Abstract
Background
Passion fruit (Passiflora edulis Sims) is an important horticultural crop in the tropics and subtropics, where it has great commercial potential due to high demand for fresh edible fruits and processed juice as well as source of raw materials in cosmetic industries. Genetic engineering shows great potential in passion fruit improvement and can compensate for the limitations of conventional breeding. Despite the success achieved in genetic modification of few passion fruit varieties, transgenic passion fruit production is still difficult for farmer-preferred cultivars. Therefore, it is important to establish a simple and fast Agrobacterium-mediated cell transformation of commercial hybrid passion fruit KPF4 (Passiflora edulis f. edulis × Passiflora edulis f. flavicarpa).
Results
In the present study, we have developed a simple and fast Agrobacterium-mediated transformation system for hybrid passion fruit KPF4 using leaf disc explants. Factors affecting the rate of transient beta (β)-glucuronidase (gusA) expression and consequently transformation efficiency were optimized as follows: Agrobacterium cell density with an OD600 of 0.5, 30 min infection time, 3 days of co-cultivation duration and the incorporation of 200 µM acetosyringone into Agrobacterium infection suspension medium. Using the optimized conditions, transgenic plants of KPF4 were produced within 2 months with an average transformation efficiency of 0.67%. The β-glucuronidase (GUS) histochemical staining confirmed the expression and integration of an intron-containing gusA gene into transformed leaf discs and transgenic plant lines of KPF4. The presence of gusA gene in the transgenic plants was confirmed by polymerase chain reaction (PCR). The results confirmed that the gusA gene was efficiently integrated into the passion fruit genome.
Conclusions
The developed transformation protocol is simple and rapid and could be useful for functional genomic studies and transferring agronomically important traits into passion fruit hybrid KPF4. This study developed a method that can be used to transfer traits such as resistance to viral diseases, low fruit quality and short storage life. To the best of our knowledge, this is the first report on genetic transformation system for commercial passion fruit hybrid KPF4.
Keywords: Passiflora edulis Sims, Agrobacterium-mediated transformation, Transient gusA expression, Transformation efficiency
Background
Passion fruit (Passiflora edulis Sims), a dicotyledonous perennial plant with shallow roots and woody vines, is widely cultivated in the sub-tropics and tropics [1]. It is an important fruit crop due to its nutritional, medicinal (as sedatives, antiplasmodic, and antibacterial) and ornamental value. Nutritionally, the ripe fruits are an important source of minerals and vitamins (including ascorbic acid), phytoconstituents and phenolic compounds [2, 3]. The use of passion fruit in cosmetic industry has also contributed to the increased production acreage in sub-tropical and tropical countries [4]. However, the low genetic variability, self and inter-specific incompatibility, high levels of ploidy, lack of pathogen resistance genes in the available cultivars and elimination of deleterious genes by many cycles of selfing and backcrossing hinder passion fruit improvement efforts by conventional breeding [5, 6]. To circumvent the limitations of conventional breeding, genetic transformation is a potential alternative and complementary strategy to accelerate the production of passion fruit cultivars with improved traits.
Agrobacterium tumefaciens and particle bombardment are commonly used methods for genetic engineering of plants. However, Agrobacterium-mediated transformation is popular in many plant species due to the integration of single-copy transgenes with minimal rearrangements, its high efficiency of transgene integration and simplicity of the equipment used [7, 8]. Genetically modified passion fruit plants by Agrobacterium-mediated transformation were first reported about 25 years ago [9]. Since then, limited progress has been achieved in the development of genetic transformation technologies and subsequent gene-function assessments in only three passion fruit germplasm. Trevisan et al. [10] reported transformation of two Brazilian yellow passion fruit (Passiflora edulis f. flavicarpa) cultivars IAC-275 and IAC-277 using a gene construct for resistance to Cowpea aphid borne mosaic virus (CABMV), which resulted in transformation efficiencies of 0.11 and 0.21%, respectively. Transformation of Passiflora alata for resistance to CABMV has also been reported with 0.89% transformation efficiency [11]. Recently, Tuhaise et al. [12] documented a transformation efficiency of 0.456% following genetic transformation of Uganda’s yellow passion fruit. The transformation efficiencies in these studies were determined by dividing the number of PCR positive plant lines by the number of explants inoculated, expressed as a percentage. These previous reports involved complicated procedures which took more than 5 months to generate transgenic seedlings. In addition, previous reports on transformation are based on model yellow passion fruit cultivars, which are not farmer-preferred due to low yield. To exploit the desirable traits of farmer-preferred passion fruit varieties, it is important to develop simple, rapid and efficient transformation system for these varieties.
The success of an Agrobacterium-mediated transformation system is influenced by many variable parameters including the type of explants, duration of pre-culture of explant, Agrobacterium strain, bacterial cell density, infection time, co-cultivation duration, concentration of acetosyringone, cultivars and antibiotic selection [13]. Here we present a transformation protocol for the popular farmer-preferred passion fruit hybrid KPF4 [purple passion fruit (Passiflora edulis f. edulis) × yellow passion fruit (Passiflora edulis f. flavicarpa)]. KPF4 is a sweet yellow passion fruit developed and released by Kenya Agricultural Research and Livestock Organization (KARLO). This variety is a farmer-preferred passion fruit due to production of large fruits with sweet and high juice content [14]. It is drought tolerant and well adapted to the coastal lowlands of Kenya [14], a region characterized by erratic rainfall. However, KPF4 is susceptible to passion fruit woodiness disease (PWD), which significantly affects passion fruit production [15], hence genetic improvement of passion fruit is required.
The aim of this study was to optimize factors that affect Agrobacterium-mediated transformation of KPF4 and establish a simple Agrobacterium-mediated protocol for commercial hybrid passion fruit KPF4. We initially established a simple and rapid plant regeneration protocol from leaf disc explants within 8 weeks. Based on this, an Agrobacterium-mediated DNA transformation method for KPF4 was developed. The method is simple and fast and could facilitate future genetic improvement of this important fruit crop. The method also provides an opportunity for introduction of agronomic traits such as resistance to viruses and longer storage of the edible fruits.
Materials and methods
In vitro seed germination to generate leaf explants
Ripe fruits of passion fruit variety KPF4 (Passiflora edulis f. edulis × Passiflora edulis f. flavicarpa) were sourced from Kenya Agricultural and Livestock Research Organization (KALRO), Thika (GPS coordinates: S0100078, E03704810, 4992 m above sea level). Mature seeds were extracted from ripe fruits of variety KPF4, rinsed with tap water and dried in the sun for 72 h. The seeds were surface-sterilized with 70% (v/v) ethanol for 5 min, followed by 2.5% sodium hypochlorite (v/v) for 20 min and then rinsed four times in double sterile distilled water. An incision of approximately 2 mm was carefully made on the lateral sides of each seed. The seeds were then placed on sterile seed germination medium (SGM; Additional file 1: Table S1). The SGM medium was prepared by dissolving Murashige and Skoog (MS) solid medium with vitamins [16], 2% (w/v) sucrose and the pH was adjusted to 5.8 using 0.1 N NaOH, followed by addition of 0.24% (w/v) gelrite. The medium was then autoclaved at 121 °C for 15 min. The jars with surface-sterilized seeds were incubated at 26 ± 2 °C for germination. Leaves from 21-day-old seedlings were excised, aseptically cut into 6 mm2 disks and used as explants for regeneration and transformation experiments.
