Abstract
Background
Clostridioides difficile infection (CDI) is commonly associated with outcomes like recurrence and readmission. The effect of social determinants of health, such as ‘neighborhood’ socioeconomic disadvantage, on a CDI patient’s health outcomes is unclear. Living in a disadvantaged neighborhood could interfere with a CDI patient’s ability to follow post-discharge care recommendations and the success probability of these recommendations, thereby increasing risk of readmission. We hypothesized that neighborhood disadvantage was associated with 30-day readmission risk in Medicare patients with CDI.
Methods
In this retrospective cohort study, odds of 30-day readmission for CDI patients are evaluated controlling for patient sociodemographics, comorbidities, and hospital and stay-level variables. The cohort was created from a random 20% national sample of Medicare patients during the first 11 months of 2014.
Results
From the cohort of 19,490 patients (39% male; 80% white; 83% 65 years or older), 22% were readmitted within 30 days of an index stay. Unadjusted analyses showed that patients from the most disadvantaged neighborhoods were readmitted at a higher rate than those from less disadvantaged neighborhoods (26% vs. 21% rate: unadjusted OR = 1.32 [1.20, 1.45]). This relationship held in adjusted analyses, in which residence in the most disadvantaged neighborhoods was associated with 16% increased odds of readmission (adjusted OR = 1.16 [1.04, 1.28]).
Conclusions
Residence in disadvantaged neighborhoods poses a significantly increased risk of readmission in CDI patients. Further research should focus on in-depth assessments of this population to better understand the mechanisms underlying these risks and if these findings apply to other infectious diseases.
Keywords: Clostridioides difficile, Socioeconomic disadvantage, Social determinants of health, Medicare
Introduction
Clostridioides difficile is a major cause of healthcare-associated diarrhea in the United States, responsible for nearly 500,000 cases of C. difficile infection (CDI), 30,000 deaths and over $5 billion each year [1, 2]. One reason that curtailment of CDI remains a major challenge is the high rate of CDI recurrence, which occurs in up to 30% of patients [3]. Patients with recurrent CDI may need to be subsequently readmitted to a healthcare facility, which presents an opportunity for continued transmission of CDI in the inpatient setting and new infections in susceptible hosts. Data on readmissions following an inpatient stay with CDI, while limited, show that approximately 23% of patients had at least one readmission, with approximately 32% of readmissions carrying a principal diagnosis of CDI [4]. Patients with a CDI discharge have been found to have a 16 percentage point higher rate of 30-day readmission than patients without a CDI discharge [5].
Commonly cited risk factors of recurrent CDI include older age and continued use of antibiotics [6]. Few studies that focus on risk factors of CDI recurrence consider the impact of social determinants of health. Social determinants are increasingly recognized as major contributors to readmissions for chronic conditions like congestive heart failure and myocardial infarction. However, little data exist on the relationship between social determinants of health and outcomes of acute infectious conditions (other than pneumonia-a condition that Centers for Medicare & Medicaid Services (CMS) penalizes for readmission) in developed countries such as the US. Because CDI diagnosis is associated with high rates of recurrence, it is plausible that the rate of readmission following a CDI-related stay would be similar to that for chronic conditions and that socioeconomic disadvantage would be an important contributor to the risk of recurrence and thereby readmission.
Socioeconomic disadvantage likely adversely impacts a CDI patient’s post discharge course. Challenges may include financial constraints to completing the full antibiotic course, especially as the most common treatments for CDI, oral vancomycin and fidaxomicin, can be prohibitively expensive for uninsured patients [7, 8]. Other challenges may include inability to manage environmental cleaning to reduce re-infection and spore shedding, and lack of resources such as transportation and social support to facilitate follow-up care [7, 9]. Household crowding, a common indicator of socioeconomic disadvantage, has also been linked to poorer outcomes for infectious disease patients [10]. As CDI is a disease caused by perturbation of the gut microbiome, factors that impede restoration of the gut microbiome may also affect CDI outcomes. Patients living in socioeconomically disadvantaged neighborhoods have higher rates of comorbid conditions, increasing their contact with the healthcare system and risk of CDI and/or antibiotic exposure. A growing body of literature suggests that diet is a major driver of gut microbiome composition and health [11, 12]. Patients living in socioeconomically disadvantaged neighborhoods are more likely to live in ‘food deserts,’ where access to healthy, high fiber foods may be limited [13]. Consumption of low-fiber, highly processed foods has been found to be strongly linked to socioeconomic disadvantage [14]. As these potential mechanisms impact specific characteristics of CDI, it is likely that disadvantage would adversely affect a CDI patient’s outcomes. Therefore, we hypothesized that socioeconomic disadvantage as measured by residence in a disadvantaged neighborhood would be associated with a higher risk of readmission for CDI patients.
