expression has been shown to induce ERK-mediated MITF degradation by the proteasome. Therefore, the loss of A-SMase observed during melanoma progression accounts for the upregulation of MITF as well as for some of its downstream targets CDK2, Bcl-2 and c-MET [34]. Secondly, AC ablation by the CRISPR-Cas9 technology, which is associated with the accumulation of long-chain saturated ceramides, led to a strong downregulation of MITF expression in human A375 melanoma cells, reducing their ability to form cancer-initiating cells and to undergo self-renewal [65]. Furthermore, exogenous addition of C2-ceramide was shown to reduce MITF expression in human melanocytes [35]. Reciprocally, we demonstrated that MITF expression increased in AC-overexpressing melanoma cells. However, at variance with Lai et al., we observed that melanoma cells expressing AC at high levels displayed a proliferative phenotype as compared to cells with low expression of AC that exhibited high mobility and gain of mesenchymal features [19]. Moreover, using a ChIP-Seq database, we identified AC as a new target of MITF, demonstrating that MITF and AC are part of a positive feedback loop.
SL metabolism could also modulate MITF levels by acting on signalling pathways known to regulate its expression in melanoma cells. For instance, canonical Wnt signalling through the Wnt/β-catenin pathway is a critical activator of MITF expression in melanoma cells [92], and deactivation correlates with a higher metastatic potential [88]. Interestingly, exogenous sphingosine has been shown to reduce nuclear and cytosolic β-catenin expression in SW480 and T84 colon cancer cells [93]. In accordance, pharmacological inhibition of SphK1 with SKI-II was associated to a decreased β-catenin expression in human hepatoma carcinoma [94]. As expected, FTY720, a sphingosine analogue known to inhibit SphK1 [95], led to the reduction of β-catenin as well as MITF expression in melanocytes [96].
Moreover, a switch in EMT-associated transcription factors (EMT-TFs) occurs in melanoma and drives tumour progression. This dynamic network includes Snail, Zeb and Twist families, which are major repressors of epithelial genes, and, conversely, major activators of mesenchymal genes (for review, see [87]). In particular, a reduced expression of ZEB2 and SNAIL2 in favour of an increased expression in ZEB1 and TWIST1 was linked to E-cadherin loss, increased invasion properties and poor clinical outcomes in human melanoma [97]. The EMT-TFs switch was associated with a reduction of MITF expression. Indeed, ZEB1 and TWIST1 have been shown to downregulate MITF whereas SNAIL2 or ZEB2 induce MITF expression, demonstrating that these EMT-TFs act as key players in melanoma phenotype switching.
Recent studies identified a strong connection between SL metabolism and EMT-TFs. Indeed, reduced expression of CerS6, which decreased the levels of intracellular C16-ceramide, was associated with an increased expression of SNAIL2 in SW480 colon cancer cells [98]. Hence, by controlling the expression of EMT-TFs or by altering plasma membrane fluidity, C16-ceramide could affect cancer cell motility [99]. Another SL-metabolising enzyme could also account for the effects of ceramide on EMT-TFs. Indeed, SMS2, which produces SM from ceramide, seems to stimulate the expression of mesenchymal markers and enhance migration and invasion of MCF-7 and MDA-MB-231 breast cancer cell lines. Interestingly, SMS2 expression was higher in metastatic breast cancer than in non-metastatic tumours. Mechanistically, SMS2 was shown to activate the canonical TGFβ/SMAD signalling pathway leading to the expression of its downstream target Snail [100]. In addition, previous studies have demonstrated that some ganglioside-metabolising enzymes are connected with EMT-FTs and gangliosides play a critical role in EMT [101]. For instance, ZEB1 was reported to be a direct regulator of the GM3 synthase gene (St3gal5) in mammary epithelial NM18 cells. ZEB1 also impaired the expression of miR-200a, a microRNA targeting the 3ʹUTR GM3 synthase mRNA. Knockdown of GM3 synthase partly mimicked the effects of ZEB1 inhibition, leading to increased expression of cell junction components such as E-cadherin as well as intercellular adhesion [102]. Moreover, overexpressing TWIST or SNAIL1 in transformed human mammary epithelial cells enhanced the expression of GD3 synthase [103]. GD3 synthase knockdown reduced breast cancer stem cell-associated properties and completely abrogated tumour formation in vivo. In accordance, other studies have shown that ceramide glycosylation by GCS was enhanced in breast [104] and colon [105] cancer stem cells and GCS inhibition significantly decreased the expression of ZEB1 and β-catenin [105]. Whether GCS, a ganglioside-metabolising enzyme, CerS6 or SMS2 modulates EMT-TFs in melanoma cells remains to be evaluated.
Furthermore, numerous studies have shown that TGF-β is a strong promoter of EMT in many tumours [106,107], including melanoma [88]. TGF-β signalling inhibits MITF expression through PAX3 repression and GLI2 activation. Many studies reported that the SphK1/S1P and the TGF-β signalling are interconnected. Indeed, through its binding to S1P receptors, S1P was shown to promote TGF-β receptor trans-activation leading to Smad phosphorylation and cell migration [108,109,110]. Additionally, in different cancers including melanoma, S1P was reported to increase TGF-β expression and secretion [62,111,112]. Inversely, TGF-β was able to increase SphK expression and activity, which were essential to control the effects of TGF-β on extracellular matrix remodelling, cell migration and invasion [113,114].
Finally, TEAD transcription factors (TEADs) were identified as key regulators of the invasive state in melanoma [115]. TEADs need coactivators such as YAP and TAZ, which are known effectors of the Hippo pathway. This pathway has been shown to modulate Wnt and TGF-β signalling and confer pro-invasive properties in melanoma [116]. Strikingly, multiple studies have identified S1P as an activator of YAP through S1P2 signalling [117,118,119]. Similarly, a recent study demonstrated that inhibition of SphK1 using the PF-543 inhibitor could inhibit TGFβ-induced activation of YAP [120]. As anticipated from its close structural similarity with S1P, SPC also regulated the Hippo pathway via S1P2, in a rather unclear manner as it could both inhibit and activate YAP [121].
To summarise, tight connections have been reported between SL metabolism and key players of the phenotype switching in melanoma including transcription factors such as MITF, EMT-TFs, TEADs and fundamental signalling pathways such as Wnt, TGF-β and Hippo. These observations further highlight the importance of the SL metabolism in melanoma progression.
4.3. SL Metabolism as a Major Regulator of Melanoma Aggressiveness
As discussed above, melanoma aggressiveness depends on the balance between its proliferative potential and migratory/invasive properties. S1P was reported either to activate or to inhibit melanoma cell migration depending on S1P receptor subtypes. Indeed, whereas S1P inhibited cell migration, with the concomitant inhibition of Rac and stimulation of RhoA, in S1P2-expressing B16F10 cells, it stimulated cell migration of S1P1-overexpressing cells, demonstrating a receptor subtype-specific action of S1P on melanoma cells [83]. The inhibitory effects of S1P were reversed by the S1P2-selective antagonist JTE013, which stimulated Rac and migration of B16F10 cells overexpressing either S1P1 or S1P3 [122]. Similar results were obtained in B16F10 cells treated with SPC instead of S1P [122].
In addition, AC overexpression in melanoma cells decreased tumour cell motility, whereas AC silencing had the opposite effect, as was observed for MITF [19]. We recently demonstrated that low AC expression was associated to increased FAK phosphorylation and relocation at focal adhesions instead of cytoplasm. This phenomenon led to increased expression of integrin β5 (ITGβ5) and integrin αV (ITGαV), which play a critical roles in the migratory and invasive capacity of cancer cells. As a result, the melanoma invasive behaviour induced by AC inhibition was reduced using an ITGαVβ5 blocking antibody [19].
Another study reported that lung metastases were reduced in A-SMase-deficient mice injected with B16-F10 melanoma cells. Treating B16F10 cells with exogenous A-SMase or C16-ceramide before inoculation restored lung metastatic lesions in A-SMase-deficient mice. Mechanistically, melanoma cells were shown to activate A-SMase in platelets, leading to ceramide production, which favoured the clustering and activation of α5β1 integrins at the surface of melanoma cells and therefore tumour cell adhesion in the lungs [123]. As the expression of melanoma A-SMase negatively correlates with tumour aggressiveness [34], one could speculate that A-SMase expression acts as a key factor that controls melanoma cell invasion and adhesion into the metastatic niches.
Gangliosides also likely contribute to melanoma cell dissemination. As a matter of fact, the level of GM3, which has been described as one of the major gangliosides in melanoma [124], increased in murine metastatic melanoma [125], suggesting a role for GM3 in tumour aggressiveness. Indeed, the addition of GM3 to B16LuF1 melanoma cells, i.e., B16-melanoma cells of lower metastatic potential to lungs, increased their dissemination capacity once injected in mice [126]. Liu et al. also described that de-N-acetyl GM3 (d-GM3), a derivative of ganglioside GM3, was mainly found in metastatic melanomas but not in benign nevi or most primary melanomas. d-GM3 expressing melanoma cells possess increased migratory and invasive capacities as compared to melanoma cells lacking d-GM3. Mechanistically, d-GM3 stimulated MMP-2 expression via the urokinase-like plasminogen activator (uPA) receptor [127].
Some studies indicated that GD3 also stimulates melanoma cell invasion. Indeed, human melanoma GD3-positive cells showed a markedly increased cell invasion potential as compared to GD3-negative cells. The invasive activity induced by GD3 was shown to be mediated by p130Cas or paxillin, two components of the focal adhesion cytoskeleton [72]. More recently, Ohmi et al. compared the effect of GD3 and GD2 on melanoma progression. Using GD3-high or GD2-high melanoma cells, obtained by overexpressing the respective glycosyltransferases involved in their production, they demonstrated that GD2 enhanced the adhesion properties of melanoma cells, while GD3 stimulated their invasive capacities. These findings led the authors to propose that GD2 would rather act at the primary and metastatic sites in order to promote cell proliferation and dissemination, while GD3 would favour melanoma cell invasion in order to reach a metastatic niche [86].
Finally, emerging literature indicates that tumour exosomes actively participate in tumour invasiveness and favour the formation of pre-metastatic niches in various types of cancer, including melanoma [128,129,130]. Exosomes are small extracellular vesicles (EVs) that originate from the fusion of multivesicular bodies with the plasma membrane and convey their cargo towards target cells. They carry transmembrane and cytosolic proteins, DNA and small RNAs [131]. In vitro, melanoma-derived exosomes were shown to promote the EMT-like processes in primary melanocytes. This effect occurred in an autocrine/paracrine fashion and was mediated by the microRNA Let-7i [132]. In mice, B16-F10-derived exosomes demonstrated preferential homing to lymph nodes and facilitated the seeding of intravenously injected parental cells [133]. In particular, Peinado et al. showed that, through the receptor c-MET, B16-F10-derived exosomes can educate bone marrow-derived cells, promoting angiogenesis, vascular leakiness, the growth of primary tumours and metastasis [128].