Optimization of a regeneration system from KPF4 leaf disc explants
The excised disc explants (6 mm2) were placed on shoot induction medium (SIM; MS with vitamins supplemented with 3% (w/v) sucrose and 0.24% (w/v) gelrite; pH adjusted to 5.8 using 0.1 N NaOH). The effect of 6-benzyl amino purine (BAP) at different concentrations (0, 1, 2 and 3 mg L−1) was evaluated on regeneration of shoots. Tissue cultures were incubated at 28 ± 2 °C, light intensity of approximately 60 μmole photons m−2s−1 and a 16 h photoperiod for 4 weeks. After incubation, the effect of different concentrations of BAP was evaluated based on the regeneration frequency and the number of shoots per explant. The regeneration efficiency was calculated as percentage of the number of leaf disc explants with induced shoots per total number of explants cultured. Micro-shoots were transferred onto shoot development medium (SDM; MS with vitamins containing 0.1 mg L−1 BAP, 3% (w/v) sucrose and 0.24% (w/v) gelrite, pH adjusted to 5.8 using 0.1 N NaOH) for 2 weeks. Well-developed shoots were transferred as stem cuttings with leaves to rooting initiation and development medium (RIM; MS with vitamins augmented with 0.1 mg L−1 naphthaleneacetic acid (NAA), 3% (w/v) sucrose and 0.24% (w/v) gelrite, pH adjusted to 5.8 using 0.1 N NaOH). After 2 weeks, the rooted plantlets were removed from the jars, washed with running tap water to remove agar and transferred into small pots containing sterilized forest soil and sand (1:1) for acclimatization. The humidity of the plants in the pots was maintained high by covering the pots with polyethylene bags.
Agrobacterium strain and plasmid vector
The hypervirulent Agrobacterium tumefaciens strain LBA4404 (Invitrogen, USA) containing binary vector pCAMBIA 1301 (http://www.cambia.org.au/) was used for optimization of transformation experiments. The pCAMBIA 1301 contains a hygromycin phosphotransferase (hpt) gene as a selection marker and a gusA gene with a castor bean catalase intron as a reporter gene, both driven by a CaMV35S promoter (Additional file 1: Figure S1).
Preparation of Agrobacterium suspension cultures
Hypervirulent Agrobacterium strain LBA 4404 harbouring pCAMBIA 1301 stored at −80 °C was revived by streaking on Luria–bertani (LB) medium (composed of 10 g L−1 tryptone, 5 g L−1 yeast extract, 10 g L−1 sodium chloride, 15 g L−1 agar and pH adjusted to 7.2 with 0.1 N NaOH) plates containing kanamycin (50 mg L−1) and rifampicin (50 mg L−1). Single colonies obtained from the streaked LB agar petri plates incubated at 28 °C were used to initiate 2 ml LB medium starter cultures. After 48 h shaking at 150 rpm at 28 °C, the starter culture was used as an inoculum to start a bacterial suspension of 20 ml LB with the same antibiotics and grown overnight on a shaker at 150 rpm to obtain an optical density (OD600) of 1.0. The bacterial culture was then centrifuged at 3500 rpm for 15 min, the supernatant was poured off and the pellet was re-suspended in 20 ml liquid Murashige and Skoog (MS) with vitamins medium (inoculation medium), supplemented with acetosyringone (Sigma Chemical Co.) at a concentration of 200 μM. The bacteria were further cultivated for 1 h at 25 °C with shaking at 100 rpm. The optical density (OD600) of the bacterial suspension was then adjusted to 0.5 using MS with vitamins medium and the suspension was subsequently used for transformation.
Optimization of parameters affecting Agrobacterium-mediated transformation
The factors (bacteria cell density, duration of infection, co-cultivation period and acetosyringone concentration) affecting Agrobacterium-mediated transformation were evaluated. For the optical density of bacteria, the pellet was dissolved in the inoculation medium to obtain an OD600 of 0.1, 0.25, 0.5, and 0.75. Five different durations of infection (10, 20, 30, 40 and 50 min) of the leaf disks were evaluated. The inoculated leaf disks were co-cultivated for different periods of 0, 1, 2, 3, 4 and 5 days. The effect of acetosyringone (Sigma Chemical Co.) at different concentrations (0, 50, 100, 150, 200, 450 µM) was also tested. The influence of these factors (bacteria density, duration of infection, co-cultivation period and concentration of acetosyringone) on transfer of transgene by Agrobacterium to the leaf disc explants and transformation efficiency was evaluated by monitoring transient gusA expression after co-cultivation on MS with vitamins supplemented with 3% sucrose, 2 mg L−1 benzyl amino purine, BAP, and 0.24% (w/v) gelrite, pH adjusted to 5.8 with 0.1 N NaOH. The experiments were performed using thirty (30) explants for each treatment with three replicates. The obtained data were expressed as percentage of explants showing blue coloration after GUS staining.
Transformation, selection and regeneration of putative transgenic plants
Inoculation and co-cultivation of leaf discs
After optimization of factors affecting transformation efficiency, the optimum parameters were used for subsequent Agrobacterium-mediated transformation of leaf discs to generate transgenic plants. A total of 300 leaf disc explants were used for each experiment and the experiments were done in triplicates. The leaf disc explants (approximately 6 mm2) were injured 10 times using sterile needles and immersed in Agrobacterium suspension culture (OD600 = 0.5) supplemented with 200 μM acetosyringone followed by gentle shaking at 25 rpm for 30 min at room temperature (23 °C). After inoculation, the leaf disc explants were blotted dry on sterile paper towels and cultured on co-cultivation medium (MS supplemented with 3% (w/v) sucrose, 2 mg L−1 benzylaminopurine, BAP, and 0.24% (w/v) gelrite, pH adjusted to 5.8 with 0.1 N NaOH) in Petri plates for 3 days under dark condition at 22 ± 1 °C, light intensity of 60 μmole photons m−2 s−1 and a photoperiod of 16/8 h. The leaf disc explants were washed four times with sterile liquid co-cultivation medium (MS supplemented with 2 mg L−1 BAP and 3% (w/v) sucrose, pH adjusted to 5.8 with 0.1 N NaOH) supplemented with 450 mg L−1 cefotaxime, blotted dry on sterile paper towels and cultured onto resting medium (RM; MS with vitamins supplemented with 2 mg L−1 BAP, 3% (w/v) sucrose, 2.4% (w/v) gelrite, pH adjusted to 5.8 with 0.1 N NaOH) containing 450 mg L−1 cefotaxime for 4 days, 28 °C with 16/8 h photoperiod.
Antibiotic selection and regeneration of putatively transformed plant lines
After 4 days of resting stage, the transformed explants were transferred onto fresh shoot induction medium (SIM; MS supplemented with 2 mg L−1 BAP, 3% (w/v) sucrose and 0.24% (w/v) gelrite, pH adjusted to 5.8 with 0.1 N NaOH) containing 450 and 7.5 mg L−1 of cefotaxime and hygromycin, respectively. The explants were incubated for 2 weeks at 28 °C and a 16/8 h photoperiod. The cultures were transferred to fresh SIM supplemented with 450 mg L−1 cefotaxime and 7.5 mg L−1 hygromycin and incubated further for 2 weeks at 28 °C, light intensity of 60 μmole photons m−2s−1 and a 16/8 h photoperiod. The developed micro-shoots were placed on shoot development medium (SDM; MS with vitamins supplemented with 0.1 mg L−1 BAP, 3% (w/v) sucrose and 0.24% (w/v) gelrite, pH adjusted to 5.8 with 0.1 N NaOH) containing 450 and 7.5 mg L−1 of cefotaxime and hygromycin, respectively, for 2 weeks. The stems of putatively transformed shoots were cut off and transferred onto root initiation medium (RIM; MS supplemented 0.1 mg L−1 NAA, 3% (w/v) sucrose, 0.24% (w/v) gelrite, pH adjusted to 5.8 with 0.1 N NaOH). The RIM was also supplemented with 7.5 mg L−1 hygromycin to confirm the status of the transgenic plants. The leaves of regenerated transgenic plants were used for histochemical β-glucuronidase assays and polymerase chain reaction (PCR) analysis. The transformation efficiency (%) was determined by dividing the number of PCR positive plant lines by the number of leaf disc explants inoculated and expressed as a percentage.