Methods
We conducted a retrospective observational study to evaluate the association between neighborhood socioeconomic disadvantage and risk of readmission for patients discharged after a CDI-related stay.
Data source and cohort creation
We measured the relative socioeconomic disadvantage at the Census Block Group, or ‘neighborhood,’ level using the Area Deprivation Index (ADI). ADI was developed three decades ago and subsequently has been validated at the more granular Census Block Group level [14]. ADI is a composite index that draws from several weighted indicators of neighborhood socioeconomic disadvantage, including constructs of income, employment, education and housing quality [15]. ADI is validated at the neighborhood level and has been used in several other studies [16–18].
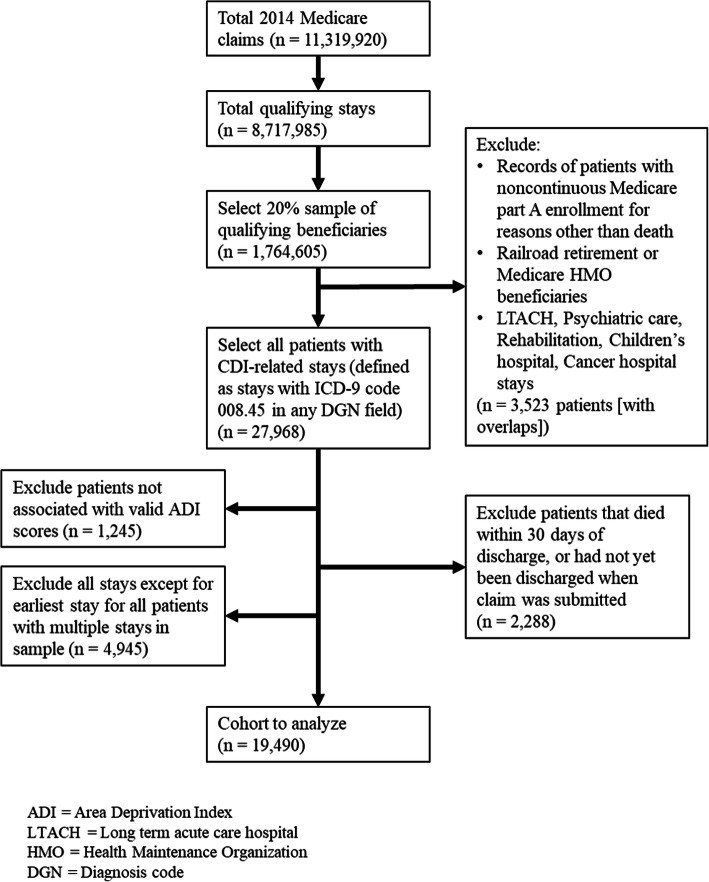
The cohort included all patients within a 20% Medicare claims national random sample who had a CDI-related index inpatient stay (ICD-9-CM = 008.45) between January 1–November 30, 2014. Beneficiaries with valid ZIP+ 4 codes (95%) were geolinked to corresponding neighborhood national ADI ranking, obtained from the UW Neighborhood Atlas [19]. As consistent with CMS readmission metric policy, patients not meeting the following criteria were excluded: stays from non-federal short-term acute hospitals, those without continuous Medicare part A and B enrollment, those who died during their index stay, or were discharged against medical advice. Other exclusions included: patients without valid geolinked ADI national percentile scores, age less than 18 years, those with railroad retirement benefits, or those enrolled in Medicare HMO. As multiple hospital stays by a single patient could invalidate the assumption of independence for all records in our dataset, we retained only the first stay for patients that had multiple hospital stays during the study period. Figure 1 describes the creation of our study cohort.
Fig. 1.