It is well known that ceramide, generated by the neutral SMase2 (nSMase2) on the cytosolic leaflet of endosomal membranes, is involved in the budding of exosomes [134]. Mechanistically, the cone-shaped structure of ceramide could induce spontaneous negative curvature by creating an area difference between the membrane leaflets [135]. Moreover, a decrease in the activity of nSMase2 induced by the GW4869 compound, resulted in the reduced release of exosomal miRNAs [136]. By controlling exosomal miRNA secretion, nSMase2 is able to promote angiogenesis as well as metastasis [137]. Furthermore, Kajimoto et al. also showed that S1P, produced by SphK2 but not Sphk1, can regulate the cargo content in exosomes [138,139] probably through the Gβγ subunit of Gi proteins coupled with S1P1 [140]. Whether nSMase2- and/or SphK2-dependent exosome formation modulates melanoma progression remains to be investigated.
All these findings clearly establish a close relationship between SL metabolism and melanoma invasion and suggest that SL metabolism could be therapeutically targeted in order to improve the outcome of melanoma patients.
5. Role of SL Metabolism in the Immune Response to Melanoma
Melanoma cells harbour an aberrant antigenic profile, which allows for an anti-tumour immune response [141]. Despite their high immunogenicity, melanoma cells eventually evade the immune system, grow and metastasise [142,143]. A growing body of evidence in the literature indicates that SLs regulate various immune processes. Thus, deciphering the role of SL metabolism in melanoma immune escape is of great clinical interest.
5.1. S1P in Lymphocyte Traffic and Differentiation
Lymphocytes sense S1P concentration via S1P1 [144,145] allowing their egress from the thymus and lymph nodes to peripheral tissues [146]. S1P1 expression is modulated cyclically during lymphocyte traffic, depending on the local S1P concentration: it is downregulated in the blood, upregulated in secondary lymphoid organs (SLO) and downregulated again in the lymph [147]. CD69, an early activation marker on lymphocyte surface, induces S1P1 internalisation and degradation [148], sequestering lymphocytes in SLO [149] and peripheral tissues [150]. S1P1 downregulation is necessary to establish a long-term memory in the skin [151,152] and CD69 is one of the markers (with CD103) for tissue-resident memory cells (TRM) [153], which play a critical role in melanoma immunosurveillance [154,155,156].
Drouillard et al. proposed that the S1P1/S1P2 ratio dictates the migration of T cells, as S1P2 inhibited the chemo-attraction of peripheral T cells [157]. Sic et al. reported that human B cells also migrate towards S1P in an S1P1-dependent manner that is inhibited by CD69 expression [158]. Interestingly, egress of natural killer (NK) cells from the bone marrow and SLO is mediated by the expression of S1P5 [159,160], which is regulated by T-box transcription factor TBX21 [161]. Increased S1P5 expression as well as downregulation of CXCR4 during NK differentiation is necessary for their egress from the bone marrow [162].
SphK activity and S1P1 expression were shown to mediate differentiation of CD4+ T cells to Th1 cells and inhibit induced Treg (iTreg) generation [163]. In accordance, in T cell-specific S1p1-transgenic mice, S1P1 oriented the differentiation of CD4+ towards the Th1 lineage when antigen-activated. Moreover, S1P1 overexpression impaired the maintenance of Foxp3 expression in naïve TGF-β-treated CD4+ T cells. The differentiation of naïve CD4+ T cells towards Th1 or iTreg appeared to be reciprocal, driven by the S1P1-mTOR axis, and dependent on the SphK activity as demonstrated with the SphK inhibitors N,N,-dimethylsphingosine (DMS) and SKI. Similarly, CD4+ T cells deficient for Sphk1 showed a lesser Foxp3 expression when cultured with IL-2 and TGF-β [164].
5.2. S1P Impairs the Immune Response in Melanoma
We reported that melanoma SphK1 plays a key role in the recruitment and phenotypic switch of TAM notably promoting their commitment to a pro-tumoral M2-like phenotype [62]. Moreover, we recently showed that high SphK1 expression in melanoma cells was associated with shorter progression-free and overall survivals in melanoma patients treated with anti-PD-1-based immunotherapy. In mice, SphK1 knockdown in melanoma tumours potently reduced the production of a number of immunosuppressive cytokines including TGF-β [22,62], limiting Treg tumour infiltration. Under these conditions, the response of melanoma cells to anti-PD-1 or anti-CTLA-4-based immunotherapy highly increased [22]. Interestingly, Chakraborty et al. also reported that tumour-infiltrating lymphocytes display higher Sphk1 expression as compared to splenocytes in B16-F10-bearing mice [164]. Melanoma antigen-specific T cells deficient for Sphk1 (pMel-SphK1−/− T cells) were shown to maintain a central memory phenotype and have a reduced propensity to differentiate into Treg as compared to wild-type T cells (pMel T cells). Tumour growth was significantly slower upon adoptive transfer of pMel-SphK1−/− T cells, as compared to mice injected with wild-type pMel T cells [164].
Recent findings also show that S1P secretion, via the S1P transporter Spinster Homologue 2 (Spns2), reduced CD8+ T cell function and therefore promoted lung metastasis. Indeed, the deletion of Spns2, either globally or in a lymphatic endothelial cell-specific manner, was associated with an increased ratio of effector T-cells to immunosuppressive Tregs, in the lungs of Spns2-deficient mice intravenously injected with B16F10 or HCmel12 murine melanoma cells. This resulted in a reduced pulmonary metastatic burden as compared to what was observed in wild-type animals [165].
5.3. Ceramide and Its Derivatives in the Immune Response
Several studies have shown that ceramide metabolism could regulate the immune response in different melanoma models. Firstly, A-SMase-deficient B16-F1 melanoma cells engrafted in mice display an inflammatory TME and are infiltrated by high levels MDSCs and Tregs and low levels of DCs. A-SMase overexpression in these cells restores CD8+ and CD4+ T cells and DCs infiltration while reducing levels of infiltrating MDSCs and Tregs, thereby reducing tumour growth [166].
Secondly, KRN7000, a synthetic alpha-galactosylceramide [167], showed promising results in enhancing NK, NKT, CD8+ T cells and M1 infiltration in the syngeneic murine B16 metastatic melanoma model [168] but further investigation needs to be conducted.
Thirdly, it was recently reported that liposomes enriched in C2-ceramide were shown to reprogram the immune TME in a PKCζ-dependent manner in B16-F10-bearing mice. Under these conditions, TAMs shifted towards an M1 phenotype and CD8+ and Th1 cells infiltration was enhanced while intra-tumour MDSCs and Tregs levels were reduced [169].
Finally, gangliosides also represent attractive targets for immunotherapies as they are abundant in melanoma cells [24] and recognised by NKT cells [170]. GM2 [171] and N-glycolyl GM3 (NGcGM3), in particular, have been the main gangliosides used as targets for the development of anti-melanoma antibodies and vaccine [172,173]. In addition, gangliosides are also known to be shed by melanoma cells [174,175] and exert a pro-apoptotic effect on DCs [176,177]. 3F8, a monoclonal anti-GD2 mAb, demonstrated anti-proliferative and pro-apoptotic activity in human melanoma cell lines [178] but clinical studies focused on neuroblastoma [179] and medulloblastoma [180] patients. More recently, GD2 has been considered as a promising target for the treatment of melanoma patients using either CAR-T cell therapy [181] or the immunocytokine hu14.18-IL2, an anti-GD2 humanised mAb linked to two molecules of IL-2 and administered to patients with recurrent resectable stage III or IV melanoma [182].
5.4. Melanoma-Derived Exosomes Are Vectors of Immunosuppression
As mentioned above, Trajkovic et al. showed that the production of ceramide by nSMase 2 was part of the mechanisms involved in exosome budding [134]. In addition to favouring progression and metastasis, melanoma-derived small extracellular vesicles, often defined as exosomes, also carry immunomodulatory molecules that impair anti-tumour immune responses. In vitro, exosomes released from B16F0 murine melanoma cells inhibited the proliferation of T cells by delivering PTPN11(SHP-2) mRNA and protein [183]. B16F10-derived exosomes can also activate the mitochondrial apoptotic pathway of CD4+ T cells in vitro and in vivo, thereby increasing tumour growth and reducing T cell infiltration [184]. The authors proposed that the miRNA cargo of exosomes (e.g., miR-690) inhibited the expression of anti-apoptotic proteins in CD4+ T cells. In addition, small EVs produced by A375 human melanoma cells were shown to be able to reduce MHC class I molecules to the cell surface of primary human monocytes and THP-1 cells and downregulate the expression of endogenous MHC class I and II molecules in DCs [185]. Exosomes from metastatic melanoma-derived cell lines inhibited TCR signalling and cytokines secretion in CD8+ T cells by transferring an array of miRNA cargo [186]. Moreover, tumour-derived exosomes harvested from melanoma patients’ plasma were shown to induce the apoptosis, inhibit proliferation and decrease the activation of CD8+ T cells. They were also able to downregulate NKG2D expression on NK cells [187]. In metastatic melanoma patients, circulating exosomal PD-L1 suppressed CD8+ T cell activity. The authors reported that the pre-treatment level of circulating exosomal PD-L1 was a better predictor of clinical response to anti-PD-1 therapy than total circulating PD-L1 [188]. Importantly, Poggio et al. showed that Pdl1 knockout or exosome depletion by knocking out Smpd3, the gene encoding nSMase2, was sufficient to restore the anti-tumour immune response and to induce an efficient anti-tumour immune-memory response in the murine TRAMP-C2 prostate cancer model [189].
The relationship between SL metabolism and exosome-mediated immunosuppression in melanoma is not well understood, yet it could be a major mechanism of resistance to immunotherapy and thus deserves further investigation.
6. Potential Therapeutic Strategies for Melanoma Patients
Historically, when tumour resection was not possible or failed, chemotherapy was used to treat melanoma. dacarbazine (DTIC) has been approved as first-line treatment for advanced-stage melanoma and has remained for more than 30 years the standard chemotherapy despite no clear overall survival benefits [190,191,192].
The identification of melanoma driven mutations such as BRAF V600E allowed for a real breakthrough in the treatment of patients with metastatic melanoma. The emergence of BRAF targeted agents such as vemurafenib [54] and dabrafenib [55] allowed tremendous progresses in the field of personalised medicine and demonstrated survival benefits in metastatic melanoma patients as compared to dacarbazine-treated patients. Subsequently, the MEK inhibitor was also approved as treatment for this pathology as it showed survival benefits for patients displaying the BRAF V600E mutation [193]. Combination therapy using BRAF and MEK inhibitors such as cobimetinib [56] is nowadays one of the first line treatment for patients with BRAF V600E metastatic melanoma. This treatment results in higher rates as well as extended duration of response and decreases the cutaneous toxicities observed with the BRAF inhibitor monotherapy. Unfortunately, such therapeutic approaches remain constrained by the inevitable emergence of resistance to single-pathway blockade [194].