Analysis of putative transgenic plant lines
Histochemical β-glucuronidase (GUS) assays
The co-cultivated explants and leaves of putatively transformed plants were utilized for β-glucuronidase histochemical assays [17]. Transient and stable gusA gene expression was driven by the cauliflower mosaic virus 35S promoter. Assays were done using transformed explants after 3 days of co-cultivation and on leaves of shoots regenerated on selection medium, respectively. Leaves of regenerated non-transformed shoot plants were used as controls. Briefly, the leaf discs were immersed in GUS staining solution [100 mM sodium phosphate buffer (pH 7.0) containing 2 mM 5-bromo-4-chloro-3-indolyl glucuronide (Duchefa Biochemie, Haarlem, The Netherlands), 0.05 mM potassium ferricyanide, 0.05 mM potassium ferrocyanide, and 0.1% (w/v) Triton X-100], and incubated in the dark at 37 °C overnight. The staining mixture was poured off, 70% (v/v) ethanol was added to remove chlorophyll and the stained plant material was photographed.
Polymerase chain reaction-based confirmation of putative transgenic lines
Two hundred (200) milligram leaves of in vitro regenerated shoots were used to isolate total genomic DNA using a cetyl trimethyl ammonium bromide (CTAB) procedure [18]. For PCR analysis, the gusA gene was amplified using gusA-specific primers to confirm the presence of the transgene in regenerated plants. The primers were designed to amplify a 542 bp product and the sequences were: forward primer 5′-TTTAACTATGCCGGGATCCATCGC-3′, and reverse primer 5′-CCAGTCGAGCATCTCTTCAGCGTA-3′.
Polymerase chain reaction (PCR) was carried out in a total amplification reaction contents of 25 µl, which contained 12.5 μl of premix (OneTaq® Quick-Load® 2 × Master Mix), 9.5 μl DNA- and RNA-free water, 1 µl of 10 µM of both forward and reverse primers and 1 μl of genomic DNA. The PCR amplification was carried out using the following thermocycling conditions: initial denaturation at 94 °C for 10 min followed by 35 cycles of 15 s denaturation at 94 °C, 40 s annealing at 62 °C and 50 s extension at 72 °C, followed by a final extension for 7 min at 72 °C. The PCR products were separated on a 0.8% (w/v) agarose gel (Duchefa Biochemie, Netherlands) containing 1 × Tris–Acetate EDTA buffer. Staining of the amplified products was carried out using 0.5 µg/ml ethidium bromide. The PCR products (4 μl) and loading dye (6 ×) were loaded into each well. The electrophoresis was run at 70 V for 45 min in a Pharmacia biotech horizontal tank. Gels visualization was carried out under a UV transilluminator and gels were photographed by a gel documentation System (Bio Rad).
Acclimatization of transgenic plants in the glasshouse
Five replicates of regenerated plants approximately 5 cm in length with more than 5 well developed roots were removed carefully from RIM and washed gently with sterilized distilled water to remove any adhered gelrite. The plantlets were transferred to plastic pots (10 cm diameter) containing sterile coconut peat (Grekkon Ltd, Kenya). All pots were covered with clear transparent polyethylene bags to allow in light to be received by the plants and to maintain humidity. The potted plantlets were placed under glasshouse conditions at 28 °C and 70% humidity. The polythene bags were opened gradually. After 2 weeks the polyethylene bags were removed completely and plantlets were watered at regular intervals. Subsequently the plantlets were transferred to larger plastic pots (30 cm × 40 cm) containing sterilized garden soil and sand (1:1) and kept under glasshouse conditions.
Statistical data analysis
All experiments were performed in three replicates and the experiments were repeated three times. Completely randomized designs (CRD) were used in all experiments. For optimization of factors affecting transformation, the percentage of GUS positive explants was calculated under different conditions of transformation. The statistical data analysis was done using GenStat® Statistical software 15th edition at P = 0.05 using analysis of variance (ANOVA) and Tukey’s test.
Results
Establishment of a simple and rapid regeneration method
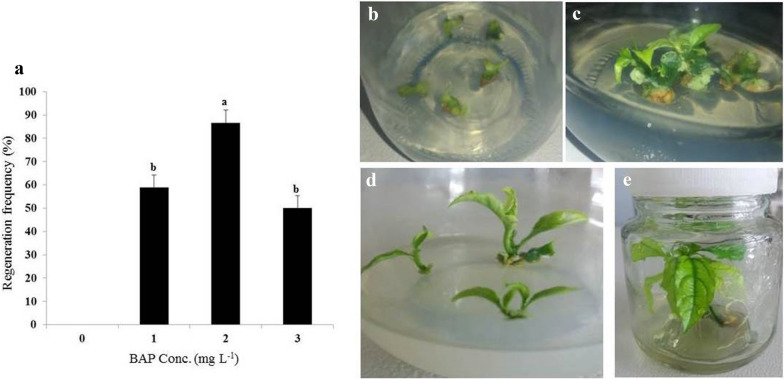
The objective of this was to establish a simple and fast regeneration procedure to facilitate Agrobacterium-mediated cell transformation of hybrid passion fruit KPF4. To establish shoot organogenesis, different BAP concentrations (0, 1.0, 2.0 and 3.0 mg L−1) were tested to obtain the optimum concentration. Shoot buds were induced in all the tested concentrations of BAP, though at different regeneration frequencies (Fig. 1a). However, on medium without BAP, there were no shoots induced from the cultured leaf disc explants. The first morphogenetic responses on the cut or injured surfaces were visible after 16–21 days, when shoot buds were observed from the leaf explant (Fig. 1b). The regeneration frequency of 0–86.67% was obtained depending on the concentration of BAP used. A concentration of 2.0 mg L−1 BAP resulted in the highest induction of shoots at 86.67 ± 5.53%, which was significantly (p < 0.05) different from the other tested concentrations of BAP (Fig. 1a). Leaf disc explants cultured on MS medium containing 3 mgL−1 BAP regenerated the lowest shoots per explant (4.5 ± 0.8 shoots per leaf disc explant) (Fig. 1a).
Fig. 1.
Effects of different concentrations of BAP on induction of shoots from leaf disc explants of passion fruit. a The regeneration efficiency on MS with vitamins supplemented with different BAP concentrations. The highest efficiency was achieved with 2 mg L−1 BAP and data are mean ± SE. Means having the same letters above bars are not significantly different at P > 0.05 using Tukey’s Honest significant difference test. b Shoot bud induction on leaf disc explants, c Shoot buds proliferating into shoots, d Elongation of induced shoots, and e The rooting of regenerated shoots
The micro-shoots (Fig. 1c) regenerated at different concentrations of BAP, did not develop further or elongate after prolonged culturing on the same media for more than 4 weeks. Elongation of micro-shoots was thereafter achieved at 0.1 mg L−1 BAP (Fig. 1d). Roots appeared on elongated shoots after 7 days on MS with vitamins augmented with 0.1 mg L−1 naphthaleneacetic acid (NAA), 3% (w/v) sucrose and 0.24% (w/v) gelrite. All plantlets had well developed roots (Fig. 1e) after 2 weeks of culture on root initiation and development medium. Fully developed rooted plantlets were transferred to the glasshouse for acclimatization. Plants regenerated through leaf discs were normal and no changes in morphology were observed in the potted plants.