Creation of the analyzed cohort and application of exclusion criteria
Variables
The primary outcome measure was odds for 30-day all-cause readmission as defined previously and which is used by CMS to inform readmission-based policies [20, 21]. We considered all-cause readmissions because of the wide range of reasons patients with recurrent CDI can be readmitted. Our key explanatory variable was neighborhood-level socioeconomic disadvantage as measured by ADI.
Covariates selected for model adjustment were chosen based on previous literature and conceptual models of readmission [22, 23]. Illness burden was captured via Elixhauser comorbidity categories in the 12 months prior to each index hospitalization [24]. Patients were considered dual Medicare-Medicaid enrolled if they were enrolled in Medicaid in any of the 12 months preceding index hospitalization. Patients were considered disabled if their reason for Medicare entitlements was disability related. Additional patient-level adjustments were made for age, race/ethnicity, and rurality via Rural-Urban Commuting Area (RUCA) codes. Age was split at 65 as advanced age is widely cited as a risk factor for incident and recurrent CDI [3, 25]. Factors associated with hospital stay included length of stay (LoS) and discharge to a skilled nursing facility (SNF) after index hospitalization. Factors related to hospital characteristics included hospital Medicare beneficiary discharge volume (grouped into tertiles), medical school affiliation (major, minor, or none), and hospital type (non-profit, for-profit, government).
Statistical analyses
We graphically depicted the 30-day readmission rate as a function of patient ADI national ranking to evaluate the unadjusted relationship between the outcome and our key explanatory variable. The cohort was split into two groupings on the basis of neighborhood disadvantage; patients living in the 85% least disadvantaged neighborhoods and those living in the 15% most disadvantaged neighborhoods. The threshold of 85 was selected based on existing literature that showed patients living in the most disadvantaged 15% of neighborhoods had significantly increased risk of readmission for common chronic conditions [22]. To test the stability of results, we conducted a sensitivity analysis on the choice of threshold.
For both ADI groupings of neighborhood disadvantage, we examined descriptive means and proportions to assess how the two groups differ on key baseline patient and hospital characteristics. We then used multivariable logistic regression techniques to further examine the relationship between ADI grouping and 30-day readmission rate.
All odds ratios and predicted probabilities were calculated twice; once using a generalized estimating equations approach with clustered standard errors at the hospital-level and once using a general linear model with robust standard errors [26, 27]. Since no difference was found between the results of the two models, we present the general linear model results. We calculated predicted probabilities using marginal standardization methods [28].
Finally, we performed a series of subgroup analyses on a-priori subgroups that may have a differential odds of readmission for those living in the most disadvantaged neighborhoods using established methods to test for interaction effects [29]. These groups were patient dual Medicare-Medicaid enrollment status, SNF discharge status and race. Race and dual Medicare-Medicaid enrollment were analyzed because of their association with increased likelihood of residence in a disadvantage neighborhood as previously reported [22]. Discharge to SNF status was analyzed to assess the impact of the patient’s post-discharge environment, as patients living in disadvantaged neighborhoods that are discharged to SNFs could be insulated from any adverse effects of their residential environment. Analyses were conducted using SAS version 9.4 (SAS Institute) and StataSE 15 (StataCorp).
IRB approval
This study has been approved by the University of Wisconsin Health Sciences Institutional Review Board.
Results
Cohort creation and characteristics
Our final cohort included 19,490 unique Medicare-insured patients with a CDI-related index stay discharged from 2855 unique health care facilities (see Fig. 1 for detailed sample derivation). Less than 0.04% of patients in the cohort were missing age and LoS data. Patients with missing age or LoS data were grouped into the 65 years and above age category or the fewer than 3 days LoS category, respectively. From our cohort, 22% were readmitted within 30 days (unadjusted). Patients living in the most disadvantaged 15% of neighborhoods were readmitted at an observed rate of 26%, while patients living in the least disadvantaged 85% were readmitted at an observed rate of 21%. Figure 2 depicts the observed readmission rate over the range of ADI national percentiles.
Fig. 2.
Unadjusted readmission rate as a function of patient neighborhood ADI. The dotted line shows the unadjusted readmission rate over ever five percentiles
Descriptively, the most disadvantaged 15% of neighborhoods had higher rates of dual Medicare-Medicaid enrollment, of patients younger than 65 years, and of patients of black race relative to the least disadvantaged 85% of neighborhoods (Table 1). This patient population also had higher rates of nearly all comorbidities, including chronic conditions such as diabetes and hypertension.