Immune checkpoint blockade (ICB) was the first therapeutic strategy to provide sustained responses and survival for advanced melanoma patients, even after treatment discontinuation [195,196,197]. Administering monoclonal antibodies targeting the immune checkpoint PD-1, alone or in combination with anti-CTLA-4 blocking antibodies is, to date, the standard of care for advanced melanoma patients. Independently of BRAF mutation status, patients treated with the anti-PD-1 and anti-CTLA-4 combo achieve at five years a progression-free survival and an overall survival of 36% and 52%, respectively [198]. Unfortunately, half of the patients do not respond or develop early resistance to ICB and exhibit severe immune-related adverse events (IRAE) [199]. Although treatment discontinuation due to adverse events seems not to affect the outcome for patients treated with ICB combination therapy [198], IRAEs tend to be associated with a better outcome for patients treated with anti-PD1 monotherapy [200].
Targeted therapies and ICB have deeply changed therapeutic management of patients with metastatic melanoma but all these therapeutic approaches still need improvement. Understanding the mechanisms that underlie resistance to these treatments is of utmost importance to improve the outcome of melanoma patients.
Here, we review some studies, which identified alterations in the SL metabolism as a cause of melanoma resistance to treatment (Table 2) and other studies using SL-related molecules as monotherapy or combined therapy to fight melanoma (Table 3).
Table 2.
SL-metabolising enzymes regulate the response of melanoma to therapy.
| Targeted SL-Metabolising Enzyme | Melanoma Cells | Experimental Strategy |
Treatment | Effects on Drug Sensitivity | Refs |
|---|---|---|---|---|---|
| A-SMase | B16-W6_pSIL10 | shRNA | Cisplatin (chemotherapy) |
Low A-SMase is associated with reduced mTOR-related autophagy and resistance to cisplatin | [201] |
| AC | A375 | AC overexpression | Dacarbazine (chemotherapy) |
AC overexpression confers resistance to dacarbazine | [66] |
| AC | G361 A375 |
ARN14988 ARN398 (AC inhibitor) |
5-FU (chemotherapy) |
AC inhibition sensitises G361 cells (proliferative phenotype) but not A375 cells (invasive phenotype) to chemotherapeutic drugs | [18] |
| SphK1 | SK-Mel-28 A375 |
FTY720 | Cisplatin (chemotherapy) |
SK1 inhibition increases cisplatin-induced apoptosis through a downregulation of the PI3K/AKT/mTOR pathway and decreases EGFR expression | [202] |
| SphK1 | UACC 903 | siRNA | Staurosporine (Apoptosis inducing agent) |
Downregulation of Sphk1 sensitises cells to staurosporine-induced apoptosis through AKT inhibition, and G0/G1 phase cell cycle arrest | [20] |
| SphK1 | A375 (overexpression) Mel-2a (downregulation) |
SphK1 overexpression or downregulation |
Doxorubicin (chemotherapy) |
Sphk1 overexpression induces resistance to doxorubicin-induced apoptosis whereas its downregulation by siRNA increases melanoma cell sensitivity to the treatment | [203] |
| SphK1 | WM115 SK-Mel-28 |
FTY720 | Vemurafenib (BRAF inhibitor) |
SK1 inhibition increases vemurafenib-induced apoptosis | [204] |
| SphK1 | WM9 | SKI-I | Vemurafenib (BRAF inhibitor) |
Sphk1 inhibition blocks BRAFi-resistant melanoma cell growth by reducing MITF and Bcl-2 expression | [64] |
| SphK1 | B16-F10 | PF-543 | ICB Adoptive transfer of melanoma antigen-specific T cells |
Sphk1 inhibition in T cells maintains Tcm phenotype, reduces Treg induction and synergises with anti-PD1 treatment | [164] |
| SphK1 | Yumm 1.7 | shRNA | ICB | SphK1 downregulation enhances ICB therapy efficacy by reducing Treg infiltration | [22] |
| GCS | B16 | PDMP | Genistein (Apoptosis inducing agent) |
Ceramide accumulation enhances genistein-induced apoptosis and growth inhibition through JNK activation and AKT inhibition |
[205] |
Table 3.
SL-related molecules used therapy in melanoma.
| SL-Related Treatment | Models | Associated Drug | Effects | Refs |
|---|---|---|---|---|
| Nanoliposomal ceramide | UACC 903 cells 1205 Lu cells Xenografts in nude mice |
Sorafenib | Inhibition of melanoma cell growth by targeting both PI3K and MAPK signalling | [206] |
| Nanoliposomal ceramide | 1205 Lu cells In vitro experiments |
None | Reduction of integrin affinity and inhibition of melanoma cell migration through PI3K and PKCζ tumour-suppressive activities |
[207] |
| KRN7000 | B16 melanoma cell graft intravenously injected in mice | None | Increase of lifespan of mice | [208] |
| OGT2378 (GCS inhibitor) |
B16 derived MEB4 melanoma cell graft in female C57BL/6 mice | None | Inhibition of tumour growth and reduction of established tumours | [69] |
| Intra-muscular GM3/VSSP vaccine | Phase I clinical trial: 26 patients with advanced (stage III and IV) melanoma | Adjuvant Montanide Isa 51 | GM3/VSSP vaccine induces anti-GM3 IgM response in 44% of patients. Serum reactivity against melanoma cells and tumour biopsies is reported | [209] |
| L612-HuMAb (Human monoclonal antibody that binds to GM3) | Phase I clinical trial: 9 patients with advanced (stage IV) melanoma | None | L612 HuMAb induces significant antitumour activity in melanoma patients | [210] |
7. Conclusions
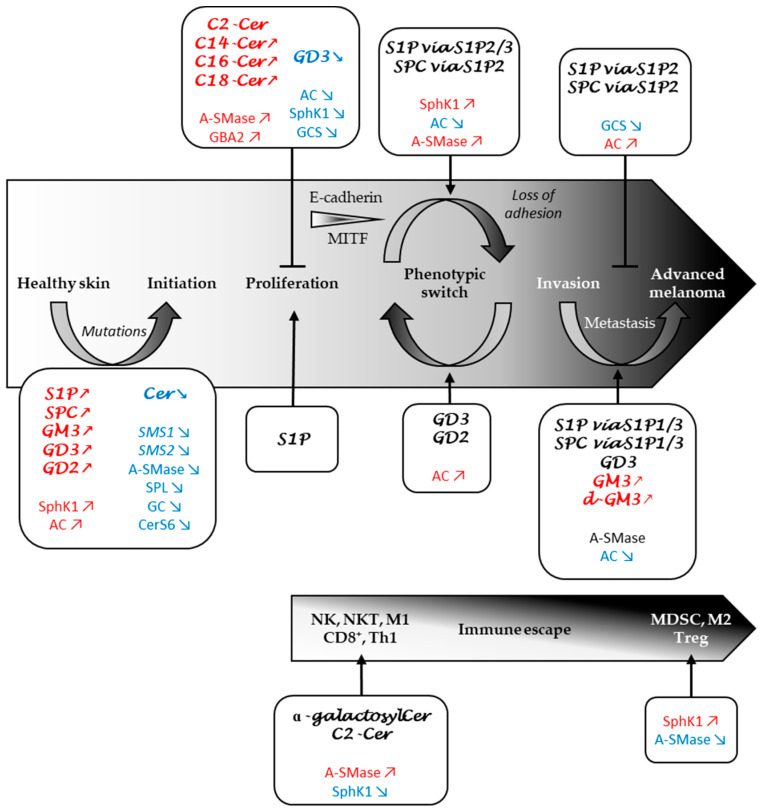
As developed in this review, changes in SL metabolism that contribute to melanomagenesis, tumour progression and therapeutic resistance are multiple (Figure 4).
Figure 4.
Role of sphingolipid metabolism in melanoma progression and immune response. SL metabolites and SL-metabolising enzymes whose levels and expression are increased, decreased or implicated are marked in red, blue or black, respectively. AC, acid ceramidase; CD8+, CD8+ T cells; Cer, ceramide; CerS, ceramide synthase; GC, glucosylceramidase; GCS, glucosylceramide synthase; M1, M1 macrophages; M2, M2 macrophages; MDSC, myeloid-derived suppressor cells; NK, natural killer cells; NKT, natural killer T cells; S1P, sphingosine 1-phosphate; S1P1/2/3, S1P receptor type 1/2/3; SMases, sphingomyelinases; SMS, sphingomyelin synthase; SPC, sphingosylphosphorylcholine; SphK, sphingosine kinase; SPL, S1P lyase; Th1, Th1 CD4+ T cells; Treg, tegulatory T cells.
The action of sphingolipids (via the enzymes that control their metabolism, and transporters) is likely mediated by modifications in key regulatory processes including the phenotypic switch and EV-mediated cell-cell communication. Interestingly, these metabolic alterations could be envisioned as potential biomarkers and be exploited to better characterise tumour progression in melanoma patients. As a matter of fact, we identified that a reduced expression of SMS1 was significantly associated with a worse prognosis in metastatic melanoma [29]. AC was also identified as a potential biomarker for the prognosis of melanoma [211]. Moreover, we recently demonstrated that human invasive melanoma cells had lower AC levels and activity than proliferative melanoma cells [19]. In accordance, high AC expression was observed in node-negative stage II melanomas [18].
It is also interesting to note that a strong association between increased serum levels of gangliosides and high Breslow index or high Clark level as well as the presence of ulceration has been reported in melanoma patients, suggesting that circulating gangliosides may serve as potential markers for melanoma staging [212].
Monitoring the expression of SL-metabolising enzymes as well as SL levels could also be used to track the response to therapy in melanoma. Indeed, we previously showed that AC expression was associated to the response of melanoma cells to dacarbazine. Whereas overexpression of AC conferred resistance to dacarbazine, AC downregulation sensitised tumour cells to the drug [66]. DTIC triggered AC degradation and this effect was accompanied with an increased ceramide/S1P ratio. Our recent results also reveal that a distinct SL profile, i.e., a tendency for increased very long-chain ceramide species, was observed in the plasma of patients with melanoma who achieve a response to a BRAF-targeted therapy as compared with patients with progressive disease [64]. Finally, we recently discovered that melanoma patients with low SphK1 expression had significantly longer progression-free survival and overall survival than those with high SphK1 expression and patients with high SphK1 expression mostly failed to respond to anti-PD-1 therapy. These findings support the hypothesis that SphK1 expression represents a potential biomarker to predict tumour progression and resistance to anti-PD-1 in metastatic melanoma patients [22].