Optimization of factors influencing Agrobacterium-mediated transformation
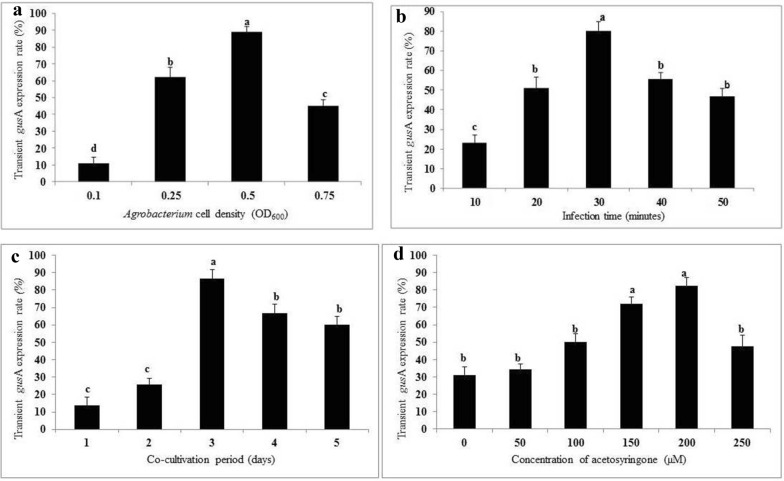
In this study, the effect of Agrobacterium cell density (LBA4404 harbouring pCAMBIA 1301), time of infection, period of co-cultivation and concentration of acetosyringone on transformation efficiency of KPF4 was evaluated based on expression of an intron-containing gusA. For Agrobacterium density, there was a significant increase in gusA expression with increasing Agrobacterium cell density with an OD600 from 0.1 to 0.5. The highest gusA expression of the inoculated leaf disc explants (88.9%) was obtained with LBA4404 at OD600 of 0.5. Therefore, bacteria cell density of OD600 = 0.5 was selected for Agrobacterium-mediated transformation of KPF4. The increase in bacterium cell density at OD600 beyond 0.5, led to a significant reduction in the percentage of leaf explants (44.9%) displaying gusA expression. The best optical cell density for delivery of the transgene into plant tissues was 0.5 at OD600 (Fig. 2a).
Fig. 2.
Factors affecting transient gusA expression of leaf disc explants of passion fruit using Agrobacterium strain LBA4404. a Effect of Agrobacterium cell density (OD600 values of 0.1, 0.25, 0.5 and 0.75); b Effect of infection time (10, 20, 30, 40 and 50 min); c Effect of co-cultivation period (1, 2, 3, 4 and 5 days); d Effect of concentration of acetosyringone (0, 50, 100, 150, 200 and 450 µM). Each experiment consisted of 30 leaf disc explants and experiments were done in triplicates. Treatments (mean ± SE) having the same letters are not significantly different at P > 0.05 using Tukey’s significant difference test
The duration of Agrobacterium infection of leaf explants had a significant influence on gusA expression. At the optimum cell density (OD600 = 0.5), the highest percentage of explants (80%) displaying gusA expression was obtained at 30 min Agrobacterium infection time. Either decrease or increase of Agrobacterium infection time beyond the optimum (30 min), significantly reduced the percentage of explants showing gusA expression (Fig. 2b).
The duration of co-cultivation had a significant effect on transient gusA expression in inoculated leaf disc explants of KPF4. There was a significant increase in transient gusA expression with increasing number of days of co-cultivation from 1 to 3. The optimum transient gusA expression (86.7%) was observed in leaf explants co-cultivated for 3 days post-infection. GusA expression of leaf explants co-cultivated for 3 days was significantly different (p≤ 0.05) from the other co-cultivation periods. The increase in co-cultivation period beyond 3 days significantly decreased the rate of transient gusA expression (Fig. 2c) and necrosis/browning of the leaf explants was observed.
The use of acetosyringone for induction of virulence genes in Agrobacterium was found to significantly influence transient gusA expression of inoculated leaf disc explants of KPF4. The concentration of acetosyringone had a significant (p ≤ 0.05) effect on transient gusA expression. The optimum transient gusA expression of leaf explants (82.2%) was obtained with acetosyringone at concentration of 200 µM. However, transient gusA expression at 200 µM acetosyringone was not significantly (p > 0.05) different from 150 µM. Acetosyringone concentration more than 200 µM in the co-cultivation medium significantly reduced the percentage of explants (47.7%) displaying transient gusA expression (Fig. 2d).
Genetic transformation and regeneration of putative transgenic plant lines
Successful Agrobacterium-mediated transformation of leaf disc explants of passion fruit hybrid KPF4 was developed based on the optimized conditions of transient gusA gene expression. Following transformation (Agrobacterium optical cell density OD600 of 0.5, 30 min of Agrobacterium infection time, 200 µM of acetosyringome and 3 days of co-cultivation), the explants were washed, placed on recovery phase (Fig. 3a) for 4 days followed by transfer to shoot bud inducing medium containing selection agent (7.5 mg L−1 hygromycin). The non-transformed leaf explants turned brown within 7 days and the transformed leaf explants had green segments (Fig. 3b) which formed shoot buds after 3 weeks of culture. After 4 weeks, induced shoot buds developed into micro-shoots (Fig. 3c). The non-transformed leaf disc explants turned necrotic and finally died when placed on selective medium (Fig. 3c). The transfer of micro-shoots onto MS augmented with reduced BAP concentration (0.1 mg L−1 BAP) and selection agent (7.5 mg L−1 hygromycin) developed into fully elongated shoots (Fig. 3d) after 2 weeks of culture. Roots were initiated after 7 days on elongated shoots cultured on root initiation and development medium and fully developed roots were formed after 2 weeks of culture (Fig. 3e). All the PCR-positive shoots formed roots in root induction medium. Following the three transformation experiments, a total of 900 leaf disc segments of P. edulis were infected with Agrobacterium, 79 explants survived on selection medium, 18 shoot buds were induced on shoot induction medium (SIM), 10 hygromycin resistant elongated shoots were obtained on shoot development medium (SDM), and 6 positive transgenic shoots developed roots on root-inducing medium (RIM). The transformation efficiency obtained in this study was 0.67% (Table 1).
Fig. 3.