Table 1.
Characteristics of Medicare patients with a CDI-related stay by ADI national percentile
| Variable | ADI National percentile < 85 (n = 17,094) | ADI National percentile ≥ 85 (n = 2396) |
|---|---|---|
| Patients | ||
| Age | ||
| Mean Age at Discharge (SD), y | 75.64 (12.83) | 71.79 (13.90) |
| 18–65 y | 16% | 26% |
| 65+ y | 84% | 74% |
| Sex | ||
| Male | 39% | 39% |
| Female | 61% | 61% |
| Race | ||
| White | 83% | 58% |
| Black | 10% | 28% |
| Other/Unknown | 8% | 14% |
| Medicaid Enrollment | ||
| Not Medicaid Enrolled | 74% | 51% |
| Medicaid Enrolled | 26% | 49% |
| Disability | ||
| Not disabled | 72% | 57% |
| Disabled | 28% | 43% |
| Patient RUCA | ||
| Urban core | 73% | 65% |
| Suburban | 9% | 6% |
| Large rural | 10% | 14% |
| Small rural | 8% | 15% |
| Elixhauser Comorbidities | ||
| Hypertension | 79% | 84% |
| Fluid and electrolyte disorders | 59% | 65% |
| Deficiency anemia | 52% | 58% |
| Diabetes (without chronic complications) | 37% | 46% |
| Renal failure | 36% | 42% |
| Chronic pulmonary disease | 34% | 42% |
| Congestive heart failure | 30% | 36% |
| Depression | 26% | 29% |
| Other neurological conditions | 25% | 28% |
| Hypothyroidism | 25% | 23% |
| Peripheral vascular disease | 22% | 26% |
| Weight loss | 20% | 25% |
| Obesity | 17% | 21% |
| Diabetes (with chronic complications) | 17% | 24% |
| Valvular disease | 15% | 14% |
| Metastatic cancer | 5% | 4% |
| Alcohol abuse | 4% | 6% |
| Drug abuse | 4% | 6% |
| Chronic blood loss anemia | 4% | 5% |
| Lymphoma | 3% | 3% |
| Acquired immune deficiency syndrome | 1% | 2% |
| Pulmonary circulation disease | 10% | 11% |
| Rheumatoid arthritis/collagen vascular disease | 8% | 9% |
| Paralysis | 8% | 10% |
| Liver disease | 7% | 9% |
| Solid tumor without metastasis | 14% | 11% |
| Psychoses | 11% | 14% |
| Coagulopathy | 14% | 15% |
| Index Stay | ||
| Length of Stay | ||
| Mean Hospital length of stay (SD), days | 9.68 (9.60) | 10.38 (11.80) |
| ≤ 2 days | 8% | 7% |
| 3–4 days | 21% | 18% |
| 5–6 days | 18% | 18% |
| 7+ days | 53% | 56% |
| SNF Discharge | ||
| Discharged to SNF | 37% | 33% |
| Not discharged to SNF | 63% | 67% |
| Index Hospital | ||
| Medical School Affiliation | ||
| Hospital affiliated with medical school | 50% | 56% |
| Minor medical school affiliated | 27% | 30% |
| Major medical school affiliated | 23% | 26% |
| Hospital Type | ||
| Non-profit hospital | 75% | 68% |
| For profit hospital | 13% | 17% |
| Government hospital | 12% | 15% |
| Discharge | ||
| Hospital discharge volume in 2014 (SD) | 6463.18 (4791.26) | 6554.34 (4968.94) |
| Hospital discharge volume: lowest tertile | 8% | 10% |
| Hospital discharge volume: middle tertile | 23% | 21% |
| Hospital discharge volume: highest tertile | 69% | 69% |
| Outcome | ||
| Rate of 30-day readmission | 21% | 26% |
| Rate of 30-day death | 13% | 13% |
ADI Area Deprivation Index
LOS Length of Stay
RUCA Rural -Urban Commuting Area
SNF skilled nursing facility
Patients living in the 15% most disadvantaged neighborhoods also had higher rates of discharge from a hospital with relatively low discharge volumes. Patients living in the 85% least disadvantaged neighborhoods had a higher rate of discharge to a SNF relative to patients living in the most disadvantaged 15% of neighborhoods. These patients also had a higher rate of residence in an urban neighborhood.