It would now be of great interest to evaluate the possible association between these SL metabolic alterations and the mutation status of oncogenes such as BRAF or NRAS as well as immune responses in metastatic melanoma patients. This will be performed in patients treated with anti-PD-1 in combination or not with anti-CTLA-4 in a prospective clinical trial (IMMUSPHINX: NCT03627026) we are currently conducting in our institute.
Funding
This research was funded by INSERM, Paul Sabatier University, Fondation Association pour la Recherche sur le Cancer [B. Ségui (R19179BB) and N. Andrieu-Abadie (R18167BB)], Société Française de Dermatologie [N. Andrieu-Abadie (R18126BB)], Fondation Toulouse Cancer Santé [B. Ségui (R19225BB)] and Institut National du Cancer [N. Andrieu-Abadie (R19243BP)]. The work also received funding from the Transcan-2 Research Program, which is a transnational R&D program jointly funded by national funding organisations within the framework of the ERA-NET Transcan-2 [N. Andrieu-Abadie (TRANS201601250)]. The APC was funded by Fondation Association pour la Recherche sur le Cancer [N. Andrieu-Abadie (R18167BB)]. L.C. is a recipient of a fellowship from Fondation pour la Recherche Médicale.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gershenwald J.E., Guy G.P. Stemming the Rising Incidence of Melanoma: Calling Prevention to Action. JNCI J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Z., Liu W., Gotlieb V. The rapidly evolving therapies for advanced melanoma—Towards immunotherapy, molecular targeted therapy, and beyond. Crit. Rev. Oncol. Hematol. 2016;99:91–99. doi: 10.1016/j.critrevonc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Crowson A.N., Magro C.M., Mihm M.C. Prognosticators of melanoma, the melanoma report, and the sentinel lymph node. Mod. Pathol. 2006;19:S71–S87. doi: 10.1038/modpathol.3800517. [DOI] [PubMed] [Google Scholar]
- 4.Shain A.H., Bastian B.C. From melanocytes to melanomas. Nat. Rev. Cancer. 2016;16:345–358. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 5.Chang A.E., Karnell L.H., Menck H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Herlyn M., Thurin J., Balaban G., Bennicelli J.L., Bondi E., Guerry D., Nowell P., Clark W.H., Koprowski H. Characteristics of Cultured Human Melanocytes Isolated from Different Stages of Tumor Progression. Cancer Res. 1985;45:8. [PubMed] [Google Scholar]
- 7.Liu J., Fukunaga-Kalabis M., Li L., Herlyn M. Developmental pathways activated in melanocytes and melanoma. Arch. Biochem. Biophys. 2014;563:13–21. doi: 10.1016/j.abb.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu M.-Y., Meier F., Herlyn M. Melanoma development and progression: A conspiracy between tumor and host. Differentiation. 2002;70:522–536. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- 9.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 10.Akbani R., Akdemir K.C., Aksoy B.A., Albert M., Ally A., Amin S.B., Arachchi H., Arora A., Auman J.T., Ayala B., et al. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodis E., Watson I.R., Kryukov G.V., Arold S.T., Imielinski M., Theurillat J.-P., Nickerson E., Auclair D., Li L., Place C., et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian C.G., Murphy G. The Genetic Evolution of Melanoma. N. Engl. J. Med. 2016;374:993–996. doi: 10.1056/NEJMc1515834. [DOI] [PubMed] [Google Scholar]
- 13.Abildgaard C., Guldberg P. Molecular drivers of cellular metabolic reprogramming in melanoma. Trends Mol. Med. 2015;21:164–171. doi: 10.1016/j.molmed.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Liu R., Cao K., Tang Y., Liu J., Li J., Chen J., Wang S., Chen Z., Zhou J. C16:0 ceramide effect on melanoma malignant behavior and glycolysis depends on its intracellular or exogenous location. Am. J. Transl. Res. 2020;12:1123–1135. [PMC free article] [PubMed] [Google Scholar]
- 15.Borodzicz S., Rudnicka L., Mirowska-Guzel D., Cudnoch-Jedrzejewska A. The role of epidermal sphingolipids in dermatologic diseases. Lipids Health Dis. 2016;15:13. doi: 10.1186/s12944-016-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garandeau D., Mrad M., Levade T., Perrotta C., Andrieu-Abadie N., Diab-Assaf M. Dysregulation of Sphingolipid Metabolism in Melanoma: Roles in Pigmentation, Cell Survival and Tumor Progression. In: Hannun Y.A., Luberto C., Mao C., Obeid L.M., editors. Bioactive Sphingolipids in Cancer Biology and Therapy. Springer International Publishing; Cham, Switzerland: 2015. pp. 123–139. [Google Scholar]
- 17.Tang Y., Cao K., Wang Q., Chen J., Liu R., Wang S., Zhou J., Xie H. Silencing of CerS6 increases the invasion and glycolysis of melanoma WM35, WM451 and SK28 cell lines via increased GLUT1-induced downregulation of WNT5A. Oncol. Rep. 2016;35:2907–2915. doi: 10.3892/or.2016.4646. [DOI] [PubMed] [Google Scholar]
- 18.Realini N., Palese F., Pizzirani D., Pontis S., Basit A., Bach A., Ganesan A., Piomelli D. Acid Ceramidase in Melanoma: EXPRESSION, LOCALIZATION, AND EFFECTS OF PHARMACOLOGICAL INHIBITION. J. Biol. Chem. 2016;291:2422–2434. doi: 10.1074/jbc.M115.666909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclerc J., Garandeau D., Pandiani C., Gaudel C., Bille K., Nottet N., Garcia V., Colosetti P., Pagnotta S., Bahadoran P., et al. Lysosomal acid ceramidase ASAH1 controls the transition between invasive and proliferative phenotype in melanoma cells. Oncogene. 2019;38:1282–1295. doi: 10.1038/s41388-018-0500-0. [DOI] [PubMed] [Google Scholar]
- 20.Madhunapantula S.V., Hengst J., Gowda R., Fox T.E., Yun J.K., Robertson G.P. Targeting sphingosine kinase-1 to inhibit melanoma: Targeting SPHK1 in melanomas. Pigment Cell Melanoma Res. 2012;25:259–274. doi: 10.1111/j.1755-148X.2012.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albinet V., Bats M.-L., Huwiler A., Rochaix P., Chevreau C., Ségui B., Levade T., Andrieu-Abadie N. Dual role of sphingosine kinase-1 in promoting the differentiation of dermal fibroblasts and the dissemination of melanoma cells. Oncogene. 2014;33:3364–3373. doi: 10.1038/onc.2013.303. [DOI] [PubMed] [Google Scholar]
- 22.Imbert C., Montfort A., Fraisse M., Marcheteau E., Gilhodes J., Martin E., Bertrand F., Marcellin M., Burlet-Schiltz O., Peredo A.G.d., et al. Resistance of melanoma to immune checkpoint inhibitors is overcome by targeting the sphingosine kinase-1. Nat. Commun. 2020;11:437. doi: 10.1038/s41467-019-14218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colie S., Van Veldhoven P.P., Kedjouar B., Bedia C., Albinet V., Sorli S.-C., Garcia V., Djavaheri-Mergny M., Bauvy C., Codogno P., et al. Disruption of Sphingosine 1-Phosphate Lyase Confers Resistance to Chemotherapy and Promotes Oncogenesis through Bcl-2/Bcl-xL Upregulation. Cancer Res. 2009;69:9346–9353. doi: 10.1158/0008-5472.CAN-09-2198. [DOI] [PubMed] [Google Scholar]
- 24.Portoukalian J., Zwingelstein G., Doré J.F. Lipid composition of human malignant melanoma tumors at various levels of malignant growth. Eur. J. Biochem. 1979;94:19–23. doi: 10.1111/j.1432-1033.1979.tb12866.x. [DOI] [PubMed] [Google Scholar]
- 25.Loganzo F., Dosik J.S., Zhao Y., Vidal M.J., Nanus D.M., Sudol M., Albino A.P. Elevated expression of protein tyrosine kinase c-Yes, but not c-Src, in human malignant melanoma. Oncogene. 1993;8:2637–2644. [PubMed] [Google Scholar]
- 26.Hamamura K., Tsuji M., Hotta H., Ohkawa Y., Takahashi M., Shibuya H., Nakashima H., Yamauchi Y., Hashimoto N., Hattori H., et al. Functional Activation of Src Family Kinase Yes Protein Is Essential for the Enhanced Malignant Properties of Human Melanoma Cells Expressing Ganglioside GD3. J. Biol. Chem. 2011;286:18526–18537. doi: 10.1074/jbc.M110.164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huitema K., van den Dikkenberg J., Brouwers J.F.H.M., Holthuis J.C.M. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaoka S., Miyaji M., Kitano T., Umehara H., Okazaki T. Expression Cloning of a Human cDNA Restoring Sphingomyelin Synthesis and Cell Growth in Sphingomyelin Synthase-defective Lymphoid Cells. J. Biol. Chem. 2004;279:18688–18693. doi: 10.1074/jbc.M401205200. [DOI] [PubMed] [Google Scholar]
- 29.Bilal F., Montfort A., Gilhodes J., Garcia V., Riond J., Carpentier S., Filleron T., Colacios C., Levade T., Daher A., et al. Sphingomyelin Synthase 1 (SMS1) Downregulation Is Associated With Sphingolipid Reprogramming and a Worse Prognosis in Melanoma. Front. Pharmacol. 2019;10:443. doi: 10.3389/fphar.2019.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi K., Kawashima M., Ichikawa Y., Imokawa G. Sphingosylphosphorylcholine is a Melanogenic Stimulator for Human Melanocytes. Pigment Cell Res. 2003;16:670–678. doi: 10.1046/j.1600-0749.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim D.-S., Park S.-H., Kwon S.-B., Park E.-S., Huh C.-H., Youn S.-W., Park K.-C. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment Cell Res. 2006;19:146–153. doi: 10.1111/j.1600-0749.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeong H.-S., Lee S.H., Yun H.-Y., Baek K.J., Kwon N.S., Park K.-C., Kim D.-S. Involvement of mTOR signaling in sphingosylphosphorylcholine-induced hypopigmentation effects. J. Biomed. Sci. 2011;18:55. doi: 10.1186/1423-0127-18-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong H.-S., Park K.-C., Kim D.-S. PP2A and DUSP6 are involved in sphingosylphosphorylcholine-induced hypopigmentation. Mol. Cell. Biochem. 2012;367:43–49. doi: 10.1007/s11010-012-1317-8. [DOI] [PubMed] [Google Scholar]
- 34.Bizzozero L., Cazzato D., Cervia D., Assi E., Simbari F., Pagni F., De Palma C., Monno A., Verdelli C., Querini P.R., et al. Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ. 2014;21:507–520. doi: 10.1038/cdd.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D.-S., Kim S.-Y., Chung J.-H., Kim K.-H., Eun H.-C., Park K.-C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell. Signal. 2002;14:779–785. doi: 10.1016/S0898-6568(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 36.Amos C.I., Wang L.-E., Lee J.E., Gershenwald J.E., Chen W.V., Fang S., Kosoy R., Zhang M., Qureshi A.A., Vattathil S., et al. Genome-wide association study identifies novel loci predisposing to cutaneous melanoma†. Hum. Mol. Genet. 2011;20:5012–5023. doi: 10.1093/hmg/ddr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Astudillo L., Therville N., Colacios C., Ségui B., Andrieu-Abadie N., Levade T. Glucosylceramidases and malignancies in mammals. Biochimie. 2016;125:267–280. doi: 10.1016/j.biochi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Dubot P., Astudillo L., Therville N., Sabourdy F., Stirnemann J., Levade T., Andrieu-Abadie N. Are Glucosylceramide-Related Sphingolipids Involved in the Increased Risk for Cancer in Gaucher Disease Patients? Review and Hypotheses. Cancers. 2020;12:475. doi: 10.3390/cancers12020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferraz M.J., Marques A.R.A., Appelman M.D., Verhoek M., Strijland A., Mirzaian M., Scheij S., Ouairy C.M., Lahav D., Wisse P., et al. Lysosomal glycosphingolipid catabolism by acid ceramidase: Formation of glycosphingoid bases during deficiency of glycosidases. FEBS Lett. 2016;590:716–725. doi: 10.1002/1873-3468.12104. [DOI] [PubMed] [Google Scholar]