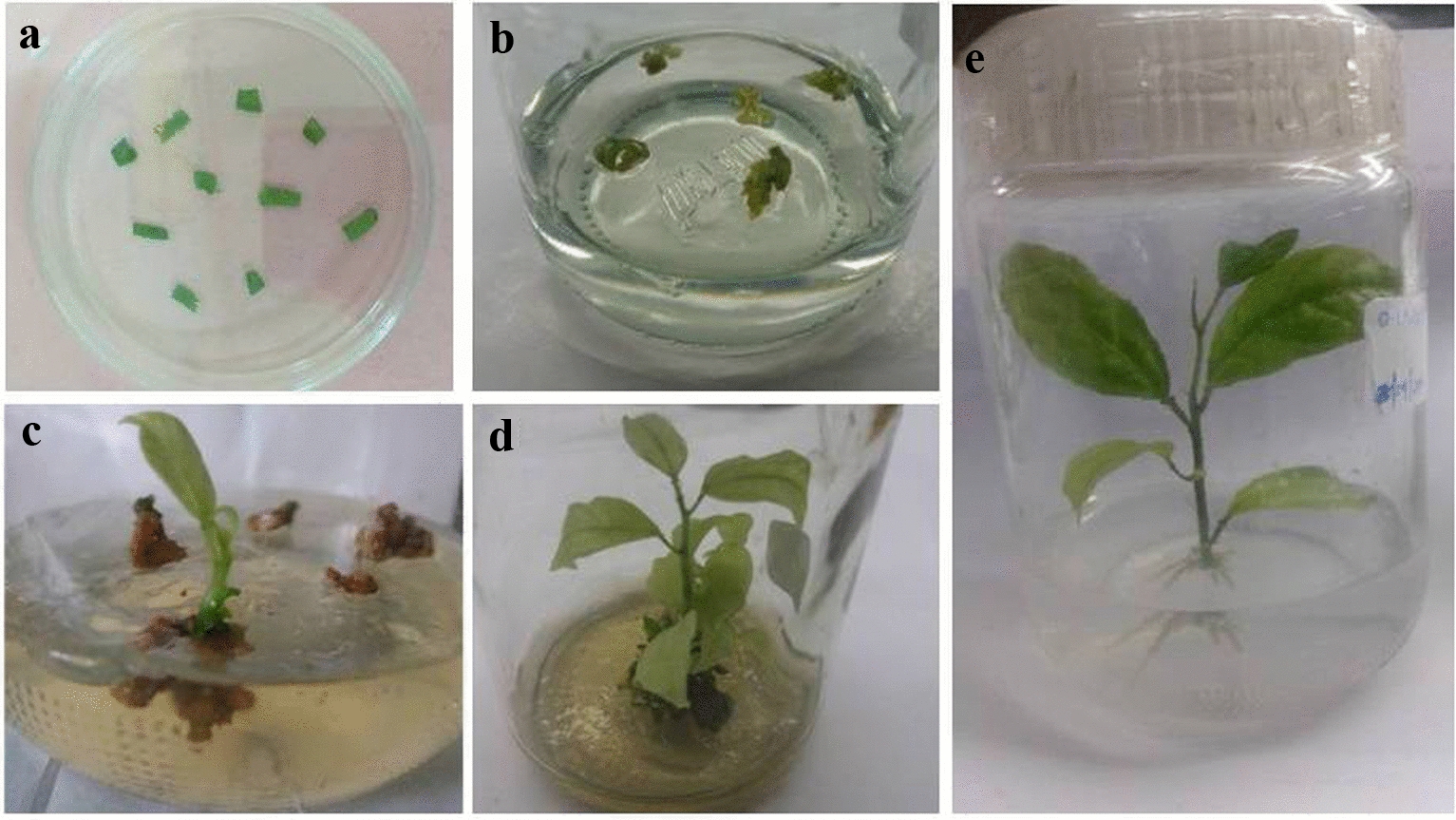
Agrobacterium-mediated transformation of leaf disc explants of passion fruit KPF4 using Agrobacterium strain LBA4404 harboring pCAMBIA1301. a Culture of inoculated leaf disc explants on resting medium; b One-week-old inoculated explants on shoot bud induction medium with selection agent (7.5 mg L−1 hygromycin); c Micro-shoots of leaf explants on SIM containing selection agent (7.5 mg L−1 hygromycin) for 3 weeks; d Elongated shoots after 4 weeks of culture on SDM containing selection agent (7.5 mg L−1 hygromycin); e Rooting test on RIM supplemented with 7.5 mg L−1 hygromycin for 2 weeks
Table 1.
Generation of transgenic plants of hybrid passion fruit KPF4
| Transformation experiment | No. of explants infected with Agrobacterium harboring pCAMBIA1301 | No. of explants surviving on selection medium | No. of explants forming shoot buds on SIM with selection agent | No. of shoots developed on SDM with selection agent | Rooting on RIM with selection agent | No. of transgenic lines with GUS expression | No of PCR-positive plant lines | Transformation efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 300 | 18 | 5 | 3 | 2 (66.67%) | 2 | 2 | 0.67 |
| 2 | 300 | 29 | 6 | 4 | 1 (25%) | 1 | 1 | 0.33 |
| 3 | 300 | 37 | 7 | 3 | 3 (100%) | 3 | 3 | 1 |
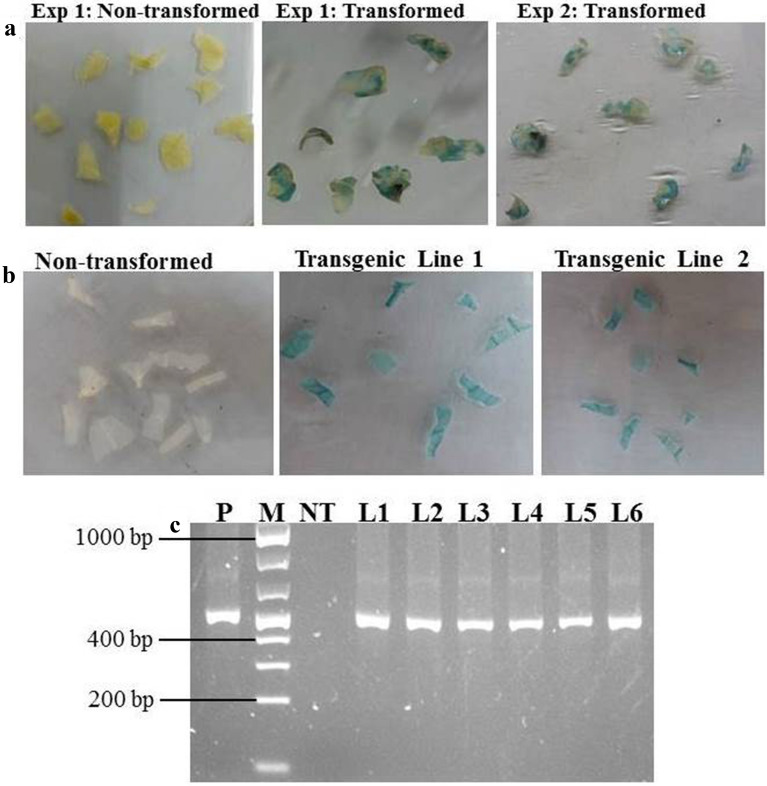
GUS expression was used to check the progress of transformation and blue coloration was detected in KPF4 leaf disc explants as early as 4 days post-infection with LBA4404 harbouring pCAMBIA1301. Transformed leaf explants were verified by histochemical GUS assay and 100% of explants showed transient gusA gene expression (Fig. 4a). A uniform blue staining (Fig. 4b) was observed in all the leaf discs of all the regenerated putatively transgenic plants. No blue staining (Fig. 4b) was obtained in leaves of non-transformed control plants regenerated without selection.
Fig. 4.
Histochemical β-glucuronidase staining and PCR analysis of transformed leaf discs and transgenic plants. a Histochemical GUS staining of leaf discs after co-cultivation for 3 days; b Stable histochemical GUS staining of leaf segments of different transgenic plant lines and non-transformed control plant; and c PCR amplification of gusA gene fragment of DNA isolated from 6 transgenic plant lines. P = plasmid DNA, M = 1 kb molecular weight marker (Fisher Thermo scientific); NT = Non-transformed control plant; and L1–L6 = Transgenic plant lines 1 to 6, respectively
PCR analysis with primers designed to amplify a fragment of the gusA reporter gene was performed on DNA isolated from control plants regenerated from leaf disc explants that did not undergo infection and putative transgenic plant lines regenerated from explants infected with LBA4404 harboring pCAMBIA1301. Expected band sizes of 542 bp equivalent to a fragment of the gusA reporter gene were amplified from genomic DNA of all putatively transformed plant lines. No amplicon was obtained in non-transgenic control plants (Fig. 4c). The PCR results showed that all the regenerated six passion fruit lines were transgenic plants.