Patient neighborhood disadvantage and 30-day readmission risk
Patients living in the most disadvantaged neighborhoods had a significantly increased odds of 30-day readmission compared to patients living in less disadvantaged neighborhoods (unadjusted OR = 1.32, 95% CI: [1.20, 1.45]) (Table 2). When adjusted for all covariates, living in the most disadvantaged neighborhoods was associated with a 16% increased odds of readmission relative to those from the least disadvantaged neighborhoods (OR = 1.16, 95% CI: [1.04, 1.28]). This translates into a 2.5% increase in the predicted probability of a readmission (from 21.6 to 24.1%). The effect is similar in magnitude to that of diabetes with chronic complications (OR = 1.12, 95% CI [1.01, 1.25]) and renal failure (OR = 1.19, 95% CI: [1.10, 1.29]) (see Supplementary Table 1). Results were robust to the choice of threshold: patients living in the least advantaged neighborhoods were consistently estimated to be at greater risk (Supplementary Table 2).
Table 2.
Odds of 30-day readmission for CDI patients by ADI score national percentile
| Variable | Odds ratio (95% CI) | Predicted Probability (%) (95% CI) |
|---|---|---|
| Unadjusted | ||
| ADI < 85 percentile | Reference | 21.3 (20.7, 21.9) |
| ADI ≥ 85 percentile | 1.32 (1.20, 1.45) | 26.3 (24.5, 28.1) |
| Adjusted | ||
| ADI < 85 percentile | Reference | 21.6 (21.0, 22.2) |
| ADI ≥ 85 percentile | 1.16 (1.04, 1.28) | 24.1 (22.4, 25.8) |
ADI Area Deprivation Index
Results of the subgroup analyses showed no evidence for a significantly modified effect by the beneficiary’s SNF discharge status, dual Medicare-Medicaid enrollment status, or race (Supplementary Table 3).
Discussion
We found that living in a disadvantaged neighborhood was associated with increased odds of readmission for patients with a CDI-related index hospital stay and this remained true after adjustment for patient-, stay-, and hospital-level variables. To our knowledge, this is the first study to explore the relationship between social determinants of health as measured by neighborhood disadvantage and risk of readmission for patients with an index CDI-related hospitalization.
Our analyses found that both neighborhood disadvantage and dual Medicare-Medicaid enrollment (a proxy for low-income individuals and common indicator of social risk) were significant predictors of readmission. This suggests ADI captures a dimension of socioeconomic disadvantage that dual Medicare-Medicaid enrollment status and potentially other individual social risk factors cannot. This ability may be driven in part by the impact of neighborhood disadvantage on a patient’s ability to follow post-discharge care and the success probability of that care.
Our findings have implications for clinicians, infection preventionists, and healthcare institutions. For clinicians, the approaches to mitigating risk to CDI patients living in disadvantaged neighborhoods may need to vary from those applied to similar patients with chronic conditions. For example, follow-ups with primary care, potentially using telemedicine resources, might need to be conducted sooner compared to other conditions. Such approaches may need to be conducted in addition to those targeting the impact of neighborhood disadvantage across all conditions. From the infection prevention perspective, placing CDI patients in contact precautions and promoting enhanced hand hygiene practices by healthcare workers is variably effective in reducing transmission, in part because of challenges in high fidelity implementation and breaches in prevention practices. Therefore, preventing readmissions as an upstream intervention is key. Additionally, unlike many chronic conditions, readmission of patients with a contagious disease such as CDI has implications not just for individual patients but for all hospitalized patients including others at risk for readmission. Asymptomatic colonized patients have been shown to contribute significantly to the overall burden of CDI in healthcare institutions, emphasizing the need to prevent unnecessary readmissions [30]. Preventing readmissions for all patients is also important as a marker for quality and because of the financial implications related to increased rates of readmission. The rates of readmission in this study of Medicare enrollees with an index stay of CDI exceeds the rate of readmission of 17% found in the general Medicare population [31]. We also found that the length of stay in our study was approximately 3 days longer than that found in the general Medicare population [32]. As the US healthcare system moves to value-based purchasing with a reduced likelihood that payers will cover costs of readmissions, and the financial penalties to healthcare institutions for CDI, it is important to understand factors that increase readmission risk. The ability to identify specific patients with increased risk for readmission could be a valuable tool to allocate resources such as transitional care programs, intensive case management, and social work to those patients.