- 40.Flanagan J., Ranes B., Brignol N., Hamler R., Clark S. The origins of glucosylsphingosine in Gaucher disease. Mol. Genet. Metab. 2013;108:S40–S41. doi: 10.1016/j.ymgme.2012.11.087. [DOI] [Google Scholar]
- 41.Lai M., La Rocca V., Amato R., Freer G., Pistello M. Sphingolipid/Ceramide Pathways and Autophagy in the Onset and Progression of Melanoma: Novel Therapeutic Targets and Opportunities. Int. J. Mol. Sci. 2019;20:3436. doi: 10.3390/ijms20143436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang W., Ogretmen B. Autophagy paradox and ceramide. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids. 2014;1841:783–792. doi: 10.1016/j.bbalip.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y., Liou B., Ran H., Skelton M.R., Williams M.T., Vorhees C.V., Kitatani K., Hannun Y.A., Witte D.P., Xu Y.-H., et al. Neuronopathic Gaucher disease in the mouse: Viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum. Mol. Genet. 2010;19:1088–1097. doi: 10.1093/hmg/ddp580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinghorn K.J., Grönke S., Castillo-Quan J.I., Woodling N.S., Li L., Sirka E., Gegg M., Mills K., Hardy J., Bjedov I., et al. A Drosophila Model of Neuronopathic Gaucher Disease Demonstrates Lysosomal-Autophagic Defects and Altered mTOR Signalling and Is Functionally Rescued by Rapamycin. J. Neurosci. 2016;36:11654–11670. doi: 10.1523/JNEUROSCI.4527-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panicker L.M., Miller D., Park T.S., Patel B., Azevedo J.L., Awad O., Masood M.A., Veenstra T.D., Goldin E., Stubblefield B.K., et al. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc. Natl. Acad. Sci. USA. 2012;109:18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown R.A., Voit A., Srikanth M.P., Thayer J.A., Kingsbury T.J., Jacobson M.A., Lipinski M.M., Feldman R.A., Awad O. mTOR hyperactivity mediates lysosomal dysfunction in Gaucher’s disease iPSC-neuronal cells. Dis. Model. Mech. 2019;12:dmm038596. doi: 10.1242/dmm.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pópulo H., Soares P., Faustino A., Rocha A.S., Silva P., Azevedo F., Lopes J.M. mTOR pathway activation in cutaneous melanoma is associated with poorer prognosis characteristics: Letter to the Editor. Pigment Cell Melanoma Res. 2011;24:254–257. doi: 10.1111/j.1755-148X.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 48.Li S., Song Y., Quach C., Guo H., Jang G.-B., Maazi H., Zhao S., Sands N.A., Liu Q., In G.K., et al. Transcriptional regulation of autophagy-lysosomal function in BRAF-driven melanoma progression and chemoresistance. Nat. Commun. 2019;10:1693. doi: 10.1038/s41467-019-09634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 50.Smalley K.S.M. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int. J. Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 51.Gorden A., Osman I., Gai W., He D., Huang W., Davidson A., Houghton A.N., Busam K., Polsky D. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–3957. [PubMed] [Google Scholar]
- 52.Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 53.Curtin J.A., Patel H.N., Cho K.-H., LeBoit P.E. Distinct Sets of Genetic Alterations in Melanoma. N. Engl. J. Med. 2005;13 doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 54.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hauschild A., Grob J.-J., Demidov L.V., Jouary T., Gutzmer R., Millward M., Rutkowski P., Blank C.U., Miller W.H., Kaempgen E., et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 56.Larkin J., Ascierto P.A., Dréno B., Atkinson V., Liszkay G., Maio M., Mandalà M., Demidov L., Stroyakovskiy D., Thomas L., et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 57.Ribas A., Daud A., Pavlick A.C., Gonzalez R., Lewis K.D., Hamid O., Gajewski T.F., Puzanov I., Wongchenko M., Rooney I., et al. Extended 5-Year Follow-up Results of a Phase Ib Study (BRIM7) of Vemurafenib and Cobimetinib in BRAF -Mutant Melanoma. Clin. Cancer Res. 2020;26:46–53. doi: 10.1158/1078-0432.CCR-18-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel H., Yacoub N., Mishra R., White A., Yuan L., Alanazi S., Garrett J.T. Current Advances in the Treatment of BRAF-Mutant Melanoma. Cancers. 2020;12:482. doi: 10.3390/cancers12020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitson S.M., Xia P., Leclercq T.M., Moretti P.A.B., Zebol J.R., Lynn H.E., Wattenberg B.W., Vadas M.A. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J. Exp. Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leclercq T., Pitson S. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life Int. Union Biochem. Mol. Biol. Life. 2006;58:467–472. doi: 10.1080/15216540600871126. [DOI] [PubMed] [Google Scholar]
- 61.Francy J.M., Nag A., Conroy E.J., Hengst J.A., Yun J.K. Sphingosine kinase 1 expression is regulated by signaling through PI3K, AKT2, and mTOR in human coronary artery smooth muscle cells. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2007;1769:253–265. doi: 10.1016/j.bbaexp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Mrad M., Imbert C., Garcia V., Rambow F., Therville N., Carpentier S., Ségui B., Levade T., Azar R., Marine J.-C., et al. Downregulation of sphingosine kinase-1 induces protective tumor immunity by promoting M1 macrophage response in melanoma. Oncotarget. 2016;7:71873–71886. doi: 10.18632/oncotarget.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pyne N.J., Pyne S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 64.Garandeau D., Noujarède J., Leclerc J., Imbert C., Garcia V., Bats M.-L., Rambow F., Gilhodes J., Filleron T., Meyer N., et al. Targeting the Sphingosine 1-Phosphate Axis Exerts Potent Antitumor Activity in BRAFi-Resistant Melanomas. Mol. Cancer Ther. 2019;18:289–300. doi: 10.1158/1535-7163.MCT-17-1141. [DOI] [PubMed] [Google Scholar]
- 65.Lai M., Realini N., La Ferla M., Passalacqua I., Matteoli G., Ganesan A., Pistello M., Mazzanti C.M., Piomelli D. Complete Acid Ceramidase ablation prevents cancer-initiating cell formation in melanoma cells. Sci. Rep. 2017;7:7411. doi: 10.1038/s41598-017-07606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bedia C., Casas J., Andrieu-Abadie N., Fabriàs G., Levade T. Acid Ceramidase Expression Modulates the Sensitivity of A375 Melanoma Cells to Dacarbazine. J. Biol. Chem. 2011;286:28200–28209. doi: 10.1074/jbc.M110.216382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han W.S., Yoo J.Y., Youn S.W., Kim D.S., Park C., Kim S.Y., Kim K.H. Effects of C2-ceramide on the Malme-3M melanoma cell line. J. Dermatol. Sci. 2002:10. doi: 10.1016/S0923-1811(02)00044-0. [DOI] [PubMed] [Google Scholar]
- 68.Deng W., Li R., Guerrera M., Liu Y., Ladisch S. Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology. 2002;12:145–152. doi: 10.1093/glycob/12.3.145. [DOI] [PubMed] [Google Scholar]
- 69.Weiss M., Hettmer S., Smith P., Ladisch S. Inhibition of melanoma tumor growth by a novel inhibitor of glucosylceramide synthase. Cancer Res. 2003;63:3654–3658. [PubMed] [Google Scholar]
- 70.Sorli S., Colié S., Albinet V., Dubrac A., Touriol C., Guilbaud N., Bedia C., Fabriàs G., Casas J., Ségui B., et al. The nonlysosomal β-glucosidase GBA2 promotes endoplasmic reticulum stress and impairs tumorigenicity of human melanoma cells. FASEB J. 2013;27:489–498. doi: 10.1096/fj.12-215152. [DOI] [PubMed] [Google Scholar]
- 71.Nakano J., Raj B.K., Asagami C., Lloyd K.O. Human melanoma cell lines deficient in GD3 ganglioside expression exhibit altered growth and tumorigenic characteristics. J. Invest. Dermatol. 1996;107:543–548. doi: 10.1111/1523-1747.ep12582802. [DOI] [PubMed] [Google Scholar]
- 72.Hamamura K., Furukawa K., Hayashi T., Hattori T., Nakano J., Nakashima H., Okuda T., Mizutani H., Hattori H., Ueda M., et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA. 2005;102:11041–11046. doi: 10.1073/pnas.0503658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furukawa K., Kambe M., Miyata M., Ohkawa Y., Tajima O., Furukawa K. Ganglioside GD3 induces convergence and synergism of adhesion and hepatocyte growth factor/Met signals in melanomas. Cancer Sci. 2014;105:52–63. doi: 10.1111/cas.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G., Satyamoorthy K., Herlyn M. D ynamics of C ell I nteractions and C ommunications during M elanoma D evelopment. Crit. Rev. Oral Biol. Med. 2002;13:62–70. doi: 10.1177/154411130201300107. [DOI] [PubMed] [Google Scholar]
- 75.Walko G., Castañón M.J., Wiche G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015;360:529–544. doi: 10.1007/s00441-015-2216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haass N.K., Smalley K.S.M., Herlyn M. The Role of Altered Cell–Cell Communication in Melanoma Progression. J. Mol. Histol. 2003;35:309–318. doi: 10.1023/B:HIJO.0000032362.35354.bb. [DOI] [PubMed] [Google Scholar]