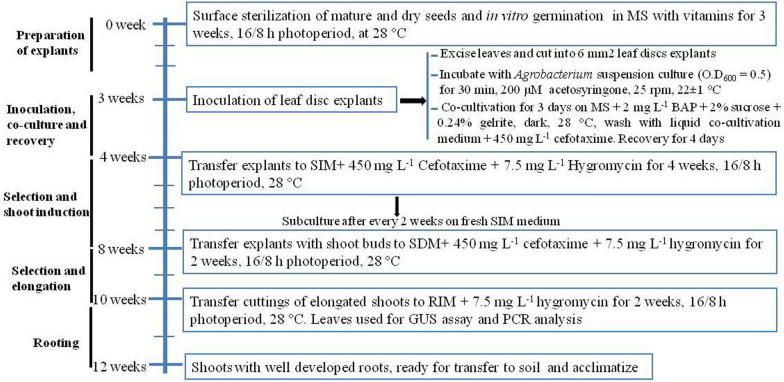
Based on the findings obtained in the current study, we present a simple and fast protocol for Agrobacterium-mediated transformation of Passiflora edulis Sims using leaf disc explants. After Agrobacterium infection, it takes approximately 8 weeks for transgenic lines to be ready for transfer to the soil. A summary of the established protocol is shown in Fig. 5.
Fig. 5.
A flow diagram for Agrobacterium-mediated transformation method of passion fruit hybrid KPF4 using leaf disc explants
Discussion
A fundamental first step for the successful establishment of a genetic transformation system in plants is an efficient plant regeneration procedure. In this study, we developed a simple and quick regeneration system of hybrid passion fruit KPF4. Plantlets formed on the leaf disc segments within 4 weeks. BAP concentrations tested had the potential to successfully induce shoots, nevertheless, the induction results indicated that 2.0 mg L−1 BAP was the optimum concentration with a higher efficiency on shoots induction compared to the other concentrations. BAP has been reported as an efficient cytokinin that induces regeneration of shoots in many plants including passion fruit species such as P. edulis f. flavicarpa, P. caerulea, P. alata and P. setacea [19–21]. However, on medium containing 2.0 mg L−1 BAP, the induced shoots of KPF4 did not proliferate or elongate and the problem became severe after six weeks of culture. To optimize the protocol, the leaf explants were cultured on medium containing 2.0 mg L−1 BAP to induce shoot buds for 4 weeks and then transferred to MS medium with reduced BAP (0.1 mg L−1) for elongation of shoots.
Agrobacterium cell density is a fundamental factor influencing genetic transformation system [22]. Different Agrobacterium cell densities used to inoculate the leaf disc explants of KPF4 had an effect on the level of transient GUS expression in hybrid passion fruit KPF4. The optimum transient GUS expression was achieved with bacterium OD600 of 0.5. At higher concentration of Agrobacterium, browning/necrosis was observed in leaf disc explants and finally death. This confirms previous reports that higher densities of A. tumefaciens results in tissue damage and hence reduction in the transformation efficiency [23, 24]. Higher bacterium cell density can cause uncontrolled growth of Agrobacterium thus limiting the survival of the explants and subsequent reduction in transformation efficiency. Alfenas et al. [25] used optical density of 0.4 to successively transform yellow passion fruit. In studies of other plant species such as rice, wheat, maize, soybean, tea and jute, the optical densities of Agrobacterium suspension cultures ranged from 0.1 to 1.0 depending on the genotype, Agrobacterium strain and plant species [26–32].
The transfer of T-DNA from Agrobacterium to the plant genome during Agrobacterium-mediated genetic transformation process is time-dependent and therefore both time of infection and co-cultivation duration affect the transformation efficiency [30]. Varying the inoculation durations had an influence on the transfer of T-DNA from Agrobacterium to plant cells and 30 min infection time at an OD600 of 0.5 was optimum for KPF4 leaf disc explants, similar to reports by Manders et al. [9] in yellow passion fruit, Jha et al. [33] and Zhao et al. [34] in Pennisetum glaucum and rice, respectively. In a previous study, Trevisan et al. [10] reported 20 min as the most suitable infection time for successful transformation of yellow passion fruit. This difference in infection time would be due to the different Agrobacterium strains used. Therefore, it is important to optimize the infection times for different Agrobacterium strains for each plant species and cultivar and it is better to select a low concentration with high infection ability. Diverse infection times in other plant species such as sorghum, maize and wheat has been reported ranging from 5 min (for sorghum, maize) to 50 min (for wheat) indicating that infection time varies with the plant species under investigation.
Co-cultivation period is important in Agrobacterium-mediated transformation because during this step the T-DNA is transferred into the genome of host plants [35, 36]. The co-cultivation period significantly influenced the rate of transient gusA expression in KPF4 leaf disc explants. Three days of co-cultivation resulted in the highest transient gusA expression rate, similar to previous reports in passion fruit [9, 10, 12]. Shorter co-cultivation periods (1 to 2 days) resulted in lower rates of transient gusA expression probably due to inadequate time for maximum transfer of T-DNA from Agrobacterium into the plant genome [36]. Co-cultivation for more than 3 days resulted to bacterial overgrowth and browning/necrosis of the leaf disc explants. Co-cultivation periods ranging from 2 to 5 days have been reported to be suitable in several plant transformation studies such as okra [30], yam [37], rice [38], wheat [30], maize [39] and soybean [31]. However, a longer co-cultivation period of 15 days has been documented in a study on sunflower (Helianthus annuus L.).
Acetosyringone, a phenolic product produced from plant wounds and has been frequently used in Agrobacterium-mediated transformation of plant species during infection and co-cultivation to improve transformation efficiency [40–43]. Acetosyringone induces the activation and expression of virulence genes in Agrobacterium required for plant genetic transformation [43]. Depending on the plant species and Agrobacterium strain, the concentration of acetosyringone used for transformation varied from 20 to 200 µM [27, 41, 42, 44]. In the present study, addition of acetosyringone into the Agrobacterium infection medium significantly increased the rate of transient gusA expression of inoculated leaf disc explants. Exogenous addition of 200 µM acetosyringone resulted in the highest transient gusA expression and this is in agreement with previous findings in passion fruit [10] and other plant species including sweet potato [45], orchid [46] and banana [47].
The optimization of factors affecting Agrobacterium-mediated transformation based on the rate of transient gusA expression allowed the establishment of a protocol for stable transformation of commercial hybrid passion fruit KPF4. The genetic transformation efficiency in present study was 0.67%. Other studies reported that transformation efficiencies of the two Brazilian passion fruit varieties IAC-275 and IAC-277 were 0.11% and 0.21%, respectively [10]. Monteiro-Hara et al. [48], documented transformation efficiencies of 0.67% and 0.19% for the same Brazilian varieties reported by Trevisan et al. [10]. A higher transformation efficiency (0.89%) was obtained by Correa et al. [11] on Passiflora alata. Recently, Tuhaise et al. [12] reported a transformation efficiency of 0.46% for Ugandan yellow varieties of passion fruit. All these previous reports involved complicated procedures which require more than 5 months to regenerate transgenic plants. The protocol established in this study for passion fruit hybrid KPF4 is simple and quick as it takes 3 months to regenerate transgenic plants.