Our findings are supported by other studies of readmissions in CDI that report rates of 25–30% readmission and prolonged duration of hospitalization. In a retrospective cohort study of 385,682 initial CDI hospitalizations identified between years 2009 and 2013 in the 4 states included in the State Inpatient Database (AHRQ), 25.7% of patients required readmission; among these, 36.8% had recurrent CDI as the principal diagnosis at the time of readmission [33]. A study of data from the Healthcare Cost and Utilization Project (HCUP) saw that patients with a primary or secondary diagnosis of CDI had a 30-day readmission rate of 29.1% [34].
We were not able to determine the extent to which recurrent CDI was the cause of the readmissions. Given the high recurrence rate associated with CDI, it is plausible that recurrent CDI contributed to the readmissions for at least some patients. Prediction models for recurrent CDI have been developed but have had variable performance to consistently predict patients at risk for CDI [35–38]. These models have largely focused on patient level factors such as severity of CDI or comorbidities that may increase readmission risk. Our study examining the relationship between neighborhood disadvantage and readmission extends the knowledge base in this area and offers an opportunity to develop and test interventions targeting social determinants of health in the Medicare-enrolled CDI population. Most other studies of interventions designed to prevent readmissions have focused on acute myocardial infarction, pneumonia, and congestive heart failure [22, 39]. Infectious conditions (other than pneumonia) have not been included in these interventions. In the case of CDI, where symptoms may be prolonged or recurrent and the implications of readmissions extend beyond the individual patient, additional interventions like those used for chronic conditions may be useful.
To develop such interventions, further research is required to understand the precise mechanisms by which neighborhood disadvantage affects readmission risk in CDI patients. These mechanisms require additional research specifically designed to explore them since those proposed in this study remain conceptual. The actual mechanisms of increased risk, which could resemble those proposed earlier, likely differ somewhat from those affecting chronic conditions because of the infectiousness and recurrence patterns of CDI. Future research efforts should subsequently focus on developing and testing interventions to prevent readmissions of CDI patients living in disadvantaged neighborhoods such as specialized allocation of resources to improve the transition of care after hospitalization and access to follow-up in the outpatient setting. Studies should then examine the impact of these new interventions on CDI rates in healthcare institutions and how these interventions affect other healthcare-associated infections. This is especially important regarding other infections associated with high rates of recurrence, or with extensive or crucial post-discharge care procedures.
Our study has limitations. We did not have patient data on treatment factors, lab tests and vital signs to include in the analyses, any of which could explain the relationship between ADI and readmission risk. Given the lack of data on direct quality measures that may impact the risk of readmission, we could not analyze the quality of care as a marker for readmission in patients with a CDI-related index stay. Our choice of the 85th ADI national percentile as the threshold to split our cohort may have also impacted the findings from our study. However, other studies have used various methods for grouping their cohorts by ADI national percentile, often focusing on the 85th ADI national percentile and up [22, 40]. Another limitation of this study is the reliance on ICD-9 codes to indicate CDI, rather than lab data. However, ICD-9 codes have been shown to have reasonable sensitivity and specificity for indicating a diagnosis of CDI [41]. This dataset also does not allow us to identify planned readmissions. Planned readmissions (for non-CDI related purposes) could bias the effect size of any of our independent variables if planned readmissions are not uniformly distributed across our covariates; however planned readmissions in patients with CDI are not common. Focusing this study on Medicare patients may also be a limitation. However, as advanced age is considered a risk factor for CDI susceptibility, it is likely that the Medicare population well reflects the overall CDI susceptible population. Finally, similar to most other studies focusing on the Medicare population, we considered all cause readmissions and did not determine relatedness to CDI [18, 22]. A justification for this approach is that CDI may influence readmission even if it is not considered as the primary cause of it, as might occur in patients with partially resolved CDI at the time of discharge. Anorexia, dehydration, and weakness related to CDI may exacerbate other chronic comorbidities and lead to readmission. These limitations notwithstanding, this study is among the first to show that neighborhood disadvantage is associated with an increased risk of readmission in inpatients with an acute infectious transmissible condition such as CDI.