- 77.Haass N.K., Smalley K.S.M., Li L., Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 78.Hsu M.-Y., Meier F.E., Nesbit M., Hsu J.-Y., Van Belle P., Elder D.E., Herlyn M. E-Cadherin Expression in Melanoma Cells Restores Keratinocyte-Mediated Growth Control and Down-Regulates Expression of Invasion-Related Adhesion Receptors. Am. J. Pathol. 2000;156:1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamashiro P.M., Furuya H., Shimizu Y., Kawamori T. Sphingosine kinase 1 mediates head & neck squamous cell carcinoma invasion through sphingosine 1-phosphate receptor 1. Cancer Cell Int. 2014;14:76. doi: 10.1186/s12935-014-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H., Ma Y., He H.-W., Zhao W.-L., Shao R.-G. SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal transition by promoting the autophagy-linked lysosomal degradation of CDH1/E-cadherin in hepatoma cells. Autophagy. 2017;13:900–913. doi: 10.1080/15548627.2017.1291479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milara J., Navarro R., Juan G., Peiró T., Serrano A., Ramón M., Morcillo E., Cortijo J. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- 82.Kono Y., Nishiuma T., Nishimura Y., Kotani Y., Okada T., Nakamura S., Yokoyama M. Sphingosine Kinase 1 Regulates Differentiation of Human and Mouse Lung Fibroblasts Mediated by TGF-β1. Am. J. Respir. Cell Mol. Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 83.Yamaguchi H., Kitayama J., Takuwa N., Arikawa K., Inoki I., Takehara K., Nagawa H., Takuwa Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem. J. 2003;374:715–722. doi: 10.1042/bj20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Braga V.M.M., Machesky L.M., Hall A., Hotchin N.A. The Small GTPases Rho and Rac Are Required for the Establishment of Cadherin-dependent Cell–Cell Contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohkawa Y., Miyazaki S., Hamamura K., Kambe M., Miyata M., Tajima O., Ohmi Y., Yamauchi Y., Furukawa K., Furukawa K. Ganglioside GD3 Enhances Adhesion Signals and Augments Malignant Properties of Melanoma Cells by Recruiting Integrins to Glycolipid-enriched Microdomains. J. Biol. Chem. 2010;285:27213–27223. doi: 10.1074/jbc.M109.087791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohmi Y., Kambe M., Ohkawa Y., Hamamura K., Tajima O., Takeuchi R., Furukawa K., Furukawa K. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE. 2018;13:e0206881. doi: 10.1371/journal.pone.0206881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hodorogea A., Calinescu A., Antohe M., Balaban M., Nedelcu R.I., Turcu G., Ion D.A., Badarau I.A., Popescu C.M., Popescu R., et al. Epithelial-Mesenchymal Transition in Skin Cancers: A Review. Anal. Cell. Pathol. 2019;2019:1–11. doi: 10.1155/2019/3851576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoek K.S., Schlegel N.C., Brafford P., Sucker A., Ugurel S., Kumar R., Weber B.L., Nathanson K.L., Phillips D.J., Herlyn M., et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006;19:290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 89.Levy C., Khaled M., Fisher D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Goding C.R., Arnheiter H. MITF—The first 25 years. Genes Dev. 2019;33:983–1007. doi: 10.1101/gad.324657.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levy C., Lee Y.-N., Nechushtan H., Schueler-Furman O., Sonnenblick A., Hacohen S., Razin E. Identifying a common molecular mechanism for inhibition of MITF and STAT3 by PIAS3. Blood. 2006;107:2839–2845. doi: 10.1182/blood-2005-08-3325. [DOI] [PubMed] [Google Scholar]
- 92.Yasumoto K., Takeda K., Saito H., Watanabe K., Takahashi K., Shibahara S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002;21:2703–2714. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmelz E.M., Roberts P.C., Kustin E.M., Lemonnier L.A., Sullards M.C., Dillehay D.L., Merrill A.H. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001;61:6723–6729. [PubMed] [Google Scholar]
- 94.Liu H., Zhang C.-X., Ma Y., He H.-W., Wang J.-P., Shao R.-G. SphK1 inhibitor SKI II inhibits the proliferation of human hepatoma HepG2 cells via the Wnt5A/β-catenin signaling pathway. Life Sci. 2016;151:23–29. doi: 10.1016/j.lfs.2016.02.098. [DOI] [PubMed] [Google Scholar]
- 95.White C., Alshaker H., Cooper C., Winkler M., Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget. 2016;7:23106–23127. doi: 10.18632/oncotarget.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J.E., Kim S.Y., Jeong Y.-M., Yun H.-Y., Baek K.J., Kwon N.S., Park K.-C., Kim D.-S. The regulatory mechanism of melanogenesis by FTY720, a sphingolipid analogue: The regulation of melanogenesis by FTY720. Exp. Dermatol. 2011;20:237–241. doi: 10.1111/j.1600-0625.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 97.Caramel J., Papadogeorgakis E., Hill L., Browne G.J., Richard G., Wierinckx A., Saldanha G., Osborne J., Hutchinson P., Tse G., et al. A Switch in the Expression of Embryonic EMT-Inducers Drives the Development of Malignant Melanoma. Cancer Cell. 2013;24:466–480. doi: 10.1016/j.ccr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 98.Lu P., White-Gilbertson S., Nganga R., Kester M., Voelkel-Johnson C. Expression of the SNAI2 transcriptional repressor is regulated by C 16 -ceramide. Cancer Biol. Ther. 2019;20:922–930. doi: 10.1080/15384047.2019.1579962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edmond V., Dufour F., Poiroux G., Shoji K., Malleter M., Fouqué A., Tauzin S., Rimokh R., Sergent O., Penna A., et al. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene. 2015;34:996–1005. doi: 10.1038/onc.2014.55. [DOI] [PubMed] [Google Scholar]
- 100.Zheng K., Chen Z., Feng H., Chen Y., Zhang C., Yu J., Luo Y., Zhao L., Jiang X., Shi F. Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin. Cell Death Dis. 2019;10:157. doi: 10.1038/s41419-019-1303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levade T., Andrieu-Abadie N., Micheau O., Legembre P., Ségui B. Sphingolipids modulate the epithelial-mesenchymal transition in cancer. Cell Death Discov. 2015;1:15001. doi: 10.1038/cddiscovery.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mathow D., Chessa F., Rabionet M., Kaden S., Jennemann R., Sandhoff R., Gröne H.-J., Feuerborn A. Zeb1 affects epithelial cell adhesion by diverting glycosphingolipid metabolism. EMBO Rep. 2015;16:321–331. doi: 10.15252/embr.201439333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Battula V.L., Shi Y., Evans K.W., Wang R.-Y., Spaeth E.L., Jacamo R.O., Guerra R., Sahin A.A., Marini F.C., Hortobagyi G., et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012;122:2066–2078. doi: 10.1172/JCI59735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta V., Bhinge K.N., Hosain S.B., Xiong K., Gu X., Shi R., Ho M.-Y., Khoo K.-H., Li S.-C., Li Y.-T., et al. Ceramide glycosylation by glucosylceramide synthase selectively maintains the properties of breast cancer stem cells. J. Biol. Chem. 2012;287:37195–37205. doi: 10.1074/jbc.M112.396390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hosain S.B., Khiste S.K., Uddin M.B., Vorubindi V., Ingram C., Zhang S., Hill R.A., Gu X., Liu Y.-Y. Inhibition of glucosylceramide synthase eliminates the oncogenic function of p53 R273H mutant in the epithelial-mesenchymal transition and induced pluripotency of colon cancer cells. Oncotarget. 2016;7:60575–60592. doi: 10.18632/oncotarget.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hao Y., Baker D., ten Dijke P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019;20:2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsubakihara Y., Moustakas A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β. Int. J. Mol. Sci. 2018;19:3672. doi: 10.3390/ijms19113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xin C., Ren S., Kleuser B., Shabahang S., Eberhardt W., Radeke H., Schäfer-Korting M., Pfeilschifter J., Huwiler A. Sphingosine 1-Phosphate Cross-activates the Smad Signaling Cascade and Mimics Transforming Growth Factor-β-induced Cell Responses. J. Biol. Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- 109.Sauer B., Vogler R., von Wenckstern H., Fujii M., Anzano M.B., Glick A.B., Schäfer-Korting M., Roberts A.B., Kleuser B. Involvement of Smad Signaling in Sphingosine 1-Phosphate-mediated Biological Responses of Keratinocytes. J. Biol. Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- 110.Radeke H.H., von Wenckstern H., Stoidtner K., Sauer B., Hammer S., Kleuser B. Overlapping Signaling Pathways of Sphingosine 1-Phosphate and TGF-β in the Murine Langerhans Cell Line XS52. J. Immunol. 2005;174:2778–2786. doi: 10.4049/jimmunol.174.5.2778. [DOI] [PubMed] [Google Scholar]
- 111.Zeng Y., Yao X., Chen L., Yan Z., Liu J., Zhang Y., Feng T., Wu J., Liu X. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/syndecan-1/TGF-β autocrine loop. Oncotarget. 2016;7:63324–63337. doi: 10.18632/oncotarget.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y.-N., Zhang H., Zhang L., Cai T.-T., Huang D.-J., He J., Ni H.-H., Zhou F.-J., Zhang X.-S., Li J. Sphingosine 1 phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T cell expansion: Leading to poor survival in bladder cancer. Cell Death Dis. 2019;10:50. doi: 10.1038/s41419-018-1298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller A.V., Alvarez S.E., Spiegel S., Lebman D.A. Sphingosine Kinases and Sphingosine-1-Phosphate Are Critical for Transforming Growth Factor β-Induced Extracellular Signal-Regulated Kinase 1 and 2 Activation and Promotion of Migration and Invasion of Esophageal Cancer Cells. Mol. Cell. Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamanaka M., Shegogue D., Pei H., Bu S., Bielawska A., Bielawski J., Pettus B., Hannun Y.A., Obeid L., Trojanowska M. Sphingosine Kinase 1 (SPHK1) Is Induced by Transforming Growth Factor-β and Mediates TIMP-1 Up-regulation. J. Biol. Chem. 2004;279:53994–54001. doi: 10.1074/jbc.M410144200. [DOI] [PubMed] [Google Scholar]
- 115.Verfaillie A., Imrichova H., Atak Z.K., Dewaele M., Rambow F., Hulselmans G., Christiaens V., Svetlichnyy D., Luciani F., Van den Mooter L., et al. Decoding the regulatory landscape of melanoma reveals TEADS as regulators of the invasive cell state. Nat. Commun. 2015;6:6683. doi: 10.1038/ncomms7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nallet-Staub F., Marsaud V., Li L., Gilbert C., Dodier S., Bataille V., Sudol M., Herlyn M., Mauviel A. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J. Invest. Dermatol. 2014;134:123–132. doi: 10.1038/jid.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller E., Yang J., DeRan M., Wu C., Su A.I., Bonamy G.M.C., Liu J., Peters E.C., Wu X. Identification of Serum-Derived Sphingosine-1-Phosphate as a Small Molecule Regulator of YAP. Chem. Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Yu F.-X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J., Yuan H., Tumaneng K., Li H., et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pors S.E., Harðardóttir L., Olesen H.Ø., Riis M.L., Jensen L.B., Andersen A.S., Cadenas J., Grønning A.P., Colmorn L.B., Dueholm M., et al. Effect of sphingosine-1-phosphate on activation of dormant follicles in murine and human ovarian tissue. Mol. Hum. Reprod. 2020:gaaa022. doi: 10.1093/molehr/gaaa022. [DOI] [PubMed] [Google Scholar]