In the current study, 6 out of the 10 shoots were positive in the rooting test. The non-transgenic shoots did not root in RIM supplemented with 7.5 mg L−1 hygromycin. This confirms the reliability of rooting procedure as a rapid second stage selection of transgenic plants as reported in studies of other plant species [49]. Confirming the transgenic plants by use of GUS assays and PCR analysis are other effective ways of eliminating false positive transformants. All the rooted plants in this study showed gusA expression as determined by GUS staining and the presence of the transgene was confirmed by PCR. This indicates that the intron-containing gusA gene was successfully inserted into the genome of the 6 obtained KPF4 lines. To the best of our knowledge, this is the first report of the successful transformation of commercial passion fruit hybrid KPF4. The establishment of transformation protocol for KPF4 will be critical in initiating its genetic improvement through transgenic approaches.
Conclusions
The present study established a simple and rapid Agrobacterium-mediated transformation protocol for passion fruit hybrid KPF4. Stable transgenic plants which showed presence and expression of gusA were regenerated within 12 weeks from leaf disc explants. The protocol can be used to insert beneficial traits into passion fruit hybrid KPF4 to create transgenic plants. This method will also be useful for stacking traits such as virus resistance and longer storage of edible fruits.
Supplementary information
Additional file 1: Table S1. Composition of various media used for in vitro regeneration and Agrobacterium-mediated transformation of KPF4 leaf explants. Figure S1. Representation of T-DNA region of the binary vector pCAMBIA1301 used for genetic transformation of Passiflora edulis Sims. NOS PolyA Nopaline synthase polyadenylation signal (terminator), gusA β-glucuronidase reporter gene containing an intron, 35SP CaMV35S promoter, MCS multiple cloning site, hpt hygromycin phosphotransferase gene, and 35S PolyA polyA cauliflower mosaic virus 35S terminator. Both the selection marker and reporter gene are under the control of the CAMV35S promoter.
Acknowledgements
The authors would like to thank the Department of Biochemistry, University of Nairobi for the research facilities.
Authors’ contributions
LKA carried out all the experiments and drafted the manuscript. ENN, ROO, and ROO were involved in experimental design, co-ordination and supervision of the work and review of the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the National Research Fund of Kenya through a PhD research grant (Financial Year 2016/2017) to the first author.
Availability of data and materials
The datasets used and analyzed in this study are available from corresponding author on reasonable request. KPF4 variety seeds are available from Kenya Agricultural Research and Livestock Organization (KARLO). LBA4404 and pCAMBIA 1301 are available from Department of Biochemistry, University of Nairobi.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13007-020-00684-4.
References
- 1.Cerqueira-Silva CBM, Jesus ON, Santos ESL, Correa RX, Souza AP. Genetic breeding and diversity of the Genus Passiflora: Progress and perspectives in molecular and genetic studies. Int J Mol Sci. 2014;15:14122–14152. doi: 10.3390/ijms150814122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramaiya SD, Bujang JS, Zakaria MH, King WS, Shaffiq Sahrir MA. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J Sci Food Agric. 2013;93:1198–1205. doi: 10.1002/jsfa.5876. [DOI] [PubMed] [Google Scholar]
- 3.Zas P, John S. Diabetes and medicinal benefits of Passiflora edulis. World J Pharm Res. 2016;5:453–465. [Google Scholar]
- 4.FAOSTAT. 2016. Database for Food and Agriculture Organization of the United Nations. http://apps.fao.org.
- 5.Varassin IG, Trigo JR, Sazima M. The role of nectar production, flower pigments and oduor in the pollination of four species of Passiflora (Passifloraceae) in south-eastern Brazil. Bot J Linn Soc. 2001;136:139–152. [Google Scholar]
- 6.Petri C, Burgos L. Transformation of fruit trees. Useful breeding tool or continued future prospect? Review. Transgenic Res. 2005. 14:15–26. [DOI] [PubMed]
- 7.Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67(1):16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imani J, Kogel KH. Plant transformation techniques: agrobacterium and microparticle- mediated gene transfer in cereal plants. Methods Mol Biol. 2020;2124:281–294. doi: 10.1007/978-1-0716-0356-7_15. [DOI] [PubMed] [Google Scholar]
- 9.Manders G, Otoni WC, dUtra vaz EB, Blackhall NW, Power JB, Davey MR. Transformation of passion fruit (Passiflora edulis fv flavicarpa Degener.). Plant Cell Rep. 1994. 13:697 – 702. [DOI] [PubMed]
- 10.Trevisan F, Mendes BM, Maciel SC, Vieira ML, Meletti LM, Rezende JA. Resistance to Passion fruit woodiness virus in transgenic passionflower expressing the virus coat protein gene. Plant Dis. 2006;90(8):1026–1030. doi: 10.1094/PD-90-1026. [DOI] [PubMed] [Google Scholar]
- 11.Correa MF, Pinto AP, Rezende JA, Harakava R, Mendes BM. Genetic transformation of sweet passion fruit (Passiflora alata) and reactions of the transgenic plants to Cowpea aphid borne mosaic virus. Eur J Plant Pathol. 2015;143(4):813–821. [Google Scholar]
- 12.Tuhaise S, Nakavuma J, Adriko J, Ssekatawa K, Kiggundu A. Establishment of a transformation protocol for Uganda’s yellow passion fruit using the gus gene. Afr J Biotechnol. 2019;18(20):416–425. [Google Scholar]
- 13.Madhulatha P, Pandey R, Hazarika P, Rajam MV. High transformation frequency in Agrobacterium-mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiol Mol Biol Plants. 2007;13:191–198. [Google Scholar]
- 14.Kenya Agricultural Research Institute. KARI sweet yellow passion that withstand critical diseases that ravaged purple passion. Hortfresh J . Tech Era Communications. Kenya: Nairobi; 2014. [Google Scholar]
- 15.Kilalo DC. 2012. Molecular detection of viruses associated with passion fruit (Passiflora edulis sims) woodiness disease, monitoring and management of aphid vectors in Kenya. Doctoral dissertation, University of Nairobi. Homepage address: erepository.uonbi.ac.ke.
- 16.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. [Google Scholar]
- 17.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soni R, Murray JA. Isolation of intact DNA and RNA from plant tissues. Anal Biochem. 1994;218(2):474–476. doi: 10.1006/abio.1994.1214. [DOI] [PubMed] [Google Scholar]
- 19.Vieira LM, Rocha DI, Taquetti MF, da Silva LC, de Campos JMS, Viccini LF, Otoni WC. In vitro plant regeneration of Passiflora setacea DC (Passifloraceae): the influence of explant type, growth regulators, and incubation conditions. Vitro Cell Dev Biol-Plant. 2014;50(6):738–745. [Google Scholar]
- 20.Rosa YBCJ, Monte-Bello CC, Dornelas MC. 2016. In vitro organogenesis and efficient plant regeneration from root explants of Passiflora suberosa L. (Passifloraceae). Vitro Cell Dev Biol-Plant. 52(1):64-71.
- 21.de Faria RB, de Carvalho IF, Rossi AAB, de Matos EM, Rocha DI, Pinto DLP, Otoni WC, da Silva ML. High responsiveness in de novo shoot organogenesis induction of Passiflora cristalina (Passifloraceae), a wild Amazon passion fruit species. Vitro cell Dev Biol-Plant. 2018;54:166–174. [Google Scholar]
- 22.Binka A, Orczyk W, Nadolska-Orczyk A. The Agrobacterium-mediated transformation of common wheat (Triticum aestivum L.) and triticale (x Triticaosecale Wittmack): Role of the binary vector system and selection cassettes. J Appl Genet. 2012. 53:1-8. [DOI] [PMC free article] [PubMed]
- 23.Amoah BK, Wu H, Sparks C, Jones HD. Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot. 2001;52(358):1135–1142. doi: 10.1093/jexbot/52.358.1135. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Cong Y, Liu Y, Wang T, Shuai Q, Chen N, Gai J, Li Y. Optimization of Agrobacterium-mediated transformation in soybean. Front Plant Sci. 2017;8:246. doi: 10.3389/fpls.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfenas PF, Braz ASK, Torres LB, Santana EN, Nascimento AVSD, Carvalho MGD, Zerbini FM. Transgenic passion fruit expressing RNA derived from cowpea aphid-borne mosaic virus is resistant to passion fruit woodiness disease. Fitopatol Bras. 2005;30(1):33–38. [Google Scholar]
- 26.Mondal T, Bhattacharya A, Ahuja P, Chand P. Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep. 2001. 20(8):712-720.