Conclusions
Residence in a disadvantaged neighborhood significantly increases the risk of readmission in patients with an index CDI-related hospital stay. The effect size of neighborhood disadvantage was similar to those of chronic conditions and individual dual Medicare-Medicaid enrollment. Interventions that target the aggravating mechanisms of neighborhood disadvantage on CDI outcomes should be considered. Programs that are designed to reduce unwanted readmissions for chronic conditions, several of which are already in place in many healthcare institutions, may also benefit patients with CDI and should be evaluated for their impact on this population.
Supplementary information
Additional file 1: Supplementary Table 1. Odds of 30-day readmission for CDI patients by patient characteristic and ADI score national percentile.
Additional file 2: Supplementary Table 2. Sensitivity analysis of risk of readmission by ADI grouping for varying threshold.
Additional file 3: Supplementary Table 3. Odds of 30-day rehospitalization by subgroup.
Acknowledgements
Not applicable.
Abbreviations
- CDI
Clostridioides difficile infection
- CMS
Centers for Medicare & Medicaid Services
- ADI
Area deprivation index
- SNF
Skilled nursing facility
Authors’ contributions
ES, NS, WRP, AJHK, OA, DS formulated the study concept and design. ES, WRP, JB, OA acquired, analyzed, and interpreted the data considered in this study. AJHK and NS obtained funding for this study. ES, WRP, JB provided administrative, technical, and/or material support. NS, WRP, AJHK, DS supervised this study. ES and NS drafted the manuscript. All authors critically revised the manuscript for important intellectual content. The author(s) read and approved the final manuscript.
Funding
This work was supported by a National Institute on Minority Health and Health Disparities Award (R01MD010243 [PI Kind]). This material is the result of work also supported with the resources and the use of facilities at the University of Wisconsin Department of Medicine Health Services and Care Research Program. Dr. Nasia Safdar was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number DP2AI144244. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
The Medicare data that support the findings of this study are available from the Centers for Medicare & Medicaid Services but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The neighborhood disadvantage datasets analyzed during the current study are available in the University of Wisconsin Madison Neighborhood Atlas, https://www.neighborhoodatlas.medicine.wisc.edu/.
Ethics approval and consent to participate
This study has been approved by the University of Wisconsin Health Sciences Institutional Review Board. Informed consent was not required as this study used only de-identified, retrospective data. Delivery of the de-identified Medicare data was approved and conducted by the University of Wisconsin under a data use agreement with the Centers for Medicare & Medicaid Services Privacy Board. The data use agreement complied with HIPAA requirements.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12879-020-05481-x.
References
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382:1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Inf Secur. 2009;58:403–410. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande A, Pant C, Olyaee M, Donskey CJ. Hospital readmissions related to Clostridium difficile infection in the United States. Am J Infect Control. 2018;46:346–347. doi: 10.1016/j.ajic.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 5.Chopra T, Neelakanta A, Dombecki C, Awali RA, Sharma S, Kaye KS, et al. Burden of Clostridium difficile infection on hospital readmissions and its potential impact under the hospital readmission reduction program. Am J Infect Control. 2015;43:314–317. doi: 10.1016/j.ajic.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell KL, Danziger LH, Johnson S. Economic Barriers in the Treatment of Clostridium difficile Infection With Oral Vancomycin. Open Forum Infect Dis. 2017;4:ofx078. doi: 10.1093/ofid/ofx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher JC, Reilly JP, Navalkele B, Downham G, Haynes K, Trivedi M. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother. 2015;59:7007–7010. doi: 10.1128/AAC.00939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt J, Bathija P. Ensuring access to quality health Care in Vulnerable Communities. Acad Med J Assoc Am Med Coll. 2018;93:1271–1275. doi: 10.1097/ACM.0000000000002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Housing and health guidelines. https://www.who.int/publications/i/item/who-housing-and-health-guidelines. Accessed 2 Jun 2020.