- 120.Huang L.S., Sudhadevi T., Fu P., Punathil-Kannan P.-K., Ebenezer D.L., Ramchandran R., Putherickal V., Cheresh P., Zhou G., Ha A.W., et al. Sphingosine Kinase 1/S1P Signaling Contributes to Pulmonary Fibrosis by Activating Hippo/YAP Pathway and Mitochondrial Reactive Oxygen Species in Lung Fibroblasts. Int. J. Mol. Sci. 2020;21:2064. doi: 10.3390/ijms21062064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kemppainen K., Wentus N., Lassila T., Laiho A., Törnquist K. Sphingosylphosphorylcholine regulates the Hippo signaling pathway in a dual manner. Cell. Signal. 2016;28:1894–1903. doi: 10.1016/j.cellsig.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 122.Arikawa K., Takuwa N., Yamaguchi H., Sugimoto N., Kitayama J., Nagawa H., Takehara K., Takuwa Y. Ligand-dependent Inhibition of B16 Melanoma Cell Migration and Invasion via Endogenous S1P 2 G Protein-coupled Receptor: REQUIREMENT OF INHIBITION OF CELLULAR RAC ACTIVITY. J. Biol. Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- 123.Carpinteiro A., Becker K.A., Japtok L., Hessler G., Keitsch S., Požgajovà M., Schmid K.W., Adams C., Müller S., Kleuser B., et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol. Med. 2015;7:714–734. doi: 10.15252/emmm.201404571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsuchida T., Saxton R.E., Morton D.L., Irie R.F. Gangliosides of human melanoma. J. Natl. Cancer Inst. 1987;78:45–54. doi: 10.1093/jnci/78.1.45. [DOI] [PubMed] [Google Scholar]
- 125.Sawada M., Moriya S., Shineha R., Satomi S., Miyagi T. Comparative study of sialidase activity and G(M3) content in B16 melanoma variants with different metastatic potential. Acta Biochim. Pol. 1998;45:343–349. doi: 10.18388/abp.1998_4229. [DOI] [PubMed] [Google Scholar]
- 126.Saha S., Mohanty K.C. Enhancement of metastatic potential of mouse B16-melanoma cells to lung after treatment with gangliosides of B-16-melanoma cells of higher metastatic potential to lung. Indian J. Exp. Biol. 2003;41:1253–1258. [PubMed] [Google Scholar]
- 127.Liu J.-W., Sun P., Yan Q., Paller A.S., Gerami P., Ho N., Vashi N., Le Poole I.C., Wang X.-Q. De-N-acetyl GM3 Promotes Melanoma Cell Migration and Invasion through Urokinase Plasminogen Activator Receptor Signaling-Dependent MMP-2 Activation. Cancer Res. 2009;69:8662–8669. doi: 10.1158/0008-5472.CAN-09-1099. [DOI] [PubMed] [Google Scholar]
- 128.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C.M., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao D., Barry S., Kmetz D., Egger M., Pan J., Rai S.N., Qu J., McMasters K.M., Hao H. Melanoma cell–derived exosomes promote epithelial–mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. 2016;376:318–327. doi: 10.1016/j.canlet.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hood J.L., San R.S., Wickline S.A. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 134.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., Simons M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 135.López-Montero I., Vélez M., Devaux P.F. Surface tension induced by sphingomyelin to ceramide conversion in lipid membranes. Biochim. Biophys. Acta BBA Biomembr. 2007;1768:553–561. doi: 10.1016/j.bbamem.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 136.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral Sphingomyelinase 2 (nSMase2)-dependent Exosomal Transfer of Angiogenic MicroRNAs Regulate Cancer Cell Metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kajimoto T., Okada T., Miya S., Zhang L., Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 139.Mohamed N.N.I., Okada T., Kajimoto T., Nakamura S.-I. Essential Role of Sphingosine Kinase 2 in the Regulation of Cargo Contents in the Exosomes from K562 Cells. Kobe J. Med. Sci. 2018;63:E123–E129. [PMC free article] [PubMed] [Google Scholar]
- 140.Kajimoto T., Mohamed N.N.I., Badawy S.M.M., Matovelo S.A., Hirase M., Nakamura S., Yoshida D., Okada T., Ijuin T., Nakamura S. Involvement of Gβγ subunits of G i protein coupled with S1P receptor on multivesicular endosomes in F-actin formation and cargo sorting into exosomes. J. Biol. Chem. 2018;293:245–253. doi: 10.1074/jbc.M117.808733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 142.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 143.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mandala S., Hadju R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G.-J., Card D. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 145.Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 146.Resop R.S., Douaisi M., Craft J., Jachimowski L.C.M., Blom B., Uittenbogaart C.H. Sphingosine-1-phosphate/sphingosine-1-phosphate receptor 1 signaling is required for migration of naive human T cells from the thymus to the periphery. J. Allergy Clin. Immunol. 2016;138:551–557.e8. doi: 10.1016/j.jaci.2015.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lo C.G., Xu Y., Proia R.L., Cyster J.G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bankovich A.J., Shiow L.R., Cyster J.G. CD69 Suppresses Sphingosine 1-Phosophate Receptor-1 (S1P 1 ) Function through Interaction with Membrane Helix 4. J. Biol. Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shiow L.R., Rosen D.B., Brdičková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 150.Mackay L.K., Braun A., Macleod B.L., Collins N., Tebartz C., Bedoui S., Carbone F.R., Gebhardt T. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 151.Skon C.N., Lee J.-Y., Anderson K.G., Masopust D., Hogquist K.A., Jameson S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amsen D., van Gisbergen K.P.J.M., Hombrink P., van Lier R.A.W. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 2018;19:538–546. doi: 10.1038/s41590-018-0114-2. [DOI] [PubMed] [Google Scholar]
- 153.Gebhardt T., Wakim L.M., Eidsmo L., Reading P.C., Heath W.R., Carbone F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 154.Park S.L., Buzzai A., Rautela J., Hor J.L., Hochheiser K., Effern M., McBain N., Wagner T., Edwards J., McConville R., et al. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature. 2019;565:366–371. doi: 10.1038/s41586-018-0812-9. [DOI] [PubMed] [Google Scholar]
- 155.Menares E., Gálvez-Cancino F., Cáceres-Morgado P., Ghorani E., López E., Díaz X., Saavedra-Almarza J., Figueroa D.A., Roa E., Quezada S.A., et al. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat. Commun. 2019;10:4401. doi: 10.1038/s41467-019-12319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hochheiser K., Aw Yeang H.X., Wagner T., Tutuka C., Behren A., Waithman J., Angel C., Neeson P.J., Gebhardt T., Gyorki D.E. Accumulation of CD103 + CD8 + T cells in a cutaneous melanoma micrometastasis. Clin. Transl. Immunol. 2019;8 doi: 10.1002/cti2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Drouillard A., Neyra A., Mathieu A.-L., Marçais A., Wencker M., Marvel J., Belot A., Walzer T. Human Naive and Memory T Cells Display Opposite Migratory Responses to Sphingosine-1 Phosphate. J. Immunol. 2018;200:551–557. doi: 10.4049/jimmunol.1701278. [DOI] [PubMed] [Google Scholar]
- 158.Sic H., Kraus H., Madl J., Flittner K.-A., von Münchow A.L., Pieper K., Rizzi M., Kienzler A.-K., Ayata K., Rauer S., et al. Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J. Allergy Clin. Immunol. 2014;134:420–428.e15. doi: 10.1016/j.jaci.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 159.Walzer T., Chiossone L., Chaix J., Calver A., Carozzo C., Garrigue-Antar L., Jacques Y., Baratin M., Tomasello E., Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 160.Drouillard A., Mathieu A.-L., Marçais A., Belot A., Viel S., Mingueneau M., Guckian K., Walzer T. S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J. Allergy Clin. Immunol. 2018;141:2265–2268.e1. doi: 10.1016/j.jaci.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 161.Jenne C.N., Enders A., Rivera R., Watson S.R., Bankovich A.J., Pereira J.P., Xu Y., Roots C.M., Beilke J.N., Banerjee A., et al. T-bet–dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mayol K., Biajoux V., Marvel J., Balabanian K., Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 2011;118:4863–4871. doi: 10.1182/blood-2011-06-362574. [DOI] [PubMed] [Google Scholar]
- 163.Liu G., Yang K., Burns S., Shrestha S., Chi H. The S1P1-mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nat. Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chakraborty P., Vaena S.G., Thyagarajan K., Chatterjee S., Al-Khami A., Selvam S.P., Nguyen H., Kang I., Wyatt M.W., Baliga U., et al. Pro-Survival Lipid Sphingosine-1-Phosphate Metabolically Programs T Cells to Limit Anti-tumor Activity. Cell Rep. 2019;28:1879–1893.e7. doi: 10.1016/j.celrep.2019.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sanger Mouse Genetics Project. van der Weyden L., Arends M.J., Campbell A.D., Bald T., Wardle-Jones H., Griggs N., Velasco-Herrera M.D.C., Tüting T., Sansom O.J., et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature. 2017;541:233–236. doi: 10.1038/nature20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Assi E., Cervia D., Bizzozero L., Capobianco A., Pambianco S., Morisi F., De Palma C., Moscheni C., Pellegrino P., Clementi E., et al. Modulation of Acid Sphingomyelinase in Melanoma Reprogrammes the Tumour Immune Microenvironment. Mediators Inflamm. 2015;2015:1–13. doi: 10.1155/2015/370482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Coerdt I., Full-Scharrer G., Hösl E. Long-term results in surgical treatment of cleft lips and palates. Prog. Pediatr. Surg. 1977;10:1–3. [PubMed] [Google Scholar]
- 168.Nakagawa R., Serizawa I., Motoki K., Sato M., Ueno H., Iijima R., Nakamura H., Shimosaka A., Koezuka Y. Antitumor activity of alpha-galactosylceramide, KRN7000, in mice with the melanoma B16 hepatic metastasis and immunohistological study of tumor infiltrating cells. Oncol. Res. 2000;12:51–58. doi: 10.3727/096504001108747521. [DOI] [PubMed] [Google Scholar]