- 27.Zhao ZY, Gu W, Cai T, Tagliani L, Hondred D, Bond D, Schroeder S, Rudert M, Pierce D. High-throughput genetic transformation mediated by Agrobacterium tumefaciens in maize. Mol Breed. 2002;8(4):323–333. [Google Scholar]
- 28.Hu T, Metz S, Chay C, Zhou HP, Biest N, Chen G, Cheng M, Feng X, Radionenko M, Lu F, Fry J. Agrobacterium-mediated large-scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Rep. 2003. 21(10):1010-1019. [DOI] [PubMed]
- 29.Saha P, Datta K, Majumder S, Sarker C, China SP, Sarker SN, Sarker D, Datta SK. Agrobacterium mediated genetic transformation of commercial jute cultivar Corchorus capsularis cv. JRC 321 using shoot tip explants. Plant cell Tissue and Organ Cult. 2014. 118(2):313-326.
- 30.Markandan M, Subramanyam K, Ishwarya R, Elayaraja D, Ganapathi A. Asssessment of factors influencing the tissue culture-independent Agrobacterium-mediated in planta genetic transformation of okra [Abelmoschus esculentus (L) Moench] Plant Cell Tissue and Organ Cult. 2015;123(2):309–320. [Google Scholar]
- 31.Yang XF, Yu XQ, Zhou Z, Ma WJ, Tang GX. A high- efficiency Agrobacterium tumefaciens mediated transformation system using cotyledonary node as explants in soybean (Glycine max L.). Acta Physiol Plant. 2016. 38(3):60.
- 32.Kumar R, Mamrutha HM, Kaur A, Venkatesh K, Sharma D, Singh GP. Optimization of Agrobacterium-mediated transformation in spring bread wheat using mature and immature embryos. Mol Bio Rep. 2019;46(2):1845–1853. doi: 10.1007/s11033-019-04637-6. [DOI] [PubMed] [Google Scholar]
- 33.Jha P, Rustagi A, Agnihotri PK, Kulkarni VM, Bhat V. Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explant source. Plant Cell Tiss Organ Cult. 2011. 107(3):501-512.
- 34.Zhao W, Zheng S, Ling HQ. An efficient regeneration system and Agrobacterium-mediated transformation of Chinese upland rice cultivar Handao297. Plant Cell Tiss Organ Cult. 2011;106(3):475. [Google Scholar]
- 35.Han JL, Wang H, Ye HC, Liu Y, Li ZQ, Zhang Y, Zhang YS, Yan F, Li GF. Hi efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci. 2005. 168(1): 73-80.
- 36.Rahman ZA, Seman ZA, Basirun N, Julkifle AL, Zainal Z, Subramaniam S. Preliminary investigations of Agrobacterium-mediated transformation in indica rice MR219 embryogenic callus using gusA gene. Afr J Biotechnol. 2011;10(40):7805–7813. [Google Scholar]
- 37.Nyaboga E, Tripathi JN, Manoharan R, Tripathi L. Agrobacterium-mediated genetic transformation of yam (Dioscorea rotundata): an important tool for functional study of genes and crop improvement. Front Plant Sci. 2014;5:463. doi: 10.3389/fpls.2014.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toki S. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Report. 1997;15:16–21. [Google Scholar]
- 39.Horn ME, Harkey RL, Vinas AK, Drees CF, Barker DK, Lane JR. Use OF Hi II-elite inbred hybrids in Agrobacterium- based transformation of maize. Vitro Cell Dev Biol-Plant. 2006;42(4):359–366. [Google Scholar]
- 40.Sheikholeslam SN, Weeks DP. Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol Biol. 1987;8(4):291–298. doi: 10.1007/BF00021308. [DOI] [PubMed] [Google Scholar]
- 41.Mishra S, Sangwan RS, Bansal S, Sangwan NS. Efficient genetic transformation of Withania coagulans (Stock) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture. Protoplasma. 2013;250(2):451–458. doi: 10.1007/s00709-012-0428-0. [DOI] [PubMed] [Google Scholar]
- 42.Xi J, Patel M, Dong S, Que Q, Qu R. Acetosyringone treatment duration affects large T-DNA molecule transfer to rice callus. BMC Biotechnol. 2018;18(1):1–8. doi: 10.1186/s12896-018-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stachel SE, Messens E, Van Montagu M, Zambryski P. Indentification of signal molecules produced by wounded plant cells that activate t-DNA transfer in Agrobacterium tumefaciens. Nature. 1985;318:624–629. [Google Scholar]
- 44.Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006;25(3):206–213. doi: 10.1007/s00299-005-0048-7. [DOI] [PubMed] [Google Scholar]
- 45.Xing Y, Yang Q, Ji Q, Luo Y, Zhang Y, Gu K, Wang D. Optimization of Agrobacterium-mediated transformation parameters for sweet potato embryogenic callus using β-glucuronidase (gus) as a reporter. Afr J Biotechnol. 2007;6(22):2578–2584. [Google Scholar]
- 46.Subramaniam S, Rathinam X. Emerging factors that influence efficiency of T-DNA gene transfer into Phalaenopsis violacea orchid via Agrobacterium tumefaciens-mediated transformation system. Int J Biol. 2010;2(2):64–73. [Google Scholar]
- 47.Subramanyam K, Subramanyam K, Sailaja KV, Srinivasulu M, Lakshmidevi K. Highly efficient Agrobacterium-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. Plant Cell Rep. 2011. 30(3):425-436. [DOI] [PubMed]
- 48.Monteiro-Hara AC, Jadão AS, Mendes BM, Rezende JA, Trevisan F, Mello AP, Vieira ML, Meletti LM, De S. Piedade SM. Genetic transformation of passionflower and evaluation of R1 and R2 generations for resistance to Cowpea aphid borne mosaic virus. Plant Dis. 2011. 95(8):1021-1025. [DOI] [PubMed]
- 49.Bull SE, Owiti JA, Niklaus M, Beeching JR, Gruissem W, Vanderschuren H. Agrobacterium-mediated transformation of friable embryogenic calli and regeneration of transgenic cassava. Nat Protoc. 2009;4(12):1845. doi: 10.1038/nprot.2009.208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Composition of various media used for in vitro regeneration and Agrobacterium-mediated transformation of KPF4 leaf explants. Figure S1. Representation of T-DNA region of the binary vector pCAMBIA1301 used for genetic transformation of Passiflora edulis Sims. NOS PolyA Nopaline synthase polyadenylation signal (terminator), gusA β-glucuronidase reporter gene containing an intron, 35SP CaMV35S promoter, MCS multiple cloning site, hpt hygromycin phosphotransferase gene, and 35S PolyA polyA cauliflower mosaic virus 35S terminator. Both the selection marker and reporter gene are under the control of the CAMV35S promoter.
Data Availability Statement
The datasets used and analyzed in this study are available from corresponding author on reasonable request. KPF4 variety seeds are available from Kenya Agricultural Research and Livestock Organization (KARLO). LBA4404 and pCAMBIA 1301 are available from Department of Biochemistry, University of Nairobi.