- 11.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 13.Franco M, Diez Roux AV, Glass TA, Caballero B, Brancati FL. Neighborhood characteristics and availability of healthy foods in Baltimore. Am J Prev Med. 2008;35:561–567. doi: 10.1016/j.amepre.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez Steele E, Baraldi LG, Louzada ML da C, Moubarac J-C, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6:e009892. doi: 10.1136/bmjopen-2015-009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. doi: 10.2105/AJPH.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddox KEJ, Reidhead M, Hu J, Kind AJH, Zaslavsky AM, Nagasako EM, et al. Adjusting for social risk factors impacts performance and penalties in the hospital readmissions reduction program. Health Serv Res. 2019;54:327–336. doi: 10.1111/1475-6773.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lantos PM, Maradiaga-Panayotti G, Barber X, Raynor E, Tucci D, Hoffman K, et al. Geographic and racial disparities in infant hearing loss. Otolaryngol Neck Surg. 2018;159:1051–1057. doi: 10.1177/0194599818803305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33:493–501. doi: 10.1177/1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible — the neighborhood atlas. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsey K, Grady JN, Horwitz LI, Li Z, Herrin J, DeBurh J, et al. Hospital-level 30-day risk-standardized readmission measure. 2016. [Google Scholar]
- 21.Centers for Medicare & Medicaid Services . CCW Technical Guidance: Getting Started with CMS Medicare Administrative Research Files. 2017. [Google Scholar]
- 22.Kind AJH, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day Rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kangovi S, Grande D. Hospital readmissions—not just a measure of quality. JAMA. 2011;306:1796–1797. doi: 10.1001/jama.2011.1562. [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18:21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 26.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 27.Prentice RL, Zhao LP. Estimating equations for parameters in means and Covariances of multivariate discrete and continuous responses. Biometrics. 1991;47:825–839. doi: 10.2307/2532642. [DOI] [PubMed] [Google Scholar]
- 28.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–970. doi: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med. 2013;369:1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Characteristics of 30-Day All-Cause Hospital Readmissions, 2010–2016 #248. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb248-Hospital-Readmissions-2010-2016.jsp. Accessed 29 Apr 2019.
- 32.U.S. Department of Health and Human Services . 2016 CMS Statistics. 2017. [Google Scholar]
- 33.Zilberberg MD, Nathanson BH, Marcella S, Hawkshead JJ, 3rd, Shorr AF. Hospital readmission with Clostridium difficile infection as a secondary diagnosis is associated with worsened outcomes and greater revenue loss relative to principal diagnosis: A retrospective cohort study. Medicine (Baltimore) 2018;97:e12212. doi: 10.1097/MD.0000000000012212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elixhauser A, Steiner C, Gould C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. Readmissions following Hospitalizations with Clostridium difficile Infections, 2009: Statistical Brief #145. [PubMed] [Google Scholar]
- 35.Reveles KR, Mortensen EM, Koeller JM, Lawson KA, Pugh MJV, Rumbellow SA, et al. Derivation and validation of a Clostridium difficile infection recurrence prediction rule in a National Cohort of veterans. Pharmacotherapy. 2018;38:349–356. doi: 10.1002/phar.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaBarbera FD, Nikiforov I, Parvathenani A, Pramil V, Gorrepati S. A prediction model for Clostridium difficile recurrence. J Community Hosp Intern Med Perspect. 2015;5:26033. doi: 10.3402/jchimp.v5.26033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med. 2014;9:418–423. doi: 10.1002/jhm.2189. [DOI] [PubMed] [Google Scholar]
- 38.Hu MY, Katchar K, Kyne L, Maroo S, Tummala S, Dreisbach V, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–1214. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 39.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 40.Jung D, Kind A, Robert S, Buckingham W, DuGoff E. Linking neighborhood context and health in community-dwelling older adults in the Medicare advantage program. J Am Geriatr Soc. 2018;66:1158–1164. doi: 10.1111/jgs.15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubberke ER, Reske KA, McDonald LC, Fraser VJ. ICD-9 codes and surveillance for Clostridium difficile–associated disease. Emerg Infect Dis. 2006;12:1576. doi: 10.3201/eid1210.060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Odds of 30-day readmission for CDI patients by patient characteristic and ADI score national percentile.
Additional file 2: Supplementary Table 2. Sensitivity analysis of risk of readmission by ADI grouping for varying threshold.
Additional file 3: Supplementary Table 3. Odds of 30-day rehospitalization by subgroup.
Data Availability Statement
The Medicare data that support the findings of this study are available from the Centers for Medicare & Medicaid Services but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The neighborhood disadvantage datasets analyzed during the current study are available in the University of Wisconsin Madison Neighborhood Atlas, https://www.neighborhoodatlas.medicine.wisc.edu/.