- 169.Ghosh S., Juin S.K., Nandi P., Majumdar S.B., Bose A., Baral R., Sil P.C., Majumdar S. PKCζ mediated anti-proliferative effect of C2 ceramide on neutralization of the tumor microenvironment and melanoma regression. Cancer Immunol. Immunother. CII. 2020;69:611–627. doi: 10.1007/s00262-020-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tiwary S., Berzofsky J.A., Terabe M. Altered Lipid Tumor Environment and Its Potential Effects on NKT Cell Function in Tumor Immunity. Front. Immunol. 2019;10:2187. doi: 10.3389/fimmu.2019.02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bay S., Fort S., Birikaki L., Ganneau C., Samain E., Coïc Y.-M., Bonhomme F., Dériaud E., Leclerc C., Lo-Man R. Induction of a melanoma-specific antibody response by a monovalent, but not a divalent, synthetic GM2 neoglycopeptide. ChemMedChem. 2009;4:582–587. doi: 10.1002/cmdc.200900032. [DOI] [PubMed] [Google Scholar]
- 172.Fernandez L.E., Gabri M.R., Guthmann M.D., Gomez R.E., Gold S., Fainboim L., Gomez D.E., Alonso D.F. NGcGM3 ganglioside: A privileged target for cancer vaccines. Clin. Dev. Immunol. 2010;2010:814397. doi: 10.1155/2010/814397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pérez K., Osorio M., Hernández J., Carr A., Fernández L.E. NGcGM3/VSSP vaccine as treatment for melanoma patients. Hum. Vaccines Immunother. 2013;9:1237–1240. doi: 10.4161/hv.24115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bernhard H., Meyer zum Büschenfelde K.H., Dippold W.G. Ganglioside GD3 shedding by human malignant melanoma cells. Int. J. Cancer. 1989;44:155–160. doi: 10.1002/ijc.2910440127. [DOI] [PubMed] [Google Scholar]
- 175.Portoukalian J., Zwingelstein G., Abdul-Malak N., Doré J.F. Alteration of gangliosides in plasma and red cells of humans bearing melanoma tumors. Biochem. Biophys. Res. Commun. 1978;85:916–920. doi: 10.1016/0006-291X(78)90630-7. [DOI] [PubMed] [Google Scholar]
- 176.Péguet-Navarro J., Sportouch M., Popa I., Berthier O., Schmitt D., Portoukalian J. Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis. J. Immunol. Baltim. Md 1950. 2003;170:3488–3494. doi: 10.4049/jimmunol.170.7.3488. [DOI] [PubMed] [Google Scholar]
- 177.Bennaceur K., Popa I., Chapman J.A., Migdal C., Péguet-Navarro J., Touraine J.-L., Portoukalian J. Different mechanisms are involved in apoptosis induced by melanoma gangliosides on human monocyte-derived dendritic cells. Glycobiology. 2009;19:576–582. doi: 10.1093/glycob/cwp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tsao C.-Y., Sabbatino F., Cheung N.-K.V., Hsu J.C.-F., Villani V., Wang X., Ferrone S. Anti-proliferative and pro-apoptotic activity of GD2 ganglioside-specific monoclonal antibody 3F8 in human melanoma cells. Oncoimmunology. 2015;4:e1023975. doi: 10.1080/2162402X.2015.1023975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kushner B.H., Cheung I.Y., Modak S., Basu E.M., Roberts S.S., Cheung N.-K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing With Granulocyte-Macrophage Colony-Stimulating Factor in Patients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018;4:1729–1735. doi: 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kramer K., Pandit-Taskar N., Humm J.L., Zanzonico P.B., Haque S., Dunkel I.J., Wolden S.L., Donzelli M., Goldman D.A., Lewis J.S., et al. A phase II study of radioimmunotherapy with intraventricular 131 I-3F8 for medulloblastoma. Pediatr. Blood Cancer. 2018;65 doi: 10.1002/pbc.26754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu J., Wu X., Yan J., Yu H., Xu L., Chi Z., Sheng X., Si L., Cui C., Dai J., et al. Anti-GD2/4-1BB chimeric antigen receptor T cell therapy for the treatment of Chinese melanoma patients. J. Hematol. Oncol. J. Hematol. Oncol. 2018;11:1. doi: 10.1186/s13045-017-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Albertini M.R., Yang R.K., Ranheim E.A., Hank J.A., Zuleger C.L., Weber S., Neuman H., Hartig G., Weigel T., Mahvi D., et al. Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma. Cancer Immunol. Immunother. CII. 2018;67:1647–1658. doi: 10.1007/s00262-018-2223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu Y., Deng W., McGinley E.C., Klinke D.J. Melanoma exosomes deliver a complex biological payload that upregulates PTPN11 to suppress T lymphocyte function. Pigment Cell Melanoma Res. 2017;30:203–218. doi: 10.1111/pcmr.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou J., Yang Y., Wang W., Zhang Y., Chen Z., Hao C., Zhang J. Melanoma-released exosomes directly activate the mitochondrial apoptotic pathway of CD4+ T cells through their microRNA cargo. Exp. Cell Res. 2018;371:364–371. doi: 10.1016/j.yexcr.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 185.Düchler M., Czernek L., Peczek L., Cypryk W., Sztiller-Sikorska M., Czyz M. Melanoma-Derived Extracellular Vesicles Bear the Potential for the Induction of Antigen-Specific Tolerance. Cells. 2019;8:665. doi: 10.3390/cells8070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vignard V., Labbé M., Marec N., André-Grégoire G., Jouand N., Fonteneau J.-F., Labarrière N., Fradin D. MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma. Cancer Immunol. Res. 2020;8:255–267. doi: 10.1158/2326-6066.CIR-19-0522. [DOI] [PubMed] [Google Scholar]
- 187.Sharma P., Diergaarde B., Ferrone S., Kirkwood J.M., Whiteside T.L. Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci. Rep. 2020;10:92. doi: 10.1038/s41598-019-56542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Poggio M., Hu T., Pai C.-C., Chu B., Belair C.D., Chang A., Montabana E., Lang U.E., Fu Q., Fong L., et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177:414–427.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Falkson C.I., Ibrahim J., Kirkwood J.M., Coates A.S., Atkins M.B., Blum R.H. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: An Eastern Cooperative Oncology Group study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998;16:1743–1751. doi: 10.1200/JCO.1998.16.5.1743. [DOI] [PubMed] [Google Scholar]
- 191.Middleton M.R., Grob J.J., Aaronson N., Fierlbeck G., Tilgen W., Seiter S., Gore M., Aamdal S., Cebon J., Coates A., et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 192.Avril M.F., Aamdal S., Grob J.J., Hauschild A., Mohr P., Bonerandi J.J., Weichenthal M., Neuber K., Bieber T., Gilde K., et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: A phase III study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 193.Flaherty K.T., Robert C., Hersey P., Nathan P., Garbe C., Milhem M., Demidov L.V., Hassel J.C., Rutkowski P., Mohr P., et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 194.Tian Y., Guo W. A Review of the Molecular Pathways Involved in Resistance to BRAF Inhibitors in Patients with Advanced-Stage Melanoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020;26:e920957. doi: 10.12659/MSM.920957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O., Patt D., Chen T.-T., Berman D.M., Wolchok J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 197.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 198.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 199.Postow M.A. Managing immune checkpoint-blocking antibody side effects. Am. Soc. Clin. Oncol. Educ. Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 200.Suo A., Chan Y., Beaulieu C., Kong S., Cheung W.Y., Monzon J.G., Smylie M., Walker J., Morris D., Cheng T. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncologist. 2020;25:438–446. doi: 10.1634/theoncologist.2019-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cervia D., Assi E., De Palma C., Giovarelli M., Bizzozero L., Pambianco S., Di Renzo I., Zecchini S., Moscheni C., Vantaggiato C., et al. Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin. Oncotarget. 2016;7 doi: 10.18632/oncotarget.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ishitsuka A., Fujine E., Mizutani Y., Tawada C., Kanoh H., Banno Y., Seishima M. FTY720 and cisplatin synergistically induce the death of cisplatin-resistant melanoma cells through the downregulation of the PI3K pathway and the decrease in epidermal growth factor receptor expression. Int. J. Mol. Med. 2014;34:1169–1174. doi: 10.3892/ijmm.2014.1882. [DOI] [PubMed] [Google Scholar]
- 203.Bektas M., Jolly P.S., Müller C., Eberle J., Spiegel S., Geilen C.C. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: Role of Bcl-2 expression. Oncogene. 2005;24:178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 204.Takahashi T., Abe N., Kanoh H., Banno Y., Seishima M. Synergistic effects of vemurafenib and fingolimod (FTY720) in vemurafenib-resistant melanoma cell lines. Mol. Med. Rep. 2018;18:5151–5158. doi: 10.3892/mmr.2018.9537. [DOI] [PubMed] [Google Scholar]
- 205.Ji C., Yang Y.-L., He L., Gu B., Xia J.-P., Sun W.-L., Su Z.-L., Chen B., Bi Z.-G. Increasing ceramides sensitizes genistein-induced melanoma cell apoptosis and growth inhibition. Biochem. Biophys. Res. Commun. 2012;421:462–467. doi: 10.1016/j.bbrc.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 206.Tran M.A., Smith C.D., Kester M., Robertson G.P. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:3571–3581. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 207.Zhang P., Fu C., Hu Y., Dong C., Song Y., Song E. C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCζ tumor-suppressive activities and regulating integrin affinity modulation. Sci. Rep. 2015;5:9275. doi: 10.1038/srep09275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kobayashi E., Motoki K., Uchida T., Fukushima H., Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 209.Guthmann M.D., Bitton R.J., Carnero A.J.L., Gabri M.R., Cinat G., Koliren L., Lewi D., Fernandez L.E., Alonso D.F., Gómez D.E., et al. Active specific immunotherapy of melanoma with a GM3 ganglioside-based vaccine: A report on safety and immunogenicity. J. Immunother. 2004;27:442–451. doi: 10.1097/00002371-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 210.Irie R.F., Ollila D.W., O’Day S., Morton D.L. Phase I pilot clinical trial of human IgM monoclonal antibody to ganglioside GM3 in patients with metastatic melanoma. Cancer Immunol. Immunother. CII. 2004;53:110–117. doi: 10.1007/s00262-003-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Musumarra G., Barresi V., Condorelli D.F., Scirè S. A bioinformatic approach to the identification of candidate genes for the development of new cancer diagnostics. Biol. Chem. 2003;384:321–327. doi: 10.1515/BC.2003.037. [DOI] [PubMed] [Google Scholar]
- 212.Ene (Nicolae) C.-D., Nicolae I. Gangliosides and Antigangliosides in Malignant Melanoma. In: Murph M., editor. Melanoma—Current Clinical Management and Future Therapeutics. Intech; London, UK: 2015. [Google Scholar]